Highlights
-
•
Urinary tract infection (UTI) caused by extended-spectrum beta-lactamase (ESBL)-producing organisms is a serious concern in the adult population.
-
•
Escherichia coli is the primary organism causing ESBL-UTI.
-
•
Avoiding indiscriminate use of antibiotics may reduce the incidence of ESBL-UTI.
Keywords: ESBL, Urinary tract infections, Extended-spectrum beta-lactamase, Antibiotics
Abstract
Background
Community-acquired urinary tract infection (UTI) is the most common infection caused by extended-spectrum beta-lactamase (ESBL)-producing organisms.
Aim
to estimate the prevalence of ESBL-UTI in adults and to identify potential risk factors that may predispose to ESBL-UTI.
Methods
A retrospective study involving adult patients with UTI caused by ESBL-producing organisms was undertaken. Patients with UTI caused by non-ESBL-producing organisms represented the control group.
Results
In total, 1100 UTI isolates were included in the study, 277 of which were ESBL positive. The prevalence rate was 25.2%. The mean age of patients was 55.87 years. On univariate analysis, prior history of UTI or ESBL-UTI, invasive urological procedure within preceding 3 months, hospital admission within preceding 3 months, and exposure to antibiotics were found to be significant risk factors for ESBL-UTI. On multi-variate analysis, use of cephalosporins [adjusted odds ratio (OR) 1.61, P=0.048], previous ESBL-UTI (adjusted OR 2.67, P<0.001), and invasive urological procedure in the preceding year (adjusted OR 1.61, P=0.022) were found to be independent risk factors for ESBL-UTI.
Conclusions
In Qatar, the prevalence of ESBL-UTI in adults is modest. Recent exposure to antibiotics, previous ESBL-UTI and invasive urological procedures were found to be independent risk factors for ESBL-UTI.
Introduction
Urinary tract infection (UTI) is one of the most common conditions among adults presenting at the emergency department and primary healthcare visits. The clinical manifestations of UTI include asymptomatic bacteriuria, pyelonephritis and sepsis (Calbo et al., 2006; Fan et al., 2014). The most common organism causing UTI in adults is Escherichia coli, which accounts for 75–90% of bacterial isolates (Hoban et al., 2011; Martin et al., 2016). Most patients with UTI are treated empirically with conventional antibiotics. However, in the recent past, extended-spectrum beta-lactamase (ESBL)-producing pathogens have been reported increasingly as a cause of UTI. Physicians face a difficult task in treating ESBL-UTI because these organisms are resistant to all penicillins, cephalosporins and aztreonam. Furthermore, high resistance rates of these organisms to trimethoprim-sulfamethoxazole (TMP-SMX) and fluoroquinolones have been reported (Meier et al., 2011).
According to a World Health Organization report published in 2021, ESBL-producing Enterobacteriaceae (ESBL-EB) are part of the group posing the highest risk to public health (World Health Organization, 2021). According to a previous report, E. coli resistance to third-generation cephalosporins is approximately 15.1% in Europe, whereas Klebsiella pneumoniae resistance is approximately 31.7% (EARS-Net, 2018). In contrast, a survey of inpatients in the USA found that the prevalence of resistant ESBL-EB isolates was approximately 12.6% nationwide (Gupta et al., 2019).
Many risk factors for ESBL-UTI have been reported, including older age (Colodner et al., 2004; Rodríguez-Baño et al., 2008; Tüzün et al., 2019), male gender (Colodner et al., 2004; Ben-Ami et al., 2009; Martin et al., 2016; Søgaard et al., 2017; Tüzün et al., 2019), previous UTI (Inns et al., 2014; Rogers et al., 2014; Søgaard et al., 2017), international travel (Freeman et al., 2008; Søraas et al., 2013; Rogers et al., 2014), prior use of antibiotics (Colodner et al., 2004; Rodríguez-Baño et al., 2008; Søraas et al., 2013; Inns et al., 2014; Rogers et al., 2014; Søgaard et al., 2017; Tüzün et al., 2019), diabetes mellitus (Colodner et al., 2004; Rodríguez-Baño et al., 2008; Søraas et al., 2013; Inns et al., 2014), and prior use of proton pump inhibitors (Søgaard et al., 2017). Other factors, such as the presence of renal disease, chronic obstructive pulmonary disease, malignancy, immunosuppressive medication and freshwater swimming (Søraas et al., 2013), were also found to be risk factors for ESBL-E. coli UTI (Søgaard et al., 2017). Published studies found that hospital admission in the preceding 3 months, healthcare-associated UTI, upper UTI, recurrent UTI (more than three times per year), and presence of a urinary catheter were risk factors for ESBL-E coli UTI. Eating fish regularly was found to be protective against ESBL-UTI (Søraas et al., 2013; Tüzün et al., 2019).
Aim of the study
The aim of this study was to estimate the prevalence of ESBL-UTI in adults, and to identify potential risk factors that may predispose to ESBL-UTI.
Methods
Study design and study setting
A retrospective case–control study was conducted at Hamad General Hospital, Hamad Medical Corporation, Qatar from January 2020 to December 2020.
Study population
Inclusion criteria
Adult patients aged >18 years diagnosed with ESBL-EB-UTI based on positive urinary culture growth of a single pathogen with >105 colony-forming units between October 2018 and September 2019 were included in the study. In patients with multiple episodes of UTI, the first visit was taken as the index episode in this study.
Exclusion criteria
Patients with signs and symptoms of UTI with insignificant growth, negative urine culture, or mixed growth in urine culture were excluded from the study.
Controls
Patients with UTI due to non-ESBL-producing organisms matched for demographic features represented the control group.
