Abstract
Background
There are many accepted airway clearance techniques (ACTs) for managing the respiratory health of people with cystic fibrosis (CF); none of which demonstrate superiority. Other Cochrane Reviews have reported short‐term effects related to mucus transport, but no evidence supporting long‐term benefits. Exercise is an alternative ACT thought to produce shearing forces within the lung parenchyma, which enhances mucociliary clearance and the removal of viscous secretions.
Recent evidence suggests that some people with CF are using exercise as a substitute for traditional ACTs, yet there is no agreed recommendation for this. Additionally, one of the top 10 research questions identified by people with CF is whether exercise can replace other ACTs.
Systematically reviewing the evidence for exercise as a safe and effective ACT will help people with CF decide whether to incorporate this strategy into their treatment plans and potentially reduce their treatment burden. The timing of this review is especially pertinent given the shifting landscape of CF management with the advent of highly‐effective small molecule therapies, which are changing the way people with CF are cared for.
Objectives
To compare the effect of exercise to other ACTs for improving respiratory function and other clinical outcomes in people with CF and to assess the potential adverse effects associated with this ACT.
Search methods
On 28 February 2022, we searched the Cochrane Cystic Fibrosis Trials Register, compiled from electronic database searches and handsearching of journals and conference abstract books. We also searched the reference lists of relevant articles and reviews.
We searched online clinical trial registries on 15 February 2022.
We emailed authors of studies awaiting classification or potentially eligible abstracts for additional information on 1 February 2021.
Selection criteria
We selected randomised controlled studies (RCTs) and quasi‐RCTs comparing exercise to another ACT in people with CF for at least two treatment sessions.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias for the included studies. They assessed the certainty of the evidence using GRADE. Review authors contacted investigators for further relevant information regarding their publications.
Main results
We included four RCTs. The 86 participants had a wide range of disease severity (forced expiratory volume in one second (FEV1) ranged from 54% to 95%) and were 7 to 41 years old. Two RCTs were cross‐over and two were parallel in design. Participants in one RCT were hospitalised with an acute respiratory exacerbation, whilst the participants in three RCTs were clinically stable. All four RCTs compared exercise either alone or in combination with another ACT, but these were too diverse to allow us to combine results. The certainty of the evidence was very low; we downgraded it due to low participant numbers and high or unclear risks of bias across all domains.
Exercise versus active cycle of breathing technique (ACBT)
One cross‐over trial (18 participants) compared exercise alone to ACBT. There was no change from baseline in our primary outcome FEV1, although it increased in the exercise group before returning to baseline after 30 minutes; we are unsure if exercise affected FEV1 as the evidence is very low‐certainty. Similar results were seen for other measures of lung function. No adverse events occurred during the exercise sessions (very low‐certainty evidence). We are unsure if ACBT was perceived to be more effective or was the preferred ACT (very low‐certainty evidence). 24‐hour sputum volume was less in the exercise group than with ACBT (secondary outcome). Exercise capacity, quality of life, adherence, hospitalisations and need for additional antibiotics were not reported.
Exercise plus postural drainage and percussion (PD&P) versus PD&P only
Two trials (55 participants) compared exercise and PD&P to PD&P alone. At two weeks, one trial narratively reported a greater increase in FEV1 % predicted with PD&P alone. At six months, the other trial reported a greater increase with exercise combined with PD&P, but did not provide data for the PD&P group. We are uncertain whether exercise with PD&P improves FEV1 as the certainty of evidence is very low. Other measures of lung function did not show clear evidence of effect. One trial reported no difference in exercise capacity (maximal work rate) after two weeks. No adverse events were reported (1 trial, 17 participants; very low‐certainty evidence). Adherence was high, with all PD&P sessions and 96% of exercise sessions completed (1 trial, 17 participants; very low‐certainty evidence). There was no difference between groups in 24‐hour sputum volume or in the mean duration of hospitalisation, although the six‐month trial reported fewer hospitalisations due to exacerbations in the exercise and PD&P group. Quality of life, ACT preference and need for antibiotics were not reported.
Exercise versus underwater positive expiratory pressure (uPEP)
One trial (13 participants) compared exercise to uPEP (also known as bubble PEP). No adverse events were recorded in either group (very low‐certainty evidence). Trial investigators reported that participants perceived exercise as more fatiguing but also more enjoyable than bubble PEP (very low‐certainty evidence). There were no differences found in the total weight of sputum collected during treatment sessions. The trial did not report the primary outcomes (FEV1, quality of life, exercise capacity) or the secondary outcomes (other measures of lung function, adherence, need for antibiotics or hospitalisations).
Authors' conclusions
As one of the top 10 research questions identified by clinicians and people with CF, it is important to systematically review the literature regarding whether or not exercise is an acceptable and effective ACT, and whether it can replace traditional methods. We identified an insufficient number of trials to conclude whether or not exercise is a suitable alternative ACT, and the diverse design of included trials did not allow for meta‐analysis of results. The evidence is very low‐certainty, so we are uncertain about the effectiveness of exercise as an ACT. Longer studies examining outcomes that are important to people with CF are required to answer this question.
Plain language summary
Exercise versus airway clearance for people with cystic fibrosis
Review question
Can exercise replace other methods of airway clearance for people with cystic fibrosis (CF)?
Background
CF affects many systems in the body, mainly the respiratory system. It causes a build‐up of thick, sticky mucus in the lungs which causes irritation and damage to the lining of the airways. CF treatment involves chest physiotherapy, also called airway clearance, which uses a range of devices or techniques to get rid of this mucus. It has been suggested that exercise may have a similar effect. Exercising results in a person taking different volumes and depths of breaths. This leads to pressure changes and forces within the airways that move secretions out of the lungs. We compared the effect on lung function of exercise versus other techniques, to see if exercise is a suitable alternative for people with CF. We wanted to answer our review question to potentially reduce their treatment burden.
Search date
The evidence is current to 15 February 2022.
Study characteristics
We searched the literature for studies where people received at least two treatment sessions of exercise or another airway clearance technique, and report on four studies including 86 people with CF in the review. The people in the studies were aged between 7 and 41 years and had varying degrees of disease severity. Three studies included people who were clinically well and one study included people admitted to hospital for a chest infection. The studies lasted between four days and six months and compared exercise (alone or in combination with another airway clearance technique) to other techniques. Two studies compared exercise with postural drainage and percussion (PD&P), one study compared exercise with the active cycle of breathing technique (ACBT) and one study compared exercise with underwater positive expiratory pressure (uPEP), also known as bubble PEP. Three studies received financial support from funding bodies such as the Cystic Fibrosis Trust, the Buffalo Foundation and the Romanian National Council for Scientific Research in Higher Education.
Key results
We did not find enough evidence to conclude whether or not exercise can replace other methods of airway clearance. We did not find any evidence to suggest that exercise was either better or worse than other methods to improve lung function or clear mucus from the airways, although exercising did improve people's exercise ability, and it was the preferred choice of treatment in one study. None of the studies reported any negative effects of exercise therapy. None of the studies evaluated quality of life or the need for extra antibiotic treatment. One study did suggest that exercise alone was less effective at clearing sputum than ACBT.
Exercise versus ACBT
One study (18 participants) found that a measure of lung function temporarily (up to 30 minutes) increased in the exercise group only, otherwise there was no difference between the ACBT or the exercise group. No adverse events were reported, and it is not certain if ACBT was thought to be more effective or was preferred. The exercise group produced less sputum than the ACBT group. The study did not report on exercise capacity, quality of life, adherence, hospitalisations and need for additional antibiotics.
Exercise plus PD&P versus PD&P alone
Two studies (55 participants) compared exercise plus PD&P to PD&P alone. At two weeks, one trial described a greater increase in lung function with PD&P alone, while at six months the second study reported a greater increase with exercise plus PD&P (but did not provide data for the PD&P group). One study reported no side effects at all, and also reported no difference between groups in exercise capacity (maximal work rate), sputum volume or the average length of time spent in hospital. Conversely, the second study reported fewer hospitalisations due to exacerbations in the exercise and PD&P group. Neither study reported on quality of life, preference and the need for antibiotics.
Exercise versus uPEP
One study (13 participants) compared exercise to uPEP (also known as bubble PEP). No adverse events were recorded in either group and investigators reported that those taking part thought that, while exercise was more tiring, it was also more enjoyable than bubble PEP. We found no differences in the total weight of sputum collected during treatment sessions. The study did not report on lung function, quality of life, exercise capacity, adherence, need for antibiotics or hospitalisations.
Certainty of the evidence
Overall, we had very little confidence in the evidence because all four studies had few participants and two studies only presented results as a shortened report given at a conference.
We do not think the fact that participants and people measuring the outcomes knew which treatment the participants were receiving influenced the results of outcomes such as lung function and sputum weight. We do not think the fact that these studies were financed should influence the interpretation of the results in this review.
Summary of findings
Summary of findings 1. Exercise compared with ACBT for people with cystic fibrosis.
| Exercise compared with ACBT for people with cystic fibrosis | ||||||
|
Patient or population: adolescents and adults with cystic fibrosis Settings: outpatients Intervention: exercise (cycling at 60% VO2 max), 20 minutes twice daily Comparison: ACBT, 20 minutes twice daily | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| ACBT | Exercise | |||||
| Pulmonary function: absolute change FEV1 % predicted Follow‐up: immediately after treatment |
The trial did not report this outcome as % predicted. There was no significant change from baseline to 30 minutes post‐treatment in FEV1 (L) in the ACBT group. There was an increase in FEV1 (L) in the exercise group (P < 0.05) immediately after the 20‐minute exercise session, but this returned to baseline values after 30 minutes (Bilton 1992). |
18 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | The trial presented very limited data and only reported changes in FEV1 L. |
||
| Change in VO2 peak during maximal exercise |
This outcome was not measured. | |||||
| Quality of life |
This outcome was not measured. | |||||
| Adverse effects Follow‐up: end of treatment day |
No adverse effects in heart rate or oxygen saturation were observed during the study. | 18 (1 study) | ⊕⊝⊝⊝
very lowa,b |
No data were available to report. | ||
| Participant preference: VAS score for treatment effectiveness and preference. Follow‐up: end of the 4‐day study period |
ACBT was perceived to be more effective than exercise alone and was the preferred treatment modality (Bilton 1992). | 18 (1 study) | ⊕⊝⊝⊝ very lowa,b | This was a three‐arm cross over trial in which participants were asked to rate the effectiveness of each treatment modality and state which they would prefer to continue at home. | ||
| Adherence |
This outcome was not measured. | |||||
| Need for extra antibiotics (days) | This outcome was not measured. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACBT: active cycle of breathing technique; CI: confidence interval; FEV1: forced expiratory volume in one second; VAS: visual analogue score; VO2 max: maximal oxygen consumption | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded twice due to risk of bias within the single included trial. Risk of bias was unclear across six of eight domains, particularly the domains of sequence generation and allocation concealment. There was a high risk of bias across the blinding domains, and authors felt that knowledge of allocation could have affected outcomes. bDowngraded once due to imprecision caused by a low number of participants. cDowngraded due to indirectness as this outcome was not measured as specified in the protocol (change in FEV1 % predicted), but measurement in litres is still relevant to the results.
Summary of findings 2. Exercise plus PD&P compared with PD&P only for people with cystic fibrosis.
| Exercise plus PD&P compared with PD&P only for people with cystic fibrosis | ||||||
|
Patient or population: children and young adults with cystic fibrosis Settings: inpatients and outpatients Intervention: exercise plus PD&P Comparison: PD&P | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| PD&P only | Exercise plus PD&P | |||||
| Pulmonary function: absolute change FEV1 % predicted Follow‐up: 2 weeks to 6 months |
After a 2‐week treatment period, FEV1 % predicted increased in both groups. The increase in the PD&P only group was greater (18.4%) than the exercise plus PD&P group (11.3%) (Cerny 1989). After a 6‐month treatment period, there was a 13.5% increase in FEV1 % predicted in the exercise plus PD&P group. The trial did not report values for the PD&P group, but the paper stated that the difference between groups was statistically significant (P = 0.043) (Almajan‐Guta 2011). |
55 (2 studies) |
⊕⊝⊝⊝ very lowa,b | Both studies presented very limited data. We have reported results narratively, directly from the papers. The 6‐month study gave the increase in the intervention group but did not provide data for the control group, only the P value. |
||
| Change in VO2 peak during maximal exercise | This outcome was not measured. | The 2‐week study reported exercise capacity in terms of maximal work rate and found no differences between groups (Cerny 1989). | ||||
| Quality of Life: change in CFQ‐R score Follow‐up: 6 months |
This outcome was not measured. | One study used a non‐validated tool to assess participation at school and activities of daily living before and after treatment, with 60% participation in school in the exercise plus PD&P group compared with 48% constant participation in school activities in the control group; investigators also reported 16% fatigue versus 42% fatigue during daily activities in the exercise plus PD&P group and PD&P alone group, respectively (Almajan‐Guta 2011). | ||||
| Adverse effects Follow‐up: 2 weeks |
There were no negative effects of exercise therapy reported. | 17 (1 study) | ⊕⊝⊝⊝ very lowb,c | The study presented no data so we have reported results narratively, directly from the paper (Cerny 1989). | ||
| Participant preference | This outcome was not measured. | |||||
| Adherence |
All PD&P treatments were completed as required for the study and 96% of the scheduled exercise therapy sessions were completed. | 17 (1 study) |
⊕⊝⊝⊝ very lowb,c | No data were available so we have reported adherence results narratively, directly from the paper (Cerny 1989). | ||
| Need for extra antibiotics (days) | This outcome was not measured. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFQ‐R: Cystic Fibrosis Questionnaire ‐ Revised; CI: confidence interval; FEV1: forced expiratory volume in one second; PD&P: postural drainage and percussion; VO2 max: maximal oxygen consumption. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded twice due to risk of bias within the included studies for this outcome. Both studies were at high or unclear risk of bias across all domains. bDowngraded once due to imprecision caused by low participant numbers. cDowngraded twice due to risk of bias within the included study for this outcome. The risk of bias was either high or unclear across all domains.
Summary of findings 3. Exercise compared with underwater PEP (uPEP; also known as bubble PEP) for people with cystic fibrosis.
| Exercise compared with uPEP for people with cystic fibrosis | ||||||
|
Patient or population: children and adults with cystic fibrosis Settings: outpatient Intervention: exercise (cycle ergometer constant effort 1/2 W/kg) 1 x 30‐minute session per week Comparison: uPEP 1 x 30‐minute session per week | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| uPEP | Exercise | |||||
| Pulmonary function: absolute change FEV1 % predicted | This outcome was not measured. | |||||
| Change in VO2 peak during maximal exercise | This outcome was not measured. | |||||
| Quality of life: change in CFQ‐R score | This outcome was not measured. | |||||
| Adverse effects Follow‐up: 2 weeks |
There was no difference between the exercise group and the uPEP group in terms of peak SaO2 (%) or desaturation. |
13 (1 study) | ⊕⊝⊝⊝
very lowa,b |
|||
| Participant preference: VAS test and a questionnaire to evaluate acceptability of technique to participants Follow‐up: 2 weeks |
The exercise test was perceived by participants as more fatiguing but was also considered more amusing. | 13 (1 study) | ⊕⊝⊝⊝
very lowa,b |
The study gave very limited information on the scale used or the results. We have reported results reported directly from the paper. | ||
| Adherence | This outcome was not measured. | |||||
| Need for extra antibiotics (days) | This outcome was not measured. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFQ‐R: Cystic Fibrosis Questionnaire ‐ Revised; CI: confidence interval; FEV1: forced expiratory volume in one second; SaO2: arterial oxygen saturation; uPEP: underwater positive expiratory pressure; VAS: visual analogue score; VO2 max: maximal oxygen consumption. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded twice due to high or unclear risk of bias across all domains in this single cross‐over study. bDowngraded once due to imprecision caused by very low participant numbers.
Background
Description of the condition
Cystic fibrosis (CF) is a genetic condition inherited in an autosomal recessive fashion (when two mutated genes are inherited, one from each parent). It is a multisystem disorder predominantly affecting the respiratory, gastrointestinal, endocrine and reproductive systems. The condition is more prevalent in populations of Northern European descent (Farrell 2018), with an incidence of 1 in every 2381 live births (Farrell 2008).
CF is the result of a mutation in the cystic fibrosis transmembrane conductance regulator gene (CFTR) which leads to poorly functioning exocrine glands and reduced ion transport across epithelia, resulting in reduced airway surface liquid and a build‐up of copious mucus. These viscous secretions cause inflammation and irreversible damage to the airway epithelia, which in turn leads to bronchiectasis that then progresses to respiratory failure and is the reason for CF being life‐limiting (Koch 1993).
Mortality rates have decreased with recent innovations in immunomodulator therapy and potentiators such as ivacaftor, which demonstrated absolute improvement in forced expiratory volume in one second (FEV1) and a decreased number of pulmonary exacerbations in people with the G551D mutation (Skilton 2019). The current median survival is 52 years in males and 49 years in females (Keogh 2018). As the life expectancy of people with CF continues to rise, disease demographics have changed. Healthcare teams now face the challenge of maintaining health and optimising the quality of life (QoL). This highlights the important contribution of exercise and physiotherapy in managing CF.
