Abstract
For poor sleep quality (SQ) as well as major depressive disorder (MDD) and burnout, a dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis has been identified. Although poor SQ is often reported as an early symptom of MDD or burnout, it is not clear whether HPA axis-related hormones can influence the association between SQ and MDD or burnout. This manuscript addresses this question by examining HPA axis-related hormones as potential moderators influencing the association between SQ and MDD or burnout.
In the fourth annual examination wave of the Dresden Burnout Study, we measured general SQ (including sleep duration and efficiency), depressive and burnout symptoms, and obtained hair samples for quantification of long-term integrated steroid concentrations (cortisol [hC], cortisone [hCn], dehydroepiandrosterone [hDHEA]) from 462 participants (67% female). Data on SQ, depressive and burnout symptoms were available from 342 participants from the preceding examination wave (average time span between examinations 13.2 months).
Cross-sectional analyses showed that the negative association between sleep duration and depressive symptoms was buffered by higher levels of hC, and hCn, whereas the negative association between sleep duration and burnout symptoms was buffered by higher levels of hDHEA. The negative association between sleep efficiency and burnout symptoms was intensified by higher levels of hC and hC/hCn ratio and the negative association between general SQ and burnout symptoms was intensified by higher levels of hC/hCn ratio. With regard to longitudinal data, a significant interaction effect between sleep duration and hC/hCn ratio could be detected for burnout symptoms.
Our results suggest opposed moderation effects of hair glucocorticoids on the association between SQ and depressive or burnout symptoms. This points toward opposed glucocorticoid receptor functioning in depression and burnout. To fully elucidate the negative consequences of poor SQ on MDD and burnout, the complex underlying mechanisms of action including HPA axis-related hormones need to be investigated in MDD and burnout separately.
Keywords: Sleep disorders, Sleep quality, Depressive disorders, Burnout, Cortisol, DHEA
Highlights
-
•
hC and hDHEA are not directly related to depression, burnout, or sleep symptoms.
-
•
Higher hC levels buffer the relation between poor sleep quality (SQ) and depression.
-
•
Higher hC levels intensify the relation between poor SQ and burnout.
-
•
Higher hDHEA levels buffer the relation between poor SQ and burnout.
-
•
hC and hDHEA exhibit contrary effects on the relation between SQ and burnout.
1. Introduction
Sleep quality (SQ) is considered an important marker of psychological health [16], while sleep disorders, often encountered in the form of insomnia, are defined by persisting difficulties falling asleep, sleeping through, or reduced SQ [55]. Sleep disorders are one of the most common health impairments affecting up to 20% of the population worldwide [14,41], while they are an early symptom of depressive disorders or burnout [46].
Lifetime prevalence of major depressive disorder (MDD) is 20.6% [25] and is therefore comparable to prevalence of sleep disorders. MDD is characterized in the Diagnostic and Statistical Manual of mental disorders 5 (DSM-5) by one of its two cardinal symptoms (depressed mood or loss of pleasure and interest) in combination with at least four out of seven additional symptoms. Out of those seven additional symptoms, insomnia or hypersomnia is very frequently reported by patients suffering from MDD [5]). Bidirectional cross-sectional and longitudinal associations between depressive symptoms and SQ are well established (e.g. [46]).
The burnout syndrome is characterized as a work-related construct described by three dimensions: emotional exhaustion, depersonalization, and reduced professional efficacy [36]. Due to the lack of clear diagnostic criteria and resulting large heterogeneity of assessment methods, attempts to systematically identify prevalence rates of burnout in the general population have failed [45]. Since the burnout syndrome is not considered an independent mental disorder, and shows overlapping symptoms with MDD, there is much debate, whether burnout could be a MDD subtype [9]. With regard to sleep, Giorgi et al. [21] showed a link between impaired SQ, sleep disturbances, and resulting daytime dysfunction and a higher risk for burnout syndromes in nurses. With regard to the direction of association between SQ and burnout symptoms, Rothe et al. [46] reported no higher risk for a burnout syndrome due to sleep disturbances, while burnout symptoms predict sleep disturbances. By contrast, Armon et al. [7] showed that the burnout syndrome predicts insomniac symptoms and vice versa.
Previous research identified associations between SQ and neuroendocrine dysregulations, like adaptations in the hypothalamus-pituitary-adrenal (HPA) axis activity. The HPA axis is one of the primary stress systems in the human organism, secreting both cortisol (metabolized into cortisone), as well as dehydroepiandrosterone (DHEA). The HPA axis influences SQ via the control of the sleep-wake cycle with clear circadian rhythmicity exhibiting lowest cortisol levels during the night, while with awakening a steep increase is observable with subsequent continuously declining levels over the day [48]. Similarly, DHEA shows a diurnal pattern following that one of cortisol but being less pronounced and responsive to external stressors [29]. Although behavioral modulation by DHEA is still insufficiently understood, DHEA as an androgenic and anabolic compound, has been suggested to act as a counter-regulatory agent buffering the effects of catabolic glucocorticoids [18,35].
A recent systematic review examining long-term integrated cortisol levels from hair (hC) in individuals with MDD or burnout, describes mixed results for MDD-healthy control contrasts [47]. For burnout the one existing study suggests increased hC levels in individuals with severe burnout symptoms compared to individuals with no or moderate burnout symptoms [42]. With regard to hair DHEA concentration (hDHEA), a negative association between depressive symptoms and levels of hDHEA was reported for a large female sample [52]. Studies examining hDHEA in individuals with burnout or relating it to continuous burnout measures are lacking.
The REM (rapid eye movement) sleep emotional homeostasis hypothesis by Goldstein and Walker [22] substantiated an interaction between SQ and cortisol for the development and maintenance of MDD. The included assumption of REM sleep recalibration means that REM sleep before an emotional event recalibrates the sensitivity of the brain to an emotional event. During REM sleep, the activity of the locus coeruleus is reduced resulting in a decreased release of noradrenaline [34]. This leads to a reduced medial prefrontal cortex top-down control of the amygdala. Therefore, sensitivity to non-salient information is increased and emotional salience discrimination is impaired [22]. In turn, changes in emotion perception are associated with depressive symptoms [32,44]. Studies showed that individuals with MDD show a faster start into and a longer duration of REM sleep [6,23] strengthening this cascade. In addition, previous research indicated that administration of ACTH and cortisol leads to reduced REM sleep [11,28]. Consequently, we hypothesized, that a negative association between SQ and depressive symptoms could be decreased by higher levels of cortisol due to the associated reduction of REM sleep. Although literature examining the effects of DHEA on REM sleep is scarce, a prior study observed an increase of REM sleep after DHEA administration suggesting higher levels DHEA to be associated with more REM sleep [19]. However, it is important to emphasize that regardless of hormonal correlates, reduced SQ increases depressive or burnout symptoms directly due to increased fatigue and reduced work or interaction capacity. Therefore, stress-associated hormones such as cortisol or DHEA can only be understood as moderators of this relationship.
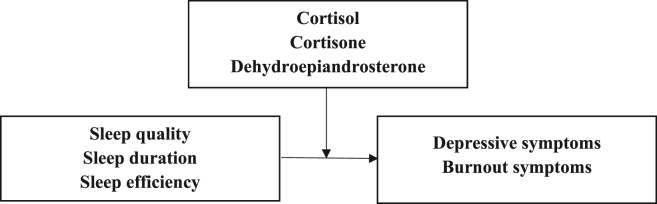
However, in an exploratory cross-sectional study including 68 female hospital workers, Wang et al. [53] examined hC as a potential mediator for the association between emotional exhaustion or depersonalization and insomnia. Although the authors report that hC resulted as a mediator between burnout symptoms and sleep quality, these findings must be viewed critically because numerous relevant covariates such as tobacco use, alcohol consumption, medication intake, or hair treatments, which have been shown to influence hC, were not controlled due to the small sample size. Due to the global effects of cortisol and DHEA in the human organism, no direct mediation assumptions but only moderation assumptions can be made. We, therefore, aimed to examine moderating effects of various HPA axis-related hormones in a significantly larger sample with more control of confounding factors than Wang et al. [53] and complement it with longitudinal analyses (see Fig. 1).
Fig. 1.
Conceptual framework of moderation model - sleep quality dimensions as independent variables and psychological constructs as dependent variables, stress-related hormones as moderator variables.
