Abstract
Background
Diverticulitis is a complication of the common condition, diverticulosis. Uncomplicated diverticulitis has traditionally been treated with antibiotics, as diverticulitis has been regarded as an infectious disease. Risk factors for diverticulitis, however, may suggest that the condition is inflammatory rather than infectious which makes the use of antibiotics questionable.
Objectives
The objectives of this systematic review were to determine if antibiotic treatment of uncomplicated acute diverticulitis affects the risk of complications (immediate or late) or the need for emergency surgery.
Search methods
For this update, a comprehensive systematic literature search was conducted in Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, ClinicalTrials.gov and WHO International Clinical Trial Registry Platform on February 2021.
Selection criteria
Randomised controlled trials (RCTs), including all types of patients with a radiologically confirmed diagnosis of left‐sided uncomplicated acute diverticulitis. Comparator and interventions included antibiotics compared to no antibiotics, placebo, or to any other antibiotic treatment (different regimens, routes of administration, dosage or duration of treatment). Primary outcome measures were complications and emergency surgery. Secondary outcomes were recurrence, late complications, elective colonic resections, length of hospital stay, length to recovery of symptoms, adverse events and mortality.
Data collection and analysis
Two authors performed the searches, identification and assessment of RCTs and data extraction. Disagreements were resolved by discussion or involvement of the third author. Authors of trials were contacted to obtain additional data if needed or for preliminary results of ongoing trials. The Cochrane Collaboration's tool for assessing risk of bias was used to assess the methodological quality of the identified trials. The overall quality of evidence for outcomes was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. Effect estimates were extracted as risk ratios (RRs) with 95% confidence intervals. Random‐effects meta‐analyses were performed with the Mantel‐Haenzel method.
Main results
The authors included five studies. Three studies compared no antibiotics to antibiotics; all three were original RCTs of which two also published long‐term follow‐up information. For the outcome of short‐term complications there may be little or no difference between antibiotics and no antibiotics (RR 0.89; 95% CI 0.30 to 2.62; 3 studies, 1329 participants; low‐certainty evidence). The rate of emergency surgery within 30 days may be lower with no antibiotics compared to antibiotics (RR 0.47; 95% CI 0.13, 1.71; 1329 participants; 3 studies; low‐certainty evidence). However, there is considerable imprecision due to wide confidence intervals for this effect estimate causing uncertainty which means that there may also be a benefit with antibiotics.
One of the two remaining trials compared single to double compound antibiotic therapy and, due to wide confidence intervals, the estimate was imprecise and indicated an uncertain clinical effect between these two antibiotic regimens (RR 0.70; 95% CI 0.11 to 4.58; 51 participants; 1 study; low‐certainty evidence). The last trial compared short to long intravenous administration of antibiotics and did not report any events for our primary outcomes. Both trials included few participants and one had overall high risk of bias.
Since the first publication of this systematic review, an increasing amount of evidence supporting the treatment of uncomplicated acute diverticulitis without antibiotics has been published, but the total body of evidence is still limited.
Authors' conclusions
The evidence on antibiotic treatment for uncomplicated acute diverticulitis suggests that the effect of antibiotics is uncertain for complications, emergency surgery, recurrence, elective colonic resections, and long‐term complications. The quality of the evidence is low. Only three RCTs on the need for antibiotics are currently available. More trials are needed to obtain more precise effect estimates.
Plain language summary
Antibiotics for uncomplicated diverticulitis
Diverticulitis is a condition with inflammation of the so‐called diverticulae. A diverticulae is a weakness in the bowel wall. Diverticulae are common in the population, especially in the elderly above the age of 60 years, and are often asymptomatic. Diverticulitis may present as abdominal pain and tenderness accompanied by signs of infection, such as fever. In most cases, diverticulitis resolves without complications, however, some patients develop complications and may need emergency surgery.
Uncomplicated acute diverticulitis is the focus of this review. It has traditionally been regarded as an infection with bacterial overgrowth in the large intestine and has been treated with antibiotics. Recently, it has been argued that diverticulitis is more likely to be an inflammatory rather than an infectious condition, making the use of antibiotics questionable. Consequently, a shift towards the use of therapeutic regimens without antibiotics has been seen. This present review investigates whether there is any existing clinical evidence supporting the use of antibiotics for uncomplicated diverticulitis.
Five clinical trials in hospitalised patients were assessed. One trial investigated two different antibiotic treatments and a second study investigated the duration of intravenous antibiotic treatment. Three trials investigated the actual need for antibiotics when compared to no antibiotics of which two trials had published long‐term follow‐up results as separate records. None of the studies found a statistical difference in the tested antibiotic regimens. Comparing no antibiotic versus antibiotic treatment did not demonstrate any differences in the occurrence of complications like abscesses and perforations of the large intestine, or in the need for emergency surgery.
Antibiotics can cause serious adverse effects, including life‐threatening allergic reactions or super‐infections of the intestine. Growing antibiotic resistance is an increasing problem rendering some infections impossible to treat with possible fatal outcomes. Therefore, strong arguments in favour of limiting the current use of antibiotics exist. Only three randomised controlled trials on the need of antibiotics are currently available and more are needed in order to obtain strong and reliable evidence. However, the newest evidence shows that the use of antibiotics for the treatment of uncomplicated acute diverticulitis is not superior to treatments that do not include antibiotics.
Summary of findings
Background
Description of the condition
Acute diverticulitis is a complication of colonic diverticulosis. The prevalence of diverticulosis increases with age and ranges from 35% in those aged under 50, to 40% for those between 51‐60, and 58% in the over 60 years (Peery 2016). Most people remain asymptomatic, but 4 per cent develop acute diverticulitis based on colonoscopy and computed tomography (CT) (Shahedi 2013).
Acute diverticulitis is a common cause of hospitalisation, and is among the ten most frequent diagnoses in patients presenting with acute abdominal pain (Viniol 2014), and the fifth most common reason for outpatient visits among lower gastrointestinal diseases in the United States (Everhart 2009). Outpatient visits often result in prescriptions for antibiotics (AB) and pain relievers. Acute diverticulitis, therefore, represents a heavy economic burden on healthcare providers (Everhart 2009).
Uncomplicated acute diverticulitis is characterised by the presence of localised inflammation with or without small abscess formation confined to the large bowel wall (Stollman 2004). Complicated diverticulitis, on the other hand, includes pericolonic abscess, peritonitis, obstruction or fistula. Complications are relatively uncommon and a progression to complicated diverticulitis develops in approximately 12% of cases with uncomplicated acute diverticulitis (Bharucha 2015). Recurrent acute diverticulitis is frequent (22%) but is mostly uncomplicated (Bharucha 2015). Risk of readmission is higher within the first year following remission and risk of recurrence is more than doubled after two earlier episodes (Hupfeld 2017). Previously, acute diverticulitis was believed to be a progressive disease, but newer studies have shown that complications occur more often at the first episode than at recurrence (Humes 2012).
Diverticulae are herniations of the mucosal layer through the muscular layer (Slack 1962; Stollman 2004). The aetiology is incompletely understood and may include complex interactions between genetic factors, microbiota, and diet, including the long‐time consumption of low‐residue diet (Humes 2014, Violi 2018). Acute diverticulitis is probably caused by faecal obstruction ‐ a longstanding but unproven theory (Berman 1968, Morson 1975, Wolf 1957). Subsequent abrasion of the mucosa results in inflammation, bacterial overgrowth of colonic flora, localised ischaemia and perforation (Jacobs 2007).
Acute diverticulitis has been associated with chronic inflammation, due to the overlap in risk factors with other diseases believed to be caused by chronic inflammation i.e. obesity (Strate 2009), physical inactivity (Strate 2019) and Western diet with high consumption of red meat, refined grains and fat (Cao 2018; Strate 2017). These risk factors all cause elevation of inflammatory biomarkers (Strate 2019). Other risk factors include smoking (Aune 2017) and regular use of aspirin or nonsteroidal anti‐inflammatory drugs (NSAIDs) (Strate 2011). The latter may result from mucosal damage with impaired barrier function of the colonic mucosa, allowing the translocation of bacteria that provoke inflammation (Humes 2014).
In non‐Asian societies, acute diverticulitis mostly affects the left side of the large bowel involving the distal descending and sigmoid colon (Kang 2004; Peery 2016). In contrast, 70% of diverticulae in Asia are located within the right side of the caecum and ascending colon (Miura 2000; Nakaji 2002). It is likely that right‐sided diverticulitis has a different pathophysiology than left‐sided diverticulitis (Ryan 1983; Stollman 2004).
The typical clinical presentation of acute diverticulitis is left‐sided abdominal pain accompanied by localised tenderness increasing to abdominal guarding, usually in combination with fever and leucocytosis (Roberts 1995). CT is the recommended diagnostic modality but, alternatively, ultrasound can also be used at centres with the proper expertise (Francis 2019). Colono‐ or sigmoidoscopy are recommended when inflammation has subsided to confirm diagnosis, to rule out malignancy, and to identify possible late complications to acute diverticulitis (Stollman 2015; Szojda 2007). It is currently being discussed whether routine colonoscopy can be omitted in patients with uncomplicated diverticulitis if CT imaging is convincing and without suspicion of alternative diagnoses (Rottier 2019). The severity of acute diverticulitis is primarily graded after the modified Hinchey’s criteria, that categorise the depth of colonic inflammation, abscess location and perforation (Sher 1997) and according to Ambrosetti’s criteria based on CT findings (Ambrosetti 2016).
Description of the intervention
Uncomplicated acute diverticulitis has routinely been treated with antibiotics (Friend 2011, Moreno 2007), even though the evidence is sparse and of low quality (De Korte 2011). Non‐randomised observational studies have found that non‐antibiotic management for uncomplicated acute diverticulitis appears to be safe and does not increase the likelihood of adverse effects (Brochmann 2016; Hjern 2007; Isacson 2014). It has also been argued that acute diverticulitis may be an inflammatory rather than an infectious condition, questioning the use of routine antibiotics (Rezapour 2018). Recently, a shift in therapeutic regimens towards no use of antibiotics has been seen in clinical guidelines. The American Gastroenterological Association (AGA) suggests selective rather than routine use of antibiotics, taking the severity of disease, presence of immunosuppression, pregnancy and significant comorbidity into consideration (Stollman 2015). An increasing number of European surgical and gastroenterological associations no longer recommend antibiotics for uncomplicated acute diverticulitis, and advocate for selective use if risk factors are present (Andersen 2012; Kruis 2014). The Italian Society of Colon and Rectal Surgery recommends avoiding antibiotics for uncomplicated acute diverticulitis because it may not improve short‐ or long‐term outcomes and, therefore, suggests a case‐by‐case assessment (Binda 2015). However, at the European Association for Endoscopic Surgery (EAES) and Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) consensus conference in 2018, there was no consensus reached on the recommendation on a non‐antibiotic therapy strategy in immunocompetent individuals presenting with uncomplicated acute diverticulitis (Francis 2019).
Antibiotic treatment has several drawbacks including cost and risk of adverse events such as allergic drug reactions or clostridium difficile superinfection, which may eventually cause toxic megacolon with high risk of mortality (Goldstein 2011). Furthermore, antimicrobial resistance has become a global threat jeopardising the future clinical effectiveness of antibiotics, including treatment of aerobe and anaerobic microbes associated with diverticulitis (Chabok 2010, Sartelli 2010). Global occurrence of antibiotic resistance has escalated due to the overuse of antibiotic treatments (Dellit 2007; WHO 2019). Thus, the medical incentive for limiting antibiotic use is ever increasing.
How the intervention might work
If antibiotics are effective, they are believed to prevent the development of complications, lower the need for surgery, shorten the duration and severity of symptoms, and possibly also lower the rate of recurrences of acute diverticulitis.
Why it is important to do this review
To clarify the evidence of the use of antibiotics in the treatment of uncomplicated acute diverticulitis. This review is an update of the previously published Cochrane systematic review (Shabanzadeh 2012).
Objectives
The objectives of this systematic review were to determine if treatment of uncomplicated acute diverticulitis with antibiotics has an impact on the development of complications (immediate or late) or on need of emergency surgery. Interventions accepted were all available antibiotic compounds, doses and ways of administration. For the above outcomes, we assessed whether the choice of antibiotic compounds, route of administration, dosage or duration of treatment had an effect.
Methods
Criteria for considering studies for this review
Types of studies
Only RCTs were included. All proposed details on study design, participants, interventions, comparators, primary outcome, search strategies and data analysis were predefined in a published study protocol (Shabanzadeh 2011).
Types of participants
Inclusion criteria were left‐sided uncomplicated acute diverticulitis confirmed by radiological modalities. Acute diverticulitis was considered complicated if there was sign of pelvic or other distant abscess, fistula, stricture, peritonitis and sepsis. No restrictions regarding the inclusion of participants were defined. Therefore, both initial and recurring cases, as well as all ages, genders, races and comorbidity were included.