The timing of this review is especially pertinent given the shifting landscape of CF management with the advent of highly effective small molecule therapies becoming available to a broader section of the population, which are changing the way people with CF are cared for and their future prognosis (Middleton 2019).
Description of the intervention
There is evidence from systematic reviews, including Cochrane Reviews, that exercise and airway clearance are important for maintaining respiratory health, even during early stages of the condition (Flume 2009).
There are many established airway clearance techniques (ACTs) that have been evaluated in Cochrane Reviews, including manual techniques such as postural drainage and percussion (PD&P) (Main 2005); breathing techniques such as autogenic drainage (AD) (Burnham 2021) and the active cycle of breathing technique (ACBT) (Mckoy 2016); oscillating devices (Morrison 2020), and use of positive expiratory pressure (PEP) devices (McIlwaine 2019). There are other interventions that train respiratory muscles (Irons 2019; Stanford 2020), but since the effects of respiratory muscle training are beyond the scope of this review, these will not be presented as comparator interventions in this review. To date, the evidence surrounding the use of exercise to aid secretion clearance has not been assessed.
It has been shown that in both children and adults with CF, habitual physical activity and exercise can decrease pulmonary exacerbations and hospitalisations, while also improving physical function, endurance and energy levels (Boas 1997; Cerny 2013; LeBlanc 2014; Radtke 2017; Rand 2012; Shoemaker 2008; Wheatley 2011). Increasing levels of exercise and physical activity have also been associated with a slower rate of decline in lung function (Cox 2018; Schneiderman 2014).
Habitual physical activity has been defined as bodily movement produced regularly by the contraction of skeletal muscles that results in a substantial increase in resting energy expenditure (Pescatello 2014). This should be differentiated from exercise, which is planned, structured and repetitive bodily movement performed to improve or maintain one or more components of physical fitness (Pescatello 2014). Within exercise there are also different subgroups, including aerobic exercise, anaerobic exercise, resistance, strength, balance and flexibility. This review assesses the evidence surrounding all types of exercise mentioned as a form of airway clearance for people with CF. This does not include respiratory muscle training, due to the similarities and overlap between this and traditional ACT.
How the intervention might work
Exercise is thought to promote clearance of mucus in a multi‐mechanistic way, including mechanical vibration, hyperventilation, coughing and changing the viscosity of sputum (Hebestreit 2001; Radtke 2017; Wilson 2019). This increased mucus clearance leads to the removal of infected secretions within the airways, reducing the release of inflammatory cytokines which cause direct effect and damage to the airway epithelia.
In comparison to routine ACTs, exercise has been shown to increase aerobic capacity, the level of activity (Selvadurai 2002), and potentially train the muscles of respiration (Dassios 2013; Stanford 2020). Aerobic capacity is thought to be one of the best predictors of survival for people with CF (Hebestreit 2019; Nixon 1992; Pianosi 2005). Exercise capacity and participation in physical training in people with CF have been related to improved posture, bone density (Hind 2008; Sawyer 2004), mental health, and QoL (Klijn 2004), and to a reduction in the number of antibiotic days (Urquhart 2012). Consequently, there are additional benefits to prescribing this form of airway clearance.
Treadmill exercise has been shown to improve mucus clearance mechanisms in CF by increasing peak expiratory flow (PEF) and reducing sputum mechanical impedance (Dwyer 2011). Theoretically, varying breath volumes during exercise and through vigorous activity produce shearing forces which enhance mucociliary clearance. This facilitates the removal of secretions, improves ventilation and reduces inflammation in the lungs, thus limiting damage to airways. Other studies have also suggested that exercise should be combined with forced expiratory techniques (FET) and cough to exert potential benefits in airway clearance (Dwyer 2021; Ward 2020).
Why it is important to do this review
People with CF consider airway clearance to be burdensome (Rowbotham 2020). Other Cochrane Reviews have been published demonstrating non‐superiority between other forms of ACTs such as AD (Burnham 2021), oscillatory devices (Morrison 2020), ACBT (Mckoy 2016), PEP (McIlwaine 2019) and conventional chest physiotherapy (Main 2005). As non‐superiority exists, there is no globally agreed definitive treatment strategy for ACTs and their prescription.
Currently, aerobic exercise is recommended for people with CF as an adjunctive therapy for airway clearance (Flume 2009), but not prescribed for this purpose alone. A recent Australian survey, however, showed that 44% of people with CF are using exercise as a substitute for traditional ACTs, suggesting a potential preference for this mode of therapy (Ward 2019). In addition, participants using exercise as a substitute for other ACTs were found to have a significantly higher FEV1 % predicted, lower perceived severity of respiratory disease and lower sputum load than other participants (Ward 2019).
A key research question identified from people with CF, their caregivers and clinicians, in a priority setting exercise with the James Lind Alliance (JLA), is whether exercise can be used to replace airway clearance in CF (Rowbotham 2018). Adults with CF report spending an average of 108 minutes on treatment and activities each day, the majority of that time performing airway clearance and exercise (Sawicki 2009). It is important that we evaluate the evidence in order to help reduce the large treatment burden imposed on people with CF. There is a range of evidence available from different sources; this review summarises the high‐quality evidence available from randomised controlled trials (RCTs) or quasi‐RCTs (including cross‐over trials) that evaluate ACT and exercise for people with CF.
This review aims to address this gap in the literature. If exercise does prove to be as effective as other ACTs, it is a safe, easily accessible and cost‐effective strategy to improve the respiratory health and QoL of people with CF (Williams 2013).
Objectives
To compare the effect of exercise to other ACTs for improving respiratory function and other clinical outcomes in people with CF and to assess the potential adverse effects associated with this ACT.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) or quasi‐RCTs (including cross‐over trials) that evaluated exercise and ACTs in people with CF.
We excluded trials involving a single intervention (i.e. one exercise session). Although the inhibition of luminal sodium conductance is thought to increase the water content of mucus during exercise in the acute phase (Hebestreit 2001), this review did not set out to evaluate outcomes associated with transient responses to exercise. Therefore, we deemed trials where therapy was performed only once as inappropriate for this review.
Types of participants
We included children (over four years of age) and adults with CF with a diagnosis based on sweat testing (sweat chloride over 59 mmol/L), genetic testing or a combination of these. We did not have any restrictions based on disease severity or exacerbation status.
Types of interventions
This review included trials that compared exercise to other recognised ACTs (as described below), either as a single technique or in combination.
Whilst a single exercise session has been reported to inhibit epithelial sodium channels and normalize transepithelial potential difference (Hebestreit 2001), the aim of this review was to establish whether exercise can replace airway clearance, rather than explore the mechanism whereby exercise leads to improved mucociliary clearance. Benefits of exercise for airway clearance and to improve lung function, ventilation and aerobic capacity are likely to be evident after multiple treatment episodes (Cholewa 2012); therefore, we only included studies that used an ACT for at least two treatment episodes.
We considered interventions of variable duration, and separated these into short‐, medium‐ and long‐term trials according to the term of intervention (e.g. up to 14 days, 15 days to 12 weeks, over 12 weeks).
Exercise
Exercise is an adjuvant to current methods and is thought to promote mechanical clearance of mucus through increasing minute ventilation and PEF (Dwyer 2011). This helps to slow the decline in lung function (Schneiderman 2014), and has been associated with improved survival (Hebestreit 2018; Nixon 1992). Various types of exercise can be beneficial, including cardiovascular, strength and flexibility training, which this review accounted for. While we included trials of planned, formal, intensity‐specific exercise, we considered those trials that looked at physical activity in a more unstructured way, with lower levels of intensity, and excluded any that were not completed on a regular basis.
Postural drainage and percussion
PD&P was introduced for managing CF in the 1950s and uses positioning, manual vibration and gravity to move mucus within the airways (Main 2005; Wilson 2019).
Autogenic drainage
This is a method of controlled breathing in the expiratory phase which helps move secretions from the smaller to larger airways (Burnham 2021). Secretions are cleared by adjusting the speed and depth of breathing according to where secretions are heard and felt by the individual. This can be performed independently without a device or a trained healthcare professional but requires commitment and training.
Active cycle of breathing technique
This technique is characterised by a combination of relaxation and breathing control, using thoracic expansion exercises with FET to achieve mucociliary clearance (Mckoy 2016).
Positive expiratory pressure
A PEP device is a mask or mouthpiece used to provide back pressure to the airways during expiration (McIlwaine 2019). There is a valve within the device that increases resistance to expiratory airflow between 10 cm to 20 cm of water (H2O). This is commonly referred to as underwater PEP (UPEP) or bubble PEP. This stimulates mucociliary clearance by building up gas behind mucus via collateral ventilation, preventing small airway collapse through stenting of airways and temporarily increasing functional residual capacity (FRC) (Groth 1985). Hi‐PEP uses full forced expiration against the PEP mask's expiratory resistor using pressures of between 40 cm to 140 cm of H2O (Prasad 1993).
Oscillatory devices
There are two main types of oscillatory devices; oscillatory PEP (O‐PEP) devices and those using external thoracic high‐frequency chest wall oscillation (HFCWO). O‐PEP devices include, but are not limited to, the RC‐Cornet, Flutter, Acapella, AerobiKA, Quake and intrapulmonary percussive ventilation (IPV). The PEP element of these therapies increases FRC and augments collateral ventilation (Groth 1985). These devices also generate intrathoracic oscillation through varying expiratory flow resistance during exhalation. This combined action helps to mobilise secretions by reducing sputum viscosity and creating small bursts of air that move secretions centrally and facilitate expectoration (McIlwaine 2006). HFCWO uses an inflatable garment that covers the chest and is attached to an air pulse‐generating compressor, which rapidly inflates and then deflates the garment, producing oscillations to manipulate the chest wall. It is proposed that HFCWO enhances mucociliary transport by creating a cough‐like expiratory flow bias that shears mucus from the airway walls by enhancing ciliary beat frequency (Hansen 1994), and by altering the rheological properties of mucus (Dasgupta 1998).
Types of outcome measures
We assessed the following outcome measures.
Primary outcomes
-
Lung function
FEV1 (per cent (%) predicted) absolute change from baseline values and final value
FEV1 (L) absolute change from baseline values and final value
-
Exercise capacity
peak oxygen uptake (VO2 peak) in L, mL/kg body weight or fat‐free mass or as % predicted (Hebestreit 2015)
maximal work rate (Wpeak) (Hebestreit 2015)
any validated field test (e.g. six‐minute walk test (metre), modified 10 metre shuttle test (level attained and distance covered)
-
QoL (self‐reported)
Cystic Fibrosis Questionnaire‐Revised (CFQ‐R) (Quittner 2009)
Cystic Fibrosis Quality of Life Questionnaire (CF‐QoL) (Gee 2000)
Chronic Respiratory Disease Questionnaire (CRQ) (Guyatt 1987)
any other validated QoL scale (e.g. Nottingham Health Profile (NHP) (Wiklund 1990), Short Form‐36 (SF‐36) (Ware 1992))
Secondary outcomes
Adverse effects (related to exercise and exercise testing)
-
Lung function
forced vital capacity (FVC) % predicted or litres (L)
mid‐forced expiratory flow (FEF25-75)
lung clearance index (LCI)
Participant preference
-
Adherence
electronic data capture
participant diary
-
Sputum weight
wet weight (g)
dry weight (g)
-
Hospital admissions due to exacerbation as defined by (Rosenfeld 2001)
number of hospital admissions
duration of hospital admission
-
Need for extra antibiotics (days)
oral
intravenous (IV)
inhaled or nebulised
Search methods for identification of studies
We searched for all relevant published and unpublished trials without restrictions on language, year or publication status.
Electronic searches
The Cochrane Cystic Fibrosis and Genetic Disorders Group's Information Specialist conducted a systematic search of the Group's Cystic Fibrosis Trials Register for relevant trials using the following terms: exercise:kw and airway clearance techniques:kw.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major CF conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
The date of the latest search was 28 February 2022.
We also searched the following trial registers:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 15 February 2022);
World Health Organization International Clinical Trials Registry Platform (ICTRP Search Portal (who.int); searched 15 February 2022).
The search strategies for these are presented in the Appendix 1.
Searching other resources
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials. We also contacted experts and organisations in the field to obtain additional information on relevant trials.
Data collection and analysis
We have followed the methods set out in our protocol as far as possible (Patterson 2019).
Selection of studies
Two authors (KP and AW) independently reviewed all citations and abstracts retrieved, using the search criteria above to determine which papers were eligible for inclusion. They then reviewed the full‐text articles. There were no conflicts, but if disagreements arise in future updates of this review, the authors will resolve these by discussion or arbitration with a third author.
Data extraction and management
Two authors (KP and AW) independently extracted the data using data extraction forms specifically developed for this purpose. The extracted data included the number of participants, participant characteristics, study design (type of randomisation, allocation and concealment), details of the intervention (type of exercise, frequency, duration of session, compliance) and outcome measures. The data extraction was transferred and completed using Covidence. For one study which had incomplete data, KP attempted to contact the study investigator for further data for inclusion.
The authors used Covidence to manage the extracted data, and compiled and analysed the limited data using the Cochrane RevMan software (RevMan 2020). The authors planned to group the results based on time (i.e. up to 14 days, 15 days to 12 weeks, over 12 weeks). Since they did not combine any data, they reported most results narratively and not combined by time point. They did not consider single episodes of treatment, as it is unlikely that a single treatment will have any long‐term effect on any measured outcome. The authors presented results separately for each comparison of the different ACTs, e.g. exercise versus PEP, exercise versus ACBT, etc. The authors also planned to present separate data sets for studies where participants were hospitalised as inpatients separately from long‐term trials in those who are clinically stable. Limited data meant the authors could only present some data from a study of clinically stable participants in the analyses; the authors presented the remaining results narratively.
Assessment of risk of bias in included studies
Two authors (KP and AW) independently assessed the included trials for risk of bias using the Cochrane risk of bias tool (Higgins 2017). This tool is a domain‐based evaluation in which authors make critical assessments separately for six different domains (generation of sequence, concealment of allocation, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting and other potential threats to validity). They graded each trial as low risk of bias, unclear risk of bias or high risk of bias for each domain. There were no disagreements; however, if there are any disagreements in future updates of this review, a third author will resolve these.
Measures of treatment effect
For continuous outcomes (FEV1, exercise capacity, QoL, adherence, FVC, FEF25-75, LCI, sputum weight, duration of hospital admissions, number of antibiotic days) the authors planned to report the mean difference (MD) and 95% confidence intervals (CIs) if trials had used the same unit of measurement, otherwise they planned to use the standardised mean difference (SMD). In the case of binary outcomes or dichotomous data (e.g. participant preference, number of participants admitted to hospital) the authors planned to combine the data from the trials using risk ratios (RR) and 95% CIs (Deeks 2021). The authors were not able to employ these methods due to a lack of analysable data in the included studies, but will follow these methods if they are able to include data in future updates of the review. For this version of the review, the authors reported the trial results narratively.
Unit of analysis issues
The review authors considered the level at which randomisation occurred in the trials (Deeks 2021). They did not include cluster‐RCTs, as they do not consider this design appropriate for exercise as an intervention.
For RCTs with a cross‐over design, i.e. where all participants receive all the interventions and act as their own control, the effects of one intervention can often persist into the next treatment period, interfering with the effects of the subsequent intervention if there is not a significant washout period. This is known as the 'carry‐over effect'. When analysing such trials, the authors planned to use the methods suggested by Elbourne (Elbourne 2002). In general, the reporting of data from cross‐over trials is variable, with limited data published that are required for a paired analysis (Higgins 2017). The review authors had planned to use data from the first arm of the trial and analyse these as for a parallel trial. The included cross‐over trials presented their results in a combined format, and the review authors reported results narratively. Neither cross‐over trial stated how they analysed their data to produce their results. For one trial that had multiple treatment groups, the review authors combined all relevant control groups (where the test sequence was reversed) into a single control group to create a single pair‐wise comparison and decrease the unit‐of‐analysis error.
Dealing with missing data
In instances of missing data, the review authors contacted the trial investigators directly. Where any data remained unavailable, the review authors had planned to listed the trial under Studies awaiting classification and include it in future updates, should relevant data become available. The review authors contacted one team of investigators to request more data than were available in the published abstract, but could not obtain additional data. The authors of this review agreed to include this trial and present the limited data narratively.
Assessment of heterogeneity
For trials with similar interventions and participants, assessing similar outcomes, the review authors planned to perform a meta‐analysis to pool the data and depict this in a forest plot. They planned to assess heterogeneity using the Chi² test (which assesses whether observed differences in results are compatible with chance alone) and the I² statistic (which describes the percentage of total variation across trials due to heterogeneity rather than chance) (Higgins 2003). The values of I² lie between 0% and 100%. The authors would have categorised heterogeneity according to the Cochrane Handbook for Systematic Reviews of Interventions as below (Deeks 2021).
