Highlights
-
•
This study aimed to predict the outcomes of febrile neutropenic patients
-
•
Septic shock, anemia, AKI, and need for mechanical ventilation were mortality risks
-
•
Our prediction score is efficacious in discriminating mortality risk group
Keywords: Febrile neutropenia, Agranulocytosis, Pathogen, Prognostic factor, Mortality
Abstract
Objectives
To determine the predictors for 30-day all-cause mortality in patients with febrile neutropenia (FN) and develop a prediction score.
Methods
The electronic medical records of patients undergoing chemotherapy with FN between 2018 and 2019 were reviewed. Multivariate logistic regression was performed to identify factors associated with 30-day all-cause mortality to develop a parsimonious model. A prediction score was developed from the model's coefficients of each predictor.
Results
There were 273 FN episodes in 153 patients. The overall mortality rate was 12.5%. Pre-existing cardiovascular disease (OR 22.45), alteration of consciousness on admission (OR 18.50), anemia (OR 4.33), acute kidney injury (AKI) (OR 13.15), causative pathogen identified (OR 8.68), intensive care unit admission (OR 0.13), septic shock (OR 18.72), and the need for mechanical ventilation (OR 22.65) were associated with mortality. After exploring confounding effects between factors, septic shock, anemia, AKI, and the need for mechanical ventilation were selected to develop the prediction score which provided good sensitivity (87.88%) and specificity (90.91%) with an area under the ROC curve of 0.8939.
Conclusions
Septic shock, anemia, AKI, and the need for mechanical ventilation were associated with FN mortality. Our prediction score is effective in discriminating high and low-risk patients for mortality.
Introduction
Neutropenia is a common condition in patients with cancer, and it results from bone marrow involvement due to either cancer or complications from chemotherapy that causes patients to be susceptible to infection from a wide range of organisms. The mortality rates of febrile neutropenia (FN) were reported to be 10‒30% (Al-Tawfiq et al., 2019; Auesomwang et al., 2018; Parodi et al., 2019; Weerasubpong et al., 2016). According to previous data, the common pathogens were gram-negative bacilli, especially Enterobacteriaceae in 60‒70% of febrile neutropenic episodes, followed by Pseudomonas spp., and gram-positive organisms (e.g., Staphylococcus aureus) (Chayakulkeeree & Thamlikitkul, 2003; Parodi et al., 2019; Roongpoovapatr & Suankratay, 2010; Weerasubpong et al., 2016; Zhang et al., 2020). In addition, increasing incidences of fungal infections (Auesomwang et al., 2018; Roongpoovapatr & Suankratay, 2010) and gram-positive organisms (Leelayuthachai & N, 2010) were reported in cohort studies done in Thailand.
Studies on the common pathogens and drug sensitivity results in a hospital are essential for physicians to select the proper empirical antibiotics. Also, mortality-associated factors are important for prognostication in those patients with FN. Therefore, this study aimed to identify the risk factors of mortality to predict the outcomes of FN patients undergoing chemotherapy. Moreover, we also aimed to demonstrate the demographic data regarding the sources of infection, common pathogens, and drug sensitivity tests to consider use of proper prophylactic antibiotics to optimize the outcomes of FN patients.
Materials and methods
This was a retrospective study conducted in Songklanagarind Hospital, which is a tertiary university hospital in southern Thailand. The study was approved by the Human Research Ethics Committee of the Faculty of Medicine, Prince of Songkla University, Thailand (REC. 63-207-14-1).
Study design and participants
The electronic medical records from January 2018 to December 2019 were reviewed for patients aged 18 years old or older admitted in Songklanagarind Hospital and diagnosed as FN episodes, which were identified by an ICD-10 diagnosis of agranulocytosis (ICD10 code: D70). Subsequently, we employed the 2010 Infectious Diseases Society of America (IDSA) FN diagnostic criteria to ascertain the diagnosis of FN (Freifeld et al., 2011). According to the criteria, FN can be diagnosed when the patient records an oral temperature ≥38 °C for at least one hour or an initial temperature of ≥38.3 °C, and the absolute neutrophil count (ANC) is less than 500 cells/mm3 or showing a tendency to be less than 500 cells/mm3 in the next 48 h. The patients who did not receive chemotherapy were excluded.
Sample size calculation
The sample size was calculated by an equation to detect the difference of two independent proportions. Using the reported mortality rate of 19.7% among Thai patients with FN (Auesomwang et al., 2018), the desired power of 80%, an alpha error of 5%, a minimum detectable effect size of 15%, and a predetermined allocation ratio of 1:1, the estimated sample size was 276. We aimed to collect approximately 320 observations to accommodate around 15% of missing data.
