Abstract
Background
Dyspnoea in patients with a para-oesophageal hernia (PEH) occurs in 7% to 32% of cases and is very disabling, especially in elderly patients, and its origin is not well defined. The present study aims to assess the impact of PEH repair on dyspnoea and respiratory function.
Methods
From January 2019 to May 2021, all consecutive patients scheduled for PEH repair presenting with a modified Medical Research Council (mMRC) score ≥ 2 for dyspnoea were included. Before and 2 months after surgery, dyspnoea was assessed by both the dyspnoea visual analogue scale (DVAS) and the mMRC scale, as well as pulmonary function tests (PFTs) by plethysmography.
Results
All 43 patients that were included had pre- and postoperative dyspnoea assessments and PFTs. Median age was 70 years (range 63–73.5 years), 37 (86%) participants were women, median percentage of the intrathoracic stomach was 59.9% (range 44.2–83.0%), and median length of hospital stay was 3 days (range 3–4 days). After surgery, the DVAS decreased statistically significant (5.6 [4.7–6.7] vs. 3.0 [2.3–4.4], p < 0.001), and 37 (86%) patients had a clinically significant decrease in mMRC score. Absolute forced expiratory volume in one second (FEV1), total lung capacity, and forced vital capacity also statistically significantly increased after surgery by an average of 11.2% (SD 17.9), 5.0% (SD 13.9), and 10.7% (SD 14.6), respectively. Furthermore, from the subgroup analysis, it was identified that patients with a lower preoperative FEV1 were more likely to have improvement in it after surgery. No correlation was found between improvement in dyspnoea and FEV1. There was no correlation between the percentage of intrathoracic stomach and dyspnoea or improvement in PFT parameters.
Conclusion
PEH repair improves dyspnoea and FEV1 in a statistically significant manner in a population of patients presenting with dyspnoea. Patients with a low preoperative FEV1 are more likely to have improvement in it after surgery.
Keywords: Laparoscopic fundoplication, Para-oesophageal hernia, Hiatal hernia, Dyspnoea, Pulmonary function tests, Forced expiratory volume
Hiatal hernias (HHs) are observed in 15–20% of the population and are mostly asymptomatic. HHs with a rolling component (type II, III, or IV), called para-oesophageal hernias (PEHs) [1, 2], representing 5% of all HHs [3] and more frequent in elderly patients [4], may be particularly disabling. Indeed, this pathology not only exposes patients to the risk of gastric volvulus [5] but also strongly impairs the patient’s quality of life when symptomatic, not only occurring mainly as digestive symptoms (e.g. dyspepsia, dysphagia, or heartburn) but also as extra digestive symptoms (e.g. cough, chest pain, or dyspnoea).
Laparoscopic fundoplication (LF), nowadays considered the gold standard treatment for symptomatic PEHs, has shown a convincing effect on digestive symptoms, with a significant decrease in the frequency and intensity of dysphagia and heartburn (86.7% with excellent to good results) unlike extra digestive symptoms, for which the impact of surgery has not yet been clearly demonstrated [6, 7]. Surgery has also shown a significant improvement in quality of life evaluated by the SF36 score, with postoperative scores becoming even better than those of the general population [6, 7]. Thanks to the laparoscopic approach, postoperative morbidity and mortality have significantly decreased over time [8–12], even in patients older than 70 and 80 years old, according to Oor et al. and El Lakis et al. [13, 14].
Dyspnoea is frequently associated with PEH, especially in the elderly population, and is a disabling condition because it limits physical activity and autonomy. These dyspnoeic patients, according to their age and condition, may have a non-PEH-related cause of dyspnoea (COPD, chronic anaemia, or heart failure), but this symptom is associated with PEH in 7% to 32% of cases [6, 7, 15–17], with various causes, such as anaemia related to Cameron ulcer (ulcers due to the imprint of the hiatus on the gastric wall), reduced lung or cardiac volume due to PEH compression [18], or diaphragmatic weakness.
The impact of HH surgical repair on respiratory symptoms has not yet been studied extensively. Only a few retrospective studies have been performed with contradictory results. Both Low et al. and Carrott et al. highlighted improvements in forced expiratory volume in one second (FEV1) [19, 20], whereas Wirsching et al., Naoum et al., and Zhu et al. did not highlight any clinically and statistically significant improvements in FEV1 [21–23]. Therefore, further studies are needed to investigate the implication of HH in the respiratory disorders of patients and whether HH repair can improve pulmonary function and symptoms. For this purpose, we decided to carry out a prospective analysis studying the evolution of respiratory function before and after surgery in patients who underwent surgery for a PEH associated with dyspnoea.
Material and methods
Study design
We conducted a prospective non-interventional cohort study. All consecutive patients who received surgery for a PEH presenting with dyspnoea according to the following criteria in the Department of Digestive Surgery, Magellan Centre, Bordeaux University Hospital, from January 2019 to May 2021 were enrolled in the study.
This study was approved by the publication group of the Ethics and Research Committee of the Bordeaux University Hospital. Informed consent was obtained for each patient prior to commencement of their preoperative workup and surgery.
Patients
Inclusion criteria were as follows: (1) patients older than 18 years old scheduled for symptomatic PEH repair; (2) patients having dyspnoea with a modified Medical Research Council (mMRC) score ≥ 2 [24]; (3) type II or greater HH proven by thoracoabdominal computed tomography (CT) scan or barium swallow [1]; and (4) patient affiliated with health care insurance.
Exclusion criteria were as follows: (1) emergency presentation needing surgery with a delay < 6 h; (2) patients presenting with strangulated HH; (3) patients with type I HH; or (4) patients with an mMRC score < 2.
Preoperative assessment
Demographic characteristics, anthropomorphic measurements, medical history, symptoms leading to the diagnosis, medication use, and clinical laboratory tests were prospectively collected prior to surgery at the outpatient clinic visits.
Pulmonary function tests (PFTs) were performed according to the American Thoracic Society criteria [25] 1 month before surgery. Spirometry and plethysmography were performed in order to measure FEV1, forced vital capacity (FVC), total lung capacity (TLC), and residual volume (RV). Reversibility of airway limitation was routinely tested in all patients with administration of a bronchodilator aerosol.
Dyspnoea was evaluated by both the mMRC scale and dyspnoea visual analogue scale (DVAS) [24].
All patients also had a preoperative thoracoabdominal CT scan or barium swallow < 6 months before surgery.
The percentage of the intrathoracic stomach (IS) was precisely determined by three-dimensional (3D) modelling using 3D slicer software for patients with CT scans or by measuring the percentage of IS height for patients who had a barium swallow. Evaluation was performed by two independent observers (D.B. and P.M.) who were blinded to the outcomes (Fig. 1).
Fig. 1.
