Abstract
Minimally invasive surgery (MIS) incorporates surgical instruments through small incisions to perform procedures. Despite the potential advantages of MIS, the lack of tactile sensation and haptic feedback due to the indirect contact between the surgeon’s hands and the tissues restricts sensing the strength of applied forces or obtaining information about the biomechanical properties of tissues under operation. Accordingly, there is a crucial need for intelligent systems to provide an artificial tactile sensation to MIS surgeons and trainees. This study evaluates the potential of our proposed real-time grasping forces and deformation angles feedback to assist surgeons in detecting tissues’ stiffness. A prototype was developed using a standard laparoscopic grasper integrated with a force-sensitive resistor on one grasping jaw and a tunneling magneto-resistor on the handle’s joint to measure the grasping force and the jaws’ opening angle, respectively. The sensors’ data are analyzed using a microcontroller, and the output is displayed on a small screen and saved to a log file. This integrated system was evaluated by running multiple grasp-release tests using both elastomeric and biological tissue samples, in which the average force-to-angle-change ratio precisely resembled the stiffness of grasped samples. Another feature is the detection of hidden lumps by palpation, looking for sudden variations in the measured stiffness. In experiments, the real-time grasping feedback helped enhance the surgeons’ sorting accuracy of testing models based on their stiffness. The developed tool demonstrated a great potential for low-cost tactile sensing in MIS procedures, with room for future improvements. Significance: The proposed method can contribute to MIS by assessing stiffness, detecting hidden lumps, preventing excessive forces during operation, and reducing the learning curve for trainees.
Keywords: Minimally invasive surgery, laparoscopy, robotic surgery, tactile sensing, force sensor, angle sensor, stiffness assessment, lump detection
I. Introduction
Over the past four decades, minimally invasive surgery (MIS) has significantly evolved to the level of becoming a gold standard approach for various common surgical procedures, including cholecystectomy, prostatectomy, hernia repair, and appendectomy [1]. This approach is characterized by the use of natural orifices or small incisions to access internal cavities and perform the procedures. MIS requires specialized surgical instruments with long shafts, such as endoscopes and surgical graspers. In contrast to the traditional open approaches, MIS brings numerous advantages to patients, such as reduced pain, shorter recovery times, lower postoperative complication rates, and better cosmetic outcomes [2].
With the continuous advances of new technologies and surgical techniques, researchers are keen to explore how to improve the performance of current surgical instruments and enhance the experience for surgeons. For instance, the emerging MIS instrumentation is evolving from simple 2-dimensional visualization and limited degrees of freedom into state-of-the-art devices with improved precision, 3-dimensional visualization, increased dexterity, and user-friendly designs [3]. Additionally, the recent technological development of surgical robotic systems brought another breakthrough to MIS, where the accuracy, speed, precision, and degrees of freedom, among other surgical necessities, have been improved [4]. With the aid of robots, MIS made contributions to the field of microsurgery concerning critical organs, e.g., the reproductive organs, eye, and brain [5]. Such an application shows the promising potential of MIS and its compatibility with technology augmentation.
From the surgeons’ perspective, the lack of tactile sensation in MIS due to switching from the direct interaction between the surgeon’s hands and tissues to an indirect contact using MIS instruments remains a challenge that may limit the surgical operations [6]. In fact, the tactile sensation is essential for safely maneuvering organs, tissues, and sutures, as well as getting reliable information about the thickness and texture of the tissues. The tactile sensation is also valuable for assessing the stiffness of organs through palpation, which proved useful for tumor detection [7]. In that sense, MIS surgeons are limited in their perception of the intensity of actual forces applied on the tissues. As a result, they lack the required information about the biomechanical properties of tissues in order to assess their health condition. Additionally, the fact that having many different MIS instruments makes it difficult to develop a common intuition or anticipation of applied forces.
The demand for artificial tactile sensing in MIS has further escalated with the growing popularity of robot-assisted minimally invasive surgery (RMIS) [8]. Surgical complications may occur if excessive forces that lead to organ damage are unintentionally applied, resulting in tissue rupture and internal bleedings [9]. As a consequence, some experts argue that there were no significant benefits with RMIS. Such opinions stem from the fact that current MIS systems lack effective feedback mechanisms to provide tactile and haptic sensations based on the surgical parameters [4].
As a response, many scientists attempted to develop engineered tactile sensation by integrating force sensors at different locations of the MIS instruments, such as at the end effector [10], on the shaft [11], or at the base [12]. While doing so, both electrical and optical tactile sensing principles were investigated for MIS tactile sensing [13], yet, the development of artificial tactile sensation for MIS is still an ongoing research trend.
Among the various optical sensors, many studies reported using fiber Bragg grating (FBG) force sensors in MIS graspers [14], [15] and micro-end effectors [16], [17]. The concept behind these sensors is monitoring the grating pitch changes as a result of straining the base material, which is equipped with FBG sensors. In addition to this wavelength modulation, other categories of optical sensors can monitor either the intensity [18] or phase [19] of the light transmitted through optical fibers. Although offering high accuracy and sensitivity, optical sensors are expensive and hard to use in medical applications due to requiring optical spectrum analyzers, sophisticated software, and rigorous mathematical models to estimate the magnitude of a force [20].
On the other hand, the electrical-based force sensing principles are the most commonly used in MIS tactile sensing due to their low cost, simple circuitry, and ease of fabrication [13]. In this context, the recent developments in micro-electro-mechanical systems (MEMS) have revolutionized the tactile sensing field. Through photolithography and microfabrication approaches, silicon-based MEMS sensors and actuators can be miniaturized to the micron level and manufactured in batches with excellent signal-to-noise ratios [21]. Consequently, several MEMS-based force sensing devices were focused on the MIS application.
