Abstract
Caloric restriction (CR)-mediated organ protection has been shown to be extremely efficient in rodent models of acute kidney injury (AKI). Limited understanding of the underlying mechanisms paired with a risk of malnourishment and feasibility problems has hindered the translation of this immense potential to the patient setting. In this mini-review, the current mechanistic concepts of CR-mediated stress-resistance as potential key targets for renal protection in AKI will be highlighted.
Keywords: Caloric restriction, Acute kidney injury, Dietary preconditioning, Mechanisms, Stress-resistance
Introduction
Acute kidney injury (AKI) is a common disease with a rising incidence that bears substantial morbidity and mortality. Despite the considerable burden associated with AKI, effective therapeutic and preventive approaches are currently lacking. Since impaired cellular stress-resistance contributes to the development of AKI, preconditioning protocols that augment renal resilience, such as caloric restriction (CR), are attractive strategies in the search for organ protection. CR has originally been extensively studied in the context of aging, since it extends lifespan and improves both general and metabolic health across taxa [1]. Recently, it has been shown that CR protects from toxic AKI and renal ischemia-reperfusion injury (IRI) in rodents [2, 3, 4]. Limited understanding of the underlying mechanisms has hindered the clinical translation of this immense potential. In this mini-review, we focus on evolutionary conserved molecular mechanisms in response to CR augmenting renal stress-resistance.
Main Text
CR, that is, a reduction of 10–40% in calorie intake, is reported to be the most potent non-generic mechanism to extend lifespan, which was firstly identified in rodents and later confirmed in various model organisms including non-human primates [5]. CR has become of special interest to nephrologists, as it augments renal protection and prevents kidney damage in rodent models of IRI and toxic AKI [2, 3, 4]. However, CR has not made its way to the clinical setting, partly due to problems regarding feasibility and potential safety concerns. Lack of knowledge regarding normal food and calorie intake especially in elderly multimorbid patients makes trial design complicated. Concealed adherence problems may occur in the CR group due to difficulties regarding food intake surveillance on the one hand. On the other hand, unintended dietary restriction may occur in the control group due to expectations regarding outcome improvement. Additionally, blinding is often difficult in trials examining dietary interventions. Despite the fact that this is enabled when using formula diets, a reduction in caloric intake may easily be noticed by the participants as a consequence of the sensation of hunger. Moreover, recruitment of patients may be limited by lack of motivation to restrict dietary habits before a surgical intervention. Besides, safety concerns, especially in elderly, frail, and severely ill patients, may play a role with prolonged CR [6, 7]. Consequently, developing an increasing knowledge of the underlying molecular mechanisms orchestrating cellular stress-resistance will help to facilitate translation, for example, by identification of targets for pharmacological interventions. Current mechanistic concepts of CR-mediated resilience propose a modulation of several metabolic pathways that will be presented in the following.
A reduction in insulin/IGF-1 signaling activity has been identified as an overlapping mechanism of different modes of CR in rodent models of AKI [2]. Improved insulin sensitivity has also been observed to be shared in both genetic and dietary models of longevity and stress-resistance. CR leads to reduced renal levels of Ghr and IGF-1 mRNA and of their associated signaling pathways as well as an elevated expression of antioxidant defense enzymes in the kidney [8]. Interestingly, upon ischemic kidney damage, insulin/IGF-1 signaling pathway activity is reduced in both CR and ad libitum fed mice; however, in CR-preconditioned mice, this decline is proportionally less, and insulin signaling is restored more quickly, which may contribute to the observed increased survival in CR-preconditioned animals [2].