Types of interventions
Interventions included: 1. Antibiotics compared to placebo or non‐antibiotic treatments. 2. Different antibiotic regimens compared to each other. 3. Comparison of different routes of administration, dosage, and duration of treatment. Duration was divided into short (less than seven days) versus long (seven days or more).
Types of outcome measures
This systematic review and meta‐analysis included both primary and secondary outcomes. No study was excluded solely because no outcomes of interest were reported.
Primary outcomes
Primary outcomes were failure of antibiotics treatment with: 1. Progression from uncomplicated to complicated acute diverticulitis defined as: abscess, fistula, stricture, peritonitis or sepsis; or 2. Emergency surgery related to acute diverticulitis. Primary outcomes had to occur within 30 days of randomisation.
Secondary outcomes
Secondary outcomes were included where available: 1. Recurrences during follow‐up beyond 30 days; 2. Late complications (same as primary outcomes) during follow‐up beyond 30 days; 3. Emergency surgery during follow‐up beyond 30 days; 4. Elective colonic resection; 5. Mortality; 6. Length of hospital stay (days); 7. Length to recovery of symptoms (days); 8. All adverse events caused by treatments, including allergic reactions.
Search methods for identification of studies
Electronic searches
A comprehensive literature search (Identical to the search made in 2012, an integrated approach) was conducted to identify all published and unpublished randomised controlled trials with no language restrictions. Search strings for searches in MEDLINE, Embase and CENTRAL were designed in cooperation with an information specialist at the Cochrane Colorectal Cancer Group. Additional ongoing trials were searched using online registers. Searches were limited to only human trials. For full search strategy see Appendix 1. The following databases were searched:
Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library, issue 2) (February 2021) (Appendix 1);
Ovid MEDLINE (Epub Ahead of print, In‐Process & Other Non‐Indexed Citations, Daily and 1946 to present) (February 2021) (Appendix 2);
Ovid Embase (1974 to February 2021) (Appendix 3);
ClinicalTrials.gov (February 2021);
WHO International Clinical Trial Registry Platform (February 2021).
Searching other resources
Reference lists from relevant randomised controlled trials and reviews identified during the search were screened for additional trials.
Data collection and analysis
Selection of studies
All titles and abstracts obtained by the electronic searches were screened for identification of relevant randomised controlled trials. The full text of articles was obtained if trial eligibility could not be assessed by title or abstract, in absence of an abstract, or when eligible randomised controlled trials were identified. Identification and assessment were done by both primary and senior authors (MLD and DMS). Uncertainty about the adequacy of trials or disagreements were resolved by discussion or by involvement of the third author (SJR) until consensus was reached.
Data extraction and management
Data extraction was performed on a preformatted data extraction form by the primary author (MLD) and reviewed by the senior author (DMS). Data were extracted according to the intention‐to‐treat principle where possible.
Assessment of risk of bias in included studies
The methods described in the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1 (Higgins 2021) were used. The Cochrane Collaboration's tool for assessing risk of bias was used to assess the methodological quality of the identified trials. This tool focuses on sequence generation, allocation concealment, blinding (participants, personnel and outcome assessors), incomplete outcome data and selective outcome reporting. Studies were ranked for risk of bias as low, high or not clear.
Measures of treatment effect
The predefined outcomes were expressed in a 2 x 2 table with treatment effect calculated as risk ratio (RR) and random‐effects meta‐analysis was performed though the Mantel‐Haenzel method, where possible. The primary outcomes of this review were progression to complicated acute diverticulitis or need for emergency surgery and, therefore, a RR below 1 was interpreted in favour of the intervention and above 1 in favour of the comparator. Uncertainty was expressed as a 95% confidence interval (CI). Some secondary outcomes were continuous and extracted as means with standard deviations.
Unit of analysis issues
In this review, we included trials where participants were randomised to either no antibiotic or antibiotic. We did not identify any cluster‐ or cross‐over trials. No trials with more than two arms were identified. Therefore, no unit of analysis issues were present in the included studies, and the unit of analysis was the individual participant.
Dealing with missing data
Authors of included randomised controlled trials were contacted by email to obtain missing or supplementary data. Attrition was described in each included study. We only used the available data and no presumptions were made. An attempt to retrieve preliminary results of ongoing trials was done by contact to authors.
Assessment of heterogeneity
The identified randomised controlled trials were assessed for clinical heterogeneity by evaluating the interventions and outcomes. Statistical heterogeneity in meta‐analysis was expressed as I2 and thresholds were interpreted according to the Cochrane Handbook for Systematic Reviews for Interventions version 6 (Higgins 2021) (0‐40%: no importance, 30‐60% moderate heterogeneity, 50‐90% substantial heterogeneity, 75‐100% considerable heterogeneity).
Assessment of reporting biases
If more than ten studies would have been identified, publication bias would have been assessed by inspection of funnel plots as recommended in Cochrane (Higgins 2021)
Data synthesis
If enough homogenous randomised controlled trials were identified (> 1), meta‐analysis was preferable. The meta‐analysis presented a comparison between pairs of interventions (an experimental; non‐antibiotic management and a comparator; antibiotic) for acute uncomplicated diverticulitis. The template of RevMan 5.4.1 was used as reporting guidelines and for production of this review. In case of an insufficient number of studies for meta‐analysis, a qualitative review of identified studies would have been performed with reporting of outcome estimates from single studies.
Subgroup analysis and investigation of heterogeneity
In the presence of substantial or considerable statistical heterogeneity, subgroup analyses of first attack of uncomplicated acute diverticulitis versus recurrence were planned, because patient characteristics for first attack uncomplicated diverticulitis and recurrence diverticulitis may be different and thereby introduce a possible selection bias.
Sensitivity analysis
If specific studies had a high risk of bias, sensitivity analyses excluding these studies were planned.
Summary of findings and assessment of the certainty of the evidence
The overall certainty of evidence for primary outcomes (short‐term complications and short‐term emergency surgery) and secondary outcomes (long‐term complications, long‐term emergency surgery, recurrences, elective colonic resection, mortality, adverse events) are evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach Schünemann 2009, see Table 1.
Summary of findings 1. Comparison of observational management with antibiotics for acute uncomplicated diverticulitis .
| Observational management compared with antibiotic treatment for acute uncomplicated diverticulitis | ||||||
|
Patient or population: Patients with acute uncomplicated diverticulitis Settings: Hospital admitted patients Intervention: Observational management Comparison: Antibiotic treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed riskb | Corresponding risk | |||||
| Antibiotic | Observational | |||||
| Primary ‐ Short‐term complications (abscess, perforation, obstruction or fistula) within 30 days | 15 per 1000 | 13 per 1000 (5 to 39) | RR 0.89 (0.30 to 2.62) | 1329 (3) | ⊕⊕⊝⊝
Low |
Downgraded to low due to imprecision and risk of biasc,d |
| Primary ‐ Short‐term emergency surgery within 30 days | 11 per 1000 | 5 per 1000 (1 to 19) | RR 0.47 (0.13 to 1.71) | 1329 (3) | ⊕⊕⊝⊝ Low | Downgraded to low due to imprecision and risk of biasc,d |
| Secondary ‐ Recurrence during follow‐up beyond 30 daysa | 238 per 1000 | 240 per 1000 (193 to 298) | RR 1.01 (0.81 to 1.25) | 1024 (2) | ⊕⊕⊕⊝ Moderate | Downgraded to moderate due to risk of biasd |
| Secondary ‐Long‐term complications during follow‐up beyond 30 daysa | 42 per 1000 | 45 per 1000 (26 to 81) | RR 1.08 (0.61 to 1.93) | 1024 (2) | ⊕⊕⊝⊝ Low | Downgraded to low due to imprecision and risk of biasc,d |
| Secondary ‐ Long‐term emergency surgery during follow‐up beyond 30 daysa | 15 per 1000 | 21 per 1000 (9 to 52) | RR 1.42 (0.58 to 3.49) | 1024 (2) | ⊕⊕⊝⊝ Low | Downgraded to low due to imprecision and risk of biasc,d |
|
Secondary ‐All‐cause mortality |
48 per 1000 | 47 per 1000 (29 to 76) | RR 0.98 ( 0.60 to 1.59) | 1262 (3) | ⊕⊕⊕⊝ Moderate | Downgraded to moderate due to risk of biasd |
| Secondary ‐ Adverse events | 9 per 1000 |
1 per 1000 (0 to 10) |
RR 0.14 (0.02 to 1.13) | 1329 (3) |
⊕⊕⊕⊝ Moderate | Downgraded to moderate due to risk of biasd |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a Range from 24 months (Van Dijk 2018) to 173 months (Isacson 2019)
bThe assumed risk = total events/total included in the observational group multiplied 100 (9/665*100 = 1.4)
Quality of evidence was downgraded for concerns in each GRADE domain.
cImprecision due to the relatively few events
dRisk of bias due to the lack of blinding and high risk of attrition in the follow up study, Van Djik 2018
The evidence could be downgraded from high certainty by one level (serious concern) or two levels (very serious concern) for the following reasons: risk of bias, indirectness, inconsistency in study results, imprecise evidence, or publication bias.
Results
Description of studies
See Included studies and Excluded studies.
Results of the search
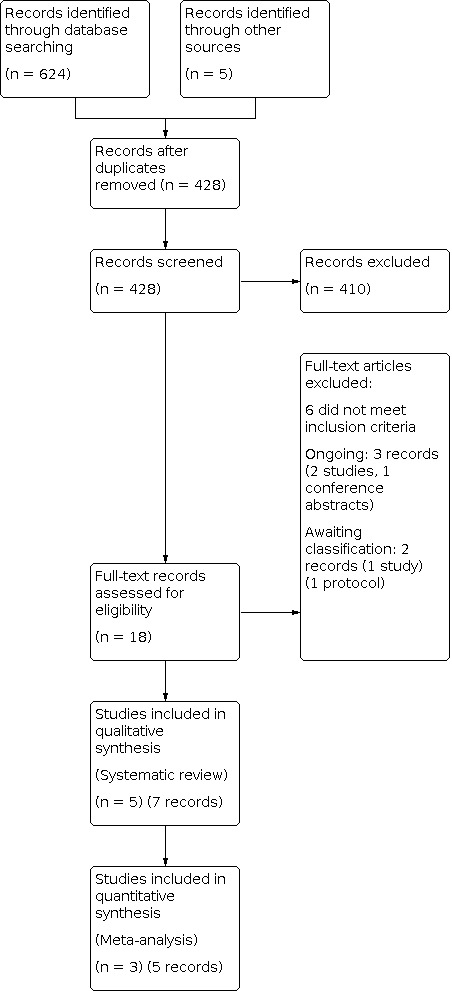
The search strategy identified 624 records from the online databases. A search for ongoing trials identified five randomised controlled trials, which were already included in the initial search results. Screening the reference lists of reviews and identified trials did not provide additional trials. Eighteen records were eligible for full‐text assessment. No records were excluded due to language restrictions. For record selection, see flow diagram Figure 1.
1.
Records identified through databases, distributed as follows: MEDLINE 147, Embase 315, Central 162
Included studies
Of the 18 eligible records, three were new ongoing records (corresponding to two studies), (Kruis 2019; Al Mansouri 2019) of which one had a published conference abstract (Al Mansouri 2019)). No additional data were obtained from the authors of these trials through email correspondence. Six further records were excluded, as they did not fulfil inclusion criteria (see Excluded studies). Initially, four randomised controlled trials had been included in the analysis (Chabok 2012; Daniels 2017; Kellum 1992; Ribas 2010) of which two groups (Chabok 2012; Daniels 2017) had also published long‐term follow‐up data (Isacson 2019; Van Dijk 2018). During the systematic review update process, two of the pre‐update ongoing trials were published, one was included (Jaung 2020) and one was assessed as awaiting classification (Mora‐Lopez 2021) (two records). Comparable data for pooling in meta‐analysis were available for only three trials (Chabok 2012; Daniels 2017; Jaung 2020) and the follow‐up studies (Isacson 2019; Van Dijk 2018). For detailed descriptions of the included studies, see Included studies. The risk of bias is reported in Figure 2.
2.

Risk of bias summary
No antibiotic versus antibiotic
Three trials (Chabok 2012; Daniels 2017; Jaung 2020) investigated the effects of antibiotic therapy in patients with CT‐diagnosed uncomplicated acute diverticulitis. The sample size ranged from 180 (Jaung 2020) to 669 (Chabok 2012) and all three were multicentre trials. Chabok 2012 and Jaung 2020 included all cases of uncomplicated acute diverticulitis, whereas Daniels 2017 only included cases with a first episode of uncomplicated acute diverticulitis. Chabok 2012 only included patients with a body temperature above 38°C and elevated white blood cell count and Jaung 2020 excluded patients with > 1 criteria for systematic inflammatory response including a body temperature above 38°C. There were no differences in baseline characteristics between the groups in any of the trials. Chabok 2012 and Daniels 2017 consisted of a non‐antibiotic intervention group versus an antibiotic‐treated control group, whereas Jaung 2020 had an antibiotic intervention group and a placebo (non‐antibiotic) group. In all trials, the participants were treated with routine broad‐spectrum antibiotic covering gram negative and anaerobic bacteria. Chabok 2012 and Daniels 2017 initially treated with intravenous antibiotics and changed to per oral administration at discharge, whereas Jaung 2020 prescribed intravenous or per oral administration depending on clinical preference. Two trials were not blinded (Chabok 2012; Daniels 2017); therefore, we assessed the studies as having high risk of performance and detection bias and one trial was triple‐blinded (Jaung 2020) and therefore had low risk of performance and detection bias. All trials conducted intention‐to‐treat analysis. In two of the trials (Chabok 2012; Daniels 2017), it was mentioned, that the funders had no involvement in trial design, conduct or reporting. Jaung 2020 was funded by the project grant from the Colorectal Surgical Society of Australia and New Zealand but it was not specified how the funders were involved in the project; details are found in Included studies. Two of the trials had published follow‐up records; Isacson 2019 had ten‐year follow‐up to Chabok 2012, and Van Dijk 2018 had two‐year follow‐up to Daniels 2017 details are found in Included studies.
