Abstract
New biomarkers for acute kidney injury (AKI) have improved our understanding of the etiology and pathogenesis of AKI. Depending on their origin, function, and kinetic profile, biomarkers have a role in screening, diagnosis, prognostication, and monitoring of AKI. This offers opportunities to improve the management of AKI, but concerns and limitations remain. In this review, we summarize the current role of new AKI biomarkers in the management of AKI and outline some of the ongoing limitations and challenges.
Keywords: Acute kidney injury, Biomarkers
Background
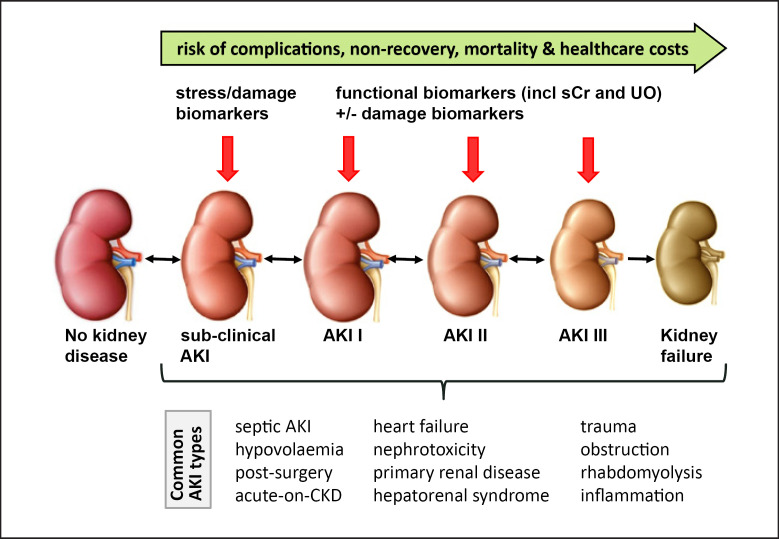
Acute kidney injury (AKI) is a syndrome with multiple diverse etiologies, pathogenic processes, and clinical phenotypes (Fig. 1). The traditional biomarkers of AKI are serum creatinine and urine output. Both are markers of kidney function but do not indicate early stress or injury of tubular cells or structural changes within the nephron (Table 1). They are not renal specific and do not differentiate between different etiologies, phenotypes of AKI, and underlying pathophysiology. Also, they can be influenced by nonrenal conditions and medications. Furthermore, dissociations between serum creatinine and histological findings on kidney biopsy have been reported [1].
Fig. 1.
Role of biomarkers in diagnosis and staging of AKI. AKI, acute kidney injury; CKD, chronic kidney disease; sCr, serum creatinine; UO, urine output.
Table 1.
Description of biomarker types (adapted from [13])
| Type of biomarker | Description |
|---|---|
| Stress marker | Marker which indicates cell stress; cell stress can resolve or progress to damage or alter renal function |
| Damage marker | Marker of structural damage which may or may not be associated with reduced renal function |
| Functional marker | Marker reflecting a change in glomerular filtration |
Role of Kidney Biomarkers
In general, biological markers (biomarkers) are indicators of normal biological processes, pathogenic processes, or responses to an intervention [2]. New molecules, biological substances, and cellular molecular patterns have been discovered in urine and serum that correlate with different types, phases, and pathways of AKI. These substances are either filtered or released from parts of the nephron in response to different stimuli (Table 2). For instance, tubular cell injury can be detected by damage biomarkers, whereas the degree of organ failure is estimated through the use of functional biomarkers such as serum creatinine and urine output. Changes in these 2 parameters − cell viability and kidney function − might occur concurrently, sequentially, or in isolation [3] (Fig. 2). Loss of kidney function may or may not be the result of kidney injury, and kidney injury may or may not lead to loss of kidney function.
Table 2.
