Abstract
Pseudomonas oleovorans ATCC 29347 was grown in chemostat culture at different dilution rates with mineral media varying in their ratios of octanoate to ammonia (C0/N0 ratio). At all dilution rates tested, three distinct growth regimes were observed: (i) carbon limitation with NH4+ in excess at low C0/N0 ratios, (ii) purely nitrogen-limited growth conditions at high C0/N0 ratios with residual octanoate in the culture supernatant, and (iii) an intermediate zone of dual-nutrient-limited growth conditions where both the concentration of octanoate and that of ammonia were very low. The dual-nutrient-limited growth zone shifted to higher C0/N0 ratios with decreasing dilution rates, and the extension of the dual-nutrient-limited growth zone was inversely proportional to the growth rate. The cells accumulated the storage compound medium-chain-length poly[(R)-3-hydroxyalkanoate] (mcl-PHA) during dual (C and N)-nutrient-limited and N-limited growth conditions. Within the dual-nutrient-limited growth zone, the cellular mcl-PHA contents increased when the C0/N0 ratio in the feed was increased, whereas the cellular mcl-PHA level was independent from the feed C0/N0 ratio during N-limited growth. The monomeric composition of the accumulated mcl-PHA was independent of both the dilution rate and the feed C0/N0 ratio and consisted of 12 mol% 3-hydroxyhexanoic acid and 88 mol% 3-hydroxyoctanoic acid. Accumulation of mcl-PHA led to an increase in the cellular C/N ratio and to changes in elemental growth yields for nitrogen and carbon.
Pseudomonas oleovorans is able to accumulate medium-chain-length poly[(R)-3-hydroxyalkanoates] (mcl-PHAs) as intracellular carbon and energy storage compounds when grown with alkanes (9, 24), mcl-alkanoates (3, 21), n-alkanols (16), or many derivatives thereof (27, 38). mcl-PHAs are hydrophobic polyesters and are attracting increasing interest for their possible application as biologically produced and inherently biodegradable substitutes for conventional plastic materials (6, 8, 10, 20, 32). Particularly, when subjected to nitrogen limitation in batch (22, 24) and chemostat culture (17, 33, 34), P. oleovorans has been reported to accumulate high cellular mcl-PHA contents.
The nature and availability of essential nutrients are important parameters in determining the extent of storage compound accumulation in P. oleovorans (23, 24). Generally, the production of microbial cell mass is limited by the restricted availability of a particular nutrient. In batch culture, the exhaustion of a specific nutrient terminates the exponential-growth phase, whereas in chemostat culture the biomass concentration is usually controlled by the permanent limitation of a single defined nutrient, typically the carbon and energy source. However, several authors have shown that two or more nutrients can simultaneously limit the production of biomass (for a summary, see reference 14). This phenomenon has also been observed for P. oleovorans, where we have found that under defined chemostat steady-state cultivation conditions, the biomass concentration and the cell composition were influenced by simultaneous limitations of the available carbon and nitrogen (12).
All of the previous reports have emphasized that dual-nutrient-limited growth depends on the ability of a microorganism to adjust its cellular composition to different degrees of nutrient limitation. If a microorganism is very flexible in this respect, an extended dual-nutrient-limited growth regime between two single-nutrient limitations should be observed in continuous culture. It is known (19) that the flexibility in cellular composition increases with decreasing growth rates. Therefore, it was predicted (14) that the range of dual nutrient limitation should also depend on the chemostat dilution rate. In order to test this hypothesis and to investigate the influence of the growth rate on the physiological adaptation and mcl-PHA accumulation pattern of P. oleovorans, cells were cultivated in the chemostat at different dilution rates and at various ratios of octanoate to ammonia in the feed medium.
MATERIALS AND METHODS
Bacterial strain, media, and growth conditions.
P. oleovorans ATCC 29347, kept as frozen stock in 15% glycerol-citrate E medium at −80°C, was used for all experiments. Modified E medium (39) contained, per liter, 3.5 g of NaNH4HPO4 · 4H2O, 7.5 g of K2HPO4, 3.7 g of KH2PO4, and 2.9 g of Na3 citrate · 2H2O. The pH was adjusted to 7.1 with 10 mM NaOH. This mineral medium was autoclaved and then supplemented with 1 ml of filter-sterilized MgSO4 (1 M) and 1 ml of MT trace element stock solution, which contained, per liter, 2.78 g of FeSO4 · 7H2O, 1.47 g of CaCl2 · 2H2O, 1.98 g of MnCl2 · 4H2O, 2.81 g of CoSO4 · 7H2O, 0.17 g of CuCl2 · 2H2O, and 0.29 g of ZnSO4 · 7H2O in 1 M HCl.
Continuous cultivation conditions.
We used a 50-ml cell suspension grown overnight at 30°C in medium E in 300-ml shaking flask batch cultures as inocula for chemostat experiments. After an initial start-up batch, the bioreactor was switched to continuous mode. The following medium was used for continuous culture cultivations; 1 g of KH2HPO4/liter 0.708 g of (NH4)2SO4/liter (equals 150 mg of N/liter), 50 ppm of silicone antifoam, and variable amounts of sodium octanoate or trisodium citrate dihydrate. After autoclaving, 1 ml of 10 mM FeSO4 · 7H2O (in 1 M HCl), 1 ml of 1 M MgSO4, and 1 ml of continuous culture trace metal (CCTM) stock solution were added to the medium by filter sterilization. CCTM stock solution consisted of 1.47 g of CaCl2 · 2H2O/liter, 1.98 g of MnCl2 · 4H2O/liter, 2.81 g of CoSO4 · 7H2O/liter, 0.17 g of CuCl2 · 2H2O/liter, 0.29 g of ZnSO4 · 7H2O/liter, and 10 g of EDTA/liter at pH 4. The continuous culture experiments were done in a 3-liter laboratory bioreactor (MBR, Wetzikon, Switzerland) with a working volume of 2 liters equipped with automated pH control, a dissolved oxygen tension electrode, a magnetic coupled stirrer, and dilution rate control by an automatic balance.
