Abstract
Women experience dramatic physiological changes during pregnancy, including changes in the production of the “stress hormone,” cortisol. Evidence has been mixed regarding whether hair cortisol concentration (HCC) can be used to accurately capture the trajectory of cortisol during this period and whether factors related to psychosocial stress are related to HCC in pregnant and postpartum women. In the current study, we collected hair samples from 85 individuals during the peripartum period (with collection occasions in pregnancy [12–37 weeks], at 3–8 weeks postpartum, and at 5–8 months postpartum) from which we derived 783 monthly observations of HCC. In addition, at each assessment individuals reported their current depressive symptoms and experiences of recent psychosocial adversity. Using piecewise mixed effects modeling, we identified significant increases in HCC across pregnancy (approximately a 2-fold rise) followed by significant decreases in HCC postpartum. Beyond these effects, however, there was substantial within-individual variability in HCC. Disaggregating between- from within-individual associations of depressive symptoms and adversity with HCC, we found that within-individual fluctuations in adversity were positively coupled with levels of HCC. Overall, the current findings suggest that measurement of cortisol in human hair captures its trajectory from conception through six months postpartum, including prenatal increases and gradual recovery of typical levels following childbirth. In addition to the overall severity of psychosocial adversity, change in women's experiences of adversity during the peripartum period merit attention.
Keywords: Hair cortisol, Pregnancy, Postpartum, Adversity, Depression
Highlights
-
•
We analyzed monthly estimates of hair cortisol concentration (HCC) across the peripartum period.
-
•
HCC increased across pregnancy and decreased through six months postpartum.
-
•
There was substantial within-person variability in HCC.
-
•
Within-person fluctuations in recent adversity were positively coupled with HCC.
-
•
Depressive symptoms were not significantly associated with HCC.
1. Introduction
Women experience dramatic physiological changes during pregnancy, including changes in the production of the “stress hormone,” cortisol. Specifically, levels of cortisol increase across gestation, supporting maturation of the fetal central nervous system [1,2]. Given the possible negative consequences of dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis for maternal–fetal health, scientists are interested in what may explain variation in prenatal cortisol [3,4], and whether variation in prenatal cortisol predicts offspring outcomes such as temperament, cognitive ability, and preterm birth [[5], [6], [7]]. Researchers are increasingly using human hair to measure cortisol secretion during pregnancy. Whereas cortisol derived from blood plasma, saliva, and urine reflects acutely circulating cortisol, cortisol from human hair is not subject to circadian changes [8], making assessment of hair cortisol concentration (HCC) a promising method for measuring long-term secretion of this hormone, including across gestation. Nonetheless, findings have been mixed with respect to whether HCC accurately captures the trajectory of prenatal cortisol production, and whether factors such as symptoms of psychopathology and psychosocial adversity are associated with prenatal HCC [9,10].
Estimation of cortisol in hair provides a “window into the past,” retrospectively assessing cumulative secretion [11]. Specifically, concentrations in the first centimeter of hair closest to the scalp reflect cortisol secretion over approximately the prior month, with successive hair segments reflecting cortisol secretion further back in time. Generally, researchers examining prenatal levels of HCC have collected 3-cm hair segments to measure the last three months of secretion, which usefully corresponds to a single trimester of pregnancy. In a recent systematic review, however, Marceau et al. [9] found that only half of the studies examining HCC across all three trimesters of pregnancy observed a substantial increase in HCC. For example, D'Anna-Hernandez et al. [12] collected 3-cm samples of hair corresponding to each trimester of pregnancy and found that levels of HCC were significantly higher in the third than in the first trimester. Similarly, Hoffman et al. [13], found that levels of HCC were significantly higher in the third trimester than in the second and first trimesters. In contrast, however, Romero-Gonzalez et al. [14] reported minimal differences in levels of HCC across trimesters. While Marceau et al. [15] identified group-level increases in HCC across pregnancy, these authors also observed substantial variability in within-person changes in HCC, suggesting that many individuals do not evidence linear increases across gestation.
Although researchers have referred to HCC as a biological marker of psychosocial stress or distress, findings have been mixed with respect to whether prenatal distress (e.g., symptoms of psychopathology) is associated with HCC or other measures of cortisol during in this period [10,16,17]. Further, data are limited regarding associations between stressors (e.g., adverse life events) and prenatal cortisol. Whereas Mustonen et al. [18] found that individuals with consistently high prenatal depressive symptoms had higher average levels of prenatal HCC than did individuals with consistently low prenatal depressive symptoms, Wikenius et al. [19] found no associations between depressive symptoms and HCC during pregnancy. In an analysis of over 3,000 pregnant women, levels of cortisol in blood serum were not related to depressive symptoms, anxiety symptoms, work stress, or parenting stress [20]. Although a meta-analysis combining data from pregnant and non-pregnant people indicated a small but significant positive association between life adversity and HCC [21], few studies have examined recent adverse experiences in relation to HCC during pregnancy. Therefore, it is not clear whether HCC or other measures of basal cortisol are, in fact, useful and valid markers of psychosocial stress or distress during pregnancy.
Addressing specific gaps in the existing research concerning HCC in the peripartum period may help to resolve these mixed findings. First, in order to model a developmental process accurately, it is critical that assessments occur at a frequency close to the pace at which change unfolds [22]. To date, most researchers have used 3-cm lengths of hair to measure overall secretion of cortisol within each trimester of pregnancy; however, change in HCC is unlikely to occur at the pace of trimesters. Moreover, trimester-level estimates of HCC fail to capture or differentiate the lowest and the highest values of HCC (i.e., at conception and close to childbirth, respectively). Therefore, a more fine-grained sampling approach would provide a more precise estimate of the pace of change in HCC, as well as the relative levels of HCC at important inflection points. Second, because few studies have examined HCC following childbirth, we lack a comprehensive characterization of HCC across the peripartum period, including the extent to which baseline levels of HCC are restored during the postpartum months. Tracing the postpartum trajectory of HCC informs basic knowledge of HPA-axis functioning across the peripartum period and may help to clarify the validity of observed increases in the prenatal period. Finally, factors related to psychosocial stress, including symptoms of psychopathology and adverse life experiences, are dynamic across the peripartum period, potentially fluctuating during pregnancy and following childbirth. By pairing longitudinal assessments of these factors with repeated measures of HCC throughout the peripartum period, we can model how fluctuations in symptoms of psychopathology or life adversity co-occur with deviations in HCC, thereby disentangling within-from between-person effects [23].