Description and characteristics of selected AKI biomarkers (adapted from [10])
| AKI biomarker | Biological role | Biological sample | Stress marker | Damage marker | Functional marker | Roles in practice | Populations studied | Limitations |
|---|---|---|---|---|---|---|---|---|
| AAP; ALP; γ-GT | Enzymes located on the brush border of proximal tubular cells; released into urine after tubular damage | Urine | X | Diagnosis and severity of AKI | ICU | Elevated in UTI, cardiovascular disease, stroke | ||
|
| ||||||||
| CCL14 | Pro-inflammatory chemokine; released into urine following tubular cell stress/damage | Urine | X | Kidney recovery | ICU | Performance might vary in different AKI phenotypes | ||
|
| ||||||||
| Chitinase 3-like protein 1 | 39-kDa intracellular protein of glycoside hydrolase family; expressed by endothelial cells, macrophages, and neutrophils and released into the urine and plasma | Urine Plasma | X | Diagnosis of AKI | Cardiac surgery ICU | Limited performance in real-world settings as a single biomarker | ||
|
| ||||||||
| Cystatin C | 13-kDa cysteine protease inhibitor produced by nucleated human cells; freely filtered | Plasma | X | Diagnosis of AKI and measurement of severity | Cardiac surgery Liver transplantation Hospitalized patients |
Confounded by age, sex, inflammatory state, diabetes, low albumin, muscle mass, high-dose steroids | ||
|
| ||||||||
| DKK3 | 38-kDa renal tubular cell-derived glycoprotein; secreted into urine under tubular stress conditions | Urine | X | Risk assessment and prediction of AKI | Cardiac surgery | Elevated in CKD | ||
|
| ||||||||
| HGF | Antifibrotic cytokine produced by mesenchymal cells; involved in tubular cell regeneration after AKI and measured in plasma | Plasma | X | Severity of AKI and renal recovery | Hospitalized | Limited performance | ||
|
| ||||||||
| Hepcidin | 2.78-kDa peptide hormone predominantly produced in hepatocytes; freely filtered into urine and plasma | Urine Plasma | X | Diagnosis of AKI and assessment of severity | Cardiac surgery ICU | Decreased in anemia and increased in inflammatory state | ||
|
| ||||||||
| TIMP-2; IGFB7 | Metalloproteinases released during tubular cell cycle arrest (cell cycle arrest biomarker) | Urine | X | Prediction and diagnosis of AKI and assessment of severity | Cardiac and noncardiac surgery ICU | Elevated in diabetes | ||
|
| ||||||||
| IL-18 | 18-kDa pro-inflammatory cytokine; released into urine following tubular cell damage | Urine | X | Prediction and diagnosis of AKI | Hospitalized patients ICU ED cardiac surgery |
Elevated in inflammatory state Lack of cutoff values |
||
|
| ||||||||
| KIM-1 | Transmembrane glycoprotein produced by proximal tubular cell; released into urine after tubular cell damage | Urine | X | Prediction and diagnosis of AKI and assessment of severity | Hospitalized patients ED cardiac surgery ICU |
Elevated in chronic proteinuria and inflammatory diseases | ||
|
| ||||||||
| L-FABP | 14-kDa intracellular lipid chaperone; freely filtered and reabsorbed in proximal tubule; excreted into urine after tubular cell damage and measured in the urine and plasma | Urine Plasma | X | Diagnosis of AKI | Cardiac surgery ICU ED |
Associated with anemia in nondiabetic patients | ||
|
| ||||||||
| NAG | >130-kDa lysosomal enzyme; released into urine after tubular damage | Urine | X | Diagnosis of AKI | Cardiac surgery Hospitalized patients |
Elevated in diabetes and albuminuria | ||
|
| ||||||||
| NGAL | At least 3 different types measured in the urine and plasma Monomeric 25-kDa glycoprotein produced by neutrophils and epithelial tissues, including tubular cells Homodimeric 45-kDa protein produced by neutrophils Heterodimeric 135-kDa protein produced by tubular cells |
Urine Plasma | X | Diagnosis of AKI and measurement of severity | Cardiac and noncardiac surgery Coronary angiography ICU hospitalized patients Posttransplant ED | Elevated in sepsis, UTI, CKD Lack of specific cutoff values | ||
|
| ||||||||
| Netrin-1 | 50–75-kDa laminin-related molecule minimally expressed in proximal tubular cells of normal kidneys; released into urine after tubular cell damage | Urine | X | Diagnosis of AKI | Cardiac surgery | Limited data in high-risk settings, for example, CKD, diabetes, critical illness | ||
|
| ||||||||
| PENK | Endogenous polypeptide hormone in adrenal medulla, immune system, and renal tissue; freely filtered and measured in plasma | Plasma | X | Diagnosis and assessment of severity of AKI and renal recovery | ICU cardiac surgery Hospitalized patients |
|||
AKI, acute kidney injury; CKD, chronic kidney disease; ED, emergency department; ICU, intensive care unit; UTI, urinary tract infection; AAP, alanine aminopeptidase; ALP, alkaline phosphatase; γ-GT, γ-glutamyl transpeptidase; CCL14, C-C motif chemokine ligand 14; DKK3, dickkopf-3; HGF, hepatocyte growth factor; TIMP-2, tissue metalloproteinase-2; IGFB7, insulin-like growth factor binding protein 7; IL-18, interleukin-18; KIM-1, kidney injury molecule-1; L-FABP, liver-type fatty acid-binding protein; NAG, N-acetyl-β-D-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; PENK, proenkephalin-A.
