Abstract
From a materials perspective, the pillars for the development of clinically translatable scaffold-based strategies for craniomaxillofacial (CMF) bone and periodontal regeneration have included electrospinning and 3D printing (biofabrication) technologies. Here, we offer a detailed analysis of the latest innovations in 3D (bio)printing strategies for CMF bone and periodontal regeneration and provide future directions envisioning the development of advanced 3D architectures for successful clinical translation. First, the principles of electrospinning applied to the generation of biodegradable scaffolds are discussed. Next, we present on extrusion-based 3D printing technologies with a focus on creating scaffolds with improved regenerative capacity. In addition, we offer a critical appraisal on 3D (bio)printing and multitechnology convergence to enable the reconstruction of CMF bones and periodontal tissues. As a future outlook, we highlight future directions associated with the utilization of complementary biomaterials and (bio)fabrication technologies for effective translation of personalized and functional scaffolds into the clinics.
Graphical Abstract
1. Introduction
Successful and predictable reconstruction of craniomaxillofacial (CMF) bones due to congenital malformations, trauma, infection, tumor resection and sport injuries remains a major clinical challenge due to the presence of sensory organs, high vasculature density, and differences in skeletal tissue characteristics.1 Moreover, severe damage to CMF bones can impair breathing, listening, chewing and speech abilities, along with a person’s esthetic features, which can lead to enduring psychological problems and poor self-esteem.2,3 Noteworthy, the management of CMF injuries is especially problematic as clinicians need to monitor bacterial infection in extremely vulnerable areas, such as the oral microenvironment.4
Some of the most prevalent pathologies that affect our oral health include tooth decay, gum disease (i.e., periodontitis), and oral cancer.5 Periodontitis, a chronic inflammatory disease that results in the loss of alveolar bone and other key tooth-supporting structures (gingiva, periodontal ligament, and cementum), affects more than 45% of the US adult population.6,7 It is described by the gradual destruction of periodontal tissues, which if left untreated, it may lead to tooth loss.8 Hence, the ultimate goal of periodontal tissue engineering has been not only the restoration of alveolar bone, but the simultaneous reestablishment of functionally oriented periodontal ligament (PDL) fibers firmly attached to the regenerated cementum and bone (Figure 1), thus extending the lifespan of the natural dentition.
Figure 1.
Schematic illustrations showing a longitudinal section through dento-gingival part of a tooth and tooth supporting structures between healthy and diseased periodontium. In particular, as the disease progresses, the periodontal complex (bone, gingiva, periodontal ligament [PDL], and cementum) is gradually destroyed and progress along the tooth root creating a deepening pocket.
Clinically speaking, the management of periodontitis demands a series of procedures that involves scaling and root planning, in addition to, in some instances, the systemic (oral) administration of antibiotics. Nonetheless, limited periodontal tissue regeneration results from this strategy.9 Meanwhile, the use of autologous (e.g., iliac crest) bone grafts has been largely employed for alveolar bone reconstruction; however, this approach brings major concerns regarding the need for multiple surgeries, associated morbidity, and a number of possible complications, including the infection of the operated area.10 Importantly, from a clinical standpoint, it is particularly difficult to shape and conform natural or synthetic bone grafts into the complex three-dimensional (3D) architecture of periodontal defects. Furthermore, bone grafts may only fill osseous defects and work inadequately in supporting true physiological regeneration of the multiple periodontal tissues.11 Remarkably, recent strides in tissue engineering in addition to the rapidly evolving field of biofabrication have not only improved clinical outcomes of regenerative therapies, but have also set new hopes for the effective translation of personalized and more predictable strategies for bone and periodontal regeneration.12–15 Nonetheless, over the last few decades, among the clinically available techniques, guided tissue regeneration (GTR) and guided bone regeneration (GBR) have invariably been the most practical approaches to manage and clinically treat the tissue destruction provoked by periodontitis.16,17
Guided tissue and guided bone regeneration (GTR/GBR) are defined as dental surgical procedures aimed at regenerating lost periodontal structures to change the prognosis of a tooth from “questionable” to “favorable”, and thus ameliorate oral health-related quality of life for millions of patients worldwide. Briefly, in these procedures, a barrier membrane is used to avert soft tissue migration into the bone defect while providing space maintenance, wound stabilization, and, ultimately, a sheltered niche for resident progenitor cells situated in the residual PDL, contiguous alveolar bone, or blood to recolonize the root area and differentiate into a new periodontal supporting apparatus.8,18
Classically, GTR/GBR membranes are divided into two groups: non-resorbable and resorbable. Non-resorbable membranes are mostly polytetrafluoroethylene-based (PTFE), such as high-density PTFE and titanium-reinforced high-density PTFE.19 Although non-resorbable materials are biocompatible and have led to positive clinical outcomes, they are biologically inert and exert only a physical barrier function. Moreover, their use demands a second surgery for its removal, which leads to added discomfort and increases the risk of infection. In this way, a variety of resorbable materials were developed as an alternative to non-resorbable options.16,19 Resorbable membranes can be synthesized from either natural, synthetic, or blends of synthetic and natural polymers.19,20 However, the well-known and intrinsic drawbacks in existing membranes, such as low attachment to adjacent tissues, lack of curative properties, and reduced regenerative capacity have propelled the field in the direction of resorbable and multifunctional polymeric membranes with controlled degradation rate, mechanical competence, and multiple therapeutic properties as to eradicate infection, ablate inflammation, and support tissue regeneration.
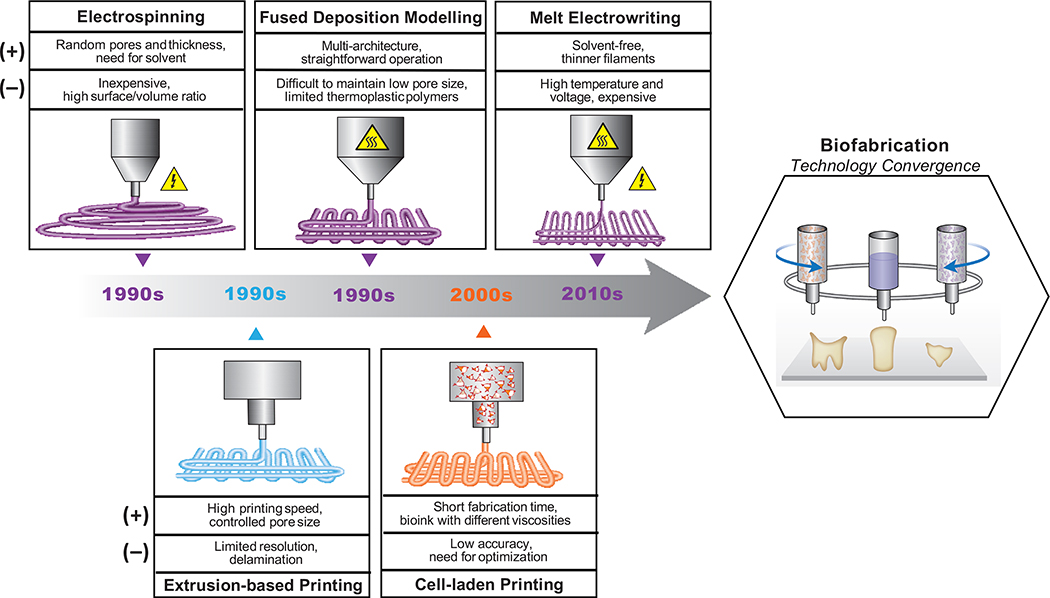
From a broad tissue engineering perspective, the pillars for the development of clinically translatable scaffold-based strategies for craniofacial bone and periodontal tissue regeneration have primarily included electrospinning and 3D printing technologies (Figure 2). In the last two decades, electrospinning has become of great importance due to its simplicity and the possibility of employing a wide range of degradable polymers in the fabrication of GTR/GBR membranes and scaffolds for periodontal regeneration.21,22 Moreover, the integration of biologically active molecules (e.g., growth factors) and therapeutic compounds,23,24 such as bioceramics and metal oxides nano/microparticles, have led to encouraging regenerative outcomes.17,25,26 Despite the tremendous advantages associated with electrospun membranes, true physiological regeneration of the periodontium still embodies one of the greatest challenges in regenerative periodontics, not only because of the varying degrees of tissue destruction produced by the disease, but more importantly due to the highly complex, 3D periodontal tissue architecture and multiplicity of tissues involved that need to be reconstructed. Accordingly, the creation of defect-specific scaffolds to recapitulate the native tissues and the carefully designed structure of the periodontium is paramount for predictable regeneration of both soft (gingiva and periodontal ligament) and hard (alveolar bone and cementum) periodontal tissues.
Figure 2.
Timeline of the development of electrospinning and extrusion-based 3D printing technologies for the generation of regenerative bone and periodontal constructs, their advantages (+) and dis-advantages (−), and outlook towards the convergence of these technologies.
3D printing, an additive manufacturing process in which a membrane or scaffold can be fabricated in a layer-by-layer fashion to obtain defect-specific and anatomically-complex constructs, has been deemed a paradigm shifting enabling technology in regenerative dentistry.11,27,28 Currently, 3D printing strategies involve designing mono- or multiphasic scaffolds presenting macro- (scaffold structure), micro- (pore size for bone zone and fiber alignment for PDL zone), and nano-scale (surface topography) features to guide the coordinated growth and regeneration of the distinct periodontal tissues.11,12,29,30 Notably, periodontal-related cell types can be 3D bioprinted, and thus distributed at specific locations of anatomically-defined scaffolds to promote the coordinated growth and simultaneous regeneration of soft and hard periodontal tissues.13,31,32
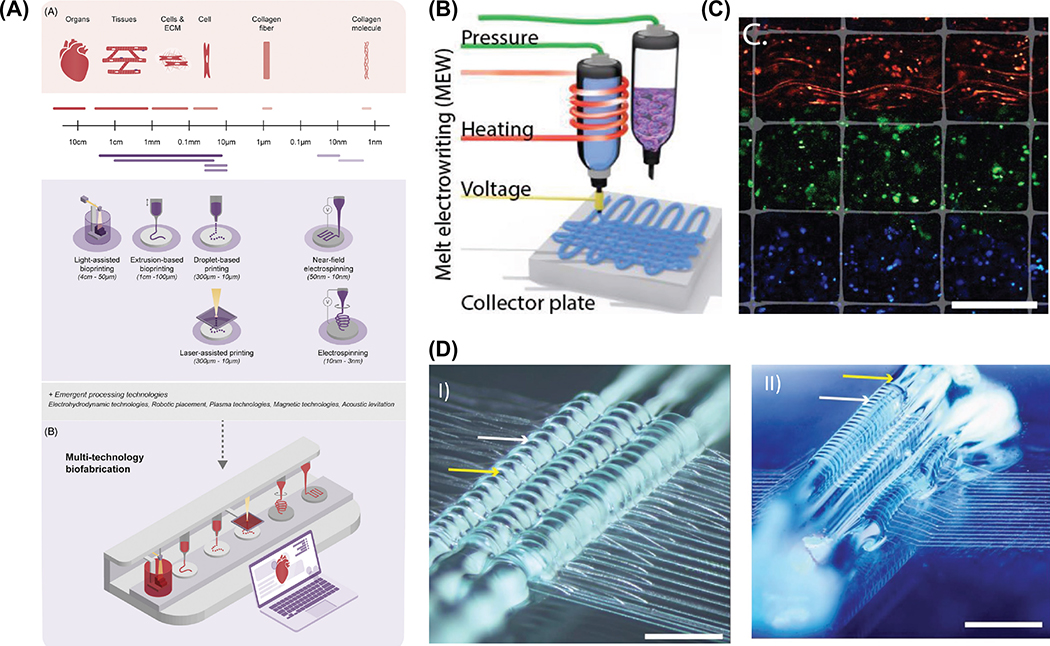
Biofabrication is a rapidly evolving field that uses 3D printing methods to engineer biologically functional and highly organized structures primarily using biomaterials and cells. In recent years, among the various tools within the biofabrication armamentarium, particularly pertaining to 3D printing and bioprinting (i.e., cell-laden scaffolds) techniques, much focus has been given to extrusion-driven processes, which even though are technologically more simple compared to other printing methods, they allow for successful processing of a wide range of cell-free or cell-laden scaffolds with significant translational potential for bone and periodontal regeneration.33–35 Succinctly put, extrusion-based printing can be classified into two major subgroups: thermal and non-thermal extrusion 3D printing. Remarkably, the latest extrusion-based technologies have also explored the benefits of coaxial and multimaterial printing to fabricate patient-specific implants mimicking complex tissue interfaces (e.g., bone-PDL, bone-cartilage, bone-ligament, etc.), which has been challenging to recapitulate using conventional scaffold fabrication methods.36,37 A promising approach in interface tissue engineering lies in the merging of electro-driven fabrication tools with extrusion-based printing into a single fabrication platform. More specifically, the combination of solution/melt electrospinning or melt electrowriting with extrusion of cell-laden hydrogels, has shown to appreciably enhance the mechanical competence of cell-laden hydrogels while enabling spatial distribution of different cell types.38,39 Overall, the combination of complementary fabrication technologies into a single platform opens up limitless and forward-thinking opportunities for engineering functional interface tissues by better resembling the mechanical and biological gradients observed in native tissue interfaces.40
In this review, we offer an analytical appraisal of the newest innovations in 3D printing and bioprinting strategies for craniofacial bone and periodontal tissue regeneration and set directions in devising highly complex 3D architectures for successful translation of personalized scaffolds for regenerative dentistry. In the first part, the principles of electrospinning applied to the generation of biodegradable nanofibrous polymeric scaffolds for use as GTR/GBR barrier membranes, as well as, tissue scaffolds for bone and periodontal tissue engineering are discussed. The second part carefully elaborates on different types of extrusion-based 3D printing technologies with a focus on how distinct biomaterial classes can be modified or functionalized to engineer scaffolds with improved regenerative capacity. This includes recent work on extrusion-based bioprinting to render cell-laden scaffolds with controlled geometry, architecture, and biological features to develop functional living tissues/organs. Next, we provide a critical assessment on the latest innovations in 3D printing and multitechnology fabrication process to enable the generation of gradient and anatomically-complex structures to allow the functional reconstruction of the alveolar bone-PDL interface and patient-specific periodontal defects. Finally, as a future outlook, we highlight upcoming directions associated with the utilization of complementary biomaterials and (bio)fabrication technologies for effective translation of personalized and functional scaffolds for predictable reconstruction of CMF bones and periodontal tissues.
2. Electrospinning
A wide range of scaffold-based tissue engineering techniques and materials have been developed for regeneration of impaired periodontal tissues. Over the last decade, researchers have focused on methods such as casting, dynamic filtration, and electrospinning to generate biodegradable polymeric membranes for periodontal tissue regeneration.16,41 Among them, electrospinning, a versatile and easy to use technique, has been widely explored to produce extracellular matrix (ECM)-mimicking nanofibrous scaffolds22 with or without the incorporation of a vast array of therapeutics for infection eradication, inflammation control, and to further boost tissue regeneration (Figure 3).
Figure 3.
Schematic representation of chemical and physical functionalization of (melt-)electrospun fibers for bone and periodontal tissue regeneration. Adapted from.42
Traditionally, the electrospinning setup has three major components, namely a syringe filled with a polymer solution and capped with a metallic needle, a high voltage supply, and a grounded collector positioned at a predetermined distance from the tip of the needle. The solution is charged by an optimized voltage while it flows through the needle and forms the so-called Taylor cone when the induced potential difference overcomes the surface tension of the polymer solution being dispensed.42,43 While traveling through the air, the polymer jet experiences instabilities and as the solvent evaporates fibers solidify and are deposited on the collector.43 Electrospinning enables the fabrication of random, aligned, or core-shell micro/nanofibers when using coaxial needle configurations. Electrospun fibers possess a high surface-to-volume ratio and allow the encapsulation and/or decoration with biomolecules,43,44 thus contributing to the generation of single (monophasic) or multilayered (multiphasic), functionally graded or not, scaffolds with therapeutic properties and tremendous impact on dental, oral, and craniofacial tissue regeneration (Table 1).16,45
Table 1.