Data collection
Demographic features, co-morbid conditions, clinical signs and symptoms, biochemical (renal function) and microbiological (urine culture/blood culture) parameters, radiological findings, complications and length of hospital stay were retrieved from the clinical information system.
Sample size
The study sample size of 1100 was derived based on the following calculation:
| Sample size for frequency in a population | |||
| Population size (for finite population correction factor) (N): | |||
| Hypothesized % frequency of outcome factor in the population (p): | |||
| Confidence limits as % of 100(absolute +/- %) (d): | |||
| Design effect (for cluster surveys): | |||
| Sample size (n) for various confidence levels | |||
| Confidence | Level (%) | Sample size | |
| 95% | 864 | ||
| 80% | 370 | ||
| 90% | 609 | ||
| 97% | 1059 | ||
Statistical analysis
Categorical data are expressed as proportions, and continuous data are presented as mean and standard deviation (SD) for normally distributed variables, or as median and interquartile range (IQR) for non-normally distributed variables. Preliminary analyses were conducted to examine the distribution of the data variables using the Kolmogorov–Smirnov test; data variables that did not show a normal distribution were transformed using logarithmic or square-root transformation as appropriate.
The aim of this study was to identify and estimate the prevalence of ESBL-UTI in adult patients diagnosed with UTI, and determine possible risk factors that may predispose to ESBL UTI. This was estimated and tested using the Z-test and the corresponding 95% confidence interval (CI) was computed to measure the precision of the point estimate value. Differences between categorical variables were compared using Chi-squared test or Fisher's exact test, as appropriate. Quantitative data between the two independent groups were analysed using unpaired t-test or Mann–Whitney U-test, as appropriate, depending on the data normality distribution. Univariate and multi-variate logistic regression methods were used to assess the predictive values of various potential predictors or risk factors associated with ESBL-UTI, and the results are reported as odds ratio (OR) and associated 95% CI. All P-values presented were two-tailed, and P<0.05 was considered to indicate statistical significance. All statistical analyses were undertaken using SPSS Version 23.0 (IBM Corp., Armonk, NY, USA) and Epi-info (Centers for Disease Control and Prevention, Atlanta, GA, USA).
Results
Prevalence
In total, 5342 urine samples were positive for UTI during the study period, of which 1556 were excluded [571 (mixed growth/contamination), 995 (colony count <105)], leaving a final cohort of 3776. From this sample, after randomization, 1100 samples were included for final analysis as per the sample size calculation.
Of the 1100 subjects with UTI included in the study, 277 had ESBL-producing organisms with a prevalence rate of 25.2% (Figure 1).
Figure 1.
Flow chart showing the inclusion of study subjects. ESBL, extended-spectrum beta-lactamase.
Profile of patients in the whole study group
The mean age of all study subjects was 55.87 (SD 19.56) years; when the distribution of patients across various age groups was examined, the age group of 18–30 years had the least number of patients (11.8%). Females accounted for 62.3% of the study subjects. Diabetes mellitus (45.6%) and chronic kidney disease (24.2%) were the most common co-morbid conditions observed. Overall, 3.7% of the study subjects were on dialysis, and 44.5% (n=490) had received antibiotics in the preceding 3 months.
Overall, 13.4% (n=147) of subjects had undergone invasive urological procedures in the preceding year, 27.5% (n=303) had a history of urinary catheterization in the preceding 3 months. 33.7% (n=371) had a history of hospital admission in the preceding 3 months, and 24% (n=264) had been admitted between 3 and 12 months before the index episode. In the previous year, 48.7% (n=536) of subjects had UTI, and 20.3% (n=223) had ESBL-UTI. The baseline characteristics of the complete cohort are detailed in Tables 1 and 2.
Table 1.
Demographic characteristics of the study population
| Characteristics | ||||
|---|---|---|---|---|
| Whole cohort | ESBL group | Non-ESBL group | P-value | |
| n (%) | n (%) | n (%) | ||
| 1100 | 277 (25.2) | 823 (74.8) | ||
| Gender | ||||
| Male | 415 (37.7) | 108 (39) | 307 (37.7) | 0.616 |
| Female | 685 (62.3) | 169 (61) | 516 (62.7) | |
| Age group (years) | ||||
| 18–30 | 130 (11.8) | 26 (9.4) | 104 (12.6) | 0.470 |
| 31–50 | 323 (29.4) | 82 (29.6) | 241 (29.3) | |
| 51–70 | 342 (31.1) | 86 (31) | 256 (31.1) | |
| >70 | 305 (27.7) | 83 (30) | 222 (27) | |
| Ethnicity | ||||
| Qatari | 388 (35.3) | 94 (33.9) | 294 (35.7) | 0.590 |
| Expatriate | 712 (64.7) | 183 (66.1) | 529 (64.3) | |
| Source of collection | ||||
| Emergency department | 466 (42.4) | 119 (43) | 347 (42.2) | 0.004 |
| Outpatient department | 357 (32.5) | 107 (38.6) | 250 (30.4) | |
| Inpatient | 277 (25.1) | 51 (18.4) | 226 (27.4) | |
| History of UTI in preceding 1 year | ||||
| Yes | 536 (48.7) | 161 (61) | 375 (47.8) | <0.001 |
| No | 513 (46.6) | 103 (39) | 410 (52.2) | |
| Number of UTIs in preceding 1 year | ||||
| NIL | 513 (46.6) | 103 (38.9) | 411 (52.2) | 0.002 |
| 1–4 | 412 (37.5) | 124 (46.8) | 288 (36.6) | |
| 5–8 | 115 (10.5) | 34 (12.8) | 81 (10.3) | |
| >8 | 11 (1.0) | 4 (1.5) | 7 (0.9) | |
| Previous ESBL-UTI in preceding 1 year | ||||
| Yes | 223 (20.3) | 97 (35.8) | 126 (15.8) | <0.001 |
| No | 848 (77.1) | 174 (64.2) | 674 (84.3) | |
| History of surgical procedures | ||||
| Invasive urological procedure in preceding 1 year | 147 (13.3) | 56 (20.2) | 91 (11.1) | <0.001 |
| History of urinary catheterization in preceding 3 months | 303 (27.5) | 76 (27.4) | 227 (27.6) | 0.963 |
| Previous hospital admissions | ||||
| <3 months | 371 (33.7) | 111 (40.1) | 260 (31.6) | 0.01 |
| 3–12 months | 264 (24.0) | 75 (27.1) | 189 (23) | 0.166 |
| Antibiotic change after culture results | ||||
| Yes | 287 (26.1) | 72 (39.3) | 215 (38.1) | 0.767 |
| No | 460 (41.6) | 111 (60.7) | 349 (61.9) | |
| Blood culture | ||||
| Positive | 61 (5.5) | 12 (12) | 49 (15.1) | 0.43 |
| Negative | 363 (33) | 88 (88) | 275 (84.9) | |
| Not available | 676 (61.5) | |||
| Recurrence of UTI after index episode | 487 (44.3) | 151 (54.5) | 336 (40.8) | <0.001 |
| Re-admission due to UTI within 30 days of index episode | 63 (5.7) | 25 (9) | 38 (4.6) | 0.006 |
UTI, urinary tract infection; ESBL, extended-spectrum beta-lactamase.