Comparison of different antibiotic agents
Kellum 1992 compared single‐compound antibiotic therapy cefoxitin to combination therapy gentamicin‐clindamycin in 77 patients diagnosed with uncomplicated acute diverticulitis.
Comparison of routes of administration and duration of therapy
Ribas 2010 compared intravenous antibiotic treatment, 24 to 48 hours versus seven days, in 50 patients with CT‐verified uncomplicated acute diverticulitis. Both groups were treated with antibiotics for 12 days in total.
Excluded studies
583 records were excluded because they were duplicate publications, not randomised controlled trials, the participants included did not have a CT‐verified diagnosis of uncomplicated acute diverticulitis or antibiotics were not assessed. Additionally, Kim 2019 was excluded because the trial investigated right‐sided diverticulitis. Schug‐Pass 2010 was excluded because the study design introduced significant selection of participants based on antibiotic treatment response before randomisation and could therefore not be considered a randomised controlled trial for this specific systematic review, although it was labelled as such. All included participants were treated with the same intravenous treatment the first four days. Randomisation was performed on day four, only if treatment had been successful. In 17 participants, the treatment had not been successful after four days, and therefore the participants where excluded from being randomised, including participants with persisting symptoms and complicated diverticulitis. This design excludes patients of interest for this review before randomisation (Excluded studies).
Risk of bias in included studies
Risk of bias for all studies can be seen in section Risk of bias in included studies with a summary in Figure 2 and Figure 3.
3.

Allocation
Kellum 1992 lacked descriptions of the randomisation process and allocation, thereby introducing a high risk for selection bias. The four other trials and their follow‐up supplied an adequate description on the allocation methods and concealment. For more details about the allocation; see Included studies.
Blinding
Jaung 2020 was the only trial without risk of performance or detection bias due to blinding of participants, healthcare providers and outcomes assessors. The other included randomised controlled trials had no blinding of participants, healthcare providers or outcome assessors, which downgraded the quality of evidence; see Table 1.
Incomplete outcome data
Attrition and exclusion of participants after randomisation was adequately reported in four trials and the two follow‐up publications (Chabok 2012; Daniels 2017; Isacson 2019; Jaung 2020; Ribas 2010; Van Dijk 2018). Isacson 2019 only included the Swedish patients from Chabok 2012 trial because the ethics committee approval for the Icelandic patients could not be obtained; all participants were identified and 67 participants were lost to follow‐up (no–AB: 34 vs. AB: 33). Daniels 2017 randomised 570 participants and excluded 42 participants with adequate reasons (no‐AB: 262 vs. AB: 266). The follow‐up data, Van Dijk 2018, were based on 468 participants, because 60 participants were lost to follow‐up (no‐AB: 35 vs. AB: 25). The follow‐up analysis, Van Dijk 2018, only included participants that completed the entire 24 months of follow‐up, and therefore attrition bias might have been introduced. Re‐analyses were done to assess the risk of attrition bias by exploring differences in baseline characteristics and clinical disease course. Baseline characteristics were comparable among the complete case analysis population and lost to follow‐up population. Second, only minimal changes in rates of complications at six months in favour of the antibiotic group were observed when the current complete case study population was compared to the original intention‐to‐treat population. Third, the time to recovery in observational participants was significantly longer in the lost to follow‐up group compared with the complete case group (median: 24 days versus 13 days, respectively; P = 0.004), whereas the time to recovery in antibiotic participants was only slightly but not significantly longer in the lost to follow‐up group (median: 15 days versus 11 days, respectively; P = 0.106). This implies that participants in the observational group tended to drop out of follow‐up sooner than participants in the antibiotic group when recovery of the initial diverticulitis episode was prolonged which, therefore, introduced risk of attrition bias; for more details see Characteristics of included studies. Kellum 1992 had inadequate reporting on attrition. Attrition during the six weeks of follow‐up was not addressed and, therefore, Kellum 1992 was deemed as having high risk for attrition bias.
Selective reporting
All included trials described the existence of a protocol. Chabok 2012, Daniels 2017 and Jaung 2020 had predefined primary and secondary outcomes and these were available throughout the whole trial period online. Ribas 2010 reported in a correspondence that the outcomes were the same in the protocol as described in the article. Kellum 1992 did not describe outcomes as predefined, creating an unknown risk for reporting bias. Kellum 1992 also excluded 34% of the randomised participants from the analysis, and some without reported reasons (Characteristics of included studies).
Other potential sources of bias
Chabok 2012; Daniels 2017 and Jaung 2020 reported sample size calculations (Characteristics of included studies). The primary outcome in Daniels 2017 was time to recovery and, in Jaung 2020, it was length of hospital admission. Thus, none of the included trials performed sample size calculations based on the primary outcome of this present study. The other included trials (Kellum 1992; Ribas 2010) did not report sample size calculations. Single‐study analyses in this present systematic review may, therefore, have been unable to show clinically relevant significant differences in outcomes.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 2. Comparison of different antibiotic agents.
| Comparison of different antibiotic agents | ||||||
|
Patient or population: Patients with acute uncomplicated diverticulitis Settings: Hospital admitted patients Intervention: Antibiotic treatment: gentamicin‐clindamycin Comparison: Antibiotic treatment: cefoxitin |
||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Cefoxitin | Gentamicin‐clindamycin | |||||
| Primary ‐ Short‐term complications (abscess, perforation, obstruction or fistula) within 30 days | No data | |||||
| Primary ‐ Short‐term emergency surgery within 30 days | 66 per 1000a | 46 per 1000 (7 to 302) | RR 0.70 (0.11 to 4.58) | 51 (1) | ⊕⊕⊝⊝ Low | Downgraded to low due to risk of biasb and imprecisionc |
| Primary ‐ Short‐term emergency surgery within 30 days | No data | |||||
| Secondary ‐ Recurrence during follow‐up beyond 30 days | No data | |||||
| Secondary ‐Long‐term complications during follow‐up beyond 30 days | No data | |||||
| Secondary ‐ Long‐term emergency surgery during follow‐up beyond 30 days | No data | |||||
| Secondary ‐All‐cause mortality | No data | |||||
|
Secondary ‐ Adverse events |
No data | |||||
aThe assumed risk = total events/total included in the cefoxitin group multiplied 100 (2/30*1000 = 66)
bOverall high risk of bias due to high attrition, no blinding and no explanation of the randomisation process
cRisk of imprecision due to wide confidence intervals
Summary of findings 3. Comparison of antibiotics for routes of administration and duration of therapy.
| Comparison of routes of administration and duration of therapy | ||||||
|
Patient or population: Patients with acute uncomplicated diverticulitis Settings: Hospital admitted patients Intervention: Antibiotic treatment: IV AB administration Comparison: Antibiotic treatment: oral AB administration |
||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral AB administration | IV AB administration | |||||
| Primary ‐ Short‐term complications (abscess, perforation, obstruction or fistula) within 30 days | No data | |||||
| Primary ‐ Short‐term emergency surgery within 30 days | No data | |||||
| Secondary ‐ Recurrence during follow‐up beyond 30 days | No data | |||||
| Secondary ‐Long‐term complications during follow‐up beyond 30 days | 46 per 1000a | 46 per 1000 (3 to 69) | RR 1.0 (0.07 to 15.00) | 44 (1) | ⊕⊕⊝⊝ Low | Downgraded to low due to risk of biasb and imprecisionc |
| Secondary ‐ Long‐term emergency surgery during follow‐up beyond 30 days | No data | |||||
| Secondary ‐All cause mortality | No data | |||||
|
Secondary ‐ Adverse events |
No data | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aThe assumed risk = total events/total included in the oral AB group multiplied 1000 (1/22*1000 = 46).
bOverall high risk of bias due to no blinding
cHigh risk of imprecision due to few participant and no events means the quality of evidence is low
No antibiotics versus antibiotics
Primary outcome
When pooled in meta‐analysis, a total of 1329 participants were included: 665 under no‐antibiotic management and 664 who received antibiotic treatment. Risk of complications within 30 days was equal in the groups (1.4% vs. 1.4%) and there may be little or no difference between the no‐antibiotic and antibiotic groups and no important heterogeneity (RR 0.89; 95% CI [0.30 to 2.62]; I2 = 19%; 3 studies, 1329 participants; low‐certainty evidence, Analysis 1.1).
1.1. Analysis.

Comparison 1: No‐antibiotic vs antibiotic group, Outcome 1: Complications (abscess, perforation, obstruction or fistula)
Emergency surgery within 30 days occurred less frequently in the no‐antibiotic group (0.5% vs. 1.0%). Estimates may indicate that there is a possible effect of no‐antibiotic management, however, there is considerable imprecision due to wide confidence intervals. The evidence is uncertain which means that there may also be a benefit with antibiotics. The evidence is without important heterogeneity (RR 0.47; 95% CI [0.13 to 1.71]; I2 = 0%; 3 studies; 1329 participants, low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: No‐antibiotic vs antibiotic group, Outcome 2: Emergency surgery
The qualities of the evidence for primary outcomes were of low quality because of the lack of blinding, leading to possible risk of detection bias and the relatively wide confidence intervals indicating imprecision.
Trial sequential analyses were performed for the primary outcome in order to assess total sample size needed or for identification of possible evidence of early futility. However, according to the parameters we had chosen (alpha 5%, beta 20%, relative risk reduction 20%), we knew too little (2%) based on the diversity‐adjusted required information size to perform analyses.
Secondary outcomes
The meta‐analyses of secondary outcomes were based on two long‐term follow‐up studies (Isacson 2019; Van Dijk 2018). A total of 1024 participants were included: 502 in the no‐antibiotic group and 522 in the antibiotic group. Risk of recurrence beyond 30 days likely results in little to no difference between the groups and with no important heterogeneity (RR 1.01; 95% CI [0.81 to 1.25]; I2 = 0 %; 2 studies; 1024 participants, moderate‐certainty evidence; Analysis 1.3). The evidence suggests that no‐antibiotic management compared to antibiotic management results in little to no difference in late complications beyond 30 days and no important heterogeneity (RR 1.08; 95% CI [0.61 to 1.93]; I2= 0%; 2 studies; 1024 participants, low‐certainty evidence; Analysis 1.4). There were more cases of emergency surgery beyond 30 days in the no‐antibiotic group (2.2 vs. 1.5 %), but the evidence suggests that no‐antibiotic management results in little to no difference in emergency surgery and no important heterogeneity (RR 1.42; 95% CI [0.58 to 3.49]; I2 = 0 %; 2 studies; 1024 participants, low‐certainty evidence; Analysis 1.5). Elective colonic resections were performed equally in the groups and no‐antibiotic management may have little to no effect on elective colonic resection rate but the evidence is very uncertain among other because of substantial heterogeneity (RR 1.1; 95% CI [0.41 to 2.97]; I2= 68%; 2 studies; 1024 participants, very low‐certainty evidence; Analysis 1.6). All‐cause mortality was based on the two long‐term follow‐up studies and Jaung 2020, with 1262 participants in total, 631 in each group. All‐cause mortality was the same in both groups; no‐antibiotic management likely results in little to no difference in all‐cause mortality with no important heterogeneity (RR 0.98; 95% CI [0.60 to 1.59]; I2= 0 %; 3 studies; 1262 participants moderate‐certainty evidence; Analysis 1.7). Chabok 2012 reported that three participants allocated to the antibiotic group terminated their antibiotic treatment because of allergic side effects. The same was applicable in Daniels 2017, where three participants in the antibiotic group were discontinued antibiotics because of side effects or allergic reactions, including an anaphylactic reaction in one participant. No allergic reactions were reported in participants allocated to the non‐antibiotic group in the other studies. Jaung 2020 did not report any allergic reactions in either of the groups. No‐antibiotic management likely results in a large reduction in adverse events (RR 0.14; 95% CI [0.02 to 1.13]; I2= 0%; 3 studies; 1329 participants, moderate‐certainty evidence, Analysis 1.8). For all results, see Table 4 and Table 1.
