Highlights
-
•
DM patients have a high TB risk, and TB patients can develop DM during their treatment.
-
•
Around 4.14% of DM patients in Ethiopia have TB.
-
•
Around 12.7% of TB patients in Ethiopia develop DM.
-
•
Type 1 DM patients have a higher TB risk.
-
•
Old age and a family history of DM are risk factors for DM in TB patients.
Keywords: tuberculosis, diabetes, Ethiopia, co-occurrence, risk factors
Abbreviations: BMI: body mass index, DM: diabetes mellitus, EPTB: extrapulmonary tuberculosis, HIV: human immunodeficiency virus, IFG: impaired fasting glucose, MDR-TB: multidrug-resistant tuberculosis, PTB: pulmonary tuberculosis; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analyses, OR: odds ratio, TB: tuberculosis, WHO: World Health Organization
Abstract
Objective
The aim of our study was to estimate the pooled prevalence of tuberculosis (TB) and diabetes mellitus (DM) co-occurrences, and associated risk factors, in Ethiopia.
Methods
In total, 392 articles were identified, and 14 were included in the analysis. The study's quality was assessed using the JBI tool. Data were analyzed using STATA version 16. The pooled prevalences of TB among DM patients and of DM among TB patients, with 95% CI, were estimated. In addition, the pooled OR values for each risk factor were estimated. The study's heterogeneity was assessed using forest plots and the I2 heterogeneity test. Publication bias was assessed using funnel plots and Egger's regression test.
Results
The pooled prevalence of TB among DM patients was 4.14% (95% CI 2.45–5.83%). The pooled prevalence of DM among TB patients was 12.77% (95% CI 6.91–18.62%). Type 1 DM patients had a higher TB risk (OR 2.70, 95% CI 1.41–3.99). Older age (OR 2.25, 95% CI 1.38–3.13) and family DM history (OR 3.65, 95% CI 1.89–5.41) were associated with DM co-occurrence among TB patients.
Conclusions
Higher prevalences of TB among DM patients and of DM among TB patients were noted in Ethiopia, when compared with the general population. Thus, active screening of DM patients for TB and vice versa is recommended.
Introduction
Even though one quarter of the global population is estimated to have been infected with Mtb, only 5–10% have a lifetime risk of falling ill with tuberculosis (TB). The risk of TB is higher among immune-compromised individuals and those with certain underlying diseases (Walker et al., 2013). Those with compromised immune systems, such as people living with HIV, malnutrition, or diabetes (DM), or smokers, have a higher risk of falling ill with TB (WHO, 2020). Many new cases of TB are attributable to five risk factors: undernutrition, HIV infection, alcohol use, smoking, and DM (WHO, 2020). In 2019, an estimated 2.2, 0.76, 0.72, 0.70, and 0.35 million TB cases were attributable to undernutrition, HIV infection, alcohol use disorders, smoking, and DM, respectively (WHO, 2020).
Aside from the traditional risk factors, DM is increasingly being recognized as an independent risk factor for TB, and the two often coexist (Yorke et al., 2017). The interaction between DM and TB is a major public health concern because of the rapidly rising levels of DM. DM increases the risk of TB infection by two to three times (Harries et al., 2013). Immune mechanisms contributing to the increased susceptibility of DM patients to TB are based on defects in bacterial recognition, phagocytic activity, and cellular activation that results in impaired production of chemokines and cytokines (Ayelign et al., 2019). The relationship between TB and DM is bidirectional, whereby TB patients also develop new DM cases during their treatment (Niazi & Kalra, 2012)Niazi & Kalra, 2012). The World Health Organization (WHO) recommends three intervention strategies: establishing mechanisms of collaboration between TB and DM control programs; early detection and management of TB in patients with DM; and early detection and management of DM in TB patients (WHO, 2015).
TB is a major public health problem in Ethiopia, with an incidence of 140/100 000 population. Ethiopia is included under high TB-, TB/HIV-, and MDR-TB-burden countries across the globe (WHO, 2020). In 2018, 114 233 TB cases were notified in the country, with a case/fatality ratio of 17% (9–25%) (WHO, 2019). Moreover, the burden of non-communicable diseases, including DM, is increasing in Ethiopia, with 3.2% in adults (IDF, 2019), ranging from 2.0% to 6.5% (Bishua et al., 2019).
Diabetic patients are susceptible to TB. Individual studies conducted in Ethiopia have also confirmed this. A TB prevalence among DM patients of more than 5.0% has been reported in Ethiopia (Gedfew et al., 2020; Abera & Ameya, 2018). Studies have also reported that DM is a common phenomenon among TB patients in Ethiopia, with a prevalence of up to 15.8% (Damtew et al., 2014). However, the findings of individual studies are inconclusive, and there is a scarcity of updated data on the status of TB and DM co-occurrences in the country. Thus, our study aimed to assess the burden of TB and DM co-occurrences, and associated risk factors, in Ethiopia.
Methods
Search strategy
Systematic article searching was conducted using electronic databases (PubMed, CINAHL, DOAJ, African Index Medicus) and other gray literature sources (Google, Google Scholar, WorldCat). This was performed independently by two authors (AA and GD) under the consultation of a senior librarian at the Ethiopian Public Health Institute. Inconsistencies were resolved by a third author (ZWB). The keywords used for searching included tuberculosis, diabetes, co-occurrences, risk factors, associated factors, determinants, predictors, and Ethiopia. These keywords were used in conjunction with the Boolean operators AND and OR. The search string for the PubMed database was ((((”Tuberculosis” [Mesh]) OR (TB)) AND (“Diabetes Mellitus” [Mesh] OR “Diabetes Mellitus, Type 1” [Mesh])) OR (DM)) AND (“Ethiopia” [Mesh]) (Additional file 1).
Study selection procedure
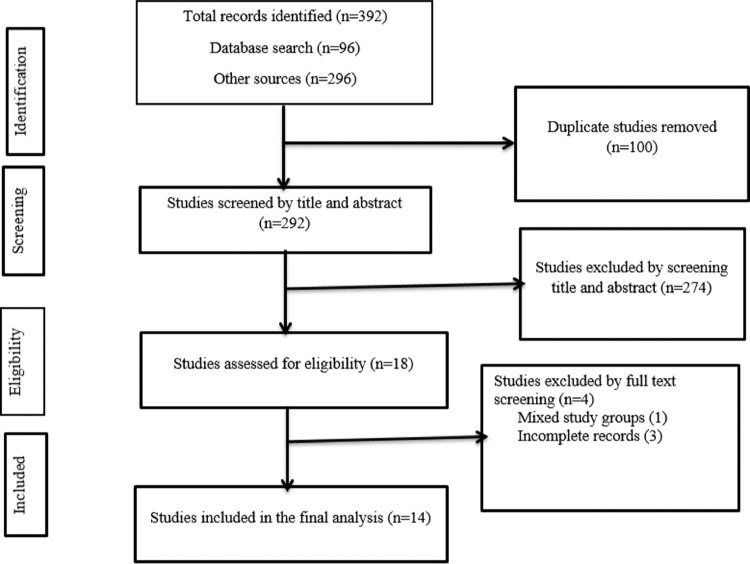
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting checklist (Hutton et al., 2015; Knobloch et al., 2011) (Additional file 2). A stepwise approach was followed to select the eligible articles included in the final analysis. The article selection procedure was conducted independently by two authors (AA and GD) using predefined inclusion criteria, and the inconsistencies were resolved by a third author (ZWB). Initially, all the identified articles (n = 392) were exported to the EndNote X8 citation manager, and 100 duplicates were removed. Next, 292 articles were screened by title and abstract. Around 18 articles that passed the first stage were assessed through a full-text review. During this review, the study subjects, study design, study quality, and outcome were considered. Finally, 14 articles became eligible for data extraction. During article eligibility assessment, the PICOS (Population, Intervention, Comparison, Outcome, Study design, Study setting) criteria were assessed (Figure 1).