In the present study, we pursued the following objectives: First, to examine cross-sectional associations between sleep dimensions and depressive or burnout symptoms and hypothesized negative associations between these constructs. Second, to investigate cross-sectional associations between sleep dimensions and HPA axis-related hormones (hC, hair cortisone [hCn], hDHEA), and between these hormones and depressive or burnout symptoms. Based on the previously reported mixed results, we hypothesized that there would be no associations between these hormones and constructs. Third, to analyze potential moderation effects of HPA axis-related hormones on associations between sleep dimensions and depressive or burnout symptoms in cross-sectional and longitudinal models. Considering our assumption with regard to the REM sleep emotional homeostasis hypothesis, we hypothesized a buffering effect of higher levels of hC for the associations between reduced SQ and depressive symptoms and an intensifying effect of hDHEA for these associations. With regard to burnout symptoms, no clear assumptions could be drawn resulting in exploratory examinations.
2. Methods
2.1. Study procedure and participants
To investigate the formulated research questions, data from preceding online assessments as well as the fourth annual wave of the in-person biomarker assessment, took place from October to December 2018, of the Dresden Burnout Study (DBS) was used. Study design, recruitment strategy and data collection are described in detail by Penz et al. [43]. In brief, the DBS, a prospective cohort study, approved by the local ethics committee and conducted in accordance with the Declaration of Helsinki, investigates biopsychosocial determinants of burnout. Inclusion criteria for participation in the DBS are age between 18 and 68 years and good German language knowledge to be able to complete questionnaires. Registration and online data collection started in January 2015, whereas the first wave of the biomarker assessment started in October 2015. Following an annual schedule, the fourth wave of biomarker assessment was conducted from October to December 2018. A week prior the biomarker assessment, where hair samples were collected, participants completed online questionnaires for assessing sociodemographic, health-related and hair-specific variables, as well as SQ and depressive and burnout symptoms after login in to the study homepage (www.dresdner-burnout-studie.de) by use of a personalized login. Participants were reimbursed with a cinema voucher or cash worth 15 € for study participation.
Participants were included in data analysis, if they were employed in a full-time or part-time job. In addition, only participants with complete data on questionnaires for depressive or burnout symptoms were included in the analyses. From initially 526 participants presenting a subsample of the examinations by Rothe et al. [46] n = 63 were not working in full- or part-time, and one participant did not answer the questionnaires. This resulted in a study sample of N = 462 (Mage = 43.7; SDage = 11.6; nfemale = 319 ≙ 69,0%). Study sample characteristics are described in Table 1. Data on SQ, depressive and burnout symptoms were available from 342 participants from preceding examination waves. Thus, for the longitudinal analyses, questionnaire data of n = 342 (Mage = 43.9; SDage = 11.6; nfemale = 242 ≙ 70.8%) participants collected during a preceding online assessment was used. For a detailed comparison of the 342 participants providing longitudinal data with the 120 participants only providing cross-sectional data stemming from the fourth wave of biomarker sampling see supplementary Table 1. No differences in continued and added participants regarding sociodemographic, health-related or clinical data or hair hormone concentrations assessed during the biomarker assessment could be detected. To limit the variance in time differences in answering the online questionnaires for longitudinal analyses, only participants were included that completed questionnaires at least six to a maximum of 18 months prior the fourth wave of biomarker assessment. On average, the participants filled out the questionnaires M = 400.7 days (SD = 77.5; nmin = 200; nmax = 533) ≙ 13.2 months before.
Table 1.
Sociodemographic, health-related and hair treatment data and descriptive results of Pittsburgh Sleep Quality Index total score and dimensions sleep duration and efficiency, Patient Health Questionnaire 9 total score, Maslach Burnout Inventory-General Survey total score and hair steroids of the total sample (N = 462).
| Range | ||
|---|---|---|
| Sociodemographics | ||
| Age in years1, M (SD) | 43.7 (11.6) | 20–70 |
| Sex, female, n (%) | 319 (69.0) | |
| Employment, n (%) | ||
| Full-time | 308 (66.7) | |
| Part-time (>50%) | 121 (26.2) | |
| Part-time (≤50%) | 33 (7.1) | |
| Health-related factors | ||
| Body mass indexa, M (SD) | 25.7 (5.1) | 17.1–49.2 |
| <18.5, n (%) | 8 (1.7) | |
| 18.5–24.99, n (%) | 232 (50.3) | |
| 25–29.99, n (%) | 145 (31.5) | |
| 30–34.99, n (%) | 44 (9.5) | |
| 35–39.99, n (%) | 24 (5.2) | |
| 40 ≤, n (%) | 8 (1.7) | |
| Alcohol usea four times per week or more, n (%) | 29 (6.3) | |
| Tobacco useb currently yes,n (%) | 57 (12.4) | |
| Caffeine intakec currently yes, n (%) | 404 (88.0) | |
| Medicationd currently yes, n (%) | 229 (50.2) | |
| Oral contraceptives, n (%) | 29 (6.3) | |
| Antidepressants, n (%) | 36 (7.8) | |
| Other psychopharmacological treatment (e.g. anxiolytics, sedatives), n (%) | 7 (1.5) | |
| Hair treatment | ||
| Washes per weeke, M (SD) | 3.4 (1.9) | 0–14 |
| Treatment currently yes, n (%) | 197 (42.6) | |
| Clinical characteristics | ||
| Depressive symptoms (PHQ-9total), M (SD) | 7.0 (4.6) | 0–21 |
| Depression groups (PHQ-9group) | ||
| Nod, n (%) | 161 (34.8) | |
| Mildd, n (%) | 176 (38.1) | |
| Moderated, n (%) | 90 (19.5) | |
| Pronouncedd, n (%) | 28 (6.1) | |
| Severed, n (%) | 7 (1.5) | |
| Burnout symptoms (MBI-GStotal), M (SD) | 2.1 (1.1) | 0.0–4.8 |
| Burnout groups (MBI-GSgroup), n (%) | ||
| Nob, n (%) | 157 (34.0) | |
| Moderateb, n (%) | 245 (53.0) | |
| Severeb, n (%) | 60 (13.0) | |
| Sleep quality (PSQItotalf), M (SD) | 6.1 (3.0) | 0–19 |
| Sleep quality groups (PSQIgroupf) | ||
| Goodsq, n (%) | 227 (50.0) | |
| Poorsq, n (%) | 227 (50.0) | |
| PSQIduration in hours, M (SD) | 6.8 (1.1) | 3.0–11.0 |
| PSQIefficiencyg, M (SD) | 87.9 (11.7) | 27.3–137.1 |
| Hair steroids | ||
| hCh, M (SD) | 6.9 (4.3) | 0.1–19.9 |
| hCni, M (SD) | 20.1 (10.0) | 0.4–50.4 |
| hDHEAj, M (SD) | 9.0 (5.6) | 0.8–25.1 |
Note. Treatment = coloration, tinting, permanent waves; PHQ-9total = Patient Health Questionnaire 9 total score; PHQ-9group = Patient Health Questionnaire 9 group variable with 5 subgroups: Nod (cut-off ≤ 4), Mildd (cut-off 5–9), Moderated (cut-off 10–14), Pronouncedd (cut-off 15–19), Severed (cut-off ≥ 20); MBI-GStotal = Maslach Burnout Inventory weighted total score; MBI-GSgroup = Maslach Burnout Inventory group variable with 3 subgroups: Nob (cut-off ≤ 1.49), Moderateb (cut-off 1.5–3.49), Severeb (cut-off ≥ 3.5); PSQI(total, duration, efficiency) = Pittsburgh Sleep Quality Index (total score, duration, efficiency); PSQIgroup = Pittsburgh Sleep Quality Index group variable with 2 subgroups: Goodsq (cut-off ≤ 5), Poorsq (cut-off ≥ 6); hC = hair cortisol concentration; hCn = hair cortisone concentration; hDHEA = hair dehydroepiandrosterone concentration; insertion of non-detectable values (for hC und hDHEA) and exclusion of outliers and extreme values (for all hair steroid variables).
n = 461.
n = 458.
n = 459.
n = 456.
n = 418.
n = 454.
n = 460.
n = 367.
n = 361.
n = 366.