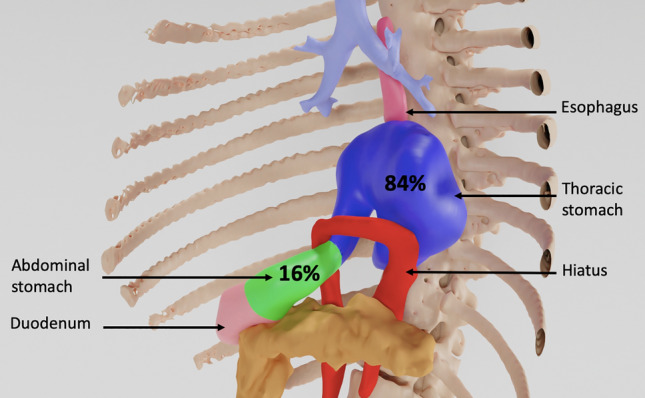
Example of volumetric determination of intrathoracic stomach percentage
Surgery
Patients were operated on by two senior surgeons (C.G. and D.C.). Interventions were performed through 5-port laparoscopy. The HH was dissected, and the hernia content was reduced. This was followed by dissection and resection of the hernial sac and section of the upper short gastric vessels. No oesophageal lengthening procedures, such as the Collis–Nissen procedure, were necessary. A posterior herniorrhaphy was systematically performed using interrupted non-absorbable sutures.
The vagus nerves were systematically identified and preserved.
Left crura plication was performed only when the hiatal orifice remained enlarged after closure of the posterior crura. Hiatal closure was calibrated by the easy passage of a 10-mm instrument through it to avoid stenosis. A partial posterior Toupet fundoplication (270°) was systematically performed (rather than Nissen fundoplication), because this technique would cause less dysphagia with the same efficacy on reflux [26]. No patient had placement of a prosthesis.
Postoperative assessment
Information on postoperative complications according to the Clavien–Dindo classification was prospectively collected [27]. Patients were seen by surgeons at 2 months ± 10 days postoperative in an outpatient clinic. A review of symptomatic outcomes and systematic physical examination was conducted. Both the mMRC scale and DVAS were systematically measured. PFTs were also systematically performed with the same method as preoperatively.
Our primary endpoint was an improvement in FEV1 of more than 10%. This cutoff was established according to the minimal clinically important difference (MCID), which was 100 mL or 10%, in COPD studies [28]. Although this is a cutoff used for a disease other than PEH, it is the only FEV1 cutoff known to determine clinical relevance. We therefore used this percentage to distinguish patients with a good PFT response from those without. For the DVAS, the MCID was 1 to 2 points in a previous study [29]. Therefore, we decided to set a 2-point DVAS decrease to consider that the patient had a good clinical response after surgery.
Potential confounding factor integration
Anaemia, which is a common symptom in patients with PEH, potentially responsible for dyspnoea [30] and is known to be healed after PEH repair [31], was considered as a potential confounding factor. Preoperatively, haemoglobin was routinely measured, and in patients suffering from anaemia, causes of anaemia other than PEH were investigated and treated when necessary. Patients with PEH-related anaemia had iron supplementation before surgery. A comparison of characteristics, dyspnoea scales, and PFT parameters was made between patients with and without anaemia.
Statistical analysis
A data monitoring and a steering committee composed of two surgeons, a pulmonologist, an epidemiologist, and a biostatistician were assembled in order to analyse the association between surgery and respiratory function.
According to the preliminary results, the sample size for a hypothesized increase of 10% in FEV1 with an estimated standard deviation of 16% and a power of 0.80 was predicted to be 41.
The FEV1 variation in the overall population was presented as the mean and standard deviation.
Data are presented with numbers and percentages for qualitative variables. As the numbers of subjects in groups of comparison were low, continuous variables were presented as medians [interquartile ranges] (and in order to preserve homogeneity in the presentation of the results, all continuous data were presented this way). Also, the nonparametric Wilcoxon test was used to compare them. Qualitative variables were compared with the Chi-squared test, unless expected counts were under 10, in which case Fisher's exact test was used. Preoperative and postoperative data were compared using paired function. Patients with significant FEV1 improvement (10% or more) and patients with significant DVAS decrease (at least 2 points) were compared to others.
The Spearman rank correlation between the DVAS and FEV1 improvement, DVAS improvement, percentage of IS and FEV1 improvement, and IS percentage were calculated.
A threshold of α = 0.05 was considered statistically significant for the final model. Analysis was performed with RStudio software version 3.5.1.
Results
Patients
Amongst 126 patients operated on for HH during the inclusion period, 45 patients presented with a PEH (type II–IV HH) and mMRC score ≥ 2 for dyspnoea. Amongst these patients, two patients were excluded from the present study, one because of an intellectual disability preventing him from undergoing PFTs in correct conditions and one refusing to perform PFTs. All 43 patients that were included had complete follow-up and were analysed. Pre- and postoperative dyspnoea scale measurements and PFTs were obtained for all included patients.
Descriptive data
The baseline clinical characteristics and postoperative outcomes of included patients are presented in Table 1.
Table 1.
Clinical, pre-, and postoperative characteristics of included patients
| Characteristics n (%) or median [IQR] | Overall population |
|---|---|
| n = 43 | |
| Age (years) | 70.0 [63.0, 73.5] |
| BMI (kg/m2) | 29.1 [27.4, 31.9] |
| Sex (F/M) | 37/6 (86.0/14.0) |
| ASA score | |
| 1 | 6 (14.0) |
| 2 | 29 (67.4) |
| 3 | 8 (18.6) |
| Comorbidities | |
| Asthma | 6 (14.0) |
| COPD | 2 (4.7) |
| Ischaemic cardiopathy | 3 (7.0) |
| Atrial fibrillation | 4 (9.3) |
| Type II diabetes | 4 (9.3) |
| Obstructive sleep apnoea | 5 (11.6) |
| Pulmonary hypertension | 0 (0) |
| Bronchodilator therapy | 7 (16.3) |
| Smoking history | 4 (12.9) |
| Previous history of abdominal surgery | 25 (58.1) |
| Previous history of fundoplication | 3 (7.0) |
| Preoperative symptoms | |
| Dyspnoea | 43 (100) |
| Heartburn | 19 (44.2) |
| Dyspepsia | 15 (34.9) |
| Dysphagia | 7 (16.3) |
| Vomiting | 4 (9.3) |
| Chest pain | 12 (27.9) |
| Cough | 5 (11.6) |
| Anaemia | 10 (23.3) |
| Intrathoracic stomach | 59.9 [44.2, 83.0] |
| Postoperative complication | |
| Clavien–Dindo I | 3 (7.0) |
| Clavien–Dindo II | 1 (2.3) |
| Clavien–Dindo IIIa | 2 (4.7) |
| Clavien–Dindo IIIb-V | 0 (0) |
| Length of stay (days) | 3.0 [3.0, 4.0] |
ASA American Society of Anesthesiologists, BMI Body Mass Index, COPD chronic obstructive pulmonary disease
Four (9.3%) patients had a preoperative assessment of IS percentage preoperatively by barium swallow, whereas the other 39 patients had thoracoabdominal CT scans (90.7%).