Selected references on electrospinning for tissue engineering strategies in periodontal tissue regeneration
| Reference | Advantages/Disadvantages | Most Relevant Findings |
|---|---|---|
|
| ||
| Monophasic Electrospun Membranes | ||
| [62] | (+) biodegradable, nano-size diameter | Good osteoinductive ability as a result of the characteristics of nHA. |
| [63] | (+) various types of synthetic polymers can be used to form fibers | High potential for osteoconduction and mineralization of ECM with PLLA membranes and better osteoinductivity with pisPLLA membranes. |
| [65] | (+) readily functionalized with bioactive molecules | Increased proliferation of preosteoblasts as compared to Epiguide and membranes without BG. |
| [66] | (+) fibers can also be produced from natural polymers | Enhanced osteogenic gene expression; able to promote periodontal tissue regeneration in the furcation defect of dogs. |
| [53] | (+) biodegradable, two types of antibiotics were incorporated into nanofibers | Prolonged release of MET and CIP and significant inhibition growth of bacteria with CIP including nanofibers. |
| [54] | (−) requirement of additional step to prevent the burst release of hydrophilic drug (CIP HCl) | Controlled release of CIP from nanofibers thanks to antimicrobial oligomer developed. |
| [55] | (+) blending with gelatin improved stretching ability of membranes under wet conditions | Antibacterial activity against periodontopathogens. |
| [59] | (−) burst release depending on nHA content | Slow release of AMX, and biomineralization owing to nHA |
| [58] | (−) burst release of MX | Achieved prolonged drug release, high proliferation rate. |
| [67] | (+) aligned and random fiber production | Proliferation and osteogenic differentiation of stem cells, sustained release of DEX thanks to aligned fibers. |
| Coaxial Nano/microfibrous Electrospun Membranes | ||
| [51] | (+) sustained release of hydrophilic drug (−) complex process of coaxial fiber production |
Controlled release of TET-HCl for 75 days for periodontal regeneration. |
| [48] | (+) release behaviour might be tuned by core:shell flow ratio | The release as sustained over 4 days. |
| [72] | (+) maintaining bioactivity of GFs, (+) incorporation of different bioactive agents in core and shell |
Bone regeneration was improved by 43% compared with single systems. |
| [70] | (+) protecting sensitive GFs from toxic solvents used (−) complex process of coaxial fiber production |
Osteoconductivity and osteoinductivity can be modified due to the sustained release of factors loaded. |
| Multiphasic and Functionally Graded Electrospun Membranes | ||
| [47] | (+) straightforward production compared to coaxial electrospinning | Sustained release were achieved for 28 days and regeneration of alveolar ridge was improved. |
| [42] | (+) the multiphasic and most advanced constructs achieved by electrospinning | nHA and MET provides the osteoinductivity and antibacterial activity to the membrane for periodontal regeneration, respectively. |
2.1. Monophasic electrospun membranes with therapeutic properties
Membranes for GTR/GBR are among the most positive methods to treat the destruction caused by periodontitis; as they provide enough time for resident progenitor cells to populate the affected area while excluding epithelial/connective tissue cells from the periodontal defect. However, the regeneration of periodontal tissues might be thwarted without proper mechanisms to control and ultimately eradicate infection.16,42 With this in mind, antimicrobial-releasing electrospun fibers have been engineered as an alternative to systemically administered antibiotics to promote localized bacterial load reduction in the periodontal defect. Most of the published studies report the use of metronidazole (MET) as the medicament of choice.46–49 For instance, MET-releasing polymeric fibers were able to inhibit the growth of known periodontopathogens (e.g., Porphyromonas gingivalis).46 In addition, other researchers addressed the potential of equally important antibiotics, such as tetracyclines50–52 and ciprofloxacin53,54 and stated similar antimicrobial outcomes – attesting for the versatility of polymeric electrospun fibers as drug delivery systems for periodontal infection ablation.53
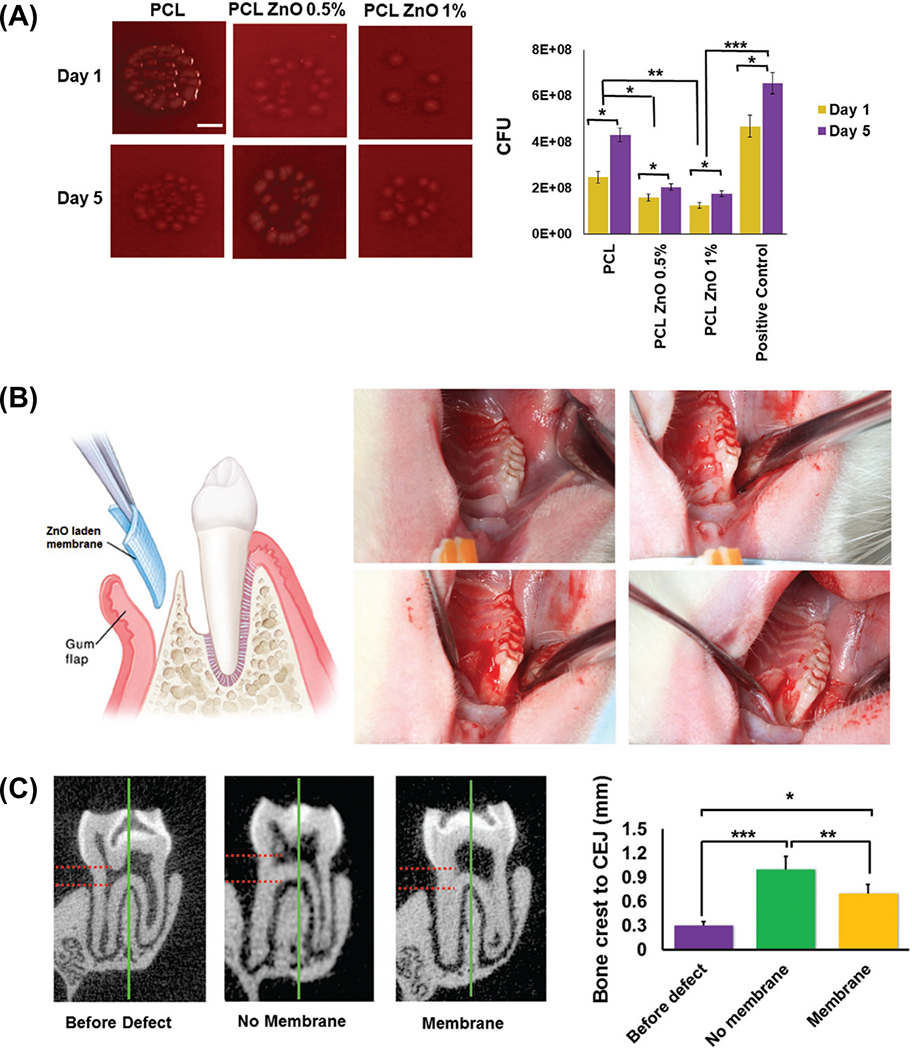
As previously highlighted, the modification of electrospun polymeric scaffolds with a wide variety of metal oxides as means to impair multifunctionality have been pursued.55 Interestingly, in a recent study, zinc oxide (ZnO)-doped poly(ε-caprolactone) nanofibers displayed not only antimicrobial activity (Figure 4a), but more importantly led to the upregulation (in vitro) of bone-related genes and reduced the size of an osseous defect in vivo (Figure 4b-c).56 Electrospinning was used to generate branched-shaped ZnO-doped PCL membranes. The unique fiber morphology, similar to a rose stem, led to antimicrobial effects against Pseudomonas aeruginosa, improved the membranes’ strength, and did not compromise epithelial cells’ attachment or viability. Moreover, the unique rose stem-like fiber morphology enhanced adhesion to soft tissue, which, in combination with bacterial inhibition, suggests that these scaffolds could be an interesting approach for regenerative strategies in CMF sites prone to infections.57 Meanwhile, alternative strategies have also focused on the combination of antimicrobial agents with bioceramics.58,59 For instance, PCL fibers loaded with hydroxyapatite (HAp) particles and amoxicillin (AMX) led to mineral precipitation, controlled drug release, and antimicrobial action.59 Similarly, to afford anti-inflammatory properties and induce bone formation, chitosan, polyvinyl alcohol, and HAp-based composite membranes containing meloxicam (MX) were also reported.58 Altogether, the aforementioned strategies prove the opportunities associated with the electrospinning technique for the development of tissue-specific scaffolds with drug delivery abilities. Nonetheless, one should bear in mind that, incorporation of antimicrobial and regenerative therapeutics often demands systematic screening on their mechanisms of action to provide the most beneficial synergistic effect.
Figure 4.
(a) Colony forming unit (CFU) qualitative and quantitative characterization of the antimicrobial properties of PCL engineered membranes containing different concentrations of ZnO nanoparticles. (b) Rat periodontal defect model depicting the surgical procedure and placement of membrane, and (c) Micro-CT analysis and semi-quantitative measurements (in mm) from the bone crest to the cemento-enamel junction (CEJ) at each time interval, before and after defect, as well as after the membrane implantation. Adapted from.56
Since the early 2000s, electrospun membranes have been developed for periodontal tissue engineering. Calcium phosphate (CaP)-based ceramics have been widely used as bioactive inorganic materials in electrospun membranes for GTR/GBR applications.17,60–64 For instance, the incorporation of nano-hydroxyapatite (nHAp) particles into PCL led to cytocompatible fibers capable to induce mineral precipitation and stimulate osteoblast-like cells to upregulate the expression of an early marker of osteogenic differentiation (alkaline phosphatase).17 Similarly, type I collagen/PCL nanofibers doped with HAp favorably supported periodontal ligament cells proliferation and upregulated osteogenic-related gene expression.62 Alternative biodegradable polymers, such as poly-L-lactic acid or poly(isosorbide succinate-co-L-lactide) have also been successfully blended with collagen and HAp leading to favorable regenerative outcomes.63 The effects associated with the use of bioactive glasses (BGs) have also been demonstrated.65,66 Composite electrospun membranes consisting of collagen, chitosan, and BG were shown to favor osteogenic gene expression, antimicrobial action, and in vivo periodontal tissue regeneration in a clinically-relevant Class II furcation defect model.66 Another study67 revealed that the incorporation of a magnesium silicate (fosterite) enhanced the therapeutic potential of dexamethasone (DEX) to stimulate osteogenic differentiation of stem cells from human exfoliated deciduous teeth.67 Concerning the well-established value of DEX as an osteogenic factor, in a recent study from our group, poly(lactic-co-glycolic acid (PLGA) electrospun nanofibers were loaded with DEX-β-cyclodextrin inclusion complex (DEX-β-CD) based on the ability of β-CD to improve the solubility and control the release of lipophilic drugs.68 PLGA/DEX-β-CD nanofibrous membranes demonstrated a sustained release of DEX and stimulated dental pulp stem cells to express important osteogenic markers with promising potential for bone tissue regeneration.68
An interesting approach was recently reported using gelatin methacryloyl hydrogel (GelMA) to fabricate CaP-doped electrospun fibers to mimic the periosteum structure, and thus hasten bone regeneration.69 GelMA/CaP electrospun fibers exhibited superior bioactivity when immersed in simulated body fluid (SBF), induced mineralization of pre-osteoblasts, and led to a vasculogenic response of endothelial cells. Altogether, these outcomes reinforce the possibilities of devising hybrid biomaterials to mimic the synergistic activity between organic and inorganic components of native bone and periodontal tissues.69
To further improve their biofunctionality, growth factors (GFs) have been added to electrospun membranes and scaffolds.23,70–72 Growth factors are natural proteins that modulate cellular functions, such as proliferation, migration, differentiation, and the formation of extracellular matrix (ECM). In a study by Chen et al. (2016), poly(ethylene glycol) (PEG)-stabilized amorphous calcium phosphate (ACP) nanoparticles containing recombinant human cementum protein 1 (rhCEMP1) were formulated and then utilized to fabricate, via electrospinning, multiphasic scaffolds constituted of PCL, type I collagen, and rhCEMP1/ACP.23 From a regenerative standpoint, the engineered scaffold, when evaluated in a relevant in vivo periodontal defect model, led to cementum-like tissue formation after 8 weeks, thus supporting the ability of tissue regeneration when proper molecules are encapsulated into nanofibers.23 Nonetheless, GFs are sensitive molecules and caution needs to be exercised in the electrospinning process to maintain their bioactive profile.16
2.2. Coaxial nano/microfibrous electrospun membranes
Coaxial electrospinning consists of two separate solutions that form core-shell fibers, where the core and shell portions can house distinct therapeutic cargos, especially when aiming at defined spatiotemporal release patterns based on the regenerative goal. For instance, by using coaxial electrospinning, it is feasible to have an osteoinductive molecule loaded within the core and an antimicrobial medication in the shell.48,49 The aforesaid arrangement is of significant relevance in periodontal regeneration once the outer layer (shell) containing the antimicrobial drug releases first to help eradicate infection, while the osteoinductive molecule in the inner layer (core), leaches out at a later stage, thus stimulating new tissue formation in an infection-free environment. Moreover, as previously alluded, coaxial electrospinning can also be used to shelter sensitive molecules from organic and harsh solvents commonly used to obtain polymeric solutions that would negatively affect their bioactivity before nanofibers’ production.
It is well-established that the regeneration of bone tissue is controlled by both osteogenic and angiogenic GFs, which are expressed in a synchronized fashion. An elegant demonstration of the opportunities involved in the creation of controlled co-delivery of growth factors through core-shell nanofibers has been recently reported. Cheng et al. (2019) used a dual growth factor-release system to achieve time-controlled of GFs release to amplify vascularized bone regeneration. In that study, core-shell nanofibrous scaffolds were fabricated using coaxial electrospinning and layer-by-layer (LBL) techniques, where bone morphogenetic protein 2 (BMP2) was loaded into the core of the nanofibers and connective tissue growth factor (CTGF) was attached onto the surface (Figure 5a-d). The findings confirmed the sustained release of BMP2 and a rapid release of CTGF. Importantly, in vitro and in vivo data showed improvements in bone regeneration when the dual-drug release system was used (Figure 5e).72 Even though the literature emphasized the relevance of bioactive molecules (e.g., growth factors) for bone regeneration and the well-understood involvement of these biological cues in a plethora of regenerative approaches, the need for a suitable carrier is essential to guarantee their efficacy.16,43,44 Due to the low stability of these molecules and their inactivation by other proteins in physiologic conditions, scaffold-based bioinspired immobilization mechanisms have been conceived to control release profiles and specific targets of GFs in regenerative strategies.73 Therefore, these scaffolds and controlled mechanisms of release may help to surpass the challenges involved with the use of bioactive molecules and bring the possibility of translating laboratory research into practice.73,74
Figure 5.
(a) Schematic illustration of the Co-axial electrospinning, (b) TEM (A) and SEM (B) images of core−shell structure nanofiber membrane, and (c) Masson’s trichrome staining of cranial defects in the at 4 and 8-weeks after implantation. Residual scaffolds are marked by a green asterisk, and newly formed bone is outlined by a yellow line. Bar represents 500 μm for all panels. Adapted from.72
2.3. Multiphasic and functionally graded electrospun membranes
Although single layer membranes and scaffolds, created by conventional and/or coaxial electrospinning, present notable advantages and translational potential with fiber diameters close to the ones found in the ECM of native tissues, they do not provide perfect hierarchical organization and spatial gradient found in living tissues. In periodontal tissue regeneration, GTR and GBR membranes face a complex environment where the soft tissue is more prone to be an open path for bacterial colonization in case of dehiscence, while the hard tissue needs the proper cell source and regenerative stimuli without being disturbed by soft tissue invagination or bacterial infection. Research has therefore led to the development of graded structures that allow not only for tissue-specific compositions, but also to endow multifunctionality, such as infection control and regenerative cues to stimulate regeneration. Our group reported on the fabrication of a spatially designed and functionally graded membrane with unique therapeutic properties (Figure 6).42 The innovative membrane was designed and processed to display a core layer (CL) and two functional surface layers (SLs) to interface with hard and soft tissues. The CL was engineered by spinning a poly(DL-lactide-co-ε-caprolactone) (PLCL) layer surrounded by two composite layers consisting of a gelatin/polymer blend. Hydroxyapatite nanoparticles were incorporated to enhance bone formation on the SL facing the bone defect and metronidazole (MET) was added to inhibit bacterial colonization on the SL facing the epithelial tissue. Worth noting, no delamination of the multilayered structure was observed upon mechanical loading, thus potentially assuring suitable surgical handling and physiologic loading in vivo.42 In a similar study, a poly(L-lactide-co-d,l-lactide)-based functionally graded membrane was developed using platelet-derived growth factor as the SL facing the bone defect and MET facing the soft tissue.47 Along with 28 days of sustained GF release, alveolar ridge regeneration was reported.47
Figure 6.
Schematic illustration of the spatially designed and functionally graded (FGM) periodontal membrane. (a) Membrane placed in a guided bone regeneration scenario and (b) details of the core-layer (CL) and the functional surface-layers (SL) interfacing bone (nano-hydroxyapatite, n-HAp) and epithelial (metronidazole, MET) tissues. Note the chemical composition step-wise grading from the CL to SLs, i.e., polymer content decreased, and protein content increased. (c) Cross-section SEM micrographs of the FGM processed via multilayering electrospinning. Adapted from.42
In sum, the development of GTR/GBR membranes by electrospinning holds significant translation potential, as it allows the use of a wide variety of polymers for fabrication of nanofibers and provides an ease of encapsulating additives such as drugs, antibacterial agents, and growth factors key in bone and periodontal tissue regeneration. Importantly, functionally graded and/or multilayered scaffold processing strategies, in addition to core-shell electrospinning approaches, can be leveraged to develop tissue-specific (hard vs. soft tissue) membranes with multifunctional properties.
Despite the impressive contribution made by electrospinning to the development of membranes and scaffolds for bone and periodontal regeneration, this technique presents intrinsic limitations. For example, electrospun fibers are generally closely packed in two-dimensional (2D) mat-like structures, which can adversely affect cell infiltration limiting tissue ingrowth and vascularization. To support true physiological regeneration of the periodontal tissues, the scaffold must facilitate cell penetration and blood vessels formation to improve healing. Additionally, limited control while designing defect-specific scaffolds and the use of hazardous solvents to solubilize the polymer(s) of interest, have prompted the search to more suitable technologies with the potential to realize anatomically complex personalized scaffold geometries. Melt electrowriting (MEW), a relatively new additive manufacturing technology, provides a more biologically compatible alternative for processing nano/microfibrous scaffolds. The fibers can be precisely positioned to control the shape, porosity, fiber diameter and mesh-width in three-dimensions.75,76 This processing versatility is especially important for bone and periodontal tissue engineering and has been recently exploited to customize structural gradients or zonal tissue constructs.77,78
3. Three-dimensional (3D) printing
3D printing has emerged as a unique manufacturing toolset within the fast-evolving field of regenerative medicine. This class of materials processing technologies allow the generation of patient- and defect-specific scaffolds and/or living constructs with finely tuned internal and external morphologies that approximate to the native tissue architectures. Not surprisingly, the utilization of different biomaterial classes to engineer cell-free or cell-laden 3D scaffolds or implants has experienced a significant increase in recent years to fulfil the complexity and efficiency requirements for clinical translation.14,79 A key advancement compared to more traditional processing methods of scaffold development is the intrinsic ability to place cells and/or biomolecules at predefined locations within the generated 3D scaffold microenvironment. Clinically speaking, recent advances in 3D printing technologies could, in the near future, eliminate the need for bone transplantation in large and geometrically complex CMF reconstructions, thus avoiding the creation of secondary bone defects.
3D printing comprises a wide array of fabrication technologies of which the most established are light-laser-, droplet-, and extrusion-assisted printing.27,80 To date, extrusion-based printing has been the most employed method for bone and periodontal tissue applications, due to its versatility and affordability.81 As the name indicates, extrusion printing involves the dispensing of molten polymers, bioceramic pastes, or hydrogels through an extrusion nozzle via pneumatic, piston-driven, or screw-driven force, resulting in the printing of continuous filaments to create predesigned architectures.33 This technique can be used to print a wide range of porous and geometrically complex scaffolds with controlled mechanical and biological properties.12,13,28,33,81
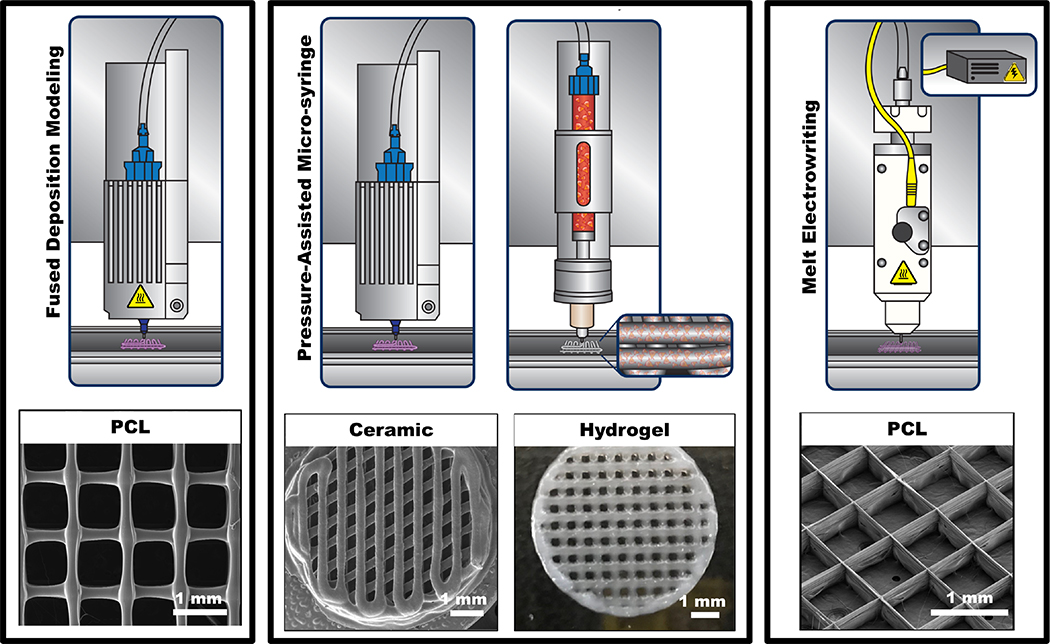
The incorporation of biomolecules (e.g., growth factors) or therapeutic compounds within 3D printed scaffolds further provide additional means to help guide and ultimately improve the regenerative outcome.82–84 Of note, coaxial and multimaterial dispensing systems have proven to be compatible with extrusion-based technologies.31,85,86 One important advantage of this method relates to its ability to generate reproducible heterogeneous structures (e.g., shape, size, porosity, pore geometry, etc.) with multiple cell types at physiological densities carefully positioned at different locations on a complex scaffold/construct architecture.13,31,37 Worth noting, the method of printing live cells to create tissue-like structures that imitate native tissues is known as bioprinting. During bioprinting, a biomaterial solution in the form of a hydrogel or its precursor embedded with the desired cell type(s), termed the bioink, is utilized to create tissue-like structures in a layer-by-layer fashion. The aqueous 3D microenvironment provided by hydrogels simulates the natural ECM and then considered excellent candidates for the incorporation of cells.87 However, given the high degree of freedom to use diverse biomaterials and cell types to engineer 3D constructs, in this section, we will discuss the most common cell-free and cell-laden extrusion-based printing techniques for craniofacial bone and periodontal regeneration. Extrusion-based printing can be classified into two main categories: thermal and non-thermal extrusion printing (Figure 7). Fused deposition modelling (FDM) is the major representative of polymer-based thermal extrusion; whereas melt electrowriting (MEW), which combines thermal extrusion and an applied electrical field, has been introduced to increase the resolution of the polymeric filaments down to the micron and sub-micron range. This technology has been deemed as a more refined version of the FDM method and has gained the spotlight in regenerative medicine due to its potential to engineer highly ordered fibrous scaffolds, capable of replicate extracellular microenvironment functions. Amongst the non-thermal extrusion-based 3D printing technologies, the pressure-assisted microsyringe is the most common approach and has been utilized to print bioceramic pastes and cell-laden hydrogel-based scaffolds (Table 2).