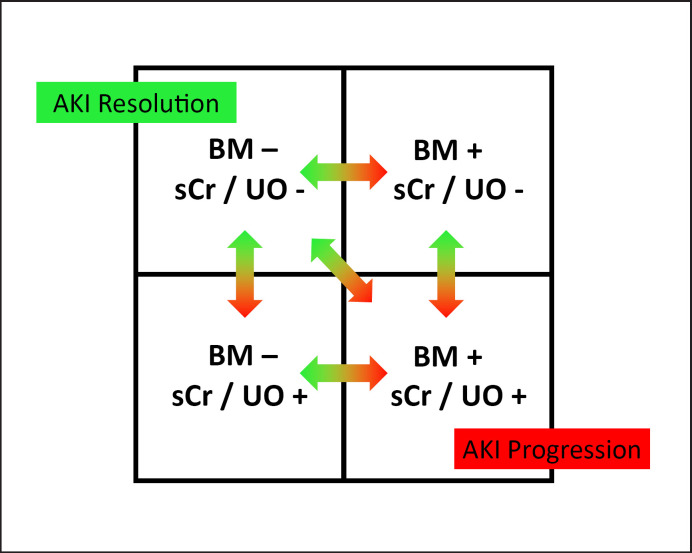
Fig. 2.
Integration of new biomarkers into the diagnosis of AKI. AKI, acute kidney injury; BM, biomarker; sCr, serum creatinine; UO, urine output. Reprinted from Acute Disease Quality Initiative 23 (https://www.ADQI.org) and used with permission; revised from [13].
In general, damage and functional biomarkers complement each other. Together, they provide information that is not available from using serum creatinine and urine output alone. Appropriate use and correct interpretation of the results and temporal pattern offer new scientific, diagnostic, and therapeutic opportunities.
Prediction of AKI Risk
New biomarkers have emerged that may allow better prediction of AKI risk. A study of 733 patients undergoing cardiac surgery demonstrated that the preoperative urinary concentration of dickkopf-3, a urinary cytokine and tubular stress biomarker, was able to identify patients with high risk of postoperative AKI and loss of kidney function (area under the receiver operating characteristics curve 0.78) [4].
Diagnosis of Early-Stage AKI
Numerous new biomarkers have been identified that indicate AKI before a rise in serum creatinine occurs. They offer the opportunity to guide management and initiate renoprotective strategies in those considered at high risk of progressive AKI. Three RCTs have confirmed a beneficial role for the implementation of a postoperative AKI care bundle in biomarker-positive patients [5, 6, 7]. In the single-center “Prevention of AKI (PrevAKI) trial” and subsequent multicenter randomized controlled PrevAKI-2 trial, patients with a positive cell cycle arrest biomarker (urinary [TIMP-2] × [IGFBP7] >0.3) 4 h after cardiac surgery were randomized to usual care versus a bundle of renoprotective strategies, including optimization of volume status and hemodynamics, correction of hyperglycemia, minimization of contrast exposure, and nephrotoxin management [5, 7]. In the PrevAKI trial (n = 276 patients), the incidence of moderate/severe AKI at 72 h was significantly lower in patients randomized to the intervention group compared to the control group (29.7% vs. 44.9%, p = 0.009). Applying the same inclusion criteria, the PrevAKI-2 trial enrolled 278 patients and again showed a lower incidence of moderate/severe AKI in the intervention cohort compared to the control group (14.0% vs. 23.9%, p = 0.03). The “Biomarker-guided Intervention to Prevent Acute Kidney Injury After Major Surgery (BigpAK)” study was conducted in patients at risk of AKI following major noncardiac surgery [6]. Patients with a positive biomarker result (urinary [TIMP-2] × [IGFBP7] >0.3) were randomized to an intervention strategy versus usual care. The overall incidence of AKI was not different at 7 days, but there was a 13% absolute reduction in AKI stage 2 and 3 in the intervention group (p = 0.04). These studies demonstrate that utilization of novel AKI biomarkers to identify patients with early AKI/kidney stress allows focused personalized management in high-risk patients.