The carbon source octanoate was not included in the mineral medium, but filter-sterilized octanoate was injected directly into the culture with a syringe pump (Perfusor Secura; B. Braun, Melsungen, Germany) through a thin needle (diameter, ca. 0.5 mm) to ensure a continuous carbon supply. The C0/N0 ratio of the medium was calculated from the pump flow rate and the measured nitrogen concentration in the mineral medium.
Sample preparation.
Cell samples were taken in ice-cooled flasks and spun down at 10,000 × g for 15 min at 4°C, and the culture supernatant was analyzed for residual nutrient concentrations. The cell pellet was washed twice with 10 mM MgCl2, frozen in nanopure water, and lyophilized.
Dry weight of cells.
The biomass concentration (measured in dry weight of cells [CDW]; also abbreviated as X) in the culture was measured by filtering an appropriate amount of cell suspension (3 to 8 mg, CDW) through a preweighed 0.2-μm-pore-size polycarbonate filter. The filter was washed once with 10 ml of a 10 mM MgCl2 solution and dried overnight at 105°C. The weight difference after cooling the filter in the exsiccator over silica gel was measured to calculate the concentration of CDW in the culture. All CDW measurements of chemostat experiments were done in triplicate. The relative error was below 5%. CDW determinations in batch cultures were done only once per sample because of the limiting amount of culture volume. Biomass concentration measurements were double-checked by optical density measurements at 450 nm and CDW determination. The results of the two techniques agreed well.
Analysis of substrates in medium and culture supernatant.
Ammonium was measured according to the indophenol method described by Scheiner (37). The detection limit of this method was 0.12 mg of N/liter. The method was linear up to concentrations of 2 mg of N/liter. Sample dilutions were done in nanopure water if necessary. The identity of acetate was verified by an enzymatic standard assay (Boehringer, Mannheim, Germany). Octanoate and acetate were measured by a gas chromatography (GC) method by direct injection of acidified aqueous samples (column, Permabond FFAP-0.35, 25 m by 0.32 mm; Macherey-Nagel, Basel, Switzerland). Butyric acid served as the internal standard, and a calibration curve for sodium octanoate in E medium was used as the external standard. Samples and standards were mixed in a 1:1 ratio with a solution containing 1 g of butyric acid/liter and 15 vol% orthophosphoric acid. The detection limit of this method was 50 mg of octanoic acid and acetic acid/liter, and the calibration curve was linear up to 4 g/liter. Dissolved organic carbon (DOC) in culture supernatants was measured using a TOCOR 2 total organic carbon analyzer (Maihak AG, Hamburg, Germany). For this purpose 10 ml of sample was acidified with 150 μl of concentrated HCl and stripped from CO2 with nitrogen gas. The linear concentration range was 5 to 100 mg of DOC/liter, and dilutions, if necessary, were made in nanopure water.
Elemental analysis.
The elemental cell composition was measured with a Carlo Erba (CHNS) elemental analyzer (model EA1108; Carlo Erba, Milan, Italy).
PHA analysis.
The PHA content and monomeric composition of the cells were analyzed by a GC method initially described by Braunegg et al. (4) and adapted for mcl-PHAs by Lageveen et al. (24). Methylbenzoate served as the internal standard, and pure mcl-PHA obtained by chloroform extraction and methanol precipitation was used as a methanolysation standard. This standard PHA was weighed and treated in the same way as the lyophilized cell samples. The identities of the monomers were checked with GC-mass spectrometry (MS) measurements (GC-MS MD 800; Brechbühler, AG, Schlieren, Switzerland).
Gas analysis.
The exhaust gas stream of the chemostat experiments was continuously analyzed for its CO2 (by infrared spectroscopy; IR-Binos; Leybold-Heraeus, Hanau, Germany) and O2 (paramagnetically) contents with a standard gas analyzer (Oxymat 3; Siemens, Erlangen, Germany).
RESULTS
P. oleovorans was grown in chemostat culture at five different dilution rates ranging from 0.05 to 0.4 h−1 with a mineral medium and octanoate as the sole source of carbon and energy. The carbon-to-nitrogen ratio of the supplied growth medium (C0/N0, given in moles per moles) was adjusted by keeping the concentrations of ammonium (N0 = 10.7 mM) and all other inorganic nutrients constant and by varying the concentration of octanoate stepwise (by varying the octanoate feed pump flow rate). Up to at least 2 g of dry PHA-free biomass liter−1, this medium was clearly limited by either C or N under the experimental conditions used (42). Culture parameters, such as residual concentrations of octanoate and ammonium, biomass produced, and cellular PHA contents were assessed under steady-state conditions (defined as constant concentrations of residual substrates and biomass concentrations).
Three distinct nutritional growth limitations.