Postoperative complications occurred in six (13.9%) patients. Four patients had medical complications: two phlebitis (Clavien–Dindo I), one spontaneously resolving gas bloat syndrome (Clavien–Dindo I), and one pneumonia (Clavien–Dindo II). Two patients had pneumothoraxes requiring drainage (Clavien–Dindo IIIa). There were no surgical revisions or deaths (Table 1).
No patient had clinical signs of recurrence at 2-month follow-up.
Outcome data
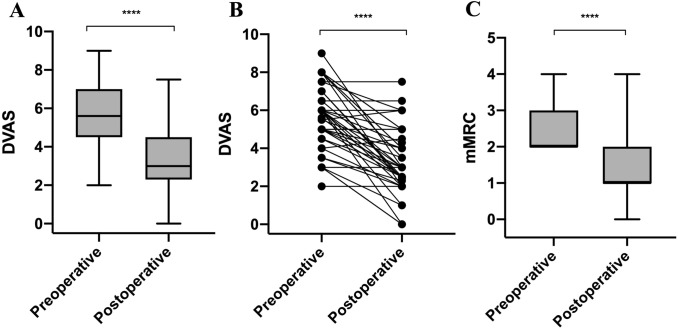
In this cohort, patients had a statistically significant regression of dyspnoea when quantified by the mMRC score (from 2.0 [2.0, 3.0] to 1.0 [1.0, 2.0], p < 0.001) and when quantified by the DVAS (from 5.6 [4.7, 6.7] to 3.0 [2.3, 4.4], p < 0.001) (Table 2, Fig. 2).
Table 2.
Preoperative and postoperative values of dyspnoea scales and PFT parameters
| Variables n (%) or median [IQR] | Preoperative | Postoperative | Difference | p-value |
|---|---|---|---|---|
| Dyspnoea scales | ||||
| mMRC | 2.0 [2.0, 3.0] | 1.0 [1.0, 2.0] | + 1.0 [+ 1.0, + 2.0] | < 0.001 |
| 0 | 0 (0) | 9 (20.9) | ||
| 1 | 0 (0) | 22 (51.2) | ||
| 2 | 28 (65.1) | 11 (25.6) | ||
| 3 | 14 (32.6) | 0 (0) | ||
| 4 | 1 (2.3) | 1 (2.3) | ||
| DVAS | 5.6 [4.7, 6.7] | 3.0 [2.3, 4.4] | + 2.0 [+ 1.0, + 3.5] | < 0.001 |
| Preoperative | Postoperative | % change | p-value | |
|---|---|---|---|---|
| PFT | ||||
| FEV1 | ||||
| (L) | 1.9 [1.6, 2.1] | 2.0 [1.7, 2.2] | + 7.1% [+ 1.6%, + 18.0%] | < 0.001 |
| (% predicted) | 87.0 [77.5, 101.5] | 102.0 [90.5, 109.5] | + 8.2% [+ 1.6%, + 18.2%] | < 0.001 |
| TLC | ||||
| (L) | 4.7 [4.2, 5.4] | 4.9 [4.5, 5.9] | + 4.3% [-2.8%, + 10.1%] | 0.002 |
| (% predicted) | 96.0 [91.0, 105.5] | 104.0 [94.0, 117.0] | + 4.7% [-2.6%, + 12.8%] | 0.002 |
| FVC | ||||
| (L) | 2.3 [2.1, 2.7] | 2.5 [2.2, 2.9] | + 9.6% [+ 3.2%, + 19.9%] | < 0.001 |
| (% predicted) | 94.0 [85.0, 103.0] | 102.0 [95.0, 117.0] | + 11.1% [+ 4.1%, + 20.2%] | < 0.001 |
| RV | ||||
| (L) | 2.3 [1.9, 2.6] | 2.3 [2,0, 2.8] | + 0.5% [− 10.6%, + 17.4%] | 0.553 |
| (% predicted) | 115.0 [105.2, 131.0] | 113.0 [103.0, 133.5] | − 1.0% [− 12.6%, + 16.9%] | 0.846 |
DVAS dyspnoea visual analogue scale, FEV1 forced expiratory volume in one second, FVC forced vital capacity, mMRC Modified Medical Research Council, PFT pulmonary function test, RV residual volume, TLC total lung capacity
Fig. 2.
Preoperative and postoperative dyspnoea scales. A DVAS; B DVAS with line graph; C mMRC. ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05
The mMRC score did not increase in any patients. It was stable in six (14%) patients, decreased by 1 point in 21 (48%) patients, decreased by 2 points in 15 (34.9%) patients, and decreased by 3 points in one patient (2.3%) (Table 2).
Consequently, the mMRC score improved in a clinically relevant way, according to the MCID, in 37 (86%) patients.
The mean postoperative DVAS (3.31 [SD 1.74]) was statistically significant lower than the mean preoperative DVAS (5.62 [SD 1.69]), which corresponds to an average decrease of 2.30 (SD 1.83) (p < 0.001) (Table 2).