Identification of AKI Subtypes
AKI is a syndrome which includes multiple types and etiologies (Fig. 1). Although serum creatinine elevation might be similar in a variety of kidney diseases, the anatomical pattern of injury, underlying pathogenesis, and genetic, molecular, and cellular responses vary.
RNA sequencing methods have confirmed activation of different genetic pathways in different experimental AKI models [3]. For instance, volume depletion activates metabolic pathways and anti-inflammatory molecules, whereas ischemic clamping of the kidney pedicle leads to activation of genes of inflammatory, coagulation, and epithelial repair pathways. The discovery of new biomarkers of kidney injury has enabled a more precise delineation of the site, pathophysiology, mechanisms, and severity of injury. For example, urinary concentrations of neutrophil gelatinase-associated lipocalin (NGAL) were found to be raised in acutely ill patients with prolonged AKI, whereas patients with acute heart failure and AKI receiving diuretics did not express NGAL [8, 9].
It is likely that the ability to identify different etiologies, mechanisms, and types of AKI will be critical in developing targeted therapies and designing pharmacological trials to enable more personalized preventive or therapeutic interventions. The expert panel of the 23rd Acute Disease Quality Initiative conference concluded that clinical information enriched by damage and functional biomarkers could lead to more sensitive AKI definitions [10]. Importantly, this concept does not suggest substituting serum creatinine for new biomarkers but rather to incorporate the information provided by each factor (Fig. 2).
Prediction of Progression of AKI/Nonrecovery
Biomarkers predictive of progressive AKI have potential to complement clinical decision-making, including monitoring and clinical care, identification of patients for clinical trials, and initiation of renal replacement therapy (RRT) [10]. The Translational Research Investigating Biomarker Endpoints in AKI (TRIBE-AKI) study included patients after cardiac surgery and showed an association between plasma NGAL and interleukin-18 on the day of AKI diagnosis and AKI progression [11]. A study in patients with septic AKI showed that those with major adverse kidney events, persistent AKI, or worsening of kidney function were characterized by significantly higher proenkephalin-A concentrations [12]. The rise in proenkephalin-A concentrations preceded elevation of serum creatinine. Similarly, a recent study in 331 critically ill patients with AKI stage 2 or 3 demonstrated that urinary C-C motif chemokine ligand 14 was predictive of persistent AKI [13].
Biomarkers indicating progression of AKI could complement clinical decision-making regarding initiation of RRT in the future [10]. A meta-analysis of 14 studies including over 15,000 patients concluded that several new biomarkers showed reasonable prediction of RRT use for critically ill patients with AKI, but currently the strength of evidence precludes their routine use to guide decision-making on when to initiate RRT [14]. Further studies using newer biomarkers including C-C motif chemokine ligand 14 are awaited.
Diagnosis of Nephrotoxicity
Panels of tubular damage markers have been approved for assessment of tubular injury in nonclinical and clinical drug studies to help guide safety assessments of new potential therapies [3, 15].
Prediction of Long-Term Outcomes
Survivors of AKI are at increased risk of CKD and premature end-stage kidney disease, but it remains unclear who should be followed up and what optimal after-care encompasses. It is hoped that new kidney biomarkers will serve to identify high-risk patients. A study of 1,207 patients discharged alive from the ICU showed that patients with elevated kidney biomarker results (i.e., plasma cystatin C, plasma NGAL, urinary NGAL, and plasma proenkephalin-A) at ICU discharge had worse 1-year outcomes than patients with normal biomarker results, including those with low serum creatinine at ICU discharge [16]. Again, this highlights the fact that serum creatinine is dependent on nonrenal factors, including muscle mass, and may be low despite kidney disease after a period of immobility. Another study showed that in patients who developed AKI within 72 h after ICU admission, urinary (TIMP-2) × (IGFBP7) levels displayed a stepwise increase in their association with the composite endpoint of death or receipt of RRT at 9 months [17].