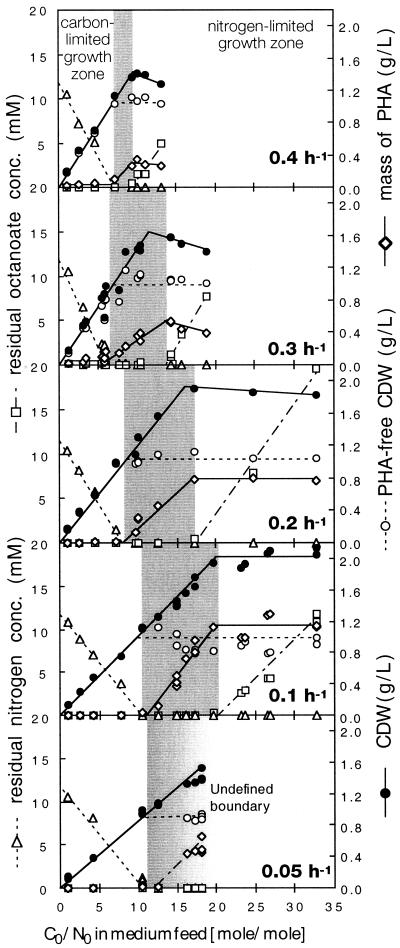
When the C0/N0 ratio of the inflowing growth medium was increased from low to high values, a typical pattern was observed at all dilution rates tested (Fig. 1): in addition to pure carbon limitation at low C0/N0 ratios in the medium feed with unutilized ammonium in the culture supernatant and pure nitrogen limitation with residual octanoate at high C0/N0 ratios, an intermediate zone of dual nutrient limitation was observed. Under these conditions both carbon and nitrogen were used to completion, and their concentrations in the culture supernatant were below the detection limit. The data clearly demonstrate that the width and location of the dual-nutrient-limited growth zone (Fig. 1) were functions of the growth rate. At a dilution rate of 0.4 h−1, the width of the zone was only 1.6 C0/N0 units, whereas at 0.1 h−1, the zone extended over 9.8 C0/N0 units. Both the lower and the upper boundaries of the dual-nutrient-limited growth zones shifted to higher C0/N0 values as the growth rate decreased.
FIG. 1.
Growth and PHA content of P. oleovorans in chemostat culture at different dilution rates, as a function of the carbon-to-nitrogen ratio of the medium feed (C0/N0, in moles per moles) with octanoate as the sole source of carbon and energy and ammonium as the only source of nitrogen. The concentrations of nitrogen and all other inorganic nutrients in the feed were kept constant (N0 = 10.7 mM), whereas the concentration of octanoate was varied. The zone of dual-nutrient-limited growth (shaded) was calculated from the slopes of residual nitrogen (lower boundary) and octanoate (upper boundary). The upper boundary at a D of 0.05 h−1 could not be determined due to by-product formation and unstable growth (see the text).
Determination of the boundaries of dual-nutrient-limited growth.
The boundaries of dual-nutrient-limited growth were estimated by two different procedures.
First, the boundary between carbon limitation and dual nutrient limitation (the lower boundary) was calculated by linear regression of residual ammonium versus the C0/N0 ratio in the feed medium and determination of the C0/N0 ratio at an NH4+ concentration of zero. Likewise, the boundary between dual-nutrient-limited growth and pure nitrogen limitation (the upper boundary) was calculated as the intercept of the best linear fit of the residual octanoate versus the C0/N0 ratio in the feed medium with the C0/N0 axis.
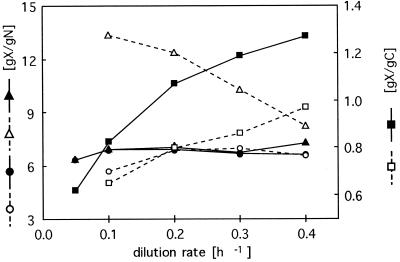
Second, elemental yield coefficients obtained under single-nutrient-limited growth conditions were used to calculate the boundaries of the dual-nutrient-limited growth zone according to the method of Egli and Quayle (13). For a particular dilution rate, the growth yield coefficients for nitrogen (YX/N) and carbon (YX/C) were constant under single-nutrient-limited conditions and changed within the dual-nutrient-limited growth zone: YX/C decreased, whereas YX/N increased (12). Additionally, the yield factors were dependent on the dilution rate (Fig. 2). YX/C increased with increasing growth rates both for carbon- and for nitrogen-limited growth conditions. Under carbon limitation, YX/N remained almost constant at all growth rates, but YX/N decreased by 38% for cells grown under nitrogen limitation when the growth rate was increased from 0.1 to 0.4 h−1 (Fig. 2).
FIG. 2.
Elemental yield coefficients for nitrogen and carbon determined for P. olevorans during growth in the chemostat with octanoate and ammonium under either nitrogen- or carbon-limited conditions, as a function of the dilution rate. Symbols: ▴, YX/N for carbon-limited growth; ▵, YX/N for nitrogen-limited growth; ■, YX/C for carbon-limited growth; □, YX/C for nitrogen-limited growth; ●, YX/N of PHA-free biomass for carbon-limited growth; ○, YX/N of PHA-free biomass for nitrogen-limited growth.
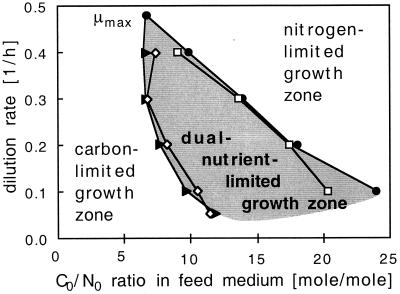
Figure 3 depicts the zone of dual-nutrient-limited growth as a function of the dilution rate and the C0/N0 ratio in the growth medium. The boundaries obtained from residual nutrient concentrations are compared to the values determined from elemental growth yield coefficients.