1.3. Analysis.

Comparison 1: No‐antibiotic vs antibiotic group, Outcome 3: Recurrence
1.4. Analysis.

Comparison 1: No‐antibiotic vs antibiotic group, Outcome 4: Long‐term complications
1.5. Analysis.

Comparison 1: No‐antibiotic vs antibiotic group, Outcome 5: Long‐term emergency surgery
1.6. Analysis.

Comparison 1: No‐antibiotic vs antibiotic group, Outcome 6: Elective colonic resections
1.7. Analysis.

Comparison 1: No‐antibiotic vs antibiotic group, Outcome 7: All‐cause mortality
1.8. Analysis.

Comparison 1: No‐antibiotic vs antibiotic group, Outcome 8: Adverse events
1. Outcomes no antibiotics vs antibiotics.
| Outcomes | No antibiotics | Antibiotics | RR (95 % CI) |
| Complications within 30 days | 9/665 | 10/664 | 0.89 (0.30 to 2.62) |
| Emergency surgery within 30 days | 3/665 | 7/664 | 0.47 (0.13 to 1.71) |
| Recurrence beyond 30 days | 121/502 | 124/522 | 1.01 (0.81 to 1.25) |
| Complications beyond 30 days | 23/502 | 22/522 | 1.08 (0.61 to 1.93) |
| Emergency surgery beyond 30 days | 19/502 | 13/522 | 1.42 (0.58 to 3.49) |
| Elective colonic resection | 26/502 | 24/522 | 1.10 (0.41 to 2.97) |
| Mortality | 12/630 | 16/639 | 0.98 (0.60 to 1.59) |
| Adverse events | 0/664 | 6/665 | 0.14 (0.02 to 1.13) |
Hospital stay was measured by both means and medians and could, therefore, not be pooled in a meta‐analysis. Chabok 2012 found no difference in mean hospital stay (2.9 vs. 2.9 days, P = 0.71) and Jaung 2020 found no difference in median hospital stay (46 vs. 40 hours, P = 0.15). Daniels 2017 showed a significant difference in median hospital stay, favouring no antibiotics but it was also concluded that the longer hospital stay was due to antibiotic treatment allocation. Length to recovery was only assessed in Daniels 2017, with no significant differences identified (14 (6 to 35) vs. 12 (7 to 30) days, P = 0.113).
The quality of the evidence for secondary outcomes was of moderate to low quality because of the lack of blinding, leading to possible risk of detection bias and because of wide confidence intervals in some of the outcomes possibly leading to imprecision. For elective colonic resections, the quality of evidence was very low because of an additional concern for inconsistency due to the substantial heterogeneity.
However only one study, Jaung 2020, included in meta‐analyses of antibiotics versus no antibiotics had no risk of bias with the remaining having high risk of bias due to no blinding.
Overall, the outcomes of the present studies had no important heterogeneity, except elective colonic resections where heterogeneity was substantial. The outcome was only based on two studies and therefore we did not identify a sufficient number of studies for subgroup analysis.
Comparison of different antibiotic agents
The estimates were only based on Kellum 1992. The calculated estimate for emergency surgery had wide confidence intervals, and the evidence indicates an uncertain clinical effect between these two antibiotic regimens (RR 0.70; 95% CI [0.11 to 4.58]; I2= not applicable; 1 study; 51 participants, low‐certainty evidence, Analysis 2.1). For the secondary outcomes, the length of hospital stay was similar in both groups and the different antibiotic agents may result in little to no difference in hospital stay (8.1 +/‐0.7 vs. 9.0 +/‐1.0 days). There was one adverse event in the gentamicin‐clindamycin group (0 vs. 4.8%). The authors of the study end up concluding that there were no differences between the two antibiotic treatment arms in effectiveness.
2.1. Analysis.

Comparison 2: Cefoxitin vs. gentamicin‐clindamycin, Outcome 1: Emergency surgery
Kellum 1992 had an overall high risk of bias due to high attrition, no blinding and no explanation of the randomisation process and further risk of imprecision due to wide confidence intervals making the quality of evidence low in analysis of different antibiotic compounds.
Comparison of routes of administration and duration of therapy
The estimates were only based on Ribas 2010. Treatment failure was equal in the groups and no significant differences were found (4.5 vs. 2.3, P = 1). A correspondence with the author confirmed that none of the participants with treatment failure were re‐evaluated with CT or received emergency surgery, but were unable to be discharged, solely due to persistent pain. Therefore, this trial did not report any events for our primary outcomes. However, secondary outcomes were reported. Colonoscopy four to six weeks after discharge revealed late complications with strictures in one participant from each group but no significant differences were found (RR 1.0; 95% CI [0.07 to 15.00]; I2= not applicable; 1 study; 44 participants, low‐certainty evidence, Analysis 3.1). Short intravenous treatment of 24 to 48 hours was therefore not inferior to long intravenous treatment of seven days, when treated with antibiotics for 12 days in total.
3.1. Analysis.

Comparison 3: Short vs. long‐term IV antibiotic treatment , Outcome 1: Long‐term complications
Ribas 2010 also had an overall high risk of bias due to no blinding and high risk of imprecision due to few participants and no events, making the quality of evidence low.
Discussion
Summary of main results
This present systematic review, based on RCTs, explored whether antibiotic treatment of uncomplicated acute diverticulitis had an impact on the development of complications or emergency surgery. According to the identified records, no significant effects of the tested antibiotic therapies were identified. There was no effect of antibiotics compared to antibiotic‐free treatment, no difference between single‐compound compared to double‐compound therapy, and no difference between short versus long intravenous antibiotic therapy identified. Meta‐analyses comparing no antibiotics versus antibiotic treatment, including approximately 1300 participants showed no statistical differences in clinical outcomes.
Overall completeness and applicability of evidence
Five randomised controlled trials and two follow‐up studies investigating participants with CT‐verified uncomplicated acute diverticulitis were identified to assess the outcomes of this systematic review. Five of these studies investigated the role of no antibiotics compared to antibiotics. Chabok 2012 only included participants with a body temperature above 38°C and elevated white blood cell count and Jaung 2020 excluded participants with greater than one criterion for systematic inflammatory response including a body temperature above 38°C. Both studies, therefore, included more selected participant groups.
Evidence for the need of antibiotics for uncomplicated acute diverticulitis is being emphasised in a number of ongoing randomised controlled trials at the moment. One group is investigating the efficacy and safety of ambulatory treatment of uncomplicated acute diverticulitis without antibiotic compared to antibiotic treatment in 460 participants, by assessing causes for readmissions and ambulatory visits, pain control and progression to complicated diverticulitis (NCT02785549). Like Jaung 2020, NCT02785549 is triple‐blinded (participant, care provider, outcomes assessors), possibly lowering risk of performance and detection bias. This trial could strengthen or weaken the overall evidence depending on the direction of the results because of the relatively high number of participants included. A smaller pilot trial including only 11 participants also investigated outpatient treatment of uncomplicated acute diverticulitis with no‐antibiotic versus antibiotic treatment (Al Mansouri 2019; NCT03146091), however no treatment failures were seen in either of the groups during the 60 days of follow‐up.
Quality of the evidence
The primary outcome was explored in three RCTs and two follow‐up studies exploring no antibiotics versus antibiotics (Chabok 2012; Daniels 2017; Isacson 2019; Jaung 2020; Van Dijk 2018); one study was assessed at low risk of bias (Jaung 2020) and the other four (Chabok 2012; Daniels 2017; Isacson 2019; Van Dijk 2018) were assessed at high risk of bias due to the lack of blinding, leading to possible risk of detection bias. The follow‐up analysis, Van Dijk 2018, also had high risk of attrition bias, because it only included participants that completed the entire 24 months of follow‐up. The 95% confidence intervals were relatively wide for primary outcomes, indicating a small sample size with few events. The presence of type II error in the analyses of no antibiotics versus antibiotics can therefore not be ruled out. Trial sequential analysis could not be performed due to the low number of included participants indicating that the available sample size is far from reaching futility. The certainty of the evidence for the primary outcome of no antibiotics versus antibiotics was, therefore, of low certainty due to possible risk of performance bias and imprecision. Secondary outcome analyses for recurrence and long‐term complications had acceptable 95% confidence intervals, indicating a true non‐inferiority and precise estimates. The low I2 in the meta‐analyses suggests low clinical heterogeneity, thereby indicating consistent results, and the certainty for these two secondary outcomes was moderate. The other two included randomised controlled trials (Kellum 1992; Ribas 2010) included very small numbers of participants, they could not be pooled in meta‐analysis and therefore also had risk of type II error and imprecision. Further, Kellum 1992 had an overall high risk of bias due to high attrition, suggesting that the certainty of evidence was low in the analysis of different antibiotic compounds. An increasing amount of evidence supporting no use of antibiotics in uncomplicated acute diverticulitis has been published since the first publication of this review (Shabanzadeh 2012), however, the total body of evidence is still limited.
Potential biases in the review process
The methodology to evaluate the evidence was carried out according to Cochrane’s tool for assessing risk of bias, resulting in a uniform and strict analysis of each RCT. Two authors performed the study selection and data extraction. All authors agreed upon study inclusion and exclusion. Six records were excluded, due to the review's inclusion criteria. Reasons for exclusion are outlined in Excluded studies. Meta‐analyses were performed by pooling data from Chabok 2012; Daniels 2017; and Jaung 2020 and the follow‐up studies, Isacson 2019 and Van Dijk 2018. Hence, this systematic review process has very low risk of potential bias.
Agreements and disagreements with other studies or reviews
A meta‐analysis (Mege 2019) based on seven randomised and non‐randomised studies (2321 participants) explored different management strategies for CT‐verified uncomplicated acute diverticulitis. It demonstrated that antibiotic‐free treatment was not inferior to antibiotic treatment in the rates of emergency surgery or recurrence of diverticulitis. A subgroup analysis of two RCTs (Chabok 2012 and Daniels 2017) revealed an increase in elective surgery during follow‐up in the antibiotic‐free group. This present systematic review demonstrated no significant differences in elective surgery (evidence is very uncertain), based on meta‐analyses of the follow‐up studies (Isacson 2019 and Van Dijk 2018). Another meta‐analysis (Desai 2019), based on almost the same seven randomised and non‐randomised studies as Mege 2019, also concluded that there were no significant differences between non‐antibiotic and antibiotic treatment, in either recurrence of diverticulitis, progression to complicated diverticulitis or colonic resection rates. These two published meta‐analyses may be methodologically inferior as they included non‐randomised studies, however, they reached the same overall conclusion in regard to primary outcomes as this present systematic review. Mege 2019 also compared oral versus intravenous treatment based on four studies (355 participants) and showed no significant differences in outcome.
The included studies reported allergic reactions and one anaphylactic reaction due to antibiotic treatment and no comparable events were reported in the non‐antibiotic groups. The possible serious adverse effects of antibiotics, and increasing antibiotic resistance, call for a reduction in the clinical use of antibiotics.
Several medications have been tested under the premise that diverticulitis is an inflammatory disorder (Strate 2019). Mesalazine, an anti‐inflammatory drug in the 5‐aminosalicylic‐acid‐group, is recommended in some guidelines for the prevention of uncomplicated acute diverticulitis recurrence (Andeweg 2013). A systematic review investigated mesalazine in diverticular disease and a subgroup analysis found that mesalazine did not prevent recurrence after uncomplicated acute diverticulitis (Iannone 2018). A meta‐analysis based on RCTs demonstrated that 5‐aminosalicyclic acid agents did not prevent recurrent diverticulitis (Urushidani 2018). Other pharmaceutical agents have been tested for prevention of recurrent diverticulitis, including the antibiotic rifaximin and probiotics (Dughera 2004, Lanas 2013), however, both studies were small and of low quality. None of the tested medical treatments have been found effective for the prevention of acute diverticulitis.
Authors' conclusions
Implications for practice.
The newest evidence on antibiotic treatment for uncomplicated diverticulitis suggests that the effect of antibiotics is uncertain for complications, emergency surgery, recurrence, elective colonic resections and long‐term complications. These results were obtained from meta‐analyses of mostly low‐certainty evidence RCTs and follow‐up studies. The evidence suggest that no‐antibiotic treatment for uncomplicated diverticulitis seems to be safe.
Implications for research.
Only three RCTs on the need of antibiotics are currently available and more are needed in order to obtain precise effect estimates. New trials should perform blinded outcome assessment in order to minimise risk of bias. At least two ongoing RCTs will provide new evidence to the management of uncomplicated diverticulitis and antibiotic treatment in the years to come.
What's new
| Date | Event | Description |
|---|---|---|
| 1 March 2021 | Amended | Peer reviewers and editors comments were incorporated. |
| 1 October 2020 | New search has been performed | An ongoing RCT was published and included in the meta‐analysis. |
| 4 September 2019 | New citation required but conclusions have not changed | Review updated and new search has been performed. |
History
Protocol first published: Issue 4, 2011 Review first published: Issue 11, 2012
Acknowledgements
Thanks and appreciation goes to Marija Barbatoskovic for her assistance with the electronic search strategies and to Cochrane's Colorectal group for supervision. Thanks to Dr. Brett Doleman and Dr. Anders Peter Skovsen for peer‐reviewing comments.