Recommendations
There are several consensus statements and guidelines from different organizations regarding the use of biomarkers in clinical practice. The ADQI expert group suggests that routine clinical assessment should be combined with damage and functional biomarkers to risk stratify, discriminate etiologies, assess the severity, plan the management, and predict the duration and recovery of AKI [10]. The Japanese 2016 Clinical Practice Guideline for AKI supports the utility of biomarkers for the early diagnosis of AKI (urine NGAL and L-FABP) in postcardiac surgery and ICU settings, predicting AKI severity and mortality (urine NGAL) and differentiating prerenal AKI from renal AKI (urine NGAL) [18]. A Critical Care expert panel published recommendations for the use of urinary (TIMP-2) × (IGFBP7) [19]. Proposed target populations include patients undergoing major surgery, those with hemodynamic instability, and patients with sepsis, especially within 72 h after ICU admission. It was suggested that positive results may lead to an integrated care bundle including nephrotoxic drug management and fluid therapy. Importantly, negative results are also informative as they may lead to “fast-track protocols” and de-escalation of monitoring.
Limitations of New Biomarkers
Only a small number of new biomarkers have been integrated into routine clinical practice. For example, NGAL is available in Europe, the use of L-type fatty acid-binding protein is covered by public health insurance in Japan, and urinary (TIMP-2) × (IGFBP7) is commercially available in the USA and Europe. Barriers include the use of different cutoff values in published reports, risk of confounding by acute and chronic comorbidities, and concerns about costs. Another limitation is that the performance of new biomarkers is best in conditions where the time of renal insult is known, for instance, postcardiac surgery or coronary angiography, compared to situations where the onset of kidney injury is less clear, for instance, in sepsis.
Conclusions
New biomarkers have improved our understanding of the pathogenesis of AKI and can allow the identification of high-risk patients and AKI subphenotypes. Depending on their origin, function, and kinetic profile, evidence is emerging that biomarkers can be used for screening, diagnosis, prognostication, and monitoring of AKI. This offers opportunities to improve the management of AKI. However, gaps in knowledge remain, and more research is necessary.
Statement of Ethics
The study is exempt from ethics committee approval because it is a summary of existing data. No new patients were recruited.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received.
Author Contributions
M.O. wrote the first draft. N.L. and E.K. edited the draft and developed the table. All authors approved the final draft.
Acknowledgments
We would like to thank the research nurses in the critical care department at Guy's & St Thomas' Hospital for supporting the research studies in acute kidney injury.
Contribution from the AKI and CRRT 2021 Symposium at the 26th International Conference on Advances in Critical Care Nephrology, A Virtual/Hybrid Event from San Diego, CA, USA, February 28–March 5, 2021. This symposium was supported in part by the NIDDK-funded University of Alabama at Birmingham-University of California San Diego O'Brien Center for Acute Kidney Injury Research (P30DK079337).