Figure 1.
Flowchart describing the selection of studies for the systematic review and meta-analysis of TB and DM co-occurrences in Ethiopia
PICOS criteria
Participants: TB/DM patients
Intervention: Not applicable
Comparator: DM patients without TB/TB patients without DM
Outcome: TB among DM patients/DM among TB patients
Study design: Observational studies
Study setting: Any setting across Ethiopia
Inclusion and exclusion criteria
Articles that reported TB prevalence among DM patients, or DM co-occurrence among TB patients, or articles that reported associated factors for TB and DM co-occurrences in Ethiopia, and that were published in the English language were included. Articles without full text, or that did not separately report the prevalence for each group, or that were not objectively designed to assess TB prevalence among DM patients or DM prevalence among TB patients were excluded.
Data extraction
Data were extracted independently by two authors (AA and GD), and the inconsistencies were resolved by a third author (ZWB). The extracted data included study characteristics, such as author, publication year, regional state, study setting, data collection period, study participants’ age range, sample size, number of DM patients with TB, and number of TB patients with DM. Demographic and behavioral data, and clinical factors for TB and DM co-occurrences, were also extracted. The data were summarized using Microsoft Excel 2016 spreadsheets (Tables 1 and 2).
Table 1.
Characteristics of the individual studies on tuberculosis infection among diabetes patients in Ethiopia included in the current systematic review and meta-analysis
| Authors, year | Study region | Study setting | Study period | Study design | Study age group | DM patients |
DM type |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total number | TB infected | Type of TB | Type 1 |
Type 2 |
||||||||
| Number | TB infected | Number | TB infected | |||||||||
| Abera & Ameya, 2018 | SNNP | Hawassa Adare Hospital | Mar to May, 2015 | Cross-sectional | 17–95 | 207 | 11 | All PTB | – | – | – | – |
| Amare et al., 2013 | Amhara | Dessie Referral Hospital | Feb to Apr, 2012 | Cross-sectional | 12–82 | 225 | 14 | All PTB | 40 | 3 | 185 | 11 |
| Andualem & Malede, 2021 | Amhara | Debre Tabor General Hospital | Mar to May, 2019 | Cross-sectional | 16–71 | 258 | 7 | All PTB | – | – | – | – |
| Feleke et al., 1999 | Addis Ababa | Tikur Anbessa Specialized Teaching Hospital | Sep 1989 to 1996 | Cross-sectional | – | 1352 | 78 | PTB = 56 EPTB = 8 Disseminated TB = 7 |
619 | 54 | 733 | 17 |
| Gedfew et al., 2020 | Amhara | Debre Markos Referral Hospital | Jan 2013 to Dec 2017 |
Retrospective cohort | 18–79 | 433 | 26 |
PTB = 20 EPTB = 6 |
224 | 19 | 209 | 7 |
| Jerene et al., 2017 | Amhara and Oromia | Bishoftu, Shashemene, Debrebirhan, Debretabor Hospitals | Feb to June, 2015 | Cross-sectional | – | 888 | 6 | – | – | – | – | – |
| Tiroro et al., 2015 | Addis Ababa | Tikur Anbessa Specialized Teaching Hospital | Jan 2010 to Jan 2014 | Cross-sectional | 18–88 | 681 | 26 | PTB = 24 EPTB = 2 |
121 | 10 | 551 | 16 |
SNNP: Southern Nations Nationalities and Peoples, DM: diabetes mellitus, TB: tuberculosis, PTB: pulmonary tuberculosis, EPTB: extrapulmonary tuberculosis
Table 2.
Characteristics of individual studies on diabetes mellitus among tuberculosis patients in Ethiopia included in the current systematic review and meta-analysis
| Author, year | Study region | Place/setting | Study period | Study design | Study age group | TB patients |
Type of TB |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total number | Had DM | Had IFG | PTB |
EPTB |
||||||||
| Number | Had DM | Number | Had DM | |||||||||
| Ashebir, 2015 | Addis Ababa | St Paul Millennium Medical College | June 2014 to Feb 2015 | Cross-sectional | - | 205 | 17 | 53 | 205 | 17 | – | – |
| Damtew et al., 2014 | Addis Ababa | St. Peter Specialized Hospital | Feb to May 2014. | Cross-sectional | 15–86 |
120 | 19 | 32 | 120 | 19 | – | – |
| Getachew et al., 2013 | Amhara | Gondar University Hospital | Oct 2011 to Nov 2012 | Cross-sectional | 14–80 | 199 | 17 | 59 | 199 | 17 | – | – |
| Gezahegn et al., 2020 | Oromia | Bale Zone Health Institutions | Mar 30 to Apr 30, 2019 | Cross-sectional | All | 316 | 16 | – | 233 | 7 | 83 | 9 |
| Jerene et al., 2017 | Amhara and Oromia | Bishoftu, Shashemene, Debrebirhan, Debretabor hospitals | Feb to June 2015 | Cross-sectional | – | 439 | 141 | – | – | – | – | – |
| Tenaye et al., 2019 | Dire Dawa | 25 public and private health facilities in Dire Dawa | Mar 10 to Apr 15, 2017 | Cross-sectional | ≥18 | 421 | 57 | 125 | 338 | 48 | 83 | 9 |
| Workneh et al., 2016 | Amhara | Health facilities in South-Eastern Amhara | Sep 2013 to Sep 2014 | Cross-sectional | 15–89 | 1314 | 109 | 139 | 770 | 70 | 544 | 39 |
| Tulu et al., 2021 | Amhara | Felege Hiwot and Debre Tabor Hospitals | Feb 1 to Jun 30, 2017 | Cross-sectional | 18–62 | 269 | 31 | 67 | 104 | 14 | 165 | 17 |
DM: diabetes mellitus, TB: tuberculosis, PTB: pulmonary tuberculosis, EPTB: extrapulmonary tuberculosis
Risk of bias (quality) assessment of studies
The quality of individual studies was assessed using the Joanna Briggs Institute (JBI) critical appraisal checklist for prevalence studies and cohort studies (Moola et al., 2020). The questions in the checklist were equally scored and then totalled to give a final score out of 100%. The quality score was graded as low if < 60%, medium if 60–80% and high if > 80% (Porritt et al., 2014; Munn et al., 2019) (Additional File 3). Two authors (AA and GD) independently assessed the quality of the studies and the inconsistencies were resolved by a third author (ZWB). The symmetry of the funnel plots was assessed visually, and Egger's regression test was performed to assess publication bias.