The goal of the current study was to characterize the trajectory of HCC from conception through six months postpartum through analyses of repeated assessments of HCC measured at one-month increments. Specifically, participants provided 5-cm hair samples during pregnancy, at one-month postpartum, and at six months postpartum. From each of these three samples, we derived up to five observations of HCC, corresponding to cortisol secretion in each of the five previous months. Using this fine-grained approach, which yielded over 750 observations of HCC, we tested the hypothesis that HCC increases across pregnancy and subsequently decreases across the first six months postpartum. In addition, we leveraged longitudinal assessments of participants’ depressive symptoms and experiences of recent psychosocial adversity to explore the within- and between-person effects of changes in and levels of symptoms and adversity on individual variation in levels and slopes of HCC across the peripartum period. Specifically, we disaggregated effects of the average severity of depressive symptoms and psychosocial adversity on HCC from the effects of within-individual fluctuations in these factors on HCC. This approach allowed us to examine not only whether individuals with higher overall scores on these factors differ in HCC from individuals with lower scores, but also how fluctuations in these factors and in HCC unfold together across the peripartum period.
2. Method
2.1. Participants
Participants were recruited from the community to participate in the Brain and Behavior Infant Experiences (BABIES) project, a longitudinal observational study of the association between perinatal experiences and child development [24,25]. Data included observations from a sample of 90 participants recruited during pregnancy (collection occasion 1[C1]) and assessed again when their infants were ages 3–8 weeks old (collection occasion 2[C2]) and 5–8 months old (collection occasion 3[C3]). At C1, participants were between 12 and 37 gestational weeks; 52 participants were in their second trimester and 30 were in their third trimester of pregnancy. The sample size was determined by available funding. Eighty-five of these participants provided a hair sample up to 5-cm in length for the analysis of monthly levels of HCC at least once across these three collection occasions and were included in the current analysis (NC1 = 82 [5 refused hair collection, 2 were using corticosteroids, 1 sample not analyzed]; NC2 = 50 [22 did not participate at this occasion, 16 refused collection, 2 were using corticosteroids], NC3 = 29 [31 did not participate, 18 refused collection, 9 participated prior to initiation of C3 collection protocol, 2 were using corticosteroids, 1 sample was collected incorrectly]). Participants who provided data at C2 and C3 did not differ significantly from those who did not provide data at those collection occasions in terms of age, gestation length, depressive symptoms, or psychosocial adversity.
2.2. Procedure
The BABIES Project was approved by the Stanford Institutional Review Board. Participants provided informed written consent and were compensated for their time. At each collection occasion, participants attended in-person sessions during which they completed questionnaires, interviews, computer tasks, and provided a hair sample. At C2 and C3, participants also completed assessments of caregiver and infant behavior, and a subset of infants underwent magnetic resonance imaging (data not included here; see Refs. [[25], [26], [27]]. Inclusion criteria at C1 were that participants were currently pregnant with a singleton, were 18 years of age or older, were fluent in English, and had no immediate plans to leave the geographic area. Exclusion criteria were bipolar disorder, psychosis, and severe learning disabilities. Additional exclusion criteria for C2 and C3 were severe complications during birth, infant head trauma, infant premature birth (<36 weeks gestation), infant congenital, genetic, or neurological disorders, and contraindication for infant MRI.
2.3. Hair cortisol concentration
The procedure to obtain hair samples was identical at each of the three collection occasions. At each occasion, we used hair scissors to cut a hair sample 3–5 mm in thickness from the posterior vertex position of the head, as close as possible to the scalp. Hair samples were stored in aluminum foil in the dark at room temperature prior to being shipped to the Kirschbaum Laboratory at the Technical University of Dresden, Germany. For each hair sample at each of the three collection occasions (C1, C2, C3), the first 5-cm of hair closest to the scalp was segmented into 1-cm increments. Following the common rule of thumb of 1 cm of hair growth per month [11], this approach yielded up to 5 values of HCC per hair sample corresponding to each of the 5 previous months. Therefore, as depicted in Fig. 1, hair samples collected at C1 captured monthly levels of HCC for each of the previous five months of pregnancy, samples collected at C2 captured monthly levels for each of the previous 1–2 months postpartum and the final 3–4 months of pregnancy, and samples collected at C3 captured monthly levels for each of the previous 5 months postpartum. At each collection occasion, there were a small number of hair samples that yielded <5 monthly observations of HCC. Specifically, at C1, 5 monthly observations of HCC were available from 71 of the participants, 4 observations from 8 participants, and 3 observations from 3 participants; at C2, 5 observations were available from 49 of the participants, and 4 observations were available from 1 participant; at C3, 5 observations were available from 26 participants of the participants, 3 observations from 2 participants, and 3 observations from 1 participant. In total, our sampling approach yielded 783 monthly observations of HCC across the peripartum period, capturing HCC spanning the first weeks of pregnancy to 6 months postpartum.
Fig. 1.
Distributions of gestational age captured by hair samples from each collection occasion. Each hair sample from each of the three collection occasions was segmented into up to five 1-cm increments where each increment reflects 1 month of HCC. Therefore, C1 hair samples captured monthly levels of HCC across the previous five months of pregnancy; C2 samples captured HCC across the previous 1–2 months postpartum and the final 3–4 months of pregnancy, and C3 sample captured HCC across the previous 5 months postpartum. Panel A shows distributions of gestational age captured at each collection occasion as kernel density estimates, which are smoothed versions of histograms. Panel B shows the range of gestational age captured for each participant at each collection occasion. Each line represents a single hair sample from a participant and each dot represents a single monthly observation of HCC from that hair sample. The dotted vertical lines in both panels correspond to childbirth.
