Abstract
Different populations of immune cells rely on their distinct migration patterns for immunosurveillance, immune regulation, tissue specific differentiation, and maturation. It is important to clarify whether cells are recirculating or tissue resident, or whether tissue-specific cells are derived from blood-borne precursors or a tissue-resident population. Though migration or tissue residency of immune cells critically depends on expression of different homing molecules (chemokine receptors, tissue retention molecules etc.), characterization solely based on expression of homing molecules may not faithfully reflect migration patterns of immune cells. Therefore, a more reliable method to clarify migration patterns of immune cells is required. Parabiosis is a surgical connection of two mice, resulting in a shared circulatory system, allowing reliable distinction of tissue resident, and circulating cells. Here, we describe a protocol of parabiosis including technique details, pitfalls, and suggestions for optimization and troubleshooting.
Keywords: Parabiosis, Resident, Recirculating, Immune cells
INTRODUCTION:
The immune system plays a pivotal role in protecting the host against a universe of pathogenic microbes. Central to this role of the immune system is its ability to mobilize or distribute immune cells in different tissues to effectively respond to an invading pathogen or malignant self. Different populations of immune cells are present within blood, lymph, primary lymphoid tissues (such as thymus and bone marrow), secondary lymphoid organs (SLOs) and nonlymphoid tissues (NLTs) for immunosurveillance, maintenance, and differentiation [1,2]. Based on their migration patterns, immune cells are characterized as either recirculating or tissue resident cells[2,3]. For instance, effector memory T cells (Tem) recirculate among nonlymphoid tissues, lymph, lymph nodes, and blood, while central memory T cells (Tcm) recirculate among blood, secondary lymphoid organs, and lymph[1,2]. In contrast, tissue resident memory T cells (Trm) do not enter the circulation but are rather confined to single tissues[4,5]. Due to their unique trafficking patterns, these cells contribute differently to immune responses. Tem cells provide a quick, but not sustained, response at the time of pathogen reentry, while Tcm cells sustain the recall response through proliferation and differentiation into effector cells in SLOs[2,6]. Trm cells are predominantly found in mucosal sites, providing the most immediate response and are highly protective during localized infections[3,4]. Beside memory T cells, invariant natural killer T (iNKT) cells are also resident in various tissue sites and show immediate response upon antigen encounter[3,7,8]. In addition to mature T cells that integrate their migrations and functions, tissue-resident macrophages and monocyte-derived macrophages play distinct roles in their local microenvironment[9]. Tissue-resident macrophages originate from the yolk sac and fetal liver populations during development and persist in tissues via self-renewal[9,10]. So, they do not leave or reenter tissue, and are characterized as tissue resident. Monocyte-derived macrophages arise from recirculating monocytes that are recruited into tissues from blood[9]. It has been suggested that tissue-resident macrophages facilitate tissue homeostasis, and monocyte-derived macrophages usually exhibit a more robust inflammatory response during tissue injury[9,10]. Moreover, a recent study using parabiosis in mice that were exposed to diverse microbes shows that the majority of CD45+ leukocytes in various NLTs are tissue resident, suggesting tissue residency may be a widely shared feature in multiple innate and adaptive immune cell types [11].
Considering the drastically different functional properties of recirculating and tissue resident cells, it is critical to accurately distinguish the trafficking patterns of cells. However, multiple barriers impede our understanding of their trafficking patterns. Organs are complex structures, containing blood and lymphatic vessels – some being heavily vascularized, such as liver and lung. This complicates interpretation due to circulating, vascular-associated cells being included in cell populations isolated from tissues. Perfusion to remove blood-borne cells does not adequately prevent this – while this procedure efficiently flushes red blood cells from tissues, leucocytes can become trapped in small capillaries within tissues, as would be expected from the much greater size of leukocytes than red blood cells[4,12,13]. So, leukocytes navigate much slower than red blood cells, and perfusion is not effective at removing them from vascular compartments[14,15]. In vivo intravascular staining accurately distinguishes leukocytes in vascular compartments from those in the tissue parenchyma, abrogating the need for perfusion[12,13]. However, tissue resident cells are defined by their migration properties, therefore, stringent evaluations of in vivo recirculation of cells are required. These include photoconversion, tissue transplantation, parabiosis surgery, etc.[16–21] Such an approach is needed when first addressing the tissue residency of cells and validating markers for tissue resident cells.
Parabiosis surgery involves anastomosis of two mice together along the flank skin[22,23]. In this way, over the next few days after surgery, microvasculature is formed at the connection between two mice. The circulatory systems of the mice will fuse and allow redistribution of circulating cells between the parabiotic pair. This is a classical technique used to assess whether certain lymphocyte subsets and/or lymphocytes in certain tissue locations exchange within the circulating pool of lymphocytes[11,19–21,24,25]. For instance, by allowing the parabiotic pair to remain linked for several weeks (usually 4 weeks), one can assess whether cells in certain locations act as long-term tissue resident cells[11,19–21,24,25]. In addition, the surgery is reversible allowing for studies in which mice are conjoined, circulating (but not resident) cells allowed to equilibrate and then the animals are separated prior to extended maintenance and/or other immunological challenges. Here, we describe a detailed protocol for parabiosis in mice that we have used in the lab to investigate the induction, maintenance, and function of tissue resident immune cells (Fig. 1).
Fig. 1. Schematic overview of potential use of parabiosis approaches in immunology research on mice.