Outcomes
The presence of TB and DM co-occurrences was the primary outcome. This was assessed by estimating the pooled prevalence of TB among DM patients and the pooled prevalence of DM among TB patients. The associated factors contributing to the presence of TB and DM co-occurrences formed the secondary outcome. The pooled odds ratio for each factor was estimated.
Operational definitions
For this study, diabetes was operationalized as having a fasting blood glucose level of 126 mg/dl or more, while prediabetes/impaired fasting blood glucose was operationalized as having a fasting blood glucose level of 100–125 mg/dl. Older age was taken as the higher age group in the primary studies; in the majority of the studies this was over 45 years.
Data synthesis and statistical analysis
The data summarized in Microsoft Excel 2016 were exported to STATA version 2016 for statistical analysis. The pooled proportion of DM patients infected with TB and the pooled proportion of TB patients who had DM were estimated, along with 95% CIs. To assess the associated factors for TB and DM co-occurrences, the pooled ORs along with 95% CIs were estimated. The pooled estimates were presented as forest plots. The forest plots and I2 heterogeneity tests were examined to assess heterogeneity among the studies. I2 values of 25%, 50%, and 75% were interpreted as the presence of the low, medium, and high heterogeneity, respectively (Sterne & Egger, 2001; Riley et al., 2011). In addition, the presence of publication bias was assessed through visual inspection of the funnel plots and Egger's regression test. Asymmetry of the funnel plots and statistical significance of Egger's regression test (p < 0.05) were considered to represent the presence of publication bias.
Results
Study characteristics of included studies
After systematic searching, 14 studies (Gedfew et al., 2020; Abera & Ameya, 2018; Damtew et al., 2014; Amare et al., 2013; Andualem & Malede, 2021; Feleke et al., 1999; Jerene et al., 2017; Tiroro et al., 2015; Ashebir, 2018; Getachew et al., 2014; Gezahegn et al., 2020; Tenaye et al., 2019; Workneh et al., 2016; Tulu et al., 2021) were included in the final analysis (Figure 1). Six studies (Gedfew et al., 2020; Abera & Ameya, 2018; Amare et al., 2013; Andualem & Malede, 2021; Feleke et al., 1999; Tiroro et al., 2015) reported on the prevalence of TB among DM patients, while the other seven studies (Damtew et al., 2014; Ashebir, 2018; Getachew et al., 2014; Gezahegn et al., 2020; Tenaye et al., 2019; Workneh et al., 2016; Tulu et al., 2021) were conducted to determine the prevalence of DM among TB patients. The one remaining study (Jerene et al., 2017) assessed both TB prevalence among DM patients and DM prevalence among TB patients. The studies that determined the prevalence of TB among DM patients were reported from Amhara (four studies), Addis Ababa (two studies), Oromia (one study), and SNNP (one study) regions. All of these studies were hospital-based. The study period for these studies ranged from 1989 (Feleke et al., 1999) to 2019 (Andualem & Malede, 2021). Six out of seven studies were conducted after January 2010 (Gedfew et al., 2020; Abera & Ameya, 2018; Damtew et al., 2014, Amare et al., 2013; Andualem & Malede, 2021; Jerene et al., 2017; Tiroro et al., 2015). Most (6/7) of the studies were cross-sectional (Abera & Ameya, 2018; Damtew et al., 2014, Amare et al., 2013; Andualem & Malede, 2021; Feleke et al., 1999; Jerene et al., 2017; Tiroro et al., 2015), while the other used a retrospective cohort study design (Gedfew et al., 2020). The age of DM patients ranged from 12 years (Amare et al., 2013) to 95 years (Abera & Ameya, 2018). The studies that assessed DM co-occurrence among TB patients were reported from Amhara (four studies), Oromia (two studies), Addis Ababa (two studies), and Dire Dawa (one study) regions. All these studies were conducted in healthcare facilities. The study period for these studies ranged from October 2011 (Getachew et al., 2104) to April 2019 (Gezahegn et al., 2020), and all the studies were cross-sectional (Tables 1 and 2).
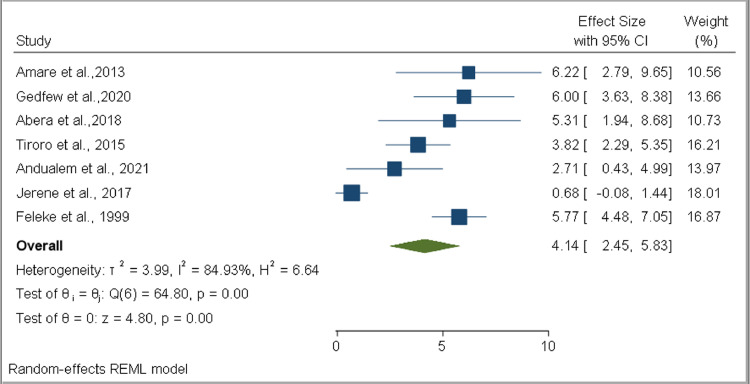
Tuberculosis among diabetes mellitus patients