Table 2.
Co-morbidities and medication use in patients with urinary tract infections caused by extended-spectrum beta-lactamase (ESBL)-producing organisms vs non-ESBL-producing organisms
| Co-morbidities | ESBL group (n=277) | Non-ESBL group (n=823) | P-value |
|---|---|---|---|
| Diabetes mellitus | 135 (48.7) | 367 (44.6) | 0.231 |
| Chronic kidney disease | 75 (27.1) | 191 (23.2) | 0.194 |
| Malignancy | 31 (11.2) | 75 (9.1) | 0.311 |
| Pregnancy | 4 (1.4) | 21 (2.5) | 0.31 |
| Post partum | 2 (0.7) | 7 (0.8) | 0.868 |
| Liver cirrhosis | 5 (1.8) | 23 (2.8) | 0.37 |
| Benign prostate hyperplasia | 37 (13.3) | 93 (11.3) | 0.457 |
| History of skin infection | 18 (6.5) | 58 (7.0) | 0.755 |
| Neurogenic bladder | 16 (5.8) | 35 (4.2) | 0.299 |
| Urolithiasis | 35 (12.6) | 106 (12.9) | 0.916 |
| Vesicouritic reflux | 9 (3.2) | 13 (1.6) | 0.093 |
| Renal transplant | 17 (6.1) | 43 (5.2) | 0.563 |
| Haemodialysis | 13 (4.7) | 28 (3.4) | 0.329 |
| Peritoneal dialysis | 5 (1.8) | 11 (1.3) | 0.575 |
| Medication history | |||
| Corticosteroids in preceding 1 month | 29 (10.5) | 67 (8.1) | 0.236 |
| Chemotherapy in preceding 1 month | 4 (1.4) | 8 (0.9) | 0.516 |
| Immunosuppressive medications | 26 (9.4) | 65 (7.9) | 0.437 |
| Antibiotics in preceding 3 months | 151 (54.5) | 339 (41.2) | <0.001 |
| Antibiotics used in preceding 3 months | |||
| No antibiotics | 124 (44.8) | 441 (53.6) | |
| Penicillin | 13 (4.7) | 75 (9.1) | 0.127 |
| Cephalosporin | 33 (11.9) | 70 (8.5) | 0.096 |
| Fluoroquinolone | 20 (7.2) | 38 (4.6) | 0.033 |
| Nitrofurantoin | 29 (10.5) | 50 (6.1) | 0.004 |
| Trimethoprim-sulphamethoxazole | 7 (2.5) | 26 (3.1) | 0.921 |
| Others | 9 (3.2) | 26 ( 3.1) | 0.603 |
Comparison of patient profiles between ESBL and non-ESBL groups
The two groups had comparable distributions in terms of gender, age and nationality. In both groups, the majority of urine samples were collected in the emergency department (P=0.004). The co-morbid conditions in the two groups were not significantly different. The ESBL group had a higher rate of UTIs in the preceding year (61% vs 47.8%; P<0.001) and a higher rate of invasive urological procedures in the preceding year (20.2% vs 11.1%; P<0.001) compared with the non-ESBL group; these differences were significant (Tables 1 and 2).
Organisms isolated
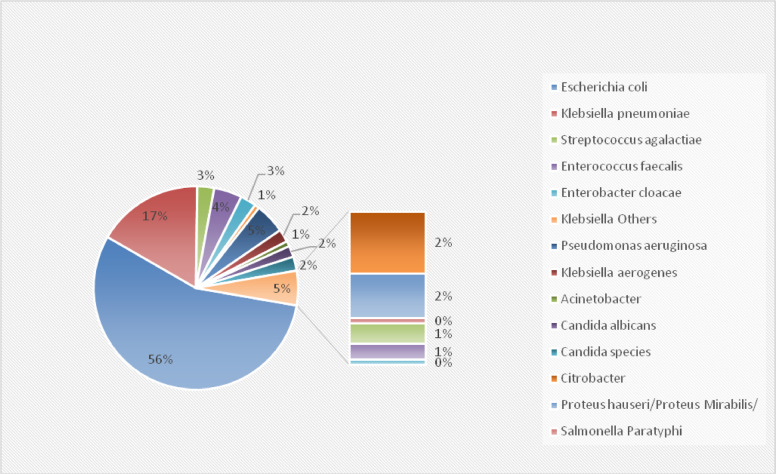
The most common organisms isolated in the whole cohort were E. coli (54.5%) and Klebsiella spp. (16.5%). The same organisms were found to be the most common isolates in the ESBL and non-ESBL sub-groups: E. coli (79% and 46.4%, respectively) and Klebsiella spp. (18% and 16.6%, respectively). Figures 2 and 3 illustrate the details of the organisms that were isolated.