For example, polyvinylidene fluoride (PVDF) MEMS-based sensors were investigated for MIS use [22], [23]. The PVDF sensors can cover the entire surface of the grasping tip to capture the magnitude and position of applied forces and measure the compliance of grasped tissues [24]. However, there are quite a few issues concerning such piezoelectric-based sensors, as they require charge amplifiers and complicated electronics setup while being limited to measuring dynamic loadings [25]. Similarly, capacitive and piezoresistive MEMS-based force sensors were considered for integration with MIS tools, but issues with hysteresis and flexibility are present [26]. A comprehensive review of conventional and emerging tactile sensors for MIS can be found in our recent review paper [27].
As a commercially available substitute for Silicon MEMS-based force sensors, force-sensitive resistors (FSRs) are thin and flexible devices that rely on the force-induced resistance change of a piezoresistive sheet to measure the magnitude of applied forces [28]. Common FSRs are either ‘thru mode’ or ‘shunt mode’; both available as off-the-shelf sensors. Shunt mode sensors involve two sensing electrodes with interlaced traces placed on top of a resistive ink-coated membrane [29]. Applied forces tend to increase the contact between the resistive layer and electrodes and, correspondingly, decrease the electrical resistance. Alternatively, thru mode FSRs consist of a thin pressure-sensitive layer sandwiched between two metallic sheets [30]. The electrical resistivity of the intermediate piezoresistive layer decreases under loading, and the amount of resistance change corresponds to the magnitude of applied forces. Between the two, thru mode shows enhanced performance in terms of linearity, sensor drift, and dynamic measurement accuracy [31].
Compared to other force sensors, off-the-shelf FSRs are cheaply available and can be used as plug-and-play with MIS tools. In addition to being disposable, these commercially available sensors are flexible and can accommodate different sizes and shapes of grasping jaws. Also, they have an extensive working range and can measure from milli-to tens of newtons. Besides, tunneling magneto-resistors (TMR) are standard off-the-shelf angle sensors that measure the rotational angle based on the change in the magnetic field orientation of a rotating magnet on top. TMRs are widely utilized for applications requiring quick and reliable angle measurements [32].
In this work, we propose a simple approach for MIS tactile sensing based on Hooke’s law and modulus of elasticity,
 . As per the mechanics of materials, the stiffness can be found by studying its force (stress) vs deformation (strain) characteristics [33], see Fig. 1(a). Basically, soft materials undergo higher deformation than the harder ones with respect to the same force. Objects with high stiffness tend to resist the deformation more, requiring higher force magnitudes to deform as much as objects with lower stiffness.
. As per the mechanics of materials, the stiffness can be found by studying its force (stress) vs deformation (strain) characteristics [33], see Fig. 1(a). Basically, soft materials undergo higher deformation than the harder ones with respect to the same force. Objects with high stiffness tend to resist the deformation more, requiring higher force magnitudes to deform as much as objects with lower stiffness.
FIGURE 1.
(a) COMSOL Multiphysics® simulation showing the cross-sectional deformation of two ellipsoid 3-D objects with different stiffness induced by the same amount of vertically applied force (softer object on the left,
 MPa, and stiffer one on the right,
MPa, and stiffer one on the right,
 MPa). (b) Schematic of the grasping tip of surgical forceps acting on two objects with different stiffness. The initial angle when the grasper is fully open is zero. When the jaw closes, the value of the angle increases. In case 1, the same amount of applied force induces higher deformation in the soft material. In case 2, reaching the same deformation level requires a higher amount of applied force on the hard object.
MPa). (b) Schematic of the grasping tip of surgical forceps acting on two objects with different stiffness. The initial angle when the grasper is fully open is zero. When the jaw closes, the value of the angle increases. In case 1, the same amount of applied force induces higher deformation in the soft material. In case 2, reaching the same deformation level requires a higher amount of applied force on the hard object.
By measuring and analyzing the grasping forces and angles, we can easily predict the stiffness of grasped objects, as illustrated in Fig. 1(b). Therefore, we integrate a commercially available laparoscopic tool with two off-the-shelf sensors; an FSR located at one of the grasper jaws and a TMR positioned on the tool’s handle (off-surgery site). Together, the two sensors provide feedback on the relative stiffness of grasped objects. This simple concept in mechanics can be powerful in translational engineering in medicine.
This work proves that conventional surgical tools can be made smart with force feedback features, on-demand, and in plug-and-play format. By having libraries of the available force and angle sensors in the market, specific sensors can be matched and integrated seamlessly with specific laparoscopic tools on-demand, based on the surgery, patient, and surgeon—a LEGO-like approach.
This paper is organized as follows. Section II presents the design and assembly of the integrated system, specifications of the two off-the-shelf sensors, experimental setup, and preparation of samples. Section III presents and describes the results. In Section IV, the strengths and limitations of this study are highlighted, and future work is discussed. Finally, we conclude this paper in Section V. A preliminary version of this work has been reported [34].
II. Methods and Procedures
Here, we present the integration of a universal laparoscopic grasper with off-the-shelf sensors. As surgeons rely on long surgical instruments to reach internal body tissues through small incisions, there is an overwhelming number of available laparoscopic tools on the market, each designed for a specific task and surgical operation. In this study, we targeted the standard laparoscopic grasping forceps as one of the most commonly used tools in MIS. The two grasping jaws at the selected forceps end effector are 6 mm wide, and the opening angle is manually controlled via the tool’s handle.
Two commercially available sensors were mounted on the laparoscopic grasper to measure the grasping force and angle. In particular, an FSR was installed on one jaw of the grasping tip for the measurement of applied forces during gripping, manipulation, and other MIS actions, and a TMR was fixed off-the-jaws at the pivot (center of rotation) of the tool’s handle to measure the corresponding jaws’ angle without interacting with the grasped organs. This eliminates the need for sterilizations and the biocompatibility of the angle sensor.
The two sensors were connected to an Arduino Uno microcontroller (Arduino, Italy) that translates the input signals into meaningful grasping force and angle measurements. Then, the calculated values are displayed on a small liquid crystal display (LCD) and recorded in a log file. Fig. 2 presents the developed tool with figure insets zooming in on the force sensor, angle sensor, and the electrical connection inside the electronics acrylic box.
FIGURE 2.