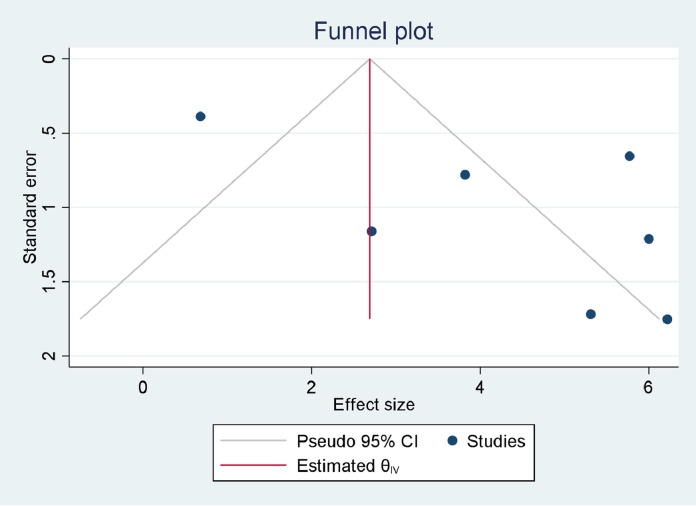
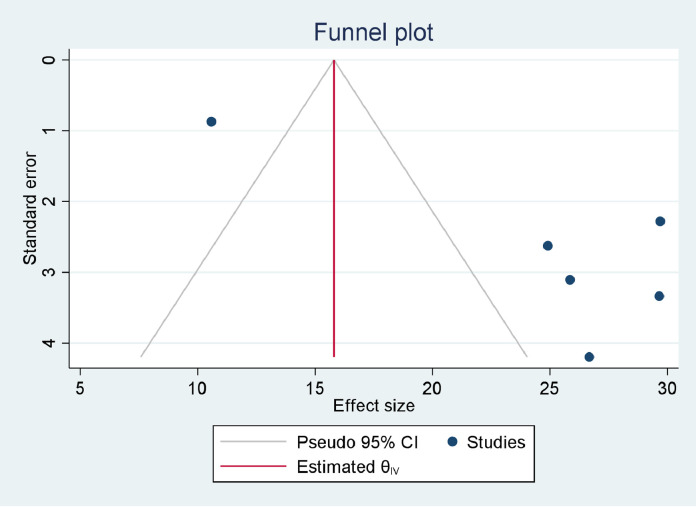
Estimates of the pooled prevalence of TB among DM patients were based on seven studies. The sample size ranged from 207 (Abera & Ameya, 2018) to 1352 (Feleke et al., 1999). Three studies (Abera & Ameya, 2018; Amare et al., 2013; Andualem & Malede, 2021) assessed the prevalence of pulmonary TB (PTB), while the other three studies assessed both PTB and extrapulmonary TB (EPTB) (Gedfew et al., 2020; Feleke et al., 1999; Tiroro et al., 2015). However, one study did not specify whether its was assessing PTB, EPTB, or both types (Jerene et al., 2017). Four studies reported TB prevalence based on DM type (Gedfew et al., 2020; Amare et al., 2013; Feleke et al., 1999; Tiroro et al., 2015). The highest prevalence was reported as 6.2% (Amare et al., 2013), while the smallest reported prevalence was 0.68% (Jerene et al., 2017). However, four of the seven studies reported prevalences of ober 5% (Gedfew et al., 2020; Abera & Ameya, 2018; Amare et al., 2013; Feleke et al., 1999). Estimates of pooled prevalences of TB were based on data collected from 4044 DM patients, of whom 168 developed TB. Based on the random effect model, the pooled prevalence of TB among DM patients was estimated as 4.14% (95% CI 2.45–5.83%, I2 = 84.93%) (Figure 2). Publication bias was not detected (p = 0.1090) (Figure 3). The pooled prevalences of TB among type 1 DM and type 2 DM patients were estimated as 8.56% (95% CI 6.74–10.38%, I2 = 0.00%) and 2.80% (95% CI 1.93–3.66%, I2 = 0.00%), respectively. Publication bias was not detected in either group (p = 0.76687 and p = 0.0836, respectively). The pooled prevalences of PTB and EPTB among DM patients, based on the available articles that specifically reported the site of TB, were estimated as 4.05% (95% CI 3.31–4.78%, I2 = 0.00%) and 0.53% (95% CI 0.19–0.88%, I2 = 0.00%), respectively. Publication bias was not detected in either group (p = 0.3895 and p = 0.2453, respectively) (Supplementary figure 1).
Figure 2.
Forest plot for the pooled prevalence of tuberculosis in patients with diabetes mellitus in Ethiopia
Figure 3.
Funnel plot for the pooled prevalence of tuberculosis in patients with diabetes mellitus in Ethiopia
Prevalence of DM among TB patients
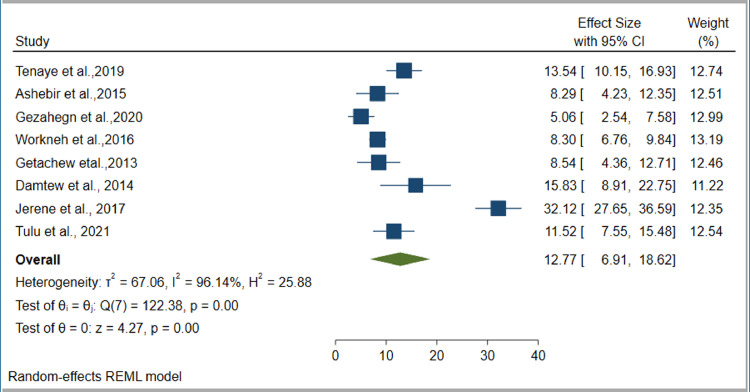
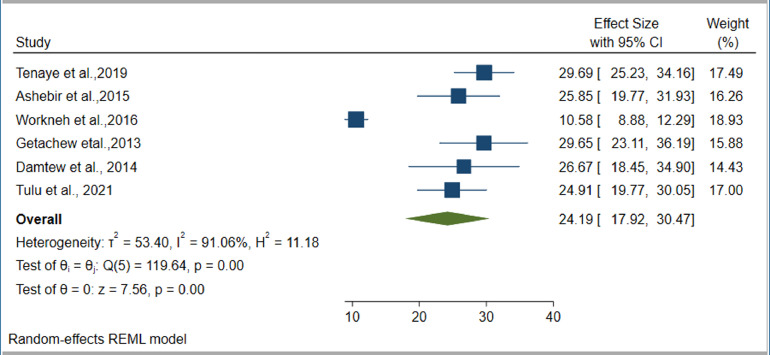
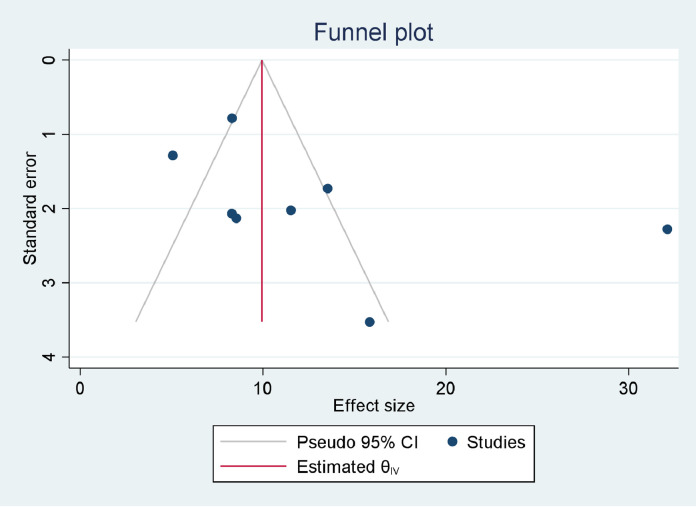
Our estimate of the pooled prevalence of DM co-occurrence among TB patients was based on eight individual studies. The sample sizes ranged from 120 (Damtew et al., 2014) to 1314 (Workneh et al., 2016). Six studies (Damtew et al., 2014; Ashebir, 2018; Getachew et al., 2014; Tenaye et al., 2019; Workneh et al., 2016; Tulu et al., 2021) reported the prevalence of IFG among TB patients. The prevalence of DM per type of TB was reported by four studies (Gezahegn et al., 2020; Tenaye et al., 2019; Workneh et al., 2016; Tulu et al., 2021). The highest prevalence of DM was reported as 32.4% (Jerene et al., 2017), while the smallest reported prevalence was 5.1% (Gezahegn et al., 2020). However, seven out of eight studies (Damtew et al., 2014; Jerene et al., 2017; Ashebir, 2018; Getachew et al., 2014; Tenaye et al., 2019; Workneh et al., 2016; Tulu et al., 2021) reported DM prevalences of over 8%. The pooled prevalence of DM among TB patients was estimated based on data collected from 3293 TB patients, of whom 407 had DM. The pooled prevalence of IFG among TB patients was estimated by data collected from 2528 TB patients, of whom 475 had IFG. Based on the random effect model, the pooled prevalence of DM and IFG among TB patients was estimated as 12.77% (95% CI 6.91–18.62%, I2 = 96.14%) (Figure 4) and 24.19% (95% CI 17.92–30.41%, I2 = 91.06%), respectively (Figure 5). Publication bias was not detected for DM (p = 0.2440) (Figure 6), but was detected for IFG (p = 0.0110) (Figure 7). Specific to the site of TB, the pooled prevalences of DM among PTB and EPTB patients were estimated as 9.79% (95% CI 6.49–13.09%, I2 = 81.88%) and 9.92% (95% CI 8.01–11.82%, I2 = 0.00%), respectively. Publication bias was detected among the PTB cases (p = 0.0456) but not among the EPTB cases (p = 0.6554) (Supplementary figure 1).
