Abstract
Background
Studies in regard to womens’ neural reactivity to erotic and other positive emotional cues in association with sexual hormones are relatively rare and findings rather inconclusive. Concerning the neural reactions towards erotic stimuli, the late positive potential (LPP) is seen as the most relevant ERP-component: More positive amplitudes are supposed to reflect larger motivational salience and higher arousal in reaction to the presented stimuli. Therefore, it was expected that the LPP in reaction to erotic pictures would be more pronounced during fertile periods of the menstrual cycle around ovulation, as well as to be associated with estradiol-levels. A similar pattern was hypothesized to be present with testosterone-levels, whereas no association with progesterone was expected.
Method
N = 35 free-cycling women completed an Erotic picture Stroop task (neutral, positive, and erotic stimuli, with three neutral- and three erotic subcategories) during follicular phase, ovulation and luteal phase, while EEG was recorded. Subjects provided saliva samples in order to determine estradiol-, progesterone-, and testosterone levels at each measuring time, and affective states were assessed using the Positive and Negative Affect Scale (PANAS).
Results
LPPs in reaction to erotic-compared to positive- and neutral pictures were larger in every cycle phase. LPPs in reaction to erotic couples were strongest in comparison to every other (sub-) category. During ovulation, higher estradiol-concentrations were associated with lower LPP-amplitudes towards erotic-couples- than to neutral pictures. No effects of progesterone, no direct effect of testosterone, as well as no effects of cycle phase, were evident.
Conclusion
Results partly contradict our hypotheses, as estradiol was expected to be positively associated with LPP during fertile stages. Possible differences between stimulus-entities (words v. pictures) and ideas for further research are being discussed.
Keywords: ERP, LPP, Menstrual cycle, Estradiol, Progesterone, Testosterone, Stroop
Highlights
-
•
Associations of LPP-amplitudes in reaction to erotic (vs. neutral) pictures with estradiol.
-
•
Higher Estradiol concentrations are associated with blunted LPP-amplitudes towards erotic pictures during ovulation.
-
•
Progesterone is not associated with the reactivity towards erotic pictures in the LPP.
-
•
Cycle phase is not associated with the reactivity towards erotic pictures in the LPP.
1. Introduction
Influence of the menstrual cycle on modulation of emotional processing has gained more attention during the last decades, as it can affect women’s behavior as well as women’s mental health. Regardless of the importance of this topic, it still remains a research field with a lot of blank spots [1]. From an evolutionary point of view, it seems likely to make a lot of sense for the reactivity towards positive cues – and especially – those with erotic content – to vary during menstrual-cycle [2,3]. Important brain areas in terms of sexual desire- and regulation of sexual behavior involve the hypothalamus, the amygdala as well as the mesolimbic reward circuit [4,5]. Still, in which way the neural reactivity towards erotic cues is associated with females’ menstrual cycle and with its hormonal fluctuations, mostly still remains unclear. Particularly concerning event-related-potentials (ERPs), most studies concentrate on effects of negative stimuli as well as on progesterone and ERPs [[6], [7], [8]]. In a recent study investigating free cycling women and their reaction to positive and erotic words in association with estradiol and progesterone, it has been reported that reaction in the late positive potential (LPP), which is related to motivational salience [9], was associated with estradiol concentration during fertile phases of the menstrual cycle [10].
1.1. Menstrual cycle phases and emotional reactivity: The role of sex steroids
Estrogen-, progesterone-, and testosterone receptors are localized in various regions of the brain–e all of them being involved in i.a. emotion- and cognitive regulation, and stress-reactivity [1,[11], [12], [13], [14], [15], [16], [17], [18], [19]]. However, there are diverging results regarding different influences of hormonal variations on emotion and cognition. This might be due to the high variance in studies concerning different assessment methods (between-subject vs. within-subject design), as well as some only relying on participants’ self-report, which is often unreliable [[20], [21], [22]]. Therefore, a within-subject design with several EEG-testing-times during females’ cycle as well as hormonal analyzes seems most promising.
While associations of progesterone with increased sensitivity to threat-associated facial expressions [[23], [24], [25]], and increased amygdala reactivity, as well as emotional memory performance in the luteal phase (LU) have been reported [11,26,27], an association with progesterone and reactivity towards positive and/or erotic stimuli has not been described so far. In one of our preceding studies, progesterone was not associated with differences in reactivity to erotic words in the LPP [10]. Wang & Johnston [28] showed associations with higher pleasantness-ratings to emotional stimuli during phases with high estradiol levels, whereas the ERP-component “P3” was more positive in response to baby-faces during LU. In an fMRI study by Rupp et al. [29], activity in the orbitofrontal-cortex in response to male faces was elevated during FO. Moreover, this response was correlated with estradiol/progesterone-ratios.
Research regarding testosterone also largely concentrates on reactivity towards negative emotional stimuli. Van Peer et al. [30] analyzed early ERP components which were recorded during an Emotional Stroop-paradigm (ESP) and reported that one-time administration of testosterone in women led to blunted responses towards angry faces in the face-relevant ERP called “N170”. Testosterone has also been reported to modulate anger- and threat processing in both sexes [31]. Research on the influence of testosterone in women on neural reactivity towards positive stimuli is rather rare. Zhang et al. [7] found negative correlations between testosterone concentration during early follicular and luteal phase and LPP-amplitudes in response to neutral, but not to positive stimuli.
One of the most promising components to investigate ERPs in association with emotional processing is the LPP. It is a wide positive potential over centro-parietal scalp areas with an onset at approximately 400–500 ms post stimulus, which can last for several hundred milliseconds [32]. Whereas the localization of the LPP’s origin is not clear, it is postulated that it is associated with activity in the limbic system and the mesolimbic dopamine system: LPP-amplitudes increase after presentation of emotional- and reward-related stimuli [10,[33], [34], [35], [36]]. Furthermore, the emotional impact (i.e. the higher positivity) on the LPP is related to the stimulus’ emotional intensity- and salience [9]. fMRI studies have shown that relevant brain areas activated during observation of erotic stimuli are predominantly areas belonging to the limbic system, whereas results according to associations with menstrual cycle revealed heterogeneous results [37,38]. In contrast to the reactivity towards negative stimuli, reward-related areas belonging to the mesolimbic dopamine system, also play a role regarding viewing of sexual stimuli [4], in line with the idea of “wanting” (anticipation of reward) postulated by Berridge und Robinson [39].