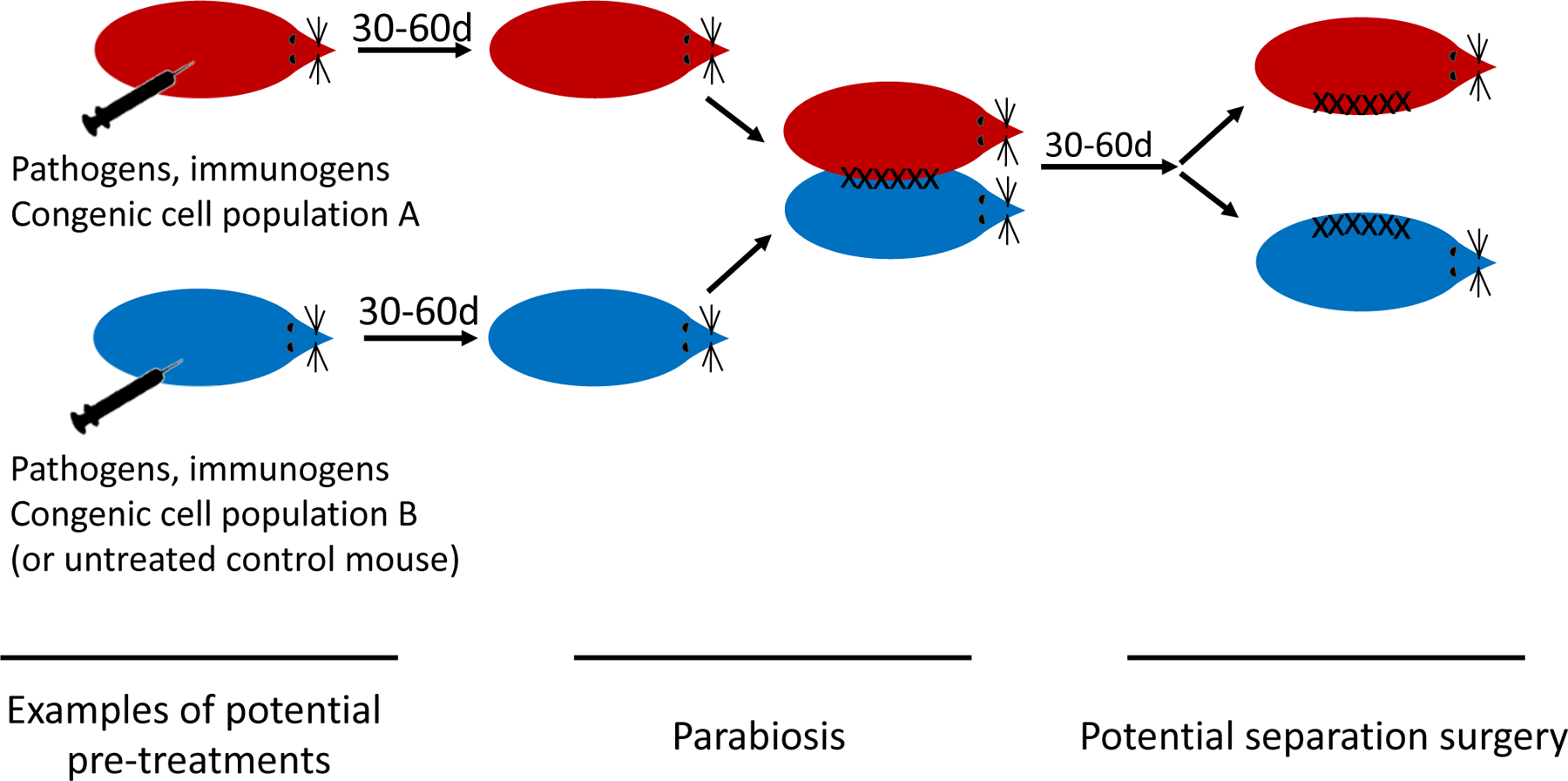
Depending on goals, an immune response may be induced in one or both of the two mice, e.g. by infection or immunization. Alternatively, or additionally, immune cell populations may be transferred into one or both mice. Often these cell populations (indicated as “A” and “B”) will differ by congenic markers used to distinguish them later by flow cytometry and/or immunofluorescence. Parabiosis entails surgical conjoining of two mice, which leads to a shared circulation over a few weeks. In some studies, parabiosis is reversed by separation surgery.
BASIC PROTOCOL 1
Basic protocol title:
Preparation of mice prior to surgery
Introductory paragraph:
After parabiosis surgery, a pair of mice is connected throughout the entire experiment before separation. This causes additional stress to mice, increases the chances of fighting or bullying, which may cause laceration of surgical sites or disruption of feeding. Therefore, it is critical to ensure that the pairs of mice are of similar age and weight and can maintain a harmonious cohabitation before surgery.
Materials:
Mice: e.g. 2 month old C57BL/6J (B6) females from Jackson Laboratories (strain #000664) or from Charles River (strain # 027). See comments under Step 1.
Diet gel (DietGel® Recovery, ClearH2O, 72-06-5022)
35mm petri dish (35mm x 10mm Petri Dish, Sterile, Celltreat, 229638)
Optional: Baytril (enrofloxacin) (Midwest Veterinary Supply, #6068906670)
Protocol steps with step annotations:
-
Select mice from the same genetic background (e.g. C57BL/6J). The selected pair of mice should be of the same sex and similar age, size, and weight. Put each pair of mice to co-house in the same cage and monitor for at least one week to ensure harmonious cohabitation (put only one pair per cage). Aim to generate at least 5 pairs of mice for typical studies.
It is very important that the pair of mice are of similar weight: paired mice should be within a 10% difference in weight (i.e. +/− 2g for a 20g mouse) if possible, although parabiosis has been successful even with a 25% difference in weight, in our hands. Female mice are preferred due to their less aggressive behavior. In some cases, these animals will differ by a congenic marker, allowing us to track migration of multiple cell types between the two individuals. In other cases, the animals will have received an adoptive transfer of TCR transgenic T cells (distinguished from both host animals by congenic markers). We will use congenic B6 strains differing by allelic markers (CD45.1/ CD45.2 or Thy1.1/Thy-1.2) – we have considerable experience with use of adoptive transfer of cells with these allelic differences and anticipate no graft-versus-host disease (GVHD) or rejection issues.
-
Introduce diet gel (in a 35mm petri dish) into the cages for each pair of mice when starting cohousing.
This helps enhance their caloric and water intake, as well as habituating them to the diet gel prior to surgery. Additionally, enriched feed (such as breeder feed) can be used instead of normal mouse chow.