Figure 7.
Schematic diagram of common extrusion-based methods with or without thermal extrusion for the printing of various biomaterials (polymer, ceramic, hydrogel) with high level of consistency.
Table 2.
Selected references on 3D (bio)printing strategies in craniofacial bone and periodontal tissue engineering
| Reference | Materials | Advantages/Disadvantages | Most Relevant Findings |
|---|---|---|---|
|
| |||
| [94] | PCL | (+) production of resorbable membranes and tunable porosity | 3D printed PCL with 30% porosity showed improved mechanical properties and osteogenic differentiation. |
| [82] | PCL/PLGA/Collagen | (+) short- or long-term release of BMP-2 can be achieved depending on the polymer used | Scaffold loaded with rhBMP-2 showed higher osteoinductivity compared to PCL/PLGA/gelatin loaded with rhBMP-2 or individual scaffold. |
| [93] | PLA | (−) requirement of the use of a post-modification method | CaP treatment of printed scaffold increased the roughness and hydrophilicity thereby positively impacting the proliferation of the osteoblasts. |
| [85] | PCL/PLGA/β-TCP | (+) similar levels of biocompatibility and bone regeneration as collagen membranes | 3D printed membrane showed bone regeneration performance similar to that of collagen membranes during a GBR procedure performed in peri-implant defects. |
| [96] | PEU and HA | (+) capability of printing various types of polymers | 3D printed HA-containing PEU composites promote higher bone regeneration compared to pure HA scaffold. |
| [99] | PLLA and β-TCP | (+) readily incorporation of bioactive tricalcium phosphate | In vivo bone formation driven by the PLLA + TCP30 scaffold with MG-63 cells was significantly greater than PLLA or TCP30 with MG63. |
| [100] | PCL/β-TCP/ | (+) phlorotannin made the composites hydrophilic | Phlorotannin composites showed higher initial cell attachment and mineralization than non-phlorotannin composite. |
| [103] | PCL-50 wt% of 45s5 bioglass or strontium substitute bioactive glass | (+) biaxially rotating bioreactor cellular homogeneity throughout the scaffolds. | Release of ions (Sr, Zn, Mg, and Si) from scaffold accelerate angiogenesis and stimulate the osteogenic differentiation of mesenchymal stem cells (MSCs) |
| [84] | PCL-poloxamine | (+) tunable bioerosion rate and DEX release | Varying osteogenic activities from human mesenchymal stem cells cultured onto scaffolds composed of the various blends are demonstrated. |
| [104] | PLA | (−) difficulty in achieving biomimetic nano resolution | Angiogenesis and osteogenesis are successively induced by delivering dual growth factors with sequential release using PLA. |
| [83] | PCL/PLGA/β-TCP | (+) slow release of BMP-2 | 3D printed GBR membrane loaded rhBMP-2 exhibited significantly greater amount of new bone in the rabbit calvarial defect model compared to the membrane without rhBMP-2. |
| [112] | PCL | (+) combination of melt and solution electrospinning | The multiphasic construct with large and small pores electrospinning to develop biphasic scaffolds to supporting bone formation and cell sheets. |
| [106] | PCL, HA | (+) multiphasic scaffold to imitate native periodontium (−) ectopic peridontium formation |
3D printed seamless scaffold with region-specific microstructure and spatial delivery of proteins resulted into putative dentin/cementum, PDL, and alveolar bone complex regeneration |
| [30] | PCL-PLGA | (+) printing complex structures by combination of FDM and electrospinning | Triphasic scaffold by combination of 3D printing (FDM) and electrospinning exhibited enhanced ALP activity and GAG amount. |
| [108] | PCL | (−) polymer of choice is limited because of the high melting point and biodegradability | MEW scaffold coated with calcium phosphate enhanced the osteogenic gene and protein expression of hOBs. |
| [109] | PLA-PEG_PLA and PLA | (+) tailoring scaffold architectures with high precision | 5% BG did not affect the processing adversely. |
| [110] | PCL and HA | (+) precise and complex porous 3D fibrous structures & tunable porosity | Incorporation of HA in PCL increased the cell spreading and migration. |
| [111] | P-(Є-CL-co-AC) | (+) production of fibrous scaffolds with sinusoidal patterns and micron-sized diameter mimicking the ligament and tendons | MEW printed P-(Є-CL-co-AC) is cytocompatible and qualitatively mimicked the mechanical characteristics of tendon and ligament tissue. |
| [113] | Star PEG heparin hydrogel/PCL | (+) multiphasic scaffold design in combination with different human cell type | Tissue-engineered periosteum constructs loaded with HUVECs and BMMSC enhance the vascularization and retained the BM-MSCs in undifferentiated state in vivo. |
| [78] | PCL | (+) heterogenous porosity of scaffold increase cell attachment (−) study needs to be confirmed in an appropriate in vivo model |
MEW scaffold with gradient pore size and fiber offset significantly improved the osteogenic potential. |
| [114] | PEOT, PBT, PCL | (+) designing structural porosity gradients | The construct with a discrete gradient in pore as a strategy to support differentiation supported the osteogenic differentiation of hMSCs. |
| [12] | PCL and GelMA | (+) reinforcing effect of meshes could be further (−) lack of detailed cross-linked kinetics of GelMA modified AMP. |
Fiber-reinforced (PCL meshes processed via MEW) membranes in combination with therapeutic agent(s) embedded in GelMA offer a robust, highly tunable platform to amplify bone regeneration not only in periodontal defects, but also in other craniomaxillofacial sites. |
| [38] | GelMA, PCL | (+) convergent approach to combine extrusion-based printing of hydrogels and MEW | Mechanically stable constructs with the spatial distributions of different cell types without compromising cell viability and differentiation. |
| [127] | Calcium silicate | (+) low-temperature rapid prototyping of C3S offers drug/GF incorporation during in printing process | Controllable nanotopography of printed structure into phosphate aqueous solution improve bone regeneration in vivo. |
| [134] | HA and Gelatin | (+) shape can be easily adjustable, in wet conditions, to that of the bone defect during surgery. | The osteogenic differentiation of MC3T3-E1 on silicon-doped HA scaffold was higher compared to HA only. |
| [102] | 13–93 Bioactive glass/alginate | (+) tunable pore size and porosity | With increase in BG/SA mass ratio, the pore size and porosity also increased. Furthermore, scaffolds exhibited in vitro apatite mineralization and osteogenic differentiation of rBMSCs. |
| [29] | PCL | (+) Printed membrane-supported periodontal ligament fibrous cell sheets under both stationary and dynamic fluid conditions (−) standardized cell source for preparing the decellularized cell sheets. |
Printed scaffolds improve the handling of the cell sheet during decellularization protocols. |
| [135] | Sr-MBG | (+) Microfill perfusion assay to determine blood vessel. (−) in depth understanding of synergistic osteogenic/angiogenic effect of Sr and Si ions released. |
Sr-ions from scaffolds create a better microenvironment activating the angiogenesis and osteogenesis pathway for the enhanced in vitro and in vivo bone formation. |
| [136] | βTCP-collagen | (+) heterophasic construct design | Scaffold allowed the proliferation of DPC and increased the differentiation towards osteoblasts. |
| [137] | Akermanite- βTCP | (+) repair of load-bearing bone defects. | Akermanite had better mechanical properties and a higher rate of new bone formation than the pure TCP scaffold. |
| [141] | Sodium alginate, Pluronic F-127, Bredigite bioceramic | (+) Better oxygen and nutrient delivery for cell activity | Enhanced vascularized bone formation due to synergistic effect of 3D printed hollow-pipe structure and release of bioactive ions. |
| [125] | Akermanite, Sodium alginate, Pluronic F-127 | (+) scaffold developed with different raw materials including ceramics, metal and polymer. | Lotus root-like biomimetic materials significantly improved in vitro and in vivo osteogenesis and angiogenesis. |
| [144] | Calcium Phosphate | (+) fabrication of humidity-set scaffold | CPC containing VEGF maintains hMSC viability and bioactivity of HDMEC. |
| [145] | βTCP, Alginate and Gelatin | (+) Printable bioink at room temperature to load drugs/GF | Cell adhesion and ALP expression was enhanced by scaffold containing PLGA microspheres with VEGF. |
| [147] | Collagen, chitosan, HA | (+) tailored scaffold property for long-term controlled drug release and bone regeneration | The bone regeneration capacity of HA scaffold coated with collagen/chitosan microsphere with rhBMP-2 was higher than the HA scaffold coated with collagen or pure HA. |
| [148] | Mesoporous silica/calcium phosphate | (+) well-interconnected macropores and ordered mesopores | MS/CPC/rhBMP-2 scaffolds induced the osteogenic differentiation and vascularization in vitro and in vivo. |
| [149] | β-TCP | (+) use of large translational animal model | Delivery of dipyridamole improved the osseoconduction in sheep calvarial defect model resulting in significant increase in bone formation. |
| [140] | Alginate, Pluronic F-127, bioceramic | (+) migration of cells in the inner part of the scaffolds due to high porosity and surface area | HSP demonstrated more new bone formation compared with a solid-struts-packed scaffold. |
| [126] | β-TCP, Wollastonite, Bredigite | (+) Scaffold stable in aqueous medium for a long period of time. | CSi-Mg10 scaffolds displayed improved flexural strength and higher osteogenic capability in rabbit mandibular defect. |
| [124] | β-TCP | (+) scaffold strut and porosity designed to elicit bone-healing behavior. | 3D printed beta-TCP induce new bridging bone formation in full-thickness mandibulectomy defects after 8 weeks without the use of osteogenic inducers. |
| [156] | PLGA-TCP | (+) Bone was able to remodel under physiological loading | Phyto-molecule icariin exhibited improved biodegradability, biocompatibility, and osteoinductivity both in vitro and in vivo. |
| [36] | Calcium phosphate, Alginate and Methylcellulose | (+) post-plotting regime, to prevent microcrack formation inside CPC strands | Biphasic scaffold showed migration of cells towards CPC from alg/mc after 7 days. |
| [153] | Alginate, Lutrol F-127, Poloxamer 407, Matrigel, Agrose, Methylcellulose | (+) two distinct cell populations printed within a single scaffold | No difference in cell proliferation and viability of 3D printed and unprinted hydrogel scaffolds. |
| [31] | α-TCP and type 1 collagen | (+) two step printing process to develop cell-loaded bioceramics scaffold. | 3D printed scaffold showed improved physical properties, metabolic activity and mineralization, compared with those of the controls. |
| [13] | AMP and synthetic peptide gel | (+) Fast degradation of AMP microparticles | AMP-modified constructs favored in vitro and in vivo mineralization without the use of a chemical inducer. |
| [163] | Collagen, β-TCP | (+) unique fibrillogenesis of collagen to produce a bioink laden with cell and bioceramics | hASC-laden composite structure (20 wt% of β-TCP) demonstrated significant osteogenic gene expression compared to control cultured using an osteogenic media |
| [164] | GelMA,k- Carrageenan,Laponite | (+) NICE bioink produce fabricate patient specific, 3D implantable scaffold | bone tissue formation was a result of endochondral differentiation of hMSCs |
| [32] | PCL, Alginate | (+)MtoBS is a promising system for regeneration of heterogeneous tissue | Multi-Arm BioPrinter enabling dispensing of human chondrocytes and MG63 cells to biofabricate osteochondral tissue. |
| [152] | Agrose hydrogel | (+) computationally designed spatial patterns of cells | 3D printed constructs with specific spatial pattern and varying cell densities improves cell viability |
| [37] | PCL, Alginate, Collagen, HA | cytocompatible multi-layered construct formed by stacking different types of printable extracellular matrix (ECM) bioink | 3D printing of construct with different types of ECM hydrogels encapsulated stem cells allowed the differentiation towards chondrogenic and osteogenic lineages |
| [182] | Alginate, chitosan | The coating improved the retention and release efficacy of drug | The coating of 3D printed alginate construct with chitosan improved cell proliferation and result into elongated cells. |
| [177] | β-TCP | (+) Scaffold preserved the cranial suture patency. | Large scaffold pore (500 νm) coated in 1000 μM dipyridamole yielded the most bone growth and faster degradation within the defect. |
| [14] | PCL | (+) first clinical case of 3D printed scaffold for periodontal regeneration. (−) Slow scaffold resorption, at 13 months, the scaffold became exposed |
The implanted 3D scaffold showed n signs of chronic inflammation or dehiscence upto 12 months. |
| [183] | PCL | (+) scaffolds combined hierarchical mesoscale and microscale features can align cells in vivo. | Combination of gene therapy and topographical guidance cues showed osseous tissue formation and oriented collagen fibers for treatment of periodontal osseous defects. |
| [77] | GelMA, PCL | (+) convergence of MEW and bioprinting, for fabrication of flat to anatomical relevant structures. | MEW process allowed the fabrication of a complete condyle-shaped biological construct. |
| [101] | PCL, MBG-58S | (+) use of clinically relevant post-menopausal mode; for bone regeneration | MBG-PCL scaffold promoted new bone formation at both the peripheral and the inner parts of the scaffolds with thick trabeculae and a high vascularization degree. |
| [123] | PCL, Mesorporous calcium silicate and bioactive glass | (+) two different scaffolds with highly properties to avoid the interference of the comparing osteogenic potential | MBG/PCL scaffolds exhibited better bioactivty than MCS/PCL scaffolds for bone regeneration. |
| [129] | Magnesium phosphate | (+) DAHP solution eliminate the conventional sintering process to extend the usefulness of loading drug | MgP scaffold showed good pore structural conditions, mechanical property and cell affinity. |
| [131] | α-TCP | (+) fabrication of thermally instable and degradable matrices of secondary calcium phosphates | The printed sample strength increase after treatment of phospharic acid give rise to brushite with minor phases of unreacted TCP. |
| [130] | TCP, DCPA | (+) complete conversion of all components involved in the production process (raw powder and binder solution) to a cement matrix minimizing risk of harmful residues | Scaffolds was printed with >96.5% of dimensional accuracy. Cell proliferation was higher on biphasic calcium phosphate when compared to HA. |
| [166] | CaP, PCL, GelMA | (+) Multi-material, multi-scale 3D printing approach (hydrogel-thermoplastic-bioceramic composite) | multi-scale composite osteochondral plugs results in the formation of cartilage-like matrix in vitro with 3.7-fold increase in strength of the interconnection at the bone-cartilage interface. |
| [172] | Calcium silicate, β-TCP | (+) engineering of pro-angiogenic microenvironment in vitro | Co-culture of HUVECs and hBMSCs on porous 5% CS/β-TCP accelerates vascularization and osteogenesis in ectopic bone formation model. |
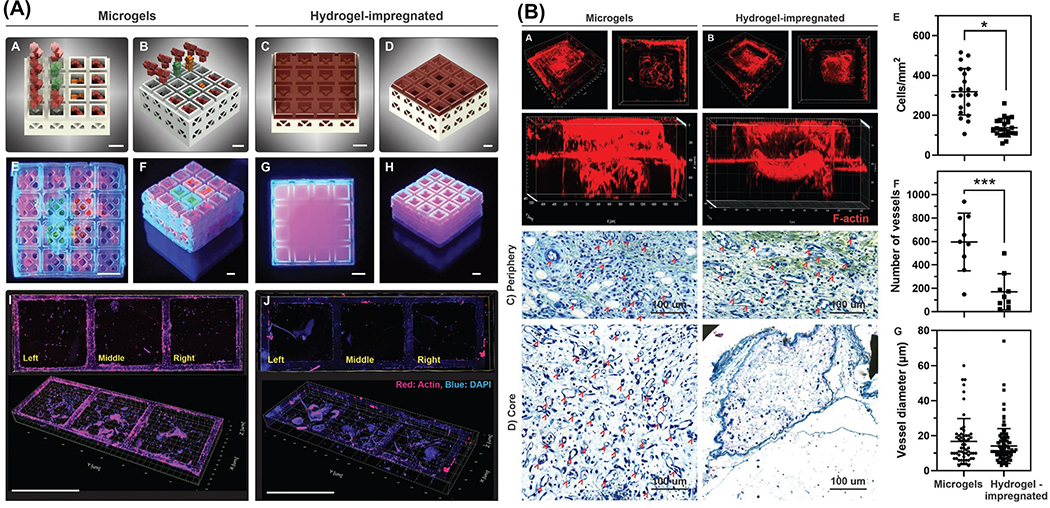
| [175] | GelMA | (+) hollow, stackable miniaturized microcage modules, resembling the features of toy interlocking building blocks | 3D printed microcages loaded with microgels supplemented with growth factors enhanced cell invasion into the core of assembled constructs in a controllable manner, thus accelerating the process of new tissue formation and healing. |
| [176] | β-TCP | (+) scaffolds do not cause premature closure of sutures and preserve normal craniofacial growth | Regeneration of vascularized bone with mechanical characteristics comparable to native bone. |
| [184] | PCL | (+) high adaptability to the created defect geometry | The ligament cells displayed highly predictable and controllable orientations along microgroove patterns on 3D biopolymeric scaffolds. |
| [178] | β-TCP | (+) Dipyridamole as a viable cost-effective osteogenic agent | Resorbable, β-TCP scaffolds treated with DIPY increased bone regeneration qualitatively and quantitatively. |
| [174] | Akermanite | (+) Development of Haversian bone–mimicking scaffolds (−) multicellular synergistic including bone-resident cells such as osteoblasts, osteoclasts, and macrophages need to be explored |
The scaffold showed the potential for multicellular delivery by inducing osteogenic, angiogenic, and neurogenic differentiation in vitro and accelerated the ingrowth of blood vessels and new bone formation in vivo. |
| [133] | TCP and anhydrocus dicalcium phosphate | (+) discrete deposition of pharmaceutical agents on bioceramics scaffold using multijet 3D printing | rhBMP-2 and vancomycin by loading the drug within the 3D printed scaffold. |
| [173] | PCL | (+) deferoxamine loaded PCL showed mechanical property similar to cancellous bone | The deferoxamine-printed scaffold had no effect on cell attachment or proliferation, but it significantly increased vascularization, which was accompanied by increased bone growth. |
3.1. Thermal extrusion-based printing
3.1.1. Fused deposition modeling
Fused deposition modeling (FDM) was invented and patented by Scott Crump in the late 1980s.88 The FDM method is based on the layer-by-layer printing of thermoplastic polymers. In this technique, the polymer of interest is heated above its melting point and due to a solid-semisolid state transition, is then extruded through a nozzle according to the predefined computer-aided-design (CAD) design.89 After the first surface layer has been deposited, the nozzle rises or the platform descends one layer in the Z-axis direction, and the process is repeated until the designed geometry is completed. The polymer fuses to create a cohesive layer until solidifying at room temperature. FDM printers usually built 3D structures with a filament diameter ranging from 160–700 μm depending on the polymer and pattern of the structure.89 The quality of the extruded filament can be modified by adjusting the printing speed, construct orientation, and layer thickness. This technique has several advantages, such as high printing speed and the potential to print multiple materials simultaneously when working with a multi-nozzle printhead.11,90 Furthermore, the FDM platform eliminates any possible toxicity induced by generally harsh organic solvents that are required for the solubilization of polymers for electrospinning.91 Nonetheless, limitations of this technique include low resolution and surface finish because material spreads out before it cools and hardens to the desired shape.92 Yet, it is still possible to obtain resolutions similar to those achieved by other 3D printing techniques when utilizing small diameter nozzles.92
Biodegradable synthetic polymers, such as poly(ε-caprolactone) (PCL), poly(lactic acid) (PLA), and poly(lactic-co-glycolic acid) (PLGA) have been widely explored in FDM to generate scaffolds with tunable mechanical, chemical, and biological properties.92–94 In a study by Shim et al. (2017), PCL membranes with various porosities and pore sizes (up to 700 μm) were printed via thermal extrusion. After 14 days of in vitro culture, the membrane with 30% porosity showed higher osteogenic differentiation of pre-osteoblasts than those cultured on 50% and 70% porosities. Further, in vivo experiments were carried out to assess the regenerative efficacy of PCL membranes with different pore sizes upon implantation into rabbit calvaria defects. After 4 weeks, membranes with 30% porosity showed extensive bone formation, along with newly formed blood vessels.94 In a different study, another interestingly described approach involved the utilization of cold atmospheric plasma to control nanoscale roughness of 3D printed PLA membranes aiming to enhance osteoblast and mesenchymal stem cells attachment and function.93