Appendices
Appendix 1. CENTRAL search strategy
Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library, Issue 2 2021)
#1 MeSH descriptor: [Diverticulitis] explode all trees
#2 MeSH descriptor: [Diverticulitis, Colonic] explode all trees
#3 Diverticulit* (Word variations have been searched)
#4 #1 or #2 or #3
#5 MeSH descriptor: [Anti‐Infective Agents] explode all trees
#6 Antibiotic* (Word variations have been searched)
#7 Anti‐bacterial agent* (Word variations have been searched)
#8 Amoxicillin* or Ampicillin* or Carbapenem* or Cefadroxil* or Cefotaxime* or Cefoxitin* or Cefpodoxime* or Ceftriaxone* or Cefuroxime* or Cephalosporin* or Ciprofloxacin* or Clavulanic* or Clindamycin* or Cotrimoxazole* or Doxycycline* or Ertapenem* or Flouroquinolone* or Gentamicin* or Gentamycin* or Imipenem* or Levofloxacin* or Meropenem* or Mesalazine* or Metramidazole* or Metronidazole* or Piperacillin* or Quinolone* or Rifaximin* or Tazobactam* or Trimethoprim* (Word variations have been searched)
#9 #5 or #6 or #7 or #8
#10 #4 and #9
Appendix 2. MEDLINE search strategy
Ovid MEDLINE, (sensitivity‐maximizing filter), (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present)
1. exp diverticulitis/
2. exp diverticulitis, colonic/
3. diverticulit*.mp.
4. 1 or 2 or 3
5. exp anti‐infective agents/
6. antibiotic*.mp.
7. *bacterial infections/dt
8. anti‐bacterial agent*.mp.
9. (Amoxicillin* or Ampicillin* or Carbapenem* or Cefadroxil* or Cefotaxime* or Cefoxitin* or Cefpodoxime* or Ceftriaxone* or Cefuroxime* or Cephalosporin* or Ciprofloxacin* or Clavulanic* or Clindamycin* or Cotrimoxazole* or Doxycycline* or Ertapenem* or Flouroquinolone* or Gentamicin* or Gentamycin* or Imipenem* or Levofloxacin* or Meropenem* or Mesalazine* or Metramidazole* or Metronidazole* or Piperacillin* or Quinolone* or Rifaximin* or Tazobactam* or Trimethoprim*).mp.
10. 5 or 6 or 7 or 8 or 9
11. 4 and 10
12. randomized controlled trial.pt
13. controlled clinical trial.pt.
14. randomized.ab.
15. placebo.ab.
16. clinical trials as topic.sh.
17. randomly.ab.
18. trial.ti.
19. 12 or 13 or 14 or 15 or 16 or 17 or 18
20. exp animals/ not humans.sh.
21. 19 not 20
22. 11 and 21
Appendix 3. Embase search strategy
Ovid Embase (1974 to 2021 February)
1. exp diverticulitis/
2. exp colon diverticulitis/
3. diverticulit*.mp.
4. 1 or 2 or 3
5. exp antiinfective agent/
6. antibiotic*.mp.
7. *bacterial infection/dt
8. antibacterial agent*.mp.
9. (Amoxicillin* or Ampicillin* or Carbapenem* or Cefadroxil* or Cefotaxime* or Cefoxitin* or Cefpodoxime* or Ceftriaxone* or Cefuroxime* or Cephalosporin* or Ciprofloxacin* or Clavulanic* or Clindamycin* or Cotrimoxazole* or Doxycycline* or Ertapenem* or Flouroquinolone* or Gentamicin* or Gentamycin* or Imipenem* or Levofloxacin* or Meropenem* or Mesalazine* or Metramidazole* or Metronidazole* or Piperacillin* or Quinolone* or Rifaximin* or Tazobactam* or Trimethoprim*).mp.
10. 5 or 6 or 7 or 8 or 9
11. 4 and 10
12. CROSSOVER PROCEDURE.sh.
13. DOUBLE‐BLIND PROCEDURE.sh.
14. SINGLE‐BLIND PROCEDURE.sh.
15. (crossover* or cross over*).ti,ab.
16. placebo*.ti,ab.
17. (doubl* adj blind*).ti,ab.
18. allocat*.ti,ab.
19. trial.ti.
20. RANDOMIZED CONTROLLED TRIAL.sh.
21. random*.ti,ab.
22. 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21
23. (exp animal/ or exp invertebrate/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans or man or men or wom?n).ti.)
24. 22 not 23
25. 11 and 24
Data and analyses
Comparison 1. No‐antibiotic vs antibiotic group.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Complications (abscess, perforation, obstruction or fistula) | 3 | 1329 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.30, 2.62] |
| 1.2 Emergency surgery | 3 | 1329 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.13, 1.71] |
| 1.3 Recurrence | 2 | 1024 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.81, 1.25] |
| 1.4 Long‐term complications | 2 | 1024 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.61, 1.93] |
| 1.5 Long‐term emergency surgery | 2 | 1024 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.58, 3.49] |
| 1.6 Elective colonic resections | 2 | 1024 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.41, 2.97] |
| 1.7 All‐cause mortality | 3 | 1262 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.60, 1.59] |
| 1.8 Adverse events | 3 | 1329 | Odds Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.13] |
Comparison 2. Cefoxitin vs. gentamicin‐clindamycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Emergency surgery | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.11, 4.58] |
Comparison 3. Short vs. long‐term IV antibiotic treatment .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Long‐term complications | 1 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.07, 15.00] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chabok 2012.
| Study characteristics | ||
| Methods | RCT, multicentre, inclusion from 10 hospitals in Sweden and one in Iceland, October 2003 to January 2010 Isacson 2019: Ten‐year follow‐up Furthermore, all participants received a Quality of Life questionnaire with five dimensions; mobility, self‐care, usual activity, pain/discomfort and anxiety/depression; and rated their health on Quality‐of‐life questionnaires, used as a quantitative measure of health outcomes. |
|
| Participants | 669 patients randomised, 46 excluded with adequate reasons (309 in no‐AB vs. 314 in AB group) Inclusion: CT with uncomplicated acute diverticulitis and lower abdominal pain, temperature 38°C or more, elevated white blood cells Exclusion: CT with complicated diverticulitis (abscess, fistula, free air), signs of other diagnosis on CT, receiving antibiotic or immunosuppressive therapy, pregnancy, high fever, affected general condition, peritonitis or sepsis Isacson 2019: Including only the Swedish participants; all were identified 67 participants lost to follow‐up (no–AB: 34 vs. AB: 33) Ethics committee approval for the Icelandic patients could not be obtained. |
|
| Interventions | Intervention: No‐AB group (IV fluids only) Control: AB group. Broad‐spectrum ABs were used according to the participating centres’ routines, covering gram‐negative and anaerobic bacteria. Treatment was initiated with an IV combination of a second‐ or third generation cephalosporin (cefuroxime or cefotaxime) and metronidazole, or with carbapenem antibiotics (ertapenem, meropenem or imipenem) or piperacillin–tazobactam. Orally administrated ABs such as ciprofloxacin or cefadroxil combined with metronidazole were initiated subsequently on the ward or at discharge. The total duration of AB was at least 7 days. | |
| Outcomes |
Primary: Complications were defined as bowel perforation with free air, abscess or fistula. Emergency surgery during hospital stay. Length of hospital stay. 10 participants allocated to no‐AB group were started on AB because of increasing abdominal pain, fever or CRP level. 3 participants allocated in the AB group had their AB terminated because of allergic side‐effects. One year follow‐up: Surgery during follow‐up. Readmission with recurrence after 12 months was assessed by questionnaires Isacson 2019 Data extraction included any admission for recurrence or complications of diverticulitis, surgery for diverticulitis, and the development of colorectal cancer that occurred more than 1 month after inclusion in the AVOD trial. Recurrent diverticulitis was defined as clinical or CT‐verified recurrence diagnosed at a hospital. Each participant was included only once in the database, but all of their complications and operations were included. |
|
| Notes | Financial support for the study was provided by the Uppsala and Örebro Regional Research Foundation. The Foundation had no involvement in the design and conduct of the study, data analysis or publication.
The authors declared no other conflict of interest. Isacson 2019 The work was supported by research grants from the County of Västmanland and Uppsala–Örebro Regional Research Council, Sweden. The authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in blocks of four and stratified by the centres. The sizes of the blocks were unknown to the participating units. At each centre, a local investigator was responsible for recruiting participants to the trial and controlling the randomisation process. From author: The centre for clinical research performed the randomisation. This centre was independent from the clinic and was not involved in participant recruitment. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were opened, distributed by the Centre for Clinical Research in Väasterås. From author: Envelopes were not possible to see through in order to figure out randomisation group. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not performed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 669 patients were randomised; 46 excluded with adequate reasons (No‐AB: 25 vs. AB: 21). 41 participants were lost to follow‐up after 12 months (No‐AB: 19 vs. AB: 22). In total 87 randomised participants did not complete the trial corresponding to an attrition rate of 13%. This attrition rate is acceptable and reasons for exclusion are all justified. Group sizes with α = 0·05 and a power of 80 per cent were calculated and reported to be 240 in each group which was fulfilled for the analysed study population. Isacson 2019 669 patients were randomised; 46 excluded with adequate reasons (No‐AB: 25 vs. AB: 21). 76 participants were lost to follow‐up. In total, 122 randomised participants did not complete the trial corresponding to an attrition rate of 18.2%. This attrition rate was acceptable and reasons for exclusion were all justified. |
| Selective reporting (reporting bias) | Low risk | Method and outcomes were specified and were available during the study period on clinicaltrials.gov (NCT01008488) In the protocol, there was no planned follow‐up, but the follow‐up assessed the same outcomes as the original study. |
Daniels 2017.
| Study characteristics | ||
| Methods | RCT, multicentre; 22 clinical sites in the Netherlands, from 1 June 2010 to 14 October 2012 Van Dijk 2018: two‐years follow‐up study |
|
| Participants | 570 patients randomised; 42 patients excluded with adequate reasons (no‐AB: 262 vs. AB: 266) Inclusion: Left‐sided, CT‐verified diverticulitis Exclusion: Previously US‐ or CT‐verified diverticulitis, inflammatory bowel disease, complicated diverticulitis, conditions with expected survival < 6 months, pregnancy, breastfeeding, ASA > III, clinical suspicion of sepsis, immunocompromised, AB in four weeks before inclusion. Van Dijk 2018: Sixty participants were lost to follow‐up (No‐AB: 35 vs. AB: 25). Participants included: 468 (No‐AB: 227 vs. AB: 241) |
|
| Interventions |
Intervention: No‐AB. Could be treated directly in an outpatient setting
Control: AB‐group: Amoxicillin‐clavulanic acid, 10‐day course, IV 1200 mg x 4 for at least 48 hours → oral 625 mg x 3 ciprofloxacin and metronidazole The AB treatment led to admission of all participants on the premise that treatment was started IV. |
|
| Outcomes |
Primary: Time to recovery during 6 months of follow‐up (median days) Criteria: discharge from hospital, normal diet, temperature less than 38°C, VAS pain score below 4 (with no use of daily pain medication), and resumption of pre‐illness working activities as assessed by a daily patient diary Secondary: At 2 and 6 months, the participant visited the outpatient clinic; follow‐up at 12 and 24 months was by telephone. A standard case record form was used for collection of study variables. Oracle® Clinical, with internet‐based remote data capture, version 4.5.3 (Oracle, Redwood Shores, California, USA), was used for entering, managing and validating data. 1. Proportion of time outside hospital in the 6‐month period 2. Readmission rate within 6 months 3. Complicated diverticulitis within 6 months (abscess, perforation, obstruction/stricture, diverticular bleeding or fistula) 4. Ongoing diverticulitis within 6 months 5. Recurrent diverticulitis within 6 months 6. Need for sigmoid resection within 6 months:
7. Need for sigmoid resection within 12 months of follow‐up 8. Adverse events 9. All‐cause mortality Van Dijk 2018: Follow‐up was performed by telephone at 12 and 24 months and all hospital records were reviewed. Recurrent diverticulitis was assessed clinically with or without imaging. |
|
| Notes | The DIABOLO trial was funded by the Netherlands Organization for Health Research and Development (ZonMw; 171002303) and the Digestive Diseases Foundation (Maag Lever Darm Sticht‐ ing, MLDS WO08‐54). The funders had no involvement in trial design, conduct or reporting.