0% to 40% as little heterogeneity
30% to 60% as moderate heterogeneity
50% to 90% as substantial heterogeneity
over 75% as considerable heterogeneity
Using the null hypothesis of homogeneity, the review authors would have deemed a P value of 0.1 to be significant. However, authors have only been able to report narrative results in this version of the review.
Assessment of reporting biases
The authors did not identify a sufficient number of trials of different sizes to create a funnel plot for assessment of reporting bias. For review updates the authors plan to use RevMan to create funnel plots for the assessment of reporting bias.
The review authors also assessed outcome reporting bias. This is when results for planned outcomes listed in the methodology are not published in the final paper, giving rise to misleading results (Pocock 1987; Tannock 1996). To reduce this type of bias, the review authors attempted to identify the relevant trial protocol for each included trial, e.g. from online trial registries. If these were not available, the review authors compared the methods section of the final publication to the results section to ensure all variables were reported.
Data synthesis
The review authors planned to analyse data using the fixed‐effect model (Mantel‐Haenszel methods) programmed into RevMan software (Greenland 1985; Mantel 1959; RevMan 2020). In future updates, if at least substantial heterogeneity (I² value over 50%) exists between identified trials, they will use the random‐effects model for analysis (Deeks 2021).
Subgroup analysis and investigation of heterogeneity
If the review had included sufficient data, the authors planned to investigate heterogeneity by performing a separate subgroup analysis based on the participant characteristics:
age (four years to 17 years compared with adults);
gender;
disease severity based on lung function (FEV1 % predicted, over 90%, 50% to 89%, below 50%).
Sensitivity analysis
If authors had been able to combine data from multiple trials, they planned to perform a sensitivity analysis, presented in the form of a summary table, to depict the robustness of the results. This would analyse the effect of trial design (parallel versus cross‐over), allocation concealment (high risk of bias versus low risk of bias) and loss to follow‐up (high risk of bias versus low risk of bias) on the results.
Summary of findings and assessment of the certainty of the evidence
The review authors determined and rated the certainty of the evidence for each outcome by using the GRADE approach (Schünemann 2021). This is presented in the summary of findings (SoF) tables (one for each comparison). The outcomes selected for each individual comparison are those that the authors feel are patient‐important outcomes.
Pulmonary function ‐ absolute change FEV1 % predicted
Exercise capacity ‐ change in VO2 peak during maximal exercise (mL/min per kg body weight)
QoL ‐ CFQ‐R
Adverse effects (related to exercise and exercise testing)
Participant preference
Adherence
Need for any extra antibiotics (oral, IV, inhaled or nebulised) in days
Results
Description of studies
Please see the following tables for additional information: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
The electronic search retrieved a total 115 citations representing 98 trials. Following screening, we considered five citations representing four trials to be eligible for inclusion (Almajan‐Guta 2011; Balestri 2004; Bilton 1992; Cerny 1989). Two of these were published in abstract form only (Almajan‐Guta 2011; Balestri 2004); however, the review authors felt there was sufficient detail to determine that they met the inclusion criteria. There was a paucity of research into long‐term outcomes, so we were only able to include three trials with short‐term interventions (less than 28 days) (Balestri 2004; Bilton 1992; Cerny 1989) and one longer‐term trial (more than 28 days) (Almajan‐Guta 2011). We excluded a total of 29 references representing 17 trials (Alexander 2019; Aquino 2006; Baldwin 1994; Dwyer 2019; Falk 1988; Kaak 2011; Lannefors 1992; Montero‐Ruiz 2020; NCT00609050; NCT00792194; NCT03295201; Radtke 2018; Reix 2012; Rodriguez 2017; Salh 1989; Santana‐Sosa 2014; Zeren 2019).
We also listed one trial (one reference) as ongoing (NCT03273959), and one trial (two references) is awaiting classification (Ward 2018).
We have documented the process of the search and trial selection in the PRISMA diagram (Figure 1).
1.
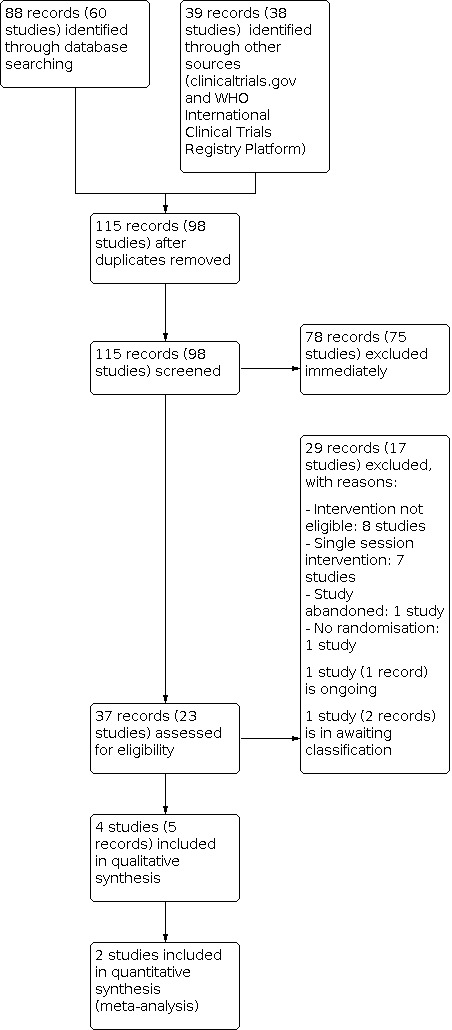
PRISMA flow diagram of study selection process
Included studies
This review only includes four trials (Almajan‐Guta 2011; Balestri 2004; Bilton 1992; Cerny 1989). Of these, two were published as abstracts only (Almajan‐Guta 2011; Balestri 2004) and two were published as full journal articles (Bilton 1992; Cerny 1989). One study was published as both an abstract and journal article (Bilton 1992).
Design
All of the included trials were randomised, although the process of randomisation was not discussed. Two trials had a parallel design (Almajan‐Guta 2011; Cerny 1989) and two were cross‐over trials (Balestri 2004; Bilton 1992). Neither of the cross‐over trials described a washout period. The total trial duration varied between four days (Bilton 1992) and six months (Almajan‐Guta 2011). Three trials were conducted over a period of up to 14 days ‐ deemed short‐term (Balestri 2004; Bilton 1992; Cerny 1989) ‐ and one long‐term trial lasted six months (Almajan‐Guta 2011). All trials were single‐centre. Three were carried out across Europe: Romania (Almajan‐Guta 2011), Italy (Balestri 2004), the UK (Bilton 1992); and one was conducted in the USA (Cerny 1989).
Participants
There were a total of 86 participants in the four included trials. Two trials reported the sex of participants, with a split of eight females to 23 males (Balestri 2004; Bilton 1992). The age of participants ranged from seven years (Almajan‐Guta 2011) to 41 years (Balestri 2004). One trial was conducted exclusively in children under 14 years of age (Almajan‐Guta 2011). The remaining three trials included both adults and children. One trial made no reference to disease severity (Almajan‐Guta 2011). Two trials reported mean FEV1 at baseline; one with a range of 54% to 95% (Balestri 2004), and one with a mean (SD) of 2.3 (1.1) L (Bilton 1992). The remaining trial commented on the pulmonary function score (PFS) of participants on admission (Cerny 1989). Participants in three trials were described as clinically stable (Almajan‐Guta 2011; Balestri 2004; Bilton 1992); the remaining trial included participants admitted for an acute exacerbation of pulmonary disease (Cerny 1989). All participants included in one trial were colonised with Pseudomonas aeruginosa (Bilton 1992), but the remaining three trials did not comment on the microbiological colonisation status of the included participants (Almajan‐Guta 2011; Balestri 2004; Cerny 1989). As all included trials were published prior to the introduction of small molecule therapies, the authors can infer that no participants were on modulators.
Interventions
The four included trials varied in their treatment comparisons and interventions. One trial compared exercise alone with ACBT (Bilton 1992) ‐ the trial had four treatment arms, but only two arms were included for the purpose of this review; two trials compared exercise plus PD&P to PD&P alone (Almajan‐Guta 2011; Cerny 1989); and one trial compared exercise alone to underwater PEP (bubble PEP) (Balestri 2004). Three of the four included trials used a cycle ergometer at varying intensity as the exercise intervention (Balestri 2004; Bilton 1992; Cerny 1989), while one trial evaluated participation in ‘sport activities,’ which were undefined (Almajan‐Guta 2011). Three of the four trials reported session duration, which varied between 20 and 30 minutes per session (Balestri 2004; Bilton 1992; Cerny 1989). Intervention duration varied greatly from one day (Bilton 1992) to six months (Almajan‐Guta 2011).
Outcomes
There was a large diversity in terms of the outcomes measured. The two most commonly reported outcomes were lung function and sputum volume. Three trials measured lung function including change in FVC and FEV1 from baseline (Almajan‐Guta 2011; Bilton 1992; Cerny 1989). Two of these trials also reported the FEF25-75 (Almajan‐Guta 2011; Cerny 1989). Two trials evaluated the effect on exercise capacity, peak load/peak heart rate and VO2max, respectively (Bilton 1992; Cerny 1989). Three trials monitored for adverse events by using heart rate and oxygen saturation monitoring throughout treatment (Balestri 2004; Bilton 1992; Cerny 1989). Two trials used a visual analogue scale (VAS) to measure participant preference (Balestri 2004; Bilton 1992). Less commonly reported outcomes were the number of hospitalisations (Almajan‐Guta 2011), duration of hospitalisation (Cerny 1989), and adherence (Cerny 1989). No trial measured QoL and the need for extra antibiotics.
Excluded studies
We excluded 17 trials (29 references) in total. Seven trials were ineligible as they described a single session intervention (Aquino 2006; Baldwin 1994; Dwyer 2019; Falk 1988; Lannefors 1992; Radtke 2018; Reix 2012). We excluded nine trials as we deemed their interventions inappropriate for this review (Alexander 2019; Kaak 2011; Montero‐Ruiz 2020; NCT00609050; NCT03295201; Rodriguez 2017; Santana‐Sosa 2014; Zeren 2019; Ward 2018). Of these, one trial investigated the effect of, 'whole body vibration training' (Alexander 2019). In another trial, the intervention was a ‘pulmonary rehabilitation program’, which was not further defined (Rodriguez 2017). Three trials compared different combinations of interventions; one trial compared physiotherapy to physiotherapy plus didgeridoo playing (Kaak 2011); one trial compared PEP plus exercise with exercise alone (Ward 2018), and one trial evaluated music therapy as an adjunct to other ACTs (Montero‐Ruiz 2020). One trial compared self‐regulated exercise combined with telephone reinforcement versus exercise without telephone reinforcement (NCT00609050). Two trials assessed the effect of inspiratory muscle training (Santana‐Sosa 2014; Zeren 2019). The exercise intervention in the final trial was thoracic vertebra mobilization, pectoralis stretching and core exercises, which the review authors agreed was of too low an intensity to be classified as ‘exercise’ for the purpose of this review (NCT03295201).
We excluded one trial as it had been abandoned; this was confirmed by the lead investigator (NCT00792194). The final trial had an ineligible study design; we contacted one of the co‐authors of this publication on 3 February 2021, and they confirmed that the trial was not randomised (Salh 1989).
Studies awaiting classification
We have listed one trial as awaiting classification (Ward 2018). This single‐centre RCT from Australia compares exercise to daily PEP plus exercise in adults with CF. The daily exercise intervention comprises 30 minutes of moderate‐ to strong‐intensity exercise such as walking or jogging or alternatively six cycles of five minutes of step‐ups using an aerobic step and two to three huffs every five minutes. The daily PEP intervention comprises six cycles of 15 breaths per cycle and two to three huffs after each cycle. The trial includes 13 participants and has a duration of 12 weeks. Outcomes include respiratory function tests and health‐related QoL.
Ongoing Studies
We have listed one parallel trial as ongoing (NCT03273959). This trial compares conventional ACT to conventional ACT plus an exercise protocol (punching, kicking, climbing stairs, sit to stand, etc.) in children and adolescents with CF admitted to hospital with a respiratory exacerbation. Outcomes include functional capacity (six‐minute walk test), pulmonary functional capacity (spirometry), physical fitness/health, clinical score (Schwachman‐Kulczycki), bacteriology and nutritional assessment (body mass index). This trial appears to be in the recruitment phase, and we found no full‐text publication associated with this trial. We contacted the trial investigators for additional data.
Risk of bias in included studies
We assessed the risk of bias in the following domains for each trial: generation of sequence; concealment of allocation sequence; blinding of the trial participants, personnel, and outcome assessors; incomplete outcome data; selective outcome reporting; and other potential sources of bias. We used the approach for assessing the risk of bias in included trials recommended by Cochrane (Higgins 2017). Risk of bias judgements are summarised in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Sequence generation
The trial authors stated that participants were randomly allocated to either the intervention in the two parallel trials (Almajan‐Guta 2011; Cerny 1989) or the treatment order in the two cross‐over trials (Balestri 2004; Bilton 1992). However, none of the trials described the process of randomisation, hence the risk of bias with respect to randomisation for all trials was unclear.
Allocation concealment
Concealment of allocation was not discussed in any of the trials, so we judged them all to have an unclear risk of bias (Almajan‐Guta 2011; Balestri 2004; Bilton 1992; Cerny 1989).
Blinding
Due to the nature of the intervention requiring physical participation or co‐operation in using manual devices, the blinding of participants or personnel supervising treatment was not possible in any of the included trials. Participant awareness of the intervention may have led to some impact on the reporting of subjective measures, such as QoL, due to pre‐existing perceptions or preferences on the benefits of exercise versus traditional ACTs. However, it is unlikely to confer any impact on objective outcome measures such as lung function and exercise testing. It is therefore difficult to objectively assess the overall risk of bias of each of the included trials. Overall, we conclude that the risk of bias secondary to lack of blinding in these trials is unclear with respect to objective outcomes, and high in terms of subjective outcomes, including QoL. None of the included trials explored whether their participants had any preconceptions or preferences about the efficacy of either traditional ACTs or exercise as a form of ACT (Almajan‐Guta 2011; Balestri 2004; Bilton 1992; Cerny 1989).
Despite the obvious challenges in blinding trial participants, it would be both achievable and desirable for outcome assessors to be blinded to the intervention received at the time of analysis. The blinding of outcome assessors is not mentioned in any of the four included trials, so we deemed the risk of bias from this source to be unclear (Almajan‐Guta 2011; Balestri 2004; Bilton 1992; Cerny 1989).
Incomplete outcome data
None of the trials refers to any consideration of intention‐to‐treat analysis in their methods. In three trials, interventions were directly observed, with immediate assessment of outcome measures, effectively excluding concerns about non‐compliance (Balestri 2004; Bilton 1992; Cerny 1989). In the remaining trial, the intervention was carried out over a six‐month period and no data were reported in the abstract concerning assessment of participant compliance (Almajan‐Guta 2011).
One trial confirmed that there were no withdrawals, and as a result we deemed it to have a low risk of bias. (Bilton 1992).
Only one of the included trials referred to incomplete outcome data, reporting that 100% of postural drainage sessions and 96% of exercise sessions were completed (Cerny 1989). However, no reason was given for the incomplete sessions, and there was no discussion of how this impacted on data analysis. The remaining two trials gave no mention of participant withdrawal or degree of compliance (Almajan‐Guta 2011; Balestri 2004). For this reason, we deemed three trials to have unclear risk of bias due to insufficient available information (Almajan‐Guta 2011; Balestri 2004; Cerny 1989).
Selective reporting
None of the included trials had protocols available to allow the comparison of planned and reported outcomes. The authors assessed selective reporting by comparing planned outcomes in the ‘Methods’ section with those reported in the ‘Results’ section of any publications.
We assessed two trials that were only available as conference abstracts to have an unclear risk of bias, as they did not provide sufficient information regarding methodology for us to make this assessment (Almajan‐Guta 2011; Balestri 2004).
We also considered the remaining two trials to have an unclear risk of reporting bias, as the authors reported all but one of the outcomes in detail (Bilton 1992; Cerny 1989). Bilton 1992 stated that spirometry was performed “prior to each treatment session, immediately following each treatment and 30 minutes after each session”. However, the authors did not provide numerical data, only narrative statements. Another trial reported the detailed recording of sputum expectorated (daily sputum volume, wet weight and dry weight (units/hr)) within the methods section, but presented no quantitative data and only a narrative statement on daily sputum volume and dry weight to reflect this (Cerny 1989).
Other potential sources of bias
Two of the trials were short‐term cross‐over trials, so trial design included a washout period between interventions (Balestri 2004; Bilton 1992). The ideal duration of washout periods is unknown, but carry‐over effects that may introduce bias are more likely with shorter washout periods and shorter trial durations. One trial involved the use of four treatments over two weeks in a random order (Balestri 2004). Each treatment was performed on two separate occasions and the allocation of treatment days was random. Only one treatment was used per day, but investigators did not specify either the number of days between each treatment or which ACTs the participants had used at baseline, meaning the washout period was unknown. The second trial involved the use of four treatments over four consecutive days, with each day involving two treatment sessions (Bilton 1992). The order of interventions was randomly determined. Given the short washout period and small number of treatment sessions in both trials, we deemed them both to have an unclear risk of bias in respect of carry‐over effects.