1.1.1. LPP and menstrual cycle
Although studies regarding associations of the LPP and sexual hormones are sparse, in a recent study (between-subject-design) 23 women were asked to rate emotional stimuli in terms of valence and arousal, while recording the EEG: No significant associations between LPP and sexual hormones could be found, however [6]. Zhang et al. [7] instead, reported negative correlations of the LPP with positive emotional pictures and progesterone levels during FO, as well as positive correlations of the overall LPP and progesterone levels in LU (within-subject-design). An earlier Study by Krug et al. [3], reported larger LPPs (within-subject-design) towards sexual stimuli in a visual attention task during ovulation (OV), pointing to associations with estradiol. However, hormonal concentrations had not directly been assessed, but rather been estimated out of subjects’ self-reported cycle phase. Therefore, main weaknesses of past publications in this research area are, firstly that hormonal concentrations were not assessed, but estimated by cycle phase; and, secondly, a general lack of neurophysiological studies concerning the reactivity towards positive emotional and erotic cues, with the focussing more strongly on behavioral measures [40].
As we reported associations between estradiol (i.e. follicular phase and ovulation) and LPP-reactivity towards erotic words in an Emotional Stroop paradigm during fertile cycle phases [10], we wanted to further elucidate this association in a within-subject-designed study with N = 35 free cycling women and the reactivity towards emotional pictures instead of emotional words. In contrast to the last study, we did not only assess estradiol and progesterone, but also testosterone: In order to find out if associations with estradiol and LPPs might be even more related to testosterone, with testosterone being the direct precursor of estradiol. Additionally, ovulation was validated using tests that indicate luteinizing hormone (LH) which peaks shortly before the actual ovulation takes place.
1.2. Objectives
1.2.1. Endocrinological measures: Hormonal concentrations across the cycle
In order to validate self-reports on cycle phase, differences between follicular phase (FO), ovulation (OV), and luteal phase (LU) in progesterone and estradiol were analyzed if they were in the expected distribution (estradiol higher during follicular phase and peaking during ovulation, progesterone increasing after ovulation). Furthermore, it was tested if testosterone followed a similar secretion-pattern as estradiol.
1.2.2. Positive and negative affective states during the cycle phases and hormonal variations
Possible differences in positive and negative affect across cycle phases could be evident (i.e. higher positive affect in fertile phases, increasing negative affect in LU, and be associated with estradiol, progesterone, and/or testosterone.
1.2.3. LPP towards emotional pictures across the cycle
Serving as manipulation check, the general reactivity towards erotic pictures in the LPP is expected to be higher than towards neutral ones, independent of cycle phase.
In association with sex hormones, we expected:
-
a)
Progesterone: Negative relationship between progesterone and LPP-amplitude towards erotic stimuli in the luteal phase
-
b)
Estradiol: Positive correlations between LPP-amplitude towards erotic stimuli and estradiol during FO & OV are expected
-
c)
Testosterone: A similar reaction pattern as with estradiol towards erotic pictures is expected
2. Methods
2.1. Overview and study design
The current study consisted of a counter-balanced repeated measurement approach (three measuring times: follicular-, and luteal phase, and ovulation) to further elucidate the relationship between fluctuations of ovarian hormones during menstrual cycle and the reactivity towards positive and erotic pictures, operationalized with mean LPP-amplitude. Additionally, associations of current affective state with hormonal levels were investigated.
Each subject was measured three times (EEG, saliva samples, PANAS). Subjects underwent another EEG-paradigm (passive viewing task of food pictures), at each measuring time in a counter-balanced order to prevent sequence effects, which was part of a cooperation with the Department of Psychotherapy and Systems Neuroscience at the University of Giessen. Results regarding this paradigm are going to be reported elsewhere. One measurement was conducted during follicular phase (mean day = 8.06, SD = 0.91), one during ovulation (mean day = 15.23, SD = 2.65) and one during luteal phase (mean day = 25.89, SD = 1.79). The cycle phase of the first measurement was randomized. Appointments were scheduled based on the self-disclosed first day of their current cycle (i.e. day on which menstruation started). Eleven subjects were first assessed in luteal phase, eleven during ovulation, 14 were first assessed in follicular phase. For further scheduling, these subjects contacted the experimenter on the first day of their new menstrual cycle. Testing appointments during ovulation were terminated using indicator tests of luteinizing hormone (LH) (OneStep, AIDE Ovulationstest, Shanghai International Holding Corporation, Hamburg). The used tests measure the LH concentration in the urine with a sensitivity of 20miu/ml. An LH-peak takes place ten to 12 h prior to ovulation and LH-tests hence can be used to predict the time of ovulation quite accurately. Subjects were instructed to use these tests at home twice a day for a duration of six days. Testing appointments during ovulation were scheduled within 48 h after subjects announced a positive LH-test result. Special attention was paid to schedule all three testing appointments at approximately the same time of the day (i.e. morning 08–10:00am, noon 12–2:00 p.m., afternoon 2–4:00 p.m., evening 4–6:00 p.m.). We did not check for an overall time effect, because most of the subjects were tested during 2–4pm. Therefore, a difference between subjects was not expected, as variance was not big enough.
2.2. Participants
Thirty-five native German-speaking Caucasian females between 18 and 34 years of age participated in this study (mean age ± SD: 24.7 ± 3.29 years) and had a Body-Mass-Index (BMI) of 22.33 (±3.09). All subjects had normal or corrected-to normal vision and were right-handed. The absence of current or chronic physical and psychological illness was confirmed via self-report in a questionnaire asking about medication intake and various acute and chronic diseases. Subjects reported no intake of hormonal contraceptives for at least 12 months, no regular medication-intake, no substance-abuse, and subjects had to have a regular menstrual cycle between 25 and 32 days (mean ± SD: 27.87 ± 1.47 days). All subjects gave their written informed consent before testing and received a monetary compensation of 8€/h for their participation in the project. Before and after EEG-testing, participants provided saliva samples for hormonal analyses. The study complies with the Declaration of Helsinki and was approved by the local ethics committee of the University of Giessen, Dept. of Psychology (file: 2017–0038).
2.3. Subjective affective ratings
Subjective affective ratings were assessed at each measuring time (FO, OV, LU) before EEG-testing, using the German version [41]of the Positive and Negative Affect Scale (PANAS; [42]).
2.4. Endocrine measurements
Saliva samples were collected in SaliCaps (IBL, Hamburg, Germany) two times per measuring time for estradiol (17β-estradiol; E2), progesterone (P), and testosterone (T), respectively, with one saliva sample being collected before, and one being collected after EEG-testing. Saliva was collected using the SaliCap included straws until at least 50% of the tube was filled. Afterwards, samples were placed in a freezer at − 20C°. E2, P and T-samples were then averaged (mean of the samples before and after EEG-testing) and analyzed using enzyme-linked-immunosorbent assay- (ELISA-) kits (IBL, Hamburg, Germany), which are all validated by LC-MS according to the manufacturer. All samples were analyzed in one run with assay-kits from the same lot to decrease inter-assay-variability. Moreover, all samples were analyzed in duplicates. Mean intra-assay coefficient across the whole range of standards was 1.83% for E2, 1.41% for P, and 1.55% for T, respectively. Inter-assay coefficients ranged from 0.89 to 1.24% for E2, from 2.14% to 5.36% for P, and from 3.77 to 10.77% for T, respectively. The standard curves were of expected slope and shape. Mean concentration for low E2-control was 3.45 pg/ml (CV 1.24%), and for high E2-control 12.30 (CV 0.89%). Mean concentration for low P-control was 67.75 pg/ml (CV 5.46%), and for high P-control 644.35 pg/ml (CV 2.14%). Mean concentration for low T-control was 25.42 pg/ml (CV 10.57%), and for high T-control 197.40 pg/ml (CV 3.77%).