Image of the developed ‘smart laparoscopic grasping forceps’ and zoomed-in insets on the different integrated components: the A201 FlexiForce FSR installed on the grasping tip, the ASR002 Smart TMR and rotating magnet integrated on the rotation point of the handle, and the electrical connections between the Arduino microcontroller and sensors inside the electronics box with an LCD.
Based on the sensors, the ratio of the force-to-angle-change during grasping events will be used in this work to obtain the stiffness index for grasped tissues. This fulfills one of the main requirements of MIS tactile sensing. Once thriving, this new integrative LEGO-like concept can be applied to other fields, such as robotic manipulators.
A. Force Sensor
To measure the applied forces while grasping organs, a thru mode FSR, serving as a force sensor, was attached to one jaw of our prototype’s grasping tip. The FSR of choice is FlexiForce A201 (Tekscan, USA), with a sensing area of 9.7 mm and 0.1–111 N working range. This FlexiForce sensor is thin, flexible, cost-effective, and customizable and can be easily wired to a data acquisition board through a voltage divider circuit with minimal power requirements. A decrease in the electrical resistance
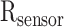 due to external forces causes a voltage increase at the divider output node [30].
due to external forces causes a voltage increase at the divider output node [30].
The measured force can be calculated using:
 |
where
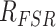 is the resistance of the force sensor, CF is the calibration factor, and
is the resistance of the force sensor, CF is the calibration factor, and
 is the offset value. This sensor has better force measurement accuracy than most other off-the-shelf force sensors [31]. The force sensor was mounted on the jaw using double-sided adhesive tape, while a 7 mm circular puck was added on top of the sensor to concentrate the load onto the sensing area when grasping wide objects. Since the sensor’s surface area was wider than the jaw, epoxy glue was used to support the hanging part of the sensor. Later, the FSR was calibrated to achieve relatively accurate measurements of actual grasping forces.
is the offset value. This sensor has better force measurement accuracy than most other off-the-shelf force sensors [31]. The force sensor was mounted on the jaw using double-sided adhesive tape, while a 7 mm circular puck was added on top of the sensor to concentrate the load onto the sensing area when grasping wide objects. Since the sensor’s surface area was wider than the jaw, epoxy glue was used to support the hanging part of the sensor. Later, the FSR was calibrated to achieve relatively accurate measurements of actual grasping forces.
B. Force Sensor Calibration
The force sensor went through a calibration process to ensure the accuracy and consistency of force readings as well as validate outputs per universal standards [35]. The calibration process was carried out by applying known loads and correlating the force sensor reading with the actual testing loads. Quantified loads were applied vertically using the Instron 5540 Series electromechanical testing system (Instron Inc., USA) equipped with a 50 N load cell capable of ±0.5% reading accuracy down to 1/250 of the cell capacity (200 mN). Under the Instron load frame, controlled loading was applied entirely within the sensing area of the sensor at a slow rate. Once a compressive load of 20 N is reached, the Instron load frame retracted to its initial level. After repeating the loading process multiple times, a correlation between the sensor outputs and the applied forces was obtained by concurrently updating the calibration factor and the offset value of equation (1) in the Arduino code. The calibration curve is presented in Fig. 3, which shows the output voltage of the voltage divider circuit vs force. This force sensor serves the purpose of proof-of-concept. Thus, the slight nonlinearity of the FSR will not impact the stiffness index calculations. Testing the repeatability and reliability of these sensors is beyond this study’s scope.
FIGURE 3.
Calibration curve for the FlexiForce A201 sensor showing the measured outputs from the voltage divider circuit
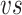 applied forces.
applied forces.
C. Angle Sensor
The opening angle of the jaws was indirectly determined by measuring the angle of the MIS tool’s handle using an angle sensor since these two angles are proportional. The jaws’ rotation is twice the handle’s rotation. In this work, we only report the rotation angle of the handle. The ‘zero’ angle corresponds to a fully open jaw (initial point), and it increases as the jaws close. Among the various off-the-shelf sensors, TMRs are ubiquitous angle sensors that translate the magnetic field orientation induced by a rotating magnet on top into a meaningful angle measurement.
We mounted an ASR002 Smart Angle Sensor (NVE Corporation, USA) off-the-jaws onto the fixed part of the handle, while the magnet was placed on the moving part of the handle. The resolution of this angle sensor is
 . By having the force and angle sensors, the stiffness of grasped organs can be estimated from the ratio of the two grasping parameters, i.e., force-to-angle-change.
. By having the force and angle sensors, the stiffness of grasped organs can be estimated from the ratio of the two grasping parameters, i.e., force-to-angle-change.
D. Preparation of Samples
After integrating the laparoscopic forceps with smart sensors, two types of samples were prepared: silicone-based rubber objects with different stiffness and animal tissue samples. These samples serve as models to test and characterize our prototype with a wide range of sample compositions and sources, including real flesh taken from animal sources.
The elastomeric samples are based on polydimethylsiloxane (PDMS), a silicone-based organic polymer widely used in chemistry and microfluidics research laboratories due to its ease of fabrication, low cost, and biocompatibility. Typically, PDMS is produced by mixing a pre-polymer base material (A) with a cross-linker curing agent (B). The mixing ratio of those two components (A:B) controls the mechanical properties of the resulting object, where a higher weight percentage of the curing agent yields a greater material strength.
First, four PDMS mixtures were prepared with a weight of 25 grams and A:B ratios of 10:1, 20:1, 27:1, and 40:1. Then, each mixture was poured into an individual Petri dish (5 cm in diameter), filling a 1 cm thickness. Afterward, the four circular molds were placed inside a 60
 oven overnight. Once fully cured, the samples were removed from the molding dishes and identified as: “hardest,” “hard,” “soft,” and “softest,” with respect to the mixing ratios mentioned earlier. The compression elastic moduli of those samples range from 1.75 to 0.17 MPa, respectively [36]. These samples are prepared for the PDMS samples palpation test.
oven overnight. Once fully cured, the samples were removed from the molding dishes and identified as: “hardest,” “hard,” “soft,” and “softest,” with respect to the mixing ratios mentioned earlier. The compression elastic moduli of those samples range from 1.75 to 0.17 MPa, respectively [36]. These samples are prepared for the PDMS samples palpation test.