2.2. Self-reported measures
2.2.1. Sleep quality
For the first time in the framework of laboratory assessment of the DBS, the Pittsburgh Sleep Quality Index (PSQI; [13]) was used to evaluate SQ in the fourth wave of the biomarker assessment. The PSQI is a self-assessment questionnaire, which records the subjective SQ of the prior month and consists of 19 items based on the subjects’ self-disclosure and five further items which are intended to be answered by the subject’s partner or flat-mate. The latter is not included in the cumulated sum score. The 19 items form seven principal components such as subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, sleep medication and daytime dysfunction are weighted from 0 to 3. A detailed description of weighting is reported in Buysse et al. [13]. The cumulated sum score of the seven principal components can be converted into a total score which can vary between 0 and 21. Low SQ is indicated by a high overall score (values > 5) and allows the discrimination between ‘good’ and ‘poor’ sleepers [13]. Previous studies show a good retest reliability of r = 0.89 [8]. The internal consistency in our study was assessed by Cronbach’s α using total component scores [8] and was acceptable (α = 0.71).
2.2.2. Depressive symptoms
The German version of the Patient Health Questionnaire 9 (PHQ-9; [33]) is a self-assessment questionnaire, based on the subjects’ self-disclosure and was used to evaluate depressive symptoms. The PHQ-9 aims to record the nine diagnostic criteria of a MDD based on the Diagnostic and Statistical Manual of Mental Disorders 4 (DSM-IV; [4]) by using nine corresponding items. To define a screening of the general major depressive severity a cumulative sum score can be generated, using the scores of the nine items. The cumulative sum score can vary between 0 and 27 as each item can be coded with 0 (‘not at all’), 1 (‘on single days’), 2 (‘on more than half of the days ´) and 3 (‘nearly every day’). This sum score was used to examine the associations between SQ and depressive symptoms [33]. The internal consistency in our study was good (α = 0.85).
2.2.3. Burnout symptoms
Burnout was evaluated by using the German version of the Maslach Burnout Inventory-General Survey (MBI-GS; [12]). The MBI-GS is a self-assessment questionnaire and consists of 16 items. Subjects evaluate how often each item apply to them on a scale from 0 (‘never’) to 6 (‘daily’). The items form the three sub-scores emotional exhaustion (EE), cynicism (CY) and reduced efficacy (PEr). The total score can be generated and allows a weighted calculation including all items. In order to assess the associations between SQ and burnout symptoms we used the total score. The internal consistency in our study was good (α = 0.91).
2.2.4. Confounders
Several confounding variables, as sociodemographic (age, sex), hair-specific (washes per week, hair treatment [permanent wave, coloration, tint]) and health-related (body mass index [BMI], smoking status, alcohol consumption, caffeine consumption, medication intake [oral contraceptives, antidepressants, other psychopharmacological treatment [hypnotics, antipsychotics, anxiolytics, sedatives]) variables were assessed by self-report. Age and hair washes per week are included in the analyses as metric variables, whereas the other variables are included as dichotomous ones (e.g. intake of medication yes/no). BMI was dummy-coded into six groups (see Table 1) thus a linear connection with the other constructs is not required.
2.3. Hair hormone analysis
To investigate the activity of the HPA axis, serum and salivary samples are the most common methods, but are very susceptible to external influences, such as food intake or the day-night cycle. Therefore, hair samples, as a new long-term marker for endocrine activity [49] were collected. Three hair strands per participant (maximum diameter of a hair strand of 3 mm) were cut as close as possible to the scalp at the posterior vertex position. It is assumed that the hair grows 1 cm per month [54]. To be able to make a statement about the cumulative hair hormone concentration of the last three months, hair cortisol concentration (hC), hair cortisone concentration (hCn) and hair DHEA concentration (hDHEA) are determined from the first 3 cm close to the scalp of the hair samples. Besides hC, hCn and hDHEA, ratios such as hC/hCn, as well as hC/hDHEA will be examined in this manuscript. hC/hCn ratio is a marker for the enzymatic activity (11β-HSD2) and therefore for the irreversible conversion of cortisol to cortisone. The higher the level of the ratio is the lower the enzymatic activity can be considered and vice versa. Since high levels of cortisol can have cytotoxic properties, the conversion of the bioactive cortisol into its biologically inactive form cortisone can provide important insight into the organisms’ capacity to deal with high levels of cortisol released upon a stressor. hC/hDHEA ratio presents a ratio for hormones with glucocorticoid versus anti-glucocorticoid effects. While cortisol is the main effector of the HPA axis and a potent glucocorticoid, DHEA has been suggested as an anti-glucocorticoid agent protecting the organism against the adverse effects of chronically raised cortisol levels. The laboratory protocol including hair samples washing procedure and hormone extraction is described by Gao et al. [20]. All hair samples were analyzed by liquid chromatography in combination with tandem mass spectrometry (LC-MS/MS).
2.4. Data analysis
All statistical analyses were done using SPSS (version 22 to 26) for Windows (IBM SPSS Statistics for Windows; [31]) and Macintosh (IBM SPSS Statistics for Macintosh; [30]). Moderation analyses were conducted by using PROCESS macro for SPSS [26]. To examine our a priori formulated research questions, regression analytical methods were used. At beginning, hormone data lower than the detection limit (hC: n = 3 ≙ 0.77%; hCn: n = 0; hDHEA: n = 35 ≙ 8.93%) were replaced by values with detection limit/2 such as recommended for < 15% of non-detectable values [51], resulting in n = 392. Afterwards, we excluded outliers and extreme values by using boxplots in SPSS. To identify outlying values SPSS uses the limit of 1.5 ∗ interquartile range. This leaded to an exclusion for analysis with HPA axis-related hormones of n = 25 (6.38%) cases for hC, of n = 31 (7.91%) cases for hCn and n = 26 (6.63%) cases for hDHEA. At last hair hormone values were log-transformed by using LG10. Additionally, to analyses with one hormone, we conducted analyses with hC/hCn and hC/hDHEA ratios to make statements about basically metabolic processes. For all research questions we did a two steps procedure. At first, simple regression analyses were performed with the independent variables PSQItotal, PSQIduration (sleep time per night in hour), or PSQIefficiency (ratio between sleep time and bedtime) and dependent variables PHQ-9total or MBI-GStotal. PSQIduration and PSQIefficiency are not reverse coded as PSQItotal, whereas higher scores mean a higher sleep duration or efficiency. Afterwards, these analyses were repeated as multiple linear regression analyses, regarding described confounders above. For moderation analyses independent variables, including confounders, were automatically mean centered by PROCESS. For significant interaction effects PROCESS constructed a syntax for moderation charts. In longitudinal moderation analyses data for variables PSQItotal, PSQIduration and PSQIefficiency were used of the online assessment completed prior the fourth wave of the biomarker assessment. All other variables (dependent and moderator variable, confounders) were used from the fourth wave of biomarker assessment. Additional confounders, time difference between the two assessment points (in days) and baseline (bl) value for dependent variables, were included in longitudinal analyses. A p-level of 0.05 for two-tailed tests was set for all analyses.
3. Results
3.1. Descriptive results
Sociodemographic, health-related and hair-specific data and descriptive results with respect to PHQ-9total, MBI-GStotal PSQItotal, PSQIduration and PSQIefficiency are shown in Table 1. Distributions of PHQ-9total, MBI-GStotal, PSQItotal, PSQIduration, PSQIefficiency scores and HPA axis-related hormone concentrations are presented in supplementary Fig. 1 to 3. Moreover, information about shifts from one to another symptom subgroup with regard to depressive and burnout symptoms, as well as SQ from the preceding online assessment to the biomarker assessment is described in the supplementary Table 4. Overall, most participants remained in the initial syndrome subgroup from the preceding online assessment.
3.2. Associations between sleep quality and depressive or burnout symptoms
Simple regression analyses showed positive associations between PSQItotal (βi = 0.68; SE = 0.05; p < 0.001) and PHQ-9total, and negative associations between PSQIduration (βi = −0.27; SE = 0.19; p < 0.001) or PSQIefficiency (βi = −0.43; SE = 0.02; p < 0.001) and PHQ-9total. Furthermore, PSQItotal (βi = 0.51; SE = 0.01; p < 0.001), PSQIduration (βi = −0.16; SE = 0.05; p < 0.01) or PSQIefficiency (βi = −0.27; SE = 0.00; p < 0.001) were significantly associated with MBI-GStotal. Addition of confounders in multiple linear regression analyses did not significantly change the results. Detailed information is presented in supplementary Tables 8 and 9. Notably, higher scores in the PSQItotal represent a worse general sleep quality, while higher scores on the subscales PSQIduration and PSQIefficiency represent longer sleep durations and better sleep efficiency, respectively.