2.5. ERP recordings and analyzes
EEG signals were amplified using a 0.1–80 Hz band-pass filter (BrainAmpDC amplifier; Brain Products GmbH, Gilching, Germany) and a sampling rate of 250 Hz at 32 scalp sites according to the 10-20-system. Active Ag/AgCl electrodes were used (actiCAP 32Ch, Brain Products GmbH, Herrsching, Germany). Impedances were kept below 10 kΩ. Vertex electrode was used as online-reference. The data were processed digitally offline, using Brain Vision Analyzer software (Brain Products GmbH, Gilching, Germany). Data were filtered using a 30 Hz (12dB/oct per order) low-pass Butterworth IIR filter, manually inspected for artifacts that were not caused by eye movements and, subsequently, excluded. To remove eye-movement artifacts, an Independent Component Analyses (ICA-) based correction algorithm was applied as implemented in the Brain Vision Analyzer. Thereafter, data were re-referenced to an average reference, filtered using a 0.5 Hz (12dB/oct per order) high-pass Butterworth IIR and a 50 Hz notch filter and, subsequently, segmented (stimulus-locked; −200 ms–1000 ms). Segments were averaged for each experimental condition and individual; consisting of 61 trials on average (SD = 3.1), and baseline-corrected (−200 to stimulus-onset). For statistical analyzes of the LPP, mean amplitudes in the time window between 400 and 900 ms were extracted at the centro parietal electrodes CP1 and CP2. The electrode choice was based on visual inspection of the grand average waves and in accordance with the typical centro parietal topography of the LPP.
2.6. Emotional Stroop Paradigm (ESP)
The ESP was used to examine processing of emotional stimuli. Whereas the regular emotional Stroop task contains words [43], pictures from different emotional categories were used in this paradigm, presented in different colors. Picture main categories were erotic, divided into three subcategories (couples, men in underwear, women in underwear), positive (pictures of happy people), and neutral, again divided into three subcategories (neutral couple pictures, single persons, pictures of trees). Stimuli category selection was based on results published from Ciardha und Gormley [44,45], with pictures of high motivational salience causing interference in the subjects’ response. However, picture categories were adapted to this research question, as we studied heterosexual healthy female subjects, whereas Ciardha und Gormley [44,45] studied hetero- and homosexual men and male sexual offenders. Stimulus selection will be described more detailed in section 2.6.1 below. Presented pictures were colored in red, green, yellow and blue. Stimuli had a size of 640 x 480px and were presented centrally in front of a black background. After stimulus onset, the subjects’ task was to indicate the picture’s color as fast and as accurately as possible using a response panel with four respectively colored buttons (Cedrus RB-730, Cedrus Corp., San Pedro, Calif., USA). According to button arrangement, they were instructed to use their right index finger for the yellow and the blue button and the left index finger for green and red, respectively. Participants were seated in a comfortable chair at a 60 cm distance to the screen. Stimulus presentation and response recording were done using Presentation 16.3 software (Neurobehavioral Systems Inc., Albany, Calif., USA) and a Pentium (Intel Corp., Santa Clara, Calif., USA)-based personal computer. SPSS Statistics 23 was used for statistical analyses (IBM Corp., Somer, N·Y., USA).
Each of the seven subcategories consisted of eight images and each of these images was presented two times in each color. Consequently, the ESP consisted of 448 trials (7 ∗ 8 ∗ 2 ∗ 4) that were divided into four blocks, each consisting of 112 trials. Each block was separated from the next by a 30 s break. After initial instruction, subjects executed 40 training trials consisting of colored squares in the above-mentioned colors. An additional twelve training trials were presented before block two to four, respectively. Picture presentation endured until subjects reacted by pressing the colored button. Afterwards a between-trial fixation cross was presented for a randomized duration (1000–1500 ms). Overall duration of the ESP was app. 20 min (in dependence of the participants’ speed). Fig. 1 illustrates the paradigm.
Fig. 1.
Emotional Stroop paradigm. Subjects were asked to react to the color of the presented picture as fast, and as accurate as possible. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.6.1. ESP stimulus selection
For initial stimulus selection, 364 pictures corresponding to the above-mentioned categories were collected from www.shutterstock.com. These pictures were then grayscaled. They were inserted into a preliminary online survey where they were rated on two visual analogue scales (VAS) reaching from 0 to 100. For the arousal scale, 0 indicated that the picture was not at all arousing, 100 indicated that it was very arousing. Correspondingly, 0 indicated extremely unpleasant and 100 extremely pleasant on the valence scale. The aim of the online survey was to select pictures for each stimulus category that fulfilled the following criteria: a valence rating of approximately 50 and low arousal ratings for the neutral stimulus category; high valence ratings and an arousal rating of approximately 50 for the positive stimulus category and high valence as well as arousal ratings for the erotic stimulus category. Selection was conducted based on means and standard deviations of all ratings. Afterwards, balance of male and female pictures was evaluated for the person neutral and person positive categories. Overall N = 144 heterosexual women (age: M = 25.11, SD = 7.52) participated in the online survey.
Ratings in the preliminary study confirmed that erotic couples were rated as more arousing and more pleasant compared to all other picture categories including erotic men, valence: Mdiff = 12.42, p = .003; arousal: Mdiff = 4.62, p = .035, and women, valence: Mdiff = 20.34, p < .001, arousal: Mdiff = 11.81, p < .001. Pictures in the person positive category were rated as more pleasant and more arousing compared to pictures showing neutral persons, valence: Mdiff = 17.46, p < .001; arousal: Mdiff = 27.63, p < .001, neutral couples, valence: Mdiff = 19.75, p = < .001; arousal: Mdiff = 27.32, p < .001, and trees, valence: Mdiff = 19.18, p < .001; arousal: Mdiff = 25.54, p < .001. There were no significant within-category differences neither in valence nor arousal ratings.
2.7. Procedure
Subjects were instructed to not eat, smoke or drink coffee/energy drinks for 1 h prior to the experiment and to not use hairspray/hair gel on the day of the experiment. On their first appointment, subjects were informed about EEG testing and gave written informed consent for participation. Subjects completed the PANAS and provided saliva samples, as they also did after completion of the EEG-experiment.
2.8. Statistical analysis
2.8.1. Participants
Possible associations of subjects’ hormone-concentrations and age were tested, and if proofed significant, added as covariate into analyses of hypotheses where hormone concentration was part of the calculations.