Figure 2.
Organisms identified in the whole cohort.
Figure 3.
Organisms identified in the extended-spectrum beta-lactamase-producing group.
Risk factors
Conventional risk factors
Univariate analysis revealed that subjects aged >70 years had the highest risk of developing ESBL-UTI (OR 1.49, 95% CI 0.90–2.46; P=0.113) compared with other groups. Prior history of UTI (OR 1.70, 95% CI 1.28–2.27; P<0.001), ESBL-UTI in the preceding year (OR 2.98, 95% CI 2.18–4.07; P<0.001) and invasive urological procedures in the preceding 3 months (OR 2.03, 95% CI 1.41–2.93; P<0.001) were all found to be risk factors for ESBL-UTI, and the associations were highly significant. Hospital admission within the preceding year appeared to be a risk factor for ESBL-UTI, with admission during the 3 months preceding the index episode having the highest risk (OR 1.44, 95% CI 1.09–1.91; P=0.010).
Presence of vesico-ureteric reflex (OR 2.09, 95% CI 0.88–4.95; P=0.093), neurogenic bladder (OR 1.38 95% CI 0.75–2.53; P=0.299), haemodialysis (OR 1.39 95% CI 0.71–2.73; P=0.329), peritoneal dialysis (OR 1.35 95% CI 0.46–3.94; P=0.575), exposure to corticosteroids (OR 1.31 95% CI 0.83–2.08; P=0.236) and chemotherapy (OR 1.49 95% CI 0.44–4.99; P=0.516) in the preceding month showed a positive association with ESBL-UTI, but these associations were not significant (Table 3).
Table 3.
Comparison of risk factors for urinary tract infections (UTIs) caused by extended-spectrum beta-lactamase (ESBL)-producing organisms vs non-ESBL-producing organisms
| Variables | ESBL group n (%) |
Unadjusted OR (95% CI for OR) | P-value |
|---|---|---|---|
| Gender | |||
| Male (n=415) | 108 (26) | ||
| Female (n=685) | 169(24.7) | 0.93 (0.70–1.23) | 0.616 |
| Age group (years) | |||
| 18–30 (n=130) | 26 (20) | 0.473 | |
| 31–50 (n=323) | 82 (25.4) | 1.36 (0.82–2.23) | 0.225 |
| 51–70 (n=342) | 86 (25.1) | 1.34 (0.82–2.20) | 0.241 |
| >71 (n=305) | 83 (27.2) | 1.49 (0.90–2.46) | 0.113 |
| Ethnicity | |||
| Qatari (n=388) | 94 (24.2) | ||
| Expatriate (n=712) | 183 (25.7) | 1.08 (0.81–1.44) | 0.590 |
| Source of collection | |||
| Emergency department (n=466) | 119 (25.5) | 0.005 | |
| Outpatient department (n=357) | 107 (30) | 1.24 (0.91–1.69) | 0.158 |
| Inpatient (n=276) | 51 (18.5) | 0.66 (0.45–0.95) | 0.028 |
| Co-morbidities | |||
| Diabetes (n=502) | 135 (26.9) | 1.18 (0.89–1.55) | 0.231 |
| Chronic kidney disease (n=266) | 75 (28.2) | 1.22 (0.90–1.67) | 0.194 |
| Malignancy (n=106) | 31 (29.2) | 1.25 (0.80–1.95) | 0.311 |
| Pregnancy (n=25) | 4 (16) | 0.57 (0.19–1.68) | 0.310 |
| Post partum (n=9) | 2 (22.2) | 0.87 (0.18–4.25) | 0.868 |
| Liver cirrhosis (n=28) | 5 (17.9) | 0.63 (0.24–1.69) | 0.370 |
| Benign prostate hyperplasia (n=130) | 37 (28.5) | 1.19 (0.74–1.90) | 0.457 |
| Skin infection (n=76) | 18 (23.7) | 0.91 (0.53–1.58) | 0.755 |
| Neurogenic bladder (n=51) | 16 (31.4) | 1.38 (0.75–2.53) | 0.299 |
| Urolithiasis (n=141) | 35 (24.8) | 0.97 (0.65–1.47) | 0.916 |
| Vesicouritic reflux (n=22) | 9 (40.9) | 2.09 (0.88–4.95) | 0.093 |
| Renal transplant (n=60) | 17 (28.3) | 1.18 (0.66–2.11) | 0.563 |
| Haemodialysis (n=41) | 13 (31.7) | 1.39 (0.71–2.73) | 0.329 |
| Peritoneal dialysis (n=16) | 5 (31.3) | 1.35 (0.46–3.94) | 0.575 |
| Medications | |||
| Corticosteroids in preceding 1 month (n=96) | 29 (30.2) | 1.31 (0.83–2.08) | 0.236 |
| Chemotherapy in preceding 1 month (n=12) | 4 (33.3) | 1.49 (0.44–4.99) | 0.516 |
| Immunosuppressive medications (n=91) | 26 (28.6) | 1.20 (0.75–1.94) | 0.437 |
| Antibiotics in preceding 3 months (n=490) | 151 (30.8) | 1.75 (1.32–2.32) | <0.001 |
| Antibiotics used in preceding 3 months | |||
| No antibiotics (n=565) | 124 (21.9) | ||
| Penicillin (n=88) | 13 (14.8) | 0.61 (0.33–1.14) | 0.127 |
| Cephalosporin (n=103) | 33 (29.2) | 1.46 (0.93–2.30) | 0.096 |