3.3. Associations between sleep quality and HPA axis-related hormones
No significant associations between general sleep quality or sleep quality dimensions and HPA axis-related hormones were identified, whether in simple, nor in multiple regression analyses. Detailed information is presented in supplementary Tables 10 to 14.
3.4. Associations between HPA axis-related hormones and depressive or burnout symptoms
No significant associations could be identified between HPA axis-related hormones and depressive or burnout symptoms, except for multiple regression analyses between hCn and MBI-GStotal (βi = −0.13; SE = 0.24; p < 0.05). However, in simple regression analyses the association was not significant (βi = −0.10; SE = 0.23; p = 0.06). Detailed information is presented in supplementary Tables 15 and 16.
3.5. Cross-sectional moderation analyses
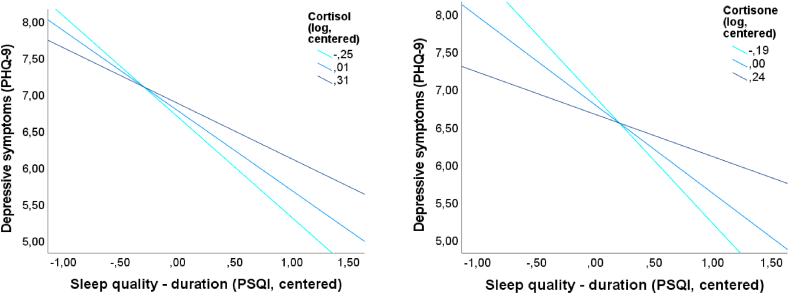
In simple moderation analyses with the dependent variable PHQ-9total, significant interactions between PSQIduration and hCn (ΔR2 = 0.01; ΔF1,357 = 5.12; p < 0.05; b = 2.14; t = 2.26; p < 0.05), hDHEA (ΔR2 = 0.01; ΔF1,362 = 5.92; p < 0.05; b = 1.41; t = 2.43; p < 0.05) or hC/hDHEA ratio (ΔR2 = 0.02; ΔF1,340 = 7.61; p < 0.01; b = 0.16; t = 2.76; p < 0.01) were determined. In multiple moderation analyses, there are significant interactions between PSQIduration and hC (ΔR2 = 0.01; ΔF1,307 = 4.47; p < 0.05; b = 1.10; t = 2.11; p < 0.05) or hCn (ΔR2 = 0.02; ΔF1,301 = 7.68; p < 0.01; b = 2.60; t = 2.77; p < 0.01). Fig. 2 presents these interaction effects, suggesting that the negative association between PSQIduration and PHQ-9total was buffered by higher levels of hC or hCn. The interaction between PSQIduration and hDHEA in simple moderation analyses could not be confirmed by multiple moderation analyses (ΔR2 = 0.01; ΔF1,308 = 2.73; p = 0.10; b = 0.94; t = 1.65; p = 0.10). Detailed information is presented in supplementary Tables 17 and 18.
Fig. 2.
Diagrams for cross-sectional multiple moderation analyses for significant moderation effects of centered and logarithmized levels of cortisol (left) and cortisone (right) for the associations between centered Pittsburgh Sleep Quality Index (PSQI) duration and depressive symptoms, measured by Patient Health Questionnaire-9 (PHQ-9)total score.
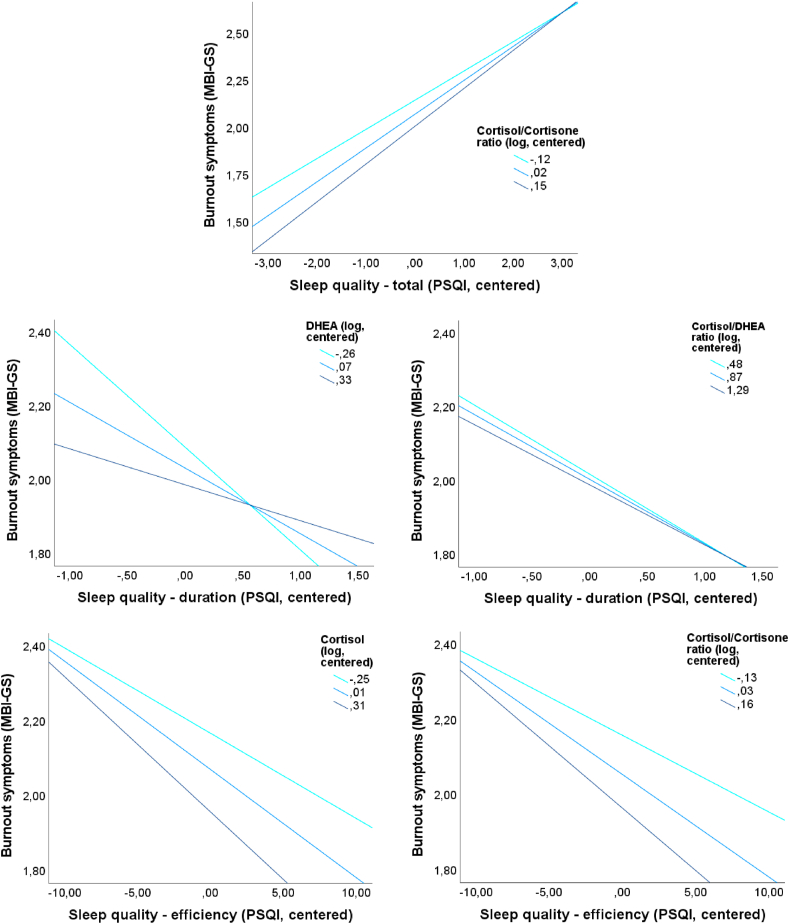
For simple moderation analyses with the dependent variable MBI-GStotal significant interactions were found for PSQIduration and hDHEA (ΔR2 = 0.02; ΔF1,362 = 6.55; p = 0.01; b = 0.37; t = 2.56; p = 0.01) or hC/hDHEA ratio (ΔR2 = 0.01; ΔF1,340 = 5.38; p < 0.05; b = 0.03; t = 2.32; p < 0.05). For multiple moderation analyses with the dependent variable MBI-GStotal significant interactions were found for PSQItotal and hC/hCn ratio (ΔR2 = 0.01; ΔF1,292 = 3.95; p < 0.05; b = 0.16; t = 1.99; p < 0.05), suggesting an intensifying effect of a higher hC/hCn ratio for the negative relation between sleep quality and burnout symptoms. Furthermore, the interactions between PSQIduration and hDHEA (ΔR2 = 0.01; ΔF1,308 = 4.49; p < 0.05; b = 0.31; t = 2.12; p < 0.05) or hC/hDHEA ratio (ΔR2 = 0.01; ΔF1,286 = 4.41; p < 0.05; b = 0.03; t = 2.10; p < 0.05) are suggesting a buffering of the negative association between PSQIduration and MBI-GStotal. Additionally, the negative association between PSQIefficiency and MBI-GStotal was intensified by higher levels of hC (ΔR2 = 0.01; ΔF1,306 = 4.34; p < 0.05; b = −0.02; t = −2.08; p < 0.05) or hC/hCn ratio (ΔR2 = 0.02; ΔF1,294 = 7.32; p < 0.01; b = −0.05; t = −2.71; p < 0.01). An overview about all cross-sectional multiple moderation analyses will be given in Table 2. The interaction effects are presented in Fig. 3. Detailed information is presented in supplementary Tables 19 and 20.
Table 2.