Furthermore, stress-resistance modulated by CR in renal IRI has been identified to be associated with repression of mammalian target of rapamycin (mTOR) and AMP-activated protein kinase (AMPK) activation prior to renal IRI in rodents [9]. mTOR is an evolutionary conserved kinase involved in growth and metabolism which is strongly modulated by nourishment and starvation as well as insulin signaling across taxa. Although there has been profound basic and translational research over the last decades, the role of mTOR in AKI and preconditioning remains controversial. mTOR consists of 2 distinct complexes, the mechanistic target of rapamycin complex 1 (mTORC1) and 2 (mTORC2), with different functions. mTORC1 is an upstream inhibitor of autophagy and can be inhibited by rapamycin pharmacologically, resulting in lifespan extension and increased stress-resistance in various longevity models [10]. As mTORC1 has a critical role in sensing nutrients and insulin, it appears reasonable to assume that CR benefits depend on reduced mTORC1 levels, too. Of note, CR does not extend lifespan in yeast, worm, and flies with reduced mTORC1 activity suggesting overlapping mechanisms of resilience by CR and mTORC1, such as an increase in autophagy and a decrease in mRNA translation and protein metabolism limiting proteotoxic and oxidative stress [10]. In contrast, mTORC2 is not affected by rapamycin and contributes to the CR-mediated improvement of insulin sensitivity, but its disruption in an adipose-specific RICTOR knockout mouse inducing insulin resistance does not alter the CR-induced increase in metabolic health and lifespan [11]. Overall mTOR activity is low in kidney tissue. However, its activity is significantly induced during the reperfusion period after renal IRI in rats, which can reasonably be assumed to be a consequence of the sudden availability of growth factors, amino acids, and adenosine triphosphate [12]. Inhibition of mTORC1 by rapamycin during reperfusion in renal IRI delays recovery of kidney function in rats and − in line with this finding − is associated with delayed graft function in humans [12]. Therefore, inhibition of mTOR by CR or rapamycin may be beneficial in renal IRI, for example, by induction of autophagy when applied before damage but appears to impair the natural response to IRI and regeneration [13]. However, it has to be noted that data on postconditioning by, for example, rapamycin treatment in AKI models are lacking. In contrast to these findings, the beneficial effects of mTOR inhibition appear to be more straightforward in sepsis or cisplatin-induced AKI models in mice and mainly employ the induction of tubular cell autophagy and the alleviation of cell death [14, 15]. Taken together, the role of mTOR inhibition in nephroprotection and AKI appears to depend on its exact timing as well as the type of damage and may also be subject to species-dependent differences.
AMPK signaling is an additional pathway that is closely connected to both mTOR and CR. AMPK is a key regulator of cellular and mitochondrial health, as it serves as an energy sensor regulating cellular metabolism and homeostasis. Additionally, AMPK promotes both mito- and autophagy. Under conditions of CR, mTORC1 is suppressed allowing activation of AMPK and the induction of autophagy. Additionally, activated AMPK suppresses the regulatory-associated protein of mTOR(RAPTOR) inhibiting mTORC1. AMPK activates its downstream target, the nicotinamide adenine dinucleotide (NAD)-dependent type III deacetylase sirtuin 1 (SIRT1), by increasing cellular NAD levels. Of note, CR also causes an increased NAD/NADH ratio itself thus promoting SIRT1 and leading to backward activation of AMPK. Promoting autophagy by pharmacological inhibition of mTORC1 or activation of AMPK as well as by CR protects from both ischemic and toxic AKI in rodents [16]. Besides, considering the growing knowledge on the impact of NAD in renal IRI, it will be intriguing to characterize a potential role of CR in this context [17].
Beyond the impact on mTOR and AMPK signaling described above, CR activates the transsulfuration pathway (TSP) resulting in the generation of hydrogen sulfide (H2S), which is associated with anti-inflammation, reduction of reactive oxygen species (ROS), and adaptations in lipid metabolism. The TSP-mediated generation of H2S by CR is evolutionary conserved across taxa as determined in different longevity models [1]. Furthermore, the endogenous generation of H2S has been identified to be essential for CR benefits in a rodent model of hepatic IRI [18]. Supplementation of the sulfur-containing amino acids (SAA) methionine and cysteine, mTORC1 activation, or inhibition of the TSP enzyme cystathionine γ-lyase (CGL) decrease the production of H2S and counteract CR-orchestrated stress-resistance in rodents. The SAA methionine and cysteine are metabolized through the TSP. Their restriction results in an elevation of endogenous TSP activity generating H2S in the kidney. Besides, dietary restriction of SAA has additionally been widely studied in the context of longevity and age-associated diseases across taxa, as it efficiently augments stress-resistance revealing various shared mechanisms with CR [1]. Of note, H2S generation by the gut microbiota in response to a high-SAA diet was recently described to protect from uremic toxicity and disease progression in a rodent CKD model [19]. In contrast to human tissue including the kidney, liver, and brain, where SAA shortage leads to the endogenous production of H2S through the activated TSP, cysteine desulfhydrases in gut bacteria directly convert cysteine to H2S, pyruvate, and ammonia explaining these seemingly conflicting observations [18, 19].
Gut microbiota have been recognized as a central modulator of cardiovascular and renal disease during the last decade. Consequently, the impact of CR on microbiota is expected to be an important aspect. Mechanistically, CR results in a decreased expression of bacterial key enzymes of lipid A biosynthesis, a critical lipopolysaccharide (LPS) building component in rodents. LPSs are cell wall components of gram-negative bacteria that contribute to inflammation and can exacerbate sepsis-induced AKI. Consequently, decreased levels of LPS are associated with improved immune tolerance and a reduction in inflammation contributing to CR-induced metabolic health [20].