| Fluoroquinolone (n=58) | 20 (34.5) | 1.87 (1.05–3.33) | 0.033 |
| Nitrofurantoin (n=79) | 29 (36.7) | 2.06 (1.25–3.39) | 0.004 |
| Trimethoprim-sulphamethxazole (n=33) | 7 (21.2) | 0.95 (0.40–2.25) | 0.921 |
| Other (n=35) | 9 (25.7) | 1.23 (0.56–2.69) | 0.603 |
| History of UTI in preceding 1 year | |||
| Yes (n=526) | 161 (30) | 1.70 (1.28–2.27) | <0.001 |
| Number of UTIs in preceding 1 year | |||
| 0 (n=514) | 103 (20) | ||
| 1–4 (n=412) | 124 (30.1) | 1.71 (1.27–2.32) | <0.001 |
| 5–8 (n=115) | 34 (29.6) | 1.77 (1.06–2.64) | 0.026 |
| >8 (n=11) | 4 (36.4) | 2.28 (0.65–7.93) | 0.195 |
| Previous ESBL-UTI in preceding 1 year | |||
| Yes (n=223) | 97 (43.5) | 2.98 (2.18–4.07) | <0.001 |
| Surgical procedures | |||
| Invasive urology procedure in preceding 1 year (n=147) | 56 (38.1) | 2.03 (1.41–2.93) | <0.001 |
| Urinary catheter in preceding 3 months (n=303) | 76 (25.1) | 0.99 (0.73–1.34) | 0.963 |
| Previous hospital admissions | |||
| <3 months (n=371) | 111(29.9) | 1.44 (1.09–1.91) | 0.010 |
| 3–12 months (n=264) | 75 (28.4) | 1.24 (0.91–1.70) | 0.166 |
| Blood culture | |||
| Positive (n=61) | 12 (19.7) | 1.30 (0.66–2.56) | 0.438 |
OR, odds ratio; CI, confidence interval.
This study found little or no association between ESBL-UTI and gender, nationality (Qatari nationals vs expatriates), urinary catheterization in the preceding 3 months, history of urolithiasis, renal transplant, pregnancy or presence of diabetes mellitus.
Antibiotics as novel risk factors
The use of antibiotics in the 3 months preceding the index episode yielded an OR of 1.75 (95% CI 1.32–2.32; P<0.001) for ESBL-UTI compared with non-ESBL-UTI, which was highly significant. Subanalysis of antibiotic exposure showed that the use of nitrofurantoin carried the highest risk for ESBL-UTI (OR 2.06, 95% CI 1.25–3.39; P=0.004) followed by exposure to fluoroquinolones (OR 1.87, 95% CI 1.05–3.33; P=0.033) and cephalosporins (OR 1.46, 95% CI 0.93–2.30; P=0.096) (Table 3).
Multi-variable logistic regression analysis indicated that the use of cephalosporins (adjusted OR 1.61, 95% CI 1.00–2.58; P=0.048), previous ESBL-UTI (adjusted OR 2.67, 95% CI 1.89–3.76; P<0.001), and invasive urological procedure in the preceding year (adjusted OR 1.61, 95% CI 1.07–2.42; P=0.022) remained significantly associated with increased risk of ESBL-UTI after adjusting for all other potential confounders and predictors (Table 4). Therefore, a prediction model was computed to evaluate the discriminative ability of potentially significant variables with P<0.10 on ESBL-UTI. Multi-variate analysis using an intermethod approach (including all variables identified on univariate analysis) provided area under the curve (AUC) of 0.678 (95% CI 0.63–0.71), which was very similar to the predictive accuracy obtained in the stepwise method. Multi-variate logistic regression (stepwise variable selection approach) indicated that the final model demonstrated modest accuracy (AUC=0.652, 95% CI 0.61–0.69) (Figure 4).
Table 4.
Risk factors associated with urinary tract infection caused by extended-spectrum beta-lactamase-producing organism (ESBL-UTI): multi-variate logistic regression analysis
| Variables | Adjusted OR | 95% CI for OR | P-value |
|---|---|---|---|
| Antibiotic use | |||
| No antibiotics | 1.0 (ref) | ||
| Penicillin | 0.59 | 0.30–1.15 | 0.124 |
| Cephalosporin | 1.61 | 1.00–2.58 | 0.048 |
| Fluoroquinolone | 1.58 | 0.83–2.99 | 0.160 |
| Nitrofurantoin | 1.71 | 0.99–2.96 | 0.054 |
| Trimethoprim-sulphamethoxazole | 0.88 | 0.36–2.15 | 0.793 |
| Previous ESBL-UTI in preceding 1 year | |||
| Yes | 2.67 | 1.89–3.76 | <0.001 |
| No | 1.0 (ref) | ||
| Invasive urology procedure in preceding 1 year | |||
| Yes | 1.61 | 1.07–2.42 | 0.022 |
| No | 1.0 (ref) |
OR, odds ratio; CI, confidence interval.
Figure 4.
Receiver operating characteristic (ROC) curve.