Overview about significant moderation models (including confounders) with PSQI as independent variables, PHQ-9total or MBI-GStotal as dependent variables and HPA axis-related hormones as moderators.
| b | SE | t | P | ||
|---|---|---|---|---|---|
| cross-sectional | PHQ-9total | ||||
| Constant | 8.27 | 1.29 | 6.41 | < 0.001 | |
| PSQIduration | −1.10 | 0.22 | −5.01 | < 0.001 | |
| hC | 0.33 | 0.76 | 0.43 | 0.67 | |
| PSQIduration∗hC | 1.10 | 0.52 | 2.11 | 0.04 | |
| Constant | 8.74 | 1.29 | 6.76 | < 0.001 | |
| PSQIduration | −1.18 | 0.22 | −5.39 | < 0.001 | |
| hCn | −0.53 | 0.94 | −0.57 | 0.57 | |
| PSQIduration∗hCn | 2.60 | 0.94 | 2.77 | < 0.01 | |
| cross-sectional | MBI-GStotal | ||||
| Constant | 2.40 | 0.30 | 7.96 | < 0.001 | |
| PSQItotal | 0.17 | 0.02 | 9.17 | < 0.001 | |
| hC/hCn | −0.50 | 0.29 | −1.74 | 0.08 | |
| PSQItotal∗hC/hCn | 0.16 | 0.08 | 1.99 | < 0.05 | |
| Constant | 2.02 | 0.35 | 5.83 | < 0.001 | |
| PSQIduration | −0.20 | 0.06 | −3.45 | < 0.001 | |
| hDHEA | −0.17 | 0.16 | −1.09 | 0.27 | |
| PSQIduration∗hDHEA | 0.31 | 0.15 | 2.12 | 0.03 | |
| Constant | 2.07 | 0.35 | 5.86 | < 0.001 | |
| PSQIduration | −0.20 | 0.06 | −3.40 | < 0.001 | |
| hC/hDHEA | −0.04 | 0.02 | −1.95 | 0.05 | |
| PSQIduration∗hC/hDHEA | 0.03 | 0.01 | 2.10 | 0.04 | |
| Constant | 2.25 | 0.32 | 6.96 | < 0.001 | |
| PSQIefficiency | −0.03 | 0.01 | −5.31 | < 0.001 | |
| hC | −0.37 | 0.20 | −1.89 | 0.06 | |
| PSQIefficiency∗hC | −0.02 | 0.01 | −2.08 | 0.04 | |
| Constant | 2.36 | 0.33 | 7.23 | < 0.001 | |
| PSQIefficiency | −0.03 | 0.01 | −4.90 | < 0.001 | |
| hC/hCn | −0.69 | 0.28 | −2.45 | 0.01 | |
| PSQIefficiency∗hC/hCn | −0.05 | 0.02 | −2.71 | < 0.01 | |
| longitudinal | MBI-GStotal | ||||
| Constant | 0.51 | 0.36 | 1.39 | 0.17 | |
| PSQIduration-bl | −0.02 | 0.05 | −0.36 | 0.72 | |
| hC/hCn | −0.07 | 0.22 | −0.33 | 0.74 | |
| PSQIduration-bl∗hC/hCn | −0.42 | 0.21 | −2.05 | 0.04 |
Note. b = regression coefficient; SE = standard error; PSQI(total) (-bl) = Pittsburgh Sleep Quality Index (total score) (-baseline); hC = hair cortisol concentration; hCn = hair cortisone concentration; hDHEA = hair dehydroepiandrosterone concentration; hC/hCn = ratio of hair cortisol concentration and hair cortisone concentration; hC/hDHEA = ratio of hair cortisol concentration and hair dehydroepiandrosterone concentration; included confounders (age, gender, body mass index, smoking status, alcohol consumption, caffeine consumption, medication intake [oral contraceptives, antidepressants, other psychopharmacological treatment], hair washes per week, hair treatment [tint, coloration, permanent wave], in longitudinal analyses additionally time interval [days] between assessment points); the presentation of confounders statistics has been omitted for reasons of space.
Fig. 3.
Diagrams for cross-sectional multiple moderation analyses for significant moderation effects of centered and logarithmized levels of (first line) cortisol/cortisone ratio for the associations between centered Pittsburgh Sleep Quality Index (PSQI) total score and burnout symptoms, measured by Maslach Burnout Inventory-General survey (MBI-GS) total score; (second line) DHEA (dehydroepiandrosterone, left) and cortisol/DHEA ratio (right) for the associations between centered PSQI duration and MBI-GS total score; (third line) cortisol (left) and cortisol/cortisone ratio (right) for the associations between centered PSQI efficiency and MBI-GS total score.
3.6. Longitudinal moderation analyses
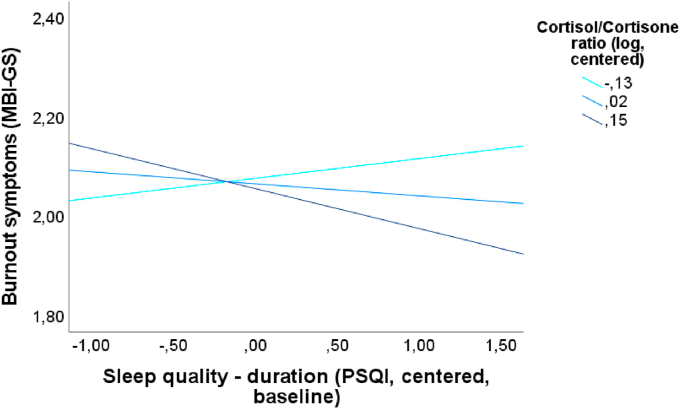
No significant interaction effects between sleep dimensions and HPA axis-related hormones with regard to depressive symptoms were found whether in simple (adjusted only for baseline depressive symptoms) nor in multiple moderation analyses. However, in analyses adjusted for baseline burnout symptoms and several confounders, with the dependent variable MBI-GStotal a significant interaction between PSQIduration-bl and hC/hCn ratio was found (ΔR2 = 0.01; ΔF1,205 = 4.22; p < 0.05; b = −0.42; t = −2.05; p < 0.05). However, PSQIduration-bl was not a significant predictor for MBI-GStotal (p = 0.72). These results indicate that the main effect of the variables has no incremental validity after considering the interaction effect. The moderation is shown in Fig. 4 and shows that higher levels of hC/hCn ratio lead to an intensified negative association between PSQIduration and MBI-GStotal, whereas lower levels of hC/hCn ratio seem to buffer this negative association. Detailed information is presented in supplementary Tables 21 to 24.
Fig. 4.
Diagram for longitudinal multiple moderation analysis (adjusted for baseline burnout symptoms and several confounders) for significant interaction effect of centered and logarithmized cortisol/cortisone ratio for the association between centered Pittsburgh Sleep Quality Index (PSQI) duration and burnout symptoms (MBI-GS total score).
3.7. Additional analyses
To get a deeper insight into the analyzed data, further additional analyses with regard to HPA axis-related hormones and syndrome groups regarding PHQ-9total, MBI-GStotal and PSQItotal were conducted (see supplementary Tables 2 and 3). We tested whether individuals with a clinically relevant depressive or burnout symptomatology differed with regard to hC, hCn, or hDHEA, as well as individuals with clinically relevant sleep disturbances showed different hormone levels than individuals without sleep disturbances. No differences in HPA axis-related hormone concentrations between depressive or burnout symptomatology subgroups were identified. Furthermore, individuals with good versus poor SQ did not differ in HPA axis-related hormone concentrations. Moreover, participants suffering from clinically relevant depressive, but not burnout symptoms versus participants suffering from clinically relevant burnout but not depressive symptoms did not differ in HPA axis-related hormones as well. Detailed information about classification of the groups is presented in the notes of the supplementary Tables 2 and 3.
4. Discussion
The present study adds new insight into the hormonal modulation of the association between SQ and depressive or burnout symptoms. Supporting the prior postulated moderation effects, no direct associations between HPA axis-related hormones and depressive or burnout symptoms, nor SQ were identified, except for hCn and burnout symptoms in multiple regression analysis. For the association between SQ and depressive symptoms some interesting moderation effects were detected: hC and its metabolite hCn moderated the association between SQ operationalized as PSQIduration and depressive symptoms suggesting higher glucocorticoid levels to weaken the negative association. Regarding our exploratory analyses of moderating effects on the association between SQ and burnout symptoms, for the associations between SQ, operationalized as PSQItotal or PSQIefficiency, and burnout symptoms, hC and hC/hCn ratio emerged as moderators. Higher levels in hC and hC/hCn ratio intensified the negative association. hDHEA further emerged as a moderator buffering the negative association between PSQIduration and MBI-GStotal. To our knowledge, this is the first study going beyond examining direct associations between SQ, depressive and burnout symptoms, and HPA axis-related hormones by investigating potential cross-sectional and longitudinal moderation effects of HPA axis-related hormones in hair on these associations.