The authors declared no conflict of interest. Van Dijk 2018: The authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation controlled centrally by computer system; block randomisation in sizes 2 or 4 |
| Allocation concealment (selection bias) | Low risk | Outcome was generated automatically, thereby preserving concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In total, 570 participants randomised. 70 were excluded with adequate reasons (No‐AB: 34 vs. AB: 36). 16 participants were lost to follow‐up (No‐AB: 6 vs. AB: 10). In total, 86 participants did not complete the trial corresponding to an attrition rate of 15% which was acceptable and reasons for exclusion were justified. Group sizes with α = 0·05 and a power of 99 per cent were calculated and reported to be 262 in each group; which gave sufficient power. Van Dijk 2018: The authors believed that attrition bias could have been introduced in the multiple analyses that were done. They showed an association with earlier dropout of follow‐up in the no‐AB group compared to the AB group, when recovery of the initial diverticulitis episode was prolonged. The authors suggested that the reason for the correlation was the open‐label design. However, baseline characteristics in the 6‐month and the 2‐years follow‐up were comparable. 570 patients randomised. 42 were excluded with adequate reasons and 60 were lost to follow‐up. In total, 102 participants did not complete the trial corresponding to an attrition rate of 17.9% which was acceptable and reasons for exclusion were justified. |
| Selective reporting (reporting bias) | Low risk | Method and primary and secondary outcomes were specified, published and available during the study (Unlu 2010). |
Jaung 2020.
| Study characteristics | ||
| Methods | RCT, multicentre; 4 clinical sites, international (New Zealand and Australia), from December 2015 to May 2019 | |
| Participants | 180 patients randomised in no‐AB (95, 1 withdrawal) and AB (85, 1 withdrawal) Inclusion: Left‐sided, CT‐verified diverticulitis, Hinchey 1a uncomplicated diverticulitis Exclusion: > 1 criterion for systemic inflammatory response syndrome upon presentation to hospital (temperature < 36°C or > 38° C, heart rate > 90 beats/min, respiratory rate > 20 breaths/min or PaCO2 < 32 mmHg, white cell count < 4 or > 12 109/L), unable to give consent or answer symptom‐related questions due to language barrier or cognitive impairment, previous drug reactions to the antibiotics used, lactose allergy, used steroids for > 5 days prior to presentation, administered regular immunomodulators or biologics within the 6 months prior to presentation, NSAID > 1 week prior to presentation, administered > 1 dose of IV or > 2 doses of oral AB during this illness but prior to enrolment in the study, pregnancy, ASA > 3, CT‐verified complicated acute diverticulitis, could not start taking the study medication within 24 hours of their admission into hospital |
|
| Interventions | Placebo‐controlled, double‐blinded, comparing AB with placebo Intervention: AB‐group: Initially IV cefuroxime 750 mg every 6 hours and oral metronidazole 400 mg 3 times a day and oral antibiotics amoxicillin/clavulanic acid 625 mg 3 times a day Control: No‐AB‐group: placebo Participants were prescribed the intravenous or oral regimen at the discretion of the surgical team, with a minimum treatment duration of 5 days of the oral regimen and a maximum treatment duration of 48 hours for the IV regimen and 5 days for the oral regimen (a total of 7 days of study medication). Participants requiring longer durations of treatment were regarded as having delayed recovery and started on conventional management, which included antibiotics. Participants were discharged when they were afebrile on oral study medication, able to tolerate oral diet, able to manage pain exclusively with oral analgesia, and able to mobilise safely and manage their activities of daily living. The final decision on discharge was made by the clinical team who were blinded to allocation status. |
|
| Outcomes |
Primary: Length of hospital admission in hours from registration in emergency department to discharge into the community Secondary: 1. Participant dropout or withdrawal rate 2. Occurrence af adverse events 3. Readmission within 1 week 4. Readmission within 30 days 5. Procedural intervention 6. Mortality 7. Change in serum markers of inflammation 8. Patient‐reported pain score at 12 and 24 hours |
|
| Notes | Rebekah Jaung is a doctoral candidate funded by the Auckland Medical Research Foundation, and the STAND study was funded by a project grant from the Colorectal Surgical Society of Australia and New Zealand. The authors disclosed no conflicts. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based random number generator. Randomisation was blocked into groups of 4 to ensure a comparable allocation to treatment and control groups. |
| Allocation concealment (selection bias) | Low risk | Allocation performed by the external pharmacy where the study medications were manufactured. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, study investigators, and clinical staff were blinded to treatment allocation. The antibiotics and placebo were packaged in identical vials and bottles and labelled with a study identification number. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 180 participants were randomised; 0 were excluded after randomisation. 3 participants were lost to follow‐up (AB: 2 vs. placebo: 1). In total, 3 participants did not complete the trial corresponding to an attrition rate of 1.7% which was acceptable and reasons for exclusion were justified. Group sizes with α = 0·05 and a power of 99 per cent were calculated and reported to be 262 in each group which gave sufficient power. |
| Selective reporting (reporting bias) | Low risk | Method and outcomes were specified and were available during the study period on clinicaltrials.gov, Jaung 2015 |
Kellum 1992.
| Study characteristics | ||
| Methods | Open‐label, RCT, multicentre | |
| Participants | 77 patients randomised, 30 vs. 21 participants Inclusion: Abdominal tenderness and signs of infection: fever/leucocytosis (white blood cells = 9.5 cells/mm3 or more), radiological (CT: colonic wall thickening/increased density of pericolic fat or contrast enema with intramural/extramural tracking/abscess), surgical (3 participants) or pathologic (1 participant) evidence, 2 participants with clinical diagnosis Exclusion: Requirement of immediate emergency surgery, admission creatinine of 3 mg/dL or more, need of additional antibiotics not permitted by the study protocol |
|
| Interventions | Intervention: IV Cefoxitin: 1‐2 g/6h Control: IV gentamicin/clindamycin (1.7 mg/kg followed by 1‐1.4 mg/kg/8h maintenance dose)/(2.4‐2.7 mg/d) Duration of treatment was determined by attending physician based on clinical assessment. | |
| Outcomes | Cured: Resolved clinical findings and discharged with no recurrence for at least 6 weeks or alternatively a candidate for elective surgery with primary anastomosis and no septic complications (wound infection, intra‐abdominal abscess or anastomotic leak) Failure: At least 48 h of AB with subsequent need of emergency surgery or switch of AB. Alternatively, the participants had undergone elective surgery with septic complications following a successful AB | |
| Notes | This study was supported in part by the grant from Merck Sharp and Dohme, West Point, Pennsylvania. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised to receive either cefoxitin (CFX) or a combination of gentamicin and clindamycin (G/C) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26 were deemed non‐evaluable (CFX: 10 vs. G/C: 16). Seven received additional antibiotics not permitted by study protocol. Interpretation: 34% of randomised participants were excluded from analysis. The exclusion was described in numbers and reasons adequately for 19 participants. The reason for the exclusion of the last 7 participants was not reported. Lost to follow‐up during the six weeks described in the methods section was not addressed in the results section. The questionable exclusion of participants, the big exclusion rate, the missing reporting on lost to follow‐up, and the overall small sample size of the study all contributed to high risk attrition bias. Frequency and time points for white blood cell count measurements was not described. This suggests that the stated significant conclusion, that leucocytosis resolved more rapidly in the single‐compound group than in the combination group, questionable. All of the above factors resulted in a high risk of reporting bias. |
| Selective reporting (reporting bias) | Unclear risk | A protocol was mentioned once in the results section. Specification of outcomes was provided in the methods section but they were never described as predefined. The recurrence outcome was never addressed in the results section although mentioned in the methods section. |
Ribas 2010.
| Study characteristics | ||
| Methods | RCT, parallel, multicentre (2 hospitals in Spain), pilot study | |
| Participants | 50 patients randomised, 44 analysed, 22 from each group Inclusion: Abdominal pain localised to left lower quadrant and tenderness at examination, CT (within 24‐48 h) with bowel wall thickness and pericolic fat infiltration Exclusion: Complicated diverticulitis on CT or clinical suspicion |
|
| Interventions |
Intervention: Short IV: IV AB for 24‐48 hours + 10 days of oral AB
Control: Long IV: IV AB for 7 days + 5 days of oral AB Amoxicillin‐clavulanic acid was used for both administration routes and intervention groups with the same dose (1 g/8h). Total duration of treatment was 12 days in both groups. |
|
| Outcomes |
Failure of treatment: (Short IV: 1 vs. long IV: 2) 1. Not able to discharge participant because of symptoms on fourth (short‐IV) or eighth (long‐IV) day 2. Emergency admission after discharge for reasons related to diverticulitis 3. Hospital readmission with the same diagnosis within 30 days Late complications: Colonoscopy 4‐6 weeks after discharge (Short IV: 1 vs. long IV: 1) |
|
| Notes | This study was supported by a grant from the Fundació Joan Costa Roma of the Consorci Sanitari de Terrassa. The authors declare no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by a computer‐generated randomisation list that was prepared by an external observant |
| Allocation concealment (selection bias) | Low risk | Allocated by means of numbered sealed envelopes that corresponded to the randomisation list. The envelopes were opened after the written consent was provided. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout after randomisation: 6 excluded because of withdrawal of consent or different CT diagnosis. No participants were excluded from analysis. 3 participants (Short‐IV: 2 vs. long‐IV: 1) had failure of treatment and could not be discharged. From author: None of the 3 participants required surgery or had re‐evaluation on CT. The participants had persistent pain and therefore could not be discharged on scheduled day. Interpretation: Failure of treatment in participants was not because of complications that required emergency surgery. |
| Selective reporting (reporting bias) | Low risk | No specification of a protocol in the study. All defined outcomes in method section are reported and assessed. From author: "a document [protocol] was written in Catalan. The article summarizes quite well what we did, the inclusion and exclusion criteria, the two groups with different treatments, as well as the outcomes". Interpretation: Relevant outcomes and relevant outcome reporting when combining article and comments plus data from author. |
AB: antibiotic ASA: American Society of Anaesthesiologists physical status classification AVOD: Antibiotika Vid Okomplicerad Divertikulit – Swedish for ‘antibiotics in uncomplicated diverticulitis’ DIABOLO: DIverticulitis AntiBiotic Or cLose Observation IV: intravenous vs.: versus NSAID: nonsteroidal anti‐inflammatory drugs PaCO2: The partial pressure of carbon diaoxid in arterial blood; RCT: randomised controlled trial STAND: Selective Treatment with Antibiotics for Non‐complicated Diverticulitis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Biondo 2014 |
Study description: Multicentre, randomised controlled trial, including patients with CT‐verified acute uncomplicated left‐sided diverticulitis. There were 2 strategies of management: hospitalisation (group 1) and outpatient (group 2). Both groups had the same antibiotic regimen. Reasons for exclusion: Investigating outpatient management and not observational versus antibiotic treatment |
| Colecchia 2007 |
Study description: Multicentre, open‐label, prospective, randomised study. Comparing rifaximin (400 mg for 7 days every month) plus dietary fibre supplementation (at least 20 g/day) and simply dietary fibre supplementation (at least 20 g/day). The study duration was 24 months. Reasons for exclusion: No distinction between acute diverticulitis and diverticular disease |
| Estrada 2016 |
Study description: Patients with mild acute diverticulitis with/or without comorbidities received outpatient treatment without antibiotics. Patients with moderate acute diverticulitis were admitted for 48 hours and then managed as outpatients; they received 10 days of antibiotics. Reasons for exclusion: Not a randomised controlled trial. Investigating outpatient management and not antibiotic treatment |
| Kim 2019 |
Study description: Single‐centre, open‐label, randomised controlled trial, investigating CT‐verified uncomplicated acute diverticulitis in the right‐sided colon. Participants were randomised to observational (bowel rest and IV fluids) and antibiotic (bowel rest, IV fluids, and antibiotics). Clinicians, the data manager, and participants were masked to treatment assignment. The primary outcome was the treatment failure, and secondary outcomes were the length of hospital stay and total admission costs. 61 participants vs. 64 participants. Concluded observational and antibiotic management of uncomplicated right‐sided colonic diverticulitis showed similar treatment failure rates and length of hospital stay. Observational management was associated with lower hospital costs, compared with standard antibiotic treatment. Therefore, observational management can be considered as a safe treatment option. Reasons for exclusion: investigating right‐sided colon |
| Ridgway 2008 |
Study description: randomised controlled trial, comparing intravenous versus oral antibiotic treatment Reasons for exclusion: Diagnosis was based on clinical symptoms only with no radiographic confirmation. |
| Schug‐Pass 2010 |
Study description: Randomised controlled trial comparing treatment with intravenous ertapenem for 4 days vs. 7 days in patients with CT/US‐verified diagnosis of uncomplicated acute diverticulitis. The primary outcome was successful treatment. Reasons for exclusion: All included participants were treated with the same intravenous treatment the first 4 days. Randomisation was performed on day 4, if treatment had been successful. Because the treatment had not been successful after 4 days, 17 participants were excluded from being randomised at day 4, including participants with persisting symptoms and complicated diverticulitis. This design excluded participants of interest for this review before randomisation. The study design introduced selection bias by undermining the concept of randomisation prior to intervention start and by selecting participants for randomisation. Therefore, this study was not considered a randomised controlled trial for this review and did not fulfil the inclusion criteria. |
Vs.: versus
Characteristics of studies awaiting classification [ordered by study ID]
Mora‐Lopez 2021.