For washing of hair and steroid extraction, the protocol of Davenport, Tiefenbacher, Lutz, Novak, and Meyer [28] was used; HCC in each 1-cm segment was estimated using a commercially available immunoassay with chemiluminescence detection (CLIA, IBL-Hamburg, Germany; intra- and inter-assay coefficients of variance <8%). Each 1-cm segment was dated in weeks since conception based on the infant's birth date (or due date, if birth date was not provided because the participant did not complete a postnatal assessment). Observations that reflected the period prior to pregnancy as well as observations of HCC that corresponded to periods in which participants reported using corticosteroids were removed from the analyses. Given positive skew, HCC values were log-transformed prior to analyses.
At each hair collection occasion, participants reported whether they had dyed, heat-treated, or chemically-straightened their hair in the past five months (missing from 4 participants at C1) and how frequently they washed their hair on a scale from 1 (<1x/week) to 4 (daily) (missing from 9 participants). We also identified the season in which each hair sample was collected. Cortisol concentration derived from hair is reliable with monthly integrated measures of salivary cortisol [29,30].
2.4. Depressive symptoms
At each hair collection occasion (C1, C2, C3), participants completed the Center for Epidemiological Studies Depression Scale (CES-D; [31]. Participants were instructed to consider their past week and respond to each of 20 items on a 4-point scale from 0 (rarely or none of the time) to 3 (most or all of the time), with higher scores representing greater symptoms of depression (Cronbach's αC1 = 0.89; αC2 = 0.88; αC3 = 0.92).
2.5. Recent psychosocial adversity
At each hair collection occasion (C1, C2, C3), participants completed the Crisis in Family Systems-Revised (CRISYS; [32]. Participants were instructed to consider the past six months and indicate whether each of 72 possible events had happened to them, their friends, or their family. These events included changes to their finances, employment, or housing, problems affording key resources, legal problems, experiences of discrimination, intimate partner discord, deaths of friends or family, community crime and violence, and illness of self, friends, or family. We summed participants responses to quantify the total number of events they had experienced in the prior six months. Two participants did not complete the CRISYS at C1.
2.6. Data analysis
Models were fit using the “lme4” package in R [33], using the “lmerTest” package to calculate degrees of freedom and p-values [34]. Scripts and analyzed data are available at: https://github.com/lucysking/hair_cortisol.
To characterize the trajectory of HCC from across the peripartum period, we used piecewise linear mixed effects modeling. Specifically, we regressed standardized log-transformed HCC values onto two terms: (1) time in weeks from conception to childbirth (hereafter, “prenatal slope”) corresponding to each monthly prenatal observation of HCC; and (2) time in weeks from childbirth to the final assessment at six months postpartum (hereafter, “postnatal slope”) corresponding to each monthly postnatal observation of HCC. In analyses examining the reliability of hair segments more distal from the scalp with proximal hair segments, we found high rank-order concordance of HCC in proximal and distal segments reflecting overlapping time periods (Pearson's r = .95), but also lower absolute values or evidence of “washout” of HCC in distal segments (see Supplementary Material). Therefore, to control for the influence of “washout,” we included in our models a random slope for hair segment (coded from 0 to 4 for each successive 1-cm from scalp), accounting for individual variation in HCC due to proximity to the scalp. This model was fit as follows, controlling for the random effects of participant intercept, prenatal slope, postnatal slope, and hair segment:
In this context, a significant effect of prenatal slope indicates change in log(HCC) across the prenatal period and a significant effect of postnatal slope indicates change in log(HCC) across the postnatal period. All variables were standardized to allow for model convergence (rescaling variables often resolves model convergence issues in the “lme4” package). Based on previous literature (for a review see Ref. [9], we evaluated the following covariates as main effects in separate models: hair chemical exposure/heat treatment, average frequency of hair washing, participant age at C1, and season at time of hair collection.
To distinguish within-from between-individual effects of depressive symptoms on variation in levels of HCC across the peripartum period, we regressed standardized log-transformed HCC values onto two terms: (1) fluctuations in depressive symptoms, modeled by centering depressive symptoms at each assessment around each participant's mean score across C1–C3 (i.e., person-mean-centering); and (2) average levels of depressive symptoms relative to other participants, modeled by taking each participant's mean score across the three assessments [23,35]. This model was fit as follows, controlling for the random effects of participant intercept, prenatal slope, postnatal slope, and hair segment:
In this context, a significant effect of fluctuations in depressive symptoms indicates that when depressive symptoms are higher (or lower) for a given individual, log(HCC) is also higher (or lower). In contrast, a significant effect of mean depressive symptoms indicates that average levels of depressive symptoms are associated with levels of log(HCC). We repeated this modeling process to examine within- and between-individual effects of psychosocial adversity on log(HCC) across the peripartum period, substituting fluctuations in psychosocial adversity and mean psychosocial adversity. As above, the variables for log(HCC), prenatal slope, and the postnatal slope were standardized; fluctuations in depressive symptoms/adversity were in raw units, and mean depressive symptoms/adversity were mean-centered.
Finally, we examined the within- and between-individual effects of depressive symptoms on variation in the prenatal and postnatal slopes of HCC. To reduce the complexity of these analyses, we conducted separate models corresponding to the prenatal and postnatal periods. For the prenatal period, fluctuations in depressive symptoms were modeled by centering depressive symptoms at C1 and C2 around each participant's mean score across C1–C2, and mean levels of depressive symptoms relative to other participants were modeled by taking each participant's mean score across C1–C2. We then tested interactions between fluctuations in depressive symptoms and the prenatal slope, and mean levels of depressive symptoms and the prenatal slope. This model was fit as follows, controlling for the random effects of participant intercept, prenatal slope, and hair segment:
In this context, a significant interaction between fluctuations in depressive symptoms and the prenatal slope indicates that increases (or decreases) in symptoms from pregnancy to shortly after childbirth are associated with the steepness of the prenatal slope; a significant interaction between mean levels of depressive symptoms and the prenatal slope indicates that average levels of depressive symptoms across this time period are associated with the steepness of the prenatal slope. For the postnatal period, we conducted similar models but instead focused on fluctuations in and average levels of depressive symptoms across C2 and C3. We then repeated this modeling process to examine the within- and between-individual effects of recent psychosocial adversity on variation in the prenatal and postnatal slopes of HCC.