Regrettably, although 3D printed polyester-based scaffolds with controlled porosity and surface modifications show good biomechanical and space maintenance properties for use in GTR/GBR applications, they present low bioactivity, and thus, lack therapeutic elements critical to the regenerative process. To amplify the regeneration capacity, bioceramics and/or growth factors (GFs) have been utilized to create composite scaffolds with improved bioactivity. Such a strategy was explored by Kim et al. (2010), whereby a novel anatomically shaped rat incisor PCL/nHAp scaffold was fabricated via a layer-by-layer approach to deliver stromal-derived factor-1 and bone morphogenetic protein 7 (BMP-7). The scaffold was implanted into the extraction socket for orthotopic tooth regeneration and, after 9 weeks, scaffold within the socket showed new bone trabeculae-like structures with new periodontal ligament consisting of fibroblast-like cells and collagen-like structures immediately adjacent to new bone. The study highlighted that carefully selected GFs may recruit multiple cell lineages into the scaffold for regeneration of a putative periodontal ligament and newly formed alveolar bone.95 Similar approaches where employed by others, where nHAp ranging from 10% to 40%, combined with 1,6-hexanediol L-phenylalanine-based poly(ester urea), were printed with 75% porosity and 300 μm pore size to enhance mechanical competence and impart bioactivity.96 The composite scaffold with 40% nHAp led to accelerated osteogenic differentiation; however, there was a decrease in the scaffold’s compressive modulus when the temperature increased to a physiological value.96 Several other investigations have followed the same approach by blending nHAp to increase osteogenesis of polymer-based 3D printed biodegradable scaffolds.97,98
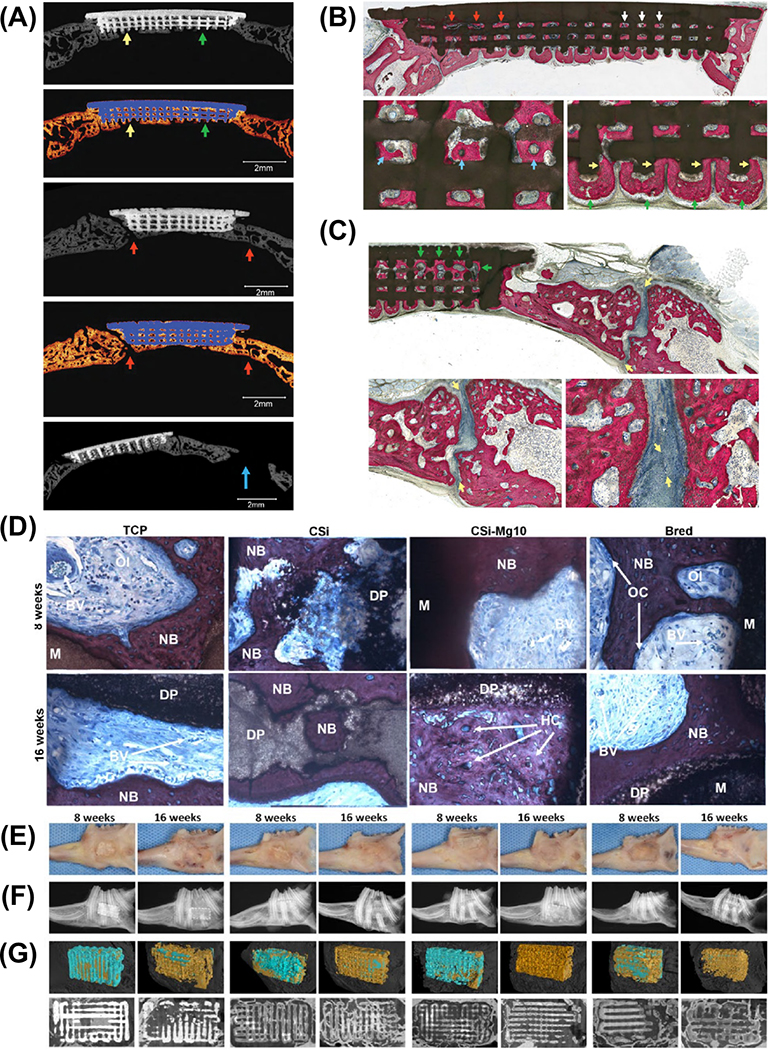
β-tricalcium phosphate (β-TCP) is another important bioceramic due to its intrinsic osteoconductivity and faster degradation rate when compared to traditional HAp. Distinct amounts (10–30%) of TCP were mixed with poly(lactic acid) to obtain scaffolds with an orthogonal design and 100 μm pore size. After 12 weeks in vivo, the degradation of the scaffolds matched that of new bone formation (Figure 8a-b).99 A similar strategy was used to print β-TCP/PCL scaffolds. The scaffold with 30% TCP, under mechanical stimulation, led to the upregulation of bone-related genes. From a mechanical standpoint, the compressive modulus of the fabricated scaffold was comparable to human trabecular bone.100 Meanwhile, synthetic biodegradable polymers have also been modified with bioactive glasses (BGs) due to their ability to form an intermediate apatite layer and stimulate osteogenic differentiation.101,102 In a recent study, 3D printed PCL-based scaffolds modified with 50% 45S5 BG or Sr-substituted BG were able to stimulate osteogenic differentiation of bone marrow stromal cells without additional chemical inducers.103
Figure 8.
(a) Images of the prepared scaffold (1) photograph, (2) micrograph, and (3) scanning electron microscopy, (b) Micro-CT images from rats that received the scaffold at 0–12 weeks after implantation. Adapted from.99 (c) Imaging of scaffold’s architecture and surface morphology by stereomicroscopy. (d-f) Confocal imaging of live (calcein, green) and dead (propidium iodide, red) human mesenchymal stem cells cultured onto the scaffolds. Adapted from.84
The incorporation of therapeutic agents has also been explored in the development of 3D printed scaffolds for vascularized bone regeneration.82,83 As an example, dexamethasone-loaded 3D printed scaffolds made of PCL and poloxamine demonstrated to be a viable option to locally deliver biomolecules to induce osteogenic differentiation of resident mesenchymal stem cells (MSCs) (Figure 8c-d).84 In another study, 3D printed PLA scaffolds with defined pore size (200 μm) were coated by a layer-by-layer nanocoating technique for sequential release of angiogenic and osteogenic GFs to generate vascularized bone constructs. The co-culture of human umbilical vein endothelial cells and bone marrow-derived MSCs on 3D printed nanocoated scaffold exhibited excellent bone forming potential with well-developed microvascular networks.104 A unique approach was employed by Shim et al. (2014) in the fabrication of PCL/PLGA/β-TCP 3D printed membranes with controlled pore size (250 μm), following which a collagen/rhBMP-2 mixture was dispensed into the membrane’s empty pores. The collagen/rhBMP-2 solution was physically gelled at 37°C prior to implantation. Upon euthanasia, the animals receiving the collagen/rhBMP-2 filled constructs showed 70% of total new bone formation compared to 40% in the case of unfilled (control) scaffolds.83
Although the aforementioned scaffold strategies are promising to regenerate vascularized craniofacial bone, they fall short of their ability to facilitate the coordinated regeneration of multiple periodontal tissues, which includes not only alveolar bone, but also cementum and periodontal ligament (PDL). In the complex architecture of the periodontium, the regeneration of bone is correlated with the neoformation of cementum and PDL. Therefore, to allow multi-tissue periodontal regeneration, multiphasic scaffolds with structural arrangement, and unique architectural and chemical characteristics, should be devised to mimic the periodontium’s highly organized zonal structure. To this end, Vaquette et al. developed biphasic 3D printed scaffolds made of PCL and 20% β-TCP with 70% porosity and 100% interconnectivity to form the bone zone. The periodontal ligament (PDL) zone was created by electrospinning of a PCL solution to support and deliver mature PDL cell sheets. The aforementioned biphasic scaffold was coupled with a dentin slice and implanted in an athymic subcutaneous rat model. The investigators observed that 67% of the test group with PDL cell sheets showed new cementum-like tissue on the dentin surface, compared to 17% for the group without PDLC sheets.105 Taken together, the regenerative outcomes achieved with biphasic scaffolds have opened up new horizons in terms of how the meticulous design of bone and PDL zones are critical in the process of periodontal tissue regeneration. Nonetheless, cementogenesis, i.e., the neoformation of cementum remains a challenging issue, as it relies on endogenous cells to facilitate cementum deposition on the root dentin surface. In this way, the consideration and implementation of a third tissue-specific zone has shown to play a key role in enabling the regeneration of cementum and supporting the controlled alignment of PDL fibers. In this context, triphasic PCL/HAp composite scaffolds have been designed with a tissue-specific zonal arrangement presenting distinct microchannel diameter (100, 600, and 300 μm for cementum, PDL, and bone zones, respectively), thus allowing the creation a hierarchical tissue-specific zonal scaffold. Importantly, PLGA microspheres loaded with regenerative cues, such as amelogenin (cementum), connective tissue growth factor (PDL), and BMP-2 (bone), were introduced into each particular zone to enhance tissue-specific cell differentiation. In vitro studies were performed by seeding alveolar bone, periodontal ligament, and dental pulp stem cells along with zone-specific GFs coupling, led to the formation of new bone and cementum-like tissue. Scaffolds seeded with DPSCs generated aligned PDL-like collagens adjacent to newer bone and cementum, which shows that the successful spatio-temporal delivery of distinct proteins can differentiate individual cells into multitissue periodontal complex in vivo.106 In another example, Criscenti et al. (2016) combined 3D printed PCL scaffolds and electrospun PLGA nanofibers to obtain triphasic scaffolds to mimic the zonal microenvironment of the bone-to-ligament interface. First, PCL scaffold, designed with a 700 μm fiber diameter and 150 μm fiber spacing, was printed to serve as a bone zone and then, two-thirds of this scaffold was coated with electrospun aligned PLGA nanofibers to mimic the ligament for the regeneration of bone-ligament interface. 3D printed, hybrid, and electrospun regions were the three regions in the aforementioned triphasic scaffold. This scaffold mimicked the gradient structure of bone-ligament. MSCs were distributed homogenously, not only at the interfaces, but also throughout the entire scaffold. Glycosaminoglycan (GAG) analysis showed higher GAG content for the triphasic scaffold than the controls, indicating improved ligamentogenesis. The results clearly indicated that hMSCs behavior is dependent on the scaffold fabrication method. Briefly, a combination of two techniques (3D printing and electrospinning) provides advantages for interface tissue engineering by mimicking the bone-ligament interface in terms of mechanical, structural, and biological properties.30
3.1.2. Melt electrowriting
Apart from the traditional thermal extrusion methods of polymer printing, a technique named melt electrowriting (MEW), that combines the principles of thermal polymer extrusion and electrospinning, was established to print filaments, which are at least 10 orders of magnitude thinner than conventional FDM. Notably, MEW allows printing highly-ordered porous scaffolds/constructs with filaments ranging from ~ 2–3 μm to 30 μm12,75,89, with recent research demonstrating the possibility to obtain nano-sized polymeric filaments when using acupuncture needles as the spinneret.76
A wide array of degradable polymers has been used to generate MEW scaffolds, with PCL being the most investigated one. In order to achieve higher resolution and overall control of the construct’s architecture, key processing parameters have been identified, such as flow rate of the polymer melt, speed of the collector, spinneret diameter, distance from the needle tip to collector, and applied electric voltage. Optimization of the aforementioned variables allows the formation of a stable, continuously drawn polymer melt directly from the spinneret over the translating collector to realize highly-ordered 3D architectures.76,107 A strategic driver for the latest advances associated with MEW is the rapidly evolving field of regenerative engineering, including, hard and soft tissue regeneration. In one of the seminal studies, PCL scaffolds were prepared using MEW and then modified with a CaP coating to increase cell attachment and osteoinductive properties.108 Human osteoblasts (HOBs) seeded on these scaffolds showed upregulation of an osteogenic-related gene (osteocalcin); whereas, upregulation of fibronectin and vitronectin was reported for PCL scaffolds etched with sodium hydroxide.108 Similarly, melt electrowritten poly(lactide-block-ethylene glycol-block-lactide) (PLA-PEG-PLA) scaffolds containing 45S5 BG were recently reported as another viable alternative to endow bioactive properties to polymeric MEW scaffolds for bone tissue engineering applications.109
Porosity is one of the main factors promoting cell migration, proliferation, and most importantly vascularization of engineered tissues. As previously described, the optimum pore size for bone regeneration is in the range of 150–300 μm. In a study by Abdalla Abdal-hay et al. (2018), composite scaffolds composed of PCL and HAp nanoparticles (3–7%) were produced via MEW to mimic the morphological and structural properties of bone. As the amount of HAp increased, cell spreading and migration also improved. Incorporation of HAp caused scaffold degradation to be faster than in alkaline conditions, which is the pH during the healing of wounds. Scaffolds with large (190 μm) interconnected pores with a high porosity (96–97%), and the presence of HAp was observed to facilitate cell infiltration and growth.110 Alternatively, in a study by Hochleitner et al. (2018), photocrosslinkable poly(L-lactide-co-ε-caprolactone-co-acryloyl carbonate) and poly(ε-caprolactone-co-acryloyl carbonate) were used to produce a sinusoidal pattern to mimic the ultrastructural and mechanical properties of native ligament and tendon tissues. The slightly sinusoidal engineered fibers were crimped enough to approximate to the non-linear stress-strain response of native tendon and ligament. This sinusoidal scaffold exhibited an improvement in cyclic fatigue testing as compared to the scaffolds composed of straight fibers thanks to their geometry. As a result, MEW technology can generate scaffolds that mimic the biomechanical behavior of crimped collagen type I found in natural tendon and ligament tissues.111
As previously highlighted, the engineering of zonal, tissue-specific scaffolds in terms of both composition and architecture has been deemed crucial to the coordinated growth and regeneration of both soft and hard periodontal tissues. In a study by Vaquette et al. (2019), matured cell sheets, including periodontal ligament cells (PDLC), gingival cells and bone marrow-derived MSCs delivery system was developed using a biphasic scaffold fabricated by a combination of solution electrospinning and melt electrowriting (MEW). The bone zone with macroscopic pores was obtained via MEW, whereas the periodontal region was created by a flexible electrospun mesh to support cell sheet delivery. The scaffolds were placed in vivo in artificially-created periodontal defects in a sheep model and revealed excellent tissue integration between bone and PDL. Importantly, a greater bone fill was seen in the PDLC group after 10 weeks. Histological analysis demonstrated the formation of new bone, cementum, and obliquely inserted PDL fibers.112 Similarly, tubular PCL scaffolds were obtained by using MEW as a tissue-engineered periosteum construct. Periosteum, which provides blood supply to the cortical bone and osteogenic niche, is of great importance for bone homeostasis and regeneration. A new concept was introduced by using MEW 3D printed tubular scaffolds and different cell types for periosteal regeneration in vivo. Star-shaped poly(ethylene glycol)-heparin hydrogel incorporated with HUVECs and PCL tubular scaffolds seeded with BMSCs were combined to mimic periosteum’s multiphasic structure. The scaffolds were placed around the femur in contact with bone, aiming at recapitulating both the vascular and osteogenic niches. The results demonstrated that human bone marrow-derived MSCs maintained its undifferentiated phenotype over 30 days of in vivo culture, which was mainly attributed to the properties of the hydrogel; whereas, HUVECs developed into mature vessels with increased vascularization compared to the cell-free approach.113
The generation of scaffolds with a porous and porosity gradient can lead to 3D architectures that resemble the structure of native bone tissue with gradually increasing pore size from one layer to the next mimicking the changes in mineral density from cortical to cancellous bone.114 Abbasi et al. (2019) developed highly-ordered MEW constructs with different pore sizes (250, 500, and 750 μm), two offset constructs of 30% and 50% layout, and a gradient construct of 250–500-750 μm (Figure 9a). The constructs were modified with CaP to boost osteogenic differentiation. The findings indicated that gradient constructs led to more pronounced ALP activity, while mineralized matrix deposition (Figure 9b) was higher in the 50% offset constructs. The expression of osteogenic genes was also upregulated in offset and gradient constructs.114
Figure 9.
(a) SEM images of the porous MEW scaffold structures. (b) Immunocytochemistry analysis of osteogenitor markers (collagen I, osteopontin and osteocalcin) for human osteoblast cells cultured in (A, B, C) Osteogenic medium (D, E, F) Basal medium after 14 and 30 days. Adapted from.78 (c) SEM and confocal micrographs of MEW PCL mesh and its interaction with hMSCs after 3 days. (d) Macrophotographs (A) detailing the steps involved in the fabrication of highly porous MEW PCL meshes with well-controlled 3D architecture and infusion with GelMA+AMP using a custom-made mold. (B-E) Representative cross-section SEM micrograph of the GelMA-PCL (GP) membrane showing the hydrogel phase uniformly infiltrated into the pores of the polymer mesh. (e) Masson’s trichrome stained sections of implanted membrane showing the remnants of the GelMA surrounded by newly generating ossifying bone after 8 weeks in the bioactive hydrogel infused in the PCL mesh and bioactive hydrogel group, indicating slower degradation of GelMA in presence of AMP and PCL mesh. Adapted from.12
Another interesting strategy examined the reinforcing effect of MEW PCL meshes upon integration with gelatin methacryloyl (GelMA) hydrogels.12,115,116 As an example, our research group recently reported on a multifunctional membrane for guided bone regeneration (Figure 9c-d) with predictable mechanical competence and therapeutic features using a highly porous MEW PCL fiber-reinforced hydrogel laden with amorphous magnesium phosphate (AMP). The incorporation of AMP and MEW fibers into the hydrogel provided tunable mechanical strength and enhanced the osteogenic potential. The results showed that the presence of MEW PCL mesh retards the degradation and successfully prevent soft tissue invasion into the osseous defect (Figure 9e). It was concluded that the membrane acted as a bioactive barrier as the presence of AMP led to improved bone formation.12 Although it was demonstrated that such composite scaffolds have improved mechanical and biological properties, the preparation of these constructs involved a two-step fabrication procedure, i.e., the generation of the reinforcing fibrous mesh followed by infusion of the hydrogel, which limited the freedom of designing microfibrous architectures, and the use of multiple materials and cell types in a controlled fashion. One important development in the evolution of the manufacturing of fiber reinforced cell-laden hydrogels is the potential to combine both hydrogel extrusion and MEW processes into a single biofabrication platform.38,40
3.2. Non-thermal extrusion-based printing
The major limitation of thermal extrusion methods is the use of high temperatures, which may ultimately lead to denaturation of proteins and biochemical factors.33,117 To solve this issue, extrusion-driven 3D printing of solutions and/or pastes (e.g., bioceramics), without material melting, has been proposed. Pressure-assisted microsyringe (PAM) uses a pneumatic-driven system to deposit, at room temperature, cell-free or cell-laden biomaterials on a substrate avoiding the denaturation of temperature-sensitive biomolecules and/or cell death.118 In this method, a semi-solid formulation with the necessary printing properties is used as the starting material to generate physically and mechanically stable 3D constructs. Therefore, the rheological characteristics of the biomaterial is an important property that affects the printing process and quality, and depends on the quantity and type of additives used to obtain the semi-solid formulation. A pressurized valve-free or valve-based air piston is usually the dispensing mechanism. Notably, the improved precision of the latter has motivated its widespread use.81,119 Nonetheless, non-thermal extrusion-based 3D printing approach has been employed in the generation of bioceramics, hydrogels, and cell-laden scaffolds at low or physiological temperatures.13,120,121 Next, we provide an overview of the most current work pertaining to non-thermal extrusion 3D printing opportunities for the fabrication of cell-free and cell-laden scaffolds with controlled geometry, architecture, and biological features for applications in craniofacial bone and periodontal regeneration.