The two parallel RCTs included in the review did not include any data on the baseline characteristics of their two groups of participants (Almajan‐Guta 2011; Cerny 1989). Not knowing the demographics and potential prognostic variables could affect the generalisability of the results and may have influenced trial outcomes. We therefore deemed this an unclear risk of bias in the study that was abstract only, allowing for the fact there may have been a limit in word count (Almajan‐Guta 2011), but a high risk of bias in the study that was published in full (Cerny 1989).
In terms of recruitment, none of the included trials considered a power calculation with the required sample size of participants necessary to achieve significance. It is therefore difficult to be certain whether a lack of effect is due to insufficient study population size or genuine lack of intervention efficacy, and so we considered these to have an unclear risk (Almajan‐Guta 2011; Balestri 2004; Bilton 1992; Cerny 1989).
In one trial, bronchodilator therapy was administered prior to conventional ACT sessions, but not in the intervention arm of exercise by cycle ergometer (Cerny 1989). Bronchodilator therapy would be anticipated to enhance airway clearance via any mechanism. Furthermore, the durations of the two interventions in this trial were not similar, with exercise set to a "target duration of 15 to 20 minutes," compared with "postural drainage with chest percussion and vibration in six positions for 20 to 40 minutes". As such, we deemed this trial to have a high risk of bias in favour of the conventional ACT arm.
Only one trial reported on QoL (Almajan‐Guta 2011). This trial did not use a validated questionnaire; therefore, we considered the credibility and comparability of the data gathered to be an unclear source of other bias.
Three of the trials reported sponsorship by the CNCSIS (National University Research Council of Romania); UK Cystic Fibrosis Trust and NIH grant and the Buffalo foundation respectively (Almajan‐Guta 2011; Bilton 1992; Cerny 1989). Sponsorship by any of these institutions would not be anticipated to introduce conflicts of interest, meaning the risk of bias from this source is low. The remaining trial does not report any sponsorship information, rendering the risk of bias from this source unclear (Balestri 2004).
Effects of interventions
See: Table 1; Table 2; Table 3
We have included four studies in this Cochrane Review. In one trial, only one arm looked at the impact of exercise exclusively and compared this to traditional ACT (ACBT) alone (Bilton 1992). We excluded the other arms of this cross‐over study as they evaluated exercise versus exercise plus ACT (ACBT), assessing the effect of the ACBT rather than exercise (Bilton 1992).
Limited data have resulted in a largely narrative summary.
Please see the summary of findings tables for the explanations of the judgements for the certainty of the evidence (Table 1; Table 2; Table 3).
Exercise versus ACBT
One trial (18 participants) reported on the comparison of exercise (cycle ergometer at 60% of VO2max) with ACBT (Bilton 1992). Key results are shown in Table 1.
Primary outcomes
1. Lung function
a. FEV1 (% predicted) absolute change from baseline
The included trial did not report on this outcome (Bilton 1992).
b. FEV1 (L) absolute change from baseline
Investigators narratively reported that there was no difference in FEV1 (L) from baseline at 30 minutes post‐treatment in either group, although they did report that immediate values following 20 minutes of exercise alone showed a significant increase in FEV1 (P < 0.05) but values returned to baseline levels after 30 minutes (very low‐certainty evidence) (Bilton 1992).
2. Exercise capacity
The included trial did not report on our prespecified measures for this outcome: VO2 peak in L, mL/kg body weight or fat‐free mass or as % predicted; or maximal work rate (Wpeak); or any other validated field test (e.g. six‐minute walk test, modified 10‐metre shuttle test) (Bilton 1992).
3. QoL
The included trial did not report on our prespecified self‐reported questionnaires for assessing QoL (CFQ‐R (Quittner 2009), CF‐QoL (Gee 2000), CRQ (Guyatt 1987), NHP (Wiklund 1990) or SF‐36 (Ware 1992)) (Bilton 1992).
Secondary outcomes
1. Adverse effects (related to exercise and exercise testing)
No adverse effects in either heart rate or oxygen saturation were observed during the exercise sessions (very low‐certainty evidence) (Bilton 1992).
2. Lung function
a. FVC (% predicted or L)
This trial reported there was no change in FVC (L) in either the exercise or ACBT group from baseline to 30 minutes post‐treatment (Bilton 1992).
b. FEF25-75
Although this trial did not specifically report on FEF25-75, investigators reported Vmax25% which is equivalent. Again, there were no differences between groups post‐treatment, but Vmax25% increased significantly (P < 0.05) in the exercise group only, returning to baseline levels after 30 minutes; no such increase was observed in the ACBT group. Investigators additionally stated that there was a positive correlation (r = 0.59, P < 0.01) between baseline FEV1 and an absolute increase in Vmax25% (Bilton 1992).
c. LCI
The included trial did not report on this outcome (Bilton 1992).
3. Participant preference
Participants were asked to score each treatment for effectiveness on a VAS and then asked which treatment they would prefer to continue for long‐term use at home. Any treatment combination including ACBT was perceived to be more effective than exercise alone and was the preferred treatment modality in this trial (very low‐certainty evidence) (Bilton 1992).
4. Adherence
The included trial did not report on adherence using either electronic data capture or participant diaries (Bilton 1992).
5. Sputum weight
The trial authors did not distinguish between wet and dry sputum weight and reported the mean 24‐hour sputum volume. The total sputum weight in 24 hours for exercise was significantly less than ACBT (P < 0.001). There was no significant difference between expectorated sputum volume during the non‐treatment times for either group (Bilton 1992).
6. Hospital admissions due to exacerbation
The included trial did not report on the number or duration of hospital admissions (Bilton 1992).
7. Need for extra antibiotics (days)
The included trial did not report on the need for additional antibiotics, either oral, IV, inhaled or nebulised (Bilton 1992).
Exercise plus PD&P versus PD&P alone
Two trials (55 participants) compared exercise with PD&P to PD&P alone (Almajan‐Guta 2011; Cerny 1989). As insufficient data existed for analysis and only narrative results were available, the review authors have reported these data together. However, one trial lasted up to 14 days in participants experiencing a pulmonary exacerbation (Cerny 1989), and the second trial lasted over 12 weeks in participants who were clinically stable (Almajan‐Guta 2011). For the purpose of this narrative account, the terms, ‘conventional therapy’ and ‘PD&P’ have been used interchangeably. Key results are shown in Table 2.
Primary outcomes
1. Lung function
a. FEV1 (% predicted) absolute change from baseline
In the trial evaluating treatment effect during a two‐week hospital admission (n = 17), FEV1 increased from baseline in both groups but the increase in the PD&P control group was greater (18.4%) than in the PD&P plus exercise group (11.3%). There were no between‐group differences reported. Standard deviations for these changes were reported in graphical but not numerical format (Cerny 1989). The six‐month trial (n = 38) reported a 13.5% increase in FEV1 from baseline in the exercise plus conventional therapy group; values for the PD&P control group were not given, but the trial report described the increase in the exercise plus PD&P group to be statistically significant compared to PD&P control (P = 0.043). As only an abstract of this trial was available, the review authors were unable to verify this claim (very low‐certainty evidence) (Almajan‐Guta 2011).
b. FEV1 (L) absolute change from baseline
The included trials did not report on this outcome (Almajan‐Guta 2011; Cerny 1989).
2. Exercise capacity
a. peak oxygen uptake (VO2 peak) in L, mL/kg body weight or fat‐free mass or as % predicted
The included trials did not report on this outcome (Almajan‐Guta 2011; Cerny 1989).
b. Wpeak (W/kg)
This outcome was recorded in the two‐week trial, and no significant between‐group differences were observed. An increase in Wpeak of 0.26 W/kg (P < 0.01) was reported in the control group compared to an increase of 0.44 W/kg (P < 0.02) in the exercise and PD&P group (Cerny 1989).
c. any validated field test (e.g. six‐minute walk test (metre), modified 10‐metre shuttle test (level attained and distance covered)
The included trials did not report on this outcome (Almajan‐Guta 2011; Cerny 1989).
3. QoL (self‐reported)
Only one trial measured QoL, but it did not use a validated tool (Almajan‐Guta 2011). The longer‐term trial evaluated participation at school and an activities of daily living (ADL) questionnaire before and after treatment, with 60% participation in school in the exercise plus PD&P group compared with 48% constant participation in school activities in the control group; investigators also reported 16% fatigue versus 42% fatigue during daily activities in the exercise plus PD&P group and PD&P alone group, respectively (Almajan‐Guta 2011).
Neither trial reported on QoL using electronic data capture (Almajan‐Guta 2011; Cerny 1989).
Secondary outcomes
1. Adverse effects (related to exercise and exercise testing)
One trial evaluated potential adverse effects by continuously monitoring arterial oxygen saturation, electrocardiographic activity and heart rate (Cerny 1989). No quantitative analysis or figures were presented, but the authors of this trial reported that there were “no negative effects of exercise therapy performed by inpatients” (very low‐certainty evidence) (Cerny 1989).
2. Lung function
a. FVC (% predicted or L)
Both trials reported FVC % predicted (Almajan‐Guta 2011; Cerny 1989). The longer‐term trial only reported an increase of 14.8% in FVC from baseline in the exercise group, but no values were given for the control group (Almajan‐Guta 2011). The trial of hospitalised participants reported a lower mean increase of 14.6% in FVC for the exercise and conventional therapy group compared to a 22.4% improvement in the conventional therapy group after two weeks (Cerny 1989).
b. FEF25-75
Both trials reported FEF25-75 % predicted (Almajan‐Guta 2011; Cerny 1989). The longer‐term trial only reported values for the exercise and conventional ACT group, showing an increase of 11.2% from baseline (Almajan‐Guta 2011). While no values were reported for the PD&P control group, the result for exercise plus PD&P was reported to represent a statistically significant difference at six months compared to the conventional therapy control (P = 0.036). The authors were unable to verify this claim as the trial was only published in abstract form. The second trial reported an improvement in FEF25-75% for both groups after two weeks; 4.11% in the exercise plus PD&P group compared to 9.6% in the PD&P control group (Cerny 1989).
c. LCI
The included trials did not report on LCI (Almajan‐Guta 2011; Cerny 1989).
3. Participant preference
The included trials did not report on participant preference (Almajan‐Guta 2011; Cerny 1989).
4. Adherence
Only the trial of hospital inpatients narratively reported on this outcome, and stated that "all postural drainage treatments were completed as required for the study and 96% of the scheduled exercise therapy sessions were completed" (Cerny 1989). The reason for lack of adherence in the exercise group is not discussed further (very low‐certainty evidence).
5. Sputum weight
One trial reported the measurement of 24‐hour sputum volume, wet weight and dry weight (units/hr); results were calculated after four days of drying in an oven (Cerny 1989).
a. wet weight (units/hr)
While investigators narratively reported on the 24‐hour sputum volume and dry weight (see below), they did not present any results (quantitative or qualitative) for sputum wet weight.
b. dry weight (units/hr)
Investigators reported that there were no differences in the 24‐hour sputum volume and dry weight. The greatest volume collected was after the morning treatment session, and there were no differences between the PD&P and exercise groups (Cerny 1989). No quantitative results were presented (very low‐certainty evidence).
6. Hospital admissions due to exacerbation
a. number of hospital admissions
This outcome was reported in one trial, which found that after six months, 4 out of 19 (21%) participants in the exercise plus PD&P group had been hospitalised compared to 6 out of 19 (32%) participants in the control group (Almajan‐Guta 2011). When analysed, these data showed no difference between groups (RR 0.67, 95% CI 0.22 to 1.99; Analysis 1.1).
1.1. Analysis.

Comparison 1: Exercise plus PD&P versus PD&P alone, Outcome 1: Hospitalisations
b. duration of hospital admission
The short‐term trial of participants hospitalised with a respiratory exacerbation reported the mean (SD) duration of hospitalisation in days (Cerny 1989); when analysed, data showed no difference between groups (MD 0.00, 95% CI ‐2.71 to 2.71; Analysis 1.2).
1.2. Analysis.

Comparison 1: Exercise plus PD&P versus PD&P alone, Outcome 2: Duration of hospitalisations
7. Need for extra antibiotics (days)
The included trials did not report on the need for additional antibiotics, either oral or IV or inhaled or nebulised (Almajan‐Guta 2011; Cerny 1989).
Exercise versus underwater PEP (uPEP)
One trial (13 participants) compared exercise (cycle ergometer under anaerobic threshold) to uPEP (also known as bubble PEP) (Balestri 2004). Key results are shown in Table 3.
Primary outcomes
1. Lung function
The included trial did not report on lung function measured either as FEV1 % predicted or FEV1 L, presented either as absolute change from baseline or final post‐treatment value (Balestri 2004).
2. Exercise capacity
The included trial did not report on our prespecified measures for this outcome: VO2 peak in L, mL/kg body weight or fat‐free mass or as % predicted; or maximal work rate (Wpeak); or any other validated field test (e.g. six‐minute walk test, modified 10‐metre shuttle test) (Balestri 2004).
3. QoL
The included trial did not report on our prespecified self‐reported questionnaires for assessing QoL (CFQ‐R (Quittner 2009), CF‐QoL (Gee 2000), CRQ (Guyatt 1987), NHP (Wiklund 1990) or SF‐36 (Ware 1992)) (Balestri 2004).
Secondary outcomes
1. Adverse effects (related to exercise and exercise testing)
In this trial, participants were monitored for heart rate and oxygen saturation (SaO2) (%) during each session. The cross‐over design demonstrated no significant difference (P = 0.194) in the peak SaO2 (%) desaturation (SD) between the exercise (92.86 (1.89)) and the uPEP (91.85 (1.96)) group (very low‐certainty evidence) (Balestri 2004). No data for heart rate were reported.
3. Participant preference
Before and after each session, a VAS test was completed by each participant, but the publication did not specify further details. A questionnaire was also submitted to evaluate the acceptability of each ACT to participants (Balestri 2004). The contents or validity of this questionnaire have not been included in this review; however, Balestri concluded that, “the exercise test perceived by patients as more fatiguing was also considered more amusing” (very low‐certainty evidence) (Balestri 2004).
4. Adherence
The included trial did not report on adherence either using electronic data capture or participant diaries (Balestri 2004).
5. Sputum weight
a. wet weight (g)
In this trial, participants were encouraged to cough and expectorate during treatment sessions. During each session the expectorated sputum was collected and weighed, and results were recorded before and after discarded supernatant. From this cross‐over trial, there was no significant difference (P = 0.897) observed in the mean (SD) amount of sputum expectorated during exercise 3.81 g (3.26) when compared with uPEP 3.2 g (2.44), but no data suitable for analysis in this review were available (Balestri 2004).
b. dry weight
The included trial did not report on this outcome (Balestri 2004).
6. Hospital admissions due to exacerbation
The included trial did not report on the number or duration of hospital admissions (Balestri 2004).
7. Need for extra antibiotics (days)
The included trial did not report on the need for additional antibiotics, either oral or IV or inhaled or nebulised (Balestri 2004).
Discussion
The aim of this review was to answer an important question, identified by the James Lind Alliance, for people with CF, “Can exercise replace airway clearance?” by comparing exercise to other ACTs (Rowbotham 2018). People with CF experience a high treatment burden, much of which can be attributed to daily airway clearance. Exercise confers numerous additional benefits to both mental and physical health, but it remains unclear whether it can be considered equivalent to conventional ACTs. We excluded single‐treatment trials from the review as airway clearance is a lifelong requirement for people with CF, thus rendering one‐off interventions irrelevant to these individuals and our prioritised outcomes of lung function and QoL. This review included trials using ACBT, uPEP (also known as bubble PEP) and PD&P as controls. These forms of ACT and others, including AD and oscillatory devices (Flutter, Cornet and HFCWO), have been assessed in other Cochrane Reviews (Burnham 2021; Main 2005; McIlwaine 2019; Mckoy 2016; Morrison 2020); and this Cochrane Review completes the series comparing different ACTs in CF. Future trials will need to consider advancements in modulator therapy and a changing CF population, and this may render many of the outcome measures included in this review less applicable; for example, sputum weight.
Summary of main results
Of the four trials included in this review two were available as abstracts only (Almajan‐Guta 2011; Balestri 2004), and two studies were published as full papers (Bilton 1992; Cerny 1989). The included trials compared exercise to ACBT, to uPEP and to conventional physiotherapy (PD&P), and duration varied from four days to six months.
The four trials enrolled a total of 86 participants (sample size ranging from 13 to 38) from both paediatric and adult CF populations, with ages ranging from seven to 41 years. None of the trials reported comprehensive baseline data regarding the severity of the participants' lung disease, nor their usual ACT. The resulting heterogeneity in the data and the small number of trials meeting the inclusion criteria meant that numerical data analyses were not possible for most outcome measures.