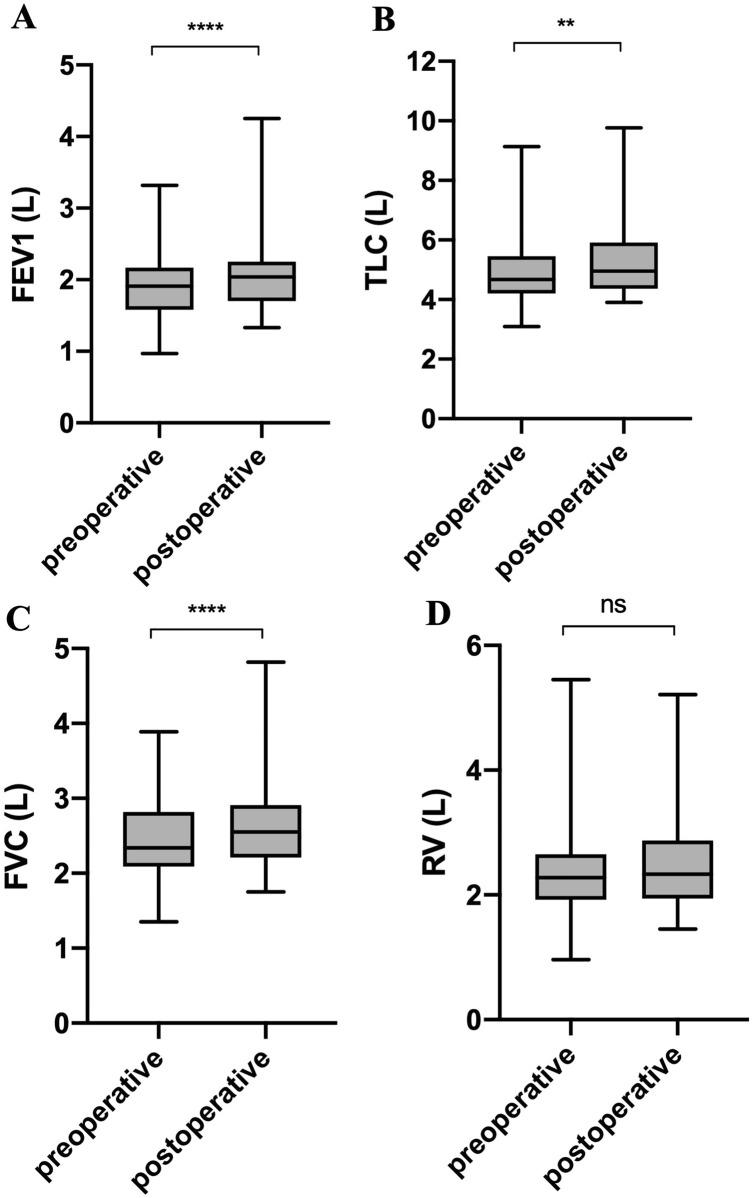
Absolute FEV1, TLC, and FVC also statistically significantly increased after surgery by an average of 11.2% (SD 17.9), 0.19 L (SD 0.25), p < 0.001; 5.0% (SD 13.9), 0.29 L (SD 0.56), p = 0.002; and 10.7% (SD 14.6), 0.25 L (SD 0.31), p < 0.001, respectively (Table 2). The absolute RV did not show any statistically significant change after surgery (10.3% [SD 35.3], 0.1 L [SD 0.6], p = 0.553) (Table 2, Fig. 3).
Fig. 3.
Preoperative and postoperative PFT parameters. A FEV1; B TLC; C FVC; D RV. ****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05
Subgroup analyses according to the clinical and functional responses
Patients with and without clinically significant FEV1 improvement
Seventeen (39%) patients had an improvement of more than 10% in FEV1. They were compared with patients that had a less than 10% improvement. No difference was found between these two groups with regard to demographics, clinical characteristics, or preoperative symptoms. Postoperative dyspnoea and PFT parameters are shown in Table 3. Preoperative FEV1 (L) was statistically significantly lower in patients with clinically significant FEV1 improvement when compared to that in the other patients (1.6 [1.3, 1.9] vs. 2.0 [1.8, 2.2], p = 0.029) (Table 3).
Table 3.
Characteristics of patients with and without significant FEV1 improvement
| Characteristics n (%) or median [IQR] | < 10% FEV1 improvement | ≥ 10% FEV1 improvement | p-value |
|---|---|---|---|
| Number of patients | 26 | 17 | |
| Intrathoracic stomach | 57.3 [36.1, 78.6] | 60.0 [55.7, 94.1] | 0.145 |
| Postoperative complication | 0.527 | ||
| Dindo I | 2 (7.7) | 1 (5.9) | |
| Dindo II | 1 (3.8) | 0 (0.0) | |
| Dindo IIIa | 2 (7.7) | 0 (0.0) | |
| Dindo IIIb–V | 0 (0) | 0 (0) | |
| Length of stay (days) | 3.5 [3.0, 4.0] | 3.0 [3.0, 4.0] | 0.347 |
| Baseline dyspnoea scale | |||
| mMRC | 2.0 [2.0, 3.0] | 2.0 [2.0, 3.0] | 0,295 |
| DVAS | 6.00 [5.00, 6.38] | 5.00 [3.50, 7.50] | 0.416 |
| Dyspnoea scale gain | |||
| mMRC | + 1.0 [+ 1.0, + 2.0] | + 1.0 [+ 1.0, + 2.0] | 0.169 |
| Worsening | 0 (0.0) | 0 (0) | 0.182 |
| Statu quo | 5 (19.2) | 1 (5.9) | |
| 1 | 13 (52.0) | 8 (47.1) | |
| 2 | 8 (32.0) | 7 (41.2) | |
| 3 | 0 (0.0) | 1 (5.9) | |
| DVAS | + 2.0 [+ 1.0, + 3.4] | + 2.0 [+ 1.2, + 3.5] | 0.803 |
| Baseline PFT | |||
| FEV1 (L) | 2.0 [1.8, 2.2] | 1.6 [1.3, 1.9] | 0.029 |
| TLC (L) | 4.9 [4.4, 5.4] | 4.3 [3.9, 4.9] | 0.074 |
| FVC (L) | 2.5 [2.2, 2.8] | 4.3 [3.9, 4.9] | 0.054 |
| RV (L) | 2.3 [1.9, 2.6] | 4.3 [3.9, 4.9] | 0.502 |
| PFT % improvement | |||
| FEV1 (L) | + 3.5% [− 1.6%, + 6.2%] | + 21.9% [+ 15.0%, + 26.4%] | < 0.001 |
| TLC (L) | + 0.4% [− 4.5%, + 8.5%] | + 5.7% [+ 1.3%, + 10.6%] | 0.13 |
| FVC (L) | + 4.5% [− 0.9%, + 10.5%] | + 18.8% [+ 11.4%, + 22.2%] | 0.002 |
| RV (L) | − 1.3% [− 10.9%, + 29.2%] | + 1.0% [-10.0%, + 11.9 +] | 0.619 |
DVAS dyspnoea visual analogue scale, FEV1 forced expiratory volume in one second, FVC forced vital capacity, mMRC Modified Medical Research Council, PFT pulmonary function test, RV residual volume, TLC total lung capacity
Patients with and without clinically significant DVAS improvement
Twenty-four (56%) patients had an improvement in DVAS of ≥ 2 points and were therefore considered as “good clinical responders”. They were compared with patients who had an improvement in DVAS of < 2 points. No difference was found between these two groups with regard to demographics, clinical characteristics, or preoperative symptoms. Postoperative dyspnoea and PFT parameters are shown in Table 4.
Table 4.