3.2.1. Cell-free scaffolds
Bioceramics refer to a class of ceramic materials made up of inorganic crystalline oxide materials that are intrinsically biocompatible and can be processed compositionally and structurally to mimic native bone.122 It is well-established that bioceramic scaffolds (e.g., hydroxyapatite [HAp] and beta-tricalcium phosphate [β-TCP]) have stimulating effects on stem cell proliferation and differentiation. Moreover, their tunable degradation permits them to be processed to provide structural support and guide bone regeneration.36,123
Hydroxyapatite is the major inorganic component of bone and is responsible for its mechanical strength and, together with other CaP-based bioceramics, have been the most widely employed in bone tissue engineering.120 Depending on their chemical composition, bioceramics can be inert or bioactive (i.e., ability to form hydroxyl apatite mineral on its surface both in vitro and in vivo). Over the last decades, bioceramics have been extensively utilized to formulate pastes and cements that can be injected into complex osseous defects that are challenging to realize with traditional bone grafting.124–128 However, such complex bone defects can hardly be treated by pasty bone materials, but require the implantation of preformed scaffolds with complex and customized geometries. Thus, 3D printing has been deemed as a promising technology to fabricate patient-specific bone grafts from bioceramics.34,35 Indeed, previous works have demonstrated the ability to manufacture, via 3D printing, customized bioceramic scaffolds129–132 and also suggested the potential to incorporate additional bioactive additives.35,133
In a study employing HAp and tricalcium silicate, a self-setting tricalcium silicate scaffold was fabricated with an up-and-down staggered structure using mechanical extrusion to facilitate subsequent cell seeding. The nHAp structure was created by immersing the printed structure in a predetermined phosphate aqueous solution at 37°C for 3 days. The presence of HAp on 3D printed tricalcium silicate scaffolds showed higher ability to induce osteogenic differentiation and bone formation in vitro and in vivo, respectively, compared to the pure 3D printed tricalcium silicate scaffold.127 Research conducted by Martinez et al. (2015) reported 3D printed scaffold of gelatin with Si doped HA microparticles using a pneumatic-driven extrusion method. The scaffolds exhibited compression strength close to that of trabecular bone. Mouse calvaria pre-osteoblasts seeded on these hybrid scaffolds upregulated the expression of osteogenic genes, such as runt-related transcription factor 2 and osteocalcin when compared with gelatin-free scaffolds.134 Similarly, 3D printed composite scaffolds consisting of 13–93 BG and sodium alginate improved rat MSCs attachment and osteogenic differentiation.102 Further, the release of bioactive ions from the scaffold contributed to apatite mineralization in vitro.102 In another study employing BGs, osteoinductive and angiogenic properties were enhanced by printing Sr-incorporated mesoporous BG.135 Sr-doped BG scaffolds implanted into critical size defects in rat calvaria led to superior bone formation compared to BG scaffolds lacking strontium (Sr) doping. Microcomputed tomography showed improved vascularization for the Sr-doped scaffolds, compared to non-doped scaffolds in vivo.135
Beta-tricalcium phosphate (β-TCP) scaffolds were printed in a layer-by-layer fashion, with deliberate geometric design (250 μm struts and 330 μm pore) and evaluated in a clinically relevant translational in vivo model.124 After 8 weeks of scaffold implantation in critically-sized full-thickness (i.e., rabbit mandible), improved bone formation was observed in the absence of an osteogenic agent, and there was no significant difference in the baseline bone area occupancy between an unoperated mandible section (55.8 ± 4.4%) and new bone (54.3 ± 11.7%).124 To achieve improved osteogenic action, a multiphasic construct consisted of a 3D printed β-TCP scaffold with an interconnected porosity similar to that of bone was infused with a collagen matrix to mimic bone’s extracellular matrix (ECM) to support the adhesion and osteogenic differentiation of dental pulp stem cells (DPSCs). After 21 days of in vitro culture, a marked increase in ALP activity of DPSCs was seen when compared to 3D printed β-TCP scaffolds devoid of collagen.136
It is well-established that successful bone reconstruction in load-bearing areas requires a mechanically competent and bioactive scaffold. In this context, the addition of akermanite (Ca2Mg(Si2O7) to β-TCP led to the fabrication of a 3D printed scaffold with improved mechanical and regenerative (bone formation) properties than pure β-TCP control scaffolds.137 Along with controlled mechanical and degradation properties, scaffolds’ macrostructure also plays a role in bone regeneration.138,139 Hollow strut-packed bioceramic-based scaffolds printed using a modified coaxial strategy with defined macropores and multi-oriented hollow channels led to greater bone formation compared to solid struts-packed scaffold.140 Meanwhile, in another study, a core-shell printed structure of 1000 μm and 500 μm in diameter, respectively was realized to obtain scaffolds with a hollow pipe-stacked bioceramic structure (bredigite, Ca7Mg(SiO4)4). The implantation of the uniquely-designed (i.e., hollow pipe) scaffold in a radial segmental defect led to the early formation of new blood vessels and bone regeneration and remodeling within the bone defect.141 A similar approach reported improved in vitro cell functions and in vivo angiogenesis for a lotus root-like scaffold with internal microfeatures (e.g., pores and microchannels) (Figure 10a-b). The proposed structure is beneficial for transferring oxygen, nutrition, and cell migration into the inner portion of the scaffold. After 12 weeks of implantation, the lotus root-like scaffolds led to greater angiogenic and osteogenic activity compared to conventional 3D printed scaffolds (Figure 10c-9).125 These studies have shown that macrostructures, mechanical strength, and ions’ release positively affect cell penetration and proliferation, thus supporting osteogenesis and improved vascularization. In addition, magnesium-based materials have recently received particular interest, due to their ability to chemically degrade in vivo and their high potential to promote bone regeneration. A striking example recently introduced by Golafshan and colleagues (2020), demonstrated the non-thermal extrusion printing of magnesium (Mg)-based 3D scaffolds (Figure 11a-b) with compressive strength and toughness similar to native bone, that led to mineralized tissue in vitro bone formation without the need of supplementation with osteoinducing components. In a 6-month in vivo validation in an equine model, the authors confirmed the osteopromotive potential and easy surgical handling of the novel Mg-based scaffolds (Figure 11c-d).35
Figure 10.
(a-b) SEM and confocal images for the morphology and cytoskeleton of BMSC cultured in TSSP, 1CSP, 2CSP, 3CSP, and 4CSP-AKT bioceramic scaffolds for 3 days. (c-d) The adhesion and proliferation of BMSC at different incubation time showing enhanced adherence and proliferation of cells with increase in channel numbers in the biomimetic scaffolds. (e) Fluorescence image of histological sections of biomimetic scaffolds stained with DAPI optical microscope image of 3CSP biomimetic scaffolds with blood vessels perfused by microfill. (f-g) Typical 3D reconstruction micro-CT images of the edges between materials and rabbit calvarial defects and analysis of the volume ratio of the newly formed bone to the defect regions (BV/TV). (h) The undecalcified histological sections stained with Van Gieson’s picrofuchsin, newly formed bone tissues (in red) can be well observed (blue arrows point to the new bone). Adapted from.125
Figure 11.
(a) Designed and printed scaffolds with various shapes of MgPSr-PCL30. (b) Schematic representation of the implantation of cylindrical constructs in the equine tuber coxae. (c) Histological assessment of new bone (*) within the MgPSr-PCL30 scaffolds after 6 months. Representative haematoxylin and eosin, basic fuchsin/methylene blue-stained MMA samples, immunohistochemical staining for collagen type I (brown region), and picrosirius red–stained tissue sections of defects filled by MgPSr-PCL30 scaffolds (S) and of empty defects. The scale bar is 50 μm. Adapted from.35
Recently, various attempts have been made to enhance the healing of bone and periodontal tissues by the utilization of growth factors to directly influence tissue morphogenesis.82–84 Hence, the appropriate release of therapeutics from 3D printed scaffolds can provide key biochemical cues to boost tissue regeneration. Currently available 3D printing technologies have been able to generate geometrically-complex scaffolds loaded with a wide variety of antimicrobial drugs and growth factors. In fact, the tunable porous structure of ceramics is suitable to the loading and release of biomolecules, such as proteins, polypeptides, or amino acids.142,143 Akkineni et al. (2015) demonstrated the feasibility of printing, via pneumatic extrusion, CaP cement composite scaffolds premixed with chitosan/dextran sulphate microparticles for localized delivery of vascular endothelial growth factor (VEGF).144 Scaffolds loaded with VEGF demonstrated higher cell viability compared to control (VEGF-free) scaffolds and higher ALP activity of MSCs cultured on the scaffolds for up to 21 days.144 Similarly, 3D printed β-TCP/gelatin/alginate composite scaffolds encapsulated with VEGF-releasing poly(lactic-co-glycolic acid) (PLGA) microspheres showed improved cell proliferation and attachment of HUVECs and osteoblasts when compared to control scaffolds.145 Of note, VEGF-laden scaffolds promoted significantly greater ALP activity compared to control scaffolds after 7 and 14 days of in vitro culture.145
Bone morphogenetic protein 2 (BMP-2) belongs to the transforming growth factor superfamily and plays an important role in bone regeneration.146 Numerous studies have reported the use of BMP-2 to repair critical size bone defects; however, a carefully designed delivery system is critical to avoid adverse events, such as ectopic bone formation.82,83 In a study conducted by Wang et al. (2016), a HAp scaffold was first obtained via 3D printing and then coated with collagen/chitosan microspheres loaded with BMP-2 to obtain continuous growth factor release. After 8 weeks of in vivo implantation, ectopic bone formation was observed only in the HAp scaffold coated with BMP-2.147 Meanwhile, 3D printed mesoporous silica (MS)/CaP cement-based scaffolds loaded with BMP-2 showed increased bone formation and angiogenesis compared to BMP-2 lacking control scaffolds.148 In addition to growth factors, alternative therapeutic drugs have been incorporated into 3D printed scaffolds. In a study by Bekisz et al. (2018), the local delivery of dipyridamole using 3D printed β-TCP/HAp scaffolds in a highly translational sheep calvarial defect model led to significant new bone.149
3.2.2. Cell-laden scaffolds
Over the last decade, 3D printing technologies have received substantial consideration due to the remarkable disruptive potential in regenerative dentistry by providing personalized solutions to reconstruct dental, oral, and craniofacial tissues compromised by trauma, disease and/or congenital deformities.14,27,28 Nonetheless, to satisfy tissue-specific needs it is important to imitate the biologic and functional hierarchy of the native tissues. In this way, bioprinting has materialized as a new and innovative 3D printing method for the generation of biologically functional tissue constructs with pre-programmed structures and patient-specific geometries. Bioprinting is defined as cell-laden biomaterial printing with precise positioning of cells into three-dimensionally organized structures using computer-aided layer-by-layer deposition.150 Noteworthy, well-defined and geometrically complex structures laden with single or heterogenous cell populations can be realized to generate constructs physiologically and morphologically similar to relevant living tissues.151 To date, depending on the cell type and bioink composition, extrusion-based bioprinting techniques have resulted in cell viability ranging from 80% to 90% within the bioprinted construct.13,31,152–154 Hydrogel precursors are commonly used as bioink because they can provide a highly hydrated and permeable 3D polymeric structure that affords improved nutrient and oxygen diffusion, and thus enhancing cell survival and function.155 In essence, hydrogels provide an ECM-like microenvironment, and can be tuned to enable cells to preferentially migrate within the hydrogel structure, unlike in native ECM.156–158
Recently, cell-laden hydrogels modified with carefully selected growth factors have been deployed as highly translatable bioprinting strategies to generate vascularized bone tissue constructs with a high degree of reproducibility.36,152 For instance, a pneumatic dispensing system was used to print bone marrow-derived MSCs (BMSCs) embedded in Poloxamer 407 and alginate.153 There was no significant decrease in cell viability upon printing when compared to the non-printed cell-laden hydrogel counterparts. After 2 weeks, 3D bioprinted BMSCs expressed early markers of osteogenic differentiation, indicating that the printed cells were able to differentiate within the constructs following in vitro culture.153
Traditionally, the combination of hydrogel with bioceramic is encouraged as the closest replicate of the extracellular matrix (ECM) composition of native bone.159 Moreover, the incorporation of bioceramics into hydrogels has the ability to augment the bioactivity of the loaded cells.160,161 As an example, a composite scaffold was devised by the combination of printable CaP cement, together with a cell-laden bioink consisting of 3% alginate and 9% methylcellulose (MC).36 There was no difference in cell viability between pristine alginate/MC scaffolds compared to CaP-modified bioprinted constructs. More importantly, after 7 days of in vitro culture, human MSCs started to migrate from alginate/MC bioink towards CaP cement strands.36 Moreover, a model of osteochondral tissue grafts was fabricated by combining CaP cement, alginate/MC, and biphasic scaffold (consisting of CaP cement and alginate/MC bioink).36 In a different study, Kim and colleagues proposed cell-laden β-TCP and collagen hydrogels using a two-step printing process. Briefly, micron-sized α-TCP/collagen struts were printed to provide a mechanically stable structure, upon which a cell-laden collagen bioink was bioprinted onto the α-TCP/collagen struts. The composite scaffold showed significantly higher cellular activities compared to the cell-laden collagen scaffolds (Figure 12a) along with improved osteogenic differentiation (Figure 12b).31
Figure 12.
(a) In vitro cell-activities and live/dead images of the cell-laden scaffolds. (b) Osteogenic activities (Alizarin Red S and Osteopontin staining) of cell-laden scaffolds at day 14. Adapted from.31 (c) Calcein AM (green) and PI (red) staining assay for live and dead analysis of DPSCs after 1, 3, and 5 days in the cell-laden ECM and ECM/AMP-bioprinted constructs (red arrows: dead cells). DPSCs show an elongated morphology in AMP-modified constructs. (d) Micro-CT analysis of rat skull 3D rendering at 4- and 8-weeks. Calvarial defects that were left empty did not heal spontaneously for the duration of the study. In contrast, bone healing was gradually achieved when the defects were filled with either ECM or ECM/1.0AMP. Adapted from.13
As previously alluded to, the modification of bioinks with growth factors has been shown to significantly heighten osteogenesis.82,106,145 BMP-2 serves as an important growth factor for bone regeneration; however, its half-life in the body is extremely limited and has some key safety concerns that are currently far from being addressed.162 To surmount this drawback, our group developed a bioink that combines micron-sized amorphous magnesium phosphate (AMP) particles and an extracellular matrix (ECM) hydrogel containing 2% octapeptide FEFEFKFK (F/phenylalanine; E/glutamic acid; and K/lysine) and 98% water with excellent printability and shape fidelity. The encapsulated cells in AMP-modified bioprinted constructs showed elongated morphology and viability similar to AMP-free hydrogel (Figure 12c). Notably, cell-free AMP-laden constructs demonstrated increased bone formation when implanted in calvarial defects for 8 weeks when compared to the AMP-free constructs, indicating AMP’s chemical ability to trigger osteogenic differentiation of endogenous cells surrounding the bone defect (Figure 12d).13 In a similar study, the incorporation of β-TCP particles into a collagen-based bioink led to 3D constructs with improved stiffness and enhanced osteogenic differentiation of encapsulated adipose stem cells.163
The translation of cell-printing technologies to preclinical and clinical models is still challenging due to the decreased shape fidelity of cell-laden constructs post-printing. To overcome this challenge, nanoengineered ionic covalent entanglement (NICE), a covalently crosslinkable gelatin methacryloyl (GelMA); ionically crosslinkable kappa-carrageenan; and electrostatically-charged nanosilicates were used to formulate a novel bioink (Figure 13a-b). The 3D printing of hMSCs encapsulated in growth factor-free conditions showed osteo-related mineralized ECM (Figure 13c-d).164 Hence, it is expected that cell-laden bioprinting can result in the development of tissue constructs with structural integrity and clinically relevant anatomical geometries for craniofacial bone and periodontal regeneration.
Figure 13.
(a) The 3D printability of each NICE bioink formulation was quantified using screw-driven extrusion printer to fabricate a 3 cm tall, 1 cm wide hollow tube. (b) Histology show progressive changes in the ECM of 3D bioprinted structures. Masson trichrome (bone tissue) Safranin O (cartilage tissue) Alcian Blue (connective tissue). Adapted from.164
Collectively, the aforementioned findings show, that extrusion-based bioprinting can render scaffold or tissue constructs with prominent shape fidelity. Remarkably, a variety of printhead designs can be used to load and print diverse materials to ensure control in terms of biological, physicochemical, and mechanical properties. The spatiotemporally controlled release of growth factor(s) from 3D bioprinted constructs and the incorporation of vascular-like channels demonstrated the potential therapeutic benefit of regenerating large bone defects with improved vascularization. The simultaneous printing of multiple materials using distinct extrusion-based methods can facilitate the engineering of scaffolds with compositional and structural gradients to mimic native tissues and tissue interfaces. Moreover, extrusion-based bioprinting has resulted in adequate cell survival and function, particularly within small bone tissue constructs. In sum, extrusion-based bioprinting has made sizeable advances in the development of in vitro model systems and in vivo therapeutics with translational potential for applications in craniofacial bone and periodontal tissue reconstruction.
4. Convergence bioprinting technologies for fabrication of architecturally complex constructs
Living tissues are architecturally complex structures with different cells and matrices arranged in three dimensions, and thus engineering of bone and periodontal tissues to replace damaged tissues demands a tissue-specific and personalized approach. In this sense, the dynamics of 3D printing and bioprinting technologies have enhanced the perspective for complete regeneration of craniofacial bone and periodontal tissues through the fabrication of defect-specific designs to mimic structure(s) damaged or lost due to trauma, resection, or chronic inflammatory diseases, such as periodontal disease.