FIG. 3.
Extension of the dual (carbon and nitrogen)-nutrient-limited growth zone for the cultivation of P. oleovorans with octanoate and ammonium as a function of the dilution rate and the C0/N0 ratio (in moles per moles) of the medium feed. Measured boundaries were obtained from the slopes of either residual nitrogen (lower boundary) or octanoate (upper boundary) versus the C0/N0 ratio in the medium feed. Alternatively, the boundaries from the carbon and nitrogen yield coefficients were calculated according to the method of Egli and Quayle (13). The growth yield coefficients for the maximum growth rate (μmax) were determined in batch culture as described in reference 12 and refer to the non-PHA biomass produced during exponential growth. Lower boundary: ▴, calculated from yield coefficients; ◊, determined from residual nitrogen concentration pattern. Upper boundary: ●, calculated from yield coefficients; □, determined from residual octanoate concentration pattern.
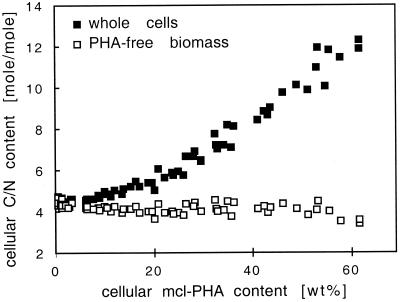
Elemental cell composition.
The elemental cell composition with respect to carbon and nitrogen was strongly dependent on the cellular PHA content (Table 1 and Fig. 4). The C/N ratio of the biomass was constant under single-nutrient-limited growth conditions and increased with increasing cellular PHA contents. In contrast, the calculated C/N ratio of the non-PHA biomass remained almost constant. This indicates that some of the surplus octanoate supplied to the culture in the dual-nutrient-limited growth zone was accumulated as mcl-PHA, and accumulation of this storage compound was the most significant factor of the change in the cellular composition with respect to carbon and nitrogen.
TABLE 1.
Culture parameters, elemental growth yields, elemental cellular composition, cellular PHA content, and PHA composition during growth of P. oleovorans in continuous culture at different dilution rates with octanoate and ammonium under either carbon- or nitrogen-limited conditions
| Parameter | Abbrev.a | Unit | Dilution rate or specific growth rateb
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.05 h−1
|
0.10 h−1
|
0.20 h−1
|
0.30 h−1
|
0.40 h−1
|
μmax (h−1) (batch)c | ||||||||
| C-lim. | N-lim. | C-lim. | N-lim. | C-lim. | N-lim. | C-lim. | N-lim. | C-lim. | N-lim. | ||||
| Observed C/N borders | C0/N0 | mol mol−1 | 11.6 | —d | 10.6 | 20.4 | 8.3 | 17.4 | 6.8 | 13.5 | 7.5 | 9.1 | — |
| Calculated C/N borderse | C0/N0 | mol mol−1 | 12.0 | — | 9.8 | 24.0 | 7.8 | 18.0 | 6.7 | 13.9 | 6.7 | 9.9 | 6.9 |
| Carbon-based growth yield | YX/C | g g−1 | 0.62 | — | 0.83 | 0.65 | 1.07 | 0.8 | 1.19 | 0.86 | 1.27 | 0.97 | 1.23 |
| Nitrogen-based growth yield | YX/N | g g−1 | 6.4 | — | 6.94 | 13.36 | 7.15 | 12.37 | 6.85 | 10.26 | 7.29 | 8.28 | 7.30 |
| Specific carbon consumption ratef | qs | g (g · h)−1 | 0.08 | — | 0.12 | 0.15 | 0.19 | 0.25 | 0.25 | 0.35 | 0.31 | 0.41 | 0.39 |
| Cellular PHA content | wt% | 1.6 | — | 1.4 | 56.1 | 2.2 | 42.3 | 10.1 | 31.2 | 7.6 | 20.9 | — | |
| Specific PHA accumulation rateg | qPHA | g (g · h)−1 | 0.001 | — | 0.001 | 0.133 | 0.005 | 0.147 | 0.038 | 0.137 | 0.035 | 0.108 | 0.031 |
| PHA yield coefficienth | YPHA/C | g g−1 | 0.01 | — | 0.01 | 0.25 | 0.02 | 0.23 | 0.09 | 0.18 | 0.07 | 0.15 | 0.046 |
| Cellular C content | wt% | 48.7 | — | 49.0 | 57.4 | 48.7 | 54.6 | 48.9 | 52.3 | 48.5 | 49.6 | — | |
| Cellular N content | wt% | 12.7 | — | 12.7 | 5.8 | 12.5 | 7.3 | 11.7 | 8.9 | 12.1 | 10.8 | — | |
| Cellular H content | wt% | 7.1 | — | 7.2 | 8.0 | 7.1 | 7.3 | 6.8 | 7.7 | 7.1 | 7.4 | — | |
| C/N ratio of dry cell mass | mol mol−1 | 4.5 | — | 4.5 | 11.6 | 4.4 | 8.7 | 4.9 | 6.8 | 4.7 | 5.4 | — | |
| C/N ratio of PHA-free biomass | mol mol−1 | 4.4 | — | 4.4 | 3.8 | 4.3 | 4.2 | 4.1 | 4.1 | 4.2 | 3.8 | — | |
Abbrev., abbreviation.
C-lim., carbon-limited growth; N-lim., nitrogen-limited growth.