Data collection
Upon recruitment, the following baseline clinical data were collected: age; gender; comorbidities; presence or absence of bone marrow disease which was defined as primary diseases of the bone marrow such as leukemia or aplastic anemia; or metastatic disease to the bone marrow such as lymphoma with bone marrow involvement or solid organ tumor with bone marrow metastasis; immunosuppressive drug use (i.e., corticosteroids where the dose had to be at least equivalent to prednisolone 20 mg/day for more than four weeks); the causes of neutropenia being categorized into chemotherapy-induced (the onset of neutropenia occurred within 14 days after chemotherapy) and due to bone marrow diseases; use of granulocyte colony-stimulating factor (G-CSF); previous history of FN; previous antibiotic use three months prior to admission; previous episodes of invasive fungal infection (IFI); use of antibiotics 48 h before the onset of FN; and presence of indwelling central venous catheter (e.g., Hickman catheter).
During an episode of FN, the collected data included the etiologies of neutropenia, total duration of neutropenia, duration from onset of neutropenia to the onset of FN, duration from onset of fever to hospital admission, vital signs and oxygen saturation on admission, level of consciousness on admission, and the presence or absence of mucositis. In addition, we reviewed the complete blood count within 72 h of the diagnosis of FN, presence or absence of metabolic acidosis (serum HCO3‒ <15 mEq/L) on admission, presence or absence of acute kidney injury (AKI) during admission, the need for mechanical ventilation, the diagnosis of septic shock according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) criteria (Singer et al., 2016), acute respiratory distress syndrome (ARDS), and intensive care unit (ICU) admission.
We reviewed the data on the causative pathogens, settings of the infections (i.e., either community-acquired or hospital-acquired infection defined as infection occurring at least 48 h after hospital admission or within 72 h after discharge) (Inweregbu et al., 2005), presence or absence of polymicrobial infection, presence or absence of multiple sites of infection, presence or absence of bacteremia or fungemia, presence or absence of breakthrough infection (defined as microbiologically-proven infection that occurred at least 48 h after the onset of FN), and the diagnosis of IFI defined by the European Organization for Research and Treatment of Cancer (Donnelly et al., 2020). FN episodes were categorized into three groups: (1) clinically documented infection (CDI) defined as fever and local inflammation without proven pathogenic organisms, (2) microbiologically documented infection (MDI) defined as fever with detected infectious organisms from blood cultures, specimens from the foci of infection, or serological results, and (3) fever of unknown origin (FUO) defined as isolated fever without signs or symptoms suggestive of clinical infection and microbial documentation.
We also reviewed the data on the timing of antibiotic administration after the diagnosis of FN, the duration from antibiotic administration to fever resolution (defined as body temperature <38 °C for at least 48 h), use of empirical vancomycin at the onset of FN, and the appropriateness of empirical treatment defined as the isolated bacteria being susceptible to at least one empirical antimicrobial agent administered as the first dose or 24 h later (Lueangarun & Leelarasamee, 2012). We assessed all-cause mortality that occurred 30 days after the diagnosis of FN as the outcome measure.
Statistical analysis
The data analysis was performed using STATA 16.1 (StataCorp LLC, TX, USA). Subjects with at least one piece of missing data were eliminated from the dataset before the analysis. The predictor variables are shown in Table S1. For each predictor variable, comparisons between survivors and non-survivors were performed using the χ2 test or Fisher's exact test as appropriate. All variables underwent univariate analysis using simple logistic regression to preliminarily determine their associations with the outcome measure. The variables with at least a moderate degree of correlation with outcomes from univariate analysis (p < 0.2) were selected for multivariate logistic regression analysis. Using the stepwise backward elimination method (p-value cut-off point for model selection: 0.1), candidate variables for a prediction model were selected. Confounding effects between all candidate variables were explored using an all-possible-estimates method (R). The parsimonious prediction model was chosen based on the Akaike and Bayesian information criteria. The model was used to develop a prognostic score to stratify patients into high- and low-risk groups. Survival rates between high- and low-risk groups were compared using log rank test and cox regression method. A p-value less than 0.05 was considered significant in all tests.
Results
Demographic data
The demographic data of 273 FN episodes among 153 patients, which included 82 (53.6%) males and 71 (46.4%) females, were reviewed for the descriptive statistics. The mean age was 53.7 ± 16.3 years in a range of 17–89 years. Of all FN episodes, 236 episodes were chemotherapy-induced, of which hematologic malignancy (204 episodes) and solid tumor (32 episodes) were the primary diseases. The most common associated comorbidity for solid organ malignancy was lung cancer, while non-Hodgkin's lymphoma and acute myeloid leukemia were the main comorbidities in hematologic diseases. The numbers and types of FN episodes were 120 FUO episodes (44.0%), 123 MDI episodes (45.1%), and 30 CDI episodes (11.0%), which were mainly comprised of gastrointestinal tract infection (12 episodes), lower respiratory tract infection (9 episodes), and soft tissue and skin infection (5 episodes) (Table S2). Of the 123 episodes of MDI, 184 isolates were identified by culture, pathologic results, or serological tests (Table 1).
Table 1.