By following the same steps, another set of PDMS samples (10:1, 15:1, 20:1, 25:1, 30:1) was molded into centrifuge tubes to form solid cylindrical pieces with 1 cm diameter and 2.5 cm height. These elastomeric cylinder blocks were used in a semi-clinical experiment, in which three surgeon subjects were asked to sort them based on their relative stiffness.
In addition to the elastomeric samples, we aimed to test our prototype against two biological samples. The first animal samples are represented by chicken meat slices. Initially, a frozen chicken breast was bought from a grocery store and left at room temperature for a few hours to defrost. Then, a total of three samples were prepared with a 1 cm thickness. A raw and 10-minute pan-fried slices are used in the palpation test, and a raw portion embedded with a small, pan-fried piece representing a hidden lump is used in the lump detection test.
The other set of animal samples included three digestive organs, i.e., a stomach, a bowel, and a colon, of a 1-year-old local sheep obtained from a nearby butcher shop. The purpose of those sheep samples is to report the typical minimum, average, and maximum forces applied by an expert laparoscopic surgeon during grasping and manipulating organs, in a way similar to the actual MIS surgical actions. All animal samples were enclosed by a thin cling plastic wrap to avoid contaminating the tool.
III. Results
After integrating the sensors and preparing the samples, different experiments and tests were performed using the developed smart laparoscopic grasping forceps to validate the proposed concept and prove the capability and benefits of our working prototype. Participants with different levels of experience have participated in this study. For experiments in subsections A, B, and C, two participants with no experience in laparoscopic surgery conducted tests on the prototype for configuration and validation. The experiments in subsection D involved three experienced surgeons.
A. PDMS Samples Palpation
The first set of experiments is basic repetitive grasp-release events (palpation) carried out on the first set of PDMS samples shown in Fig. 4(a). These samples have varying hardness based on varying the A:B mixing ratios at the time of fabrication. After running the test, the output signals from the two integrated sensors were displayed on the same graph as shown in Fig. 4(b). At each grasp, the angle sensor reflects the opening angle of the grasping jaws, and the force sensor measures the amount of normal force being applied. This result is in agreement with the theory of Young’s Modulus, which relates the amount of deformation to the magnitude of the compressive load and the mechanical properties of the material [37]. Relating this to our study, the toughest sample (far-right end of the graph) required the most significant force to reach the same compression angle, and softer ones show lower force amplitudes. These results were consistent over repetitive tests.
FIGURE 4.

PDMS samples palpation: (a) Image of the first four PDMS samples. (b) Plot of force and angle output signals from repetitive grasp-release events performed using our smart laparoscopic grasping forceps on the four PDMS samples. (c) Grasping force exerted on the four PDMS samples while securing the grasps at a constant angle. (d) Bar chart showing the average force-to-angle-change ratio of the four PDMS samples out of 10 repetitive grasps for each, reflecting the stiffness index,
 , with standard deviation error bars.
, with standard deviation error bars.
The variation in force signals among the four samples was also noticeable while securely grasping them for a while at a constant angle, as shown in Fig. 4(c). As anticipated, the softer samples were easily deformed and reached high angle changes with small magnitudes of applied forces, and vice versa for harder samples. In addition, the constant grasping force over time shows the elastic nature of the PDMS.
The stiffness of grasped objects can be estimated by grasping the object and calculating the ratio of the maximum force,
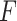 , reached during the grasp to the change in angle,
, reached during the grasp to the change in angle,
 . The latter is calculated by subtracting the initial angle at which the sample is in contact with both upper and lower jaws from the maximum angle reached during that grasp. We refer to this ratio as the stiffness index,
. The latter is calculated by subtracting the initial angle at which the sample is in contact with both upper and lower jaws from the maximum angle reached during that grasp. We refer to this ratio as the stiffness index,
 , given by:
, given by:
 |
Consequently, our prototype allows to remarkably distinguish between grasped samples based on their estimated stiffness. In Fig. 4(d), the bar chart shows that the stiffness index calculated using equation (2) is low for the soft sample and increases as the sample’s stiffness increases, in agreement with their actual stiffness. Moreover, the small standard deviation error bars show the accuracy of our proposed method and reveal a high distinction certainty between the soft and hard samples, which is required for situations when objects are relatively close in stiffness levels. By taking advantage of this in MIS operations, surgeons become aware of, not only the applied force, but also the stiffness of grasped organs and tissues, which helps in making informed decisions on how to proceed with the surgery.
B. Chicken Meat Slices Palpation
After evaluating the prototype with elastomeric PDMS samples, similar testing was conducted on the raw and pan-fried chicken meat slices shown in Fig. 5(a). The sensors’ output signals out of repetitive grasp-release events are displayed in Fig. 5(b). As anticipated, the cooked meat requires a significantly larger force than the raw meat to reach the same compression angle. This implies that the cooked chicken meat is the stiffer of the two, in line with the biochemical nature of the cooked sample resulting from the lowered water holding capacity and decreased tenderness [38].
FIGURE 5.
Chicken meat slices palpation: (a) Image of the raw and pan-fried chicken meat slices. (b) Plot of force and angle output signals from repetitive grasp-release testing performed using the smart laparoscopic grasping forceps on the two chicken meat samples. (c) Grasping force exerted on the two chicken meat slices while securing the grasps at a constant angle (the viscoelastic properties of the chicken meat show a decrease in the grasping force over time).
In addition, the two chicken meat slices were grasped for a while at a constant angle, and the data are presented in Fig. 5(c). Again, this test confirmed that the required force to deform the two samples equally is conspicuously different, being higher for the cooked sample. Unlike the elastomeric testing models, the viscoelastic properties of the chicken meat are observed as a decrease in the value of normal force over time. Similarly, the force-to-angle-change ratio of those biological samples can be used in the calculation of their stiffness index, as previously shown in the PDMS samples palpation. In actual MIS practices, surgeons can utilize this in discerning tissues of different material stiffness.
