Highlights
-
•
An overall UTI prevalence of 9.2% (90/982) was detected in diabetic patients in Ghana.
-
•
Multidrug-resistant E. coli and Klebsiella spp. are a cause for concern in diabetic patients.
-
•
Phylo-groups B2 and D were prevalent, followed by group C.
-
•
The predominant virulence genes observed were iutA (17.9%) and KpsMTIII (14.3%).
-
•
Phylo-group B2 had the highest number of VFs, and was resistant to most of the tested antibiotics.
Keywords: urinary tract infections (UTI), Escherichia coli, virulence factor, phylogenetic group, Ghana
Abstract
Objectives
Our study aimed to determine the etiology of urinary tract infections (UTIs), resistance profiles of isolated bacteria, and virulence factors of Escherichia coli associated with bacteriuria in diabetic patients in Ghana.
Methods
Midstream urine samples from 982 diabetic patients were tested for uropathogens at the National Diabetes Management and Research Centre in Ghana, using standard bacteriological methods, with antibiogram testing of the isolates using the Kirby–Bauer disk diffusion, as per CLSI guidelines. Polymerase chain reaction (PCR) was used to investigate the phylogenetic groupings and virulence factor (VF) genes of isolated E. coli.
Results
The overall prevalence of UTIs was 9.2%, and the main uropathogens were Klebsiella spp. (55.6%) and Escherichia coli (31.3%). Age, duration of diabetes, and a previous history of UTIs were risk factors associated with UTI (p-value < 0.05). High levels of antibacterial resistance to cefuroxime (84%), ampicillin (80%), and gentamicin (70.7%) were observed. The distribution of VFs in each phylogenetic group revealed that sfa-iutA-KpsTMII-KpsTMIII genes were associated with group B2, and iutA-ibe were associated with group D.
Conclusions
The isolated uropathogens were highly resistant, and the E. coli isolates possessed varying VFs. Continuous monitoring of bacteria associated with UTI in diabetics is highly recommended.
INTRODUCTION
Diabetes mellitus (DM) is a polygenic metabolic disorder characterized by increased blood glucose levels (hyperglycemia) resulting from a defect in insulin secretion or insulin action, or both (Foxman, 2010; Hall et al., 2011). It is a major predisposing factor for urinary tract infections (UTI), with the principal site of infection in diabetics being the urinary tract. UTI are the most common bacterial infection affecting people of all ages, leading to considerable morbidity and costly health problems globally (Foxman, 2010). Diabetic patients have a higher incidence of UTI due to: nerve damage caused by high blood glucose levels, which affects the ability of the bladder to sense the presence of urine; high glucose levels in urine, enabling the growth of the bacteria; or abnormalities of the host defense system (Geerlings et al., 2000; Johnsson et al., 2013; Nitzan et al., 2015; Szucs et al., 1960).
UTI in diabetic patients can cause varying complications, ranging from dysuria to organ damage and complicated UTI (pyelonephritis) (Walsh and Collyns, 2017). The common range of aggressive pathogens involved in UTIs in diabetics includes Escherichia coli, Klebsiella spp., enterococci, group B streptococci, Pseudomonas spp., Proteus mirabilis, Mycoplasma spp., Enterobacter spp., Staphylococcus aureus, and Candida spp. (Walsh and Collyns, 2017).
Several studies have reported the coinfection of DM with UTI. In Ethiopia, a prevalence of 13.6% of bacteriuria among symptomatic diabetic patients has been reported (Yeshitela et al., 2012). In South Africa, a UTI prevalence of 22% was reported in diabetes patients, with women more affected than men (Mgbakogu and Eledo, 2015). A UTI prevalence of 38.3% was reported in diabetic patients in Cameroun (Bissong et al., 2013), while in Kenya and Sudan, varying prevalences of 64% and 35% were reported in males and females, respectively (Mogaka, 2020; Nabaigwa et al., 2017) A slightly lower prevalence (26%) has been reported in Nigeria and Ghana, with E. coli being the commonest bacteria isolate (Mgbakogu and Eledo, 2015; Sakyi et al., 2017).
To avoid related UTI complications in diabetic patients, the successful management of UTI depends on accurate identification of pathogens and the selection of effective antibacterial therapy for treatment (Donkor et al., 2017). However, the emergence of multidrug-resistant strains has rendered the treatment of these infections ineffective, posing a major challenge for their treatment. Furthermore, virulence markers carried on a large number of pathogenic-associated islands in bacterial pathogens increase their ability to resist and overcome host immune defenses, and subsequently reduce treatment outcomes, leading to severe infections (Malekzadegan et al., 2018). The characterization of virulence factors is critical in improving our understanding of the pathogenicity of the common bacteria associated with UTI (Firoozeh et al., 2014).