4.1. Summary and integration of results
First, we could replicate positive associations between PSQItotal (higher values means worse general SQ) and depressive or burnout symptoms reported in an online only large scale sample of the DBS [46]. In the present subsample of the DBS including biomarker analysis, we complemented this investigation for two essential sleep dimensions (PSQIduration and PSQIefficiency) showing negative associations with depressive and burnout symptoms as well. These dimensions of sleep play an important role for the understanding of sleep behavior, are associated with health-related quality of life [17], and could be considered as markers for good SQ [3].
Furthermore, we investigated associations between SQ and HPA axis-related hormones. No significant associations in multiple regression analyses were found. Meerlo et al. [38] summarized in their review, that during night and in the evening of the next day after sleep deprivation there are higher glucocorticoid levels, but the higher HPA axis activity is rather small compared to effects of other stress situations. Although associations for example between sleep deprivation and glucocorticoids measured in plasma are confirmed in human and animal studies [15,50], due to general small effects and that we analyzed cumulative HPA axis-related hair hormone concentrations, it is explainable that in our study no significant associations between SQ and hair hormone concentrations could be identified. This supports further the assumption of moderating influences and exclude the possibility for potential mediation effects of these hormones between SQ and depressive or burnout symptoms in these data.
In addition, we examined associations between HPA axis-related hormones and depressive or burnout symptoms. Multiple regression analyses showed no associations between HPA axis-related hormones and PHQ-9total. Only one significant association between hCn and MBI-GStotal was found. Only one study, using also data of the DBS was identified in a recent systematic review [47] which investigated the association between MBI-GStotal and hC, hCn and hC/hCn ratio [42]. The authors reported no significant associations for continuous burnout measures, but higher cortisol and cortisone levels in individuals scoring high in the MBI-GS compared to individuals reporting no or moderate burnout symptoms. The reason, why no associations were found with the continuous MBI-GS measure was explained by a potential non-linear relationship between burnout severity and glucocorticoid exposure [42].
The main goal of this study, however, was to examine potential moderating effects of HPA axis-related hormones on the associations between SQ and depressive or burnout symptoms. In cross-sectional multiple moderation analyses for the associations between SQ and PHQ-9total, we found significant interaction effects between PSQIduration and hC or hCn. The negative association between PSQIduration and PHQ-9total seems to be diminished by higher hC and hCn levels. No other significant moderating effects were found for the association between SQ and PHQ-9total. Furthermore, in analyses for the associations between PSQItotal or PSQIefficiency and MBI-GStotal, we identified hC and hC/hCn ratio as moderators, intensifying these negative associations. The directions of moderating effects of hC depends on the predicted psychological symptoms – depression or burnout. In addition, hDHEA seems to buffer the negative association between PSQIduration and MBI-GStotal. Nevertheless, it should be noted, that the additional variance explanation (ΔR2) due to the moderating effects is small (e.g. interaction effect between PSQIduration and hC: ΔR2 = 0.01), suggesting the results to be interpreted with caution and that other potential moderators might be more relevant.
Many studies showed a higher cortisol release after sleep deprivation in individuals with depressive symptoms [37]. Our results showed a moderating effect of hC or hCn on the association between PSQIduration and PHQ-9total. Higher hC and hCn levels weaken the association between PSQIduration and depressive symptoms. This suggests a compensating influence of hC and hCn on sleep deprivation to depressive symptomatology. This might be due to the activating and energizing function of cortisol. Furthermore, previous research indicated that infusions of ACTH and cortisol leads to reduced REM sleep in healthy men [11], whereby glucocorticoid receptors (GR) are the relevant occupied receptors which are able to influence REM sleep [10]. Studies showed that individuals with MDD show a faster start into and a longer duration of REM sleep [6,23]. With regard to the REM sleep emotional homeostasis hypothesis by Goldstein and Walker [22], this in turn results in reduced activity of the locus coeruleus and a reduced release of noradrenaline [34], which is associated with a reduced top-down control of the amygdala. Consequently, the sensitivity to non-salient information can increase and emotional salience discrimination can be impaired, which is associated with depressive states [32]. Thus, a reduced REM sleep due to higher levels of cortisol may lead to higher activity of the locus coeruleus and resulting in reduced depressive symptoms. Our results, of a moderation effect of hC and hCn on the association between sleep duration and depressive symptoms support this prospective.
Interestingly, no moderation effect of HPA axis-related hormones on the association between general SQ and depressive symptoms could be identified. Regarding burnout symptoms, only one significant moderation effect was detected between general SQ and the analyzed hormones. We assume that is because general SQ, operationalized with the PSQI, is a heterogeneous construct including rather ‘objective’ variables as sleep duration or sleep efficiency providing information about time-dependent aspects of SQ, and more ‘subjective’ variables such as daytime dysfunction presenting a consequence of bad SQ. Therefore, it is not surprising that nearly no interaction effects with the PSQItotal emerged. By contrast, more interaction effects with sleep duration or efficiency could be identified. Therefore, to fully understand the relation between SQ, HPA axis hormones, and depressive or burnout symptomatology more additional and more ‘objective’ quantification of SQ such as polysomnography or actigraphy should be used.
Additionally, Rothe et al. [47] further suggest a GR resistance in individuals with MDD based on a lower number of GR or a lower affinity or sensitivity for cortisol by the GR. If a certain amount of cortisol is necessary to weaken the association between SQ and depressive symptomatology, then higher levels of cortisol are needed in individuals with MDD due to the established GR resistance. By contrast, for the associations between general SQ or sleep efficiency and burnout symptomatology, hC or hC/hCn ratio showed an intensifying effect, whereas lower levels buffered these associations. In individuals with a burnout, a glucocorticoid hypersensitivity has been suggested [47], so that a relatively small quantity of glucocorticoids in the system can already suffice to modulate the associations between SQ and burnout symptomatology. Higher levels of glucocorticoids thus intensify the burnout symptomatology. The results therefore indicate that a certain amount of GR has to be occupied to weaken the association between SQ with regard to depressive symptoms, while with regard to burnout symptomatology, higher glucocorticoid levels intensify the association between SQ and burnout pointing toward diametrically opposed GR functioning in depression and burnout [39,40]. We speculate that the time required for a clinically relevant depressive syndrome as compared to a burnout syndrome is longer. Thus, in individuals with higher depressive symptomatology, GR resistance might be established, while in individuals with burnout a hypersensitivity of GR is in place. Therefore, higher levels of cortisol weaken the association between SQ and depressive symptomatology, while higher levels of cortisol strengthen the association between SQ and burnout symptomatology.
While hC and the hC/hCn ratio intensify the associations between SQ and burnout symptomatology, for hDHEA a buffering effect was observed. Metabolites of DHEA are able to limit the binding of cortisone, so that cortisone cannot be converted into cortisol. So, DHEA exhibits an inherent anti-glucocorticoid effect [27]. Our results show, that the relationship between SQ and burnout symptoms is moderated by hC and hDHEA in an opposed way, which underlines the anti-glucocorticoid effect of DHEA suggesting DHEA to act as a counterregulatory agent balancing glucocorticoid action. Interestingly, these effects could not be observed in models with depressive symptoms suggesting a further developed maladaptation of the HPA axis in depressed compared to burned-out individuals, where the counter-regulatory properties of DHEA fail and the system’s own adaptation mechanisms no longer work. This could indicate, that burnout is a preliminary stage of MDD, which was indicated in various previous studies [2,24].
Although, these findings could not be confirmed in longitudinal moderation analyses, nevertheless, one significant interaction effect was identified. hC/hCn ratio moderated the association between sleep duration at baseline and burnout symptoms at follow-up. An explanation for observing moderating effects in cross-sectional and not in longitudinal analyses can be, that the concentration of hormones is decisive in the period when SQ was measured and that longer time periods convey too much environmental influence to maintain the effects.
4.2. Strengths and limitations
Some strengths of the study are described in the following. First, in our cross-sectional and longitudinal analyses, we included in sum N = 462 and n = 342 participants, respectively, which ensures a large diversity of our studied population with regard to sex, age, or health status. Second, it should be emphasized that in comparison to other studies investigating burnout symptoms, we do not limit the analyses to certain occupational groups, but include all employees regardless of their occupation in order to achieve generalization. Third, we could examine in a large sample the moderating role of long-term integrated HPA axis-related hair hormones for the first time, and internally validate the results with regard to SQ, depression, and burnout symptoms in longitudinal analysis.