Characteristics of patients with and without significant DVAS improvement
| Characteristics n (%) or median [IQR] | < 2 DVAS improvement | ≥ 2 DVAS improvement | p-value |
|---|---|---|---|
| Number of patients | 19 | 24 | |
| Intrathoracic stomach | 58.9 [50.5, 75.2] | 66.2 [42.2, 90.8] | 0.912 |
| Postoperative complication | 0.171 | ||
| Dindo I | 2 (10.5) | 1 (4.2) | |
| Dindo II | 1 (5.3) | 0 (0.0) | |
| Dindo IIIa | 2 (10.5) | 0 (0.0) | |
| Dindo IIIb–V | 0 (0) | 0 (0) | |
| Length of stay (days) | 4.0 [3.0, 4.0] | 3.0 [3.0, 4.0] | 0.108 |
| Baseline dyspnoea scale | |||
| mMRC | 2.0 [2.0, 3.0] | 2.0 [2.0, 3.0] | 0.87 |
| DVAS | 5.0 [4.2, 6.0] | 6.0 [5.0, 8.0] | 0.046 |
| Dyspnoea scale gain | |||
| mMRC | + 1.0 [0.0, + 1.0] | + 1.5 [+ 1.0, + 2.0] | 0.017 |
| Worsening | 0 (0) | 0 (0.0) | 0.09 |
| statu quo | 5 (26.3) | 1 (4.2) | |
| 1 | 10 (52.6) | 11 (45.8) | |
| 2 | 4 (21.1) | 11 (45.8) | |
| 3 | 0 (0.0) | 1 (4.2) | |
| DVAS | + 1.0 [0.0, + 1.5] | + 3.3 [+ 2.9, + 4.5] | < 0.001 |
| Baseline PFT | |||
| FEV1 (L) | 1.9 [1.6, 2.1] | 1.9 [1.6, 2.9] | 0.951 |
| TLC (L) | 4.8 [4.3, 6.4] | 4.5 [4.2, 5.1] | 0.203 |
| FVC (L) | 2.3 [2.2, 2.9] | 2.3 [2.1, 2.6] | 0.642 |
| RV (L) | 2.3 [2.1, 2.8] | 2.2 [1.9, 2.5] | 0.276 |
| PFT % improvement | |||
| FEV1 (L) | + 5.7% [− 0.9%, + 16.8%] | + 8.4% [+ 4.1%, + 17.7%] | 0.42 |
| TLC (L) | + 0.4% [− 3.7%, + 8.8%] | + 6.4% [− 0.3%, + 10.6%] | 0.179 |
| FVC (L) | + 8.1% [+ 4.0%, + 18.8%] | + 10.7% [+ 2.9%, + 19.8%] | 0.807 |
| RV (L) | + 0.5% [− 12.4%, + 37.5%] | + 0.5% [− 8.7%, + 13.0%] | 0.961 |
DVAS dyspnoea visual analogue scale, FEV1 forced expiratory volume in one second, FVC forced vital capacity, mMRC modified medical research council, PFT pulmonary function test, RV residual volume, TLC total lung capacity
Correlation analysis
No correlation was highlighted between DVAS and FEV1 (L) improvement (ρ = 0.163, 95% CI − 0.153 to 0.449), percentage of IS and preoperative DVAS (ρ = − 0.199, 95% CI − 0.485 to 0.124), preoperative FEV1 (L) (ρ = − 0.273, 95% CI − 0.535 to 0.038), DVAS improvement (ρ = 0.007, 95% CI − 0.310 to 0.322), and FEV1 (L) improvement (ρ = 0.189, 95% CI -0.135 to 0.476).
Potential confounding factor integration
Ten (23%) patients presented with anaemia that could be responsible for dyspnoea. Causes of anaemia other than PEH were investigated and none were found in those 10 patients. No difference was found between patients suffering from anaemia compared to those without anaemia with regard to demographics, clinical characteristics, or preoperative symptoms. Otherwise, compared to patients without anaemia, patients with anaemia tended to have greater but not statistically significant improvements in the DVAS (3.0 [2.0, 3.5] vs. 1.7 [1.0, 3.1], p = 0.784), in FEV1 (9.75 [3.6, 15.5] vs. 7.7 [1.0, 18.6], p = 0.829) and mMRC scale (p = 0.053).
Discussion
PEH repair improves dyspnoea and respiratory function
This prospective study demonstrated that PEH repair significantly improved the respiratory function of patients, with a mean increase in FEV1 of 11.2% in patients suffering from dyspnoea. This improvement in FEV1 is furthermore clinically significant, as it is greater than 10%. It was also demonstrated that this procedure decreases dyspnoea in a large number of patients, with 87% of patients achieving a clinically relevant improvement in their mMRC scores and a mean decrease in DVAS of 2.30 (1.83). A low preoperative FEV1 was the only criterion identified as predictive of improvement in FEV1 after surgery, according to our subgroup analysis.
This improvement in FEV1 is consistent with previous studies. Both Low et al. and Carrott et al., respectively, showed 16% and 10.4% improvements in FEV1 in their respective 45 and 120 patient cohorts (Table 5) [19, 20]. These two cohort studies, although one was retrospective, carry the same design as ours. The clinical characteristics, procedures, and perioperative PFTs of their included patients are very similar to those of the present study.
Table 5.
Impact of laparoscopic fundoplication for PEH on respiratory function in literature
| Author | Year | Journal | Country | Study Type | Patients | Patients with dyspnoea | PFT | Percentage IS assessment | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PFT dealy (month) | FEV1 gain (%) | FEV1 gain (L) | Examination | Method | <50% | 50–75% | 75–100% | Surgery | |||||||
| Bouriez et al | 2021 | Submitted to Surg. Endosc. | France | PCS | 43 | 43 (100%) | 2 | 11.2% | 0.19 | CT & BS | Volumentry | 13 | 15 | 13 | Toupet |
| Wirsching et al. [21] | 2019 | Dis. Esophagus | USA | RCS | 299 | 145 (48%) | 3–4 | 9.1% | BS | EER | 20 | 119 | 115 | Hill & Toupet | |
| Milito et al. [18] | 2018 | Int. J. Cardiol. | Italy | PCS | 35 | 25 (73%) | 3 | 0.08 | MRI | Volumetry | Toupet | ||||
| Naoum et al. [22] | 2017 | Clin. Respir. J. | Australia | RCS | 73 | 35 (48%) | 3 | 6% | 0.13 | BS | EER | 15 | 14 | 16 | Nissen |
| Carrott et al. [20] | 2012 | J. Thorac. Cardiovasc. Surg. | USA | PCS | 120 | 63 (53%) | 3 | 10.4% | 0.23 | BS | EER | 6 | 35 | 79 | Hill |
| Zhu et al. [23] | 2011 | Surg. Endosc. | Australia | PCS | 30 | 26 (87%) | 3–6 | 2% | 0.05 | BS | EER | Toupet | |||
| Low et al. [19] | 2002 | Ann. Thorac. Surg. | USA | RCS | 45 | 38 (84%) | 1–6 | 16% | 0.3 | BS | EER | 2 | 16 | 27 | Hill |
BS barium swallow, CT computerized tomography, EER eye estimation by radiologist, FEV1 forced expiratory volume in one second, IS intrathoracique stomach, PCS prospective cohort study, PFT pulmonary function tests, RCS retrospective cohort study
In contrast, both Zhu et al. and Naoum et al. showed an improvement in absolute FEV1 of 2% and 6% in their respective cohorts of 30 and 73 patients, which did not reach the clinical significance threshold of a 10% improvement in FEV1. In these two studies, the preoperative FEV1 was higher (2.03 L and 2.06 L, respectively), which may explain their lower improvements in FEV1 (Table 5) [22, 23]. Wirshing et al. showed a nonclinically significant improvement (9.1%), and their results were not statistically significant probably because many of their patients were lost to follow-up [21].