| Methods | Randomised controlled trial, triple‐masking (participant, care provider, investigator) |
| Participants | 460 |
| Interventions | No‐antibiotic versus antibiotic |
| Outcomes |
Primary: readmission Secondary: re‐consultation, reason for re‐consultation, reason for readmission, pain control, complications |
| Notes | Protocol for NCT02785549 Registered on clinical trials register 30 May 2016 |
Characteristics of ongoing studies [ordered by study ID]
Al Mansouri 2019.
| Study name | Outpatient treatment of uncomplicated diverticulitis with either antibiotic or nonantibiotic treatment (MUD) |
| Methods | Randomised controlled trial, quadruple‐masking (participant, care provider, investigator, outcomes assessor) |
| Participants | 40 |
| Interventions | No‐antibiotic versus antibiotic |
| Outcomes |
Primary: recruitment rate Secondary: median visual analogue pain scores at 7 days and treatment failures (defined as persistence, increase or recurrence of abdominal pain and/or fever, inflammatory bowel obstruction, need for radiological abscess drainage or immediate surgery due to complicated diverticulitis, need for hospital admission, and mortality during the first 60 days after discharge) |
| Starting date | April 9, 2018 |
| Contact information | sarah.samfaris@gmail.com |
| Notes | MUD trial, registered on clinical trials register May 9 2017 Al‐Mansouri 2019: conference abstract for NCT03146091 at 2019 Scientific Session of the Society of American Gastrointestinal and Endoscopic Surgeons, SAGES. United States |
Kruis 2019.
| Study name | Novel rifamycin Sv multi‐matrix formulation for treatment of acute uncomplicated diverticulitis: results of a prospective double‐blind, placebo‐controlled, randomized study |
| Methods | Randomised controlled trial, quadruple‐blinded (participant, care provider, investigator, outcomes assessor) |
| Participants | 201 |
| Interventions | Rifamycin 400 mg vs. rifamycin 600 mg vs. placebo (controls) |
| Outcomes | Primary: Treatment success at day 10 Secondary: Complete treatment success |
| Starting date | August 2013 |
| Contact information | Not noted |
| Notes | Completed July 2017 |
Differences between protocol and review
The title was changed from 'Antibiotics for uncomplicated acute diverticulitis' to 'Antibiotics for uncomplicated diverticulitis' because we believe the term 'acute' is superfluous since diverticulitis is always acute.
A language restriction to English, German, Danish, Norwegian and Swedish trials was stated in the protocol, but all relevant articles were identified regardless of language.
Both RCTs and quasi‐RCTs could be included, but we only identified RCTs in the present search.
No numbers needed to treat for an additional beneficial outcome (NNTB) were calculated as stated in the protocol.
The number of secondary outcomes was increased from six to eight and the phrasing was sharpened; since the protocol was published, the clinic has changed, which the updated secondary outcomes reflects. A distinction between emergency and elective colon resection and adverse events was added. Furthermore, the item, '7. Length to recovery of symptoms (days)' secondary outcome replaced two original outcomes, the '3. Time to recovery of clinical signs' and '4. Time to recovery of signs of infection (fever, leucocytosis, CRP, ESR)' in the protocol.
Contributions of authors
MLD: searches, data extraction, analyses, interpretation of results, drafting of manuscript SJR: interpretation of results, critical review of manuscript DMS: protocol (design and method), searches, data extraction, interpretation of results, critical review of manuscript
Sources of support
Internal sources
-
No source of support , Denmark
This systematic review and meta‐analysis has no source of support,
External sources
-
No source of support , UK
This systematic review and meta‐analysis has no source of support
Declarations of interest
No declarations of interest
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Chabok 2012 {published data only}
- Chabok A, Påhlman L, Hjern F, Haapaniemi S, Smedh K. Randomized clinical trial of antibiotics in acute uncomplicated diverticulitis. British Journal of Surgery 2012;99(4):532-9. [DOI] [PubMed] [Google Scholar]
- Isacson D, Smedh K, Nikberg M, Chabok A. Long-term follow-up of the AVOD randomized trial of antibiotic avoidance in uncomplicated diverticulitis. British Journal of Surgery 2019;106(11):1542-8. [DOI] [PubMed] [Google Scholar]
Daniels 2017 {published data only}
- Daniels L, Unlu C, De Korte N, Van Dieren S, Stockmann HB, Vrouenraets B, et al. Randomized clinical trial of observational versus antibiotic treatment for a first episode of CT-proven uncomplicated acute diverticulitis. British Journal of Surgery 2017;104(1):52-61. [DOI] [PubMed] [Google Scholar]
- Van Dijk ST, Daniels L, Unlu C, De Korte N, Van Dieren S, Stockmann HB, et al. Long-term effects of omitting antibiotics in uncomplicated acute diverticulitis. American Journal of Gastroenterology 2018;113(7):1045-52. [DOI] [PubMed] [Google Scholar]
Jaung 2020 {published and unpublished data}
- Jaung R, Nisbet S, Gosselink MP, Di Re A, Keane C, Lin A, et al. Antibiotics do not reduce length of hospital stay for uncomplicated diverticulitis in a pragmatic double-blind randomized trial. Clinical Gastroenterology & Hepatology 2020;19(3):503-10. [DOI] [PubMed] [Google Scholar]
Kellum 1992 {published data only}
- Kellum JM, Sugerman HJ, Coppa GF, Way LR, Fine R, Herz B, et al. Randomized, prospective comparison of cefoxitin and gentamicin-clindamycin in the treatment of acute colonic diverticulitis. Clinical Therapeutics 1992;14(3):376-84. [PubMed] [Google Scholar]
Ribas 2010 {published and unpublished data}
- Ribas Y, Bombardó J, Aguilar F, Jovell E, Alcantara-Moral M, Campillo F, et al. Prospective randomized clinical trial assessing the efficacy of a short course of intravenously administered amoxicillin plus clavulanic acid followed by oral antibiotic in patients with uncomplicated acute diverticulitis. International Journal of Colorectal Disease 2010;25(11):1363-70. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Biondo 2014 {published data only}
- Biondo S, Golda T, Kreisler E, Espin E, Vallribera F, Oteiza F, et al. Outpatient versus hospitalization management for uncomplicated diverticulitis: a prospective, multicenter randomized clinical trial (DIVER trial). Annals of Surgery 2014;259(1):38–44. [DOI] [PubMed] [Google Scholar]
Colecchia 2007 {published data only (unpublished sought but not used)}
- Colecchia A, Vestito A, Pasqui F, Mazzella G, Roda E, Pistola F, et al. Efficacy of long term cyclic administration of the poorly absorbed rifaximin in symptomatic, uncomplicated colonic diverticular disease. World Journal of Gastroenterology 2017;13(2):264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Estrada 2016 {published data only}
- Estrada Ferrer O, Ruiz Edo N, Hidalgo Grau LA, Abadal Prades M, Del Bas Rubia M, Garcia Torralbo EM, et al. Selective non-antibiotic treatment in sigmoid diverticulitis: is it time to change the traditional approach? Techniques in Coloproctology 2016;20(5):309-15. [DOI] [PubMed] [Google Scholar]
Kim 2019 {published data only}
- Kim JY, Park SG, Kang HJ, Lim YA, Pak KH, Yoo T, et al. Prospective randomized clinical trial of uncomplicated right-sided colonic diverticulitis: antibiotics versus no antibiotics. International Journal of Colorectal Disease 2019;34(8):1413-20. [DOI] [PubMed] [Google Scholar]
Ridgway 2008 {published data only}
- Ridgway PF, Latif A, Shabbir J, Ofriokuma F, Hurley MJ, Evoy D, et al. Randomized controlled trial of oral vs intravenous therapy for the clinically diagnosed acute uncomplicated diverticulitis. Colorectal Disease 2008;11(9):941-6. [DOI] [PubMed] [Google Scholar]
Schug‐Pass 2010 {published data only}
- Schug-Pass C, Geers P, Hügel O, Lippert H, Köckerling F. Prospective randomized trial comparing short-term antibiotic therapy versus standard therapy for acute uncomplicated sigmoid diverticulitis. International Journal of Colorectal Disease 2010;25(6):751-9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Mora‐Lopez 2021 {published data only}
- Mora-López L, Ruiz-Edo N, Estrada-Ferrer O, Piñana-Campón ML, Labró-Ciurans M, Escuder-Perez J, et al. Efficacy and safety of nonantibiotic outpatient treatment in mild acute diverticulitis (DINAMO-study): a multicentre, randomised, open-label, noninferiority trial. Annals of Surgery 2021;274(5):e435-42. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Mora Lopez L, Ruiz-Edo N, Serra Pla S, Pallisera Llovera A, Navarro Soto S, Serra-Aracil X. Multicentre, controlled, randomized clinical trial to compare the efficacy and safety of ambulatory treatment of mild acute diverticulitis without antibiotics with the standard treatment with antibiotics. International Journal of Colorectal Disease 2017;32(10):1509-16. [DOI: 10.1007/s00384-017-2879-4] [EMBASE: 618080522] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Al Mansouri 2019 {published and unpublished data}
- Al-Mansouri S, Salama E, Lachance S, Faris-Sabboobeh S, Savard J, Morin N, et al. A North American single-blinded pilot randomized controlled trial for outpatient non-antibiotic management of acute uncomplicated diverticulitis (MUD trial): feasibility and lessons learned. Surgical Endoscopy 2019;33:S234. [DOI: 10.1007/s00464-019-06704-2] [DOI] [Google Scholar]
- NCT03146091. Outpatient treatment of uncomplicated diverticulitis with either antibiotic or nonantibiotic treatment (MUD). clinicaltrials.gov/ct2/show/NCT03146091 (first received 9 May 2017).