Optional: one day prior to surgery, administer prophylactic antibiotics. At our facility we use Baytril (enrofloxacin) at a dose of 30 mg/kg intraperitoneally, and anecdotal evidence suggests it improves survival. We advise working with your local animal facility veterinarians to identify the appropriate antibiotic and dosing.
BASIC PROTOCOL 2
Basic protocol title:
Parabiosis surgery
Introductory paragraph:
The most critical part of parabiosis is efficient surgery to conjoin the skin of mouse pairs. In this protocol, we use surgical clips to close the skin incisions, which can be conducted more swiftly than suturing by most personnel. The wound healing of injured skins in proximity leads to angiogenesis and fusion of the skin incisions between the two mice. Therefore, the two mice connected through parabiosis surgery will share their circulatory systems in approximately 2 weeks after surgery.
Materials:
Mice (basic protocol 1)
Steri-Drapes (Fisher Scientific, NC9430913)
0.5 mL insulin syringes (EXELINT INTERNATIONAL, 26028)
Surgeon attire: hair bonnet, face mask, gown, several packs of sterile gloves (typically provided by animal research facility).
Betadine (BETADINE Surgical Scrub, 67618-151-17)
Ketamine (Akorn, VetaKet CIII, NDC: 59399-114-10)
Xylazine (Akorn, Ana-sed, NDC: 59399-110-20)
Buprenorphine (ZooPharm, Buprenorphine HCl SR, 3mg/ml)
Bupivacaine (McKesson, 707663)
Sterile Saline (Fisher Scientific, 50843139)
Ophthalmic ointment (MAJOR LubriFresh P.M., 0904-6488-38)
Sterile 4×4 Gauze Pads (Fisher Scientific, NC9676871)
Alcohol Prep Pads (McKESSON, 24-106)
Cotton swabs (McKESSON, 58-204)
Wahl Cordless Clippers (Harvard Apparatus, BS4 72-6110)
Nair depilatory cream (Nair™ Hair remover lotion. Over the counter)
Surgical blade (RAZOR BLADE CO., 27-151CTN) or scalpel.
Surgical Tools (autoclaved): 2 curved forceps (Fine Science Tool, 11063-07), 1 pair scissors (Fine Science Tool, 14060-09), 1 blunt forceps (Fine Science Tool, 11158-10) (or fine hemostat: Fine Science Tool, 13020-12).
Autoclave package (Fisher Scientific, 19160133)
9mm Autoclip Applier, Remover, Wound Clips (Fisher Scientific, 22-275998)
Refill Wound autoclips, 9mm (Fisher Scientific, 01-804-5)
Heating pad (Pure Enrichment® PureRelief™ XL (12" x 24") Electric Heating Pad), or institutionally approved method for maintain heat support (exothermic heat pads, warm water pads, etc.)
Diet gel (DietGel® Recovery, ClearH2O, 72-06-5022)
35mm petri dish (Celltreat, 229638)
Protocol steps with step annotations:
Prepare surgical area prior to surgery. Perform all surgical procedures in a clean animal surgery room. Set up equipment and tools. Sterilize surgical tools (two curved forceps, fine scissors, blunt forceps or fine hemostat) and wound clips, through autoclaving in autoclave packages. Designate an area for shaving that is not near the surgical field, an area for operation that is heated, and an area for sterile instruments. Place surgical tools on sterile surgical drapes or pads. Surgeons must scrub hands before starting the procedure and wear a hair bonnet, gown, surgical mask and sterile gloves during the entire surgical procedure. Use new gloves for each mouse.
On the day of surgery, weigh each mouse again: do not proceed with surgery if there is more than a 10% difference between mice in body weight.
-
Anesthetize animals by intraperitoneal injection of freshly prepared ketamine/xylazine to achieve the standard dose (80–120 mg/kg of ketamine, 5–10 mg/kg xylazine, prepare mix in saline).
Other anesthetic approaches can be used depending on institutional recommendations and experience of surgeon: these alternatives might include isoflurane (precision vaporizer with dual nose cones) or avertin (if permitted).
-
Assess loss of consciousness of mice by toe pinch. The loss of toe pinch reflex is expected in 1–3 minutes after injection.
If an animal fails to go under, additional ketamine (WITHOUT xylazine) can be administered at 1/3rd to 1/4th of the original dosage.
-
Apply ophthalmic ointment to each eye.
This prevents mouse eyes from drying during anesthesia period.
-
Clip the whiskers of both mice with a scissor designated for this purpose.
This avoids discomfort and aggression of parabionts due to their whiskers rubbing against the partner.
-
Shave the anesthetized mice on opposing flanks using electric clippers. Make a large surgical window, shaving skin from the hip to below the ear and from the ventral to dorsal midline.
Some procedures suggest shaving the mice a day before surgery, to shorten the time of anesthesia and surgical procedure[26], depending on institutional recommendations.
Administer sustained-release buprenorphine (2mg/kg of undiluted suspension) and bupivacaine (2mg/kg) through a single subcutaneous injection via back skin.
Administer 500μL of warmed saline subcutaneously pre-surgical procedure.
Apply Nair® to the skin of shaved area. Wait for 30 seconds and no more than 1 minute before wiping off Nair® with a sterile ethanol prep-pad.
Apply betadine to the skin of shaved area using clean cotton swab or surgical scrub, wait for 30 seconds then wipe off betadine and any residual Nair® with a sterile ethanol (70%) prep-pad. Swipe in gradually larger circular patterns from center to periphery of proposed incision site. Repeat this cycle of betadine scrub and ethanol wipes three times.
-
Make a lateral mirror-image incision through the skin (using fine scissors or blade) on the shaved flanks of the mice: on the left side of one animal, the right side of the other.
Start with a small incision in the skin, 0.3 cm caudal to the hip joint. Extend the incision, following the curvature of the spine and towards the shoulder. The final incision should run from the hip joint to ~0.5 cm caudal of the ear pinna.