Discussion
This study found that the prevalence of ESBL-UTI among the adult population in Qatar was 25.2%. This finding supports previous reports from Qatar, which found that the prevalence of ESBL-UTI in children was 26.8%, with E. coli being the most common organism (Awean et al., 2019); however, no research on the prevalence of ESBL-UTI in adults in Qatar has been published previously.
Worldwide, the prevalence of ESBL-UTI has a wide range among different nations, ranging from <2% in Norway (Søraas et al., 2013) to 74% in Iraq (Al-Mayahie and Al Kuraiashy, 2016). The prevalence rate found in the present study is lower than rates reported from neighbouring Middle Eastern countries and Middle East and North African (MENA) countries, but higher than rates reported from the Western world.
In published research from Syria and Jordan, ESBL-E. coli was found in 52% and 62% of patients with UTIs, respectively (Al-Assil et al., 2013; Al-Jamei et al., 2019). A study from Turkey found that hospital-acquired ESBL-E. coli-UTI had a prevalence rate of 50.5%, whereas community-acquired ESBL-E. coli-UTI had a prevalence rate of 38.2% (Koksal et al., 2017).
In the Western world, the lowest rates of ESBL-UTI have been reported in Norway (2%) (Søraas et al., 2013) and Australia (2.1%) (Osthoff et al., 2015). A study on the prevalence of ESBL-UTI in the USA and Canada showed that the rate increased significantly from 7.8% to 18.3% between 2010 and 2014 in the USA, and increased from 10.4% to 13% during the same period in Canada (Lob et al., 2016). In South Korea, the prevalence of ESBL-UTI among outpatients was lower (12.1%) compared with that in the present study (25.2%), but among inpatients (23.1%) the results were similar to that in the present study (Lee et al., 2010).
This study also sought to identify various risk factors predisposing to ESBL-UTI, with an emphasis on antibiotics as a novel risk factor. The findings suggest that recent antibiotic exposure (within the preceding 3 months) was associated with increased risk of ESBL-UTI. With the exception of penicillin and TMP-SMX, all of the antibiotics studied revealed a significant risk of ESBL-UTI. Compared with other antibiotics, nitrofurantoin posed the greatest risk. The use of nitrofurantoin in ESBL-UTI prophylaxis in some of the study subjects may have led to overestimation of its risk.
Søgaard et al. (2017) reported exposure to nitrofurantoin as a risk factor for ESBL-UTI. The present study confirms the results of multiple previous studies on the exposure of various antibiotics as risk factors for ESBL-UTI: quinolones (Colodner et al., 2004; Rodríguez-Baño et al., 2004; Søraas et al., 2013; Goyal et al., 2019; Tüzün et al., 2019), beta-lactams (Azap et al., 2010; Søraas et al., 2013) and cephalosporins (Colodner et al., 2004; Osthoff et al., 2015; Goyal et al., 2019; Tüzün et al., 2019).
Antibiotics are not sold over the counter in Qatar; as such, their use is restricted. This could be one of the reasons for the lower prevalence of ESBL-UTI in Qatar compared with other MENA countries. It is important to note that inappropriate antibiotic use can prevent the isolation of organisms in culture specimens (Wilson et al., 2011), and can lead to the emergence of ESBL and other multi-drug-resistant organisms.
Concerning the conventional risk factors for UTI by ESBL-producing organisms, this study found that a history of UTI or ESBL-UTI in the preceding year and a history of invasive urological procedures in the preceding 3 months were significant risk factors. This validates previous studies on recurrent UTI as a risk factor by Al-Jamei et al. (2019), Briongos-Figuero et al. (2012), Goyal et al. (2019) and Tüzün et al. (2019). Previous hospital admission was also found to be a significant risk factor on univariate analysis in the present study, which was consistent with the findings of Al-Jamei et al. (2019), Søgaard et al. (2017) and Tüzün et al. (2019). The reason for hospital admission as a risk factor can be multi-factorial. Past reports suggest that rectal colonization with ESBL-producing Enterobacteriaceae was high among residents of long-term care facilities (Hogardt et al., 2015; Ludden et al., 2015), and they may serve as significant healthcare reservoirs of the organism (Rodríguez-Baño et al., 2004; Banerjee et al., 2013). Furthermore, the prevalence of ESBL-UTI is higher among hospitalized patients than the general population.
Older age (>70 years), haemodialysis or peritoneal dialysis, chemotherapy and corticosteroids were found to be risk factors for ESBL-UTI. Although univariate analysis revealed multiple risk factors for ESBL-UTI, only recent antibiotic use, previous ESBL-UTI and invasive urological procedures in the preceding 1 year were found to be independent risk factors for ESBL-UTI on multi-variate analysis. In contrast to earlier publications, the present study did not find any association between diabetes mellitus, urinary catheterization in the preceding 3 months, benign prostate hyperplasia and ESBL-UTI on multi-variate analysis.
Limitations
This study has certain limitations. First, as the participants were not grouped into hospitalized and community settings, there was heterogeneity in the study population, which could have influenced the prevalence rate and risk factor analyses. Also, as the study was retrospective, some data on co-morbidities were missing. However, it is believed that this had little impact on the study results due to the small amount of missing data.
Conclusions
The prevalence of ESBL-UTI is modest in Qatar. It is lower than that reported from neighbouring Middle Eastern and MENA countries, but higher than in the Western world. Recent antibiotic use, previous ESBL-UTI and invasive urological procedures in the preceding 1 year were found to be independent risk factors for ESBL-UTI.
Author contributions
VAN: study design, data collection, analysis, manuscript writing, editing.
NP: study design, data collection, analysis, manuscript writing, editing.
GW: data collection, analysis, manuscript writing.
PC: study design, data analysis. editing.
PJ: data collection, analysis, manuscript writing.
ZK: data collection, editing.