3. Results
3.1. Sample characteristics
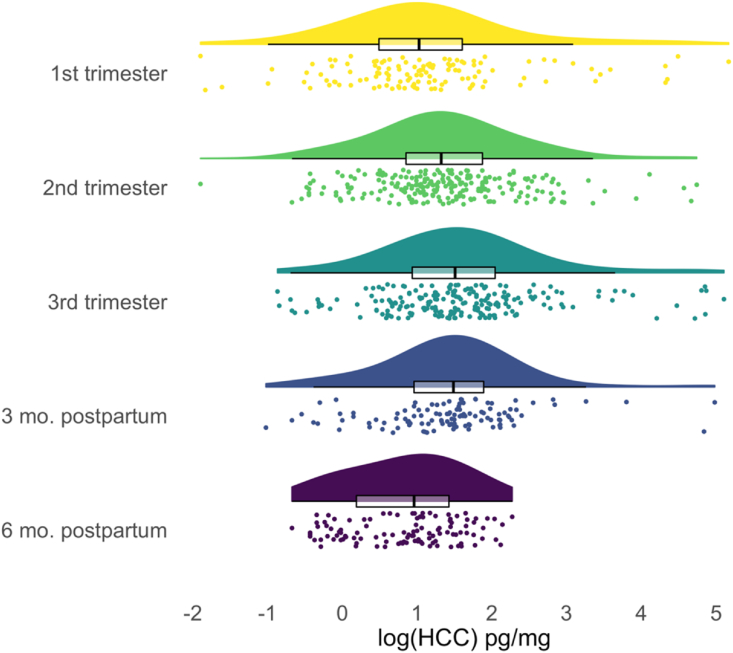
Descriptive statistics for the study sample are presented in Table 1. Based on dating of hair segments in weeks since conception, HCC values were available from 60 participants in the first trimester (122 observations), 82 in the second trimester (228 observations), 61 in the third trimester (208 observations), 57 in the three months postpartum (113 observations), and 29 in the six months postpartum (112 observations). In Fig. 2, we show the distributions of log(HCC) values during each of these phases of the peripartum period. Average levels of log(HCC) were higher in the second trimester (mean[SD] = 1.37[0.96]) than in the first (mean[SD] = 1.12[1.21]), and in the third trimester than in the second (mean[SD] = 1.60[1.05]). Average levels of log(HCC) were lower in the three months postpartum than in the third trimester (mean[SD] = 1.42[0.93]), and in the six months postpartum than in the three months postpartum (mean[SD] = 0.83[0.72]).
Table 1.
Descriptive statistics. C1 = time 1 (pregnancy). C2 = time 2 (3–8 weeks postpartum). C3 = time 3 (5–8 months postpartum). HCC = hair cortisol concentration, presented in raw units. Data for race, ethnicity, income, and education were missing from one participant.
| Variable | Mean (SD) or N | Range |
|---|---|---|
| Age (years) at C1 | 32.7(4.9) | 21.0-44.4 |
| Gestational weeks at childbirth | 39.4(1.3) | 33.3-41.6 |
| Weeks since conception | ||
| C1 | 24.2(5.4) | 12.4-37.0 |
| C2 | 45.1(1.2) | 42.1-49.3 |
| C3 | 65.9(1.7) | 62.7-72.0 |
| HCC pg/mg | ||
| C1 | 7.93 (16.83) | 0.15-175.47 |
| C2 | 9.93 (22.90) | 0.43-164.64 |
| C3 | 2.90 (2.01) | 0.36-9.70 |
| Depressive symptoms | ||
| C1 | 10.1(8.2) | 0-35 |
| C2 | 9.5(6.3) | 0-23 |
| C3 | 9.9(9.2) | 0-31 |
| Psychosocial adversity | ||
| C1 | 5.5(3.9) | 0-24 |
| C2 | 5.2(4.7) | 0-24 |
| C3 | 5.6(3.8) | 0-16 |
| Race | ||
| White | 53 | |
| Asian/Asian American | 18 | |
| Black/African American | 3 | |
| Native Hawaiian/Pacific Islander | 2 | |
| Another race | 8 | |
| Hispanic/Latinx Ethnicity | 8 | |
| Income | ||
| >$150,000 | 31 | |
| $90,001–150,000 | 29 | |
| $60,001–90,000 | 13 | |
| $30,001–60,000 | 8 | |
| $15,001–30,000 | 1 | |
| $5,001–15,000 | 0 | |
| $0–5,000 | 2 | |
Fig. 2.
Distribution and central tendency of hair cortisol concentration during each phase of the peripartum period. Half violin plots show distributions of log-transformed raw HCC values with tails trimmed to the range of the data. Box plots show distributions of log(HCC) values with hinges corresponding to the 25th and 75th percentiles, whiskers extending to 1.5 × interquartile range, and black central lines indicating the median values. Points are individual log(HCC) values. Phases of peripartum period are determined by dating of hair segments based on the fetus' birth date.