-
Use blunt dissection (using blunt forceps or hemostat) to separate the skin from the fascia during the incision. This separation should be extended to ~0.5 cm on either side of the incision, achieved by pushing between the skin and body cavity wall with blunt forceps or hemostat.
In order to separate the skin from the fascia, insert the tip of the blunt forceps or hemostat into the incision in the closed position and open the tip of the forceps/hemostat carefully. A second pair of forceps can be used to hold the skin during separation.
Position the two mice, ensuring that the heads, shoulders, and hips are aligned. Beginning at the middle point of the incisions, join the dorsal skin flaps of the mice with two curved forceps. When bringing the skin together, combine the subcutaneous layers so they are flush with the epidermis facing outwards. Using several surgical clips, secure the skin together along the length of the incision, working equally towards head and tail. Surgical clips should be spaced evenly, side-by-side without overlap or major gaps. Maintain consistent pressure when applying each clip. Clips should bite into the skin and remain stable but should not bunch or create pallor in the skin. If the latter is noted the clips are too tight and may lead to poor healing due to loss of blood supply and tissue damage.
-
Repeat for ventral skin flaps. Apply additional surgical clips to completely close the wound. As necessary, add extra clips near the forelimbs and hindlimbs to provide extra strength to securely hold the mice together and to prevent wound dehiscence.
It has been shown in previous studies that the peritoneal walls of the mice can be joined at this point[27,28]. We do not perform this procedure to minimize invasiveness of the surgery, and the effective blood exchange can still be established.
At this point, some protocols recommend suturing the leg joints between the parabiotic pairs – i.e. suturing the elbows (using olecranon bone) and knee (stifle joint) between the mice[26]. This may restrict the mobility and therefore the feeding of mice after surgery, and we currently do not perform this procedure.
The objective is to align and join the base of the skin flaps from each animal. Look for any remaining openings along the incision and add clips to achieve complete closure. Additional clips at the ends of the incision sites can be used to reduce strain on these high-tension locations, reducing the likelihood of wound dehiscence. Sutures could also be used, especially to reinforce the ends of the incisions near the joints. However, in our hands, using wound clips alone for closure is sufficient for typical experiments (30 days of parabiosis), and instances of dehiscence (wound opening) are uncommon. If dehiscence is observed, the parabiotic pair should be sacrificed.
Apply betadine using cotton swabs to the entire length of the surgical site after closure.
-
Administer 500 μL of sterile saline, warmed to room temperature, through subcutaneous injection.
This helps with hydration and recovery.
BASIC PROTOCOL 3
Basic protocol title:
Recovery and use of mice after parabiosis surgery
Introductory paragraph:
Another critical point of a parabiosis experiment is to ensure good post-operation recovery. The body temperature of mice will drop during and after the surgical procedure, primarily due to the effects of anesthesia and the large surgical incisions. Therefore, it is important to maintain the body temperature of mice both during the procedure as well as in the post-operative period. Also, easy access to food and water helps reduce movement and improve wound healing. This protocol describes the post-operative care procedure for parabiosis surgery.
Materials:
Heating pad (Pure Enrichment® PureRelief™ XL (12" x 24") Electric Heating Pad)
Diet gel (DietGel® Recovery, ClearH2O, 72-06-5022)
35mm petri dish (35mm x 10mm Petri Dish, Sterile, Celltreat, 229638)
CO2 chamber (Provided by Research Animal Resources)
Flow cytometer (BD Fortessa X-20, BD Fortessa X-30)
Prism 7, GraphPad (https://www.graphpad.com)
FlowJo v10, TreeStar Flowjo (https://www.flowjo.com/solutions/flowjo)
Protocol steps with step annotations:
-
Allow mice to recover on their bellies on a heating pad (use padding or paper towels beneath mice placed on a heating pad to prevent burns or overheating).
The animals are expected to show signs of recovery within 30 minutes of anesthetic.
-
Put the animal into a cage (no bedding) to finish recovery once mice start moving.
Place the cage with only half of it on a heating pad so the animals can move off the heat area to prevent dehydration or overheating. Conventional bedding (such as corncob) can cause irritation of the wound site – use of a cellulose-based bedding such as cellunest is recommended. Nesting and enrichment additions can be introduced, and normal bedding is reintroduced 1-week post-surgery.
After recovery, place only one pair of mice per cage and maintain in appropriate housing.
-
To minimize the need for movement and pressure on the wound, continue feeding mice with diet gel for the first week or two following surgery. Alternatively, give the animals mouse chow pellets that have been softened with a water and placed in petri dishes at the base of the cage.
Uneaten food needs to be removed every 2 days. Diet gel that enhances the caloric and water intake can be provided as a supplement to normal chow pellets.
The mice need to be checked every 24 hours for at least 1 week following surgery, ensuring that both animals are active, mobile and do not show signs of separation along the surgical site. Institutional requirements may vary but monitoring twice per day for the first 3 days after surgery is sometimes necessary. If one or both animals in the pair show signs of distress, lethargy or inattention, veterinarian input may be needed to determine whether animals should be euthanized.
Some institutions recommend removal of wound clips at a defined time point following surgery (e.g. at 14 days), but others permit the clips to remain in place in order to minimize skin stretching or twisting. Wound clips may spontaneously fall out over the first few weeks following surgery and do not need to be replaced.
-
Bleed the mice at 12- or 14-days post-surgery to test blood sharing (by assessing equilibrium of congenic markers on blood lymphocytes using flow cytometry).
To do this, briefly anesthetize the mice using isoflurane (using an induction chamber, precision vaporizer or drop jar with a divider between the mice and isoflurane-soaked cotton balls, depending on institutional recommendations). Bleed via the facial vein or retroorbital route. Apply anesthetic ophthalmic ointment (Proparacaine or equivalent) prior to the blood draw.