C. Lump Detection
Another goal of this device is to detect stiff lumps hidden inside the grasped organs and tissues. For a demonstration, the non-experienced participants conducted a grasp-release test on the raw chicken meat sample embedded with a small cooked piece representing a hidden lump. As shown in Fig. 6, six grasping positions with a 1 cm gap between each are highlighted on the sample, where the lump is implanted at position X while keeping the thickness uniform across all positions. The grasping events started at position 1 and ended at position 6, and the grasping signals are plotted in Fig. 6. As evident from the graph, the location of the implanted cooked piece can be identified based on the higher amplitude of force at a consistent grasping angle. The sudden increase in the force signal across the sample helps in locating lumps that have stiffer characteristics. Potentially, MIS surgeons can significantly benefit from this functionality in determining the location of more rigid regions in tissues and organs, which are primarily associated with unhealthy segments or even tissue tumors in some cases.
FIGURE 6.
Lump detection: plot of force and angle output signals from grasping the highlighted positions on the raw chicken slice embedded with a small cooked chicken piece located at position X.
D. Sorting Samples Based on Hardness
Our last experiment in this study aims to further showcase the benefits of having the real-time force and angle feedback. Three subjects with various experience levels in laparoscopic surgery participated in this experiment. Subject I has 20 years of experience, subject II has 10 years of experience, and subject III has a few years of experience. Each subject was asked to sort the five cylindrical PDMS samples from hardest to softest four separate times: first palpating the samples using the device with closed eyes (‘blind’), then palpating again while ‘looking’ at the samples, thirdly repeating the palpation while taking advantage of the real-time ‘feedback’ signal displayed on the small LCD screen, and lastly palpating the samples using their ‘fingers’ (without the tool). The test samples had relatively close values of stiffness and were randomly rearranged after each sorting trial.
The results of this experiment are listed in Table 1, where the sorting accuracy of the different scenarios can be compared. To summarize this, the real-time grasping force and angle feedback made the sorting tasks using the laparoscopic tool more accessible and more accurate, closer to the human fingers case. Based on this, MIS surgeons can effectively detect the force and angle of deformation while grasping organs and draw estimations about their stiffness.
TABLE 1. Sorting Samples Based on Stiffness (Samples 1 to 5 are Listed From Hardest to Softest).
| Reference | Subject I | Subject II | Subject III | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blind | Looking | Feedback | Fingers | Blind | Looking | Feedback | Fingers | Blind | Looking | Feedback | Fingers | |
| 1 | 1 | 1 | 1 | 1 | 5 | 1 | 1 | 1 | 2 | 1 | 1 | 1 |
| 2 | 3 | 2 | 2 | 2 | 4 | 2 | 2 | 2 | 5 | 3 | 2 | 2 |
| 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 2 | 3 | 3 |
| 4 | 4 | 4 | 4 | 4 | 1 | 5 | 4 | 4 | 3 | 4 | 4 | 4 |
| 5 | 5 | 5 | 5 | 5 | 2 | 4 | 5 | 5 | 1 | 5 | 5 | 5 |
| Time (s) | 75 | 30 | 45 | 30 | 70 | 30 | 55 | 60 | 60 | 55 | 140 | 25 |
| # of TRUE | 3 | 5 | 5 | 5 | 1 | 3 | 5 | 5 | 0 | 3 | 5 | 5 |
| # of FALSE | 2 | 0 | 0 | 0 | 4 | 2 | 0 | 0 | 5 | 2 | 0 | 0 |
Following that, subject I was asked to grip, hold, and manipulate three digestive organs of a sheep, namely the stomach, bowel, and colon, using our tool in a way that mimics the actual surgical tasks. The goal is to predict the typical magnitude of forces applied during tissue grasping and retracting in laparoscopic surgery. The measured grasping force was 3.7 ± 1.7 N (average ± standard deviation) out of 15 grasps-releases events, with a minimum and maximum of 2 N and 7.5 N, respectively. These tissue grasping forces are in line with other studies reporting on the surgical grasping parameters [39].
IV. Discussion
While most studies in the literature focus only on force sensing and complicated designs to measure the stiffness of tissues, we proposed integrating an additional angle sensor to measure the angle of deformation and simply calculate a stiffness index based on the force-to-angle-change ratio. This proposed concept was evaluated by performing several experiments using the in-house developed prototype on two types of samples. Overall, the elastomeric samples served as quick and clean candidates for testing our prototype. The chicken and sheep samples were just models to show the technique’s applicability with real flesh taken from biological sources. Hence, they do not necessarily reflect human tissues involved in actual surgical procedures.
The novelty of our work can be summarized as follows: Firstly, the presented technique was sensitive enough to show stiffness differences between the PDMS samples as well as between raw and cooked chicken meat slices. Secondly, a small piece of cooked chicken meat hidden inside a raw portion was detected with our integrated tool, demonstrating the ability to detect the presence of hidden lumps. Thirdly, our proposed concept makes sorting samples based on hardness easy, even for non-expert surgeons. This was validated with the help of three surgeons, who found the tool to be impressively simple and could finish the sorting task with higher accuracy and efficiency when the grasping feedback was accessible. Based on this, the same strategy of tactile sensing using force and angle sensors can be implemented for other MIS tools.
Compared to other studies in the literature [10], we provided a simple method to estimate the stiffness index of tissues based on the ratio between the applied force measured by a force sensor and the induced deformation indicated from the angle change using an angle sensor. Some complicated designs limit the grasping force to 1 N [12]. Therefore, we substituted the need for such complex designs and high-cost fabrication techniques with a simple off-the-shelf solution. Also, our study includes participants with varying levels of experience in laparoscopy to add a clinical perspective to it.
While the current prototype serves as a proof of concept, our future work should focus on several improvements. More sensitive force and angle sensors can be used to distinguish tissues having very close values of stiffness. Also, more realistic models of flesh embedded with lumps should be used and characterized. Besides, other parameters of the MIS operation need to be evaluated and discussed.