3.2. Trajectory of HCC across the peripartum period
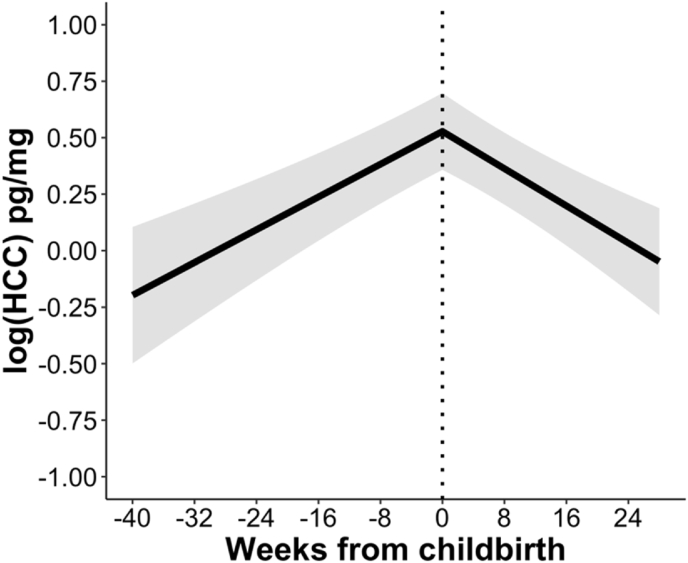
As displayed in Table 2, results of a piecewise linear mixed effects model indicated that levels of log(HCC) increased significantly during pregnancy and then decreased significantly during the postpartum period. As shown in Fig. 3, log(HCC) approximately doubled across the prenatal period; by six months postpartum, log(HCC) had returned to levels close to baseline. Nonetheless, with an intraclass correlation coefficient (ICC) of .90, within-person variability, and not the effects of time (i.e., the pre- and postnatal slopes), accounted for most of the variation in log(HCC). Comparing the random effects for the pre- and postnatal slopes, within-person variability in change across pregnancy was larger than that for change across the postpartum period.
Table 2.
Results of piecewise linear mixed effects model of trajectory of hair cortisol concentration across the peripartum period. HCC was log-transformed and all variables were then standardized prior to analysis. ICC = intraclass correlation coefficient. Prenatal slope captures rate of change in log(HCC) from conception to childbirth. Postnatal slope captures rate of change in log(HCC) from childbirth to six months postpartum. Number of participants = 85. Number of observations = 783. Marginal R2/Conditional R2 = 0.04/0.90.
| Predictors | 95% CI | p | |
|---|---|---|---|
| (Intercept) | 0.18 | 0.01, 0.36 | .041 |
| Prenatal slope | 0.24 | 0.16, 0.33 | <.001 |
| Postnatal slope | −0.20 | −0.28, −0.12 | <.001 |
| Random effects | |||
| (Intercept) | 0.71 | ||
| Prenatal slope | 0.23 | ||
| Postnatal slope | 0.05 | ||
| Hair segment | 0.08 | ||
| Residual | 0.11 | ||
| ICC | 0.90 | ||
Fig. 3.
Linear trajectories of HCC across pregnancy and the postpartum period. Data are fitted values from piecewise linear mixed effects model. log(HCC) = standardized log-transformed hair cortisol concentration.
Hair chemical exposure/heat treatment, frequency of hair washing, and age were not significantly associated with log(HCC) (all p-values > .60). Levels of log(HCC) did differ significantly, however, based on the season in which hair was collected (F(3, 320.58) = 6.03, p < .001). Levels of log(HCC) were estimated to be highest in summer, followed by the fall, winter, and spring. Estimates for the prenatal and postnatal slopes remained significant and were similar in magnitude when each of these covariates was included in the model. The results of sensitivity analyses in which we analyzed only HCC observations from the first 3-cm of hair proximal to the scalp were highly similar (see Supplementary Material).
3.3. Associations of depressive symptoms and psychosocial adversity with HCC
When added to the piecewise linear mixed effects model, neither within-individual fluctuations in depressive symptoms across C1–C3 nor between-individual mean levels of depressive symptoms across C1–C3 were associated with individual variation in levels of log(HCC) across the peripartum period (fluctuations in depressive symptoms: =<0.01, 95%CI[-0.01, 0.02], p = .602; mean depressive symptoms: = 0.01, 95%CI[-0.02, 0.03], p = .664). However, fluctuations in psychosocial adversity across C1–C3 were significantly positively associated with variation in levels of log(HCC) across the peripartum period ( =0.04, 95%CI[0.01, 0.08], p = .007), indicating that when psychosocial adversity was relatively higher within individuals, log(HCC) was also higher. Mean levels of psychosocial adversity across C1–C3 were not associated with variation in levels of log(HCC) ( =0.01, 95%CI[-0.03, 0.05], p = .617).
Neither fluctuations in depressive symptoms nor mean levels of depressive symptoms across were associated with individual variation in the prenatal slope (fluctuations in depressive symptoms: = 0.02, 95%CI[-0.01, 0.04], p = .062; mean depressive symptoms: = 0.01, 95%CI[-0.01, 0.02], p = .061) or the postnatal slope (fluctuations in depressive symptoms: = 0.01, 95%CI[-0.02, 0.03], p = .686; mean depressive symptoms: = -0.01, 95%CI[-0.02, 0.01], p = .489). Similarly, neither fluctuations in psychosocial adversity nor mean levels of psychosocial adversity were associated with individual variation in the prenatal slope (fluctuations in adversity: = -0.03, 95%CI[-0.07, 0.01], p = .151; mean adversity: = -0.01, 95%CI[-0.02, 0.02], p = .733) or the postnatal slope (fluctuations in adversity: = 0.04, 95%CI[-0.01, 0.10], p = .118; mean adversity: = 0.01, 95%CI[-0.02, 0.03], p = .863).
4. Discussion
In this study, we estimated the trajectory of HCC from conception through six months postpartum, comprehensively characterizing HPA-axis functioning throughout a critical developmental period. Leveraging over 750 monthly observations of HCC from 85 individuals derived from three samples of hair collected throughout the peripartum period, we found that HCC increased significantly across pregnancy and decreased significantly in the first six months postpartum. Disaggregating within- and between-person effects of longitudinally assessed depressive symptoms and psychosocial adversity, we found no evidence that depressive symptoms were associated with individual variation in levels or slopes of HCC during the peripartum period. Although overall levels of recent psychosocial adversity were also not associated with variation in levels or slopes of HCC, within-person fluctuations in adversity over time were significantly associated with levels of HCC.