Figure 4.
Forest plot for the pooled prevalence of diabetes mellitus co-occurrence among tuberculosis patients in Ethiopia
Figure 5.
Forest plot for the pooled prevalence of impaired fasting glucose among tuberculosis patients in Ethiopia
Figure 6.
Funnel plot for the pooled prevalence of diabetes mellitus co-occurrence among tuberculosis patients in Ethiopia
Figure 7.
Funnel plot for the pooled prevalence of impaired fasting glucose among tuberculosis patients in Ethiopia
Risk factors for tuberculosis and diabetes mellitus co-occurrences
The associated risk factors for developing TB among DM patients and DM co-occurrence among TB patients were estimated using the available studies conducted so far in Ethiopia. The pooled OR was estimated for the 13 variables associated with developing TB among DM patients, while the pooled OR was estimated for the 12 variables associated with DM co-occurrence among TB patients. These variables were chosen based on their appearance in the primary studies. The variables included socio-demographic, behavioral, and clinical characteristics.
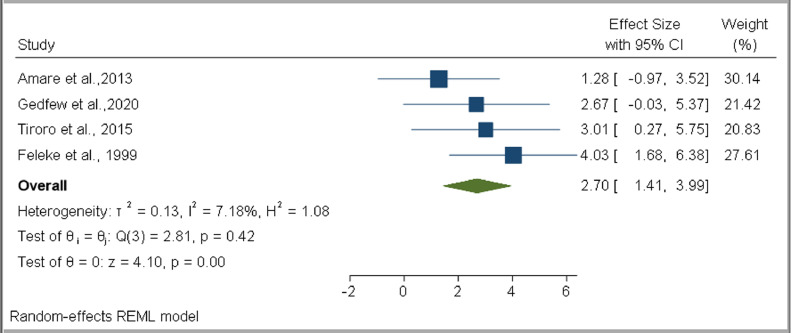
Based on the pooled estimates, only the type of DM was associated with developing TB among DM patients (OR 2.70, 95% CI 1.41–3.99, I2 = 7.18%) (Figure 8). No statistically significant association was found for male sex (OR 1.38, 95% CI 0.56–2.20, I2 = 0.00%), urban residence (OR 1.01, 95% CI 0.22–1.81, I2 = 0.00%), smoking (OR 5.63, 95% CI −2.79 to 14.06, I2 = 2.08%), alcohol consumption (OR 6.95, 95% CI 0.79–13.10, I2 = 0.00%), HIV (OR 1.42, 95% CI −0.19–3.03, I2 = 0.00%), previous TB history (OR 10.64, 95% CI −1.28 to 22.56, I2 = 8.56%), DM duration more than 10 years (OR 7.21, 95% CI −1.35 to 15.76, I2 = 0.00%), BMI < 18.5 kg/m2 (OR 2.57, 95% CI −1.18 to 6.32, I2 = 43.09%), family DM history (OR 1.42, 95% CI –0.19 to 3.03, I2 = 0.00%), close contact with a known TB patient (OR 5.73, 95% CI 0.32–11.13, I2 = 0.00%), insulin medication (OR 2.11, 95% CI 0.71–3.52, I2 = 0.00%), and poor glycemic control (OR 1.51, 95% CI 0.37–2.66, I2 = 0.00%) (Table 3 and Supplementary figure 2).
Figure 8.
Forest plot for the association of type 1 DM with TB infection among diabetes mellitus patients in Ethiopia
Table 3.
Summary of pooled estimates of OR values for associated factors for TB infection among DM patients in Ethiopia
| Variable | Odds ratio |
|||
|---|---|---|---|---|
| Number of studies | Estimate (95% CI) | Heterogeneity |
||
| I2 | p-value | |||
| Male sex | 5 | 1.38 (0.56, 2.20) | 0.00% | 0.81 |
| Urban residence | 3 | 1.01 (0.22, 1.81) | 0.00% | 0.76 |
| Previous TB history | 3 | 10.64 (−1.28, 22.56) | 8.56% | 0.46 |
| DM duration more than 10 years compared with < 5 years | 4 | 7.21 (−1.35, 15.76) | 0.00% | 0.96 |
| Type 1 DM | 4 | 2.70 (1.41, 3.99) | 7.18% | 0.42 |
| BMI < 18.5 kg/m2 | 4 | 2.57 (−1.18, 6.32) | 43.09% | 0.17 |
| HIV seropositive | 4 | 1.42 (−0.19, 3.03) | 0.00% | 0.65 |
| Family history of DM | 3 | 1.42 (−0.19, 3.03) | 0.00% | 0.66 |
| History of close contact with a known TB patient | 5 | 5.73 (0.32, 11.13) | 0.00% | 0.76 |
| Insulin medication | 3 | 2.11 (0.71, 3.52) | 0.00% | 0.81 |
| Smoking | 4 | 5.63 (−2.79, 14.06) | 2.08% | 0.39 |
| Alcohol | 3 | 6.95 (0.79, 13.10) | 0.00% | 0.74 |
| Poor glycemic control | 4 | 1.51 (0.37, 2.66) | 0.00% | 0.93 |
DM: diabetes mellitus, TB: tuberculosis, BMI: body mass index, HIV: human immunodeficiency virus
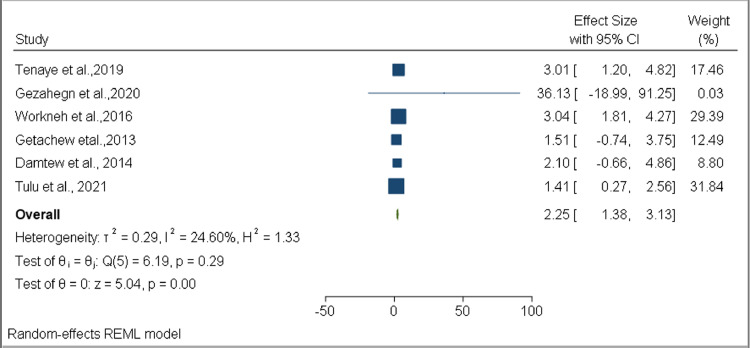
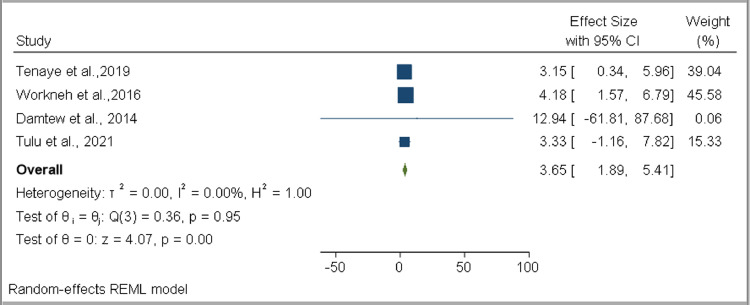
Among the variables assessed to determine the factors associated with DM co-occurrence among TB patients, two were found to have a statistically significant association: older age (OR 2.25, 95% CI 1.38–3.13, I2 = 24.60%) (Figure 9) and family history of DM (OR 3.65, 95% CI 1.89–5.41, I2 = 0.00%) (Figure 10). No statistically significant associations were found for female sex (OR 0.92, 95% CI 0.40–1.43, I2 = 79.83%), married (OR 1.39, 95% CI 0.60–2.18, I2 = 48.11%), urban residence (OR 0.68, 95% CI 0.38–0.97, I2 = 42.76%), smoking (OR 0.79, 95% CI 0.25–1.34, I2 = 0.00%), alcohol consumption (OR 0.82, 95% CI 0.49–1.14, I2 = 1.62%), khat consumption (OR 1.11, 95% CI 0.64–1.58, I2 = 30.99%), HIV (OR 0.80, 95% CI 0.14–1.47, I2 = 72.92%), overweight (OR 2.27, 95% CI 0.59–3.96, I2 = 0.00%), and smear-positive TB (OR 0.79, 95% CI 0.19–1.40, I2 = 56.02%) (Table 4 and Supplementary figure 3).