Besides CR, hypoxic preconditioning (HP) is an efficient strategy to prevent from AKI in rodents. A recent study by our group used the RNA sequencing-based comparison of CR and HP to identify common molecular mechanisms ameliorating stress-resistance prior to AKI [3]. With regard to an overlap on a single gene level, this approach resulted in a row of highly promising genes that were studied in detail with regard to their functional status in stress-resistance and AKI. To allow for optimal exploitation of this approach by researchers in the field, the data are provided as an interactive online tool allowing for visualization of gene expression regulation at the crossroads of preconditioning and IRI (http://shiny.cecad.uni-koeln.de:3838/IRaP/) [3]. As an example, acyl-CoA synthetase Acsm3 is among the genes that is downregulated commonly in response to CR and HP in murine kidneys. Acsm3 is involved in the first step of fatty acid metabolism − a key metabolic pathway in AKI. Acsm3 has been identified in the context of CR-mediated organ protection before, but has never been linked to HP [21]. Another commonly regulated gene that was enriched by CR and HP is peroxisomal Hao2, which is involved in the generation of hydrogen peroxide (H2O2). Taken together, alterations of fatty acid metabolism as well as the reduction in redox activity in peroxisomes and mitochondria limiting cellular ROS defense contribute to ischemic renal damage and both are differentially regulated by CR and HP [3].
Conclusion
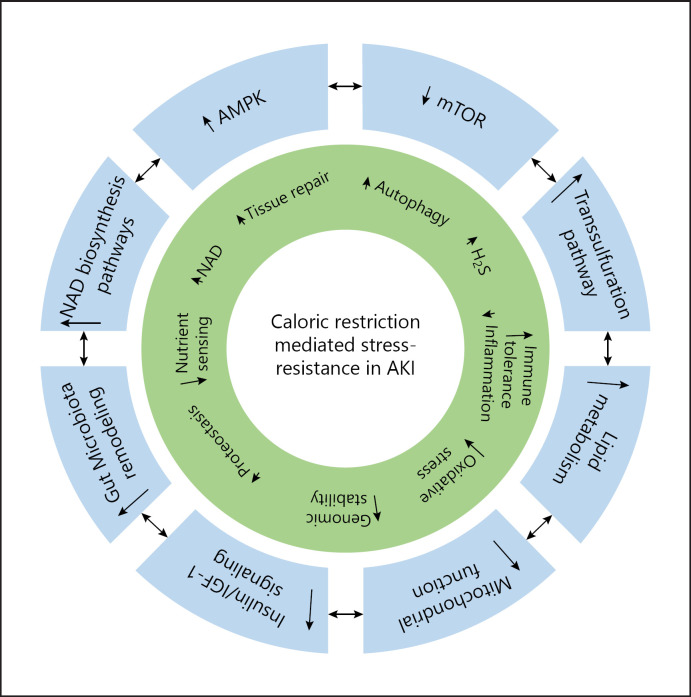
Preconditioning with CR protects efficiently from experimental AKI by increased renal stress-resistance and appears to employ a number of interlinked and evolutionary conserved signaling pathways (Fig. 1). Due to its immense potential, further studies are currently performed in order to identify the most promising targets enabling pharmacological interventions to facilitate successful translation to the clinical setting.
Fig. 1.
Caloric restriction orchestrates multiple interacting molecular, cellular, and systemic mechanisms that augment renal stress-resistance. AKI, acute kidney injury; AMPK, AMP-activated protein kinase; IGF-1, insulin growth factor 1; H2S, hydrogen sulfide; mTOR, mechanistic target of rapamycin; NAD, nicotinamide adenine dinucleotide.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
F.C.K. is supported by Else Kröner-Fresenius-Stiftung, by the German Research Foundation under Germany's Excellence Strategy − EXC 2030: CECAD − Excellent in Aging Research − Project No. 390661388 − and by the Koeln Fortune program/Faculty of Medicine, University of Cologne. M.R.S. is supported by the Koeln Fortune program and by the Cologne Clinician Scientist Program (CCSP)/Faculty of Medicine/University of Cologne, funded by the German Research Foundation (DFG, FI 773/15-1)/Faculty of Medicine, University of Cologne. R.-U.M. is supported through the Nachwuchsgruppen. NRW program of the Ministry of Science North Rhine-Westphalia and the German Research Foundation (CRU 329, MU 3629/3-1).
Author Contributions
F.C.K., M.R.S., K.J.R.H., and R.-U.M. conceived the article contents, prepared the manuscript, and endorsed the final draft submitted.
Contribution from the AKI and CRRT 2021 Symposium at the 26th International Conference on Advances in Critical Care Nephrology, A Virtual/Hybrid Event from San Diego, CA, USA, February 28–March 5, 2021. This symposium was supported in part by the NIDDK funded University of Alabama at Birmingham-University of California San Diego O'Brien Center for Acute Kidney Injury Research (P30DK079337).