MZ: data collection, analysis, manuscript writing.
MK: data collection, analysis, manuscript writing.
NJ: data collection, manuscript writing.
BA: data collection, manuscript writing.
SC: data collection, manuscript writing.
EB: data collection, manuscript writing.
SJ: data collection, manuscript writing.
Declaration of Competing Interest
None declared.
Acknowledgments
Funding
None.
Ethical approval
This study was approved by the Institutional Review Board of Medical Research Centre, Hamad Medical Corporation (Approval No. MRC-01-20-006).
References
- Al-Assil B, Mahfoud M, Hamzeh AR. Resistance trends and risk factors of extended spectrum β-lactamases in Escherichia coli infections in Aleppo, Syria. Am J Infect Control. 2013;41:597–600. doi: 10.1016/j.ajic.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Al-Jamei SA, Albsoul AY, Bakri FG, Al-Bakri AG. Extended-spectrum β-lactamase producing E. coli in urinary tract infections: a two-center, cross-sectional study of prevalence, genotypes and risk factors in Amman. Jordan. J Infect Public Health. 2019;12:21–25. doi: 10.1016/j.jiph.2018.07.011. [DOI] [PubMed] [Google Scholar]
- Al-Mayahie S, Al Kuriashy JJ. Distribution of ESBLs among Escherichia coli isolates from outpatients with recurrent UTIs and their antimicrobial resistance. J Infect Dev Ctries. 2016;10:575–583. doi: 10.3855/jidc.6661. [DOI] [PubMed] [Google Scholar]
- Awean GZA, Salameh K, Elmohamed H, Alshmayt H, Omer MRB. Prevalence of ESBL urinary tract infection in children. J Adv Pediatr Child Health. 2019;2:4–7. [Google Scholar]
- Azap OK, Arslan H, Serefhanoğlu K, Colakoğlu S, Erdoğan H, Timurkaynak F, et al. Risk factors for extended-spectrum beta-lactamase positivity in uropathogenic Escherichia coli isolated from community-acquired urinary tract infections. Clin Microbiol Infect. 2010;16:147–151. doi: 10.1111/j.1469-0691.2009.02941.x. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Johnston B, Lohse C, Porter SB, Clabots C, Johnson JR. Escherichia coli sequence type 131 is a dominant, antimicrobial-resistant clonal group associated with healthcare and elderly hosts. Infect Control Hosp Epidemiol. 2013;34:361–369. doi: 10.1086/669865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami R, Rodríguez-Baño J, Arslan H, Pitout JD, Quentin C, Calbo ES, et al. A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing Enterobacteriaceae in nonhospitalized patients. Clin Infect Dis. 2009;49:682–690. doi: 10.1086/604713. [DOI] [PubMed] [Google Scholar]
- Briongos-Figuero LS, Gómez-Traveso T, Bachiller-Luque P, Domínguez-Gil González M, Gómez-Nieto A, Palacios-Martín T, et al. Epidemiology, risk factors and comorbidity for urinary tract infections caused by extended-spectrum beta-lactamase (ESBL)-producing enterobacteria. Int J Clin Pract. 2012;66:891–896. doi: 10.1111/j.1742-1241.2012.02991.x. [DOI] [PubMed] [Google Scholar]
- Calbo E, Romaní V, Xercavins M, Gómez L, Vidal CG, Quintana S, et al. Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum beta-lactamases. J Antimicrob Chemother. 2006;57:780–783. doi: 10.1093/jac/dkl035. [DOI] [PubMed] [Google Scholar]
- Colodner R, Rock W, Chazan B, Keller N, Guy N, Sakran W, et al. Risk factors for the development of extended-spectrum beta-lactamase-producing bacteria in nonhospitalized patients. Eur J Clin Microbiol Infect Dis. 2004;23:163–167. doi: 10.1007/s10096-003-1084-2. [DOI] [PubMed] [Google Scholar]
- EARS-Net Surveillance of antimicrobial resistance in Europe. Annual report of the European Antimicrobial Resistance Surveillance Network. 2018 https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2018.pdf Available at: (accessed November 2021) [Google Scholar]
- Fan NC, Chen HH, Chen CL, Ou LS, Lin TY, Tsai MH, et al. Rise of community-onset urinary tract infection caused by extended-spectrum β-lactamase-producing Escherichia coli in children. J Microbiol Immunol Infect. 2014;47:399–405. doi: 10.1016/j.jmii.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Freeman JT, McBride SJ, Heffernan H, Bathgate T, Pope C, Ellis-Pegler RB. Community-onset genitourinary tract infection due to CTX-M-15-producing Escherichia coli among travelers to the Indian subcontinent in New Zealand. Clin Infect Dis. 2008;47:689–692. doi: 10.1086/590941. [DOI] [PubMed] [Google Scholar]
- Goyal D, Dean N, Neill S, Jones P, Dascomb K. Risk factors for community-acquired extended-spectrum beta-lactamase-producing Enterobacteriaceae infections – a retrospective study of symptomatic urinary tract infections. Open Forum Infect Dis. 2019;6:ofy357. doi: 10.1093/ofid/ofy357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Ye G, Olesky M, Lawrence K, Murray J, Yu K. National prevalence estimates for resistant Enterobacteriaceae and Acinetobacter species in hospitalized patients in the United States. Int J Infect Dis. 2019;85:203–211. doi: 10.1016/j.ijid.2019.06.017. [DOI] [PubMed] [Google Scholar]
- Hoban DJ, Nicolle LE, Hawser S, Bouchillon S, Badal R. Antimicrobial susceptibility of global inpatient urinary tract isolates of Escherichia coli: results from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program: 2009–2010. Diagn Microbiol Infect Dis. 2011;70:507–511. doi: 10.1016/j.diagmicrobio.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Hogardt M, Proba P, Mischler D, Cuny C, Kempf VA, Heudorf U. Current prevalence of multidrug-resistant organisms in long-term care facilities in the Rhine-Main district, Germany, 2013. Euro Surveill. 2015;20:21171. doi: 10.2807/1560-7917.es2015.20.26.21171. [DOI] [PubMed] [Google Scholar]