Mice may proceed to reversal surgery (next Protocol) or additional immunization steps, per experimental design. Animals are euthanized (CO2 asphyxiation) at the desired time points to collect tissues for analysis by flow cytometry, immunohistochemistry and/or functional assays. Typical experiments entail analysis of mice at 30–60 days following parabiosis surgery.
BASIC PROTOCOL 4
Basic protocol title:
Reversal of parabiotic surgery
Introductory paragraph:
For the purposes of some experiments, animals will be subjected to a second survival surgery in which the parabiotic mice are disjoined. These studies are necessary for testing whether cells that exchange between the parabiotic pairs are functionally distinct from cells that do not exchange between the animals.
Materials:
Steri-Drapes (Fisher Scientific, NC9430913)
Ketamine (Akorn, VetaKet CIII, NDC: 59399-114-10)
Xylazine (Akorn, Ana-sed, NDC: 59399-110-20)
Buprenorphine (ZooPharm, Buprenorphine HCl SR, 3mg/ml)
Bupivacaine (McKesson, 707663)
Ophthalmic ointment (MAJOR LubriFresh P.M., catalog number: 0904-6488-38)
Sterile 4×4 Gauze Pads (Fisher Scientific, NC9676871)
Alcohol Prep Pads (Fisher Scientific, 06-669-62)
Wound Clip Remover (Fisher Scientific, 01-804-15)
Heating pad (Pure Enrichment® PureRelief™ XL (12" x 24") Electric Heating Pad), or institutionally approved method for maintain heat support (exothermic heat pads, warm water pads, etc.)
Surgical Tools (autoclaved): 2 curved forceps (Fine Science Tool, 11063-07), 1 pair scissors (Fine Science Tool, 14060-09), 1 blunt forceps (Fine Science Tool, 11158-10) (or fine hemostat: Fine Science Tool, 13020-12).
Autoclip Wound Closing System: Autoclip Applier, Remover, Wound Clips (Fisher Scientific, 22-275998)
9mm Refill Wound autoclips (Fisher Scientific, 01-804-5)
Protocol steps with step annotations:
Prepare surgical area prior to surgery. Perform all surgical procedures in a clean animal surgery room. Set up equipment and tools. Sterilize surgical tools (two curved forceps, fine scissors, blunt forceps or fine hemostat) and wound clips, through autoclaving in autoclave packages. Designate an area for shaving that is not near the surgical field, an area for operation that is heated, and an area for sterile instruments. Place surgical tools on sterile surgical drapes or pads. Surgeons must scrub hands before starting the procedure and wear a hair bonnet, gown, surgical mask, and sterile gloves during the entire surgical procedure. Use new gloves for each mouse pair.
-
Anesthetize animals by intraperitoneal injection of freshly prepared ketamine/xylazine achieve the standard dose (80–120 mg/kg of ketamine, 5–10 mg/kg xylazine, prepare mix in saline).
Other anesthetic approaches can be used depending on institutional recommendations and experience of surgeon: these alternatives might include isoflurane (precision vaporizer with dual nose cones) or avertin (if permitted).
-
Assess loss of consciousness of mice by toe pinch. The loss of toe pinch reflex is expected in 1–3 minutes after injection.
If an animal fails to go under, additional ketamine (WITHOUT xylazine) can be administered at 1/3rd to 1/4th of the original dosage.
-
Apply ophthalmic ointment to each eye.
This prevents mouse eyes from drying during anesthesia period.
Remove any remaining surgical clips using a wound clip remover.
-
Cut the surgery incision of parabiotic pair. Close the skin incision of each mouse using surgical clips so that the pair is no longer connected.
There will be a need to sever some blood vessels (those that had joined between the parabiotic pairs), but these will be relatively small capillary beds, and no special procedure is needed to close these vessels following surgery.
House the same pair of parabiotic mice together after separation for the duration of experiment.
Recovery and post operation care are similar to those described in basic protocol 3.
BASIC PROTOCOL 5
Basic protocol title:
Analysis of parabionts
REAGENTS AND SOLUTIONS:
Ketamine/Xylazine mix: 100 mg/kg of ketamine, 10 mg/kg xylazine, prepare mix in saline
Saline: Sterile Phosphate Buffered Saline (Corning, 21-040-CV)
Antibodies for flow cytometry:
Below is a representative list of antibodies we have used previously [11] to identify the following major subsets of immune cells: B cells: CD45+ CD19+ B220+; CD4 T cells: CD45+ TCRβ+ CD4+; CD8 T cells: CD45+ TCRβ+ CD8α+; Dendritic cells: CD45+ CD11chigh, MHC-IIhigh; Neutrophils: CD45+ CD11b+ Ly6G+ SiglecF−; Eosinophils: CD45+ CD11b+ Ly6G− SiglecF+; Macrophages: CD45+ F4/80+ Mertk+. Also included are antibodies to Thy-1 alleles, which may be used to distinguish congenic T cell populations (e.g., in the context of adoptive transfer studies, endogenous and transferred T cells can be distinguished by differing Thy-1 genotypes, i.e. Thy-1.1/1.1, Thy-1.1/1.2 and Thy1.2/1.2).
anti-CD4 (BD Biosciences, 563331)
anti-CD8α (BD Biosciences, 563786)
anti-TCRβ (BD Biosciences, 563221)
anti-CD45.1 (BioLegend, 110738)
anti-CD45.2 (eBioscience, 11-0454-81)
anti-CD45 (eBioscience, 25-0451-82)
anti-B220 (BioLegend, 103244)
anti-CD19 (TONBO Biosciences, 60-0193-U100)
anti-CD11c (eBioscience, 47-0114-82)
anti-CD11b (eBioscience, 47-0112-82)
anti‑F4/80 (eBioscience, 47-4801-82)
anti-MerTK (BioLegend, 151504)
anti-SiglecF (BD Biosciences, 740280)
anti-Ly6G (Biolegend, 127610)
anti-MHC-II (Biolegend, 107653)
anti-Thy-1.1 (BD Biosciences, 554897)
anti-Thy-1.2 (Biolegend, 140319)
Other cell surface and intracellular antibodies will be needed to identify additional cell types, subsets and cell activation states – these will vary depending on experimental goals.