The maximum growth rate (μmax) for P. oleovorans with octanoate was 0.48 h−1; data are taken from reference 12.
—, calculation or analysis not possible.
Calculation according to reference 13.
Grams of carbon used per gram of CDW per hour.
Grams of PHA accumulated per gram of PHA-free biomass per hour.
Grams of carbon accumulated in PHA per gram of carbon used.
FIG. 4.
Cellular C/N ratio of P. oleovorans grown with octanoate at different dilution rates as a function of the mcl-PHA content. ■, C/N ratio of whole freeze-dried cells; □, C/N ratio of the non-PHA biomass.
mcl-PHA accumulation as a function of the C0/N0 ratio and dilution rate.
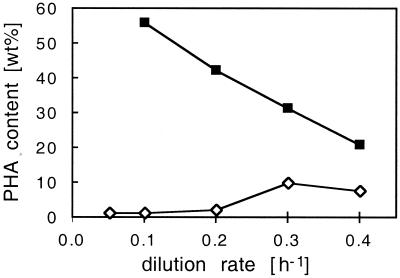
PHA accumulation was detected in cells of P. oleovorans under all growth conditions tested (Fig. 5). Low cellular PHA contents were measured even during carbon-limited growth at low C0/N0 ratios. The cellular PHA content under carbon-limited growth conditions was a function of the dilution rate. It was below 2 wt% at a D of 0.05 h−1, exhibited a maximum of more than 10 wt% at a growth rate of 0.3 h−1, and decreased again to 7.6 wt% at 0.4 h−1. For all dilution rates, the cellular PHA content increased within the dual-nutrient-limited growth zone and remained at the maximum level under pure nitrogen limitation (Fig. 5; compare also Fig. 1). For cells grown under distinct nitrogen limitation, the mcl-PHA content was inversely proportional to the growth rate and decreased from more than 56% at a growth rate of 0.1 h−1 to less than 21% at 0.4 h−1.
FIG. 5.
Cellular mcl-PHA contents of P. oleovorans grown in continuous culture with octanoate and ammonium, as a function of the growth-limiting nutrient at different dilution rates. PHA content was measured under carbon (◊)- and nitrogen (■)-limited growth conditions.
Composition of the accumulated polymer.
The monomeric composition of the accumulated mcl-PHA was independent of the dilution rate and consisted of 88 mol% 3-hydroxy-octanoic acid (3OH-8) and 12 mol% 3-hydroxy-hexanoic acid (3OH-6). Only when the cellular PHA content was below 3 wt% was no 3OH-6 detected by GC analysis. However, this was possibly due to analytical limitations of the method and not to the total absence of 3OH-6 in the polymer itself.
By-product formation under nitrogen-limited growth conditions at low dilution rates.
At dilution rates equal to or lower than 0.1 h−1 and C0/N0 ratios in the feed medium that were close to or above the upper boundary of dual nutrient limitation, significant by-product formation was detected. This excretion of by-products always went along with a visible browning of the culture supernatant and an exceptionally low YX/C. Although no distinct absorption maxima were observed, UV/visible spectrometry of the culture supernatant showed that the excreted products resulted in an increase in absorption at wavelengths below 450 nm. Size exclusion chromatography indicated that the major fraction consisted of by-products with molecular masses below 250 Da. Acetate was identified as the main by-product by ion chromatography and enzymatic assay. However, because the absolute amount of excreted carbonaceous compounds is unknown, the fraction of acetate can only be estimated to be approximately 60% of the excreted carbon. When the chemostat dilution rate was increased above 0.1 h−1 or the C0N0 ratio in the medium feed was decreased below a critical value, the brown color disappeared according to a washout curve. Due to this phenomenon, no purely nitrogen-limited steady-state conditions could be achieved at a dilution rate of 0.05 h−1.
DISCUSSION
Dual-nutrient-limited growth zones and their boundaries.
The cultivation of P. oleovorans in chemostat culture with octanoate and ammonium at different C0/N0 ratios of the medium feed led to steady-state growth not only under clearly C- or N-limited conditions. In addition, in between the two single-substrate-limited growth regimes, a stable zone of growth was observed where both nutrients were utilized to completion (referred to as a dual-nutrient-limited growth zone).
With decreasing dilution rates, the lower as well as the upper boundary of the dual-nutrient-limited growth zone shifted to higher C0/N0 ratios and the zone broadened (Fig. 3). The boundaries obtained from the residual nutrient concentrations were in good agreement with the results calculated from nutrient yield coefficients measured under single-nutrient-limited growth conditions as described by Egli and Quayle (13). They proposed a simple empirical equation (equations 1 and 2) which allows an estimation of the borders of dual nutrient limitation on a C0/N0 axis from the elemental growth yields derived from single-nutrient-limited growth under the condition that these yields are constant under the respective limitation. The steady-state biomass at the border of dual nutrient limitation (see Fig. 1) can be calculated from the elemental growth yield factor and the amount of the respective nutrient used.
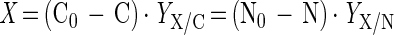 |
1 |
Assuming that the concentrations of the residual nutrients at the border of dual nutrient limitation are close to zero, the equation can be rearranged to yield the C0/N0 ratio of the feed medium at which the boundary should be observed:
 |
2 |
Equation 2 implies that the zone of dual-nutrient-limited growth should become more extended as the difference in the yield coefficients between the two single nutrient limitations becomes more pronounced, which is increasingly the case as the dilution rate is lowered (19).