In order to check if hormonal concentration was as expected, three separate repeated measurement ANOVAs with cycle phase (3 levels) and hormone concentration (3 levels, estradiol/progesterone/testosterone), were calculated.
2.8.2. Subjective affective states during cycle phases
Subjective affective state was tested each time before EEG-recording. To assess differences in emotional states between cycle phases, repeated-measurement ANOVAs with the factors cycle phase (3 levels), Emotion (2 levels, positive vs. negative emotional state) were calculated. Furthermore, it was assessed if means of self-rated emotional states were correlated with estradiol, progesterone, or testosterone-concentrations at different cycle phases.
2.8.3. Neural response towards erotic pictures in association with gonadal hormones
Mean amplitudes of the LPP were entered into three repeated-measures ANOVAs with the within-subject factors electrode (CP1 and CP2) and emotion (erotic-couple versus neutral pictures), and 1) progesterone, 2) estradiol and 3) testosterone as covariates (3 measuring times each). Greenhouse-Geisser correction was applied in case of violation of the sphericity assumption. Bonferroni corrections for multiple testing were applied in case of significant effects. In case of significant interaction-effects of hormone concentration and emotional response measured in the LPP-response, correlations were calculated, with hormone concentration at the specific cycle phase along with the difference of mean amplitudes (mean of both electrodes; erotic minus neutral) at the respective cycle phase.
3. Results
3.1. Participants
Two participants had to be excluded from EEG-and any further analyses due to artifacts during EEG-testing, leaving a final sample of 33 female subjects. Each subject was measured three times during the menstrual cycle. Groups did not differ in age, cycle duration or Body-Mass-Index (BMI). In order to reveal possible significant associations between age and concentration of gonadal steroids, correlational analyses were conducted. Age did neither correlate with estradiol during FO, OV, nor during LU (all p > .67), nor with progesterone during any phase (all p > .32), as well as neither with testosterone at any ohase (all p > .15). As no significant associations between age and hormone concentration during any cycle phase was evident, age was not added as covariate in any analysis.
3.2. Subjective affective states during cycle phases and hormonal concentrations
Subjects’ general positive affect (PA) was greater than their negative affect (NA), F(1,32) = 96.29, p < .01, ƞp2 = 0.830 (M positive affect = 2.4 ± 0.08; M negative affect = 1.7 ± 0.063). Whereas PA did not differ significantly during cycle phases, F(2,31) = 1.09, p = .340, NA affect was higher in luteal phase in comparison to ovulation, F(2,31) = 5.05, p < .05, ƞp2 = 0.129.
No associations of progesterone-concentration and positive- (r = 0.070, p = .69) or negative affect (r = 0.1356, p = .45), nor with estradiol-concentration with positive- (r = −0.284, p = .10) or negative affect (r = −0.152, p = .39), nor with testosterone and PA (r = −0.022, p = .90) or NA (r = −0.311, p = .078) in general or during different cycle phases were evident.
3.3. Endocrine measurements
3.3.1. Progesterone
Progesterone-concentrations differed significantly between cycle phases, F(1.27, 41.75) = 33.02, p < .001, ηp2 = 0.508. Pairwise comparisons showed that progesterone concentration was significantly higher during LU compared to FO, Mdiff = 72.97, p < .001, and to OV, Mdiff = 60.19, p < .001. It was also higher during OV than during FO, Mdiff = 12.79, p = .039. Fig. 2 illustrates differences in progesterone-concentrations during cycle phases.
Fig. 2.
Mean progesterone-concentrations across different cycle phases of n = 33 free-cycling women. Error bars reflects standard errors of the mean (SEM); ∗∗∗p < .001, ∗p < 05.
3.3.2. Estradiol
Repeated-measurements ANOVA revealed significant differences between estradiol-concentration during cycle phases, F(2,66) = 7.19, p = .002, ηp2 = 0.183. Pairwise comparison revealed that estradiol concentration during OV was significantly higher than during FO, Mdiff = 0.66, SEM = 0.20, p = .008. No difference was observed between OV and LU or FO and LU. Results are illustrated in Fig. 3.
Fig. 3.
Mean estradiol-concentrations across different cycle phases of n = 33 free-cycling women. Error bars reflect standard errors of the mean (SEM); ∗∗p < .01.
3.3.3. Testosterone
No differences between cycle phases were evident in testosterone concentrations, F(2,66) = 1.76, p = .180. Fig. 4 illustrates testosterone-concentrations during cycle phases.
Fig. 4.
Mean testosterone-concentrations across different cycle phases of n = 33 free-cycling women (no significant differences between cycle phases). Error bars reflect standard errors of the mean (SEM).
3.4. Neural response towards erotic pictures in association with gonadal hormones
First, general responsivity towards erotic vs. other emotional categories in the LPP were analyzed (manipulation check). Second, influences of cycle phase, and, furthermore, estradiol and progesterone and testosterone-concentrations towards the reactivity to erotic pictures were investigated.
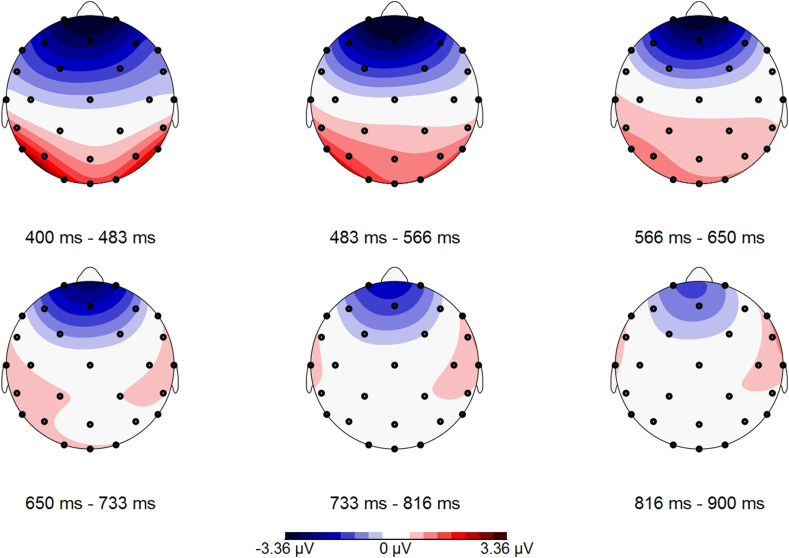
The LPP-response towards erotic pictures was in general more positive than to neutral ones (main-effect of emotional category), with F(1,33) = 53,157, p < .001, ƞp2 = 0.610, independent of cycle phase. LPPs towards erotic versus neutral pictures are illustrated in Fig. 5. A topographical map of the LPP-reactivity towards erotic pictures can be seen in Fig. 6, towards neutral ones in Fig. 7.
Fig. 5.