Exercise versus ACBT
One cross‐over trial (18 clinically stable participants) compared exercise to ACBT (Bilton 1992). Investigators found no change from baseline in lung function (either FEV1 or FVC) at 30 minutes post‐treatment in either group; although FEV1 and Vmax25% (equivalent to FEF25-75) temporarily increased in the exercise group before levels returned to baseline at 30 minutes post‐treatment. No adverse effects (either heart rate or oxygen saturation) were reported for either group. When questioned, participants perceived ACBT to be more effective than exercise alone and preferred this treatment. The total sputum weight in 24 hours collected during treatment from the exercise group was less than that from the ACBT group (P < 0.001), but there was no difference in sputum volume during the non‐treatment times for either group (Bilton 1992). This trial did not report on exercise capacity, QoL, adherence to treatment, hospitalisations or the need for antibiotics.
Exercise plus PD&P versus PD&P only
Two trials (55 participants) reported on this comparison; the first lasted 12 weeks in clinically stable participants (Almajan‐Guta 2011), while the second lasted 14 days in participants hospitalised for a pulmonary exacerbation (Cerny 1989). The short‐term trial stated that, while both groups experienced an increase in FEV1, this was greater in the group using PD&P alone (Cerny 1989). The longer trial only presented data for an increase in FEV1 for the exercise plus PD&P group, but narratively reported a significant difference in this group compared to PD&P alone (Almajan‐Guta 2011). Only the short‐term trial reported on exercise capacity (Wpeak) and stated that, while Wpeak increased in both groups from admission to discharge, there was no difference between the treatment groups (Cerny 1989). Only the longer trial evaluated any measure of QoL, but did not use a validated tool (Almajan‐Guta 2011). Investigators assessed participation at school and activities of daily living at the start and end of the trial, and reported greater participation in school and less fatigue during daily activities in the exercise plus PD&P group compared with the PD&P control group.
Only one trial assessed negative or adverse effects of exercise therapy, and reported that none were observed (Cerny 1989).
Both trials reported additional measures of lung function. In the short‐term trial of hospitalised participants, FVC improved in both groups, but there were no between‐group differences and FEF25%-75% improved only in the PD&P group (Cerny 1989). In the longer trial evaluating clinically stable participants, both FVC and FEF25%-75% improved in the exercise group compared with the control (Almajan‐Guta 2011). Only the short‐term trial commented narratively on adherence to treatment, and stated that all treatments in the PD&P group and 96% of the exercise sessions were completed (Cerny 1989). Again, only the short‐term trial reported on sputum volume and found no difference between groups over a 24‐hour period, with the highest volume of sputum being collected after the morning session in both groups (Cerny 1989). Only the six‐month trial reported on the number of hospital admissions (Almajan‐Guta 2011); when we analysed the data we found no difference between the groups (Analysis 1.1). In the trial of hospitalised participants there was no difference in the mean length of stay between groups (Analysis 1.2). Neither trial reported on the need for antibiotics.
Exercise versus uPEP
One trial (13 clinically stable participants) compared exercise to uPEP, but did not report on any of this review's primary outcome measures (FEV1, exercise capacity and QoL) (Balestri 2004). Investigators reported no negative or adverse effects of therapy after monitoring oxygen saturations and heart rate throughout the intervention period (Balestri 2004). Participant preference was measured using a non‐validated VAS and the exercise intervention was perceived to be the most fatiguing but the most enjoyable (Balestri 2004). With respect to mucociliary clearance, there was no significant difference in sputum expectorated between the exercise or uPEP sessions (Balestri 2004).
Overall completeness and applicability of evidence
Due to conventional methods of airway clearance being the accepted standard of care for people with CF and exercise being considered as an adjuvant therapy only, we anticipated identifying few trials that compared exercise alone to another ACT. Therefore, we evaluated the effect of exercise alone and exercise in combination with another form of ACT, in the hope of still being able to provide knowledge that could potentially lessen the treatment burden for people with CF. In the comparisons we have presented, the comparator intervention included exercise AND the alternative ACT. The authors accept and acknowledge that this is not ideal, and trials with direct comparisons are needed. The paucity of research available meant the studies selected were the only way to begin to examine the effect of this intervention and address the question identified by the James Lind Alliance.
Unfortunately, most eligible trials were short‐term and only one of the four included trials reported a medium‐term intervention (three times weekly over six months) (Almajan‐Guta 2011). CF is a lifelong, progressive disorder, with an ongoing and often progressively increasing requirement for airway clearance over time, meaning that medium‐ to long‐term trials are of more relevance to this population than single or short‐term treatments. A complete lack of long‐term data means that the longer‐term effects of exercise as an ACT remain unclear.
The trials in this Cochrane Review included both children and adults with CF, both clinically stable and with a respiratory exacerbation. None of the trials reported on the severity of lung disease at baseline, meaning it is difficult to judge the degree to which the trial population is representative of the CF population as a whole. One trial that evaluated the number of hospitalisations throughout the study period presented no baseline parameters in participant characteristics or number of hospital admissions for each participant in the preceding six months for comparison (Almajan‐Guta 2011). Therefore, the applicability, validity and significance of this value is very difficult to assess.
Two of the included trials were conducted approximately three decades ago (Bilton 1992; Cerny 1989); whilst ACTs in themselves have not changed much in this time, CF care as a whole has changed a great deal. As such, baseline treatments and severity of lung disease, particularly amongst the paediatric CF population, are likely to be significantly different now than they were then. The advent of disease‐modifying therapies such as CFTR modulators have revolutionised CF care, and it is to be hoped that in the coming decades the maintenance of lung health rather than management of early advanced bronchiectasis will be a mainstay of CF treatment. As a result, trials focusing on preventative and holistic management such as exercise are likely to become of increasing relevance to people with CF.
Exercise programmes in three of the included trials were individualised using cardiopulmonary exercise testing (CPET) (Balestri 2004; Bilton 1992; Cerny 1989). CPET technology and appropriately qualified physiologists and physiotherapists should be available in the majority of specialist CF centres, meaning that tailored exercise programmes should be a feasible intervention for the majority of people with CF.
Whilst three of the included trials involved supervised intervention, so compliance with therapy can be inferred (Balestri 2004; Bilton 1992; Cerny 1989), the only medium‐term trial included in the review did not include any data on adherence to the intervention (Almajan‐Guta 2011). As a result, it is challenging to assess whether the reported improvements in outcome measures were genuine, and it also brings into question the frequency of intervention required to obtain meaningful clinical improvements.
Quality of the evidence
Only four studies, with a total of 86 participants, were included in the review. In part, this is due to the feasibility of conducting studies of this kind.
A greater degree of reliability could have been placed on the findings of the included trials if trial investigators had concealed allocation and described the method of randomisation. These omissions mean that it is challenging to draw definite conclusions on the degree of bias affecting each trial, and all trials had an unclear risk of bias judgement for each of these domains.
As both exercise and other ACTs being studied require physical participation using manual devices, the blinding of participants or personnel supervising treatment was not possible in any of the included trials. Lack of blinding is unlikely to affect objective measures such as lung function, so we assessed this to have an unclear risk. The potential impact of participant awareness of the intervention having some influence over subjective outcomes, such as QoL, meant that the risk of bias judgement for all studies was high.
One trial only commented on no missing data, so we deemed this to be at low risk. None of the remaining included trials referred to intention‐to‐treat analysis in their methods, but the interventions were directly observed, so we assessed these to have an unclear risk of bias due to insufficient available information.
The two parallel RCTs included in the review did not include any data on the baseline characteristics of their two groups of participants, which may have influenced trial outcomes (Almajan‐Guta 2011; Cerny 1989). The remaining two short‐term cross‐over trials had a minimal washout period (Balestri 2004; Bilton 1992). Both trials made some attempt to assess the effects of the order of the interventions by subgroup analysis and randomisation of the order of the intervention; however, there remains a high probability of bias.
One of the main benefits of exercise is the associated improvement in QoL, which is of particular relevance to a chronic condition like CF that confers a high treatment burden. Despite this, only one of the trials assessed QoL, and even this trial did not report on outcomes from a validated QoL questionnaire.
None of the included trials included a power calculation, and without this information is impossible to be certain whether a lack of effect is due to insufficient study population size or genuine lack of intervention efficacy. We evaluated the certainty of the evidence for a number of outcomes in all comparisons using the GRADE system (Table 1; Table 2; Table 3). We judged the evidence for all outcomes assessed to be of very low certainty. We downgraded the certainty of the evidence mainly due to concerns surrounding risk of bias from a number of domains, imprecision caused by low numbers of participants, and in one case due to indirectness where we reported FEV1 in L and not as per cent predicted as specified in our protocol (Table 1).
Potential biases in the review process
Two authors undertook a thorough search of the literature (KP, AW). Due to the COVID‐19 pandemic, it is possible that trials have been undertaken that have not yet been identified or published. Two trials were published in abstract form only, and the review authors were unable to contact the trial investigators, so only limited data were available. All review authors have an active interest in promoting and encouraging participation in physical activity and exercise in their clinical practice, but have not been endorsed to provide training in this technique.
Agreements and disagreements with other studies or reviews
ACTs have been the mainstay form of treatment for people with CF (particularly those who are produce a high volume of sputum), to provide symptomatic benefit and reduce decline in lung function. There is a sufficient evidence base to support this (Warnock 2015). This review completes the series of Cochrane Reviews evaluating the efficacy of different forms of ACT for airway clearance in CF. Previous reviews comparing conventional physiotherapy (PD&P) (Main 2005), PEP (McIlwaine 2019), ACBT (Mckoy 2016), oscillating devices (Morrison 2020), and AD (Burnham 2021) have concluded non‐superiority between ACTs, which is consistent with this review.
A recent systematic review has been undertaken by an Australian team which mirrored the findings of this review (Ward 2020). Their publication employs the use of appropriate methodology and has identified all of the trials included in this review, apart from one which may not have been included due to the limited data presented in the abstract (Almajan‐Guta 2011). A further difference between the Ward 2020 review and our Cochrane Review is that we excluded single‐session interventions, whereas the Ward 2020 review included these. We excluded such trials due to our focus being on evaluating the long‐term effects of exercise on respiratory health and other outcomes for people with CF; airway clearance is a lifelong requirement for people with CF, thus rendering one‐off interventions irrelevant to these individuals and our prioritised outcomes of lung function and QoL. The Ward 2020 systematic review also compared exercise to rest, in order to evaluate the efficacy of exercise alone. The authors identified two studies that reported the peak expiratory flow (PEF) rates and peak expiratory to peak inspiratory flow ratios (PEF:PIF) between exercise and rest. Treadmill exercise resulted in a significantly higher PEF than rest, improved mucociliary clearance and increased the ease of expectoration in both studies (Dwyer 2011; Dwyer 2019; Ward 2020).
Authors' conclusions
Implications for practice.
This Cochrane Review cannot definitively answer the question of how efficacious exercise is in aiding sputum clearance, due to a lack of sufficient evidence to suggest that exercise is an inferior airway clearance technique (ACT) for respiratory health parameters compared with more traditional techniques. We can, however, infer that exercise improves aerobic capacity and has a positive effect on lung function and health‐related quality of life (Radtke 2017). This ACT may become even more necessary to adopt considering the remarkable differences already observed in people with cystic fibrosis (CF) since the introduction of modulator therapy, for example with increasing central adiposity (Bailey 2021; Litvin 2019; Volkova 2020), which has an impact on body image ‐ a recognised health‐related quality of life domain. It is also accepted that higher levels of aerobic fitness are associated with reduced disease severity, improved prognosis and survival (Hebestreit 2019; Nixon 1992). Therefore, regardless of which airway clearance technique is chosen by the individual, exercise should be prioritised and considered an essential component of airway clearance in CF. Although clinicians will not yet be able to provide people with CF the answer to the question: "Can exercise replace my usual ACT?" it is important that healthcare providers continue to educate and encourage active participation in physical activity based on the findings generated in this review.
Three trials in this review recruited participants with stable CF‐associated lung disease (Almajan‐Guta 2011; Balestri 2004; Bilton 1992), with the remaining trial investigating participants with a severe exacerbation of their lung disease (Cerny 1989). In practice, the question of whether exercise can achieve comparable efficacy of airway clearance to conventional ACTs during periods of clinical stability compared to during a pulmonary exacerbation could be considered as two separate questions. During an exacerbation, the increased volume and purulence of airway secretions, in association with acute lung inflammation and possibly systemic illness, is likely to significantly alter the response to and ability to perform airway clearance. In order to participate in therapeutic exercise the individual needs to be clinically well enough to do so, and in reality there will always be circumstances in which conventional ACTs are required. Therefore, it is important when interpreting the applicability of the review findings to consider that in clinical practice, the two techniques have complimentary roles in therapy at different times and severities of illness.
Whilst three of the included trials involved supervised intervention, so compliance with therapy can be inferred (Balestri 2004; Bilton 1992; Cerny 1989), the only medium‐term trial included in the review did not include any data on adherence to the intervention (Almajan‐Guta 2011). As a result it is challenging to assess whether the reported improvements in outcome measures were genuine, which also brings into question the frequency of intervention required to obtain meaningful clinical improvements.
Implications for research.
With the introduction of genetic modulators, CF care is vastly changing. As outcomes improve and the focus of care shifts towards maintaining lung health, exercise as a therapy is likely to play a huge role in optimising respiratory function, improving quality of life and reducing treatment burden. This review highlights the lack of evidence to support the replacement of standard ACTs with exercise. To assist in future research aiming to answer this question, we propose considerations for an optimal trial design to robustly study the effects of exercise as an intervention compared with other ACTs.
Ideally, randomised control trials (RCTs) are required to answer the specific question: "In a population of children and adults with cystic fibrosis, can exercise replace other ACTs to preserve respiratory function?" Such a trial will then be able to directly address one of the top 10 research priorities identified by people with CF in a James Lind Alliance Priority Setting Project (Rowbotham 2018). Most of the evidence in this review is from short‐term trials, but given the proposed physiological benefits of exercise and the anticipated effect on lung function, future trials should be longer‐term (i.e. over six months). This will provide stronger evidence to support patient‐centred outcomes, such as adherence and individual preference, and will imitate the chronicity and instability of CF‐lung disease. Exercise as an intervention precludes blinding of participants and trial personnel, introducing further concerns about bias within the results. The cross‐over trial design is an alternative study design that may mitigate some challenges encountered when using RCTs (for example, recruitment of fewer participants), but many cross‐over studies in this area have been too short in duration or have used insufficient washout periods. As CF is characterised by frequent exacerbations and progressive decline in lung function, cross‐over designs have limited use due to high rates of exacerbation‐related participant dropouts and change in clinical status between trial arms. Given recent therapeutic innovations and the associated reduction in lung function decline, it may be that a cross‐over design will present fewer challenges than it once did.
CF has a varied phenotypical pattern, with over 2000 CF transmembrane conductance regulator (CFTR) gene mutations identified. It is important that trials consider this variation to ensure applicability of results to the CF population as a whole. Future trial participants should have a confirmed diagnosis of CF via genetic testing or a positive sweat test, and have been clinically stable for an agreed period prior to the start of the trial. We suggest recruiting clinically stable participants due to the anticipated effect of modulator therapy in reducing the frequency and severity of pulmonary exacerbations. This also considers the potential benefits of exercise as a maintenance rather than adjunctive therapy in CF. One negative denominator of the trials included in this review was the lack of data representing participant characteristics at baseline. Due to the complex nature of CF and variety of factors known to influence outcomes, it is important to consider other predictors that may influence the effect of exercise as an ACT, such as: height, weight and body mass index (BMI). It is also particularly pertinent to record whether a participant is on modulator therapy, and if so which one, to facilitate potential subgroup analysis.
We also propose that the type of exercise should be participant‐selected. In this review, most trials evaluated cycle ergometer at varying intensities. This was suitable for a clinical trial setting but is unlikely to reflect real‐life application of exercise as an intervention. To ensure results are applicable and meaningful to the CF community, it would be preferable to offer a selection of exercise interventions that would achieve the same heart‐rate intensity, for the same duration of time. This flexibility is likely to aid recruitment, improve adherence and remove socioeconomic barriers conferred by activities that require access to expensive equipment. For the purpose of designing a trial with the sole aim of ascertaining whether exercise can replace existing ACTs, the authors recommend aligning with the current gold standard practice, which uses physiological recommendations determined by cardiopulmonary exercise testing, i.e. aerobic exercise performed at 70% to 80% of maximal heart rate or peak oxygen uptake (Radtke 2017; Swisher 2015). This is a pragmatic suggestion, and we acknowledge that this does not take into consideration the psychological impact of exercise (Greut 2021), and that not tailoring the exercise program to fit the individual's needs may affect enjoyment and long‐term engagement with this therapy (Ekkekakis 2011; Greut 2021; Hurley 2021; Lewis 2016; Williams 2008). Once this important question is addressed, future research might look at the benefits of aerobic versus anaerobic or resistance training for people with CF, or combination exercise programmes to optimise global health.