However, some limitations of our study need to be considered when interpreting the results. First, we used self-report questionnaires for SQ, depression, and burnout symptomatology. Therefore, no clinical confirmed diagnostic groups could be analyzed. Second, in our study a potential selection bias was observed (e.g. 67% of our participants were women), limiting the generalizability of our results. Third, we failed to replicate our cross-sectional findings in longitudinal analyses. We traced it back to the assessment point of HPA axis-related hormones (see above). Hair samples were collect at the fourth biomarker assessment. For longitudinal analyses we used SQ data from a proceeding online assessment 13 months prior. Forth, in further studies objective parameters for quantification of SQ (e.g. polysomnography) should be used to reduce the impact of a potential altered perception of physical or mental states, such as SQ, due to e.g. depressive symptomatology [1].
4.3. Implications
In our study we investigated the impact of HPA axis-related hormones for the associations between SQ and depressive or burnout symptoms. The opposite associations of long-term integrated glucocorticoids in hair on the relation between sleep and depressive or burnout symptomatology, suggest that burnout could be a preliminary state of MDD, because the HPA axis and in particular the GR seems to function at an intensified sensitivity in burnout, which shifts into a glucocorticoid resistance in depressive disorders. More longitudinal studies are needed, in which participants are accompanied from the onset of burnout symptoms to determine at which point of a burnout or a depression, the functioning of the HPA axis changes. To examine the potential of hormonal therapy (e.g. with DHEA) in reducing depressive or burnout symptomatology by influencing the relation between SQ and depression or burnout, administration studies are needed.
4.4. Conclusion
HPA axis-related hormones moderate the association between SQ and depressive or burnout symptoms in a different way. While higher hC buffers the association between sleep duration and depressive symptoms, it intensifies the association between general SQ and sleep efficiency and burnout symptoms. hDHEA, however, buffers the association between sleep duration and burnout symptoms. The question as to whether hormone therapies might present options for reducing the negative consequences of reduced SQ on depressive or burnout symptoms remains to be examined in randomized clinical trials.
Source of funding
This work was supported by the TU Dresden’s Institutional Strategy (“The Synergetic University”), which is funded by the Excellence Initiative of the German Federal and State Governments awarded to CK. N.R. was supported by a scholarship program for the Promotion of Early-Career Female Scientists of TU Dresden. Funders were not involved in design, collection, analysis, interpretation, and publication decisions of the data.
Author contributions
NR and AW co-wrote the first draft of the manuscript and integrated the findings. NR and AW planned and realized the fourth biomarker assessment. NR, SV and KS conducted the analyses. KS made an essential contribution to the method part. CK revised subsequent versions of the manuscript. MP and MKW constructed the design of the Dresden Burnout Study and contributed essentially to some of the research questions. WG performed the hair analyses and supported data analysis and interpretation. The manuscript was supervised by AW and CK.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We thank all of our interns for their support in preparation and realization of the fourth wave of biomarker assessment.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpnec.2021.100051.
Contributor Information
Nicole Rothe, Email: Nicole.Rothe@tu-dresden.de.
Sabrina Vogel, Email: Sabrina.Vogel@mailbox.tu-dresden.de.
Kristin Schmelzer, Email: Kristin.Schmelzer@mailbox.tu-dresden.de.
Clemens Kirschbaum, Email: Clemens.Kirschbaum@tu-dresden.de.
Marlene Penz, Email: Marlene.Penz@jku.at.
Magdalena Katharina Wekenborg, Email: Magdalena.Wekenborg@tu-dresden.de.
Wei Gao, Email: Wei.Gao@tu-dresden.de.
Andreas Walther, Email: a.walther@psychologie.uzh.ch.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Achat H., Kawachi I., Spiro A., Demolles D.A., Sparrow D. Optimism and depression as predictors of physical and mental health functioning: the normative aging study. Ann. Behav. Med. 2000;22:127–130. doi: 10.1007/BF02895776. [DOI] [PubMed] [Google Scholar]
- 2.Ahola K., Honkonen T., Kivimäki M., Virtanen M., Isometsä E., Aromaa A., Lönnqvist J. Contribution of burnout to the association between job strain and depression: the health 2000 study. J. Occup. Environ. Med. 2006;48:1023–1030. doi: 10.1097/01.jom.0000237437.84513.92. [DOI] [PubMed] [Google Scholar]
- 3.Åkerstedt T., Hume K.E.N., Minors D., Waterhouse J.I.M. The meaning of good sleep: a longitudinal study of polysomnography and subjective sleep quality. J. Sleep Res. 1994;3:152–158. doi: 10.1111/j.1365-2869.1994.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association . American Psychiatric Association; 2003. Diagnostic and Statistical Manual of Mental Disorders DSM 4 TR (Text Revision) [Google Scholar]
- 5.American Psychiatric Association . American Psychiatric Publishing; 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) [Google Scholar]
- 6.Armitage R. Sleep and circadian rhythms in mood disorders. Acta Psychiatr. Scand. 2007;115:104–115. doi: 10.1111/j.1600-0447.2007.00968.x. [DOI] [PubMed] [Google Scholar]
- 7.Armon G., Shirom A., Shapira I., Melamed S. On the nature of burnout–insomnia relationships: a prospective study of employed adults. J. Psychosom. Res. 2008;65:5–12. doi: 10.1016/j.jpsychores.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Backhaus J., Junghanns K., Broocks A., Riemann D., Hohagen F. Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J. Psychosom. Res. 2002;53:737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi R., Schonfeld I.S., Laurent E. Burnout-depression overlap: a review. Clin. Psychol. Rev. 2015;36:28–41. doi: 10.1016/j.cpr.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Born J., DeKloet E.R., Wenz H., Kern W., Fehm H.L. Gluco- and antimineralocorticoid effects on human sleep: a role of central corticosteroid receptors. Am. J. Physiol.-Endocrinol. Metabol. 1991;260:E183–E188. doi: 10.1152/ajpendo.1991.260.2.E183. [DOI] [PubMed] [Google Scholar]
- 11.Born J., Späth-Schwalbe E., Schwakenhofer H., Kern W., Fehm H.L. Influences of corticotropin-releasing hormone, adrenocorticotropin, and cortisol on sleep in normal man∗. J. Clin. Endocrinol. Metabol. 1989;68:904–911. doi: 10.1210/jcem-68-5-904. [DOI] [PubMed] [Google Scholar]
- 12.Büssing A., Glaser J. 1999. Deutsche Fassung des Maslach burnout inventory–General survey (MBI-GS-D) Munich: München, Technische Universität, Lehrstuhl für Psychologie. [Google Scholar]
- 13.Buysse D.J., Reynolds C.F., III, Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatr. Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 14.Cao X.-L., Wang S.-B., Zhong B.-L., Zhang L., Ungvari G.S., Ng C.H., Li L., Chiu H.F.K., Lok G.K.I., Lu J.-P., Jia F.-J., Xiang Y.-T. The prevalence of insomnia in the general population in China: a meta-analysis. PloS One. 2017;12 doi: 10.1371/journal.pone.0170772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapotot F., Buguet A., Gronfier C., Brandenberger G. Hypothalamo-pituitary- adrenal Axis Activity is related to the level of central arousal: effect of sleep deprivation on the association of high-frequency waking electroencephalogram with cortisol release. Neuroendocrinology. 2001;73:312–321. doi: 10.1159/000054648. [DOI] [PubMed] [Google Scholar]
- 16.Cho K.-O. Sleep duration and self-rated health are independently associated with physical activity level in the Korean population. Iran. J. Public Health. 2014;43:590–600. [PMC free article] [PubMed] [Google Scholar]
- 17.Faubel R., Lopez-Garcia E., Guallar-Castillón P., Balboa-Castillo T., Gutiérrez-Fisac J.L., Banegas J.R., Rodríguez-Artalejo F. Sleep duration and health-related quality of life among older adults: a population-based cohort in Spain. Sleep. 2009;32:1059–1068. [PMC free article] [PubMed] [Google Scholar]