LPP [μV] during the time window between 400 and 900 ms post stimulus (shaded in blue) at electrode CP1 towards erotic couples (red line) vs. the three neutral picture categories neutral person (black line), neutral couple (dark blue), and tree (purple). Pictures across three cycle phases of n = 33 free-cycling women. LPP towards erotic pictures is significantly more positive than towards all neutral categories, p < .001, reactivity towards neutral categories does not differ within them. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6.
Topographical map of the LPP in reaction to erotic pictures (400–900 ms post stimulus).
Fig. 7.
Topographical map of the LPP in reaction to neutral pictures (400–900 ms post stimulus).
Regarding specific emotional picture subcategories, analyzes revealed expected differences in LPP amplitudes, with highest LPP-amplitudes in reaction to subcategory “erotic couple” F(1,32) = 26.7, p < .001, ƞp2 = 0.440, independent of cycle phase. LPP towards erotic couples was higher than towards all the other subcategories (Mdiff p < .001 in all cases, Bonferroni corrected for multiple comparisons). Detailed results are illustrated in Fig. 8.
Fig. 8.
LPP (400–900 ms post stimulus) at electrodes CP1 and CP2 towards different emotional picture categories across three cycle phases of n = 33 free-cycling women; ∗∗∗p < .001. Results are Bonferroni corrected for multiple comparisons. Error bars reflects standard errors of the mean (SEM).
As the results reveal that LPP-manipulation regarding emotional subcategories was as expected regarding the neutral subcategories, the LPP-reaction of those will be averaged across all neutral categories. Regarding the erotic ones, only the “erotic couple” subcategory will be analyzed in further detail, as LPP towards the subcategories “female” and “male” were not as distinct as expected, with being significantly lower in LPP-amplitude than the erotic couple-subcategory. Therefore, they were excluded from further analyses.
3.4.1. LPP and cycle phase
No direct-effect of cycle phase on responsiveness towards erotic vs. neutral pictures was evident, F(2,66) = 0.254, p = .77. Therefore, LPP-amplitudes in response to erotic pictures did not differ between FO, OV, or LU, if hormonal concentrations were not taken into account.
3.4.2. Associations of LPP-responsiveness towards erotic words with ovarian hormones
-
a)
LPP and progesterone
Reactivity of the LPP towards erotic or neutral pictures did neither interact significantly with subject’s progesterone-concentrations during follicular phase, F(1,29) = 0.52, p = .47, nor during ovulation, F(1,29) = 1.47, p = .23, but showed a tendency to do so during luteal phase, F(1,29) = 3.79, p = .056.
-
b)
LPP and estradiol
Reactivity of the LPP towards erotic and neutral pictures interacted significantly with subject’s estradiol-concentrations during the Ovulation, F(1,29) = 11.94, p = .002, ƞp2 = 0.292, but not during FO, F(1,29) = 0.12, p = .67 or LU, F(1,29) = 2.32, p = .138. In order to assess the direction of the observed interaction during OV, a correlation of the subjects’ estradiol-concentration during ovulation with the LPP (difference variable erotic couple-neutral) was calculated. Results revealed a negative correlation between the difference of the LPP-reaction and estradiol-concentration during Ovulation, r(1,33) = −0.304, p = .043. Therefore, the higher the estradiol-concentration during ovulation, the lower is the difference between LPP towards erotic and neutral pictures. Results can be seen in Fig. 9.
Fig. 9.
-
c)LPP and testosterone
Reactivity of the LPP towards erotic or neutral pictures did neither interact significantly with subject’s testosterone-concentrations during follicular phase, F(1,29) = 0.0, p = .98, nor during ovulation, F (1,29) = 0.80, p = .373, nor during luteal phase, F(1,29) = 1.21, p = .279.
4. Discussion
In this study, associations of gonadal hormones (estradiol, progesterone, and testosterone) with the reactivity towards erotic pictures, operationalized with the LPP-mean-amplitude during womens’ menstrual cycle, was tested in a within-subject-design. It was expected that the reactivity of the LPP towards erotic (vs. neutral) pictures would be dependent of especially estradiol, as we reported interactions of estradiol-concentrations during FO and OV and LPP towards erotic words in a former study, and wanted to establish, if reaction patterns to pictures would be similar as those towards words [10]. Progesterone was not associated with the reactivity towards erotic pictures, which is coherent with former investigation in which it also was not associated with the reactivity towards erotic words. Furthermore, testosterone concentrations were not associated with reactivity in the LPP.
4.1. Endocrine measurements
4.1.1. Progesterone
Progesterone-concentrations were expected to be highest during luteal phase, as progesterone is mainly produced by the corpus luteum after ovulation, and was confirmed by our data. Therefore, reported concentrations are in line with expected E2-and P-concentrations during menstrual cycle.
4.1.2. Estradiol
E2-concentrations were expected to be highest during ovulation, with significant differences towards both the follicular- and luteal phase. Results indicate significant differences in E2-concentration only between the follicular-phase and ovulation. This can be explained by the subjects’ average testing date for luteal phase being on day 26 of their menstrual cycle. As estradiol fluctuates during luteal phase (Bäckström, McNeilly, Leask, & Baird, 1982), it is possible that E2 concentrations from OV to LU were not significantly different because the second E2-peak in LU was measured during our appointment for LU. As ovulation was validated with subjects using LH-tests and by making an appointment for EEG-testing after being tested positive for ovulation by it, it is, nonetheless, secure to assume that subjects were ovulating during respective EEG-measuring time. Furthermore, it is also validated by the subjects’ progesterone concentration during measurement times (see 4.1.1).
4.1.3. Testosterone
No substantial fluctuations of testosterone over the menstrual cycle were revealed. Present outcomes are in line with the results of Liening et al. [46] who found its concentrations to be unaffected by menstrual cycle phases.
As well as in the former study with erotic words [10], subjects’ positive affect was higher than negative affect, independent of cycle phase or any hormonal concentration. Negative affect was, however, significantly higher during luteal phase in comparison to ovulation, again, independent of any hormonal concentration. This finding is in line with the idea of higher depressive symptoms in the premenstrual cycle phases, or premenstrual syndrome [47,48], but one has to emphasize that investigated subjects were psychologically and physiologically healthy young women, and higher negative affect alone does not indicate premenstrual dysphoric disorder.
As no associations with subjective situational wellbeing and any gonadal hormone were evident - neither in this, nor in the former study in our lab, it is possible that pure sex-steroid concentration does not have a direct influence on mood, but rather its interaction and or its relative fluctuations during the cycle. Effects of hormonal changes during climacterium, hormone-replacement-therapy, estrogen-therapy in women with osteoporosis, or hormonal contraceptives on experienced emotions (only to name a few), clearly point to influences of sex hormones on emotional experiences [49].
4.2. Neural responses: LPP
It was postulated that general reactivity towards erotic pictures would result in higher LPP-amplitudes compared to neutral ones, as well as associations of LPP-amplitudes with menstrual cycle estradiol-concentrations, as found in an earlier study towards erotic words. Coherent with our findings in reaction to erotic words, no associations with progesterone and LPP towards erotic pictures were evident [10]. Associations of LPP with testosterone were tested on an exploratory level, as there are no studies (to our knowledge) that tested this association using this or a similar paradigm.