Ideally, the trial would be powered to detect non‐inferiority in terms of forced expiratory volume in one second (FEV1). With the changing landscape of CF care, the lung clearance index may also be an alternative outcome measure due to its ability to more sensitively identify early lung disease and any response to interventions like exercise (Perrem 2018). As the question of potentially replacing traditional ACTs with exercise is being driven by the CF community, future research might focus on more patient‐centred outcomes, such as individual preference and improvement in quality of life, ideally assessed by a validated tool such as the Cystic Fibrosis Questionnaire ‐ Revised (Quittner 2009) or the St George’s Respiratory Questionnaire (Jones 1991), rather than measures such as sputum weight.
History
Protocol first published: Issue 3, 2019
Acknowledgements
The review authors would like to sincerely thank our Managing Editor Nikki Jahnke for her assistance and support, and Dr Donald Urquhart for discussing future study design and implications for research. The authors would also like to thank Pamela McCormack for her work on the protocol.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Electronic search strategies
| ClinicalTrials.gov (www.clinicaltrials.gov/) |
RECRUITMENT STATUS: All studies CONDITION OR DISEASE: cystic fibrosis OTHER TERMS: Exercise AND (airway clearance OR oscillating devices OR postural drainage OR percussion OR active cycle OR positive expiratory pressure OR autogenic drainage) |
| WHO ICTRP (ICTRP Search Portal (who.int)) |
Advanced search Recruitment status: ALL Search 1: Condition: cystic fibrosis AND Intervention: exercise AND airway clearance Search 2: Condition: cystic fibrosis AND Intervention: exercise AND oscillating devices Search 3: Condition: cystic fibrosis AND Intervention: exercise AND postural drainage Search 4 : Condition: cystic fibrosis AND Intervention: exercise AND percussion Search 5 : Condition: cystic fibrosis AND Intervention: exercise AND active cycle Search 6: Condition: cystic fibrosis AND Intervention: exercise AND positive expiratory pressure Search 7: Condition: cystic fibrosis AND Intervention: exercise AND autogenic drainage |
Data and analyses
Comparison 1. Exercise plus PD&P versus PD&P alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Hospitalisations | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 At six months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Duration of hospitalisations | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 At six months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Almajan‐Guta 2011.
| Study characteristics | ||
| Methods | RCT Parallel design Single centre: Romanian National CF centre Duration: 6 months |
|
| Participants |
Inclusion criteria: children (age limit not defined). No further inclusion criteria specified Exclusion criteria: not specified 38 participants randomised: (19 = control group) (19 = intervention group) Baseline characteristics Age, range: 7 to 13 years Sex: not specified Disease status of participants: not specified Lung function: not specified |
|
| Interventions |
Intervention: PD&P and sport activities 3 times per week Comparator: PD&P |
|
| Outcomes | FEV1 % predicted, FVC, FEF25%-75%, number of hospitalisations, participation in school activities, fatigue during daily activities | |
| Identification |
Sponsorship source: supported by CNCSIS (Romanian National Council for Scientific Research in Higher Education) (grant TE36) Country: Romania Authors name: Bogdan Almajan‐Guta Institution: University Politehnica, Sport and Physical Education, Timisoara, Romania; National CF Centre, Timisoara, Romania Email: bogdan.almajan@efs.upt.ro Address: Bulevardul Vasile Pârvan 4, Timișoara 300223, Romania |
|
| Notes | Abstract only published. Lead author was contacted on 16 April 2019 and 5 April 2020 for further information and results. No response from authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Process of randomisation not described: "This study was conducted for 6 months, in the Romanian National CF centre with 38 patients, randomized in 2 groups." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible due to the type of intervention used. |
| Blinding of participants and personnel (performance bias ‐ subjective outcomes) Subjective outcomes | High risk | Blinding was not possible due to the type of intervention used. Participant awareness of the intervention may have led to some impact on reporting of subjective measures, such as QoL, due to pre‐existing perceptions or preferences on the benefits of exercise versus traditional airway clearance techniques. |
| Blinding of participants and personnel (performance bias ‐ objective outcomes) Objective outcomes | Unclear risk | Blinding was not possible due to the type of intervention used; however, this was unlikely to confer any impact on objective outcome measures such as lung function and exercise testing |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unknown whether or not the outcome assessors were aware of the intervention assignments. It is unclear who the personnel responsible for overseeing the intervention and those responsible for collection of data were. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Abstract only. All outcomes reported in narrative form only, with no access to raw data. No mention of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | No protocol for comparison. Only methodology available in abstract. |
| Other bias | Unclear risk | There was a unclear risk of recruitment bias in this study, as it mentioned no exclusion or specific inclusion criteria of participants or baseline characteristics. This may have been due to the limited abstract word count. There was no reported power calculation. There is an unclear risk associated with the potentially insufficient sample size. |
Balestri 2004.
| Study characteristics | ||
| Methods | RCT Cross‐over design. No mention of a washout period Single centre: Centro Fibrosi Cistica, Cesena Duration: 2 weeks |
|
| Participants |
Inclusion criteria: clinically stable Exclusion criteria: not specified 13 participants Age, range: 10 to 41 years Sex: 10 males, 3 females Disease status: all stable at time of study Lung function, FEV1 % predicted (range): 54% to 95% |
|
| Interventions |
Intervention: exercise (cycle ergometer constant effort 1/2 W/kg) 1 x 30‐minute session per week where participant undertakes 5 minutes exercise followed by a 2.5 minute break, then 3 bouts of cough, repeated 4 times Comparator: chest physiotherapy (underwater PEP), 1 x 30‐minute session per week where, for each of the 4 positions, participant performs 15 expirations against 10 cm water resistance, then 3 bouts of cough, repeated 4 times |
|
| Outcomes | Sputum expectorated during treatment (wet weight (g)), acceptability of treatment (VAS), adverse events (SPO2 % desaturation), peak heart rate | |
| Identification |
Sponsorship source: not stated Country: Italy Authors name: Elena Balestri Institution: Centro Fibrosi Cistica, Cesena Email: elenabalestri66@gmail.com Address: Centro Fibrosi Cistica, Cesena, FC, Italy |
|
| Notes | Abstract only published. Lead author was contacted on 16 April 2019 for further information and results. They confirmed that no further results are available, and no manuscript will be published. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Process of randomisation not described: "13 CF patients underwent the 4 sequences randomly assigned for the 4 treatments." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible due to the type of intervention used. |
| Blinding of participants and personnel (performance bias ‐ subjective outcomes) Subjective outcomes | High risk | Blinding was not possible due to the type of intervention used. Participant awareness of the intervention may have led to some impact on reporting of subjective measures, such as QoL, due to pre‐existing perceptions or preferences on the benefits of exercise versus traditional airway clearance techniques. |
| Blinding of participants and personnel (performance bias ‐ objective outcomes) Objective outcomes | Unclear risk | Blinding was not possible due to the type of intervention used; however, this was unlikely to confer any impact on objective outcome measures such as lung function and exercise testing. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is unknown whether or not the outcome assessors were aware of the intervention assignments. It is unclear who the personnel responsible for overseeing the intervention and those responsible for collection of data were. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All outcomes reported in narrative form only or partially reported with final mean values and standard deviations. No access to raw data. There seems to be zero attrition in this study, with all 13 participants completing the trial; however, there was no reference to participant withdrawal or intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | No protocol for comparison. Only methodology available in abstract. This may have been limited by the abstract word count. |
| Other bias | Unclear risk | Abstract only available. There is no mention of how participants were recruited into this study. The washout period used during the study was not reported, as methods stated only that 4 treatment days were completed during a 2‐week period, with no explicit statement about whether the days were conducted with fixed or variable gaps. There was no reported power calculation. There is an unclear risk associated with the potentially insufficient sample size. |
Bilton 1992.
| Study characteristics | ||
| Methods | RCT Cross‐over design Single centre: Regional Adult Cystic Fibrosis Unit, Manchester Duration: 4 days |
|
| Participants |
Inclusion criteria: not specified Exclusion criteria: not specified 18 participants Baseline characteristics Age, mean (range): 21 (16 to 34) years Sex: 13 males, 5 females Disease status: clinically stable at time of study; all colonised with Pseudomonas aeruginosa Lung function, FEV1 L, mean (SD): 2.3 L (1.1) |
|
| Interventions |
Intervention 1: chest physiotherapy (ACBT), 20 minutes 2 x daily Intervention 2: exercise (cycling at 60% VO2 max), 20 minutes 2 x daily Intervention 3: chest physiotherapy (ACBT), 10 minutes 2 x daily plus exercise (cycling at 60% VO2 max), 10 minutes 2 x daily |
|
| Outcomes | Absolute FEV1 L, adverse events, FVC L, participant preference, sputum expectorated during treatment (wet weight (g)), sputum expectorated during non‐treatment times (wet weight (g)), perceived effectiveness by high and low sputum producers | |
| Identification |
Sponsorship source: supported by the Cystic Fibrosis Trust Country: UK Setting: Regional Adult Cystic Fibrosis Unit, Monsall Hospital, Newton Heath, Manchester Authors name: Diana Bilton Institution: Faculty of Medicine, National Heart & Lung Institute, Imperial College London Email: diana.bilton@imperial.ac.uk Address: Regional Adult Cystic Fibrosis Unit, Monsall Hospital, Newton Heath, Manchester, M10 8WR |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation process not described: "The order of these was randomly allocated to each patient." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel carrying out or supervising interventions was not feasible due to the nature of the study intervention. |
| Blinding of participants and personnel (performance bias ‐ subjective outcomes) Subjective outcomes | High risk | Blinding was not possible due to the type of intervention used. Participant awareness of the intervention may have led to some impact on reporting of subjective measures, such as QoL, due to pre‐existing perceptions or preferences on the benefits of exercise versus traditional airway clearance techniques. |
| Blinding of participants and personnel (performance bias ‐ objective outcomes) Objective outcomes | Unclear risk | Blinding was not possible due to the type of intervention used; however this was unlikely to confer any impact on objective outcome measures such as lung function and exercise testing. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated who the outcome assessors were and whether or not they were blinded to allocation during data analysis. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All 18 patients completed the study." No re‐inclusions in analysis performed by review authors. No withdrawal of participants mentioned. Mean 24‐hour sputum weights for each of the 4 treatments and non‐treatment times fully reported. Full data presented for participant preference. Narrative data presented for pulmonary function only. No access to raw data. |
| Selective reporting (reporting bias) | Unclear risk | No protocol for comparison ‐ outcomes in methodology compared with available results, numerical data for spirometry not reported. |
| Other bias | Unclear risk | There were specific inclusion and exclusion criteria set for participants, but no evidence for how study participants were recruited which may be attributed to the limited abstract wordcount. There was no reported power calculation. There is an unclear risk associated with the potentially insufficient sample size. |
Cerny 1989.
| Study characteristics | ||
| Methods | RCT Parallel design Single centre: Buffalo, New York Duration: 2 weeks |
|
| Participants |
Inclusion criteria: acute exacerbation of pulmonary disease, defined as, "increased shortness of breath, coughing and sputum production and decreased lung function"; able to perform pulmonary function tests Exclusion criteria: not specified Pretreatment: no attempt was made to match participants for disease severity 17 participants: (8 = control) (9 = intervention group) Baseline characteristics PD&P Group Age, mean (SD): 15.9 (4.9) years Height, mean (SD): 151.6 (14.2) cm Weight, mean (SD): 38.4 (14.4) kg Pulmonary function score, mean (SD): 14.9 (0.9) Exercise therapy + PD&P Group Age, mean (SD): 15.4 (4.9) years Height, mean (SD): 149.5 (14.6) cm Weight, mean (SD): 35.9 (10.5) kg Pulmonary function score, mean (SD): 12.2 (1.3) |
|
| Interventions |
Intervention: cycle ergometer: on days 1 to 4, a workload of 25% to 45% of peak HRR; on day 4 to discharge, a workload of 40% to 65% of peak HRR, target duration 15 to 20 mins 2x daily AND PD&P 20 to 40 mins 1 x daily, no inhaled bronchodilator pre‐treatment Comparator: PD&P in 6 positions, 20 to 40 mins 3 x daily with inhaled bronchodilator pretreatment |
|
| Outcomes | FEV1 % predicted (change from baseline), FVC % predicted (change from baseline), duration of hospitalisation, FEF25-75, FRC % predicted, peak load (w/kg), peak heart rate, peak VE, sputum dry weight (g), sputum volume, sputum wet weight (g), adherence, adverse events, pulmonary function score at discharge | |
| Identification |
Sponsorship source: supported in part by NIH Grants 5R01AM24066 and RR‐05493C and by the Buffalo Foundation Country: USA Setting: inpatient, hospital Authors name: Frank J Cerny Institution: Department of Paediatrics, Division of Pulmonary Disease, Children's Hospital, Buffalo Email: Frank.cerny@verizon.net Address: Department of physical therapy and exercise science, State University of New York at Buffalo, 411 Kimball Tower, Buffalo, New York 14214 (USA) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method of randomisation stated: "The patients were randomly assigned to either a group that participated in two cycle ergometer sessions per day and one bronchial hygiene treatment session per day or a group that participated in three bronchial hygiene treatment sessions per day." |
| Allocation concealment (selection bias) | Unclear risk | No concealment of allocation discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants not possible. |
| Blinding of participants and personnel (performance bias ‐ subjective outcomes) Subjective outcomes | High risk | Blinding was not possible due to the type of intervention used. Participant awareness of the intervention may have led to some impact on reporting of subjective measures, such as QoL, due to pre‐existing perceptions or preferences on the benefits of exercise versus traditional airway clearance techniques. |
| Blinding of participants and personnel (performance bias ‐ objective outcomes) Objective outcomes | Unclear risk | Blinding was not possible due to the type of intervention used; however this was unlikely to confer any impact on objective outcome measures such as lung function and exercise testing. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated who the outcome assessors were and whether or not they were blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All randomised participants completed the trial with similar numbers of participants in each group: intervention (9), comparator (8). No re‐inclusions in analyses performed by the review authors. "All postural drainage treatments were completed as required for the study and 96% of scheduled exercise sessions were completed." There is no mention of how authors dealt with this missing data. Intention to treat analysis not discussed. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in the methods section were reported in numerical or graphical format or commented on in the discussion, except wet sputum weight. New outcomes were not added. Outcomes of interest were not changed throughout the study. There were no numerical data provided for daily sputum volume or dry sputum weight. |
| Other bias | High risk | Intervention length varied ‐ exercise was set to a "target duration of 15 to 20 minutes" whereas "PD with chest percussion and vibration in six positions for 20 to 40 minutes." The comparison intervention was also preceded by bronchodilator therapy. The effect of this on the outcomes is unknown. No baseline characteristics tables included in full publication. The effect of potentially unmatched groups of participants was deemed high risk. There was no reported power calculation. There is an unclear risk associated with the potentially insufficient sample size. |
ACBT: active cycle of breathing techniques CF: cystic fibrosis FEF25-75: mid forced expiratory flow FEV1: forced expiratory volume in 1 second FRC: functional residual capacity FVC: forced vital capacity HRR: heart rate reserve PD&P: exercise plus postural drainage and percussion PEP: positive expiratory pressure RCT: randomised controlled trial SPO2: oxygen saturation SD: standard deviation VAS: visual analogue scale VE: minute ventilation VO2 max: maximum rate of oxygen consumption measured during incremental exercise
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alexander 2019 | Intervention not eligible ‐ usual care vs whole body vibration training Outcomes not relevant (anthropometric data and bone parameters) |
| Aquino 2006 | Duration not eligible, single session intervention |
| Baldwin 1994 | Duration not eligible, single session intervention |
| Dwyer 2019 | Duration not eligible, single session intervention |
| Falk 1988 | Duration not eligible, single session intervention |
| Kaak 2011 | Intervention not eligible ‐ physiotherapy vs physiotherapy AND didgeridoo playing Not randomised |
| Lannefors 1992 | Duration not eligible, single session intervention |
| Montero‐Ruiz 2020 | Intervention not eligible ‐ music therapy as an adjunct to airway clearance techniques |
| NCT00609050 | Intervention not eligible ‐ 6 months self‐regulated exercise with telephone reinforcement vs 6 months self‐regulated exercise with no telephone reinforcement |
| NCT00792194 | Trial abandoned due to loss to follow‐up and difficulties with inclusion criteria (author contacted 16 April 2019) |
| NCT03295201 | Intervention not eligible ‐ thoracic vertebrae mobilization and scapula stretches considered too low intensity to fit inclusion criteria for this review |
| Radtke 2018 | Duration not eligible, single session intervention |
| Reix 2012 | Duration not eligible, single session intervention |
| Rodriguez 2017 | Intervention not eligible ‐ chest physiotherapy vs 'pulmonary rehabilitation program,' which is not specified further (no clarification on whether or not this included exercise as an intervention) |
| Salh 1989 | Study methodology not appropriate ‐ no randomisation of participants mentioned; co‐author contacted 3 February 2021 to confirm this |
| Santana‐Sosa 2014 | Intervention not appropriate to this review. Inspiratory muscle training and exercise versus no intervention. |
| Zeren 2019 | Intervention not eligible ‐ comprehensive chest physiotherapy program vs comprehensive chest physiotherapy program AND inspiratory muscle training |
vs: versus
Characteristics of studies awaiting classification [ordered by study ID]
Ward 2018.