In addition, there is a great potential for emerging tactile sensing technologies to enable low-cost and high-efficiency sensors for MIS applications [27]. For instance, microfluidic-based tactile sensing facilitates several advantages, such as enhanced flexibility and stretchability in contrast to rigid solid-state sensors [40]. One work reported on using microfluidic-based force sensing skin to detect impending tissue damage during neuroendoscopy [11].
Alternatively, sensorless techniques have been investigated for RMIS, where the sensors used for force estimation are inherent in the surgical robot [41]. In model-based approaches, the encoders and the motor current measurements are the sensors [42]. In vision-based techniques, the sensor is the visual feedback of the surgical site through mono or stereo cameras [43], [44].
Some recent work on MIS tactile sensing displayed visual information on the endoscopic screen [45], while others proposed using magneto-rheological sponge cells to deliver haptic information [46]. Such haptic-based devices can be incorporated to provide the surgeon with haptic feedback.
Whether robot-assisted or direct use of probes and endoscopes, MIS has been compatible with technological upgrades and therefore has limitless potential for the future, especially in fields that demand extra precision and accuracy, including microsurgery. Thus, the force feedback mechanism components need to be advanced to minimize errors and augment the demanding role of the surgeon.
Ultimately, MIS surgeons aided with artificial tactile sensation can avoid applying excessive forces that accidentally lead to surgical complications [8]. Also, trainees such as medical students and surgical residents would benefit from the real-time grasping feedback to fast-track their learning curves by reducing the time required for training and mastering MIS. Force feedback also helps in avoiding slippage of grasped tissues by guiding the operator towards secure grasps [47]. Potentially, MIS surgeons will regain the ability to determine the presence of stiffer tumor tissues and detect hidden lumps.
V. Conclusion
The major limitation of MIS is the loss of tactile sensation when touching or handling tissues indirectly using laparoscopic tools. This can be overcome by implementing an artificial tactile sensation into the MIS instrumentation to restore the feel of touch. Knowledge about applied forces and tissues’ stiffness enables lump detection, pressure estimation on thin walls at the tissue level, and informed decision making that boosts the surgeons’ confidence level.
This work serves as a proof-of-concept study showing the proposed concept and the smart laparoscopic grasping forceps in action, equipped with two off-the-shelf sensors to provide real-time feedback on two grasping parameters: the grasping force and the angle of deformation. The ability to estimate the stiffness of grasped samples/objects based on this feedback was demonstrated. Furthermore, the in-house developed prototype proved helpful for assisting surgeons in determining the amount of force applied to grasped tissues resulting in better hand-device coordination and more accurate sample sorting based on hardness. Additionally, this approach can obtain perceptions of hidden lumps by indicating the location of the stiffer, unhealthy segments; hence it stands promising for tumor detection developments. This ability can be amplified when used in conjunction with novel imaging devices to visualize and discern tumors and other peculiarities.
Acknowledgment
Wael Othman acknowledges NYUAD Global Ph.D. Fellowship. Kojo E. Vandyck acknowledges NYUAD Undergraduate Fellowship. Authors acknowledge technical support from the NYUAD Core Technology Platforms.
Funding Statement
This work was supported by New York University Abu Dhabi.
References
- [1].Litynski G. S., “Endoscopic surgery: The history, the pioneers,” World J. Surg., vol. 23, no. 8, pp. 745–753, Aug. 1999, doi: 10.1007/s002689900576. [DOI] [PubMed] [Google Scholar]
- [2].Velanovich V., “Laparoscopic vs open surgery: A preliminary comparison of quality-of-life outcomes,” Surgical Endoscopy, vol. 14, no. 1, pp. 16–21, Jan. 2000, doi: 10.1007/s004649900003. [DOI] [PubMed] [Google Scholar]
- [3].Antoniou S. A., Antoniou G. A., Antoniou A. I., and Granderath F.-A., “Past, present, and future of minimally invasive abdominal surgery,” J. Soc. Laparoendoscopic Surgeons, vol. 19, no. 3, 2015, Art. no. e2015.00052, doi: 10.4293/JSLS.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jiahai C., You L., Jianping G., and Yakun W., “Application of da Vinci surgical robotic system in hepatobiliary surgery,” Int. J. Surgery Med., vol. 4, pp. 22–27, Jan. 2017, doi: 10.5455/ijsm.da-Vinci-surgical-robotic-system-in-hepatobiliary-surgery. [DOI] [Google Scholar]
- [5].Gudeloglu A., Brahmbhatt J. V., and Parekattil S. J., “Robotic-assisted microsurgery for an elective microsurgical practice,” Seminars Plastic Surg., vol. 28, no. 1, pp. 11–19, Feb. 2014, doi: 10.1055/s-0034-1368162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tholey G., Desai J. P., and Castellanos A. E., “Force feedback plays a significant role in minimally invasive surgery: Results and analysis,” Ann. Surg., vol. 241, no. 1, pp. 102–109, Jan. 2005, doi: 10.1097/01.sla.0000149301.60553.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Krouskop T., Wheeler T. M., Kallel F., Garra B. S., and Hall T., “Elastic moduli of breast and prostate tissues under compression,” Ultrason. Imag., vol. 20, pp. 260–274, Oct. 1998, doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- [8].Sastry S. S., Cohn M., and Tendick F., “Milli-robotics for remote, minimally invasive surgery,” Robot. Auto. Syst., vol. 21, no. 3, pp. 305–316, Sep. 1997, doi: 10.1016/S0921-8890(96)00082-6. [DOI] [Google Scholar]