4.2.1. Manipulation check
As expected, a generally more positive LPP towards erotic than neutral pictures was evident, so that more arousing and more positive cues elicited larger LPPs. This effect was evident in comparison of all erotic picture categories (erotic -couple, -man, -women, with erotic male vs. neutral pictures F (1, 32) = 13.76, p = .001, and erotic female vs. neutral F (1, 32) = 22.48, p < .0001.), but most prominent (and significantly higher than to all subcategories) in the erotic couple-category.
4.2.2. The LPP towards erotic (vs. neutral) pictures during different cycle phases
Whereas an interaction of estradiol with reactivity towards erotic stimuli during ovulation (see 4.3.3) was evident, no main effect of cycle phase regarding LPP could be observed. This result is in line with observations regarding the emotional Stroop study with words [10]. Furthermore, these results are in line with studies that concentrated on effects of cycle phase on LPP-reactivity and reaction to emotional stimuli [[6], [7], [8]].
4.2.3. Associations of the LPP towards erotic (vs. neutral) pictures with ovarian hormones
-
a)
Progesterone
In line with results of the emotional Stroop study with erotic words, no associations of progesterone and LPP-response were evident in this study. These results deviate from other ERP studies’ results regarding the reactivity towards emotional stimuli and ovarian hormones, which reported associations with progesterone [[6], [7], [8]]. Those studies mainly focused, however, on luteal phase, on negative emotional responses, as well as associations to neuroticism, or the reactivity towards negative emotional faces [6,7,23,24].
-
b)
Estradiol
Whereas it was expected that the association of estradiol concentration and LPP towards erotic pictures would be similar to reactivity towards erotic words, with significant positive associations during follicular phase, and ovulation, results revealed a different pattern: LPP-amplitudes towards erotic (vs. neutral) pictures correlated significantly with estradiol-concentration during ovulation – however - negatively, i.a. higher estradiol-concentration went along with lower LPP-amplitude towards erotic pictures.
Although no causal interferences can be drawn from these results, it is very important to shed more light on these differing results: On one hand, it is possible that reactivity in the LPP towards words and pictures is inherently different, e.g. because processing of a written stimulus takes longer time, and more (conscious) resources than processing of a picture. Whereas the processing of words is primarily located in the left hemisphere, pictures-especially with emotionally relevant content-are processed primarily in the right hemisphere [50]. This would mean that both stimuli are processed inherently different, and should be treated differently when being analyzed. Another explanation that leads into another direction would be that pictures cause so much cognitive interference in subjects with high estradiol that the LPP-reaction is blunted because of this interference. This would somehow be in line with the findings of blunted LPP responses towards erotic cues in male subjects carrying the risk-allele of the dopamine-D2-receptor-gene-polymorphism Taq1A [36]. This again, is rather hypothetical and further studies should reveal if those results can be replicated. One approach in order to study possible distinct entity differences in the reaction towards erotic words versus pictures in the LPP, would be to study both in the same sample.
-
c)
Testosterone
Based on the findings of higher estradiol being associated with the reactivity towards erotic words during ovulation and follicular phase, it was assumed that testosterone, due to its close biological link to estradiol, also played a role in this reactivity [3,10]. Therefore, an equivalent relationship was hypothesized with the biologically closely but it could not be found. These findings are in line with Zhang et al. [7]. They used another different methodological approach, however: Testosterone concentration was determined in blood samples and the subjects completed an emotional evaluation task while the EEG was recorded in which they had to rate the presented negative, neutral, and positive stimuli regarding valence and arousal. It is possible that the neural processes occurring during the conscious emotional evaluation of the stimuli are different from those that occur during the ESP, in which a reaction task is performed independently from the content of the stimuli. Zhang et al. [7] examined the association between testosterone level and LPP-response towards affective stimuli separately in each cycle phase. Following this approach, we correlated averaged (across cycle phases) LPPs in reaction to erotic couple pictures with mean testosterone. Accordingly, there was no significant relationship between testosterone and LPP-response either (r = −0.21, p = .23).
4.3. Post hoc manipulation check: Reactivity towards pictures of other emotional categories
Post-hoc-analyzes were conducted in order to specify that the reported reaction pattern is not attributable to other positive pictures apart from erotic ones: There was neither an effect of LPP towards positive pictures (happy/laughing people) vs. neutral categories, F(1,32) = 0.18, p = .67, nor an interaction of estradiol, progesterone or testosterone at any cycle phase towards those positive pictures (p > .05) evident.
4.4. Limitations of the study
Several limitations regarding the present study have to be mentioned. Firstly, generalization of effects is difficult due to the limited representativeness of the subjects who were all students and somewhat young. Secondly, a potential selection-bias because of the stringent selection-criteria reduces representativeness even more. However, it has to be noted that limitation in variance reduces the likelihood to find significant results. Hence, it is possible that findings in a more heterogeneous sample could even lead to findings that are more robust. Moreover, comparisons with a control group would support a better interpretation and understanding of the results. One possibility would be a control group of women who take oral contraceptives, as those lower the testosterone concentration [46] and stabilize cyclical estradiol fluctuations [51]. The effects of endogenous and exogenous testosterone can be more easily interpreted with the implementation of a control group of women who receive exogenous testosterone.
5. Conclusion
The present study was supposed to be a continuation and derivative of the emotional Stroop study in reaction to words, where higher levels of estradiol during follicular phase and ovulation were association with a more positive LPP towards erotic words. Whereas it was hypothesized that reaction pattern towards erotic pictures was similar to the former one, results show a somewhat different pattern: Higher estradiol goes along with lower LPPs in reaction to erotic pictures during ovulation. This might be due to a distinct processing of words in contrast to pictures, possibly with words eliciting more own ideas and fantasy, whereas pictures might lead to different thought processes (i.e. self-esteem relevant, or other associations), that are not directly associated with motivational salience, and maybe also not positively connoted. These ideas are, however, only speculative and similar studies need to be conducted, in order to specify these issues. Nonetheless, findings regarding progesterone are in line with previous findings in our lab, with no associations towards erotic stimuli.
In contrast to the Word-Stroop-Study we validated ovulation using LH-tests, in order to make sure to actually test the subjects during ovulation. This is a major issue regarding results of other studies, as they, firstly, often do not measure any hormones, but just assume due to subjects’ calendar-method, secondly, even if they measure them, mostly only during follicular- and luteal-phase, and thirdly, seldom validate ovulation with LH-tests. Therefore, results cannot be explained by wrong interferences regarding cycle-phases. Further studies need to be conducted in order to clarify reported findings. Those should also include women who use hormonal contraception, in order to further elucidate the role of gonadal hormones and their concentrations in women’s’ emotional-, behavioral-, and neurophysiological patterns.
Role of funding sources
None.