- Inns T, Millership S, Teare L, Rice W, Reacher M. Service evaluation of selected risk factors for extended-spectrum beta-lactamase Escherichia coli urinary tract infections: a case–control study. J Hosp Infect. 2014;88:116–119. doi: 10.1016/j.jhin.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Koksal I, Yilmaz G, Unal S, Zarakolu P, Korten V, Mulazimoglu L, et al. Epidemiology and susceptibility of pathogens from SMART 2011–12 Turkey: evaluation of hospital-acquired versus community-acquired urinary tract infections and ICU- versus non-ICU-associated intra-abdominal infections. J Antimicrob Chemother. 2017;72:1364–1372. doi: 10.1093/jac/dkw574. [DOI] [PubMed] [Google Scholar]
- Lee DS, Lee CB, Lee SJ. Prevalence and risk factors for extended spectrum beta-lactamase-producing uropathogens in patients with urinary tract infection. Korean J Urol. 2010;51:492–497. doi: 10.4111/kju.2010.51.7.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lob SH, Nicolle LE, Hoban DJ, Kazmierczak KM, Badal RE, Sahm DF. Susceptibility patterns and ESBL rates of Escherichia coli from urinary tract infections in Canada and the United States, SMART 2010–2014. Diagn Microbiol Infect Dis. 2016;85:459–465. doi: 10.1016/j.diagmicrobio.2016.04.022. [DOI] [PubMed] [Google Scholar]
- Ludden C, Cormican M, Vellinga A, Johnson JR, Austin B, Morris D. Colonisation with ESBL-producing and carbapenemase-producing Enterobacteriaceae, vancomycin-resistant enterococci, and meticillin-resistant Staphylococcus aureus in a long-term care facility over one year. BMC Infect Dis. 2015;15:168. doi: 10.1186/s12879-015-0880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D, Fougnot S, Grobost F, Thibaut-Jovelin S, Ballereau F, Gueudet T, et al. Prevalence of extended-spectrum beta-lactamase producing Escherichia coli in community-onset urinary tract infections in France in 2013. J Infect. 2016;72:201–206. doi: 10.1016/j.jinf.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Meier S, Weber R, Zbinden R, Ruef C, Hasse B. Extended-spectrum β-lactamase-producing Gram-negative pathogens in community-acquired urinary tract infections: an increasing challenge for antimicrobial therapy. Infection. 2011;39:333–340. doi: 10.1007/s15010-011-0132-6. [DOI] [PubMed] [Google Scholar]
- Osthoff M, McGuinness SL, Wagen AZ, Eisen DP. Urinary tract infections due to extended-spectrum beta-lactamase-producing Gram-negative bacteria: identification of risk factors and outcome predictors in an Australian tertiary referral hospital. Int J Infect Dis. 2015;34:79–83. doi: 10.1016/j.ijid.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Baño J, Navarro MD, Romero L, Martínez-Martínez L, Muniain MA, Perea EJ, et al. Epidemiology and clinical features of infections caused by extended-spectrum beta-lactamase-producing Escherichia coli in non-hospitalized patients. J Clin Microbiol. 2004;42:1089–1094. doi: 10.1128/JCM.42.3.1089-1094.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Baño J, Alcalá JC, Cisneros JM, Grill F, Oliver A, Horcajada JP, et al. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch Intern Med. 2008;168:1897–1902. doi: 10.1001/archinte.168.17.1897. [DOI] [PubMed] [Google Scholar]
- Rogers BA, Ingram PR, Runnegar N, Pitman MC, Freeman JT, Athan E, et al. Australasian Society for Infectious Diseases Clinical Research Network. Community-onset Escherichia coli infection resistant to expanded-spectrum cephalosporins in low-prevalence countries. Antimicrob Agents Chemother. 2014;58:2126–2134. doi: 10.1128/AAC.02052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard M, Heide-Jørgensen U, Vandenbroucke JP, Schønheyder HC. Vandenbroucke-Grauls CMJE. Risk factors for extended-spectrum β-lactamase-producing Escherichia coli urinary tract infection in the community in Denmark: a case–control study. Clin Microbiol Infect. 2017;23:952–960. doi: 10.1016/j.cmi.2017.03.026. [DOI] [PubMed] [Google Scholar]
- Søraas A, Sundsfjord A, Sandven I, Brunborg C, Jenum PA. Risk factors for community-acquired urinary tract infections caused by ESBL-producing Enterobacteriaceae – a case–control study in a low prevalence country. PLoS One. 2013;8:e69581. doi: 10.1371/journal.pone.0069581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tüzün T, Sayın Kutlu S, Kutlu M, Kaleli İ. Risk factors for community-onset urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Turk J Med Sci. 2019;49:1206–1211. doi: 10.3906/sag-1902-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G, Badarudeen S, Godwin A. Antibiotic screening of urine culture as a useful quality audit. J Infect Dev Ctries. 2011;5:299–302. doi: 10.3855/jidc.923. [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2021. Global priority pathogens list of antibiotic-resistant bacteria.https://www.doherty.edu.au/news-events/news/who-global-priority-pathogens-list-of-antibiotic-resistant-bacteria Available at: (accessed December 2021) [Google Scholar]