Our study investigated the etiology, resistance profiles, and risk factors of bacteria-associated bacteriuria in diabetic patients in Ghana, as well as the types of uropathogen present in the urine of diabetic patients with good and poor glycemic control. Furthermore, the study determined the antibiotic resistance genes, phylogenetic groupings, and virulence factors of Escherichia coli isolates associated with UTI in diabetic patients.
METHODS
This cross-sectional study was carried out with 982 confirmed diabetic patients attending the diabetic clinic in Korle-Bu teaching hospital, from June 2018 to October 2019. The sample size was determined using an assumed UTI prevalence of 50%. The minimum sample size was obtained using the formula n=z²*p (1-p)/d² (Daniel, 1999). Where n is the required sample size, z is the normal deviation (= 1.96) corresponding to the 95% confidence interval, p is the proportion of the target population with desired characteristics (0.5 = 50%), and d is degrees of freedom (= 0.05). Confirmed diabetic Ghanaians at the National Diabetes Management and Research Centre (NDMRC) were included in the study, with those on antimicrobial treatments for up to 14 days excluded from this study. After obtaining informed consent from each patient, a self-administered questionnaire was used to obtain information on demographic and socio-economic characteristics. Midstream-clean-catch urine from participants was inoculated onto cysteine lactose electrolyte-deficient (CLED) agar and incubated at 37°C for 16–18 h. UTI was defined as the presence of a positive urine culture (≥ 105 colony-forming units [CFU]/ml).
Bacterial identification
Identification of isolates was performed according to morphological characteristics on the culture media, Gram reaction, and biochemical tests, including triple sugar iron, indole, urease, citrate, catalase, and coagulase tests (KonemannAllen et al., 2006). The API 20E identification system (bioMerieux SA, Marcy l'Etoile, France) was also used to confirm the Gram-negative isolates. Confirmed E. coli isolates were kept frozen in tryptic soy broth (Merck Co., Germany), containing 20% glycerol (Merck KGaA, Germany) at −70°C until further experiments.
Antibacterial susceptibility
Antibacterial susceptibility testing of the isolates was performed using the Kirby–Bauer disk diffusion method for sensitivity testing, based on guidelines and breakpoints from the Clinical and Laboratory Standards Institute (CLSI, 2015). The antibiotics tested included ampicillin (10 μg), amoxicillin/clavulanate acid (10 μg), cefotaxime (30 μg), ceftriaxone (30 μg), nalidixic acid (30 μg), trimethoprim-sulfamethoxazole (1.25 μg), gentamicin (10 μg), and ciprofloxacin (5 μg). E. faecalis ATCC 29212, E. coli ATCC 25922, and Staphylococcus aureus ATCC 29213 were used as the quality-control strains for antibacterial susceptibility testing.
Molecular characterization
Genomic DNA was extracted from the E. coli isolates by suspending colonies of fresh bacterial culture in 200 ml of sterile water. The suspension was heated at 98°C for 10 min and centrifuged at 17 900 g for 5 min. The supernatant was recovered and used as a template for polymerase chain reaction (PCR) testing.
Gene amplification was performed for 16 virulence factors relating to adhesions, toxins, iron capture systems, protectins, uropathogenic-specific protein, and the aerobactin system, as previously reported (Bingen-Bidois et al., 2002; Clermont et al., 2013; Ewers et al., 2005; Huang et al., 1995; 2000; Johnson et al., 2008; Johnson et al., 2003; Yamamoto et al., 1995; Zhao et al., 2009) using the primers listed in Supplementary Table 2. The virulence genes were amplified in five primer pools: 1 (iron, sfa, iuC, sat), 2 (Cnf1, iutA, papC, hlyD), 3 (usp, ompT, KpsMTII, papA), 4 (KpsMTIII, ibeA), and 5 (hra, ireA). For the purposes of this study, only E. coli isolates were further analyzed for virulence factors and phylogenetic groupings.
Phylogenetic analysis
Phylogenetic groups of the isolates were investigated using the quadruplex PCR method described by Clermont et al. (2013) based on the presence of the genes chuA, yjaA, TspE4.C2, and arpA. According to the amplification results, the E. coli isolates were classified into one of the major phylogenetic groups: A, B1, B2, C, D, or F.
Statistical analysis
Data were analyzed using GraphPad Prism software, version 6. Bacteriuria was defined as bacterial growth of ≥ 105 colony-forming units/ml urine sample on CLED. Associations between socio-demographic characteristics and the development of UTI, phenotypic resistance, and virulence factors were assessed using the chi-square test. A p-value < 0.05 was considered statistically significant.