The general shape of the dual-nutrient-limited growth zone presented here for the growth of P. oleovorans with octanoate and ammonium (Fig. 3) is in good agreement with the predictions made earlier by Egli (14). From reports in the literature of growth yield factors obtained for single-nutrient-limited growth of Klebsiella pneumoniae with glycerol and NH4+, this author predicted a similarly shaped zone of dual-nutrient-limited growth conditions.
From Fig. 2 and on the basis of equation 2, two main parameters can be seen to influence the extension and location of the dual-nutrient-limited growth zone.
First, under carbon limitation, the change of YX/C determines the location of the lower boundary of the dual-nutrient-limited growth regime, because under these conditions YX/N is constant at all dilution rates. YX/C is the sum of a growth-associated term and the maintenance coefficient (31), and the fraction of maintenance energy with respect to the total yield coefficient increases with decreasing growth rate. Therefore, the shift of the boundary between the C- and the C/N-limited growth zone with decreasing growth rates is predominantly caused by the maintenance energy coefficient.
Second, YX/C and YX/N obtained for N-limited conditions both changed with different dilution rates (Fig. 2). As described above, YX/C increased with increasing growth rate due to the dwindling influence of the maintenance energy coefficient. In contrast, YX/N measured under nitrogen limitation for whole cells decreased with increasing growth rates, because the cellular PHA content also decreased as the dilution rate increased. When the nitrogen-based growth yield was calculated for the non-PHA biomass (YB/N), it remained constant at all growth rates. This indicates that the accumulation of mcl-PHA was the main cause for the change in YX/N under nitrogen limitation and that this affected the position of the upper boundary of the dual-nutrient-limited growth regime.
mcl-PHA accumulation under carbon-limited growth conditions.
At growth rates approaching the maximum growth rate (μmax), the cells produced a considerable amount of mcl-PHA under purely carbon-limited growth conditions (Fig. 5). As we have shown elsewhere (12), P. oleovorans also accumulates mcl-PHA in batch culture during unrestricted growth in the exponential phase. Both observations might result from the fact that in cells cultivated with mcl-fatty acids at high growth rates, the intracellular metabolite pools relevant for PHA accumulation are saturated, and overflow metabolism directs β-oxidation metabolites into mcl-PHA precursors and polymer. A similar explanation has been put forward for the growth-associated poly (hydroxybutyric acid) (PHB) accumulation in Methylobacterium rhodesianum (1), where a postulated metabolic bottleneck diverted intermediates into PHB formation. Page and Knosp (29) explained the PHB accumulation during unrestricted growth of Azotobacter vinelandii strain UWD by its deficient capacity to oxidize NADH and the resulting high ratio of NADH to NAD+. This excess of reducing power in the cell feeds back on the tricarboxylic acid (TCA) cycle, which in turn promotes the condensation of acetoacetyl coenzyme A (CoA) that allows PHB synthesis as an alternative electron sink (see also reference 30). In both cases PHB was formed as an overflow product of central metabolic pathway intermediates. Several other authors detected growth-associated PHB or mcl-PHA accumulation in the exponential phase of batch cultures in various microorganisms (2, 4, 22, 26, 41).
mcl-PHA accumulation under nitrogen limitation.
When cells were subjected to pure nitrogen limitation, the extent of mcl-PHA stored increased with decreasing growth rates. Although the absolute PHA contents reported differed slightly, the same observation has been made previously for P. oleovorans growing under conditions of carbon excess at different dilution rates in a two-liquid-phase chemostat with octane (33). Similar observations have also been reported by Ramsay et al. (35), who cultivated P. oleovorans with octanoate at different dilution rates at a fixed C0/N0 ratio of 14.7 (this C0/N0 ratio was calculated from the experimental data reported by these authors).
The phenomenon of dilution rate-dependent PHA accumulation can be attributed to the generally observed fact that the microbial cell composition is more flexible at lower growth rates (19). The calculated specific PHA accumulation rates of the non-PHA biomass are around 14 wt%/h(Table 1) for intermediate dilution rates (0.1, 0.2, and 0.3 h−1). This indicates that P. oleovorans produces PHA at a maximum rate at these growth rates. At a dilution rate of 0.4 h−1 the specific PHA accumulation rate was below 11%/h, which suggests that at this dilution rate, which is close to μmax, the intracellular nitrogen limitation is probably not as strong as at lower dilution rates.
Dual nutrient limitation as a tool for growth medium optimization.
The phenomenon of growth under dual-nutrient-limited conditions at a constant dilution rate has been described previously (7, 11, 13, 15, 25, 28, 36, 40). It has also been conjectured that the zone of multiple-nutrient limitation should be a function of the growth rate (13, 14). However, only one report is known which actually presented some experimental evidence for this hypothesis (28). Here, we conducted the first investigation focusing on the relation between the growth rate and the extension of the dual-nutrient-limited growth zone in combination with the accumulation of a nutrient storage compound.
The work reported here has several important implications. First, the data clearly demonstrate that the design of a microbial growth medium for chemostat cultures needs close attention. For instance, a carbon-limited growth medium used for low dilution rates can become dual nutrient limited or even nitrogen limited at higher dilution rates. Second, it is obvious that the concentrations of residual nutrients are also dependent on the dilution rate. This is of special interest for inhibitory or even toxic substrates, where very low (actual) concentrations in the culture supernatant are of crucial importance. For example, the decrease of formed CDW above the dual-nutrient-limited growth zone at dilution rates of 0.4 and 0.3 h−1 may have been caused by the inhibitory effect of residual octanoate (compare reference 35). Additionally, it has been pointed out recently that for the industrial production of mcl-PHA, the amount of carbon substrate used must be minimized because the price of the carbon source has a major influence on the production costs of the end product (8, 17). Cultivation of the cells at the upper boundary of the dual-nutrient-limited growth zone satisfies both requirements: low actual concentrations of possibly unfavorable nutrients and optimum utilization of the growth substrates supplied.