References
- 1.Fontana L, Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015 Mar;161((1)):106–18. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N, van den Engel S, et al. Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell. 2010 Feb;9((1)):40–53. doi: 10.1111/j.1474-9726.2009.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnsen M, Kubacki T, Yeroslaviz A, Späth MR, Mörsdorf J, Göbel H, et al. The integrated RNA landscape of renal preconditioning against ischemia-reperfusion injury. J Am Soc Nephrol. 2020 Apr;31((4)):716–30. doi: 10.1681/ASN.2019050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spath MR, Bartram MP, Palacio-Escat N, Hoyer KJR, Debes C, Demir F, et al. The proteome microenvironment determines the protective effect of preconditioning in cisplatin-induced acute kidney injury. Kidney Int. 2019 Feb;95((2)):333–49. doi: 10.1016/j.kint.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 5.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012 Sep;489((7415)):318–21. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grundmann F, Müller RU, Reppenhorst A, Hülswitt L, Späth MR, Kubacki T, et al. Preoperative short-term calorie restriction for prevention of acute kidney injury after cardiac surgery: a randomized, controlled, open-label, pilot trial. J Am Heart Assoc. 2018 Mar;7((6)) doi: 10.1161/JAHA.117.008181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grundmann F, Müller RU, Hoyer-Allo KJR, Späth MR, Passmann E, Becker I, et al. Dietary restriction for prevention of contrast-induced acute kidney injury in patients undergoing percutaneous coronary angiography: a randomized controlled trial. Sci Rep. 2020 Mar;10((1)):5202. doi: 10.1038/s41598-020-61895-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKiernan SH, Tuen VC, Baldwin K, Wanagat J, Djamali A, Aiken JM. Adult-onset calorie restriction delays the accumulation of mitochondrial enzyme abnormalities in aging rat kidney tubular epithelial cells. Am J Physiol Renal Physiol. 2007 Jun;292((6)):F1751–60. doi: 10.1152/ajprenal.00307.2006. [DOI] [PubMed] [Google Scholar]
- 9.Robertson LT, Treviño-Villarreal JH, Mejia P, Grondin Y, Harputlugil E, Hine C, et al. Protein and calorie restriction contribute additively to protection from renal ischemia reperfusion injury partly via leptin reduction in male mice. J Nutr. 2015 Aug;145((8)):1717–27. doi: 10.3945/jn.114.199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017 Apr;168((6)):960–76. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu D, Tomasiewicz JL, Yang SE, Miller BR, Wakai MH, Sherman DS, et al. Calorie-restriction-induced insulin sensitivity is mediated by adipose mTORC2 and not required for lifespan extension. Cell Rep. 2019 Oct;29((1)):236–48.e3. doi: 10.1016/j.celrep.2019.08.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol. 2009 Dec;20((12)):2493–502. doi: 10.1681/ASN.2008111186. [DOI] [PubMed] [Google Scholar]
- 13.Cicora F, Roberti J, Vasquez D, Guerrieri D, Lausada N, Cicora P, et al. Preconditioning donor with a combination of tacrolimus and rapamacyn to decrease ischaemia-reperfusion injury in a rat syngenic kidney transplantation model. Clin Exp Immunol. 2012 Jan;167((1)):169–77. doi: 10.1111/j.1365-2249.2011.04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012 Dec;82((12)):1271–83. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howell GM, Gomez H, Collage RD, Loughran P, Zhang X, Escobar DA, et al. Augmenting autophagy to treat acute kidney injury during endotoxemia in mice. PLoS One. 2013 Jul;8((7)):e69520. doi: 10.1371/journal.pone.0069520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang C, Livingston MJ, Liu Z, Dong Z. Autophagy in kidney homeostasis and disease. Nat Rev Nephrol. 2020 Sep;16((9)):489–508. doi: 10.1038/s41581-020-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016 Mar;531((7595)):528–32. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015 Jan;160((1–2)):132–44. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobel L, Cao YG, Fenn K, Glickman JN, Garrett WS. Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science. 2020 Sep;369((6510)):1518–24. doi: 10.1126/science.abb3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabbiano S, Suárez-Zamorano N, Chevalier C, Lazarević V, Kieser S, Rigo D, et al. Functional gut microbiota remodeling contributes to the caloric restriction-induced metabolic improvements. Cell Metab. 2018 Dec;28((6)):907–21.e7. doi: 10.1016/j.cmet.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scholz H, Boivin FJ, Schmidt-Ott KM, Bachmann S, Eckardt KU, Scholl UI, et al. Kidney physiology and susceptibility to acute kidney injury: implications for renoprotection. Nat Rev Nephrol. 2021 May;17((5)):335–49. doi: 10.1038/s41581-021-00394-7. [DOI] [PubMed] [Google Scholar]