Compared to those six previously published studies [18–23], postoperative PFTs and dyspnoea evaluation were performed closer to surgery (2 months vs. 3–6 months), which could have diminished the magnitude of these results due to an incomplete postoperative recovery. The fact that results are nevertheless significant reinforces the idea that PEH repair improves lung function and dyspnoea (Table 5).
Regarding the other PFT parameters, the increase in FVC (10.7%, SD 14.6, p < 0.0001) demonstrated in this study is consistent with three of the four previously published studies that evaluated this parameter [19, 20, 22], which reinforces the consistency of our results. Zhu et al. found no statistically significant improvement in this parameter, perhaps because of a lack of power due to a too small population size [23]. Only two studies described volumes estimated by plethysmography (TLC and RV). Zhu et al. showed no statistically significant difference between pre- and postoperative measures, whilst Naoum et al. showed a 4.3% (p = 0.008) improvement in absolute TLC, which is consistent with our results [22, 23]. Also, as in the present study, no statistically significant change in RV following surgery was highlighted in these two studies [22, 23].
The six previously mentioned studies, unlike the present study, did not have only patients with preoperative dyspnoea [18–23]. It was decided in the present study to include only patients with preoperative dyspnoea to assess the improvement of this symptom more accurately and assess whether it can constitute an operative indication. To our knowledge, there is no previous study that has shown a statistically significant decrease in dyspnoea assessed by DVAS and mMRC scale scores after PEH repair in a dyspnoeic patient population.
Zhu et al., Low et al., and Naoum et al. showed a significant decrease in dyspnoea after PEH repair but in populations that did not include only dyspnoeic patients [19, 22, 23]. Furthermore, in those studies, dyspnoea was graded in four stages or with the NYHA scale, which are less precise than the DVAS.
No correlation between FEV1 and dyspnoea improvement
We did not find any correlation between improvement in dyspnoea (by DVAS and mMRC) and improvement in FEV1. Using subgroup analysis, we verified that chronic anaemia linked to PEH [30], corrected by the reduction of the herniated stomach and by a possible martial supplementation, was not a factor implicated in these results [31].
Otherwise, in COPD, it is known that the correlation between FEV1 and dyspnoea is weak [32]. Moreover, it is known that improvement of respiratory quality of life after a rehabilitation programme can be present, although respiratory functional parameters are unchanged [33]. All the above would tend to show the absence of a relationship between PFT parameters and the sensation of dyspnoea in the COPD example. It could therefore be hypothesized that this condition might also be true in PEH-related dyspnoea.
Indeed, dyspnoea is a complex subjective symptom, the causes of which are not solely pulmonary. It can be related to anaemia, heart diseases, and neuromuscular pathologies not only affecting the respiratory muscles but also other muscles and could even be due to a mismatch between ventilation and perfusion, particularly in obese patients [34]. In fact, the present study’s population is overweight (average BMI: 29 kg/m2), which could also possibly explain this lack of correlation.
Otherwise, one of the main assumptions for this absence of correlation would be a “care effect”: patients who have undergone surgery feel better on a general level and falsely attribute an improvement of their dyspnoea to the surgery. This encourages further studies investigating the correlation between improvements in respiratory symptoms and digestive quality of life after PEH repair.
Correlation between hernia size and dyspnoea or respiratory function
In the present study, we did not find any correlation between the volume of the PEH and preoperative respiratory parameters and the improvement of these parameters after surgery. However, the determination of IS percentage was done in the most accurate way possible (by volumetric measurements), which is not the case in the other studies that estimated the percentage of IS by eye on CT scans or barium swallow imaging [19–23].
A correlation between respiratory parameters and IS percentage was nevertheless highlighted in other studies. Naoum et al. demonstrated a correlation between the IS percentage and TLC or FVC but not FEV1 or between the IS percentage and improvement in PFT parameters [22]. Low et al. highlighted higher improvements in FEV1 and FVC in patients with 100% IS when compared to patients with less than 50% IS [19]. Senyk et al. showed that a higher hernia diameter was correlated with an abnormal lung ventilation and perfusion ratio, which could explain dyspnoea [35]. Milito et al. showed a correlation between PEH size and cardiac volumes but did not compare it to respiratory parameters [18]. Other studies have shown the cardiac repercussions of PEH, which may explain dyspnoea by electrocardiographic abnormalities or compression of the cardiac chambers [36–38]. A first hypothesis explaining this absence of correlation would be an insufficient statistical power of the study, preventing the demonstration of whether a correlation actually exists. Another hypothesis would be that dyspnoea in patients with PEH is not simply due to the hernia volume compressing the lung but is related to a more complicated cause. Firstly, gastroesophageal reflux in HH is known to be responsible for microaspiration, which cause bronchial spasms leading to cough, asthma, and dyspnoea [39–41]. Also, according to Senyk et al., dyspnoea could be caused by a diaphragmatic defect impairing lung expansion and inducing atelectasis, resulting in poor respiratory function [35, 42]. Further studies with imaging of the diaphragmatic course and defect or with PFTs analysing maximum inspiratory and expiratory pressures could help us better understand the cause of dyspnoea in PEH.
Limitations
Our study was single centred, and the operations were performed by only two surgeons, which could partially limit its external validity.
Also, PFTs and radiological percentage of IS were not performed in a standardized way regarding meal intake. In PEH, symptoms (dysphagia, chest pain, and dyspnoea) are often related to the postprandial period [16]. Thus, the patient’s prandial status could influence respiratory symptoms or PFT results and constitute a bias in the analysis of the results of this study. Milito et al. performed heart and stomach volume evaluations before and after a standard meal of 250 mL yogurt and demonstrated an increase in PEH volume and a decrease in cardiac cavity volume and ejection fraction [18]. They also demonstrated that PEH repair significantly improved FEV1 and FVC, but PFTs were not performed at a specific time in relation to meals. Further studies evaluating improvement in respiratory function after PEH repair in a standardized time related to meal intake are therefore needed.
Also, it might be interesting to study not only the percentage of IS but also the percentage of chest cavity volume occupied by the hernia in order to really determine the volume stolen by the stomach from the lung.
A study comparing improvement in quality of life with improvement in respiratory symptoms after PEH repair could also provide a better understanding of the origin of dyspnoea in PEH by possibly highlighting a correlation between these two conditions.
Conclusion
PEH repair significantly improves dyspnoea and FEV1 in a population of patients presenting with dyspnoea. Patients with a low preoperative FEV1 are more likely to show FEV1 improvement after surgery.