| Methods | RCT Wash‐in period of daily PEP and exercise Single centre: Adelaide, Australia Duration: 12 weeks |
| Participants |
Inclusion criteria
Exclusion criteria
Total cohort: 13 participants Sex: 6 male; 7 female Disease status: clinically stable, mild disease Lung function FEV1 % predicted (range): 83% to 115% |
| Interventions |
Intervention: daily exercise (with huffing); 30 min of moderate‐ to strong‐intensity exercise (Borg rating of perceived exertion 3 to 5), such as walking or jogging or alternatively 6 cycles of 5 min of step‐ups using an aerobic step, with the height and step rate adjusted to achieve the target exertion intensity. 2 to 3 huffs every 5 minutes Control: daily PEP (6 cycles of 15 breaths per cycle; 2 to 3 huffs after each cycle) plus exercise (as above) |
| Outcomes | Respiratory function tests; FEV1 (L); FVC (L); FEF25-75 HRQoL: CFQ‐R (respiratory); LCQ |
| Notes |
CF: cystic fibrosis CFQ‐R: Cystic Fibrosis Questionnaire ‐Revised FEF25-75: mid‐peak expiratory flow FEV1: forced expiratory volume in 1 second FVC: forced vital capacity HRQoL: health‐related quality of life LCQ: Leicester Cough Questionnaire PEP: positive expiratory pressure RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT03273959.
| Study name | Program Of Exercises During The Hospitalization Of Children And Adolescents With Cystic Fibrosis |
| Methods | Parallel RCT. Single‐blind (investigator). |
| Participants | Males and females aged 6 to 18 years with an acute pulmonary exacerbation (hospitalised) Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: routine physiotherapy and physical exercise program 5 x weekly whilst in hospital Control: routine physiotherapy |
| Outcomes |
Primary: functional capacity (6‐minute walk test) Secondary: pulmonary functional capacity (spirometry), physical fitness/health, clinical score (Schwachman‐Kulczycki), bacteriology, nutritional assessment (body mass index) |
| Starting date | 28 August 2017 |
| Contact information | Bruna Zieglert: brunaziegler@yahoo.com.br Taiane Feiten: taifeiten@gmail.com |
| Notes | Author contacted both investigators above on 16 April 2019 and 5 April 2020 to find out if further information was available. To date, no success. |
CF: cystic fibrosis FEV1: forced expiratory volume in 1 second RCT: randomised controlled trial
Differences between protocol and review
Following the publication of the protocol, it was felt that the authors needed to make a post‐hoc change to clarify the definition of a 'one session intervention'. We did this to reflect the aim of the review, which is to establish if exercise can replace more traditional forms of airway clearance in people with cystic fibrosis. For this reason, we excluded studies only completing one session of any intervention on the grounds that this would not reflect the ability of that intervention as a main form of treatment.
Contributions of authors
| Roles and responsibilities | |
| TASK | WHO UNDERTOOK THE TASK? |
| Protocol stage: draft the protocol | KP |
| Review stage: select which trials to include (2 + 1 arbiter) | KP and AW (ZJ) |
| Review stage: extract data from trials (2 people) | KP and AW |
| Review stage: enter data into RevMan | KP |
| Review stage: carry out the analysis | KP, AW, KR and ZJ |
| Review stage: interpret the analysis | KP, AW, KR, ZJ and KS |
| Review stage: draft the final review | KP, AW, KR, ZJ, and KS |
| Update stage: update the review | KP, AW, ZJ and KS |
Sources of support
Internal sources
No sources of support provided
External sources
-
National Institute for Health Research, UK
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
KH: none known.
AW: none known.
KWS: none known.
ZJ: none known.
KHR: none known.
New
References
References to studies included in this review
Almajan‐Guta 2011 {published data only}
- Almajan-Guta B, Avram C, Almajan-Guta V, Rusu A, Ciuca I, Cluci O, et al. High motivation for playing sports in cystic fibrosis - what we play is life. Journal of Cystic Fibrosis 2011;10(Suppl 1):S64. [ABSTRACT: 254] [CFGD REGISTER: PE189] [Google Scholar]
Balestri 2004 {published data only}
- Balestri E, Ambroni M, Dall' Ara S, Miano A. Efficacy of physical exercise for mucus clearance in patients with cystic fibrosis (CF). Pediatric Pulmonology 2004;38(Suppl 27):316. [CFGD REGISTER: PE150] [Google Scholar]
Bilton 1992 {published data only}
- Bilton D, Dodd M, Webb AK. The benefits of exercise combined with physiotherapy in cystic fibrosis. Pediatric Pulmonology 1990;9(Suppl 5):253-4. [ABSTRACT: 238] [CFGD REGISTER: PE41a] [Google Scholar]
- Bilton D, Dodd ME, Abbot JV, Webb AK. The benefits of exercise combined with physiotherapy in the treatment of adults with cystic fibrosis. Respiratory Medicine 1992;86(6):507-11. [CFGD REGISTER: PE41b] [DOI: 10.1016/S0954-6111(96)80012-6] [DOI] [PubMed] [Google Scholar]
Cerny 1989 {published data only}
- Cerny FJ. Relative effects of bronchial drainage and exercise for in-hospital care of patients with cystic fibrosis. Physical Therapy 1989;69(8):633-9. [CFGD REGISTER: PE13] [DOI: 10.1093/ptj/69.8.633] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alexander 2019 {published data only}
- Alexander E, Wilson C, Kapur N, Brookes D. Is telehealth as effective as face to face therapy for delivery of whole body vibration training (WBVT) as an adjunct to physiotherapy in children and young people with cystic fibrosis (CF)? Respirology 2019;24:135. [CFGD REGISTER: MH178] [Google Scholar]
Aquino 2006 {published data only}
- Aquino A, Balestri E, Dall 'Ara S, Lami I, Gobbi F, Ambroni M, et al. Efficacy of physical exercise playing a video game for mucus clearance in patients with cystic fibrosis. Journal of Cystic Fibrosis 2006;5(Suppl 1):S83. [CFGD REGISTER: PE163] [Google Scholar]
Baldwin 1994 {published data only}
- Baldwin DR, Hill AL, Peckham DG, Knox AJ. Effect of addition of exercise to chest physiotherapy on sputum expectoration and lung function in adults with cystic fibrosis. Respiratory Medicine 1994;88(1):49-53. [CFGD REGISTER: PE19] [DOI: 10.1016/0954-6111(94)90174-0] [DOI] [PubMed] [Google Scholar]
Dwyer 2019 {published data only}ACTRN12608000287336
- ACTRN12608000287336. Does exercise enhance mucociliary clearance in adults with cystic fibrosis? trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12608000287336 (date registered 4 June 2008). [CFGD REGISTER: PE274b]
- Dwyer TJ, Daviskas E, Zainuldin R, Verschuer J, Eberl S, Bye P, et al. Effects of exercise and airway clearance (positive expiratory pressure) on mucus clearance in cystic fibrosis: a randomised crossover trial. European Respiratory Journal 2019;53(4):1801793. [CFGD REGISTER: PE274a] [DOI: 10.1183/13993003.01793-2018] [DOI] [PubMed] [Google Scholar]
Falk 1988 {published data only}
- Falk M, Kelstrup M, Andersen JB, Pedersen SS, Rossing I, Dirksen H. PEP treatment or physical exercise. Effects on secretions expectorated and indices of central and peripheral airway function. A controlled study. In: 10th International Cystic Fibrosis Congress; 1988 March 5-10; Sydney. 1988:35. [CFGD REGISTER: PE36]
Kaak 2011 {published data only}
- Kaak I, Helbig I, Ankermann T. Didgeridoo playing as an adjunctive therapy to conventional physiotherapy for patients with cystic fibrosis. Zeitschrift fur Physiotherapeuten 2011;63(2):6-14. [CFGD REGISTER: PE292] [Google Scholar]
Lannefors 1992 {published data only}
- Lannefors L, Wollmer P. Mucus clearance in cystic fibrosis (CF) - a comparison between postural drainage, PEP-mask and physical exercise. In: 11th International Cystic Fibrosis Congress; 1992; Dublin, Ireland. 1992:AHP31. [CFGD REGISTER: PE46a] [PubMed]
- Lannefors L, Wollmer P. Mucus clearance with three chest physiotherapy regimes in cystic fibrosis: a comparison between postural drainage, PEP and physical exercise. European Respiratory Journal 1992;5(6):748-53. [CFGD REGISTER: PE46b] [PubMed] [Google Scholar]
Montero‐Ruiz 2020 {published data only}
- Frias JP, Montero-Ruiz A, Galvez LA F, Perez-Ruiz E, Huelamo MP, Martin-Montañez E. A music therapy intervention as an adjunct to chest physiotherapy in children with cystic fibrosis. European Respiratory Journal 2018;52(Suppl 62):PA4626. [CFGD REGISTER: PE293b] [Google Scholar]
- Montero-Ruiz A, Fuentes LA, Perez Ruiz E, Garcia-Agua Soler N, Rius-Diaz F, Caro Aguilera P, et al. Effects of music therapy as an adjunct to chest physiotherapy in children with cystic fibrosis: a randomized controlled trial. PloS one 2020;15(10):e0241334. [CFGD REGISTER: PE293a] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00609050 {published data only}
- NCT00609050. Exercise training study for patients with cystic fibrosis [Self-regulated exercise in CF: a randomized trial]. clinicaltrials.gov/ct2/show/NCT00609050 (first posted 6 February 2008).
NCT00792194 {published data only}
- NCT00792194. Improvement of aerobic capacity in cystic fibrosis patients with a one-year home training period. clinicaltrials.gov/ct2/show/NCT00792194 (first posted 17 November 2008).
NCT03295201 {published data only}
- NCT03295201. Clinical effects of exercise program added to pulmonary rehabilitation in patients with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT03295201 (first posted 27 September 2017).
Radtke 2018 {published data only}
- NCT02750722. Exercise and oscillatory positive expiratory pressure therapy in cystic fibrosis [Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusion capacity in cystic fibrosis: a randomized crossover trial]. clinicaltrials.gov/ct2/show/NCT02750722 (first posted 25 April 2016). [CFGD REGISTER: PE255d]
- Radtke T, Boeni L, Bohnacker P, Maggi-Bebba M, Fischer P, Kriemler S, et al. Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusing capacity in cystic fibrosis: a randomized, controlled, crossover trial. European Respiratory Journal 2018;52(Suppl 62):PA3418. [CFGD REGISTER: PE255e] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke T, Boeni L, Bohnacker P, Maggi-Bebba M, Fischer P, Kriemler S, et al. Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusing capacity in cystic fibrosis: a randomized, controlled, crossover trial. Journal of Cystic Fibrosis 2018;17(Suppl 3):S15. [CFGD REGISTER: PE255b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke T, Boni L, Bohnacker P, Fischer P, Benden C, Dressel H. The many ways sputum flows - dealing with high within-subject variability in cystic fibrosis sputum rheology. Respiratory Physiology & Neurobiology 2018;254:36-9. [CFGD REGISTER: PE255c] [DOI] [PubMed] [Google Scholar]
- Radtke T, Boni L, Bohnacker P, Maggi-Beba M, Fischer P, Kriemler S, et al. Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusing capacity in cystic fibrosis: a randomized, controlled, crossover trial. BMC Pulmonary Medicine 2018;18(1):99. [CFGD REGISTER: PE255a] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reix 2012 {published data only}
- NCT01509235. Self drainage in pediatric cystic fibrosis patients [Exercise coupled to self drainage in pediatric cystic fibrosis patients: an open label cross over randomised clinical trial]. clinicaltrials.gov/show/NCT01509235 (first posted 12 January 2012). [CFGD REGISTER: PE183c]
- Reix P, Aubert F, Kassai B, Bige V, Bellon G. Better satisfaction of cystic fibrosis paediatric patients with autogenic drainage associated to exercise compared to conventional chest physiotherapy. Journal of Cystic Fibrosis 2009;8(Suppl 2):S73. [ABSTRACT: 293] [CFGD REGISTER: PE183a] [DOI: 10.1016/s1569-1993(09)60288-5] [DOI] [Google Scholar]
- Reix P, Aubert F, Werck-Gallois MC, Toutain A, Mazzocchi C, Moreux N, et al. Exercise with incorporated expiratory manoeuvres was as effective as breathing techniques for airway clearance in children with cystic fibrosis: a randomised crossover trial. Journal of Physiotherapy 2012;58(4):241-7. [CFGD REGISTER: PE183b] [DOI] [PubMed] [Google Scholar]
Rodriguez 2017 {published data only}
- Rodriguez M, Cortina S, Carro M, Ruiz-de-Valbuena M, Cosmes J. A pulmonary rehabilitation program to increase adherence to airway clearance techniques for children and adults with cystic fibrosis. Journal of Cystic Fibrosis 2017;16(Suppl 1):S58. [ABSTRACT: IPD.01] [Google Scholar]
Salh 1989 {published data only}
- Salh W, Bilton D, Dodd M, Webb AK. Effect of exercise and physiotherapy in aiding sputum expectoration in adults with cystic fibrosis. Thorax 1989;44(12):1006-8. [CFGD REGISTER: PE63] [DOI: 10.1136/thx.44.12.1006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santana‐Sosa 2014 {published data only}
- NCT01706445. Combined inspiratory muscle and 'whole muscle' training in children with cystic fibrosis. clinicaltrials.gov/show/NCT01706445 (date first posted 15 October 2012). [CFGD REGISTER: PE201b]
- Santana-Sosa E, Gonzalez-Saiz L, Groeneveld IF, Villa-Asensi JR, Barrio Gomez de Aguero MI, Fleck SJ, et al. Benefits of combining inspiratory muscle with 'whole muscle' training in children with cystic fibrosis: a randomised controlled trial. British Journal of Sports Medicine 2014;48(20):1513-7. [CFGD REGISTER: PI201a] [DOI] [PubMed] [Google Scholar]
Zeren 2019 {published data only}
- NCT03375684. Effects of inspiratory muscle training on postural stability, balance, pulmonary function and functional capacity in children with cystic fibrosis. clinicaltrials.gov/show/NCT03375684 (first posted 18 December 2017). [CFGD REGISTER: PE275c]
- Zeren M, Cakir E, Gurses HN. Effects of inspiratory muscle training on postural stability, pulmonary function and functional capacity in children with cystic fibrosis: a randomised controlled trial. Respiratory Medicine 2019;148:24-30. [CFGD REGISTER: PE275a] [DOI] [PubMed] [Google Scholar]
- Zeren M, Cakir E, Gurses HN. Effects of inspiratory muscle training on postural stability, pulmonary function and functional capacity in children with cystic fibrosis: a randomised controlled trial. Turkish Thoracic Journal 2019;20:S106. [CFGD REGISTER: PE275b] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ward 2018 {published data only}
- Ward N, Stiller K, Rowe H, Morrow S, Morton J, Greville H, et al. Airway clearance by exercising in mild cystic fibrosis: clinical outcomes. Respirology 2018;23(Suppl 1):140. [CFGD REGISTER: PE257a] [DOI] [PubMed] [Google Scholar]
- Ward N, Stiller K, Rowe H, Morrow S, Morton J, Greville H, et al. Airway clearance by exercising in mild cystic fibrosis (ACE-CF): a feasibility study. Respiratory Medicine 2018;142:23-8. [CFGD REGISTER: PE257b] [DOI: 10.1016/j.rmed.2018.07.008] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT03273959 {published and unpublished data}
- NCT03273959. Program of exercises during the hospitalization of children and adolescents with cystic fibrosis [Effects of an exercise program during the hospitalization of children and adolescents with cystic fibrosis: randomized clinical test]. clinicaltrials.gov/ct2/show/NCT03273959 (first posted 6 September 2016).
Additional references
Bailey 2021
- Bailey J, Rozga M, McDonald CM, Bowser EK, Farnham K, Mangus M. Effect of CFTR modulators on anthropometric parameters in individuals with cystic fibrosis: an Evidence Analysis Center Systematic Review . Journal of the Academy of Nutrition and Dietetics 2021;121(7):1364-78. [DOI] [PubMed] [Google Scholar]
Boas 1997
- Boas SR. Exercise recommendations for individuals with cystic fibrosis. Journal of Sports Medicine 1997;24(1):17-37. [DOI] [PubMed] [Google Scholar]
Burnham 2021
- Burnham P, Stanford G, Stewart R. Autogenic drainage for airway clearance in cystic fibrosis. Cochrane Database of Systematic Reviews 2021, Issue 12. Art. No: CD009595. [DOI: 10.1002/14651858.CD009595.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cerny 2013
- Cerny F. Exercise and cystic fibrosis (CF) 2.0. Pediatric Exercise Science 2013;25(4):616-23. [DOI] [PubMed] [Google Scholar]
Cholewa 2012
- Cholewa JM, Paolone VJ. Influence of exercise on airway epithelia in cystic fibrosis: a review. Medicine and Science in Sports and Exercise 2012;44(7):1219-26. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 10 February 2019. Melbourne, Australia: Veritas Health Innovation. Available at www.covidence.org.