Isolated pathogens in FN defined as microbiologically documented infections
| Organisms | No. of isolate | Source |
||||||
|---|---|---|---|---|---|---|---|---|
| Primary bacteremia | CRBSI | UTI | Pneumonia | GI | SSI | Others | ||
| Gram-negative bacilli | ||||||||
| Escherichia coli | 35 | 19 | 2 | 9 | 4 | - | 1 | - |
| Klebsiella pneumoniaea | 32 | 14 | 4 | 4 | 5 | 5 | 1 | - |
| Pseudomonas aeruginosa | 24 | 10 | 3 | 1 | 6 | 1 | 3 | - |
| Acinetobacter baumannii | 8 | 3 | - | - | 3 | - | 1 | 1 |
| Enterobacter spp. | 8 | 2 | 1 | 2 | 2 | - | - | 1 |
| Aeromonas spp. | 4 | 2 | - | - | - | 2 | - | - |
| Serratia marcescens | 3 | 1 | 2 | - | - | - | - | - |
| Ralstonia mannitolilytica | 2 | 2 | - | - | - | - | - | - |
| Salmonella spp. | 1 | - | - | - | - | 1 | - | - |
| Proteus spp. | 2 | - | - | 1 | 1 | - | - | - |
| Others* | 3 | 1 | 1 | - | 1 | - | - | - |
| Total | 122 | 54 | 13 | 17 | 22 | 9 | 6 | 2 |
| Gram-positive organisms | ||||||||
| Enterococcus spp. | 16 | 5 | 1 | 7 | 1 | 1 | - | 1 |
| Streptococcus spp. | 6 | 3 | 1 | - | 1 | 1 | - | - |
| Staphylococcus aureusb | 4 | 1 | - | - | 1 | - | 2 | 1 |
| Staphylococcus epidermidis | 2 | 2 | - | - | - | - | - | - |
| Corynebacterium striatum | 1 | 1 | - | - | - | - | - | - |
| Total | 29 | 12 | 2 | 7 | 3 | 2 | 2 | 2 |
| Fungus and other organisms | ||||||||
| Candida spp.c | 15 | 8 | 2 | 3 | 3 | 3 | 1 | - |
| Aspergillus spp.d | 13 | - | - | - | 12 | 2 | - | - |
| Cytomegalovirus | 1 | - | - | - | - | - | 1 | - |
| Fusarium spp.e | 1 | - | - | - | 1 | - | 1 | - |
| Others (e.g., PJP, unidentified mold) | 3 | - | - | - | 1 | 1 | 1 | - |
| Total | 33 | 8 | 2 | 3 | 17 | 6 | 4 | - |
CRBSI = catheter-related bloodstream infection; UTI = urinary tract infection; GI = gastrointestinal tract infection; SSI = soft tissue and skin infection; PJP = Pneumocystis jirovecii pneumonia.
1 isolate with gastrointestinal and soft tissue and skin infection
1 isolate with pneumonia and soft tissue and skin infection
1 isolate with gastrointestinal tract infection, pneumonia and CRBSI; 1 isolate with gastrointestinal and soft tissue and skin infection; 1 isolate with urinary tract infection, pneumonia and CRBSI
1 isolate with pneumonia and gastrointestinal tract infection
1 isolate with pneumonia and soft tissue and skin infection
Others: Burkholderia pseudomallei, Morganella morganii, and Stenotrophomonas maltophilia
We found 30 drug-resistant gram-negative pathogens (24.8% of all 122 gram-negative isolates), consisting of 22 extended-spectrum beta-lactamase-producing bacteria, six carbapenem-resistant Klebsiella pneumoniae, and two multidrug-resistant Acinetobacter baumannii. In addition, two methicillin-resistant Staphylococcus epidermidis and one multidrug-resistant Corynebacterium striatum were detected (10.3% of all 29 gram-positive isolates); however, no incidence of methicillin-resistant Staphylococcus aureus or other drug-resistant gram-positive organisms was observed. Fungal infection was identified in 31 isolates (16.8% of all isolated pathogens).
Mortality rate and associated risk factors
From the total of 273 FN episodes, episodes with missing data were excluded, leaving 264 episodes for analysis. Regarding baseline characteristics prior to FN episodes, there was no significant difference between the survivors and non-survivors in terms of age, comorbidities or previous infection, as shown in Table 2. In addition, the cause of neutropenia was not associated with mortality. The univariate analysis by simple logistic regression found statistically significant associations between 30-day all-cause mortality and the presence of indwelling central venous catheter (OR 0.39; 95% CI 0.17–0.90) and use of antibiotics 48 h before FN onset (OR 2.99; 95% CI 1.08–8.28).
Table 2.