Kruis 2019 {published data only}
- Kruis W, Poskus T, Boehm G, Bunganic I, Racz I, Fratila O, et al. Novel rifamycin sv multi-matrix formulation for treatment of acute uncomplicated diverticulitis: results of a prospective double-blind, placebo-controlled, randomized study. Gastroenterology 2019;156 (6 Suppl 1):S-1110-1. [EMBUI: 2001915811] [Google Scholar]
Additional references
Ambrosetti 2016
- Ambrosetti P. Acute left-sided colonic diverticulitis: clinical expressions, therapeutic insights, and role of computed tomography. Clinical and Experimental Gastroenterology 2016;9:249-57. [DOI: 10.2147/CEG.S110428.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersen 2012
- Andersen JC, Bundgaard L, Elbrond H, Laurberg S, Walker LR, Stovring J. Danish national guidelines for treatment of diverticular disease. Danish Medical Journal 2012;59(5):C4453. [PubMed] [Google Scholar]
Andeweg 2013
- Andeweg CS, Mulder IM, Felt-Bersma RJ, Verbon A, Van der Wilt GJ, Van Goor H, et al. Guidelines of diagnostics and treatment of acute left-sided colonic diverticulitis. Digestive Surgery 2013;30(4-6):278-92. [DOI: 10.1159/000354035] [DOI] [PubMed] [Google Scholar]
Aune 2017
- Aune D, Sen A, Leitzmann MF, Tonstad S, Norat T, Vatten LJ. Tobacco smoking and the risk of diverticular disease - a systematic review and meta-analysis of prospective studies. Colorectal Disease 2017;19(7):621-33. [DOI] [PubMed] [Google Scholar]
Berman 1968
- Berman LG, Burdick D, Heitzman ER, Prior JT. A critical reappraisal of sigmoid peridiverticulitis. Surgery, Gynecology & Obstetrics 1968;127(3):481-91. [PubMed] [Google Scholar]
Bharucha 2015
- Bharucha AE, Parthasarathy G, Ditah I, Fletcher JG, Ewelukwa O, Pendlimari R, et al. Temporal trends in the incidence and natural history of diverticulitis: a population-based study. American Journal of Gastroenterology 2015;110(11):1589-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Binda 2015
- Binda GA, Cuomo R, Laghi A, Nascimbeni R, Serventi A, Bellini D, et al. Practice parameters for the treatment of colonic diverticular disease: Italian Society of Colon and Rectal Surgery (SICCR) guidelines. Techniques in Coloproctology 2015;19(10):615-26. [DOI] [PubMed] [Google Scholar]
Brochmann 2016
- Brochmann ND, Schultz JK, Jakobsen GS, Oresland T. Management of acute uncomplicated diverticulitis without antibiotics: a single-centre cohort study. Colorectal Disease 2016;18(11):1101-7. [DOI] [PubMed] [Google Scholar]
Cao 2018
- Cao Y, Strate LL, Keeley BR, Tam I, Wu K, Giovannucci EL, et al. Meat intake and risk of diverticulitis among men. Gut 2018;67(3):466-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chabok 2010
- Chabok A, Tarnberg M, Smedh K, Pahlman L, Nilsson LE, Lindberg C, et al. Prevalence of fecal carriage of antibiotic-resistant bacteria in patients with acute surgical abdominal infections. Scandinavian Journal of Gastroenterology 2010;45(10):1203-10. [DOI] [PubMed] [Google Scholar]
De Korte 2011
- De Korte N, Unlu C, Boermeester MA, Cuesta MA, Vrouenreats BC, Stockmann HB. Use of antibiotics in uncomplicated diverticulitis. British Journal of Surgery 2011;98(6):761-7. [DOI] [PubMed] [Google Scholar]
Dellit 2007
- Dellit TH, Owens RC, McGowan JE, Gerding DN, Weinstein RA, Burke JP, et al. Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clinical Infectious Diseases 2007;44(2):159–77. [DOI: 10.1086/510393] [DOI] [PubMed] [Google Scholar]
Desai 2019
- Desai M, Fathallah J, Nutalapati V, Saligram S. Antibiotics versus no antibiotics for acute uncomplicated diverticulitis: a systematic review and meta-analysis. Diseases of the Colon and Rectum 2019;62(8):1005-12. [DOI: 10.1097/DCR.0000000000001324] [DOI] [PubMed] [Google Scholar]
Dughera 2004
- Dughera L, Serra AM, Battaglia E, Tibaudi D, Navino M, Emanuelli G. Acute recurrent diverticulitis is prevented by oral administration of a polybacterial lysate suspension. Minerva Gastroenterologica e Dietologica 2004;50(2):149-53. [PubMed] [Google Scholar]
Everhart 2009
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part II: lower gastrointestinal diseases. Gastroenterology 2009;136(3):741-54. [DOI] [PubMed] [Google Scholar]
Francis 2019
- Francis NK, Sylla P, Abou-Khalil M, Arolfo S, Berler D, Curtis N, et al . EAES and SAGES 2018 consensus conference on acute diverticulitis management: evidence-based recommendations for clinical practice. Surgical Endoscopy 2019;33(9):2726-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friend 2011
- Friend K, Mills AM. Is outpatient oral antibiotic therapy safe and effective for the treatment of acute uncomplicated diverticulitis? Annals of Emergency Medicine 2011;57(6):600-2. [DOI] [PubMed] [Google Scholar]
Goldstein 2011
- Goldstein EJC. Beyond the target pathogen: ecological effects of the hospital formulary. Current Opinion in Infectious Diseases 2011;24(Suppl 1):S21-S31. [DOI: 10.1097/01.qco.0000393485.17894.4c] [DOI] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Hjern 2007
- Hjern F, Josephson T, Altman D, Holmström B, Mellgren A, Pollack J, et al. Conservative treatment of acute colonic diverticulitis: are antibiotics always mandatory? Scandinavian Journal of Gastroenterology 2007;42(1):41-7. [DOI: 10.1080/00365520600780650] [DOI] [PubMed] [Google Scholar]
Humes 2012
- Humes DJ, West J. Role of acute diverticulitis in the development of complicated colonic diverticular disease and 1-year mortality after diagnosis in the UK: population-based cohort study. Gut 2012;61(1):95-100. [DOI] [PubMed] [Google Scholar]
Humes 2014
- Humes DJ, Spiller RC. Review article: the pathogenesis and management of acute colonic diverticulitis. Alimentary Pharmacology and Therapeutics 2014;39(4):359-70. [DOI] [PubMed] [Google Scholar]
Hupfeld 2017
- Hupfeld L, Burcharth J, Pommergaard HC, Rosenberg J. Risk factors for recurrence after acute colonic diverticulitis: a systematic review. International Journal of Colorectal Disease 2017;32(5):611-22. [DOI] [PubMed] [Google Scholar]
Iannone 2018
- Iannone A, Ruospo M, Wong G, Barone M, Principi M, Di Leo A, et al . Mesalazine for people with diverticular disease: a systematic review of randomized controlled trials. Canadian Journal of Gastroenterology & Hepatology 2018;5437135:12. [DOI: 10.1155/2018/5437135] [DOI] [PMC free article] [PubMed] [Google Scholar]
Isacson 2014
- Isacson D, Andreasson K, Nikberg M, Smedh K, Chabok A . No antibiotics in acute uncomplicated diverticulitis: does it work? Scandinavian Journal of Gastroenterology 2014;49(12):1441-6. [DOI] [PubMed] [Google Scholar]
Jacobs 2007
- Jacobs DO. Clinical practice. Diverticulitis. New England Journal of Medicine 2007;357(20):2057-66. [DOI] [PubMed] [Google Scholar]
Kang 2004
- Kang JY, Melville D, Maxwell JD. Epidemiology and management of diverticular disease of the colon. Drugs & Aging 2004;21(4):211-28. [DOI: 10.2165/00002512-200421040-00001] [DOI] [PubMed] [Google Scholar]
Kruis 2014
- Kruis W, Germer CT, Leifeld L. Diverticular disease: guidelines of the German society for Gastroenterology, Digestive and Metabolic Diseases and the German Society for General and Visceral Surgery. Digestion 2014;90(3):190-207. [DOI] [PubMed] [Google Scholar]
Lanas 2013
- Lanas A, Ponce J, Bignamini A, Mearin F. One year intermittent rifaximin plus fibre supplementation vs. fibre supplementation alone to prevent diverticulitis recurrence: a proof-of-concept study. Digestive and Liver Disease 2013;45(2):104–9. [DOI: 10.1016/j.dld.2012.09.006] [DOI] [PubMed] [Google Scholar]
Mege 2019
- Mege D, Yeo H. Meta-analyses of current strategies to treat uncomplicated diverticulitis. Diseases of the Colon and Rectum 2019;62(3):371-8. [DOI] [PubMed] [Google Scholar]
Miura 2000
- Miura S, Kodaira S, Shatari T, Nishioka M, Hosoda Y, Hisa TK. Recent trends in diverticulosis of the right colon in Japan: retrospective review in a regional hospital. Diseases Of The Colon And Rectum 2000;43(10):1383-9. [DOI: 10.1007/BF02236634] [DOI] [PubMed] [Google Scholar]
Moreno 2007
- Moreno AM, Wille-Jørgensen P. Long-term outcome in 445 patients after diagnosis of diverticular disease. Colorectal Disease 2007;9(5):464-8. [DOI: 10.1111/j.1463-1318.2006.01173.x] [DOI] [PubMed] [Google Scholar]
Morson 1975
- Morson BC. Pathology of diverticular disease of the colon. Clinics in Gastroenterology 1975;4(1):37-52. [PubMed] [Google Scholar]
Nakaji 2002
- Nakaji S, Danjo K, Munakata A, Sugawara K, MacAuley D, Kernohan G, et al. Comparison of etiology of right-sided diverticula in Japan with that of left-sided diverticula in the West. International Journal Of Colorectal Disease 2002;17(6):365-73. [DOI: 10.1007/s00384-002-0403-x] [DOI] [PubMed] [Google Scholar]
NCT01008488
- NCT01008488. Antibiotic therapy of acute uncomplicated colonic diverticulitis. clinicaltrials.gov/ct2/show/NCT01008488 (first received 5 November 2009).
Peery 2016
- Peery AF, Keku TO, Martin CF, Eluri S, Runge T, Galanko JA, et al. Distribution and characteristics of colonic diverticula in a United States screening population. Clinical Gastroenterology and Hepatology 2016;14(7):980-5. [DOI: 10.1016/j.cgh.2016.01.020] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 5.4.1 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4.1. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rezapour 2018
- Rezapour M, Stollman N. Antibiotics in uncomplicated acute diverticulitis: to give or not to give? Inflammatory Intestinal Diseases 2018;3(2):75-9. [DOI: 10.1159/000489631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 1995
- Roberts P, Abel M, Rosen L, Cirocco W, Fleshman J, Leff E, et al . Practice parameters for sigmoid diverticulitis. The Standards Task Force American Society of Colon and Rectal Surgeons. Diseases of the Colon and Rectum 1995;38(2):125-32. [DOI: 10.1007/BF02052438.] [DOI] [PubMed] [Google Scholar]
Rottier 2019
- Rottier SJ, Van Dijk ST, Van Geloven AA, Schreurs WH, Draaisma WA, Van Enst WA, et al . Meta-analysis of the role of colonoscopy after an episode of left-sided acute diverticulitis. British Journal of Surgery 2019;106(8):988-97. [DOI: 10.1002/bjs.11191] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ryan 1983
- Ryan P. Changing concepts in diverticular disease. Diseases of the Colon and Rectum 1983;26(1):12-8. [DOI: 10.1007/BF02554670] [DOI] [PubMed] [Google Scholar]
Sartelli 2010
- Sartelli M. A focus on intra-abdominal infections. World Journal of Emergency Surgery 2010;5:9. [DOI: 10.1186/1749-7922-5-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Shahedi 2013
- Shahedi K, Fuller G, Bolus R, Cohen E, Vu M, Shah R, et al. Long-term risk of acute diverticulitis among patients with incidental diverticulosis found during colonoscopy. Clinical Gastroenterology and Hepatology 2013;11(12):1609-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sher 1997
- Sher ME, Agachan F, Bortul M, Nogueras JJ, Weiss EG, Wexner SD. Laparoscopic surgery for diverticulitis. Surgical Endoscopy 1997;11(3):264-7. [DOI] [PubMed] [Google Scholar]
Slack 1962
- Slack WW. The anatomy, pathology, and some clinical features of diverticulitis of the colon. British Journal of Surgery 1962;50:185-90. [DOI] [PubMed] [Google Scholar]
Stollman 2004
- Stollman N, Raskin JB. Diverticular disease of the colon. Lancet 2004;363(9409):631-9. [DOI: 10.1016/S0140-6736(04)15597-9] [DOI] [PubMed] [Google Scholar]
Stollman 2015
- Stollman N, Smalley W, Hirano I. American Gastroenterological Association Institute guideline on the management of acute diverticulitis. Gastroenterology 2015;149(7):1944-9. [DOI] [PubMed] [Google Scholar]
Strate 2009
- Strate LL, Liu YL, Aldoori WH, Giovannucci EL. Physical activity decreases diverticular complications. American Journal of Gastroenterology 2009;104(5):1221-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strate 2011
- Strate LL, Liu YL, Huang ES, Giovannucci EL, Chan AT. Use of aspirin or nonsteroidal anti-inflammatory drugs increases risk for diverticulitis and diverticular bleeding. Gastroenterology 2011;140(5):1427-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strate 2017
- Strate LL, Keeley BR, Cao Y, Wu K, Giovannucci EL, Chan AT. Western dietary pattern increases, and prudent dietary pattern decreases, risk of incident diverticulitis in a prospective cohort study. Gastroenterology 2017;152(5):1023-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strate 2019
- Strate LL, Morris AM. Epidemiology, pathophysiology, and treatment of diverticulitis. Gastroenterology 2019;156(5):1282-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Szojda 2007
- Szojda MM, Cuesta MA, Mulder CM, Felt-Bersma RJ. Review article: management of diverticulitis. Alimentary Pharmacology & Therapeutics 2007;26 (Suppl 2):67-76. [DOI] [PubMed] [Google Scholar]
Unlu 2010
- Unlu C, De Korte N, Daniels L, Consten EC, Cuesta MA, Gerhards MF, et al. A multicenter randomized clinical trial investigating the cost-effectiveness of treatment strategies with or without antibiotics for uncomplicated acute diverticulitis (DIABOLO trial). BMC Surgery 2010;10:23. [DOI: 10.1186/1471-2482-10-23.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Urushidani 2018
- Urushidani S, Kuriyama A, Matsumura M. 5-aminosalicylic acid agents for prevention of recurrent diverticulitis: a systematic review and meta-analysis. Journal of Gastroenterology & Hepatology 2018;33(1):12-9. [DOI] [PubMed] [Google Scholar]
Viniol 2014
- Viniol A, Keunecke C, Biroga T, Stadje R, Dornieden K, Bosner S, et al . Studies of the symptom abdominal pain - a systematic review and meta-analysis. Family Practice 2014;31(5):517-29. [DOI] [PubMed] [Google Scholar]
Violi 2018
- Violi A, Cambie G, Miraglia C, Barchi A, Nouvenne A, Capasso M, et al . Epidemiology and risk factors for diverticular disease. Acta Bio-Medica 2018;89(9-s):107-12. [DOI: 10.23750/abm.v89i9-S.7924] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2019
- World Health Organization. Antimicrobial resistance. www.who.int/en/news-room/fact-sheets/detail/antimicrobial-resistance (accessed 2019).
Wolf 1957
- Wolf BS, Khilnani M, Marshak RH. Diverticulosis and diverticulitis: Roentgen findings and their interpretation. American Journal of Roentgenology, Radium Therapy, and Nuclear Medicine 1957;77(4):726-43. [PubMed] [Google Scholar]
References to other published versions of this review
Shabanzadeh 2011
- Shabanzadeh DM. Antibiotics for uncomplicated acute diverticulitis. Cochrane Database of Systematic Reviews 2011, Issue 4. Art. No: CD009092. [DOI: 10.1002/14651858.CD009092] [DOI] [PubMed] [Google Scholar]
Shabanzadeh 2012
- Shabanzadeh DM, Wille-Jorgensen P. Antibiotics for uncomplicated diverticulitis. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD009092. [DOI: 10.1002/14651858.CD009092] [DOI] [PubMed] [Google Scholar]