Our finding of approximately a two-fold increase in HCC across pregnancy is consistent with previous studies that have analyzed cortisol in blood, saliva, and urine samples. For example, Jung et al. [1] found that compared to non-pregnant individuals assessed across the same period, pregnant individuals demonstrated a three-fold increase in total plasma and 24-h urinary free cortisol levels across trimesters. Similarly, D'Anna-Hernandez et al. [12] found that levels of both diurnal salivary cortisol and HCC increased by approximately one and a half times across trimesters, and further, that measures of salivary cortisol and HCC were significantly positively correlated. Increases in cortisol during pregnancy are driven by the growth of a new organ, the placenta; specifically, the rise in placental corticotropin releasing hormone over the course of gestation stimulates the release of adrenocorticotrophic hormone and ultimately the production of cortisol [36]. Some studies, however, have not identified increases in HCC across pregnancy [14] or have found increases in both pregnant and non-pregnant people across the same time period [15], raising questions concerning the use of hair to measure changes in HPA-axis functioning during pregnancy.
By using a finer-grained approach in which we analyzed monthly levels of HCC rather than averaging across trimesters, we believe we were better positioned to detect changes in HCC because our observations were spaced at a frequency closer to the pace at which change unfolds [22]. Specifically, most previous research examining changes in HCC across pregnancy has analyzed average levels estimated at the level of trimesters. Although increases may also be observed using that approach, it likely underestimates change in HCC because the lowest and highest values are not captured (i.e., values of HCC closest to conception and childbirth, respectively). Although researchers have suggested that observed increases in HCC during pregnancy are due to washout of cortisol in hair segments more distal from the scalp [9], our finding that HCC decreased in the postpartum period suggests that prenatal increases were most likely driven by developmental changes in HPA-axis functioning and not methodological noise. Specifically, if washout of HCC was responsible for any change, we would expect to see increases of HCC across time regardless of the developmental period. Decreases in HCC during the postpartum period reflect progressive recovery of typical HPA-axis functioning following childbirth. While cortisol in the postpartum period has received less attention than prenatal changes, studies that documented increases in cortisol during pregnancy also found lower cortisol after childbirth. For example, total plasma and 24-h urinary free cortisol were lower at three months postpartum than in the third trimester of pregnancy [1], as was HCC at three months postpartum compared to the third trimester [12].
Although our findings suggest that HCC does capture a biologically plausible trajectory of cortisol secretion across the peripartum period, like Marceau et al. [15]; we found that there was substantial within-person variability in HCC, especially during the prenatal period. It is possible that pregnancy leads to greater individual differences in other biological process linked to HPA-axis regulation (e.g., immune system functioning) that, in turn, generate greater variability in HCC during this period, although research is needed to examine this theory. High variability in cortisol during pregnancy does not appear to be limited to cortisol measured in hair. For example, in a recent study, researchers used growth mixture modeling, a person-centered approach, to identify subgroups of individuals who showed different plasma cortisol trajectories across pregnancy [37]. Although the largest group was characterized by a steady rise in cortisol throughout gestation, the researchers also identified a group that increased in levels of cortisol more steeply and a group with initially higher levels of cortisol that plateaued by mid-gestation. A composite measure of psychological distress (average prenatal anxiety, state anxiety, perceived stress, and depressive symptoms across gestation) was associated with these cortisol trajectory groups; specifically, individuals in the group with levels of cortisol that plateaued by mid-gestation had higher levels of psychological distress than did individuals in the other two groups.
Factors related to psychosocial stress may also help to explain variability in HCC during the peripartum period. Several research groups having examined whether levels of HCC are associated with symptoms of psychopathology (e.g., Refs. [18,19]; however, findings have been mixed, leading researchers to call for study designs that pair repeated assessments of symptoms with repeated assessments of HCC [10]. Repeated assessments allow for the separation of between-individual differences (i.e., do individuals with higher symptoms tend to have higher cortisol?) from within-individual effects (i.e., for an individual, are increases in symptoms paired with changes in cortisol?), that are otherwise confounded. This disaggregation is important because the meaning of a given symptom score or level of psychosocial adversity, and its impact on neurobiology, may depend on what is typical for a given person [23]. Consistent with meta-analytic data from pregnant and non-pregnant people documenting no robust associations between mood disorders and HCC [39] and with evidence from a systematic review indicating no consistent association between depression and prenatal cortisol measured in hair, saliva, or blood [38], we found no associations between depressive symptoms and variation in levels or slopes of HCC during the peripartum period. However, we did find that within-person fluctuations in recent psychosocial adversity over the peripartum period were associated with variation in levels of HCC. Thus, HCC did not vary based on absolute levels of adversity between individuals but was sensitive to changes in adversity within individuals. When adversity was relatively higher for a given individual, HCC was also higher. Although additional research is necessary to replicate these results, our findings may have important clinical implications; specifically, in addition to the level of adversity, change in women's experiences of adversity during the peripartum period merit attention, as these changes may track with deviations in neurobiology.
We should note five limitations of the current study. First, the sample size was relatively small. Although we obtained a large number of observations of HCC, these observations came from only 85 individuals. Attrition was also high, with only 29 individuals providing hair samples at the final collection occasion. Larger sample sizes may allow for more precise estimates of the trajectory of HCC across the peripartum period and of associations of factors related to psychosocial stress with HCC in pregnant and postpartum individuals. Second, the current sample tended to be highly educated and report high household income. Our findings may have differed in a sample that better represented a wider range of socioeconomic resources. Third, although our statistical models attempted to account for the potential for washout of cortisol along the hair shaft, we may not have fully controlled for degradation of HCC in hair segments more distal from the scalp and this could have affected our estimates. Fourth, monthly estimates of HCC were based on a fairly consistent hair growth rate of 1-cm per month; however, there may be individual variability in this growth rate [11]. Finally, although we assessed depressive symptoms and psychosocial adversity repeatedly, these assessments (at three occasions across the peripartum period) were not as fine-grained as our observations of HCC (each month of the peripartum period), precluding analysis of how monthly fluctuations in symptoms and adversity track with HCC. Future research may therefore benefit from denser sampling of adversity across the peripartum period.