- [9].Simaan N., Yasin R. M., and Wang L., “Medical technologies and challenges of robot-assisted minimally invasive intervention and diagnostics,” Annu. Rev. Control Robot. Auto. Syst., vol. 1, no. 1, pp. 465–490, May 2018, doi: 10.1146/annurev-control-060117-104956. [DOI] [Google Scholar]
- [10].Kim U., Lee D.-H., Moon H., Koo J. C., and Choi H. R., “Design and realization of grasper-integrated force sensor for minimally invasive robotic surgery,” in Proc. IEEE/RSJ Int. Conf. Intell. Robots Syst., Chicago, IL, USA, Sep. 2014, pp. 4321–4326, doi: 10.1109/IROS.2014.6943173. [DOI] [Google Scholar]
- [11].Codd P. J., Veaceslav A., Gosline A. H., and Dupont P. E., “Novel pressure-sensing skin for detecting impending tissue damage during neuroendoscopy: Laboratory investigation,” J. Neurosurgery: Pediatrics, vol. 13, no. 1, pp. 114–121, Jan. 2014, doi: 10.3171/2013.9.Peds12595. [DOI] [PubMed] [Google Scholar]
- [12].Lee D. H., Kim U., Gulrez T., Yoon W. J., Hannaford B., and Choi H. R., “A laparoscopic grasping tool with force sensing capability,” IEEE/ASME Trans. Mechatronics, vol. 21, no. 1, pp. 130–141, Feb. 2016, doi: 10.1109/Tmech.2015.2442591. [DOI] [Google Scholar]
- [13].Bandari N., Dargahi J., and Packirisamy M., “Tactile sensors for minimally invasive surgery: A review of the state-of-the-art, applications, and perspectives,” IEEE Access, vol. 8, pp. 7682–7708, 2020, doi: 10.1109/Access.2019.2962636. [DOI] [Google Scholar]
- [14].Sun K., Li M., Wang S., Zhang G., Liu H., and Shi C., “Development of a fiber Bragg grating-enabled clamping force sensor integrated on a grasper for laparoscopic surgery,” IEEE Sensors, vol. 21, no. 15, pp. 16681–16690, Aug. 2021, doi: 10.1109/Jsen.2021.3081444. [DOI] [Google Scholar]
- [15].Wang P.et al. , “Smart laparoscopic grasper integrated with fiber Bragg grating based tactile sensor for real-time force feedback,” J. Biophotonics, vol. 15, no. 5, May 2022, Art. no. e202100331, doi: 10.1002/jbio.202100331. [DOI] [PubMed] [Google Scholar]
- [16].He X. C.et al. , “Force sensing micro-forceps with integrated fiber Bragg grating for vitreoretinal surgery,” Proc. SPIE, vol. 8218, pp. 213–219, Jan. 2012, doi: 10.1117/12.909602. [DOI] [Google Scholar]
- [17].Suzuki H.et al. , “Development and testing of force-sensing forceps using FBG for bilateral micro-operation system,” IEEE Robot. Autom. Lett., vol. 3, no. 4, pp. 4281–4288, Oct. 2018, doi: 10.1109/LRA.2018.2864771. [DOI] [Google Scholar]
- [18].Bandari N., Dargahi J., and Packirisamy M., “Miniaturized optical force sensor for minimally invasive surgery with learning-based nonlinear calibration,” IEEE Sensors, vol. 20, no. 7, pp. 3579–3592, Apr. 2020, doi: 10.1109/Jsen.2019.2959269. [DOI] [Google Scholar]
- [19].Shang W. J., Su H., Li G., Furlong C., and Fischer G. S., “A Fabry–Perot interferometry based MRI-compatible miniature uniaxial force sensor for percutaneous needle placement,” in Proc. SENSORS, Baltimore, MD, USA, Nov. 2013, pp. 57–60, doi: 10.1109/ICSENS.2013.6688137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Roriz P., Frazão O., Lobo-Ribeiro A. B., Santos J. L., and Simões J. A., “Review of fiber-optic pressure sensors for biomedical and biomechanical applications,” J. Biomed. Opt., vol. 18, no. 5, May 2013, Art. no. 050903, doi: 10.1117/1.Jbo.18.5.050903. [DOI] [PubMed] [Google Scholar]
- [21].Xu Y.et al. , “Silicon-based sensors for biomedical applications: A review,” Sensors, vol. 19, no. 13, p. 2908, Jul. 2019, doi: 10.3390/s19132908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Qasaimeh M. A., Sokhanvar S., Dargahi J., and Kahrizi M., “PVDF-based microfabricated tactile sensor for minimally invasive surgery,” J. Microelectromech. Syst., vol. 18, no. 1, pp. 195–207, Feb. 2009, doi: 10.1109/JMEMS.2008.2008559. [DOI] [Google Scholar]
- [23].Xin Y.et al. , “PVDF tactile sensors for detecting contact force and slip: A review,” Ferroelectrics, vol. 504, no. 1, pp. 31–45, Nov. 2016, doi: 10.1080/00150193.2016.1238723. [DOI] [Google Scholar]
- [24].Qasaimeh M. A., Sokhanvar S., Dargahi J., and Kahrizi M., “A micro-tactile sensor for in-situ tissue characterization in minimally invasive surgery,” Biomed. Microdevices, vol. 10, no. 6, pp. 823–837, Jun. 2008, doi: 10.1007/s10544-008-9197-0. [DOI] [PubMed] [Google Scholar]
- [25].Qasaimeh M., Ramezanifard M., and Dargahi J., “An endoscopic grasper with corrugated plate-shaped tactile sensors,” J. Mech. Mater. Struct., vol. 4, no. 5, pp. 913–926, Sep. 2009, doi: 10.2140/jomms.2009.4.913. [DOI] [Google Scholar]
- [26].Naidu A. S., Patel R. V., and Naish M. D., “Low-cost disposable tactile sensors for palpation in minimally invasive surgery,” IEEE/ASME Trans. Mechatronics, vol. 22, no. 1, pp. 127–137, Feb. 2017, doi: 10.1109/Tmech.2016.2623743. [DOI] [Google Scholar]