Figure 9.
Forest plot for the association of older age with developing DM comorbidity among TB DM patients in Ethiopia
Figure 10.
Forest plot for the association of family history of DM with developing DM-comorbidity among TB DM patients in Ethiopia
Table 4.
Summary of pooled estimates of OR for associated factors for diabetes mellitus co-occurrence among tuberculosis patients in Ethiopia
| Variable | Odds ratio |
|||
|---|---|---|---|---|
| Number of studies | Estimate (95% CI) | Heterogeneity |
||
| I2 | p-value | |||
| Female sex | 7 | 0.92 (0.40, 1.43) | 79.83% | < 0.001 |
| Older age | 6 | 2.25 (1.38, 3.13) | 24.60% | 0.29 |
| Married | 5 | 1.39 (0.60, 2.18) | 48.11% | 0.11 |
| Family history of DM | 4 | 3.65 (1.89, 5.41) | 0.00% | 0.95 |
| BMI greater than 25 | 2 | 2.27 (0.59, 3.96) | 0.00% | 0.62 |
| EPTB | 4 | 0.77 (0.51, 1.03) | 0.00% | 0.63 |
| Khat chewing | 2 | 1.11 (0.64, 1.58) | 30.99% | 0.23 |
| Smoking | 4 | 0.79 (0.25, 1.34) | 0.00% | 0.69 |
| Urban setting | 7 | 0.68 (0.38, 0.97) | 42.76% | 0.19 |
| HIV seropositive | 4 | 0.80 (0.14, 1.47) | 71.92% | 0.01 |
| Alcohol | 3 | 0.82 (0.49, 1.14) | 1.62% | 0.39 |
| Smear positive TB | 5 | 0.79 (0.19, 1.40) | 56.02% | 0.08 |
DM: diabetes mellitus, TB: tuberculosis, EPTB: extrapulmonary tuberculosis, BMI: body mass index, HIV: human immunodeficiency virus.
Discussion
This systematic review and meta-analysis study assessed the burden of TB and DM co-occurrences, and associated factors, in Ethiopia. Based on data extracted from the seven available studies, the pooled prevalence of TB among DM patients was estimated as 4.14% (95% CI 2.45–5.83%), while the pooled prevalence of DM among TB patients was estimated as 12.77% (95% CI 6.91–18.62%). Our study also revealed that type of DM was associated with developing TB among DM patients, while older TB patients (OR 2.25, 95% CI 1.38–3.13) and TB patients who had a family history of DM (OR 3.65, 95% CI 1.89–5.41) had a higher risk of developing DM compared with their counterparts.
Our study revealed that around 4.14% (4140 per 100 000 population) of DM patients in Ethiopia had TB. This was higher than the estimated national TB prevalence among the general population (140/100 000 population) (WHO, 2020). In a study carried out by Wagnew et al. (2018), using studies conducted in African and Asian countries, the pooled prevalence of TB among DM patients was estimated as 4.72%, with a pooled prevalence of 5.13% in Africa alone (Wagnew et al., 2018). Workneh et al., in their systematic review, revealed that the median overall global prevalence of TB among DM patients was 4.1% (Workneh et al., 2017). Another global pooled estimate revealed that DM patients had a two-to-four-fold increased risk of TB (Al-Rifai et al., 2017).
Our study also estimated the pooled prevalence of TB based on DM type. The findings revealed an 8.56% pooled TB prevalence among individuals who had type 1 DM, compared with 2.80% for individuals with type 2 DM. The pooled OR revealed that those individuals with type 1 DM had 2.70 times the odds for developing TB compared with type 2 DM patients. Likewise, a higher prevalence of TB among children and adolescents with type 1 DM was reported from South Africa (Webb et al., 2009), while a 10.0% prevalence of culture-positive PTB among type 1 DM patients was reported from India (Nair et al., 2016). The higher risk of TB in patients with type 1 DM might be due to the longer duration and difficulties in controlling hyperglycemia in this group, in addition to the lower body weights of young people who are mainly affected by type 1 DM.
Our study also assessed the prevalence of DM among TB patients. According to data collected from 3283 TB patients, 407 developed DM, giving a pooled prevalence estimate of 12.77%. This was lower than a global pooled estimate of 15.3% reported by Noubiap et al. (Noubiap et al., 2019). Likewise, in a study conducted by Workneh et al., the global pooled median prevalence of DM among TB patients was estimated as 16%. This might have been due to the high prevalence of DM among the general population in the countries included in the above studies. This was supported by a study conducted in South Asia, where the pooled prevalence of DM among TB patients was estimated as 21% (Gautam et al., 2021). However, in a study conducted by Alebel et al., the pooled estimate of DM prevalence among TB patients in sub-Saharan Africa was estimated as 9.0%, which is lower than that found by our study (Alebel et al., 2019).
Our study estimated the pooled prevalence of prediabetes among TB patients to be 24.19%. Although there have been no previously reported pooled estimates of the prevalence of prediabetes among TB patients, it has been commonly reported in individual studies. A comparable finding of 24.5% has been reported in India (Viswanathan et al., 2012). Higher prevalences have been reported in Vietnam (29%; Hoa et al., 2018) and Kenya (37.5%; Owiti et al., 2017), with a lower prevalence reported in Eritrea (10%; Araia et al., 2021). Generally, our study and previous studies conducted in different countries have revealed that TB patients are at high risk of developing DM, which necessities early DM detection among TB patients.