Tissue isolation techniques have been reviewed elsewhere [12,13]. Intravenous administration of labeled antibodies [12,13] is critical for labeling of cells that are exposed to the vasculature (in most tissues, these are blood contaminants). While various antibodies can be used for this purpose, antibodies to CD45 (or CD45 alleles) are especially useful since this molecule is expressed on most hematopoietic cells. Antibodies used for intravenous labeling should be conjugated to large fluorochromes (e.g., PE, APC) and animals sacrificed (by CO2 inhalation or other IACUC-approved methods) within 3 minutes, to avoid rapid tissue penetration of the “i.v.” antibody.
COMMENTARY:
Background Information:
Parabiosis, means “alongside life”, is surgical anastomosis of two living organisms (in this case, pairs of mice) which results in a shared vascular system. This procedure was pioneered in 1860s by Paul Bert in white albino rats to study organ transplantation[23]. It has now become a classical method widely used in physiology, transplantation, stem cell biology and immunology research, and has contributed to breakthroughs in these fields. Though it was initially described over 150 years ago, the surgical procedure has been similar throughout time. It is performed by making skin incisions extending along the adjacent flanks of a pair of mice and connecting the skin flaps between the mice. Previous protocols mostly use sutures. Moreover, in some studies, the adjoining peritoneum is also incised and sutured together between the mice to form a shared peritoneal cavity. In the protocol described here, we use surgical clips applied by staples, instead of sutures, to close and connect skin incisions between mice, and it does not involve suturing of peritoneum.
Surgical sutures are useful to close incisions and wound cuts. One principal advantage of sutures is their flexibility compared to surgical clips. However, in the case of parabiosis surgery, in which parabiotic mice are in constant movement, the flexible nature of sutures makes it easier for connected mice to pull away from each other. The rigid surgical clips can hold the parabiotic mice together more tightly. Also, though dependent on an individual surgeon’s experience level, sutures are generally more time consuming compared to applying surgical clips. Prolonged operative time is associated with increased risk of adverse events and complications[29], which we also observed in our parabiosis surgeries. Continuous sutures tend to be faster compared to interrupted sutures, particularly for the long incisions made for parabiosis surgery. However, the wound is at greater risk of dehiscence if the suture breaks in the case of continuous sutures. Moreover, sutures may even cause ischemia of the skin flaps at incision sites, which hinders wound healing[30]. This may also occur with overly tightened clips, however, hence the need for appropriate training of surgical personnel. Taken together, we prefer using surgical clips in parabiosis surgery.
In some studies, short incisions were made through the peritoneal walls of parabiotic mice, and the openings were sutured together. We observed the blood chimerism can be effectively established by joining the skip flaps alone. To reduce operative time and minimize surgery invasiveness, we do not cut and suture peritoneum in our parabiosis surgery.
The protocol described entails parabiosis between mice of similar ages, but successful “heterochronic parabiosis” – conjoining mice of different ages, such as ~2 months and ~20 months – has been reported in several studies (reviewed in [31]). This permits analysis of dominant effects of soluble factors and circulating cells derived from young and old animals on physiology.
Critical Parameters:
Selection of mice
The pair of parabiotic mice are kept together throughout the experiment (normally more than 4 weeks), and it involves continuous exchange of blood and cells between mice. To avoid “graft-versus-host disease” or rejection issues, the parabiotic partners must be from the same genetic background. To maintain harmonious cohabitation, the pair of mice must be of similar size and body weight (≤ 10% difference) when selected for surgery, and female mice are strongly preferred. However, it has been reported that male mice could be successfully used in parabiosis experiments[32,33]. Maintaining the harmonious cohabitation is still the key for using male mice, as they were littermates and had been cohoused since weaning[32,33].
Postoperative care
Postoperative mortality is the major challenge of parabiosis experiments. It is important to follow protocol to ensure maintaining body temperature, proper pain management and hydration of mice. Heating pad should be used to help maintain body temperature during and after surgery. Administration of saline post operatively is helpful for keeping mice hydrated. Check the mice upon waking to ensure they move around the cage freely. The mice should be monitored daily for recovery characteristics including body weight, responsiveness, diet, and water feeding. According to our experience, better postoperative care could significantly improve the survival of parabiotic mice.
Troubleshooting:
Understanding Results:
Using the approach described allows discrimination of cells that recirculate freely between parabionts and those cells that are “resident” in a given tissue, defined as cells that remain associated with the animal in which they developed or differentiated. Data from a representative study are presented to illustrate typical findings (Fig. 2).
Fig. 2. Data and analysis from parabiosis study.