- [27].Othman W.et al. , “Tactile sensing for minimally invasive surgery: Conventional methods and potential emerging tactile technologies,” Frontiers Robot. AI, vol. 8, p. 376, Jan. 2022, doi: 10.3389/frobt.2021.705662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yun M. H., Kotani K., and Ellis D., “Using force sensitive resistors to evaluate hand tool grip design,” in Proc. Hum. Factors Soc. Annu. Meeting, 1992, vol. 36, no. 10, pp. 806–810, doi: 10.1177/154193129203601036. [DOI] [Google Scholar]
- [29].Parmar S., Khodasevych I., and Troynikov O., “Evaluation of flexible force sensors for pressure monitoring in treatment of chronic venous disorders,” Sensors, vol. 17, no. 8, p. 1923, Aug. 2017, doi: 10.3390/s17081923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hollinger A. and Wanderley M., “Evaluation of commercial force-sensing resistors,” in Proc. Int. Conf. New Interfaces Musical Expression, Paris, France, 2006, pp. 1–4. [Google Scholar]
- [31].Vecchi F., Freschi C., Micera S., Sabatini A., Dario P., and Sacchetti R., “Experimental evaluation of two commercial force sensors for applications in biomechanics and motor control,” in Proc. 5th Annu. Conf. (IFESS) Jun. 2000, pp. 636–643. [Google Scholar]
- [32].Kumar A. S. A., George B., and Mukhopadhyay S. C., “Technologies and applications of angle sensors: A review,” IEEE Sensors, vol. 21, no. 6, pp. 7195–7206, Mar. 2021, doi: 10.1109/Jsen.2020.3045461. [DOI] [Google Scholar]
- [33].Goodno B. J. and Gere J., Statics and Mechanics of Materials. Boston, MA, USA: Cengage Learning, 2018. [Google Scholar]
- [34].Othman W. and Qasaimeh M. A., “Smart laparoscopic grasper utilizing force and angle sensors for stiffness assessment in minimally invasive surgery,” in Proc. 43rd Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. (EMBC), Nov. 2021, pp. 7336–7339, doi: 10.1109/EMBC46164.2021.9630100. [DOI] [PubMed] [Google Scholar]
- [35].Eren H., “Calibration process,” in Handbook of Measuring System Design, Sydenham P. H. and Thorn R., Eds. Hoboken, NJ, USA: Wiley, 2005. [Google Scholar]
- [36].Sharfeddin A., Volinsky A. A., Mohan G., and Gallant N. D., “Comparison of the macroscale and microscale tests for measuring elastic properties of polydimethylsiloxane,” J. Appl. Polym. Sci., vol. 132, no. 42, pp. 1–6, Nov. 2015, doi: 10.1002/App.42680.25866416 [DOI] [Google Scholar]
- [37].Labašová E., “Determination of modulus of elasticity and shear modulus by the measurement of relative strains,” Res. Papers Fac. Mater. Sci. Technol. Slovak Univ. Technol., vol. 24, no. 39, pp. 85–92, Dec. 2016, doi: 10.1515/rput-2016-0021. [DOI] [Google Scholar]
- [38].Yusnaini S., Suryanto E., and Armunanto R., “The effect of heating process using electric and gas ovens on chemical and physical properties of cooked smoked-meat,” in Proc. 1st Int. Symp. Food Agro-Biodiversity Conducted Indonesian Food Technol. Community, vol. 3, 2015, pp. 19–26, doi: 10.1016/j.profoo.2015.01.002. [DOI] [Google Scholar]
- [39].Golahmadi A. K., Khan D. Z., Mylonas G. P., and Marcus H. J., “Tool-tissue forces in surgery: A systematic review,” Ann. Med. Surg., vol. 65, May 2021, Art. no. 102268, doi: 10.1016/j.amsu.2021.102268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gao Y.et al. , “Wearable microfluidic diaphragm pressure sensor for health and tactile touch monitoring,” Adv. Mater., vol. 29, no. 39, pp. 1–8, Oct. 2017, doi: 10.1002/adma.201701985. [DOI] [PubMed] [Google Scholar]
- [41].Stephens T. K.et al. , “Conditions for reliable grip force and jaw angle estimation of da Vinci surgical tools,” Int. J. Comput. Assist. Radiol. Surg., vol. 14, no. 1, pp. 117–127, Jan. 2019, doi: 10.1007/s11548-018-1866-8. [DOI] [PubMed] [Google Scholar]
- [42].Li Y. and Hannaford B., “Gaussian process regression for sensorless grip force estimation of cable-driven elongated surgical instruments,” IEEE Robot. Autom. Lett., vol. 2, no. 3, pp. 1312–1319, Jul. 2017, doi: 10.1109/Lra.2017.2666420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Shimonomura K., “Tactile image sensors employing camera: A review,” Sensors, vol. 19, no. 18, p. 3933, Sep. 2019, doi: 10.3390/s19183933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Marban A., Srinivasan V., Samek W., Fernández J., and Casals A., “A recurrent convolutional neural network approach for sensorless force estimation in robotic surgery,” Biomed. Signal Process. Control, vol. 50, pp. 134–150, Apr. 2019, doi: 10.1016/j.bspc.2019.01.011. [DOI] [Google Scholar]
- [45].Schostek S., Zimmermann M., Schurr M. O., and Prosst R. L., “Design and performance of a low-cost telemetric laparoscopic tactile grasper,” Surgical Innov., vol. 23, no. 3, pp. 291–297, Jun. 2016, doi: 10.1177/1553350615615440. [DOI] [PubMed] [Google Scholar]
- [46].Kim S., Kim P., Park C.-Y., and Choi S.-B., “A new tactile device using magneto-rheological sponge cells for medical applications: Experimental investigation,” Sens. Actuators A: Phys., vol. 239, pp. 61–69, Mar. 2016, doi: 10.1016/j.sna.2016.01.016. [DOI] [Google Scholar]
- [47].Overtoom E. M., Horeman T., Jansen F.-W., Dankelman J., and Schreuder H. W., “Haptic feedback, force feedback, and force-sensing in simulation training for laparoscopy: A systematic overview,” J. Surg. Educ., vol. 76, no. 1, pp. 242–261, 2019, doi: 10.1016/j.jsurg.2018.06.008. [DOI] [PubMed] [Google Scholar]