Cox 2018
- Cox NS, Alison JA, Button BM, Wilson JW, Morton JM, Holland AE. Accumulating physical activity in at least 10-minute bouts predicts better lung function after 3-years in adults with cystic fibrosis. European Respiratory Journal 2018;4(2):00095-2017. [DOI: 10.1183/23120541.00095-2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dasgupta 1998
- Dasgupta B, Brown NE, King M. Effects of sputum oscillations and rhDNase in vitro: a combined approach to treat cystic fibrosis lung disease. Pediatric Pulmonology 1998;26(4):250-5. [DOI] [PubMed] [Google Scholar]
Dassios 2013
- Dassios T, Katelari A, Doudounakis S, Dimitriou G. Aerobic exercise and respiratory muscle strength in patients with cystic fibrosis. Respiratory Medicine 2013;107(5):684-90. [DOI] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG on behalf of the Cochrane Statistical Methods Group, editor(s). Chapter 10: Analysing data and undertaking meta-analysis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). The Cochrane Collaboration, 2021. Available from training.cochrane.org/handbook.
Dwyer 2011
- Dwyer TJ, Alison JA, McKeough ZJ, Daviskas E, Bye PT. Effects of exercise on respiratory flow and sputum properties in patients with cystic fibrosis. Chest 2011;139(4):870-7. [DOI] [PubMed] [Google Scholar]
Dwyer 2021
- Dwyer T. Huff and puff of exercise for airway clearance in cystic fibrosis: how clear is the evidence? Thorax 2021;76:746-7. [DOI] [PubMed] [Google Scholar]
Ekkekakis 2011
- Ekkekakis P, Parfitt G, Petruzzello SJ. The pleasure and displeasure people feel when they exercise at different intensities: decennial update and progress towards a tripartite rationale for exercise intensity prescription. Journal of Sports Medicine 2011;41(8):641-71. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Farrell 2008
- Farrell PM. The prevalence of cystic fibrosis in the European Union. Journal of Cystic Fibrosis 2008;7(5):450-3. [DOI] [PubMed] [Google Scholar]
Farrell 2018
- Farrell P, Férec C, Macek M, Frischer T, Renner S, Riss K, et al. Estimating the age of p.(Phe508del) with family studies of geographically distinct European populations and the early spread of cystic fibrosis. European Journal of Human Genetics 2018;26(12):1832-9. [DOI: 10.1038/s41431-018-0234-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flume 2009
- Flume PA, Robinson KA, O ´ Sullivan BP, Finder JD, Vender RL, Willey-Courand DB, et al. Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respiratory Care 2009;54(4):522-37. [PubMed] [Google Scholar]
Gee 2000
- Gee L, Abbott J, Conway S, Etherington C, Webb A. Development of a disease specific health related quality of life measure for adults and adolescents with cystic fibrosis. Thorax 2000;55(11):946-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenland 1985
- Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow-up data. Biometrics 1985;41(1):55-68. [PubMed] [Google Scholar]
Greut 2021
- Gruet M, Saynor ZL, Urquhart DS, Radtke T. Rethinking physical exercise training in the modern era of cystic fibrosis: a step towards optimising short-term efficacy and long-term engagement. Journal of Cystic Fibrosis 2021 Sep 04 [Epub ahead of print]. [DOI: 10.1016/j.jcf.2021.08.004] [DOI] [PubMed]
Groth 1985
- Groth S, Stafanger G, Dirksen H, Andersen JB, Falk M, Kelstrup M. Positive expiratory pressure (PEP-mask) physiotherapy improves ventilation and reduces volume of trapped gas in cystic fibrosis. Bulletin Européen de Physiopathologie Respiratoire 1995;21(4):339-43. [PubMed] [Google Scholar]
Guyatt 1987
- Guyatt G, Berman L, Townsend M, Pugsley S, Chambers L. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987;42(10):773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 1994
- Hansen LG, Warwick WJ, Hansen KL. Mucus transport mechanisms in relation to the effect of high frequency chest compression (HFCC) on mucus clearance. Pediatric Pulmonology 1994;17(2):113-8. [DOI] [PubMed] [Google Scholar]
Hebestreit 2001
- Hebestreit A, Kersting U, Basler B, Jeschke R, Hebestreit H. Exercise inhibits epithelial sodium channels in patients with cystic fibrosis. American Journal of Respiratory Critical Care Medicine 2001;164(3):443-6. [DOI] [PubMed] [Google Scholar]
Hebestreit 2015
- Hebestreit H, Arets HG, Aurora P, Boas S, Cerny F, Hulzebos EH, et al. Statement on exercise testing in cystic fibrosis. Respiration 2015;90(4):332-51. [DOI] [PubMed] [Google Scholar]
Hebestreit 2018
- Hebestreit H, Lands LC, Alarie N, Schaeff J, Karila C, Orenstein DM, et al. Effects of a partially supervised conditioning programme in cystic fibrosis: an international multi-centre randomised controlled trial (ACTIVATE-CF): study protocol. BMC Pulmonary Medicine 2018;18(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hebestreit 2019
- Hebestreit H, Hulzebos E, Schneiderman J, Karila C, Boas SR, Kriemler S, et al. Cardiopulmonary exercise testing provides additional prognostic information in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2019;199(8):987-95. [DOI: 10.1164/rccm.201806-1110OC] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2/.
Hind 2008
- Hind K, Truscott JG, Conway SP. Exercise during childhood and adolescence: a prophylaxis against cystic fibrosis-related low bone mineral density? Exercise for bone health in children with cystic fibrosis. Journal of Cystic Fibrosis 2008;7(4):270-6. [DOI] [PubMed] [Google Scholar]
Hurley 2021
- Hurley N, Moyna NM, Kehoe B, McCaffrey N, Redmond K, Hardcastle SJ. Factors influencing physical activity in adults with cystic fibrosis. BMC Pulmonary Medicine 2021;21:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Irons 2019
- Irons JY, Petocz P, Kenny DT, Chang AB. Singing as an adjunct therapy for children and adults with cystic fibrosis. Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No: CD008036. [DOI: 10.1002/14651858.CD008036.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1991
- Jones PW, Quirk FH, Baveystock CM. The St. George's Respiratory Questionnaire. Respiratory Medicine 1991;85(Suppl 2):25-31. [DOI: 10.1016/S0954-6111(06)80166-6] [DOI] [PubMed] [Google Scholar]
Keogh 2018
- Keogh RH, Szczesniak R, Taylor-Robinson D, Bilton D. Up-to-date and projected estimates of survival for people with cystic fibrosis using baseline characteristics: a longitudinal study using UK patient registry data. Journal of Cystic Fibrosis 2018;17(2):218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klijn 2004
- Klijn PH, Oudshoorn A, Ent CK, Net J, Kimpen JL, Helders PJ. Effects of anaerobic training in children with cystic fibrosis: a randomized controlled study. Chest 2004;125(4):1299-305. [DOI] [PubMed] [Google Scholar]
Koch 1993
- Koch C, Høiby N. Pathogenesis of cystic fibrosis. Lancet 1993;341(8852):1065-9. [DOI] [PubMed] [Google Scholar]
LeBlanc 2014
- LeBlanc CM, Lands LC. Can I play? Pediatric Annals 2014;43(12):316-24. [DOI] [PubMed] [Google Scholar]
Lewis 2016
- Lewis BA, Williams DM, Frayeh A, Marcus BH. Self-efficacy versus perceived enjoyment as predictors of physical activity behaviour. Psychology & Health 2016;31:456-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Litvin 2019
- Litvin M, Yoon JC, Leey Casella J, Blackman SM, Brennan AL. Energy balance and obesity in individuals with cystic fibrosis. Journal of Cystic Fibrosis 2019;18(2):38-47. [DOI] [PubMed] [Google Scholar]
Main 2005
- Main E, Prasad A, Schans CP. Conventional chest physiotherapy compared to other airway clearance techniques for cystic fibrosis. Cochrane Database of Systematic Reviews 2005, Issue 1. Art. No: CD002011. [DOI: 10.1002/14651858.CD002011.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719-48. [PubMed] [Google Scholar]
McIlwaine 2006
- McIlwaine M. Physiotherapy and airway clearance techniques and devices. Paediatric Respiratory Reviews 2006;7(Suppl 1):S220-2. [DOI] [PubMed] [Google Scholar]
McIlwaine 2019
- McIlwaine M, Button B, Nevitt SJ. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD003147. [DOI: 10.1002/14651858.CD003147.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mckoy 2016
- Mckoy NA, Wilson LM, Saldanha IJ, Odelola OA, Robinson KA. Active cycle of breathing technique for cystic fibrosis. Cochrane Database of Systematic Reviews 2016, Issue 7. Art. No: CD007862. [DOI: 10.1002/14651858.CD007862.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Middleton 2019
- Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. Elexacaftor–tezacaftor–ivacaftor for cystic fibrosis with a single Phe508del allele. New England Journal of Medicine 2019;381:1809-19. [DOI: 10.1056/NEJMoa1908639] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 2020
- Morrison L, Milroy S. Oscillating devices for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD006842. [DOI: 10.1002/14651858.CD006842.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nixon 1992
- Nixon PA, Orenstein DM, Kelsey SF, Doershuk CF. The prognostic value of exercise testing in patients with cystic fibrosis. New England Journal of Medicine 1992;327(25):1785-8. [DOI] [PubMed] [Google Scholar]
Perrem 2018
- Perrem L, Rayment JH, Ratjen F. The lung clearance index as a monitoring tool in cystic fibrosis: ready for the clinic? Current Opinion in Pulmonary Medicine 2018;24(6):579-85. [DOI: 10.1097/MCP.0000000000000515] [DOI] [PubMed] [Google Scholar]
Pescatello 2014
- Pescatello LS, Arena R, Riebe D, Thompson PD. ACSM’s guidelines for exercise testing and prescription 9th edition. Journal of Canadian Chiropractic Association 2014;58(3):328. [Google Scholar]
Pianosi 2005
- Pianosi P, Leblanc J, Almudevar A. Peak oxygen uptake and mortality in children with cystic fibrosis. Thorax 2005;60(1):50-4. [DOI: 10.1136/thx.2003.008102] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pocock 1987
- Pocock S, Hughes MD, Lee RJ. Statistical problems in the reporting of clinical trials. A survey of three medical journals. New England Journal of Medicine 1987;317(7):426-32. [DOI] [PubMed] [Google Scholar]
Prasad 1993
- Prasad SA. Current concepts in physiotherapy. Journal of the Royal Society of Medicine 1993;86(20):23-9. [PMC free article] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Radtke 2017
- Radtke T, Nevitt SJ, Hebestreit H, Kriemler S. Physical exercise training for cystic fibrosis. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD002768. [DOI: 10.1002/14651858.CD002768.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rand 2012
- Rand S, Prasad SA. Exercise as part of a cystic fibrosis therapeutic routine. Expert Review of Respiratory Medicine 2012;6(3):341-52. [DOI] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- Review Manager (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rosenfeld 2001
- Rosenfeld M, Emerson J, Williams-Warren J, Pepe M, Smith A, Montgomery B, et al. Defining a pulmonary exacerbation in cystic fibrosis. Journal of Pediatrics 2001;139(3):359-65. [DOI] [PubMed] [Google Scholar]
Rowbotham 2018
- Rowbotham NJ, Smith S, Leighton PA, Rayner O, Gathercole K, Elliott ZC, et al. The top 10 research priorities in cystic fibrosis developed by a partnership between people with CF and healthcare providers. Thorax 2018;73(4):388-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowbotham 2020
- Rowbotham NJ, Smith SJ, Davies G, Daniels T, Elliott ZC, Gathercole K, et al. Can exercise replace airway clearance techniques in cystic fibrosis? A survey of patients and healthcare professionals.. Journal of Cystic Fibrosis 2020;19(4):19-24. [DOI] [PubMed] [Google Scholar]
Sawicki 2009
- Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. Journal of Cystic Fibrosis 2009;8(2):91-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sawyer 2004
- Sawyer MG, Reynolds KE, Couper JJ, French DJ, Kennedy D, Martin J, et al. Health-related quality of life of children and adolescents with chronic illness – a two year prospective study. Quality of Life Research 2004;13(7):1309-19. [DOI] [PubMed] [Google Scholar]
Schneiderman 2014
- Schneiderman JE, Wilkes DL, Atenafu EG, Nguyen T, Wells GD, Alarie N, et al. Longitudinal relationship between physical activity and lung health in patients with cystic fibrosis. European Respiratory Journal 2014;43(3):817-23. [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al on behalf of the Cochrane GRADEing Methods Group. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Selvadurai 2002
- Selvadurai HC, Blimkie CJ, Meyers N, Mellis CM, Cooper PJ, Van Asperen PP. Randomized controlled study of in-hospital exercise training programs in children with cystic fibrosis. Pediatric Pulmonology 2002;33(3):194-200. [DOI] [PubMed] [Google Scholar]
Shoemaker 2008
- Shoemaker MJ, Hurt H, Arndt L. The evidence regarding exercise training in the management of cystic fibrosis: a systematic review. Cardiopulmonary Physical Therapy Journal 2008;19(3):75-83. [PMC free article] [PubMed] [Google Scholar]
Skilton 2019
- Skilton M, Krishan A, Patel S, Sinha IP, Southern KW. Potentiators (specific therapies for class III and IV mutations) for cystic fibrosis. Cochrane Database of Systematic Reviews 2019, Issue 1. Art. No: CD009841. [DOI: 10.1002/14651858.CD009841.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanford 2020
- Stanford G, Ryan H, Solis-Moya A. Respiratory muscle training for cystic fibrosis. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD006112. [DOI: 10.1002/14651858.CD006112.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swisher 2015
- Swisher A, Hebestreit H, Mejia-Downs A, Lowman JD, Gruber W, Nippins M, et al. Exercise and habitual physical activity for people with cystic fibrosis: expert consensus, evidence-based guide for advising patients. Cardiopulmonary Physical Therapy Journal 2015 ;26:85-98. [Google Scholar]
Tannock 1996
- Tannock IF. False-positive results in clinical trials: multiple significance tests and the problem of unreported comparisons. Journal of the National Cancer Institute 1996;88(3-4):206-7. [DOI] [PubMed] [Google Scholar]
Urquhart 2012
- Urquhart D, Sell Z, Dhouieb E, Bell G, Oliver S, Black R, et al. Effects of a supervised, outpatient exercise and physiotherapy programme in children with cystic fibrosis. Pediatric Pulmonology 2012;47(12):1235-41. [DOI] [PubMed] [Google Scholar]
Volkova 2020
- Volkova N, Moy K, Evans J, Campbell D, Tian S, Simard C. Disease progression in patients with cystic fibrosis treated with ivacaftor: Data from national US and UK registries. Journal of Cystic Fibrosis 2020;19(1):68-79. [DOI] [PubMed] [Google Scholar]
Ward 2019
- Ward N, Stiller K, Holland AE, Australian Cystic Fibrosis Exercise Survey Group. Exercise is commonly used as a substitute for traditional airway clearance techniques by adults with cystic fibrosis in Australia: a survey. Journal of Physiotherapy 2019;65(1):43-50. [DOI] [PubMed] [Google Scholar]
Ward 2020
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PMID: ] [PubMed] [Google Scholar]
Warnock 2015
- Warnock L, Gates A. Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD001401. [DOI: 10.1002/14651858.CD001401.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wheatley 2011
- Wheatley CM, Wilkins BW, Snyder EM. Exercise is medicine in cystic fibrosis. Exercise and Sports Sciences Reviews 2011;39(3):155-60. [DOI] [PubMed] [Google Scholar]
Wiklund 1990
- Wiklund I. The Nottingham Health Profile--a measure of health-related quality of life. Scandinavian Journal of Primary Health Care Suppl 1990;1:15-8. [PMID: ] [PubMed] [Google Scholar]
Williams 2008
- Williams DM, Dunsiger S, Ciccolo JT, Lewis BA, Albrecht AE, Marcus BH. Acute affective response to a moderate-intensity exercise stimulus predicts physical activity participation 6 and 12 months later. Psycholgy of Sport and Exercise 2008;9(3):231-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2013
- Williams CA, Stevens D. Physical activity and exercise training in young people with cystic fibrosis: current recommendations and evidence. Journal of Sport and Health Science 2013;2(1):39-46. [Google Scholar]
Wilson 2019
- Wilson LM, Morrison L, Robinson KA. Airway clearance techniques for cystic fibrosis: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2019, Issue 1. Art. No: CD011231. [DOI: 10.1002/14651858.CD011231.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Patterson 2019
- Patterson KD, Walsh A, McCormack P, Southern KW. Exercise versus airway clearance techniques for people with cystic fibrosis. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD013285. [DOI: 10.1002/14651858.CD013285] [DOI] [PMC free article] [PubMed] [Google Scholar]