A leading approach for the reconstruction of highly organized living tissues and tissue interfaces, resides in the convergence of complementary 3D printing/bioprinting technologies integrated into a single biofabrication platform, recently termed multitechnology fabrication (Figure 14a). Converged biofabrication has the potential to yield scaffold systems with high printing fidelity, spatial control over cell distribution and improved biomechanical properties without compromising cell viability and functionality.40 For example, successful combined approaches of hydrogel printing and electrospinning or MEW of polymers have already been reported.38,165 In addition, Xu and colleagues pioneered the combination of inkjet bioprinting with solution electrospinning to fabricate cartilage tissue constructs.165 By alternating the deposition of electrospun polycaprolactone/pluronic fiber meshes and inkjet printed chondrocytes containing hydrogels, the authors were able to create a series of millimeter-sized constructs with promising biomechanical properties and encouraging cartilage-like tissue formation. More recently, de Ruijter and co-authors shown the successful combination of extrusion-based bioprinting and MEW in a single biofabrication platform, which allowed for the generation of living constructs with the spatial distribution of mesenchymal stromal cells and improved biomechanical functionality without jeopardizing cell viability or chondrogenic differentiation (Figure 14b-d).38 In a follow up study Diloksumpan and colleagues demonstrated another promising converged biofabrication strategy to engineer hard-to-soft tissue interfaces (Figure 15a-h).166 Altogether, the integration of fiber technologies with cell printing enhances the mechanical competences of the bioprinted constructs while simultaneously offering cues for (stem) cells to differentiate and organize tissue matrix. In a study focus on the hard-to-soft periodontal tissue interface, an osteoconductive biphasic scaffold with a bone compartment was fabricated using β-TCP/PCL printed via FDM, then coated with CaP and the periodontal compartment was electrospun through a melt electrospinning device (Figure 16a). In vivo analysis confirmed the integration of tissue between the two compartments and the formation of structurally similar tissue to native periodontal tissues. (Figure 16b-c).167
Figure 14.
(a) Schematic Illustration of multitechnology biofabrication showing typical operation length of single deposition biofabrication technologies with the size and hierarchical structure of tissues and organs. Adapted from 40 (b) Convergence of MEW (PCL) and extrusion-based bioprinting (GelMA) into a single-step approach. (c) Fluorescence staining showing spatial placements of the cells in the composite constructs. (d) The guidance of MEW fibers over a strand of hydrogel for development of complex tissue architectures l) interlocked with hydrogel, and ll) out-of-plane fiber deposition. Yellow arrows depict the hydrogel whereas the white arrows depict the PCL fiber. Scale bar = 500 μm Adapted from.38
Figure 15.
(a) Fabrication process of the osteochondral construct by using a combination of different 3D printing techniques. (b) Mechanical properties of the biomimetic PCaP scaffolds. (c) Micrograph obtained from micro-CT scanning showing a biomimetic PCaP scaffold that could be placed press-fit inside an ex vivo osteochondral defect. (Scale Bar = 1 mm.). (d) basic fuchsin and methylene blue staining reveal pattern of embedded PCL microfibres inside the non-porous layer of the C-PCaP scaffold of the constructs with osteal C-PCaP anchor. (Scale Bar = 100 μm.). (e) quantification of sGAG in hydrogel per DNA content. (f) Interfacial adhesion strength, and (g) interfacial toughness (day 1 and day 42) while applying shear force at the interface between equine ACPCs encapsulated in GelMA and C-PCaP-based bone compartment. (h) Safranin-O staining (1), collagen type II immunostaining (2) and collagen type I immunostaining (3) of paraffin embedded microfibre reinforced GelMA without osteal C-PCaP (F) and with osteal C-PCaP (G), respectively, after cultivation for 42 days. (Scale Bar = 100 μm). Adapted from.166
Figure 16.
(a) Graphical illustration of the biomimetic coating procedure, the fabrication of the biphasic scaffold, cell seeding, subsequent in vitro culture and in vivo implantation of the cellular constructs. (b) SEM images of the seeded scaffolds after 6 weeks of in vitro culture under four different conditions. N–N non-coated scaffold cultured in basal medium, N–O non-coated scaffold cultured in osteogenic medium, calcium phosphate–N calcium phosphate-coated scaffold cultured in basal medium, calcium phosphate–O calcium phosphate-coated scaffold cultured in osteogenic medium. (c) Representative H&E and immunostaining images of implanted biphasic scaffolds. Adapted from.167
Alongside this strategy, other combinatorial approaches consisting of extrusion-based printing integrated with sacrificial support materials,168 external magnetic field stimulation169,170 or atmospheric plasma functionalization171 have been described. Such strategies have the potential to generate personalized biologically relevant tissue-like materials with relevant sizes and structures that were previously deemed as “unprintable”, while affording high cell viability. These biofabrication platforms can also provide suitable mechanical and structural features to direct cell growth and differentiation. An even more enticing feature of this fabrication systems is the combination of digital design tools coupled with multitechnology biofabrication to create materials with locally defined chemical compositions, structures, and properties that can resemble biological materials and ultimately drive tissue regeneration.40 We strongly believe, this next generation of multitechnology biofabrication systems will incorporate real-time inline monitoring of the printing process through combinations of machine vision, inspection sensors, and feedback control systems supervised by artificial intelligence (AI) algorithms for high-throughput and truly functional tissue equivalents biomanufacturing.40
It is worth noting that the hierarchically organized bone and connective tissues also present micro-vascularization and innervation that should be replicated while generating scaffolds for bone and periodontal tissue engineering. Although mimicking bone microvasculature and innervation is usually neglected in regenerative therapies, recent publications have directed their focus over to these very sensitive matters for successful clinical translation of bone tissue analogs.172–174 For instance, tuning β-TCP scaffolds with 5% calcium-silicate and loading the 3D constructs with co-cultures of HUVECs and human bone marrow-derived stem cells (hBMSCs) induced vessel formation through the porous structure of the scaffolds and new bone tissue in rat models.172 The authors also highlighted that the revascularization process contributed to the supply of biomolecules involved in bone tissue regeneration. Meanwhile, another significant gain in vascularized tissue during bone regeneration was achieved by 3D printing PCL-Chitosan-deferoxamine scaffolds.173 Deferoxamine (DFO) promotes hypoxia conditions, which apparently plays a role in the modulation of genes key to angiogenesis and osteogenesis. In this way, 3D printed scaffolds modified with DFO led to the upregulation of essential genes related to vascularization and mineralization and induced high vascular volume and bone formation in femoral defects.173 Regardless of the important findings of vascularization- and bone regeneration-inductive mechanisms, these studies printed porous constructs involving no specific architecture, and the innervation aspect was not taken into account.
Remarkably, in a recently published work, the unique architecture of Haversian bone was successfully mimicked using 3D printing for multicellular delivery in bone regeneration.174 Bioceramic-based scaffolds were printed by digital laser processing (DLP) with different pore sizes and arrangements that replicate cancellous bone, Haversian, and Volkmann canals within a single construct, as evidenced in natural bone and by the amount and distribution of the channels, as the volume of cortical bone determined the strength of the scaffolds. The co-cultures of osteogenic and angiogenic or neurogenic cells were effective in proliferating and expressing specific markers of mineralization, vascularization, and innervation. Noteworthy, upon in vivo implantation the fabricated scaffolds induced new bone formation, following the design of the construct, and the endothelial cells cultured into the Haversian canals accelerated vessels’ ingrowth through the construct.174 However, the proposed co-cultures’ model still needs to be checked in vivo, including osteogenic, neurogenic, and vasculogenic cells in a single model to validate the findings. Nonetheless, the gradually arranged and highly complex architectures present in that scaffold reinforce the remarkable advances 3D printing technology has brought to the dynamics of regenerating bone.
Latest research 3D printed the stackable miniaturized microcage modules, resembling the features of toy interlocking building blocks (e.g., LEGO) loaded with different growth factors, such as VEGF, PDGF, and BMP2 to create a concentration gradient within the microcage scaffold to direct cell migration in specific fashion. Lithography-based Ceramic Manufacturing (LCM) 3D printing technique, was used to create 1.5 mm microcages with wall thickness of 230–560 μm. Digital Light Processing (DLP) 3D printing was used to build five-pointed flower-like geometry loaded with growth factors which was manually placed inside the microcage. The study found that the infiltration of cells was 2-fold greater and vascularization was 3-fold higher in microgel-loaded microcage modular scaffolds than hydrogel-impregnated composite scaffold (Figure 17a-b).175
Figure 17.
(a) (A-D) 3D diagram showing the distribution of microgels and impregnation of hydrogel in individual microcage units. (E-H) Photographs of the microcage scaffolds schematically depicted above after loading with fluorescently labeled microparticles embedded with the microgel and monolithic hydrogel. (I-K) Transwell migration assay shows distinct migration toward the microcages as confirmed by the immunofluorescence staining images (top: 2D view, bottom: 3D view) and Corresponding quantitative data of cells migration (p < 0.05) (scale bar: 1.5 mm). (b) (A-B) Angled, top, and orthogonal projections of F-actin-stained samples show the infiltration of cells throughout the microgel-loaded microcage scaffolds in comparison with the hydrogel-impregnated samples. (C-G) Histology (toluidine blue staining) images, quantification of cell distribution and vessel diameter show directed cell migration and neovascularization (indicated by arrowheads) in the periphery and core regions of the microgel-loaded microcage scaffold, when compared with the hydrogel-impregnated samples, which showed little evidence of cellularity and vasculature formation in the core regions. Adapted from.175
Craniofacial bone injuries can occur throughout the lifetime of a patient. Hence, it is critical to ponder the age and stage of bone development when planning a regenerative-based therapy. Also, congenital anomalies, such as orofacial clefts, imply special approaches and knowledge regarding tissue development and growth. Accordingly, advances in the field were recently achieved using 3D printed scaffolds as a concept extrapolation for regeneration at different stages of bone growth.176,177 For instance, β-TCP scaffolds loaded with dipyridamole, an osteoinductive drug, were previously tested in calvaria149 and to regenerate long bone defects.178 The 3D printed scaffolds coated with dipyridamole177 led to bone formation throughout the scaffolds’ porosities, regardless of pore dimensions, and preserved suture patency for therapeutic and super-dosages of the osteoinductive drug.177 Moreover, dipyridamole-loaded scaffolds induced native bone-like formation in vivo,176 and there was neither ectopic bone formation nor scaffold-induced asymmetry, and the bone sutures were not affected by the bone regeneration process (Figure 18a-c).176 Since suture patency preservation is relevant to maintaining normal development of the craniofacial skeleton, these studies informed an important path on the use of 3D printing for bone regeneration when considering different stages of life and bone growth. However, these experiments were not tested in a real craniofacial cleft defect. Ideally, that type of analyses should map the real defect based on 3D images to be converted into defect-specific constructs.
Figure 18.
(a) Micro–computed tomographic slices of new bone formation within scaffold at 8 weeks. The osseoconductive infiltration through the scaffolds (green and yellow arrows on second row) and bone regeneration in immature skeletal calvaria did not affect the suture patency (red arrows). (b-c) Histological examination showing bone formation is guided by highly osseoconductive scaffold dimensions as new bone formation is directed from scaffold pore-to-pore while interacting with scaffold struts. Adapted from.177 (d) Histological analysis of bone defect site in rabbit mandibles, ×20 (e) Images of the specimens. (f) Radiographs of the specimens. (g) Two-dimensional, 3-dimensional micro–computed tomography (CT) images of the scaffolds implanted in the rabbit alveolar defects with different time separately. Adapted from.126
The periodontium forms an important complex and comprises multiple tissues (i.e., gingival, cementum, periodontal ligament, and alveolar bone) at distinct locations that act together to allow the tooth to be attached to the jaw and to afford a defensive barrier for the underlying structures from the oral microflora. In order to overcome the challenges in regenerating multiple tissues, hydrogel-based scaffolds must contain a heterogeneous cell population at predesignated sites with a high degree of spatial organization179 to allow the fabrication of a multitissue structure comprising bone and mineralized cementum-like core enclosed within a periodontal ligament (PDL)-like tissue. An innovative approach, with significant potential in periodontal tissue regeneration, was successfully deployed using a multi-head tissue/organ building system (MtoBS) with 6 dispensing units for the biofabrication of cell-laden constructs for osteochondral tissue regeneration. The multilayered living constructs containing human turbinate-derived mesenchymal stromal cells (hTMSCs) and growth factors was printed in the empty cavity of a predesigned biodegradable polymer (PCL) framework. The hTMSCs were embedded in a hydrogel comprised of atelocollagen and supramolecular hyaluronic acid to give rise to subchondral bone, and a superficial cartilage layer, respectively.37 In addition, transforming growth factor (TGF)-β and rhBMP-2 were added to atelocollagen and supramolecular hyaluronic acid, respectively. PCL fibers were extruded from a heated nozzle of MtoBS, an in-house-developed 3D printing system. A cell-rich cylindrical 3D construct 5 mm in height was divided into a 4 mm subchondral bone layer and a 1 mm cartilage layer and was implanted into the osteochondral defect in the knee joint of a rabbit. Macroscopically, the defect was fully covered after 8 weeks with neo-tissue, exhibiting a smooth surface, and the cartilage-like neo-tissue was whiter than the adjacent native cartilage. However, cell-seeded PCL scaffolds partially covered the defect, and neo-tissue was primarily found in the interface between the native tissue and the defect.37
Over the last decade, promising steps to realize tissue-specific and geometrically complex constructs have been possible through the combination of high-resolution images from 3D computed tomography (CT) and computed software available for 3D printing, which has fostered new horizons on the development of personalized scaffolds for regenerative medicine. This combination has been used for many years by prototyping bone implants. Now, the conversion of CT images into defect-specific scaffolds has also instigated novel therapies and biofabrication approaches, for craniofacial bone and periodontal tissue regeneration.126,180 For example, mineralized collagen-loaded porous titanium scaffolds were designed based on CT images of rabbits’ large bone defects.180 The porous structure of titanium and the collagen embedded in the scaffolds not only induced new bone formation and osseointegration, but also stimulated the expression of angiogenesis markers through the porosities.180 Despite osseointegration and biocompatibility properties, titanium constructs are not degradable. Thus, this type of construct is directed to weight-bearing and large bone defects’ reconstruction. Regarding craniomaxillofacial bone defects, digital images from 3D microfocus CT were used to obtain defect-specific bioceramic constructs for repairing mandibular defects.126 Wollastonite scaffolds modified 10% Mg induced bone regeneration throughout the entire defect after 16 weeks and a reasonable degradability ratio. These scaffolds were also more efficient as a regenerative material than other CaP-based constructs (Figure 18d-g).126 These bone-regeneration outcomes were achieved in controlled defects from sound tissue models, without infection, which makes this type of construct suitable for treating fractures and resections. However, it is hard to predict the behavior of these constructs as facing possibly infected niches like it can occur in periodontal defects. Therefore, while printing cells and/or personalized constructs, drugs can also be incorporated into the system to minimize inflammation of surrounding tissue to allow for healthier bone growth.181 For instance, osteoblasts-laden alginate-based hydrogels containing diclofenac, an anti-inflammatory drug, were bioprinted using a pneumatic system. The alginate scaffold was coated with 1% chitosan scaffold to control release of diclofenac from the bioprinted construct. Taken together, the study confirmed that diclofenac suppressed secretion of inflammatory compounds and resulted in enhanced cell proliferation and calcium secretion.182
From a detailed dental perspective, periodontium comprises protective and supportive structures, and its interactive function is important for maintaining oral health. Furthermore, periodontal structures involve hard (alveolar bone and cementum) connective tissues (periodontal ligament) and their interfaces, which makes the design of personalized and defect-specific 3D constructs a challenge for the simultaneous and coordinated growth of hard and soft tissues. Theoretically, ideal constructs for periodontal tissue regeneration should present a certain level of compartmentalization, because of differences in size, arrangement, type of cells, and structural properties of the three (i.e., cementum, periodontal ligament, and alveolar bone) components of the supportive periodontium. At the same time, the system works as a bundle that cannot be dissociated, and their interfaces micron-scale organization and transitions are more complex to replicate and demand very accurate 3D printing technologies. Therefore, over the last decade, the development of scaffolds to guide periodontal ligament fibers’ alignment and proper insertion is a relevant approach to mimic the native conditions of periodontal tissues has been systematically investigated. For instance, the periodontal ligament (PDL) attaches to bone and cementum and plays an important role in biomechanical load distribution and proprioception. Each group of fibers of the PDL has a specific alignment contributing to masticatory and bite force dissipation, while it controls tooth movement and stability. Thus, controlling the fiber deposition and space between layers has fostered the development of 3D printing methods that can fabricate accurate scaffolds that replicate not only the micron-scale, but also the geometries of specific periodontal defects.
From this viewpoint, it is noteworthy that different 3D printing techniques have been purposed to develop scaffolds for PDL regeneration, but feasibility and clinical translation of these methods are still limited.183–186 Initial achievements were mostly based on the use of CT images to aid accurate size and orientation, while designing the constructs. For instance, cone-beam CT (CBCT) images from the periodontal defects of rats were used to create 3D designs with structural integration among components of supportive periodontal tissues, and, subsequently, freeze-casted PCL constructs were synthesized from those designs to guide regeneration and PDL fibers’ orientation.185 The constructs had a high adaptation ratio to the defect, while the same constructs loaded with human PDL cells (hPDL) and fibrin gel induced PDL fibers’ regeneration and proper orientation, and the bone part of the construct induced new bone formation (Figure 19a-c).
Figure 19.
(a) Micro-CT images of a periodontal defect and the design and adaptation ratio of a site-specific scaffold for regeneration. (b) SEM images evidencing dense formation of fibrous tissue for the fiber-guiding scaffolds and collagen-like fibers anchorage on the dentin structure. (c) Histological analysis (H&E) comparing tissue morphologies and organization of random and fiber-guiding scaffolds. Adapted from.185
Another interesting aspect about the PDL is that it presents different micron-scale organization according to the region of the root where it attaches. To address that specificity, micro-grooved 3D patterns were developed to guide PDL fibers formation.183,184 The freeze-casted PCL micro-grooved constructs induced PDL cells’ alignment at different angles (0°, 45°, and 90°) (Figure 20a-b).184 Also, these studies reinforced that the scaffolds and polymer nature-related factors, such as depth and roughness of the constructs, influence the organization of newly formed tissue in vivo.183,184 On one hand, designing angulated micropatterns for guiding PDL regeneration encourages further research. On the other hand, removing the construct from the mold when using this more simplistic casting-assisted approach is a very sensitive procedure and minimal errors in that step could compromise the integrity of the scaffold.
Figure 20.
(a) Micro-groove pattern designs to guide PDL orientation (0°, 45°, and 90°). SEM and confocal microscopies illustrates surface morphology and topography of the constructs according to the micro-groove orientation. (b) Fluorescence images and quantitative data for the cell and nuclear angulation of hPDL cells being influenced by the 3D micro-groove patterns after 7 and 21 days of culture. Adapted from.184 (c) 3D printing using polycaprolactone was made to fit the periosseous defect based on the patient’s cone beam computed tomography scan. The implanted 3D scaffold filled the periodontal osseous defect without clinical signs of chronic inflammation or rejection of the polycaprolactone-based material during the first year. After 14 months, due to postoperative exposure (i.e., soft tissue dehiscence over the scaffold), the patient-specific 3D printed scaffold was retrieved. Histologic analysis of the retrieved scaffold with Masson’s trichrome staining indicates small islands of new bone formation within a milieu of primarily granulomatous tissue (yellow arrowheads). Scale bar = 50 μm. Adapted from.14
Fortunately, other 3D printing technologies have been identified to design and print these complex periodontal tissue constructs. In this way, it is necessary to combine the most relevant findings of different research groups to develop prime 3D printed scaffolds for clinical translation. To date, there is a single clinical report in the literature that uses CBCT images to create a defect-specific 3D printed polymeric (PCL) scaffold for periodontal regeneration,14 designed using a selective laser sintering (SLS) approach to treat a large periodontal osseous defect.14 Specifically, SLS was utilized to fabricate a HAp-modified PCL-based composite scaffold according to the anatomical configuration of the defect. The scaffold was designed to display perforations for fixation, an internal port for delivery of recombinant human platelet-derived growth factor (PDGF-BB), and pegs perpendicularly oriented to the root to guide PDL formation. The adaptation ratio based on the methodology for PDL fiber guidance was defined based on micro-CT data. Prior to implantation, the scaffold was immersed in rhPDGF-BB, filled with autologous blood, and stabilized over the defect with resorbable pins. No clinical signs of chronic inflammation or rejection associated with the presence of the scaffold was seen during the first 12 months. A burst release of rhPDGF-BB was observed within the first 3 h in an in vitro drug release study. From a clinical standpoint, the scaffold remained covered for 12 months, revealing a 3-mm gain of clinical attachment and partial root coverage. Regrettably, scaffold exposure was noticed at 13 months and, though palliative measures were completed to save the treatment, the implanted scaffold (~ 76% of its original molecular weight) was removed (Figure 20c).14 While the success of that clinical study was far from ideal, given that complete regeneration of the periodontium was not observed, it provided key information to drive the field forward, particularly regarding the aspects associated with scaffold design and fabrication.