Of the 12 variables assessed using pooled OR estimates, two — older age and family DM history — were found to have a statistically significant association with DM in TB patients. Older TB patients had 2.25 times the odds for developing DM compared with their counterparts. A link between DM and older age was also reported in an earlier systematic review (Workneh et al., 2017). Likewise, a global meta-analysis study revealed that when the age of TB patients increased, the prevalence of DM also increased (Noubiap et al., 2019), while a nationwide cohort study in Portugual revealed that the odds for DM among TB patients increased by 4.7% per year of age (da Costa et al., 2016). The elderly are at risk of developing DM — mainly type 2 — due to decreased insulin secretion and impaired pancreatic islet functioning. A lack of physical activity and modern lifestyles, especially with regard to food choice, are the main factors resulting in obesity and consequent development of DM (Kirkman et al., 2012).
This study also revealed that TB patients with a family history of DM had 3.65 times the odds for developing DM co-occurrence compared with TB patients with no family DM history. This finding has been supported by a global systematic review (Workneh et al., 2017), while an individual study conducted in Tanzania revealed that TB patients who had a family history of DM had up to 17.5 times the odds for developing DM compared with their counterparts (Mabula et al., 2021). Other studies have also shown a family history of DM to be a major risk factor in developing DM in the general population (Zuo et al., 2014; Ismail et al., 2021).
Important limitations should be considered when interpreting the results of this study. First, the study was based on primary studies conducted only in the English language, which might have introduced bias. In addition, the majority of the studies were cross-sectional; this might have a limited the assessment of risk factors for co-occurrence. Furthermore, the small number of available primary studies might also have introduced bias. Finally, all the studies were hospital-based, which might not have reflected the nature of co-occurrences in the general population.
Conclusion
Co-occurrence of TB and DM is a major public health problem in Ethiopia. The prevalence of TB among DM patients (4140/100 000 population) estimated in our study was far higher than the national TB prevalence (140/100 000 population) among the general population. Individuals with type 1 DM had a higher TB risk (8560/100 000 population) compared with those individuals with type 2 DM (2800/100 000 population). The estimated DM prevalence among TB patients (12.77%) was far beyond the national DM prevalence among adults (3.2%). This suggests the need for an integrative approach to decreasing the dual burden. Elderly TB patients with a family history of DM had a higher risk of developing DM; this needs to be considered during anti-TB treatment follow-up. Thus, active screening of DM patients for TB, and vice versa, is recommended.
Author contributions
AA conceptualized, designed, and drafted the manuscript. AA, GD, and ZWB performed article searching, data extraction, and quality assessment. AA and ZWB conducted data analysis and wrote the manuscript. BG reviewed the final manuscript. All authors read, reviewed, and approved the final manuscript.
Funding
The authors did not receive specific funding for this work.
Availability of data and materials
All relevant data are available from the corresponding author upon request.
Ethical approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors have declared that they do not have any competing interests.
Acknowledgments
The authors would like to acknowledge those responsible for the primary studies included in this study. They also acknowledge the Ethiopian Public Health Institute and Aklilu Lemma Institute of Pathobiology, Addis Ababa University, for non-financial support, including access to internet searching.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2021.10.004.
Appendix. Supplementary materials
References
- Abera A, Ameya G. Pulmonary tuberculosis and associated factors among diabetic patients attending Hawassa Adare Hospital, Southern Ethiopia. The Open Microbiology Journal. 2018;12 doi: 10.2174/1874285801812010333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alebel A, Wondemagegn AW, Tesema C, Kibret GD, Wagnew F, et al. Prevalence of diabetes mellitus among tuberculosis patients in Sub-Saharan Africa: a systematic review and meta-analysis of observational studies. BMC Infectious Diseases. 2019;19:254. doi: 10.1186/s12879-019-3892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rifai RH, Pearson F, Critchley JA, AbuRaddad LJ. Association between diabetes mellitus and active tuberculosis: a systematic review and meta-analysis. PLoS ONE. 2017;12(11) doi: 10.1371/journal.pone.0187967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amare H, Gelaw A, Anagaw B, Gelaw B. Smear positive pulmonary tuberculosis among diabetic patients at the Dessie Referral Hospital, Northeast Ethiopia. Infectious Diseases of Poverty. 2013;2:6. doi: 10.1186/2049-9957-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andualem T, Malede E. Pulmonary tuberculosis and associated factors among diabetic patients attending health care at Debre Tabor General Hospital, Northwest Ethiopia. Ethiop J Health Dev. 2021;35(2) [Google Scholar]
- Araia ZZ, Mesfin AB, Mebrahtu AH, Tewelde AG, Osman R, Tuumzghi HA. Diabetes mellitus and its associated factors in tuberculosis patients in Maekel region, Eritrea: analytical cross-sectional study. Diabetes Metab Syndr Obes. 2021;14:515–523. doi: 10.2147/DMSO.S293557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashebir N. Prevalence of diabetes mellitus among active pulmonary tuberculosis patients in Addis Ababa. Ethiopia. American Society for Clinical Pathology. 2018;150:S135–S138. [Google Scholar]
- Ayelign B, Negash M, Genetu M, Wondmagegn T, Shibabaw T. Immunological impacts of diabetes on the susceptibility of Mycobacterium tuberculosis. J Immunol Res. 2019;2019 doi: 10.1155/2019/6196532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishua KG, JenkinsC Yebyod HG, Atsbha M, Wubayehu T, et al. Diabetes in Ethiopia: a systematic review of prevalence, risk factors, complications, and cost. Obesity Medicine. 2019;15 [Google Scholar]
- da Costa JC, Oliveira O, Baía L, Gaio R, Neves MC, Duartel R. Prevalence and factors associated with diabetes mellitus among tuberculosis patients: a nationwide cohort. Eur Respir J. 2016;48:261–264. doi: 10.1183/13993003.00254-2016. [DOI] [PubMed] [Google Scholar]
- Damtew E, Ali I, Meressa D. Prevalence of diabetes mellitus among active pulmonary tuberculosis patients at St. Peter Specialized Hospital, Addis Ababa, Ethiopia. World Journal of Medical Sciences. 2014;11(3):389–396. [Google Scholar]
- Feleke Y, Abdulkadir J, Aderaye G. Prevalence and clinical features of tuberculosis in Ethiopian diabetic patients. East African Medical Journal. 1999;76(7):361–364. [PubMed] [Google Scholar]
- Gautam S, Shrestha N, Mahato S, Nguyen TPA, Mishra SR, et al. Diabetes among tuberculosis patients and its impact on tuberculosis treatment in South Asia: a systematic review and meta‑analysis. Scientific Reports. 2021;11:2113. doi: 10.1038/s41598-021-81057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedfew M, Ayana M, Abate A, Bewket B, Haile D. Incidence and predictors of tuberculosis among adult diabetic patients, Debre Markos Referral Hospital, Northwest Ethiopia, 2018: a retrospective cohort study. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2020;13:869–878. doi: 10.2147/DMSO.S233564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew A, Mekonnen S, Alemu S, Yusuf H. High magnitude of diabetes mellitus among active pulmonary tuberculosis patients in Ethiopia. British Journal of Medicine and Medical Research. 2014;4(3):862–872. [Google Scholar]
- Gezahegn H, Ibrahim M, Mulat E. Diabetes mellitus and tuberculosis comorbidity and associated factors among Bale Zone health institutions. Southeast Ethiopia. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2020;13:3879–3886. doi: 10.2147/DMSO.S248054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries AD, Satyanarayana S, Kumar AM, Nagaraja SB, Isaakidis P, Malhotra S, et al. Epidemiology and interaction of diabetes mellitus and tuberculosis and challenges for care: a review. Public Health Action. 2013;3(Suppl 1):S3–S9. doi: 10.5588/pha.13.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoa NB, Phuc PD, Hien NT, Hoa VQ, Thuong PH, Anh PT, et al. Prevalence and associated factors of diabetes mellitus among tuberculosis patients in Hanoi, Vietnam. BMC Infectious Diseases. 2018;18(1):603. doi: 10.1186/s12879-018-3519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Annals of Internal Medicine. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- I DF; International Diabetes Federation. Diabetes atlas. Ninth edition. 2019.