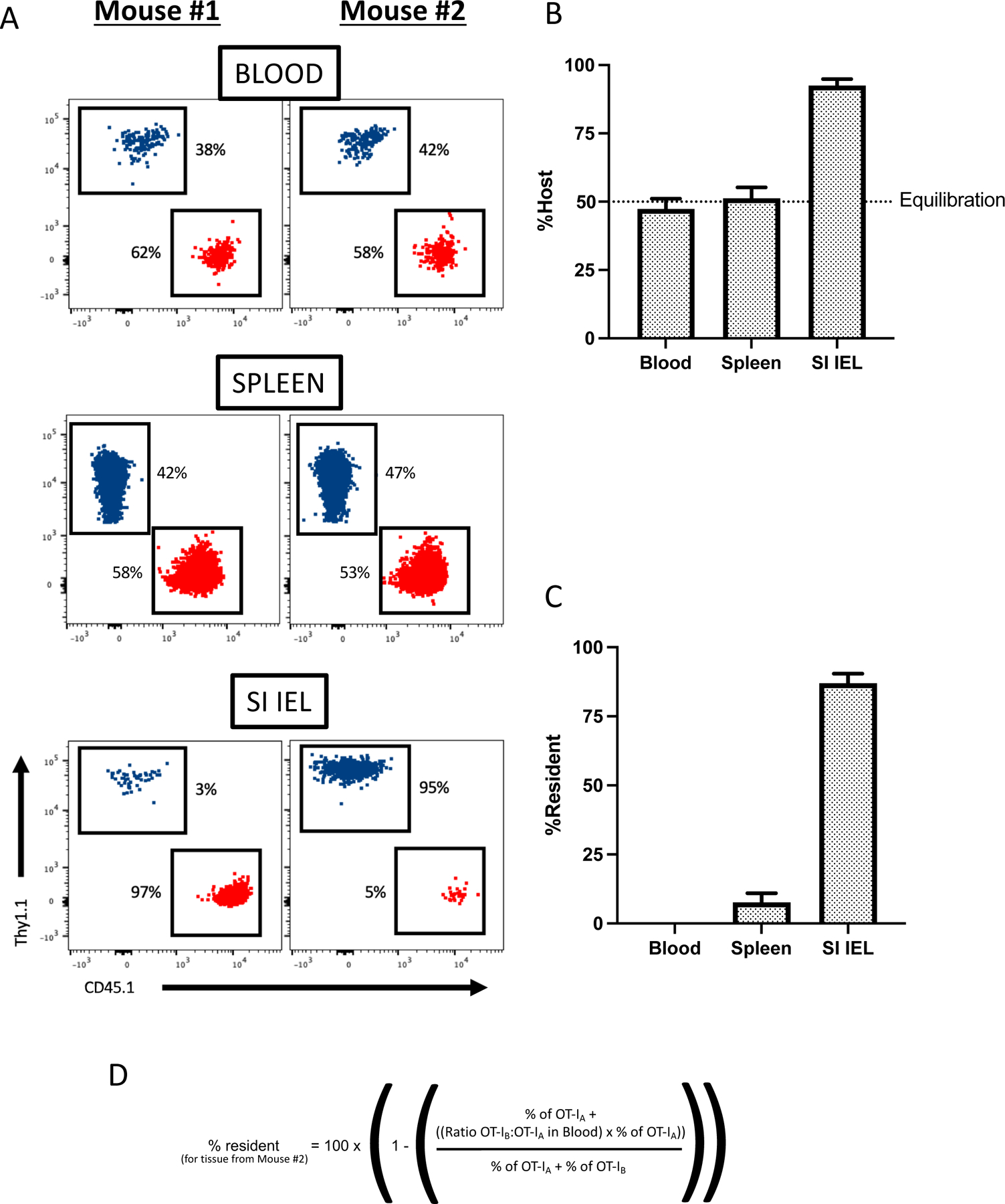
In these representative data, two C57BL/6 mice received congenically distinct populations of OT-I TCR transgenic CD8+ T cells – one mouse received Thy1.1-ve, CD45.1+ve OT-I, the other mouse Thy1.1+ve, CD45.1-ve OT-I cells (equivalent to populations “A” and “B” in Figure 1). An immune response by the donor OT-I population was induced a day later by infection with recombinant vesicular stomatitis virus containing the OVA gene (VSV-OVA). At least 30 days following infection, the mice were conjoined by parabiosis. A) shows flow cytometric data from indicated tissues (blood, spleen and small intestine intra-epithelial pool), gated on the two donor OT-I cell populations (represented as red and blue colored dots) in each mouse of the parabiotic pair (indicated as Mouse #1 and Mouse #2). The percentage of each donor population in each mouse is noted. B) shows the frequency of OT-I cells in each parabiont, represented as the frequency of cells corresponding to the original host (in this example, OT-I population “A” in Mouse #1, OT-I population “B” in Mouse #2) using compiled data from the same experiment. In C), these values are converted to “percent resident”, using the equation provided in (D).
Table 1.
Troubleshooting Guide for Parabiosis Surgery
| Problem | Possible cause | Solution |
|---|---|---|
| High mortality rate after surgery | The most common complications after surgery are dehydration, malnutrition, hypothermia, dehiscence, pain, and infections. Evidence of these conditions are noted by excessive weight loss (>5%), lethargy, unequal growth of one mouse open wounds, and possible chewing or licking at clips and incision sites. | These complications can be treated effectively if found at early phase, therefore, it is important to perform health monitoring of the mice during recovery from parabiosis surgery. If dehydration is observed, fluids should be supplemented by injecting each mouse with 500μL-1 ml of sterile body temperature saline (subcutaneous injection) twice daily for 3–7 days (volume based on severity of dehydration). If weight loss or decreasing body condition is observed, place cage on heating pad for supportive care and provide additional options for supplemental food including wet chow and diet gels. To prevent infection, house mice in autoclaved cages after surgery, provide sterile water, and the water can be supplemented with antibiotics for prophylactic treatment. Multi-modal analgesia may be considered if signs of pain are noted. In addition to buprenorphine and the local analgesic, NSAIDS such as carprofen or meloxicam may be added to the analgesic regimen. Moreover, failure to accurately match weights of mice can be a big risk factor. The weight difference should be less than 10% difference in weight at the beginning of cohousing. The mice should be weighed again before the surgery. The weight difference should still be less than 10% difference in weight. |
| Poor induction of blood sharing | The common cause of poor blood sharing is poor wound healing and low angiogenesis due to inappropriate close of incisions. | While training on closure techniques is imperative prior to starting planned surgery, new surgeons may benefit from a surgical assistant at the time of closure. One person can hold the skin flaps from the dorsal sides of both mice up and together using two curved end forceps. In the meanwhile, another person can apply clips to close incisions. To achieve better angiogenesis, it is very important to have the outer edge of skin flaps exposed and face to the clips at this step. It is important to prevent the skin or remaining hair from clipping into the body during this procedure. Make sure the inside of the skin flaps is in touch when applying clips. It is also helpful to pull skins up when holding the margins together for clipping, this can improve clip placement and avoid inclusion of deeper tissue structures. |
| Lack of mobility of forelimb and/or hindlimb | This is usually due to inappropriate clipping of tissues (e.g., muscle). | Take off clips that impinge forelimb or hindlimb, reapply new clips. In cases where clips continue to impede movement, tension relieving sutures may be applied. |
Time Considerations:
Preparation of mice prior to surgery:
1 week.