Acknowledgements
We thank Laure Davoust, native English speaker, for English reviewing of the manuscript; Isabelle Nassiet, medical assistant, for her help in scheduling patients’ work-up; Clémentine Brocard, for reviewing of the manuscript.
Abbreviations
- DVAS
Dyspnoea visual analogue scale
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- CT
Computed tomography
- HH
Hiatal hernia
- IS
Intrathoracic stomach
- MCID
Minimal clinically important difference
- mMRC
Modified Medical Research Council
- PEH
Para-oesophageal hernia
- PFTs
Pulmonary function tests
- RV
Residual volume
- TLC
Total lung capacity
Author contributions
Conceptualization and design: DB, PB, CG, and DC; methodology and acquisition of the data: DB, PM, and MB; analysis and interpretation of the data: DB, YB, PB, and CG; main writing of the manuscript: DB and CG; and supervision: DB, CG, DC, HN, and PB.
Funding
None.
Declarations
Conflict of interest
The authors Drs. Damien Bouriez, Yaniss Belaroussi, Mehdi Boubaddi, Paul Martre, Haythem Najah, Caroline Gronnier, and Denis Collet declare that they have no conflicts of interest or financial ties. Patrick Berger declares the following conflicts of interest outside the submitted work. P. Berger also reports grants and personal fees from AstraZeneca and Novartis; grants, personal fees, and non-financial support from Boehringer Ingelheim; personal fees and non-financial support from Chiesi, AstraZeneca, and Sanofi; personal fees from Menarini and TEVA. In addition, he has a delivered patent (EP N°15152886.6, i.e. New compositions and methods of treating and/or preventing Chronic Obstructive Pulmonary Disease), a submitted patent (22605-FR, i.e. Geometric characterization of airways using MRI), and a submitted patent (EP N°20173595.8, i.e. New compositions and methods of treating COVID-19 Disease)).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Caroline Gronnier and Denis Collet have equally contributed to the manuscript.
References
- 1.Landreneau RJ, Del Pino M, Santos R. Management of paraesophageal hernias. Surg Clin North Am. 2005;85:411–432. doi: 10.1016/j.suc.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Mitiek MO, Andrade RS. Giant hiatal hernia. Ann Thorac Surg. 2010;89:S2168–2173. doi: 10.1016/j.athoracsur.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Aly A, Munt J, Jamieson GG, Ludemann R, Devitt PG, Watson DI. Laparoscopic repair of large hiatal hernias. Br J Surg. 2005;92:648–653. doi: 10.1002/bjs.4916. [DOI] [PubMed] [Google Scholar]
- 4.Hazebroek EJ, Gananadha S, Koak Y, Berry H, Leibman S, Smith GS. Laparoscopic paraesophageal hernia repair: quality of life outcomes in the elderly. Dis Esophagus. 2008;21:737–741. doi: 10.1111/j.1442-2050.2008.00831.x. [DOI] [PubMed] [Google Scholar]
- 5.Kahrilas PJ, Kim HC, Pandolfino JE. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol. 2008;22:601–616. doi: 10.1016/j.bpg.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Low DE, Unger T. Open repair of paraesophageal hernia: reassessment of subjective and objective outcomes. Ann Thorac Surg. 2005;80:287–294. doi: 10.1016/j.athoracsur.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Nason KS, Luketich JD, Qureshi I, Keeley S, Trainor S, Awais O, Shende M, Landreneau RJ, Jobe BA, Pennathur A. Laparoscopic repair of giant paraesophageal hernia results in long-term patient satisfaction and a durable repair. J Gastrointest Surg. 2008;12:2066–2075. doi: 10.1007/s11605-008-0712-7. [DOI] [PubMed] [Google Scholar]
- 8.Zehetner J, Demeester SR, Ayazi S, Kilday P, Augustin F, Hagen JA, Lipham JC, Sohn HJ, Demeester TR. Laparoscopic versus open repair of paraesophageal hernia: the second decade. J Am Coll Surg. 2011;212:813–820. doi: 10.1016/j.jamcollsurg.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 9.Quinn MA, Geraghty AJ, Robertson AGN, Paterson-Brown S, Lamb PJ, Edinburgh Oesophago-Gastric Surgery Group Long-term outcomes following surgical repair of giant paraoesophageal hiatus hernia. Surg Endosc. 2019;33:1846–1853. doi: 10.1007/s00464-018-6463-y. [DOI] [PubMed] [Google Scholar]
- 10.Luketich JD, Nason KS, Christie NA, Pennathur A, Jobe BA, Landreneau RJ, Schuchert MJ. Outcomes after a decade of laparoscopic giant paraesophageal hernia repair. J Thorac Cardiovasc Surg. 2010;139(395–404):404. doi: 10.1016/j.jtcvs.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mungo B, Molena D, Stem M, Feinberg RL, Lidor AO. Thirty-day outcomes of paraesophageal hernia repair using the NSQIP database: should laparoscopy be the standard of care? J Am Coll Surg. 2014;219:229–236. doi: 10.1016/j.jamcollsurg.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen NT, Christie C, Masoomi H, Matin T, Laugenour K, Hohmann S. Utilization and outcomes of laparoscopic versus open paraesophageal hernia repair. Am Surg. 2011;77:1353–1357. doi: 10.1177/000313481107701018. [DOI] [PubMed] [Google Scholar]
- 13.Oor JE, Koetje JH, Roks DJ, Nieuwenhuijs VB, Hazebroek EJ. Laparoscopic hiatal hernia repair in the elderly patient. World J Surg. 2016;40:1404–1411. doi: 10.1007/s00268-016-3428-y. [DOI] [PubMed] [Google Scholar]
- 14.El Lakis MA, Kaplan SJ, Hubka M, Mohiuddin K, Low DE. The importance of age on short-term outcomes associated with repair of giant paraesophageal hernias. Ann Thorac Surg. 2017;103:1700–1709. doi: 10.1016/j.athoracsur.2017.01.078. [DOI] [PubMed] [Google Scholar]
- 15.Dallemagne B, Kohnen L, Perretta S, Weerts J, Markiewicz S, Jehaes C. Laparoscopic repair of paraesophageal hernia. Long-term follow-up reveals good clinical outcome despite high radiological recurrence rate. Ann Surg. 2011;253:291–296. doi: 10.1097/SLA.0b013e3181ff44c0. [DOI] [PubMed] [Google Scholar]
- 16.Maziak DE, Todd TR, Pearson FG. Massive hiatus hernia: evaluation and surgical management. J Thorac Cardiovasc Surg. 1998;115:53–60. doi: 10.1016/S0022-5223(98)70442-8. [DOI] [PubMed] [Google Scholar]