Baseline patient characteristics prior to febrile neutropenic episodes
| Characteristics | Episodes in survivors n = 231 (%) | Episodes in non-survivors n = 33 (%) | p-value |
|---|---|---|---|
| Age ≥ 60 years | 26.41 | 24.24 | 0.791 |
| Female | 44.59 | 48.48 | 0.674 |
| Comorbidities | |||
| Hematologic disease | 89.61 | 84.85 | 0.381 |
| Essential hypertension | 11.26 | 21.21 | 0.153 |
| Dyslipidemia | 12.12 | 15.15 | 0.579 |
| Chronic kidney disease | 9.96 | 6.06 | 0.750 |
| Diabetes mellitus | 9.96 | 9.09 | 1.000 |
| HIV infection | 7.79 | 6.06 | 1.000 |
| Cardiovascular disease | 3.03 | 9.09 | 0.116 |
| Autoimmune diseases | 2.16 | 6.06 | 0.213 |
| Chronic obstructive pulmonary disease | 1.73 | 3.03 | 0.490 |
| Cirrhosis | 0.87 | 3.03 | 0.331 |
| Immunosuppressive drug use | 3.90 | 9.09 | 0.178 |
| Presence of bone marrow diseases/involvement | 48.05 | 48.48 | 0.963 |
| Cause of neutropenia | 0.271 | ||
| Chemotherapy-induced | 88.31 | 81.82 | |
| Due to bone marrow disease/involvement | 11.69 | 18.18 | |
| G-CSF | 0.137 | ||
| Not received | 12.12 | 21.21 | |
| Prophylaxis dose | 62.77 | 45.45 | |
| Treatment dose | 25.11 | 33.33 | |
| Presence of indwelling central venous catheter | 45.02 | 24.24 | 0.024* |
| Previous FN | 55.41 | 42.42 | 0.162 |
| Previous fungal infection | 13.42 | 12.12 | 1.000 |
| Previous antibiotics use 3 months prior to admission | 57.14 | 69.70 | 0.171 |
| Use of antibiotics 48 hours before FN onset | 6.93 | 18.18 | 0.041* |
Abbreviations: HIV = human immunodeficiency virus; G-CSF = granulocyte colony-stimulating factor; FN = febrile neutropenia.
p < 0.05
At the onset of FN, tachypnea and tachycardia were significantly associated with non-survival status (Table S3). Non-survivors were also desaturated and had altered mental status at presentation. Furthermore, AKI and severe metabolic acidosis were more common among non-survivors compared to survivors. Non-survivors also demonstrated lower hemoglobin levels. The levels of ANC, absolute lymphocyte count, and platelet count were not statistically different between the survivors and non-survivors. Simple logistic regression analysis demonstrated associations between 30-day all-cause mortality and the following predictor variables: duration from onset of neutropenia to onset of FN >10 days (OR 3.73; 95% CI 1.31 – 10.61), systolic blood pressure <90 mmHg (OR 14.84; 95% CI 1.31–168.48), tachycardia (pulse rate ≥120 beats per min) (OR 3.16; 95% CI 1.50–6.68), tachypnea (respiratory rate ≥24 breaths per min) (OR 2.24; 95% CI 1.06–4.73), desaturation (OR 15.78; 95% CI 6.53–38.15), alteration of consciousness (OR 23.00; 95% CI 2.32 – 228.25), hemoglobin concentration less than 8 g/dL (OR 3.54; 95% CI 1.61–7.78), AKI (OR 21.97; 95% CI 9.19 – 52.52), severe metabolic acidosis (OR 11.45; 95% CI 1.84 – 71.32), ARDS (OR 20.45; 95% CI 3.79–110.38), septic shock (OR 25.20; 95% CI 10.44 – 60.84), the need for mechanical ventilation (OR 41.99; 95% CI 16.33–107.97), and ICU admission (OR 14.20; 95% CI 6.15–32.80).
Non-surviving patients tended to have bacteremia, polymicrobial infections, IFI, and breakthrough infections. Compared to survivors, infections in non-survivors also took a longer time to subside. Using simple logistic regression analysis, the following variables were associated with 30-day all-cause mortality: duration from onset of symptom to antibiotic >1 h (OR 0.48; 95% CI 0.23–0.99), causative pathogen identified (OR 5.51; 95% CI 2.30–13.22), bacteremia/fungemia (OR 3.54; 95% CI 1.67–7.51), probable IFI (OR 11.00; 95% CI 2.08–58.11), proven IFI (OR 19.80; 95% CI 6.05–64.77), breakthrough infection (OR 11.85; 95% CI 5.18–27.15), polymicrobial infection (OR 3.73; 95% CI 1.31–10.61), multiple site infection (OR 5.37; 95% CI 2.03–14.20), and the duration from onset of FN to fever resolution >72 h (OR 5.32; 95% CI 2.22–12.75) (Table S4).