5. Future outlook
Although 3D (bio)printing has been identified as a paradigm-shifting platform technology with real potential in delivering patient-specific and functional regenerative therapeutics in dentistry, it still remains in its infancy. Undoubtfully, future work in the development of personalized and functional scaffolds for craniomaxillofacial (CMF) bone and periodontal regeneration will continue to encompass high-resolution imaging (computed tomography) combined with printing technologies and biomaterials to recapitulate not only the tissue-specific architecture and function, but also to address the very challenging objective of rebuilding the different tissues and tissue interfaces found in the periodontium. To address these challenges, great efforts have been made to engineer defect-specific scaffolds mimicking the hierarchical and complex architecture of the periodontium.
Up to now, extrusion-based (bio)printing systems have shown great potential to design, using a variety of biomaterials, personalized scaffolds for CMF bone and periodontal tissue regeneration. Although this printing technology has been successfully applied to fabricate scaffolds/constructs loaded with tissue-specific cell types and/or biomolecules to amplify the predictability of the regenerative outcome; little attention has been given to antimicrobial and immunomodulatory strategies. Future work on the integration of antimicrobial drugs and/or biomaterials with immunomodulatory capability to enhance the regenerative response upon 3D (bio)printed scaffold implantation should be also explored.
In addition, ongoing improvements in 3D (bio)printing of engineered vascularized and innervated analogous bone tissues are outlining as the future of regenerative oral and orthopedic bone regeneration therapies. These developments will lay the groundwork for designing improved scaffolds and implants both from an anatomical and biological fidelity viewpoint. Nonetheless, concerns about successful and expeditious translation of 3D bio(printing) technologies for regenerative dentistry is dependent on the careful development of guidelines and standardization of clinical protocols to accelerate the required approval from regulatory agencies, such as the FDA and CE. To reach this goal, future studies should broaden the concept of technology convergence by leveraging the fast-evolving 3D (bio)printing technologies (multimaterial bioplotters) and materials/bioinks to build physiologically relevant tissues and tissue interfaces to suitably reconstruct CMF bones and periodontal tissues. The successful translation of these concepts to clinics will support multiple possibilities of regenerative therapies and quotidian patient care.
Acknowledgements
The authors are grateful for the images and cartoons designed by Kenneth Rieger (Multimedia Designer, Department, School of Dentistry, University of Michigan, Ann Arbor, MI, USA). This project was supported by funding from the National Institutes of Health (NIH, National Institute of Dental and Craniofacial Research (K08DE023552 and R01DE026578 to MCB), the Osteology Foundation (Advanced Researcher Award), OsteoScience Foundation (Peter Geistlich Research Award), the International Association for Dental Research (IADR-GSK Innovation in Oral Care Award and IADR-AO Implant Sciences Award), the American Academy of Implant Dentistry Foundation, the European Union H2020 program BRAVE (grant number 874827) and the Gravitation Program “Materials Driven Regeneration” by the Netherlands Organization for Scientific Research (024.003.013).
Footnotes
Disclosure statement
The authors declare no competing financial interest or with respect to the authorship and/or publication of this article.
References
- 1.Zhang W and Yelick PC, Cold Spring Harbor perspectives in medicine, 2018, 8(1), a025775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Futran ND and Mendez E, The Lancet Oncology, 2006, 7(3), 249–258. [DOI] [PubMed] [Google Scholar]
- 3.Genden EM, Okay D, Stepp MT, Rezaee RP, Mojica JS, Buchbinder D, and Urken ML, Archives of Otolaryngology–Head & Neck Surgery, 2003, 129(7), 775–780. [DOI] [PubMed] [Google Scholar]
- 4.Ward B, Brown S, and Krebsbach P, Oral diseases, 2010, 16(8), 709–716. [DOI] [PubMed] [Google Scholar]
- 5.Peres MA, Macpherson LM, Weyant RJ, Daly B, Venturelli R, Mathur MR, Listl S, Celeste RK, Guarnizo-Herreño CC, and Kearns C, The Lancet, 2019, 394(10194), 249–260. [DOI] [PubMed] [Google Scholar]
- 6.Eke PI, Dye B, Wei L, Thornton-Evans G, and Genco R, Journal of dental research, 2012, 91(10), 914–920. [DOI] [PubMed] [Google Scholar]
- 7.Eke PI, Borgnakke WS, and Genco RJ, Periodontology 2000, 2020, 82(1), 257–267. [DOI] [PubMed] [Google Scholar]
- 8.Bottino MC, Pankajakshan D, and Nör JE, Dental Clinics, 2017, 61(4), 689–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornman KS, Periodontology 2000, 2018, 78(1), 12–29. [DOI] [PubMed] [Google Scholar]
- 10.Reichert JC, Cipitria A, Epari DR, Saifzadeh S, Krishnakanth P, Berner A, Woodruff MA, Schell H, Mehta M, and Schuetz MA, Science translational medicine, 2012, 4(141), 141ra193–141ra193. [DOI] [PubMed] [Google Scholar]
- 11.Obregon F, Vaquette C, Ivanovski S, Hutmacher D, and Bertassoni L, Journal of dental research, 2015, 94(9_suppl), 143S–152S. [DOI] [PubMed] [Google Scholar]
- 12.Dubey N, Ferreira JA, Daghrery A, Aytac Z, Malda J, Bhaduri SB, and Bottino MC, Acta Biomaterialia, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubey N, Ferreira JA, Malda J, Bhaduri SB, and Bottino MC, ACS Applied Materials & Interfaces, 2020, 12(21), 23752–23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasperini G, Pilipchuk S, Flanagan C, Park C, Pagni G, Hollister S, and Giannobile W, Journal of dental research, 2015, 94(9_suppl), 153S–157S. [DOI] [PubMed] [Google Scholar]
- 15.Shen C, Witek L, Flores RL, Tovar N, Torroni A, Coelho PG, Kasper FK, Wong M, and Young S, Tissue Engineering Part A, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bottino MC, Thomas V, Schmidt G, Vohra YK, Chu T-MG, Kowolik MJ, and Janowski GM, Dental materials, 2012, 28(7), 703–721. [DOI] [PubMed] [Google Scholar]
- 17.Yang F, Both SK, Yang X, Walboomers XF, and Jansen JA, Acta biomaterialia, 2009, 5(9), 3295–3304. [DOI] [PubMed] [Google Scholar]
- 18.Karring T, Journal of the International Academy of Periodontology, 2000, 2(4), 101–109. [PubMed] [Google Scholar]
- 19.Gentile P, Chiono V, Tonda-Turo C, Ferreira AM, and Ciardelli G, Biotechnology journal, 2011, 6(10), 1187–1197. [DOI] [PubMed] [Google Scholar]
- 20.Jung RE, Fenner N, Hämmerle CH, and Zitzmann NU, Clinical oral implants research, 2013, 24(10), 1065–1073. [DOI] [PubMed] [Google Scholar]
- 21.Ramakrishna S, Fujihara K, Teo W-E, Yong T, Ma Z, and Ramaseshan R, Materials today, 2006, 9(3), 40–50. [Google Scholar]
- 22.Greiner A and Wendorff JH, Angewandte Chemie International Edition, 2007, 46(30), 5670–5703. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Liu Y, Miao L, Wang Y, Ren S, Yang X, Hu Y, and Sun W, International journal of nanomedicine, 2016, 11, 3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gümüşderelioğlu M, Sunal E, Demirtaş TT, and Kiremitçi AS, Journal of Materials Science: Materials in Medicine, 2020, 31(1), 4. [DOI] [PubMed] [Google Scholar]
- 25.Yang F, Wolke J, and Jansen J, Chemical Engineering Journal, 2008, 137(1), 154–161. [Google Scholar]
- 26.Elgali I, Turri A, Xia W, Norlindh B, Johansson A, Dahlin C, Thomsen P, and Omar O, Acta biomaterialia, 2016, 29, 409–423. [DOI] [PubMed] [Google Scholar]
- 27.Carter S-SD, Costa PF, Vaquette C, Ivanovski S, Hutmacher DW, and Malda J, Annals of biomedical engineering, 2017, 45(1), 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zadpoor AA and Malda J, Additive manufacturing of biomaterials, tissues, and organs. 2017, Springer. [DOI] [PubMed] [Google Scholar]
- 29.Farag A, Hashimi SM, Vaquette C, Volpato FZ, Hutmacher DW, and Ivanovski S, Archives of oral biology, 2018, 88, 67–76. [DOI] [PubMed] [Google Scholar]
- 30.Criscenti G, Longoni A, Di Luca A, De Maria C, Van Blitterswijk C, Vozzi G, and Moroni L, Biofabrication, 2016, 8(1), 015009. [DOI] [PubMed] [Google Scholar]
- 31.Kim WJ, Yun H-S, and Kim GH, Scientific Reports, 2017, 7(1), 3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shim J-H, Lee J-S, Kim JY, and Cho D-W, Journal of Micromechanics and Microengineering, 2012, 22(8), 085014. [Google Scholar]
- 33.Ozbolat IT and Hospodiuk M, Biomaterials, 2016, 76, 321–343. [DOI] [PubMed] [Google Scholar]
- 34.Jakus AE, Rutz AL, Jordan SW, Kannan A, Mitchell SM, Yun C, Koube KD, Yoo SC, Whiteley HE, and Richter C-P, Science translational medicine, 2016, 8(358), 358ra127–358ra127. [DOI] [PubMed] [Google Scholar]
- 35.Golafshan N, Vorndran E, Zaharievski S, Brommer H, Kadumudi FB, Dolatshahi-Pirouz A, Gbureck U, van Weeren R, Castilho M, and Malda J, Biomaterials, 2020, 261, 120302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahlfeld T, Doberenz F, Kilian D, Vater C, Korn P, Lauer G, Lode A, and Gelinsky M, Biofabrication, 2018, 10(4), 045002. [DOI] [PubMed] [Google Scholar]
- 37.Shim J-H, Jang K-M, Hahn SK, Park JY, Jung H, Oh K, Park KM, Yeom J, Park SH, and Kim SW, Biofabrication, 2016, 8(1), 014102. [DOI] [PubMed] [Google Scholar]
- 38.de Ruijter M, Ribeiro A, Dokter I, Castilho M, and Malda J, Advanced healthcare materials, 2019, 8(7), 1800418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei C and Dong J, Journal of Manufacturing Processes, 2014, 16(2), 257–263. [Google Scholar]
- 40.Castilho M, de Ruijter M, Beirne S, Villette CC, Ito K, Wallace GG, and Malda J, Trends in Biotechnology, 2020. [DOI] [PubMed] [Google Scholar]
- 41.Haider A, Haider S, Kummara MR, Kamal T, Alghyamah A-AA, Iftikhar FJ, Bano B, Khan N, Afridi MA, and Han SS, Journal of Saudi Chemical Society, 2020, 24(2), 186–215. [Google Scholar]
- 42.Bottino MC, Thomas V, and Janowski GM, Acta biomaterialia, 2011, 7(1), 216–224. [DOI] [PubMed] [Google Scholar]
- 43.Xue J, Wu T, Dai Y, and Xia Y, Chemical reviews, 2019, 119(8), 5298–5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uyar T and Kny E: ‘Electrospun materials for tissue engineering and biomedical applications: research, design and commercialization’; 2017, Woodhead Publishing. [Google Scholar]
- 45.Yu X, Tang X, Gohil SV, and Laurencin CT, Advanced healthcare materials, 2015, 4(9), 1268–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reise M, Wyrwa R, Müller U, Zylinski M, Völpel A, Schnabelrauch M, Berg A, Jandt KD, Watts DC, and Sigusch BW, Dental Materials, 2012, 28(2), 179–188. [DOI] [PubMed] [Google Scholar]
- 47.Ho M-H, Chang H-C, Chang Y-C, Claudia J, Lin T-C, and Chang P-C, International journal of nanomedicine, 2017, 12, 5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He M, Jiang H, Wang R, Xie Y, and Zhao C, Journal of colloid and interface science, 2017, 490, 270–278. [DOI] [PubMed] [Google Scholar]
- 49.He P, Zhong Q, Ge Y, Guo Z, Tian J, Zhou Y, Ding S, Li H, and Zhou C, Materials Science and Engineering: C, 2018, 90, 549–556. [DOI] [PubMed] [Google Scholar]
- 50.Shahi R, Albuquerque M, Münchow E, Blanchard S, Gregory R, and Bottino M, Odontology, 2017, 105(3), 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ranjbar-Mohammadi M, Zamani M, Prabhakaran M, Bahrami SH, and Ramakrishna S, Materials Science and Engineering: C, 2016, 58, 521–531. [DOI] [PubMed] [Google Scholar]
- 52.Jia L.-n., Zhang X, Xu H.-y., Hua F, Hu X.-g., Xie Q, Wang W, and Jia J, Journal of Nanomaterials, 2016, 2016. [Google Scholar]
- 53.Bottino MC, Arthur RA, Waeiss RA, Kamocki K, Gregson KS, and Gregory RL, Clinical oral investigations, 2014, 18(9), 2151–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright ME, Wong AT, Levitt D, Parrag IC, Yang M, and Santerre JP, Journal of Biomedical Materials Research Part A, 2018, 106(5), 1211–1222. [DOI] [PubMed] [Google Scholar]
- 55.Münchow EA, Albuquerque MTP, Zero B, Kamocki K, Piva E, Gregory RL, and Bottino MC, Dental materials, 2015, 31(9), 1038–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nasajpour A, Ansari S, Rinoldi C, Rad AS, Aghaloo T, Shin SR, Mishra YK, Adelung R, Swieszkowski W, and Annabi N, Advanced Functional Materials, 2018, 28(3), 1703437. [Google Scholar]
- 57.Nasajpour A, Mandla S, Shree S, Mostafavi E, Sharifi R, Khalilpour A, Saghazadeh S, Hassan S, Mitchell MJ, and Leijten J, Nano letters, 2017, 17(10), 6235–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yar M, Farooq A, Shahzadi L, Khan AS, Mahmood N, Rauf A, Chaudhry AA, and ur Rehman I, Materials Science and Engineering: C, 2016, 64, 148–156. [DOI] [PubMed] [Google Scholar]
- 59.Furtos G, Rivero G, Rapuntean S, and Abraham GA, Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2017, 105(5), 966–976. [DOI] [PubMed] [Google Scholar]
- 60.Hokmabad VR, Davaran S, Aghazadeh M, Rahbarghazi R, Salehi R, and Ramazani A, Journal of biomaterials applications, 2019, 33(8), 1128–1144. [DOI] [PubMed] [Google Scholar]
- 61.Ko E, Lee JS, Kim H, Yang SY, Yang D, Yang K, Lee J, Shin J, Yang HS, and Ryu W, ACS applied materials & interfaces, 2017, 10(9), 7614–7625. [DOI] [PubMed] [Google Scholar]
- 62.Wu X, Miao L, Yao Y, Wu W, Liu Y, Chen X, and Sun W, International journal of nanomedicine, 2014, 9, 4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goncalves F, de Moraes MS, Ferreira LB, Carreira ACO, Kossugue PM, Boaro LCC, Bentini R, Garcia C. R. d. S., Sogayar MC, and Arana-Chavez VE, PLoS One, 2016, 11(3), e0152412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Z, Liang R, Jiang X, Xie J, Cai P, Chen H, Zhan X, Lei D, Zhao J, and Zheng L, Materials Science and Engineering: C, 2019, 104, 109796. [DOI] [PubMed] [Google Scholar]
- 65.Rowe MJ, Kamocki K, Pankajakshan D, Li D, Bruzzaniti A, Thomas V, Blanchard SB, and Bottino MC, Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2016, 104(3), 594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou T, Liu X, Sui B, Liu C, Mo X, and Sun J, Biomedical Materials, 2017, 12(5), 055004. [DOI] [PubMed] [Google Scholar]
- 67.Kharaziha M, Fathi MH, Edris H, Nourbakhsh N, Talebi A, and Salmanizadeh S, Journal of Materials Science: Materials in Medicine, 2015, 26(1), 36. [DOI] [PubMed] [Google Scholar]
- 68.Daghrery A, Aytac Z, Dubey N, Mei L, Schwendeman A, and Bottino MC, Colloids and Surfaces B: Biointerfaces, 2020, 111011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu W, Bi W, Sun Y, Wang L, Yu X, Cheng R, Yu Y, and Cui W, Materials Science and Engineering: C, 2020, 110, 110670. [DOI] [PubMed] [Google Scholar]
- 70.Yin L, Yang S, He M, Chang Y, Wang K, Zhu Y, Liu Y, Chang Y, and Yu Z, Journal of Materials Science: Materials in Medicine, 2017, 28(6), 94. [DOI] [PubMed] [Google Scholar]
- 71.Aragón J, Salerno S, De Bartolo L, Irusta S, and Mendoza G, Journal of colloid and interface science, 2018, 531, 126–137. [DOI] [PubMed] [Google Scholar]
- 72.Cheng G, Yin C, Tu H, Jiang S, Wang Q, Zhou X, Xing X, Xie C, Shi X, and Du Y, ACS nano, 2019, 13(6), 6372–6382. [DOI] [PubMed] [Google Scholar]
- 73.Wang Z, Wang Z, Lu WW, Zhen W, Yang D, and Peng S, NPG Asia Materials, 2017, 9(10), e435–e435. [Google Scholar]
- 74.Kowalczewski CJ and Saul JM, Frontiers in pharmacology, 2018, 9, 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hutmacher DW and Dalton PD, Chemistry–An Asian Journal, 2011, 6(1), 44–56. [DOI] [PubMed] [Google Scholar]
- 76.Kade JC and Dalton PD, Advanced Healthcare Materials, 2020, 2001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peiffer QC, de Ruijter M, van Duijn J, Crottet D, Dominic E, Malda J, and Castilho M, Materials & design, 2020, 195, 109025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abbasi N, Ivanovski S, Gulati K, Love RM, and Hamlet S, Biomaterials research, 2020, 24(1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Igawa K, Chung U, and Tei Y, Clinical calcium, 2008, 18(12), 1737. [PubMed] [Google Scholar]
- 80.Atala A and Yoo JJ: ‘Essentials of 3D biofabrication and translation’; 2015, Academic Press. [Google Scholar]
- 81.Placone JK and Engler AJ, Advanced healthcare materials, 2018, 7(8), 1701161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shim J-H, Kim SE, Park JY, Kundu J, Kim SW, Kang SS, and Cho D-W, Tissue Engineering Part A, 2014, 20(13–14), 1980–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shim J-H, Yoon M-C, Jeong C-M, Jang J, Jeong S-I, Cho D-W, and Huh J-B, Biomedical materials, 2014, 9(6), 065006. [DOI] [PubMed] [Google Scholar]