Given that changes in cortisol secretion across the peripartum period unfold in tandem with rapid fetal development and the transition to parenthood, understanding the trajectory of cortisol during pregnancy and the postpartum months may be helpful for research examining variation in maternal–offspring functioning. Overall, the current findings suggest that measurement of cortisol in hair can capture this trajectory, including increases during pregnancy and gradual return to baseline levels six months after childbirth. Further, HCC during the peripartum period appears to be coupled with fluctuations in psychosocial adversity. Beyond these effects, however, there is substantial variability in cortisol during the peripartum period. Future research may yield insight into how differences in both levels and trajectories of cortisol across the peripartum period are related to fetal development and maternal–infant health in the first months of life.
Declaration of competing interest
The authors report no conflicts of interest.
Acknowledgements
Funding was provided by the National Institutes of Health (IHG, R21 MH111978; IHG, R21 HD090493; IHG, R37 MH101495; IHG, R03HD101714; LSK, F32 HD105385); the National Science Foundation (LSK, Graduate Research Fellowship); the Jacobs Foundation (KLH, Early Career Research Fellowship 2017-1261-05). We thank Anna Cichocki, Cheyenne Garcia, Amar Ojha, Marissa Roth, Francesca Querdasi, and Lucinda Sisk for their assistance in recruitment, project management, and data collection. We gratefully acknowledge the participants for their contributions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpnec.2021.100102.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Jung C., Ho J.T., Torpy D.J., Rogers A., Doogue M., Lewis J.G., Czajko R.J., Inder W.J. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J. Clin. Endocrinol. Metabol. 2011;96(5):1533–1540. doi: 10.1210/jc.2010-2395. [DOI] [PubMed] [Google Scholar]
- 2.Meulenberg P.M.M., Hofman J.A. Differences between concentrations of salivary cortisol and cortisone and of free cortisol and cortisone in plasma during pregnancy and postpartum. Clin. Chem. 1990;36(1):70–75. doi: 10.1093/clinchem/36.1.70. [DOI] [PubMed] [Google Scholar]
- 3.Field T., Diego M.A., Hernandez-Reif M., Figueiredo B., Ascencio A., Schanberg S., Kuhn C. Prenatal dysthymia versus major depression effects on maternal cortisol and fetal growth. Depression and Anxiety. 2008;25(6):11–16. doi: 10.1002/da.20307. [DOI] [PubMed] [Google Scholar]
- 4.Stephens J.E., Kessler C.L., Buss C., Miller G.E., Grobman W.A., Keenan-Devlin L., Borders A.E., Adam E.K. Early and current life adversity: past and present influences on maternal diurnal cortisol rhythms during pregnancy. Dev. Psychobiol. 2021;63(2):305–319. doi: 10.1002/dev.22000. [DOI] [PubMed] [Google Scholar]
- 5.Davis E.P., Glynn L.M., Dunkel-Schetter C., Hobel C.J., Chicz-Demat A., Sandman C.A. Prenatal exposure to maternal depression and cortisol influences infant temperament. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46(6):737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- 6.Davis E.P., Head K., Buss C., Sandman C.A. Prenatal maternal cortisol concentrations predict neurodevelopment in middle childhood. Psychoneuroendocrinology. 2017;75:56–63. doi: 10.1016/j.psyneuen.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Blanco A., Diago V., Serrano De La Cruz V., Hervás D., Cháfer-Pericás C., Vento M. Can stress biomarkers predict preterm birth in women with threatened preterm labor? Psychoneuroendocrinology. 2017;83(January):19–24. doi: 10.1016/j.psyneuen.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Greff M.J.E., Levine J.M., Abuzgaia A.M., Elzagallaai A.A., Rieder M.J., van Uum S.H.M. Hair cortisol analysis: an update on methodological considerations and clinical applications. Clin. Biochem. 2019;63(September 2018):1–9. doi: 10.1016/j.clinbiochem.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Marceau K., Wang W., Robertson O., Shirtcliff E.A. A systematic review of hair cortisol during pregnancy: reference ranges and methodological considerations. Psychoneuroendocrinology. 2020;122(July) doi: 10.1016/j.psyneuen.2020.104904. 104904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mustonen P., Karlsson L., Scheinin N.M., Kortesluoma S., Coimbra B., Rodrigues A.J., Karlsson H. Hair cortisol concentration (HCC) as a measure for prenatal psychological distress — a systematic review. Psychoneuroendocrinology. 2018;92(March):21–28. doi: 10.1016/j.psyneuen.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Stalder T., Kirschbaum C. Analysis of cortisol in hair - state of the art and future directions. Brain, Behavior, and Immunity. 2012;26(7):1019–1029. doi: 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 12.D'Anna-Hernandez K.L., Ross R.G., Natvig C.L., Laudenslager M.L. Hair cortisol levels as a retrospective marker of hypothalamic–pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiol.Behavior. 2011;104(2):348–353. doi: 10.1016/j.physbeh.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman M.C., Mazzoni S.E., Wagner B.D., Laudenslager M.L., Ross R.G. Measures of maternal stress and mood in relation to preterm birth. Obstetrics Gynecol. 2016;127(3):545–552. doi: 10.1097/AOG.0000000000001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero-Gonzalez B., Caparros-Gonzalez R.A., Gonzalez-Perez R., Delgado-Puertas P., Isabel Peralta-Ramirez M. Newborn infants' hair cortisol levels reflect chronic maternal stress during pregnancy. PLoS ONE. 2018;13(7) doi: 10.1371/journal.pone.0200279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marceau K., Rolan E., Robertson O.C., Wang W., Shirtcliff E.A. Within-person changes of cortisol, dehydroepiandrosterone, testosterone , estradiol, and progesterone in hair acr o ss pregnancy, with comparison to a non-pregnant reference group. Compr. Psychoneuroendocrinology. 