The multivariate logistic regression analysis included factors that demonstrated at least a moderate degree of correlation (p < 0.20) to 30-day mortality. Through a backward elimination method, cardiovascular disease, alteration of consciousness, ICU admission, septic shock, the need for mechanical ventilation, hemoglobin concentration less than 8 g/dL, AKI, bacteremia, and causative pathogen identified were selected as candidate variables for the prediction model (Table 3). The model based on these nine factors gave Akaike's information criterion (AIC) and Bayesian information criterion (BIC) scores of 98.54 and 134.30, respectively. Moreover, as some of these factors are often coincidental in clinical practice (e.g., patients with septic shock are commonly admitted to the ICU), we explored confounding effects between all candidate variables using an all-possible-estimates method (R). This method provides an overall effect estimate of the exposure of interests in the setting of all possible sets of confounders within the model. The analyses suggested that the correlations between some candidate variables, namely bacteremia, cardiovascular diseases, ICU admission, alteration of consciousness, and causative pathogen identified, and 30-day mortality are considerably subjected to their relationships with other covariates (Figure 1).
Table 3.
Multiple logistic regression for predictors of 30-days all-cause mortality
| Variables | Coefficient | SE | p-value | OR (95% CI) |
|---|---|---|---|---|
| Bacteremia | -1.82 | 1.00 | 0.068 | 0.16(0.02-1.14) |
| Pre-existing cardiovascular disease | 3.11 | 1.33 | 0.019* | 22.45(1.66 – 303.49) |
| ICU admission | -2.02 | 1.01 | 0.045* | 0.13(0.02 – 0.95) |
| Septic shock | 2.93 | 0.93 | 0.002* | 18.72(3.04-115.38) |
| Alteration of consciousness on admission | 2.92 | 1.34 | 0.030* | 18.50(1.33-258.18) |
| Causative pathogens identified | 2.16 | 0.92 | 0.019* | 8.68(1.42-52.95) |
| Hemoglobin concentration < 8 g/dL | 1.46 | 0.66 | 0.026* | 4.33(1.20-15.65) |
| Acute kidney injury | 2.58 | 0.68 | <0.001* | 13.15(3.48 – 49.75) |
| Need for mechanical ventilation | 3.12 | 0.78 | <0.001* | 22.65(4.90 – 104.78) |
Abbreviations: SE = standard errors; OR = odds ratios; ICU = intensive care unit
p < 0.05
Figure 1.
Analyses of confounding effects between candidate variables
The candidate variables were selected by a backward elimination method. Confounding effects between all candidate variables were explored using all-possible-estimates method. Odds ratios between each candidate variable and 30-day mortality and the corresponding p-values from likelihood-ratio tests were estimated from all possible set of confounders within the model.
Abbreviations: AOC: alteration of consciousness, AKI: acute kidney injury, BIC: Bayesian information criterion, CVD: cardiovascular disease, Hb: Hemoglobin, ICU: intensive care unit, OR: odds ratio.
In contrast, despite some degree of confounding effects from other variables, septic shock, hemoglobin concentration less than 8 g/dL, AKI, and the need for mechanical ventilation were highly associated with 30-day mortality. The model based on four candidate variables resulted in the comparable AIC score and lower BIC score compared to the model created from factors selected via the backward selection method (AIC = 105.53, BIC = 123.41). Therefore, to make the model less complicated, we chose septic shock, anemia (hemoglobin concentration less than 8 mg/dL), AKI, and the need for mechanical ventilation as factors highly associated with 30-day mortality for the final parsimonious model.
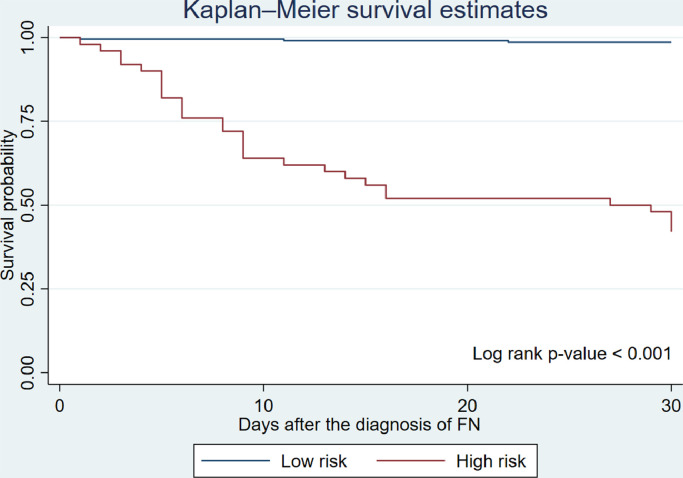
The parsimonious models were tested for model fitness. No influential outlier with effects on the estimation of coefficients was observed upon an examination of the scatter plot between the Pregibon's delta coefficient weighted delta Pearson χ2 statistics and the predicted probability. The model calibration with the Hosmer-Lemeshow χ2 test also demonstrated a good fit with the data (χ2 = 7.72, df = 10, p = 0.17). Using the rounded numbers of the coefficients derived from the parsimonious model, we developed a prognostic prediction score in the form of a point scoring system to stratify FN patients according to their risks for 30-day all-cause mortality (Table 4). The scores from each variable were summed and used to stratify patient risk. The calibration of cut-off thresholds was performed and found that a cut-off point of ≥ 3.5 resulted in the highest sensitivity (87.88%) and specificity (90.91%). The percentages of survivors and non-survivors according to their risk category are shown in Table 5. In addition, our risk stratification system produced good discrimination with the area under the ROC curve (AUC) of 0.8939. Our scoring system performed well during internal validation using data splitting and bootstrap methods. We randomly drew a split sample from the original dataset at a ratio of 50:50. There was no statistically significant difference in AUC between the original dataset and the split sample (p = 0.76). Furthermore, the assessment of model calibration and discrimination was done on 1,000 bootstrapped samples. Our prediction score produced the corrected calibration coefficient of 0.77 and the corrected discrimination coefficient of 0.89. Due to the lack of an additional dataset, external validation was not performed. A Kaplan-Meier survival curve (Figure 2) demonstrated a statistically significantly lower survival rate among the high-risk patients (p-value from a log rank test < 0.001) with a hazard ratio of 8.76 (95% CI 1.49-51.53).