Declarations of competing interest
None.
Acknowledgements
We would like to thank Nicole Tscherney for research assistance, and Saskia Oriendi for collecting the data and acquiring participants. We also thank our participants for their dedication in research.
References
- 1.Toffoletto Simone, Lanzenberger Rupert, Gingnell Malin, Sundström-Poromaa Inger, Comasco Erika. Emotional and cognitive functional imaging of estrogen and progesterone effects in the female human brain. A systematic review. Psychoneuroendocrinology. 2014;50:28–52. doi: 10.1016/j.psyneuen.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 2.Little Anthony C. The influence of steroid sex hormones on the cognitive and emotional processing of visual stimuli in humans. Front. Neuroendocrinol. 2013;34(4):315–328. doi: 10.1016/j.yfrne.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Krug R., Plihal W., Fehm H.L., Born J. Selective influence of the menstrual cycle on perception of stimuli with reproductive significance. An event-related potential study. Psychophysiology. 2000;37(1):111–122. doi: 10.1111/1469-8986.3710111. [DOI] [PubMed] [Google Scholar]
- 4.Micevych Paul E., Meisel Robert L. Integrating neural circuits controlling female sexual behavior. Front. Syst. Neurosci. 2017;11:42. doi: 10.3389/fnsys.2017.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salamone John D., Pardo Marta, Yohn Samantha E., López-Cruz Laura, SanMiguel Noem∖’∖i, Correa Mercè. Behavioral Neuroscience of Motivation. Springer; 2015. Mesolimbic dopamine and the regulation of motivated behavior; pp. 231–257. [DOI] [PubMed] [Google Scholar]
- 6.Mačiukaitė Laura, Jarutytė Lina, Rukšėnas Osvaldas. The effects of menstrual cycle phase on processing of emotional images. J. Psychophysiol. 2017;31(4):179–187. doi: 10.1027/0269-8803/a000179. [DOI] [Google Scholar]
- 7.Zhang Wenjuan, Zhou Renlai, Wang Qingguo, Zhao Yan, Liu Yanfeng. Progesterone mediates the late positive potentials evoked by affective pictures in high neuroticism females. Psychoneuroendocrinology. 2015;59:49–58. doi: 10.1016/j.psyneuen.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Wenjuan, Zhou Renlai, Ye Maolin. Menstrual cycle modulation of the late positive potential evoked by emotional faces. Percept. Mot. Skills. 2013;116(3):707–723. doi: 10.2466/22.27.PMS.116.3.707-723. [DOI] [PubMed] [Google Scholar]
- 9.Hajcak Greg, Dunning Jonathan P., Foti Dan. Motivated and controlled attention to emotion. Time-course of the late positive potential. Clin. Neurophysiol. 2009;120(3):505–510. doi: 10.1016/j.clinph.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 10.Munk Aisha J.L., Zoeller A.C., Hennig J. Fluctuations of estradiol during women’s menstrual cycle: influences on reactivity towards erotic stimuli in the late positive potential. Psychoneuroendocrinology. 2018;91:11–19. doi: 10.1016/j.psyneuen.2018.02.028. Online verfügbar unter. [DOI] [PubMed] [Google Scholar]
- 11.Andreano Joseph M., Cahill Larry. Menstrual cycle modulation of medial temporal activity evoked by negative emotion. Neuroimage. 2010;53(4):1286–1293. doi: 10.1016/j.neuroimage.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Wingen Guido, van Broekhoven Frank, Verkes Robbert Jan, Petersson Karl Magnus, Bäckström Torbjörn, Buitelaar Jan, Fernández Guillén. How progesterone impairs memory for biologically salient stimuli in healthy young women. J. Neurosci. 2007;27(42):11416–11423. doi: 10.1523/JNEUROSCI.1715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ossewaarde Lindsey, Hermans Erno J., van Wingen Guido A., Kooijman Sabine C., Johansson Inga-Maj, Bäckström Torbjörn, Fernández Guillén. Neural mechanisms underlying changes in stress-sensitivity across the menstrual cycle. Psychoneuroendocrinology. 2010;35(1):47–55. doi: 10.1016/j.psyneuen.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Melcangi Roberto C., Panzica G., Garcia-Segura L.M. Neuroactive steroids. Focus on human brain. Neuroscience. 2011;191:1–5. doi: 10.1016/j.neuroscience.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Albert Kimberly, Pruessner Jens, Newhouse Paul. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology. 2015;59:14–24. doi: 10.1016/j.psyneuen.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gangestad Steven W., Thornhill Randy. Menstrual cycle variation in women’s preferences for the scent of symmetrical men. Proc. R. Soc. Lond. B Biol. Sci. 1998;265(1399):927–933. doi: 10.1098/rspb.1998.0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bixo Marie, Bäckström Torbjörn, Winblad Bengt, Andersson Agenta. Estradiol and testosterone in specific regions of the human female brain in different endocrine states. J. Steroid Biochem. Mol. Biol. 1995;55(3):297–303. doi: 10.1016/0960-0760(95)00179-4. [DOI] [PubMed] [Google Scholar]
- 18.Cunningham Rebecca L., Lumia Augustus R., McGinnis Marilyn Y. Androgen receptors, sex behavior, and aggression. Neuroendocrinology. 2012;96(2):131–140. doi: 10.1159/000337663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrin Jennifer S., Hervé Pierre-Yves, Leonard Gabriel, Perron Michel, Pike G. Bruce, Pitiot Alain, et al. Growth of white matter in the adolescent brain. Role of testosterone and androgen receptor. J. Neurosci. : the official journal of the Society for Neuroscience. 2008;28(38):9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jukic Anne Marie Zaura, Weinberg Clarice R., Wilcox Allen J., McConnaughey D. Robert, Hornsby Paige, Baird Donna D. Accuracy of reporting of menstrual cycle length. Am. J. Epidemiol. 2007;167(1):25–33. doi: 10.1093/aje/kwm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Small Chanley M., Manatunga Amita K., Marcus Michele. Validity of self-reported menstrual cycle length. Ann. Epidemiol. 2007;17(3):163–170. doi: 10.1016/j.annepidem.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Wideman Laurie, Montgomery Melissa M., Levine Beverly J., Beynnon Bruce D., Shultz Sandra J. Accuracy of calendar-based methods for assigning menstrual cycle phase in women. Sport Health. 2013;5(2):143–149. doi: 10.1177/1941738112469930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conway C.A., Jones B.C., DeBruine L.M., Welling L.L.M., Law Smith M.J., Perrett D.I., et al. Salience of emotional displays of danger and contagion in faces is enhanced when progesterone levels are raised. Horm. Behav. 2007;51(2):202–206. doi: 10.1016/j.yhbeh.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Derntl Birgit, Hack Ramona L., Kryspin-Exner Ilse, Habel Ute. Association of menstrual cycle phase with the core components of empathy. Horm. Behav. 2013;63(1):97–104. doi: 10.1016/j.yhbeh.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson Rebecca, Lewis Michael B. Fear recognition across the menstrual cycle. Horm. Behav. 2005;47(3):267–271. doi: 10.1016/j.yhbeh.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Poromaa Inger Sundström, Gingnell Malin. Menstrual cycle influence on cognitive function and emotion processing—from a reproductive perspective. Front. Neurosci. 2014;8 doi: 10.3389/fnins.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayer Janine, Schultz Heidrun, Gamer Matthias, Sommer Tobias. Menstrual-cycle dependent fluctuations in ovarian hormones affect emotional memory. Neurobiol. Learn. Mem. 2014;110:55–63. doi: 10.1016/j.nlm.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Wang Xiao Tian, Johnston Victor S. Changes in cognitive and emotional processing with reproductive status. Brain Behav. Evol. 1993;42(1):39–47. doi: 10.1159/000114139. [DOI] [PubMed] [Google Scholar]