- Ismail L, Materwala H, Al Kaabi J. Association of risk factors with type 2 diabetes: a systematic review. Comput Struct Biotechnol J. 2021;19:1759–1785. doi: 10.1016/j.csbj.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerene D, Hiruy N, Jemal I, Gebrekiros W, Anteneh T, et al. The yield and feasibility of integrated screening for TB, diabetes and HIV in four public hospitals in Ethiopia. Int Health. 2017;9:100–104. doi: 10.1093/inthealth/ihx002. [DOI] [PubMed] [Google Scholar]
- Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in older adults. Diabetes Care. 2012;35(12):2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39(2):91–92. doi: 10.1016/j.jcms.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Mabula PL, Kazinyingia KI, Chavala EC, Mosha V, Msuya SE, et al. Prevalence and risk factors for diabetes mellitus among tuberculosis patients in Moshi Municipal Council. Kilimanjaro Tanzania. East African Health Research Journal. 2021;5(1) doi: 10.24248/eahrj.v5i1.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moola et al, 2020.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. In: JBI Manual for Evidence Synthesis. Aromataris E, Munn Z, editors. JBI; 2020. Chapter 7: Systematic review of etiology and risk.https://synthesismanual.jbi.global Available from. [Google Scholar]
- Munn Z, Aromataris E, Tufanaru C, Stern C, Porritt K, Farrow J, et al. The development of software to support multiple systematic review types: the Joanna Briggs Institute System for the Unified Management, Assessment and Review of Information (JBI SUMARI) Int J Evid Based Healthc. 2019;17(1):36–43. doi: 10.1097/XEB.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Nair A, Guleria R, Kandasamy D, Sharma R, Tandon N, Singh UB, et al. Prevalence of pulmonary tuberculosis in young adult patients with type 1 diabetes mellitus in India. Multidiscip Respir Med. 2016;11:22. doi: 10.1186/s40248-016-0058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazi AK, Kalra S. Diabetes and tuberculosis: a review of the role of optimal glycemic control. J Diabetes Metab Disord. 2012;11(1):28. doi: 10.1186/2251-6581-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noubiap JJ, Nansseu JR, Nyaga UF, Nkeck JR, Endomba FT, et al. Global prevalence of diabetes in active tuberculosis: a systematic review and meta-analysis of data from 2.3 million patients with tuberculosis. The Lancet. 2019;7 doi: 10.1016/S2214-109X(18)30487-X. [DOI] [PubMed] [Google Scholar]
- Owiti P, Keter A, Harries AD, Pastakia S, Wambugu C, Kirui N, et al. Diabetes and pre-diabetes in tuberculosis patients in western Kenya using point-of-care glycated haemoglobin. Public Health Action. 2017;7(2):147–154. doi: 10.5588/pha.16.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porritt K, Gomersall J, Lockwood C. JBI's systematic reviews: study selection and critical appraisal. Am J Nurs. 2014;114(6):47–52. doi: 10.1097/01.NAJ.0000450430.97383.64. [DOI] [PubMed] [Google Scholar]
- Riley RD, Higgins JPT, Deek JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:dS49. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- Tenaye L, Mengiste B, Baraki N, Mulu E. Diabetes mellitus among adult tuberculosis patients attending tuberculosis clinics in Eastern Ethiopia. BioMed Research International. 2019 doi: 10.1155/2019/7640836. Volume 2019, Article ID 7640836, 7 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiroro S, Enkquselassie F, Getachew S, Kebede T. The magnitude and associated factors of tuberculosis among diabetic patients at Tikur Anbessa Specialized Teaching Hospital in Addis Ababa, Ethiopia. 2015.
- Tulu B, Amsalu E, Zenebe Y, Abebe M, Fetene Y, Agegn M, et al. Diabetes mellitus and HIV infection among active tuberculosis patients in Northwest Ethiopia: health facility-based cross-sectional study. Trop Med Health. 2021;49(1):68. doi: 10.1186/s41182-021-00358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan V, Kumpatla S, Aravindalochanan V, Rajan R, Chinnasamy C, Srinivasan R, et al. Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PloS One. 2012;7(7):e41367. doi: 10.1371/journal.pone.0041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagnew F, Eshetie S, Alebel A, Dessie G, Tesema C, Abajobir AA. Meta-analysis of the prevalence of tuberculosis in diabetic patients and its association with cigarette smoking in African and Asian countries. BMC Res Notes. 2018;11:298. doi: 10.1186/s13104-018-3390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker NF, Meintjes G, Wilkinson RJ. HIV-1 and the immune response to TB. Future Virol. 2013;8(1):57–80. doi: 10.2217/fvl.12.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb EA, Hesseling AC, Schaaf HS, Gie RP, Lombard CJ, et al. High prevalence of Mycobacterium tuberculosis infection and disease in children and adolescents with type 1 diabetes mellitus. Int J Tuberc Lung Dis. 2009;13(7):868–874. [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2015. Collaborative framework for care and control of tuberculosis and diabetes. [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2019. Global tuberculosis report. [Google Scholar]
- WHO . World Health Organization; Geneva, Switzerland: 2020. Global tuberculosis report. [Google Scholar]
- Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of diabetes mellitus among tuberculosis patients in Southeastern Amhara Region, Ethiopia: a cross sectional study. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workneh MH, Bjune GA, Yimer SA. Prevalence and associated factors of tuberculosis and diabetes mellitus comorbidity: a systematic review. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorke E, Atiase Y, Akpalu J, Sarfo-Kantanka O, Boima V, Dey ID. The bidirectional relationship between tuberculosis and diabetes. Tuberc Res Treat. 2017;2017 doi: 10.1155/2017/1702578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo H, Shi Z, Hussain A. Prevalence, trends and risk factors for the diabetes epidemic in China: a systematic review and meta-analysis. Diabetes research and clinical practice. 2014;104(1):63–72. doi: 10.1016/j.diabres.2014.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are available from the corresponding author upon request.