Parabiosis surgery:
30 minutes to prepare surgical area, 30 minutes for surgery of each pair.
Recovery and use of mice after surgery:
allow at least 1 hour for mice to recover. Blood chimerism is established around 2 weeks post-surgery. The parabiotic mice usually kept for at least 4 weeks before euthanasia for experiments.
Reversal of parabiotic surgery:
30 minutes to prepare surgical area, 20–30 minutes for reversal surgery of each pair.
ACKNOWLEDGEMENTS:
This article was supported by NIH award AI38903 (SCJ).
Footnotes
CONFLICT OF INTEREST STATEMENT:
The authors declare no conflict of interests.
DATA AVAILABILITY STATEMENT:
Data are available upon request.
LITERATURE CITED:
- 1.Masopust D, Schenkel JM: The integration of T cell migration, differentiation and function. Nat Rev Immunol 2013, 13:309–320. [DOI] [PubMed] [Google Scholar]
- 2.Jameson SC, Masopust D: Understanding Subset Diversity in T Cell Memory. Immunity 2018, 48:214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masopust D, Soerens AG: Tissue-Resident T Cells and Other Resident Leukocytes. Annu Rev Immunol 2019, 37:521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schenkel JM, Masopust D: Tissue-resident memory T cells. Immunity 2014, 41:886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kok L, Masopust D, Schumacher TN: The precursors of CD8. Nat Rev Immunol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jameson SC: The Naming of Memory T-Cell Subsets. Cold Spring Harb Perspect Biol 2021, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Hogquist KA: How Lipid-Specific T Cells Become Effectors: The Differentiation of iNKT Subsets. Front Immunol 2018, 9:1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Breed ER, Lee YJ, Qian LJ, Jameson SC, Hogquist KA: Myeloid cells activate iNKT cells to produce IL-4 in the thymic medulla. Proc Natl Acad Sci U S A 2019, 116:22262–22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe S, Alexander M, Misharin AV, Budinger GRS: The role of macrophages in the resolution of inflammation. J Clin Invest 2019, 129:2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies LC, Jenkins SJ, Allen JE, Taylor PR: Tissue-resident macrophages. Nat Immunol 2013, 14:986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wijeyesinghe S, Beura LK, Pierson MJ, Stolley JM, Adam OA, Ruscher R, Steinert EM, Rosato PC, Vezys V, Masopust D: Expansible residence decentralizes immune homeostasis. Nature 2021, 592:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson KG, Sung H, Skon CN, Lefrancois L, Deisinger A, Vezys V, Masopust D: Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol 2012, 189:2702–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, et al. : Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 2014, 9:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogg JC, Coxson HO, Brumwell ML, Beyers N, Doerschuk CM, MacNee W, Wiggs BR: Erythrocyte and polymorphonuclear cell transit time and concentration in human pulmonary capillaries. J Appl Physiol (1985) 1994, 77:1795–1800. [DOI] [PubMed] [Google Scholar]
- 15.Segel GB, Cokelet GR, Lichtman MA: The measurement of lymphocyte volume: importance of reference particle deformability and counting solution tonicity. Blood 1981, 57:894–899. [PubMed] [Google Scholar]
- 16.Schmidt TH, Bannard O, Gray EE, Cyster JG: CXCR4 promotes B cell egress from Peyer’s patches. J Exp Med 2013, 210:1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR: Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 2009, 10:524–530. [DOI] [PubMed] [Google Scholar]
- 18.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, et al. : Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med 2010, 207:553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borges da Silva H, Peng C, Wang H, Wanhainen KM, Ma C, Lopez S, Khoruts A, Zhang N, Jameson SC: Sensing of ATP via the Purinergic Receptor P2RX7 Promotes CD8. Immunity 2020, 53:158–171.e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Hogquist KA: CCR7 defines a precursor for murine iNKT cells in thymus and periphery. Elife 2018, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schenkel JM, Fraser KA, Vezys V, Masopust D: Sensing and alarm function of resident memory CD8⁺ T cells. Nat Immunol 2013, 14:509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamran P, Sereti KI, Zhao P, Ali SR, Weissman IL, Ardehali R: Parabiosis in mice: a detailed protocol. J Vis Exp 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggel A, Wyss-Coray T: A revival of parabiosis in biomedical research. Swiss Med Wkly 2014, 144:w13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iijima N, Iwasaki A: T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 2014, 346:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY: Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 2015, 350:981–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spencer CA, Leung TH: Research Techniques Made Simple: Parabiosis to Elucidate Humoral Factors in Skin Biology. J Invest Dermatol 2019, 139:1208–1213.e1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, et al. : The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477:90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, et al. : Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med 2014, 20:659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng H, Clymer JW, Po-Han Chen B, Sadeghirad B, Ferko NC, Cameron CG, Hinoul P: Prolonged operative duration is associated with complications: a systematic review and meta-analysis. J Surg Res 2018, 229:134–144. [DOI] [PubMed] [Google Scholar]
- 30.Cochetti G, Abraha I, Randolph J, Montedori A, Boni A, Arezzo A, Mazza E, Rossi De Vermandois JA, Cirocchi R, Mearini E: Surgical wound closure by staples or sutures?: Systematic review. Medicine (Baltimore) 2020, 99:e20573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conboy MJ, Conboy IM, Rando TA: Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell 2013, 12:525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumagai K, Vasanji A, Drazba JA, Butler RS, Muschler GF: Circulating cells with osteogenic potential are physiologically mobilized into the fracture healing site in the parabiotic mice model. J Orthop Res 2008, 26:165–175. [DOI] [PubMed] [Google Scholar]
- 33.Brazelton TR, Blau HM: Optimizing techniques for tracking transplanted stem cells in vivo. Stem Cells 2005, 23:1251–1265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request.