ACKNOWLEDGMENT
This research was supported in part by the Swiss Priority Programme for Biotechnology, grant NF-5002-37951.
REFERENCES
- 1.Ackermann J U, Babel W. Growth-associated synthesis of poly(hydroxybutyric acid) in Methylobacterium rhodesianum as an expression of an internal bottleneck. Appl Microbiol Biotechnol. 1997;47:144–149. [Google Scholar]
- 2.Anderson A J, Williams D R, Dawes E A, Ewing D F. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in Rhodococcus ruber. Can J Microbiol. 1995;41(Suppl. 1):4–13. [Google Scholar]
- 3.Brandl H, Gross R A, Lenz R W, Fuller R C. Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl Environ Microbiol. 1988;54:1977–1982. doi: 10.1128/aem.54.8.1977-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braunegg G, Sonnleitner B, Lafferty R M. A rapid gas chromatographic method for the determination of poly-3-hydroxybutyric acid in microbial biomass. Eur J Microbiol Biotechnol. 1978;6:29–37. [Google Scholar]
- 5.Braunegg G, Lefebvre G, Renner G, Zeiser A, Haage G, Loidllanthaler K. Kinetics as a tool for polyhydroxyalkanoate production optimization. Can J Microbiol. 1995;41(Suppl. 1):239–248. [Google Scholar]
- 6.Byrom D. Polymer synthesis by microorganisms: technology and economics. Trends Biotechnol. 1987;5:246–250. [Google Scholar]
- 7.Cooney C L, Wang D I C. Transient response of Enterobacter aerogenes under a dual nutrient limitation in a chemostat. Biotechnol Bioeng. 1976;18:189–198. doi: 10.1002/bit.260180205. [DOI] [PubMed] [Google Scholar]
- 8.de Koning G J M, Kellerhals M B, van Meurs C, Witholt B. Poly(hydroxyalkanoates) from fluorescent Pseudomonads in retrospect and prospect. J Environ Polym Degrad. 1996;4:243–252. [Google Scholar]
- 9.de Smet M J, Eggink G, Witholt B, Kingma J, Wynberg H. Characterization of intracellular inclusions formed by Pseudomonas oleovorans during growth on octane. J Bacteriol. 1983;154:870–878. doi: 10.1128/jb.154.2.870-878.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi Y. Microbial synthesis, physical properties, and biodegradability of polyhydroxyalkanoates. Macromol Symp. 1995;98:585–599. [Google Scholar]
- 11.Duchars M G, Attwood M M. The influence of C:N ratio in the growth medium on the cellular composition and regulation of enzyme activity in Hyphomicrobium X. J Gen Microbiol. 1989;135:787–793. [Google Scholar]
- 12.Durner R. Feast and starvation: accumulation of bioplastic in Pseudomonas oleovorans. Ph.D. thesis. Switzerland: Swiss Federal Institute of Technology, Zürich; 1998. p. 12591. [Google Scholar]
- 13.Egli T, Quayle J R. Influence of the carbon:nitrogen ratio of the growth medium on the cellular composition and the ability of the methylotrophic yeast Hansenula polymorpha to utilize mixed carbon sources. J Gen Microbiol. 1986;132:1779–1788. [Google Scholar]
- 14.Egli T. On multiple-nutrient-limited growth of microorganisms, with special reference to dual limitation by carbon and nitrogen substrates. Antonie Leeuwenhoek. 1991;60:225–234. doi: 10.1007/BF00430367. [DOI] [PubMed] [Google Scholar]
- 15.Gräzer-Lampart S D, Egli T, Hamer G. Growth of Hyphomicrobium ZV620 in the chemostat: regulation of NH4+-assimilating enzymes and cellular composition. J Gen Microbiol. 1986;132:3337–3347. [Google Scholar]
- 16.Haywood G W, Anderson A J, Dawes E A. A survey of the accumulation of novel polyhydroxyalkanoates by bacteria. Biotechnol Lett. 1989;11:471–476. [Google Scholar]
- 17.Hazenberg W, Witholt B. Efficient production of medium-chain-length poly(3-hydroxyalkanoates) from octane by Pseudomonas oleovorans: economic considerations. Appl Microbiol Biotechnol. 1997;48:588–596. [Google Scholar]
- 18.Hazenberg W M. Production of poly(3-hydroxyalkanoates) by Pseudomonas oleovorans in two-liquid-phase media. Ph.D. thesis. Zürich, Switzerland: Swiss Federal Institute of Technology; 1997. p. 12050. [Google Scholar]
- 19.Herbert D. Stoichiometric aspects of microbial growth. In: Dean A C R, Ellwood D C, Evans C G T, Melling J, editors. Continuous culture: applications and new fields. Chichester, United Kingdom: Ellis Horwood; 1975. pp. 1–30. [Google Scholar]
- 20.Hocking P J, Marchessault R H. Biopolyesters. In: Griffin G J L, editor. Chemistry and technology of biodegradable polymers. London, United Kingdom: Chapman & Hall; 1994. pp. 48–96. [Google Scholar]
- 21.Huisman G W, de Leeuw O, Eggink G, Witholt B. Synthesis of polyhydroxyalkanoates is a common feature of fluorescent pseudomonads. Appl Environ Microbiol. 1989;55:1949–1954. doi: 10.1128/aem.55.8.1949-1954.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huisman G W, Wonink E, De Koning G J M, Preusting H, Witholt B. Synthesis of poly(3-hydroxyalkanoates) by mutant and recombinant Pseudomonas strains. Appl Microbiol Biotechnol. 1992;38:1–5. [Google Scholar]