Table 4.
Proposed clinical risk prediction score for a 30-day all-cause mortality in patients with febrile neutropenia.
| Predictors | Score | |
|---|---|---|
| The patient has anemia (hemoglobin concentration < 8 g/dL) at the onset of febrile neutropenia. | Yes | 1.5 |
| No | 0 | |
| The patient develops septic shock during neutropenia. | Yes | 1.5 |
| No | 0 | |
| The patient develops acute kidney injury during neutropenia. | Yes | 2.0 |
| No | 0 | |
| The patient develops respiratory failure requiring mechanical ventilators. | Yes | 2.0 |
| No | 0 | |
*The patient will be classified as high-risk for mortality (score ≥ 3.5) or low-risk for mortality (score < 3.5).
Table 5.
Percentage of survivors and non-survivors according to prediction score risk category
| Prediction score category | Survivors | Non-survivors | p-value |
|---|---|---|---|
| Low risk | 90.91 | 12.12 | <0.001 |
| High risk | 9.09 | 87.88 |
Figure 2.
Kaplan-Meier survival curve demonstrating survival of patients in high- and low-risk group
A Kaplan-Meier survival curve demonstrated a statistically significantly lower survival rate among the high-risk patients (p-value from a log rank test < 0.001).
Discussion
In this present study, we demonstrated that chemotherapy was the common cause of FN, which accounted for approximately 85%. The most common associated comorbidities were non-Hodgkin's lymphoma and acute myeloid leukemia for hematologic disease and lung cancer for solid organ malignancy, which was similar to previous reports (Al-Tawfiq et al., 2019; Auesomwang et al., 2018; Parodi et al., 2019; Roongpoovapatr & Suankratay, 2010). In the FN episodes, infections were identified as either MDI (45%) or CDI (11%), whereas almost half of the patients were categorized as FUO. The results were consistent with the results at King Chulalongkorn Memorial Hospital, which could identify microbiologically defined infection in 48% of all episodes (Roongpoovapatr & Suankratay, 2010).
Gram-negative bacilli constituted approximately 70% of all isolates, while gram-positive organisms constituted 15%. Interestingly, the incidence of fungal infection was 17% which was relatively high compared to previous studies that reported incidences of fungal infection of 6–7% (Auesomwang et al., 2018; Parodi et al., 2019; Roongpoovapatr & Suankratay, 2010). The increased frequency of fungal infection was possibly associated with the aggressive chemotherapy treatment and highly sensitive diagnostic tools in our cohort. Similar to the previous studies, the most common gram-negative pathogen was Enterobacteriaceae spp., including Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa (Auesomwang et al., 2018; Roongpoovapatr & Suankratay, 2010). Although Staphylococcus spp. was previously reported as the most common gram-positive pathogen among previous studies (Auesomwang et al., 2018; Parodi et al., 2019; Roongpoovapatr & Suankratay, 2010), the common gram-positive pathogen in this study was Enterococcus spp. The majority of the published guidelines emphasize the need of Pseudomonas spp. and methicillin-resistant Staphylococcus aureus coverage for empirical treatment of FN; however, in this present study, we demonstrated that the most common gram-positive organism to encounter was Enterococci spp., rather than Staphylococcus spp. Hence, the suggestion that an empirical antibiotic regimen for FN patients in Thailand should focus on antibiotics for enterococci coverage, which may differ from the European and American guidelines. Moreover, we did not find any vancomycin-resistant enterococci; in contrast, there was a report that vancomycin-resistant gram-positive organisms emerged in FN patients (Irfan et al., 2008). The possible reason is that vancomycin was not prescribed as a routine empirical antibiotic for FN in our hospital except for clinically or biologically documented infection.