References
- 1.Chu R, Li C, Wang S, Zou W, Liu G, Yang L. Assessment of KDIGO definitions in patients with histopathologic evidence of acute renal disease. Clin J Am Soc Nephrol. 2014;9((7)):1175–82. doi: 10.2215/CJN.06150613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biomarkers Definitions Working Group Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69((3)):89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 3.Desanti De Oliveira B, Xu K, Shen TH, Callahan M, Kiryluk K, D'Agati VD, et al. Molecular nephrology: types of acute tubular injury. Nat Rev Nephrol. 2019;15((10)):599–612. doi: 10.1038/s41581-019-0184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schunk SJ, Zarbock A, Meersch M, Küllmar M, Kellum JA, Schmit D, et al. Association between urinary dickkopf-3, acute kidney injury, and subsequent loss of kidney function in patients undergoing cardiac surgery: an observational cohort study. Lancet. 2019;394((10197)):488–96. doi: 10.1016/S0140-6736(19)30769-X. [DOI] [PubMed] [Google Scholar]
- 5.Meersch M, Schmidt C, Hoffmeier A, Van Aken H, Wempe C, Gerss J, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: the Prev AKI randomized controlled trial. Intensive Care Med. 2017;43((11)):1551–61. doi: 10.1007/s00134-016-4670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Göcze I, Jauch D, Götz M, Kennedy P, Jung B, Zeman F, et al. Biomarker-guided intervention to prevent acute kidney injury after major surgery: the prospective randomized BigpAK Study. Ann Surg. 2018;267((6)):1013–20. doi: 10.1097/SLA.0000000000002485. [DOI] [PubMed] [Google Scholar]
- 7.Zarbock A, Küllmar M, Ostermann M, Lucchese G, Baig K, Cennamo A, et al. Prevention of cardiac surgery-associated acute kidney injury by implementing the KDIGO guidelines in high-risk patients identified by biomarkers: the PrevAKI-multicenter randomized controlled trial. Anesth Analg. 2021 doi: 10.1213/ANE.0000000000005458. [DOI] [PubMed] [Google Scholar]
- 8.Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59((3)):246–55. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad T, Jackson K, Rao VS, Tang WHW, Brisco-Bacik MA, Chen HH, et al. Worsening renal function in patients with acute heart failure undergoing aggressive diuresis is not associated with tubular injury. Circulation. 2018;137((19)):2016–28. doi: 10.1161/CIRCULATIONAHA.117.030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostermann M, Zarbock A, Goldstein S, Kashani K, Macedo E, Murugan R, et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: a consensus statement. JAMA Netw Open. 2020;3((10)):e2019209. doi: 10.1001/jamanetworkopen.2020.19209. [DOI] [PubMed] [Google Scholar]
- 11.Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012;23((5)):905–14. doi: 10.1681/ASN.2011090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollinger A, Wittebole X, François B, Pickkers P, Antonelli M, Gayat E, et al. Proenkephalin A 119-159 (Penkid) is an early biomarker of septic acute kidney injury: the kidney in sepsis and septic shock (Kid-SSS) study. Kidney Int Rep. 2018;3((6)):1424–33. doi: 10.1016/j.ekir.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoste E, Bihorac A, Al-Khafaji A, Ortega LM, Ostermann M, Haase M, et al. Identification and validation of biomarkers of persistent acute kidney injury: the RUBY study. Intensive Care Med. 2020;46((5)):943–53. doi: 10.1007/s00134-019-05919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein SJ, Brandtner AK, Lehner GF, Ulmer H, Bagshaw SM, Wiedermann CJ, et al. Biomarkers for prediction of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 2018;44((3)):323–36. doi: 10.1007/s00134-018-5126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieterle F, Sistare F, Goodsaid F, Papaluca M, Ozer JS, Webb CP, et al. Renal biomarker qualification submission: a dialog between the FDA-EMEA and predictive safety testing consortium. Nat Biotechnol. 2010;28((5)):455–62. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- 16.Legrand M, Hollinger A, Vieillard-Baron A, Dépret F, Cariou A, Deye N, et al. One-year prognosis of kidney injury at discharge from the ICU: a multicenter observational study. Crit Care Med. 2019;47((12)):e953–e61. doi: 10.1097/CCM.0000000000004010. [DOI] [PubMed] [Google Scholar]
- 17.Koyner JL, Shaw AD, Chawla LS, Hoste EA, Bihorac A, Kashani K, et al. Tissue inhibitor metalloproteinase-2 (TIMP-2)⋅IGF-binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol. 2015;26((7)):1747–54. doi: 10.1681/ASN.2014060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doi K, Nishida O, Shigematsu T, Sadahiro T, Itami N, Iseki K, et al. The Japanese clinical practice guideline for acute kidney injury 2016. J Intensive Care. 2018;6:48. doi: 10.1186/s40560-018-0308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzzi LM, Bergler T, Binnall B, Engelman DT, Forni L, Germain MJ, et al. Clinical use of (TIMP-2) × (IGFBP7) biomarker testing to assess risk of acute kidney injury in critical care: guidance from an expert panel. Crit Care. 2019;23((1)):225. doi: 10.1186/s13054-019-2504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]