- 29.Rupp Heather A., James Thomas W., Ketterson Ellen D., Sengelaub Dale R., Janssen Erick, Heiman Julia R. Neural activation in the orbitofrontal cortex in response to male faces increases during the follicular phase. Horm. Behav. 2009;56(1):66–72. doi: 10.1016/j.yhbeh.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Peer Jacobien M., Enter Dorien, van Steenbergen Henk, Spinhoven Philip, Roelofs Karin. Exogenous testosterone affects early threat processing in socially anxious and healthy women. Biol. Psychol. 2017;129:82–89. doi: 10.1016/j.biopsycho.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Wirth Michelle M., Schultheiss Oliver C. Basal testosterone moderates responses to anger faces in humans. Physiol. Behav. 2007;90(2):496–505. doi: 10.1016/j.physbeh.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Hajcak Greg, MacNamara Annmarie, Olvet Doreen M. Event-related potentials, emotion, and emotion regulation. An integrative review. Dev. Neuropsychol. 2010;35(2):129–155. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- 33.Cuthbert Bruce N., Schupp Harald T., Bradley Margaret M., Birbaumer Niels, Lang Peter J. Brain potentials in affective picture processing. Covariation with autonomic arousal and affective report. Biol. Psychol. 2000;52(2):95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- 34.Schupp Harald T., Cuthbert Bruce N., Bradley Margaret M., Cacioppo John T., Ito Tiffany, Lang Peter J. Affective picture processing. The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37(2):257–261. [PubMed] [Google Scholar]
- 35.Schacht Annekathrin, Sommer Werner. Emotions in word and face processing. Early and late cortical responses. Brain Cognit. 2009;69(3):538–550. doi: 10.1016/j.bandc.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Munk Aisha J.L., Wielpuetz Catrin, Osinsky Roman, Müller Erik M., Grant Phillip, Hennig Jürgen. Specific reaction patterns to distinct positive emotional cues related to incentive motivation in dependence of the Taq1A-polymorphism. Molecular genetic associations of early and late event-related potentials. NPS. 2016;73(1):23–34. doi: 10.1159/000441658. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Xun, Wang Xiaoying, Parkinson Carolyn, Cai Chengxu, Gao Song, Hu Peicheng. Brain activation evoked by erotic films varies with different menstrual phases. An fMRI study. Behav. Brain Res. 2010;206(2):279–285. doi: 10.1016/j.bbr.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 38.Gizewski Elke R., Krause Eva, Karama Sherif, Baars Anneke, Senf Wolfgang, Forsting Michael. There are differences in cerebral activation between females in distinct menstrual phases during viewing of erotic stimuli. A fMRI study. Exp. Brain Res. 2006;174(1):101–108. doi: 10.1007/s00221-006-0429-3. [DOI] [PubMed] [Google Scholar]
- 39.Berridge Kent C., Robinson Terry E. What is the role of dopamine in reward. Hedonic impact, reward learning, or incentive salience? Brain Res. Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 40.Gildersleeve Kelly, Haselton Martie G., Fales Melissa R. Do women’s mate preferences change across the ovulatory cycle? A meta-analytic review. Psychol. Bull. 2014;140(5):1205. doi: 10.1037/a0035438. [DOI] [PubMed] [Google Scholar]
- 41.Egloff B., Kohlmann C.W., Tausch A., Krohne H.W. Untersuchungen mit einer deutschen Version der “Positive and Negative Affect Schedule”(PANAS)[Evaluation of a German version of the Positive and Negative Affect Schedule (PANAS)] Diagnostica. 1996;42:139–156. [Google Scholar]
- 42.Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect. The PANAS scales. J. Pers. Soc. Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 43.Stroop J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18(6):643–662. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- 44.Ciardha Caoilte Ó., Gormley Michael. Cognitive Approaches to the Assessment of Sexual Interest in Sexual Offenders. 2009. Comparing two implicit cognitive measures of sexual interest. A pictorial modified Stroop task and the implicit association test; pp. 177–201. [Google Scholar]
- 45.Ciardha Caoilte Ó., Gormley Michael. Using a pictorial-modified stroop task to explore the sexual interests of sexual offenders against children. Sexual Abuse. 2012;24(2):175–197. doi: 10.1177/1079063211407079. [DOI] [PubMed] [Google Scholar]
- 46.Liening Scott H., Stanton Steven J., Saini Ekjyot K., Schultheiss Oliver C. Salivary testosterone, cortisol, and progesterone: two-week stability, interhormone correlations, and effects of time of day, menstrual cycle, and oral contraceptive use on steroid hormone levels. Physiol. Behav. 2010;99(1):8–16. doi: 10.1016/j.physbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Gingnell Malin, Morell Arvid, Bannbers Elin, Wikström Johan, Poromaa Sundström, Inger Menstrual cycle effects on amygdala reactivity to emotional stimulation in premenstrual dysphoric disorder. Horm. Behav. 2012;62(4):400–406. doi: 10.1016/j.yhbeh.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Beddig Theresa, Kuehner Christine. Current aspects of premenstrual dysphoric disorder-A review. Psychother. Psychosom. Med. Psychol. 2017;67(12):504–513. doi: 10.1055/s-0043-113816. [DOI] [PubMed] [Google Scholar]
- 49.Richardson T.A., Robinson Menopause and depression: a review of psychologic function and sex steroid neurobiology during the menopause. Prim. Care Update OB/GYNS. 2000;7(6):215–223. doi: 10.1016/s1068-607x(00)00049-4. [DOI] [PubMed] [Google Scholar]
- 50.Wittling W., Roschmann R. Emotion-related hemisphere asymmetry: subjective emotional responses to laterally presented films. Cortex. 1993;29(3):431–448. doi: 10.1016/s0010-9452(13)80252-3. [DOI] [PubMed] [Google Scholar]
- 51.Huppelsberg Jens, Walter Kerstin. Georg Thieme Verlag; 2013. Kurzlehrbuch Physiologie. [Google Scholar]