We demonstrated an overall mortality rate of 12.5%, similar to previous studies which reported mortality rates of 10–20% (Al-Tawfiq et al., 2019; Auesomwang et al., 2018; Parodi et al., 2019; Roongpoovapatr & Suankratay, 2010). From previously published literature, the identified risk factors for mortality were older age, uncontrolled cancer, prior infection, body temperature ≥39°C, hypotension, dehydration, tachycardia, acute respiratory failure, septic shock, pneumonia, bacteremia, low lymphocyte or platelet count, and increased alanine transaminase, C-reactive protein, and procalcitonin levels (Aagaard et al., 2020; Al-Tawfiq et al., 2019; Auesomwang et al., 2018; Calik et al., 2018; García de Guadiana-Romualdo et al., 2019; Kim et al., 2017; Parodi et al., 2019). The only identified protective factor was adequate empirical antibiotics (Calik et al., 2018). In this study, we failed to demonstrate most of the aforementioned mortality predictors reported in the previous studies. Instead, according to our data, pre-existing cardiovascular disease, alteration of consciousness on admission, anemia (hemoglobin concentration less than 8 g/dL), AKI, causative pathogen identified, intensive care unit admission, septic shock, and the need for mechanical ventilation were at higher risk of 30-day all-cause mortality than patients who did not have these risk factors.
We developed a prognostic model based on septic shock, hemoglobin concentration less than 8 g/dL, AKI, and the need for mechanical ventilation which was highly associated with 30-day mortality. The model provided relatively high sensitivity and specificity, which is practical to use. The Multinational Association for Supportive Care in Cancer (MASCC) risk index score has been accepted to assess the risk of complication in patients with FN by the European Society of Medical Oncology and Infectious Disease Society of America. (Klastersky & Paesmans, 2013). Despite low specificity with a cut-off point of 21 in some reports, the score served its purpose as a screening tool for low-risk patients who could be discharged early or managed in outpatient settings (Ahn et al., 2016; Klastersky & Paesmans, 2013; Zheng et al., 2020). In contrast, our study included only patients admitted to the hospitals; thus, we aimed to use it for the inpatient setting to identify those with a high risk of mortality. Therefore, we suggested that patients in the high mortality risk group (score ≥ 3.5) should be closely monitored for clinical deterioration.
Interestingly, AKI, which is one of the modifiable factors in our model, demonstrated a strong correlation with mortality, independent of other notorious unfavorable clinical outcomes (e.g., septic shock, ICU admission, and the need for mechanical ventilation). This finding suggested that kidney insults, such as dehydration, hyperchloremia, intravenous contrast media, and nephrotoxic drugs, play a substantial role in determining patient survival in the context of FN. Efforts to minimize kidney injury in patients with FN may improve clinical outcomes.
The main limitation of this study lies within the nature of the study participants. Firstly, most of the patients recruited into the study were younger than 60 years, and the majority of them did not have underlying diseases other than cancer. Studies involving older patients with significant comorbidities may produce different outcomes. Secondly, the size of the study population was postulated from a predetermined allocation ratio of 1:1; therefore, it might lack sufficient power to identify some significant associations. Thirdly, the number of participants was slightly lower than the sample size calculation, leading to a marginally underpowered study. Finally, since we did not have an additional dataset, external validation could not be performed.
Conclusion
In patients undergoing chemotherapy with FN, septic shock, anemia, AKI, and the need for mechanical ventilation were associated with 30-day all-cause mortality. In this study, we developed a practical scoring system that can identify FN patients with an increased risk of 30-day all-cause mortality; however, further external validation with an additional dataset should be further investigated.
Conflict of interest
The authors have no conflict of interest to declare. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
Acknowledgements
We would like to thank Dr. Chutima Sereeaphinan for the data analysis.
Funding source
This study was supported by the Faculty of Medicine, Prince of Songkla University.
Ethical approval
The study was approved by the Human Research Ethics Committee of the Faculty of Medicine, Prince of Songkla University, Thailand (REC. 63-207-14-1).
Footnotes
Funding: This work was supported by the Faculty of Medicine, Prince of Songkla University
Conflicts of Interest: All authors declare that they have no conflict of interest.
Reprint requests: Jakrawadee Julamanee
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2021.09.002.