2021;5(September 2020):100024. doi: 10.1016/j.cpnec.2020.100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salacz P., Csukly G., Haller J., Valent S. Association between subjective feelings of distress, plasma cortisol, anxiety, and depression in pregnant women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012;165(2):225–230. doi: 10.1016/j.ejogrb.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 17.van den Heuvel M.I., van Assen M.A.L.M., Glover V., Claes S., Van den Bergh B.R.H. Associations between maternal psychological distress and salivary cortisol during pregnancy: a mixed-models approach. Psychoneuroendocrinology. 2018;96(December 2017):52–60. doi: 10.1016/j.psyneuen.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Mustonen P., Karlsson L., Kataja E.L., Scheinin N.M., Kortesluoma S., Coimbra B., Rodrigues A.J., Sousa N., Karlsson H. Maternal prenatal hair cortisol is associated with prenatal depressive symptom trajectories. Psychoneuroendocrinology. 2019;109(February):104383. doi: 10.1016/j.psyneuen.2019.104383. [DOI] [PubMed] [Google Scholar]
- 19.Wikenius E., Moe V., Kjellevold M., Smith L., Lyle R., Waagbø R., Page C.M., Myhre A.M. The association between hair cortisol and self-reported symptoms of depression in pregnant women. PLoS ONE. 2016;11(9):1–10. doi: 10.1371/journal.pone.0161804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleker L.S., Roseboom T.J., Vrijkotte T.G., Reynolds R.M., de Rooij S.R. Determinants of cortisol during pregnancy – the ABCD cohort. Psychoneuroendocrinology. 2017;83(April):172–181. doi: 10.1016/j.psyneuen.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Khoury J.E., Bosquet Enlow M., Plamondon A., Lyons-Ruth K. The association between adversity and hair cortisol levels in humans: a meta-analysis. Psychoneuroendocrinology. 2019;103(January):104–117. doi: 10.1016/j.psyneuen.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ram N., Gerstorf D. Time-structured and net intraindividual variability: tools for examining the development of dynamic characteristics and processes. Psychol. Aging. 2009;24(4):778–791. doi: 10.1037/a0017915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curran P.J., Hancock G.R. The challenge of modeling co-developmental processes over time. Child Devel. Perspect. 2021:1–9. doi: 10.1111/cdep.12401. 0(0) [DOI] [Google Scholar]
- 24.Humphreys K.L., King L.S., Choi P., Gotlib I.H. Maternal depressive symptoms, self-focus, and caregiving behavior. J. Affect. Disord. 2018;238:465–471. doi: 10.1016/j.jad.2017.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King L.S., Camacho M.C., Montez D.F., Humphreys K.L., Gotlib I.H. Naturalistic language input is associated with resting-state functional connectivity in infancy. J. Neurosci. 2021;41(3):424–434. doi: 10.1523/jneurosci.0779-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camacho M.C., King L.S., Ojha A., García C.M., Sisk L.S., Cichocki A.C., Humphreys K.L., Gotlib I.H. Cerebral blood flow in 5- to 8-month-olds: regional tissue maturity is associated with infant affect. Dev. Sci. 2020;23(4) doi: 10.1111/desc.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King L.S., Querdasi F.R., Humphreys K.L., Gotlib I.H. Dimensions of the language environment in infancy and symptoms of psychopathology in toddlerhood. Dev. Sci. 2021 doi: 10.1111/desc.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davenport M.D., Tiefenbacher S., Lutz C.K., Novak M.A., Meyer J.S. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen. Comp. Endocrinol. 2006;147(3):255–261. doi: 10.1016/j.ygcen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Short S.J., Stalder T., Marceau K., Entringer S., Moog N.K., Shirtcliff E.A., Wadhwa P.D., Buss C. Correspondence between hair cortisol concentrations and 30-day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology. 2016;71:12–18. doi: 10.1016/j.psyneuen.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugaya N., Izawa S., Ogawa N., Shirotsuki K., Nomura S. Association between hair cortisol and diurnal basal cortisol levels: a 30-day validation study. Psychoneuroendocrinology. 2020;116:104650. doi: 10.1016/j.psyneuen.2020.104650. July 2019. [DOI] [PubMed] [Google Scholar]
- 31.Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. J. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- 32.Berry C., Quinn K., Shalowitz M., Wolf R. Validation of the Crisis in family systems–revised, a contemporary measure of life stressors. Psychol. Rep. 2001;88(3):713–724. doi: 10.2466/pr0.2001.88.3.713. [DOI] [PubMed] [Google Scholar]
- 33.Bates D., Machler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Software. 2014:1–51. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 34.Kuznetsova A., Brockhoff P.B., Christensen R.H.B. Tests in linear mixed effects models. J. Stastical Software. 2016;82(13):1–26. doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- 35.Curran P.J., Bauer D.J. The disaggregation of within-person and between-person effects in longitudinal models of change. Ann. Rev. Psychol. 2011;62:583–619. doi: 10.1146/annurev.psych.093008.100356. The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howland M.A., Sandman C.A., Glynn L.M. Developmental origins of the human hypothalamic-pituitary-adrenal axis. Health Psychol. 2017;12(5):321–339. doi: 10.1080/17446651.2017.1356222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterson G.E., Espel E., Davis E.P., Sandman C.A., Glynn L.M. Characterizing prenatal maternal distress with unique prenatal cortisol trajectories. Health Psychol. 2020 doi: 10.1007/978-1-137-34589-9_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orta O.R., Gelaye B., Bain P.A., Williams M.A. The association between maternal cortisol and depression during pregnancy, a systematic review. Archives of Women’s Mental Health. 2018;21:43–53. doi: 10.1007/s00737-017-0777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stalder T., Steudte-Schmiedgen S., Alexander N., Klucken T., Vater A., Wichmann S., Miller R. Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology. 2017;77:261–274. doi: 10.1016/j.psyneuen.2016.12.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.