- 17.Rogers ML, Duffy JP, Beggs FD, Salama FD, Knowles KR, Morgan WE. Surgical treatment of para-oesophageal hiatal hernia. Ann R Coll Surg Engl. 2001;83:394–398. [PMC free article] [PubMed] [Google Scholar]
- 18.Milito P, Lombardi M, Asti E, Bonitta G, Fina D, Bandera F, Bonavina L. Influence of large hiatus hernia on cardiac volumes. A prospective observational cohort study by cardiovascular magnetic resonance. Int J Cardiol. 2018;268:241–244. doi: 10.1016/j.ijcard.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Low DE, Simchuk EJ. Effect of paraesophageal hernia repair on pulmonary function. Ann Thorac Surg. 2002;74:333–337. doi: 10.1016/S0003-4975(02)03718-9. [DOI] [PubMed] [Google Scholar]
- 20.Carrott PW, Hong J, Kuppusamy M, Kirtland S, Koehler RP, Low DE. Repair of giant paraesophageal hernias routinely produces improvement in respiratory function. J Thorac Cardiovasc Surg. 2012;143:398–404. doi: 10.1016/j.jtcvs.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 21.Wirsching A, Klevebro F, Boshier PR, Hubka M, Kuppusamy MK, Kirtland SH, Low DE. The other explanation for dyspnea: giant paraesophageal hiatal hernia repair routinely improves pulmonary function. Dis Esophagus. 2019;32:doz032. doi: 10.1093/dote/doz032. [DOI] [PubMed] [Google Scholar]
- 22.Naoum C, Kritharides L, Ing A, Falk GL, Yiannikas J. Changes in lung volumes and gas trapping in patients with large hiatal hernia: lung volumes and hiatal hernia. Clin Respir J. 2017;11:139–150. doi: 10.1111/crj.12314. [DOI] [PubMed] [Google Scholar]
- 23.Zhu JC, Becerril G, Marasovic K, Ing AJ, Falk GL. Laparoscopic repair of large hiatal hernia: impact on dyspnoea. Surg Endosc. 2011;25:3620–3626. doi: 10.1007/s00464-011-1768-0. [DOI] [PubMed] [Google Scholar]
- 24.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulmonary Function Testing (2021). https://www.thoracic.org/statements/pulmonary-function.php. January 2021
- 26.Koch OO, Kaindlstorfer A, Antoniou SA, Asche KU, Granderath FA, Pointner R. Laparoscopic Nissen versus Toupet fundoplication: objective and subjective results of a prospective randomized trial. Surg Endosc. 2012;26:413–422. doi: 10.1007/s00464-011-1889-5. [DOI] [PubMed] [Google Scholar]
- 27.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 28.Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2:111–124. doi: 10.1081/copd-200053377. [DOI] [PubMed] [Google Scholar]
- 29.Ries AL. Minimally clinically important difference for the UCSD shortness of breath questionnaire, borg scale, and visual analog scale. COPD. 2005;2:105–110. doi: 10.1081/copd-200050655. [DOI] [PubMed] [Google Scholar]
- 30.Gray DM, Kushnir V, Kalra G, Rosenstock A, Alsakka MA, Patel A, Sayuk G, Gyawali CP. Cameron lesions in patients with hiatal hernias: prevalence, presentation, and treatment outcome. Dis Esophagus. 2015;28:448–452. doi: 10.1111/dote.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheverie JN, Lam J, Neki K, Broderick RC, Lee AM, Matsuzaki T, Cubas R, Sandler BJ, Jacobsen GR, Fuchs K-H, Horgan S. Paraesophageal hernia repair: a curative consideration for chronic anemia? Surg Endosc. 2020;34:2243–2247. doi: 10.1007/s00464-019-07014-3. [DOI] [PubMed] [Google Scholar]
- 32.Redelmeier DA, Goldstein RS, Min ST, Hyland RH. Spirometry and dyspnea in patients with COPD. When small differences mean little. Chest. 1996;109:1163–1168. doi: 10.1378/chest.109.5.1163. [DOI] [PubMed] [Google Scholar]
- 33.Casaburi R, Porszasz J, Burns MR, Carithers ER, Chang RS, Cooper CB. Physiologic benefits of exercise training in rehabilitation of patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;155:1541–1551. doi: 10.1164/ajrccm.155.5.9154855. [DOI] [PubMed] [Google Scholar]
- 34.Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, Calverley PM, Gift AG, Harver A, Lareau SC, Mahler DA, Meek PM, O’Donnell DE, American Thoracic Society Committee on Dyspnea An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senyk J, Arborelius M, Lilja B. Respiratory function in esophageal hiatus hernia. II. Regional lung function. Respiration. 1975;32:103–111. doi: 10.1159/000193640. [DOI] [PubMed] [Google Scholar]
- 36.Hokamaki J, Kawano H, Miyamoto S, Sugiyama S, Fukushima R, Sakamoto T, Yoshimura M, Ogawa H. Dynamic electrocardiographic changes due to cardiac compression by a giant hiatal hernia. Intern Med. 2005;44:136–140. doi: 10.2169/internalmedicine.44.136. [DOI] [PubMed] [Google Scholar]
- 37.Hunt GS, Gilchrist DM, Hirji MK. Cardiac compression and decompensation due to hiatus hernia. Can J Cardiol. 1996;12:295–296. [PubMed] [Google Scholar]
- 38.Kounis NG, Zavras GM, Kitrou MP, Soufras GD, Constantinidis K. Unusual electrocardiographic manifestations in conditions with increased intrathoracic pressure. Acta Cardiol. 1988;43:653–661. [PubMed] [Google Scholar]
- 39.Kiljander TO, Salomaa E-RM, Hietanen EK, Ovaska J, Helenius H, Liippo K. Gastroesophageal reflux and bronchial responsiveness: correlation and the effect of fundoplication. Respiration. 2002;69:434–439. doi: 10.1159/000064021. [DOI] [PubMed] [Google Scholar]
- 40.Greub G, Liaudet L, Wiesel P, Bettschart V, Schaller M-D. Respiratory complications of gastroesophageal reflux associated with paraesophageal hiatal hernia. J Clin Gastroenterol. 2003;37:129–131. doi: 10.1097/00004836-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Vincent D, Cohen-Jonathan AM, Leport J, Merrouche M, Geronimi A, Pradalier A, Soulé JC. Gastro-oesophageal reflux prevalence and relationship with bronchial reactivity in asthma. Eur Respir J. 1997;10:2255–2259. doi: 10.1183/09031936.97.10102255. [DOI] [PubMed] [Google Scholar]
- 42.Senyk J, Arborelius M, Lilja B, Ohlsson NM. Respiratory function in esophageal hiatus hernia. I. Spirometry, gas distribution, and arterial blood gases. Respiration. 1975;32:93–102. doi: 10.1159/000193639. [DOI] [PubMed] [Google Scholar]