Appendix. Supplementary materials
References
- Aagaard T., Reekie J., Jørgensen M., Roen A., Daugaard G., Specht L.…Helleberg M. Mortality and admission to intensive care units after febrile neutropenia in patients with cancer. Cancer Med. 2020;9(9):3033–3042. doi: 10.1002/cam4.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S., Lee Y.S., Lee J.L., Lim K.S., Yoon S.C. A new prognostic model for chemotherapy-induced febrile neutropenia. Int J Clin Oncol. 2016;21(1):46–52. doi: 10.1007/s10147-015-0853-0. [DOI] [PubMed] [Google Scholar]
- Al-Tawfiq J.A., Hinedi K., Khairallah H., Saadeh B., Abbasi S., Noureen M.…Alkhatti A. Epidemiology and source of infection in patients with febrile neutropenia: A ten-year longitudinal study. J Infect Public Health. 2019;12(3):364–366. doi: 10.1016/j.jiph.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Auesomwang C., Suwannawiboon B., Chayakulkeeree M. Changes in etiologic microorganisms in Thai patients with chemotherapy-induced neutropenia and fever. J Med Assoc Thai. 2018;101:173–180. [Google Scholar]
- Calik S., Ari A., Bilgir O., Cetintepe T., Yis R., Sonmez U., Tosun S. The relationship between mortality and microbiological parameters in febrile neutropenic patients with hematological malignancies. Saudi Med J. 2018;39(9):878–885. doi: 10.15537/smj.2018.9.22824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayakulkeeree M., Thamlikitkul V. Risk index for predicting complications and prognosis in Thai patients with neutropenia and fever. J Med Assoc Thai. 2003;86(3):212–223. [PubMed] [Google Scholar]
- Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E.…Pappas P.G. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifeld A.G., Bow E.J., Sepkowitz K.A., Boeckh M.J., Ito J.I., Mullen C.A.…Americaa I.D.S.O. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):427–431. doi: 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]
- García de Guadiana-Romualdo L., Cerezuela-Fuentes P., Español-Morales I., Esteban-Torrella P., Jiménez-Santos E., Hernando-Holgado A., Albaladejo-Otón M.D. Prognostic value of procalcitonin and lipopolysaccharide binding protein in cancer patients with chemotherapy-associated febrile neutropenia presenting to an emergency department. Biochem Med (Zagreb) 2019;29(1) doi: 10.11613/BM.2019.010702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inweregbu K., Dave J., Pittard A. Nosocomial infections. Contin Educ Anaesth Crit Care Pain. 2005;5(1):14–17. [Google Scholar]
- Irfan S., Idrees F., Mehraj V., Habib F., Adil S., Hasan R. Emergence of Carbapenem resistant Gram negative and vancomycin resistant Gram positive organisms in bacteremic isolates of febrile neutropenic patients: A descriptive study. BMC Infectious Diseases. 2008;8(1):80. doi: 10.1186/1471-2334-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Ahn S., Kim W.Y., Sohn C.H., Seo D.W., Lee Y.S., Lim K.S. Predictive performance of the quick Sequential Organ Failure Assessment score as a screening tool for sepsis, mortality, and intensive care unit admission in patients with febrile neutropenia. Support Care Cancer. 2017;25(5):1557–1562. doi: 10.1007/s00520-016-3567-6. [DOI] [PubMed] [Google Scholar]
- Klastersky J., Paesmans M. The Multinational Association for Supportive Care in Cancer (MASCC) risk index score: 10 years of use for identifying low-risk febrile neutropenic cancer patients. Support Care Cancer. 2013;21(5):1487–1495. doi: 10.1007/s00520-013-1758-y. [DOI] [PubMed] [Google Scholar]
- Leelayuthachai T., N K. Febrile neutropenia in post-chemotherapeutic patients in medicine department, Thammasat University Hospital. J Hematol Transfus Med. 2010;20(3):197–204. [Google Scholar]
- Lueangarun S., Leelarasamee A. Impact of inappropriate empiric antimicrobial therapy on mortality of septic patients with bacteremia: a retrospective study. Interdiscip Perspect Infect Dis. 2012;2012 doi: 10.1155/2012/765205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi R.L., Lagrutta M., Tortolo M., Navall E., Rodríguez M.S., Sasia G.F.…Greca A.A. A multicenter prospective study of 515 febrile neutropenia episodes in Argentina during a 5-year period. PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0224299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roongpoovapatr P., Suankratay C. Causative pathogens of fever in neutropenic patients at King Chulalongkorn Memorial Hospital. J Med Assoc Thai. 2010;93(7):776–783. [PubMed] [Google Scholar]
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M.…Angus D.C. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerasubpong B., Makruasi N., Linasmita P., Rattanamongkolgul S. Factors Associated with Survival Outcomes of Febrile Neutropenia in Hematologic Malignancy Patients. J Med Assoc Thai. 2016;99(Suppl 8):S53–s62. [PubMed] [Google Scholar]
- Zhang Y., Zheng Y., Dong F., Ma H., Zhu L., Shi D.…Hu J. Epidemiology of Febrile Neutropenia Episodes with Gram-Negative Bacteria Infection in Patients Who Have Undergone Chemotherapy for Hematologic Malignancies: A Retrospective Study of 10 Years' Data from a Single Center. Infect Drug Resist. 2020;13:903–910. doi: 10.2147/IDR.S241263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B., Toarta C., Cheng W., Taljaard M., Reaume N., Perry J.J. Accuracy of the Multinational Association of Supportive Care in Cancer (MASCC) and Clinical Index of Stable Febrile Neutropenia (CISNE) scores for predicting serious complications in adult patients with febrile neutropenia: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2020;149 doi: 10.1016/j.critrevonc.2020.102922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.