- 18.Fiacco S., Walther A., Ehlert U. Steroid secretion in healthy aging. Psychoneuroendocrinology. 2019;105:64–78. doi: 10.1016/j.psyneuen.2018.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Friess E., Trachsel L., Guldner J., Schier T., Steiger A., Holsboer F. DHEA administration increases rapid eye movement sleep and EEG power in the sigma frequency range. Am. J. Physiol.-Endocrinol. Metabol. 1995;268:E107–E113. doi: 10.1152/ajpendo.1995.268.1.E107. [DOI] [PubMed] [Google Scholar]
- 20.Gao W., Stalder T., Foley P., Rauh M., Deng H., Kirschbaum C. Quantitative analysis of steroid hormones in human hair using a column-switching LC–APCI–MS/MS assay. J. Chromatogr. B. 2013;928:1–8. doi: 10.1016/j.jchromb.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Giorgi F., Mattei A., Notarnicola I., Petrucci C., Lancia L. Can sleep quality and burnout affect the job performance of shift-work nurses? A hospital cross-sectional study. J. Adv. Nurs. 2018;74:698–708. doi: 10.1111/jan.13484. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein A.N., Walker M.P. The role of sleep in emotional brain function. Annu. Rev. Clin. Psychol. 2014;10:679–708. doi: 10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gottesmann C., Gottesman I. The neurobiological characteristics of rapid eye movement (REM) sleep are candidate endophenotypes of depression, schizophrenia, mental retardation and dementia. Prog. Neurobiol. 2007;81:237–250. doi: 10.1016/j.pneurobio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Hakanen J.J., Schaufeli W.B. Do burnout and work engagement predict depressive symptoms and life satisfaction? A three-wave seven-year prospective study. J. Affect. Disord. 2012;141:415–424. doi: 10.1016/j.jad.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 25.Hasin D.S., Sarvet A.L., Meyers J.L., Saha T.D., Ruan W.J., Stohl M., Grant B.F. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA PsychiATR. 2018;75:336–346. doi: 10.1001/jamapsychiatry.2017.4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes A.F. Guilford Press; New York: 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. [Google Scholar]
- 27.Hennebert O., Chalbot S., Alran S., Morfin R. Dehydroepiandrosterone 7α- hydroxylation in human tissues: possible interference with type 1 11β-hydroxysteroid dehydrogenase-mediated processes. J. Steroid Biochem. Mol. Biol. 2007;104:326–333. doi: 10.1016/j.jsbmb.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 28.Holsboer F., von Bardeleben U., Steiger A. Effects of intravenous corticotropin- releasing hormone upon sleep-related growth hormone surge and sleep EEG in man. Neuroendocrinology. 1988;48:32–38. doi: 10.1159/000124986. [DOI] [PubMed] [Google Scholar]
- 29.Hucklebridge F., Hussain T., Evans P., Clow A. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology. 2005;30:51–57. doi: 10.1016/j.psyneuen.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 30.IBM SPSS Statistics for Macintosh, Version 22.0, 23.0 & 26.0. IBM Corp., Armonk, NY.
- 31.IBM SPSS, IBM SPSS Statistics for Windows, Version 25.0. IBM Corp., Armonk, NY.
- 32.Kohler C.G., Hoffman L.J., Eastman L.B., Healey K., Moberg P.J. Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatr. Res. 2011;188:303–309. doi: 10.1016/j.psychres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Löwe B., Spitzer R.L., Zipfel S., Herzog W. PHQ-D: Gesundheitsfragebogen Für Patienten; Manual Komplettversion und Kurzform. Pfizer GmbH. 2002 [Google Scholar]
- 34.Mallick B.N., Majumdar S., Faisal M., Yadav V., Madan V., Pal D. Role of norepinephrine in the regulation of rapid eye movement sleep. J. Biosci. 2002;27:539–551. doi: 10.1007/BF02705052. [DOI] [PubMed] [Google Scholar]
- 35.Maninger N., Wolkowitz O.M., Reus V.I., Epel E.S., Mellon S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front. Neuroendocrinol. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maslach C., Schaufeli W.B., Leiter M.P. Job burnout. Annu. Rev. Psychol. 2001;52:397–422. doi: 10.1146/annurev.psych.52.1.397. [DOI] [PubMed] [Google Scholar]
- 37.McEwen B.S. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism. 2006;55:S20–S23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Meerlo P., Sgoifo A., Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med. Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Menke A., Arloth J., Gerber M., Rex-Haffner M., Uhr M., Holsboer F., Binder E.B., Holsboer-Trachsler E., Beck J. Dexamethasone stimulated gene expression in peripheral blood indicates glucocorticoid-receptor hypersensitivity in job-related exhaustion. Psychoneuroendocrinology. 2014;44:35–46. doi: 10.1016/j.psyneuen.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Menke A., Arloth J., Pütz B., Weber P., Klengel T., Mehta D., Gonik M., Rex-Haffner M., Rubel J., Uhr M., Lucae S., Deussing J.M., Müller-Myhsok B., Holsboer F., Binder E.B. Dexamethasone stimulated gene expression in peripheral blood is a sensitive marker for glucocorticoid receptor resistance in depressed patients. Neuropsychopharmacology. 2012;37:1455. doi: 10.1038/npp.2011.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohayon M. Epidemiological study on insomnia in the general population. Sleep. 1996;19:S7–S15. doi: 10.1093/sleep/19.suppl_3.s7. [DOI] [PubMed] [Google Scholar]
- 42.Penz M., Stalder T., Miller R., Ludwig V.M., Kanthak M.K., Kirschbaum C. Hair cortisol as a biological marker for burnout symptomatology. Psychoneuroendocrinology. 2018;87:218–221. doi: 10.1016/j.psyneuen.2017.07.485. [DOI] [PubMed] [Google Scholar]
- 43.Penz M., Wekenborg M.K., Pieper L., Beesdo-Baum K., Walther A., Miller R., Stalder T., Kirschbaum C. The Dresden Burnout Study: protocol of a prospective cohort study for the bio-psychological investigation of burnout. Int. J. Methods Psychiatr. Res. 2018;27:e1613. doi: 10.1002/mpr.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatr. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 45.Rotenstein L.S., Torre M., Ramos M.A., Rosales R.C., Guille C., Sen S., Mata D.A. Prevalence of burnout among physicians: a systematic review. J. Am. Med. Assoc. 2018;320:1131–1150. doi: 10.1001/jama.2018.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothe N., Schulze J., Kirschbaum C., Buske-Kirschbaum A., Penz M., Wekenborg M.K., Walther A. Sleep disturbances in major depressive and burnout syndrome: a longitudinal analysis. Psychiatr. Res. 2020;286:112868. doi: 10.1016/j.psychres.2020.112868. [DOI] [PubMed] [Google Scholar]
- 47.Rothe N., Steffen J., Penz M., Kirschbaum C., Walther A. Examination of peripheral basal and reactive cortisol levels in major depressive disorder and the burnout syndrome: a systematic review. Neurosci. Biobehav. Rev. 2020;114:232–270. doi: 10.1016/j.neubiorev.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt-Reinwald A., Pruessner J.C., Hellhammer D.H., Federenko I., Rohleder N., Schürmeyer T.H., Kirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci. 1999;64:1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- 49.Stalder T., Kirschbaum C. Analysis of cortisol in hair–state of the art and future directions. Brain Behav. Immun. 2012;26:1019–1029. doi: 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Suchecki D., Lobo L., HipÓLide D., Tufik S. Increased ACTH and corticosterone secretion induced by different methods of paradoxical sleep deprivation. J. Sleep Res. 1998;7:276–281. doi: 10.1046/j.1365-2869.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- 51.U.S. Environmental Protection Agency . EPA QA/G9-S. Scholar’s Choice; Washington, DC: 2015. Data Quality Assessment. Statistical Methods for Practitioners. [Google Scholar]
- 52.Walther A., Tsao C., Pande R., Kirschbaum C., Field E., Berkman L. Do dehydroepiandrosterone, progesterone, and testosterone influence women’s depression and anxiety levels? Evidence from hair-based hormonal measures of 2105 rural Indian women. Psychoneuroendocrinology. 2019;109:104382. doi: 10.1016/j.psyneuen.2019.104382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C., Dai J., Li J. Mediating effects of hair cortisol on the mutual association of job burnout and insomnia: a retrospective exploratory study. J. Psychiatr. Res. 2019;117:62–67. doi: 10.1016/j.jpsychires.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Wennig R. Potential problems with the interpretation of hair analysis results. Forensic Sci. Int. 2000;107:5–12. doi: 10.1016/s0379-0738(99)00146-2. [DOI] [PubMed] [Google Scholar]
- 55.World Health Organization . World Health Organization; Geneva: 1992. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.