- 23.Lageveen R. Oxidation of aliphatic compounds by Pseudomonas oleovorans. Ph.D. thesis. Groningen, The Netherlands: Rijksuniversiteit Groningen; 1986. [Google Scholar]
- 24.Lageveen R G, Huisman G W, Preusting H, Ketelaar P, Eggink G, Witholt B. Formation of polyesters by Pseudomonas oleovorans: effect of substrates on formation and composition of poly-(R)-3-hydroxyalkanoates and poly-(R)-3-hydroxyalkenoates. Appl Environ Microbiol. 1988;54:2924–2932. doi: 10.1128/aem.54.12.2924-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsson C, von Stockar U, Marison I, Gustafsson L. Growth and metabolism of Saccharomyces cerevisiae in chemostat cultures under carbon-, nitrogen-, or carbon- and nitrogen-limiting conditions. J Bacteriol. 1993;175:4809–4816. doi: 10.1128/jb.175.15.4809-4816.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefebvre G, Rocher M, Braunegg G. Effects of low dissolved-oxygen concentrations on poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) production by Alcaligenes eutrophus. Appl Environ Microbiol. 1997;63:827–833. doi: 10.1128/aem.63.3.827-833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenz R W, Kim B W, Ulmer H W, Fritzsche K, Knee E, Fuller R C. Functionalized poly-beta-hydroxyalkanoates produced by bacteria. In: Dawes E A, editor. Novel biodegradable polymers. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1990. pp. 23–25. [Google Scholar]
- 28.Minkevich I G, Krynitskaya A Y, Eroshin V K. A double substrate limitation zone of continuous microbial growth. In: Kyslik P, Dawes E A, Krumphanzl V, Novak M, editors. Continuous culture. London, United Kingdom: Academic Press; 1988. pp. 171–189. [Google Scholar]
- 29.Page W J, Knosp O. Hyperproduction of poly-β-hydroxybutyrate during exponential growth of Azotobacter vinelandii UWD. Appl Environ Microbiol. 1989;55:1334–1339. doi: 10.1128/aem.55.6.1334-1339.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page W J, Manchak J. The role of β-oxidation of short-chain alkanoates in polyhydroxyalkanoate copolymer synthesis in Azotobacter vinelandii UWD. Can J Microbiol. 1995;41(Suppl. 1):106–114. [Google Scholar]
- 31.Pirt S J. Principles of microbe and cell cultivation. 2nd ed. London, United Kingdom: Blackwell; 1975. [Google Scholar]
- 32.Pool R. In search of the plastic potato. Science. 1989;245:1187–1189. doi: 10.1126/science.245.4923.1187. [DOI] [PubMed] [Google Scholar]
- 33.Preusting H, Kingma J, Witholt B. Physiology and polyester formation of Pseudomonas oleovorans in continuous two-liquid-phase cultures. Enzyme Microb Technol. 1991;13:770–780. [Google Scholar]
- 34.Preusting H, Hazenberg W, Witholt B. Continuous production of poly(3-hydroxyalkanoates) by Pseudomonas oleovorans in a high-cell-density, two-liquid-phase chemostat. Enzyme Microb Technol. 1993;15:311–316. [Google Scholar]
- 35.Ramsay B A, Saracovan I, Ramsay J A, Marchessault R H. Continuous production of long-side-chain poly-β-hydroxyalkanoates by Pseudomonas oleovorans. Appl Environ Microbiol. 1991;57:625–629. doi: 10.1128/aem.57.3.625-629.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutgers M, Balk P A, van Dam K. Quantification of multiple-substrate controlled growth—simultaneous ammonium and glucose limitation in chemostat cultures of Klebsiella pneumoniae. Arch Microbiol. 1990;153:478–484. doi: 10.1007/BF00248430. [DOI] [PubMed] [Google Scholar]
- 37.Scheiner D. Determination of ammonia and Kieldahl nitrogen by indophenol method. Water Res. 1976;10:31–36. [Google Scholar]
- 38.Steinbüchel A, Valentin H E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol Lett. 1995;128:219–228. [Google Scholar]
- 39.Vogel H J, Bonner D M. Acetylornithinase of E. coli: partial purification and some properties. J Biol Chem. 1956;218:97–106. [PubMed] [Google Scholar]
- 40.von Stockar U, Larsson C, Marison I W, Cooney M J. Calorimetry of dual limitations in yeast cultures. Thermochim Acta. 1995;250:247–258. [Google Scholar]
- 41.Yamane T, Chen X F, Ueda S. Growth-associated production of poly(3-hydroxyvalerate) from n-pentanol by a methylotrophic bacterium, Paracoccus denitrificans. Appl Environ Microbiol. 1996;62:380–384. doi: 10.1128/aem.62.2.380-384.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zinn M. Dual (C,N) nutrient limited growth of Pseudomonas oleovorans. Ph.D. thesis. Zürich, Switzerland: Swiss Federal Institute of Technology; 1998. p. 12987. [Google Scholar]