- 84.Costa PF, Puga AM, Díaz-Gomez L, Concheiro A, Busch DH, and Alvarez-Lorenzo C, International journal of pharmaceutics, 2015, 496(2), 541–550. [DOI] [PubMed] [Google Scholar]
- 85.Won J, Park C, Bae J, Ahn G, Kim C, Lim D, Cho D, Yun W, Shim J, and Huh J, Biomedical Materials, 2016, 11(5), 055013. [DOI] [PubMed] [Google Scholar]
- 86.Kim G, Ahn S, Kim Y, Cho Y, and Chun W, Journal of Materials Chemistry, 2011, 21(17), 6165–6172. [Google Scholar]
- 87.Malda J, Visser J, Melchels FP, Jüngst T, Hennink WE, Dhert WJ, Groll J, and Hutmacher DW, Advanced materials, 2013, 25(36), 5011–5028. [DOI] [PubMed] [Google Scholar]
- 88.Crump SS, Apparatus and method for creating three-dimensional objects. 1992, Google Patents. [Google Scholar]
- 89.Youssef A, Hollister SJ, and Dalton PD, Biofabrication, 2017, 9(1), 012002. [DOI] [PubMed] [Google Scholar]
- 90.Ngo TD, Kashani A, Imbalzano G, Nguyen KT, and Hui D, Composites Part B: Engineering, 2018, 143, 172–196. [Google Scholar]
- 91.Hong JM, Kim BJ, Shim J-H, Kang KS, Kim K-J, Rhie JW, Cha HJ, and Cho D-W, Acta Biomaterialia, 2012, 8(7), 2578–2586. [DOI] [PubMed] [Google Scholar]
- 92.Mwema FM and Akinlabi ET: ‘Basics of Fused Deposition Modelling (FDM)’, in ‘Fused Deposition Modeling’, 1–15; 2020, Springer. [Google Scholar]
- 93.Wang M, Favi P, Cheng X, Golshan NH, Ziemer KS, Keidar M, and Webster TJ, Acta biomaterialia, 2016, 46, 256–265. [DOI] [PubMed] [Google Scholar]
- 94.Shim J-H, Jeong J.-h., Won J-Y, Bae J-H, Ahn G, Jeon H, Yun W-S, Bae E-B, Choi J-W, and Lee S-H, Biomedical Materials, 2017, 13(1), 015014. [DOI] [PubMed] [Google Scholar]
- 95.Kim K, Lee C, Kim B, and Mao J, Journal of dental research, 2010, 89(8), 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yu J, Xu Y, Li S, Seifert GV, and Becker ML, Biomacromolecules, 2017, 18(12), 4171–4183. [DOI] [PubMed] [Google Scholar]
- 97.Oladapo BI, Zahedi S, and Adeoye A, Composites Part B: Engineering, 2019, 158, 428–436. [Google Scholar]
- 98.Goncalves EM, Oliveira FJ, Silva RF, Neto MA, Fernandes MH, Amaral M, Vallet-Regí M, and Vila M, Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2016, 104(6), 1210–1219. [DOI] [PubMed] [Google Scholar]
- 99.Kwon DY, Park JH, Jang SH, Park JY, Jang JW, Min BH, Kim WD, Lee HB, Lee J, and Kim MS, Journal of tissue engineering and regenerative medicine, 2018, 12(2), 516–528. [DOI] [PubMed] [Google Scholar]
- 100.Park SH, Park SA, Kang YG, Shin JW, Park YS, Gu SR, Wu YR, Wei J, and Shin J-W, Tissue Engineering and Regenerative Medicine, 2017, 14(4), 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gómez-Cerezo N, Casarrubios L, Saiz-Pardo M, Ortega L, De Pablo D, Díaz-Güemes I, Fernández-Tomé B, Enciso S, Sánchez-Margallo F, and Portolés M, Acta biomaterialia, 2019, 90, 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Luo G, Ma Y, Cui X, Jiang L, Wu M, Hu Y, Luo Y, Pan H, and Ruan C, RSC advances, 2017, 7(20), 11880–11889. [Google Scholar]
- 103.Poh PS, Hutmacher DW, Holzapfel BM, Solanki AK, Stevens MM, and Woodruff MA, Acta biomaterialia, 2016, 30, 319–333. [DOI] [PubMed] [Google Scholar]
- 104.Cui H, Zhu W, Holmes B, and Zhang LG, Advanced science, 2016, 3(8), 1600058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vaquette C, Fan W, Xiao Y, Hamlet S, Hutmacher DW, and Ivanovski S, Biomaterials, 2012, 33(22), 5560–5573. [DOI] [PubMed] [Google Scholar]
- 106.Lee CH, Hajibandeh J, Suzuki T, Fan A, Shang P, and Mao JJ, Tissue Engineering Part A, 2014, 20(7–8), 1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dalton PD, Current Opinion in Biomedical Engineering, 2017, 2, 49–57. [Google Scholar]
- 108.Muerza-Cascante ML, Shokoohmand A, Khosrotehrani K, Haylock D, Dalton PD, Hutmacher DW, and Loessner D, Acta biomaterialia, 2017, 52, 145–158. [DOI] [PubMed] [Google Scholar]
- 109.Hochleitner G, Kessler M, Schmitz M, Boccaccini AR, Teßmar J, and Groll J, Materials Letters, 2017, 205, 257–260. [Google Scholar]
- 110.Abdal-hay A, Abbasi N, Gwiazda M, Hamlet S, and Ivanovski S, European Polymer Journal, 2018, 105, 257–264. [Google Scholar]
- 111.Hochleitner G, Chen F, Blum C, Dalton PD, Amsden B, and Groll J, Acta biomaterialia, 2018, 72, 110–120. [DOI] [PubMed] [Google Scholar]
- 112.Vaquette C, Saifzadeh S, Farag A, Hutmacher D, and Ivanovski S, Journal of dental research, 2019, 98(6), 673–681. [DOI] [PubMed] [Google Scholar]
- 113.Baldwin J, Wagner F, Martine L, Holzapfel B, Theodoropoulos C, Bas O, Savi F, Werner C, De-Juan-Pardo E, and Hutmacher D, Biomaterials, 2017, 121, 193–204. [DOI] [PubMed] [Google Scholar]
- 114.Di Luca A, Ostrowska B, Lorenzo-Moldero I, Lepedda A, Swieszkowski W, Van Blitterswijk C, and Moroni L, Scientific reports, 2016, 6(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Visser J, Melchels FP, Jeon JE, Van Bussel EM, Kimpton LS, Byrne HM, Dhert WJ, Dalton PD, Hutmacher DW, and Malda J, Nature communications, 2015, 6(1), 1–10. [DOI] [PubMed] [Google Scholar]
- 116.Castilho M, Mouser V, Chen M, Malda J, and Ito K, Acta Biomaterialia, 2019, 95, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gu Z, Fu J, Lin H, and He Y, Asian Journal of Pharmaceutical Sciences, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vozzi G, Flaim CJ, Bianchi F, Ahluwalia A, and Bhatia S, Materials Science and Engineering: C, 2002, 20(1–2), 43–47. [Google Scholar]
- 119.Hospodiuk M, Dey M, Sosnoski D, and Ozbolat IT, Biotechnology advances, 2017, 35(2), 217–239. [DOI] [PubMed] [Google Scholar]
- 120.Du X, Fu S, and Zhu Y, Journal of Materials Chemistry B, 2018, 6(27), 4397–4412. [DOI] [PubMed] [Google Scholar]
- 121.He Y, Yang F, Zhao H, Gao Q, Xia B, and Fu J, Scientific reports, 2016, 6, 29977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Labate GFDU, Catapano G, Vitale-Brovarone C, and Baino F, Ceramics International, 2017, 43(12), 9443–9450. [Google Scholar]
- 123.Feng X, Wu Y, Bao F, Chen X, and Gong J, Microporous and Mesoporous Materials, 2019, 278, 348–353. [Google Scholar]
- 124.Lopez CD, Diaz-Siso JR, Witek L, Bekisz JM, Cronstein BN, Torroni A, Flores RL, Rodriguez ED, and Coelho PG, journal of surgical research, 2018, 223, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Feng C, Zhang W, Deng C, Li G, Chang J, Zhang Z, Jiang X, and Wu C, Advanced Science, 2017, 4(12), 1700401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shao H, Sun M, Zhang F, Liu A, He Y, Fu J, Yang X, Wang H, and Gou Z, Journal of dental research, 2018, 97(1), 68–76. [DOI] [PubMed] [Google Scholar]
- 127.Yang C, Wang X, Ma B, Zhu H, Huan Z, Ma N, Wu C, and Chang J, ACS Applied Materials & Interfaces, 2017, 9(7), 5757–5767. [DOI] [PubMed] [Google Scholar]
- 128.Christel T, Kuhlmann M, Vorndran E, Groll J, and Gbureck U, Journal of Materials Science: Materials in Medicine, 2013, 24(3), 573–581. [DOI] [PubMed] [Google Scholar]
- 129.Lee J, Farag MM, Park EK, Lim J, and Yun H.-s., Materials Science and Engineering: C, 2014, 36, 252–260. [DOI] [PubMed] [Google Scholar]
- 130.Castilho M, Dias M, Vorndran E, Gbureck U, Fernandes P, Pires I, Gouveia B, Armés H, Pires E, and Rodrigues J, Biofabrication, 2014, 6(2), 025005. [DOI] [PubMed] [Google Scholar]
- 131.Gbureck U, Hölzel T, Klammert U, Wuerzler K, Mueller FA, and Barralet JE, Advanced Functional Materials, 2007, 17(18), 3940–3945. [Google Scholar]
- 132.Castilho M, Moseke C, Ewald A, Gbureck U, Groll J, Pires I, Teßmar J, and Vorndran E, Biofabrication, 2014, 6(1), 015006. [DOI] [PubMed] [Google Scholar]
- 133.Vorndran E, Klammert U, Ewald A, Barralet JE, and Gbureck U, Advanced Functional Materials, 2010, 20(10), 1585–1591. [Google Scholar]
- 134.Martínez-Vázquez F, Cabañas M, Paris J, Lozano D, and Vallet-Regí M, Acta biomaterialia, 2015, 15, 200–209. [DOI] [PubMed] [Google Scholar]
- 135.Zhao S, Zhang J, Zhu M, Zhang Y, Liu Z, Tao C, Zhu Y, and Zhang C, Acta biomaterialia, 2015, 12, 270–280. [DOI] [PubMed] [Google Scholar]
- 136.Fahimipour F, Dashtimoghadam E, Rasoulianboroujeni M, Yazdimamaghani M, Khoshroo K, Tahriri M, Yadegari A, Gonzalez JA, Vashaee D, and Lobner DC, Dental Materials, 2018, 34(2), 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu A, Sun M, Yang X, Ma C, Liu Y, Yang X, Yan S, and Gou Z, Journal of biomaterials applications, 2016, 31(5), 650–660. [DOI] [PubMed] [Google Scholar]
- 138.De Witte T-M, Fratila-Apachitei LE, Zadpoor AA, and Peppas NA, Regenerative biomaterials, 2018, 5(4), 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Feng C, Zhang M, and Bhandari B, Critical reviews in food science and nutrition, 2019, 59(19), 3074–3081. [DOI] [PubMed] [Google Scholar]
- 140.Luo Y, Zhai D, Huan Z, Zhu H, Xia L, Chang J, and Wu C, ACS applied materials & interfaces, 2015, 7(43), 24377–24383. [DOI] [PubMed] [Google Scholar]
- 141.Zhang W, Feng C, Yang G, Li G, Ding X, Wang S, Dou Y, Zhang Z, Chang J, and Wu C, Biomaterials, 2017, 135, 85–95. [DOI] [PubMed] [Google Scholar]
- 142.Parent M, Baradari H, Champion E, Damia C, and Viana-Trecant M, Journal of Controlled Release, 2017, 252, 1–17. [DOI] [PubMed] [Google Scholar]
- 143.Paul W and Sharma CP, Journal of biomaterials applications, 2003, 17(4), 253–264. [DOI] [PubMed] [Google Scholar]
- 144.Akkineni AR, Luo Y, Schumacher M, Nies B, Lode A, and Gelinsky M, Acta biomaterialia, 2015, 27, 264–274. [DOI] [PubMed] [Google Scholar]
- 145.Fahimipour F, Rasoulianboroujeni M, Dashtimoghadam E, Khoshroo K, Tahriri M, Bastami F, Lobner D, and Tayebi L, Dental Materials, 2017, 33(11), 1205–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cao X and Chen D, Gene, 2005, 357(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang H, Wu G, Zhang J, Zhou K, Yin B, Su X, Qiu G, Yang G, Zhang X, and Zhou G, Colloids and Surfaces B: Biointerfaces, 2016, 141, 491–498. [DOI] [PubMed] [Google Scholar]
- 148.Li C, Jiang C, Deng Y, Li T, Li N, Peng M, and Wang J, Scientific reports, 2017, 7(1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bekisz JM, Flores RL, Witek L, Lopez CD, Runyan CM, Torroni A, Cronstein BN, and Coelho PG, Journal of Cranio-Maxillofacial Surgery, 2018, 46(2), 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Groll J, Boland T, Blunk T, Burdick JA, Cho D-W, Dalton PD, Derby B, Forgacs G, Li Q, and Mironov VA, Biofabrication, 2016, 8(1), 013001. [DOI] [PubMed] [Google Scholar]
- 151.Murphy SV and Atala A, Nature biotechnology, 2014, 32(8), 773–785. [DOI] [PubMed] [Google Scholar]
- 152.Carlier A, Skvortsov GA, Hafezi F, Ferraris E, Patterson J, Koç B, and Van Oosterwyck H, Biofabrication, 2016, 8(2), 025009. [DOI] [PubMed] [Google Scholar]
- 153.Fedorovich NE, De Wijn JR, Verbout AJ, Alblas J, and Dhert WJ, Tissue Engineering Part A, 2008, 14(1), 127–133. [DOI] [PubMed] [Google Scholar]
- 154.Möller T, Amoroso M, Hägg D, Brantsing C, Rotter N, Apelgren P, Lindahl A, Kölby L, and Gatenholm P, Plastic and reconstructive surgery global open, 2017, 5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Groll J, Burdick JA, Cho D-W, Derby B, Gelinsky M, Heilshorn SC, Juengst T, Malda J, Mironov VA, and Nakayama K, Biofabrication, 2018, 11(1), 013001. [DOI] [PubMed] [Google Scholar]
- 156.Lai Y, Cao H, Wang X, Chen S, Zhang M, Wang N, Yao Z, Dai Y, Xie X, and Zhang P, Biomaterials, 2018, 153, 1–13. [DOI] [PubMed] [Google Scholar]
- 157.Tibbitt MW and Anseth KS, Biotechnology and bioengineering, 2009, 103(4), 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang H-B, Xing T-L, Yin R-X, Shi Y, Yang S-M, and Zhang W-J, Chinese Journal of Traumatology, 2016, 19(4), 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Asa’ad F, Pagni G, Pilipchuk SP, Giannì AB, Giannobile WV, and Rasperini G, International Journal of Dentistry, 2016, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Swetha S, Lavanya K, Sruthi R, and Selvamurugan N, Journal of Materials Chemistry B, 2020. [DOI] [PubMed] [Google Scholar]
- 161.Sadat-Shojai M, Khorasani M-T, and Jamshidi A, Materials Science and Engineering: C, 2015, 49, 835–843. [DOI] [PubMed] [Google Scholar]
- 162.James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang X, Ting K, and Soo C, Tissue Engineering Part B: Reviews, 2016, 22(4), 284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim W and Kim G, Biofabrication, 2019, 12(1), 015007. [DOI] [PubMed] [Google Scholar]
- 164.Chimene D, Miller L, Cross LM, Jaiswal MK, Singh I, and Gaharwar AK, ACS Applied Materials & Interfaces, 2020, 12(14), 15976–15988. [DOI] [PubMed] [Google Scholar]
- 165.Xu T, Binder KW, Albanna MZ, Dice D, Zhao W, Yoo JJ, and Atala A, Biofabrication, 2012, 5(1), 015001. [DOI] [PubMed] [Google Scholar]
- 166.Diloksumpan P, de Ruijter M, Castilho M, Gbureck U, Vermonden T, van Weeren PR, Malda J, and Levato R, Biofabrication, 2020, 12(2), 025014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Costa PF, Vaquette C, Zhang Q, Reis RL, Ivanovski S, and Hutmacher DW, Journal of clinical periodontology, 2014, 41(3), 283–294. [DOI] [PubMed] [Google Scholar]
- 168.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H-J, Ramadan MH, Hudson AR, and Feinberg AW, Science advances, 2015, 1(9), e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Durmus NG, Tekin HC, Guven S, Sridhar K, Yildiz AA, Calibasi G, Ghiran I, Davis RW, Steinmetz LM, and Demirci U, Proceedings of the National Academy of Sciences, 2015, 112(28), E3661–E3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Betsch M, Cristian C, Lin YY, Blaeser A, Schöneberg J, Vogt M, Buhl EM, Fischer H, and Duarte Campos DF, Advanced healthcare materials, 2018, 7(21), 1800894. [DOI] [PubMed] [Google Scholar]
- 171.Liu F, Wang W, Mirihanage W, Hinduja S, and Bartolo P, CIRP Annals, 2018, 67(1), 229–232. [Google Scholar]
- 172.Deng Y, Jiang C, Li C, Li T, Peng M, Wang J, and Dai K, Scientific reports, 2017, 7(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yan Y, Chen H, Zhang H, Guo C, Yang K, Chen K, Cheng R, Qian N, Sandler N, and Zhang YS, Biomaterials, 2019, 190, 97–110. [DOI] [PubMed] [Google Scholar]
- 174.Zhang M, Lin R, Wang X, Xue J, Deng C, Feng C, Zhuang H, Ma J, Qin C, and Wan L, Science advances, 2020, 6(12), eaaz6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Subbiah R, Hipfinger C, Tahayeri A, Athirasala A, Horsophonphong S, Thrivikraman G, França CM, Cunha DA, Mansoorifar A, and Zahariev A, Advanced Materials, 2020, 32(36), 2001736. [DOI] [PubMed] [Google Scholar]
- 176.Wang MM, Flores RL, Witek L, Torroni A, Ibrahim A, Wang Z, Liss HA, Cronstein BN, Lopez CD, and Maliha SG, Scientific reports, 2019, 9(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Maliha SG, Lopez CD, Coelho PG, Witek L, Cox M, Meskin A, Rusi S, Torroni A, Cronstein BN, and Flores RL, Plastic and reconstructive surgery, 2020, 145(2), 337e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Witek L, Alifarag AM, Tovar N, Lopez CD, Cronstein BN, Rodriguez ED, and Coelho PG, Journal of Orthopaedic Research®, 2019, 37(12), 2499–2507. [DOI] [PubMed] [Google Scholar]
- 179.Wüst S, Müller R, and Hofmann S, Journal of functional biomaterials, 2011, 2(3), 119–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ma L, Wang X, Zhao N, Zhu Y, Qiu Z, Li Q, Zhou Y, Lin Z, Li X, and Zeng X, ACS applied materials & interfaces, 2018, 10(49), 42146–42154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li Y, Bai Y, Pan J, Wang H, Li H, Xu X, Fu X, Shi R, Luo Z, and Li Y, Journal of Materials Chemistry B, 2019, 7(4), 619–629. [DOI] [PubMed] [Google Scholar]
- 182.Lin HY, Chang TW, and Peng TK, Journal of Biomedical Materials Research Part A, 2018, 106(6), 1511–1521. [DOI] [PubMed] [Google Scholar]
- 183.Pilipchuk SP, Monje A, Jiao Y, Hao J, Kruger L, Flanagan CL, Hollister SJ, and Giannobile WV, Advanced healthcare materials, 2016, 5(6), 676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Park CH, Kim K-H, Lee Y-M, Giannobile WV, and Seol Y-J, International journal of molecular sciences, 2017, 18(9), 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Park CH, Rios HF, Taut AD, Padial-Molina M, Flanagan CL, Pilipchuk SP, Hollister SJ, and Giannobile WV, Tissue Engineering Part C: Methods, 2014, 20(7), 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Park C, Kim K, Rios H, Lee Y, Giannobile W, and Seol Y, Journal of dental research, 2014, 93(12), 1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]