RESULTS
Socio-demographic characteristics of study participants
The study participants comprised 212 males (21.6%) and 770 females (78.4%) (Table 1). The predominant age group was 51–60 years [260/982 (26.3%)], followed by 61–70 years [234/982 (23.8%)] and 41–50 years [202/982 (2.2%)] (Table 1). Most of the patients were married [870/982 (89%)], unemployed [524/982 (53.3%)], and with type 2 diabetes [912/982 (92.9%)] (Table 1).
Table 1.
Socio-demographic characteristics of study participants
| Characteristics | No. (%) | |
|---|---|---|
| Age | 20–30 | 22 (2.2) |
| 31–40 | 50 (5.1) | |
| 41–50 | 202 (20.4) | |
| 51–60 | 260 (26.3) | |
| 61–70 | 234 (23.8) | |
| 71–80 | 170 (17.3) | |
| 81–90 | 44 (4.5) | |
| Sex | Male | 212 (21.6) |
| Female | 770 (78.4) | |
| Marital status | Divorced | 20 (2) |
| Married | 870 (89) | |
| Never married | 14 (1.4) | |
| Separated | 2 (0.2) | |
| Widowed | 76 (7.7) | |
| Education status | No formal | 186 (18.9) |
| Basic | 220 (22.4) | |
| Secondary | 346 (35.2) | |
| Tertiary | 230 (23.4) | |
| Work status | Gov. employed | 182 (18.5) |
| Non-employed | 72 (7.3) | |
| Self-employed | 56 (5.7) | |
| Unemployed | 524 (53.3) | |
| Type of diabetes | Type1 | 70 (7.1) |
| Type2 | 912 (92.9) | |
| Duration of diabetes (yrs) | 1–5 | 280 (28.5) |
| 6–10 | 298 (30.3) |
Comparisons between diabetic UTI-positive and UTI-negative patients
Out of a total of 982 diabetic urine samples tested, 9.2% (90/982) had urinary tract infections. Of the 90 positive patients, 86.7% (78/90) were female and 13.3% (12/90) were male (Table 2). The predominant age range among UTI-positive patients was 61–70 years [35.6% (32/90)], followed by 41–50 years [22.2% (20/90)] and 11–20 years [2.2% (2/90)].
Table 2.
Risk factors associated with urinary tract infections
| Total No. of UTI-positive patients (n = 90) | Total No. of UTI-negative patients (n = 892) | |||
|---|---|---|---|---|
| Characteristics | No. (%) | No. (%) | Odds ratio | p-value |
| Age in years | ||||
| < 60 | 42 (15.3) | 232 (84.7) | 0.417 | 0.003 |
| ≥ 60 | 48 (6.8) | 660 (93.2) | ||
| Sex | ||||
| Male | 12 (5.7) | 200 (94.3) | 1.89 | 0.158 |
| Female | 78 (10.1) | 692 (89.9) | ||
| Previous UTI | ||||
| Positive | 10 (15.7) | 160 (84.2) | 0.437 | 0.013 |
| Negative | 30 (7.6) | 732 (92.4) | ||
| Hypertension | ||||
| Positive | 68 (24.3) | 212 (75.7) | 0.077 | 0.001 |
| Negative | 22 (3.1) | 680 (96.9) | ||
| Kidney stone | ||||
| Positive | 4 (14.3) | 24 (85.7) | 0.595 | 0.062 |
| Negative | 86 (9.1) | 868 (97.3) | ||
| Burning sensation | ||||
| Positive | 16 (17) | 78 (8.7) | 0.445 | 0.062 |
| Negative | 74 (8.4) | 810 (91.6) | ||
| Frequent urination | ||||
| Positive | 58 (19.6) | 119 (80.4) | 0.189 | 0.001 |
| Negative | 32 (4.4) | 347 (95.6) | ||
| Rush urine | ||||
| Positive | 42 (42.9) | 28 (57.1) | 0.077 | 0.001 |
| Negative | 48 (5.4) | 418 (94.6) | ||
| Type of diabetes | ||||
| Type1 | 16 (22.9) | 27(77.1) | 0.298 | 0.009 |
| Type2 | 74 (8.1) | 419 (91.9) | ||
| Duration of diabetes | ||||
| < 5 yrs | 40 (14.3) | 120 (85.7) | 0.46 | 0.013 |
| > 5 yrs | 50 (7.1) | 326 (92.9) |
There were significant associations between UTI and the following variables: age (OR = 0.417, p = 0.003), previous UTI (OR = 0.437, p = 0.013), hypertension (OR = 0.077, p = 0.001), frequent urination (OR = 0.189, p = 0.001), type 2 diabetes mellitus (OR = 0.298, p = 0.009), and duration of diabetes > 5 yrs (OR = 0.46, p = 0.013) (Table 2). However, gender (OR = 1.89, p = 0.158) did not show any significant association with urinary tract infection in this study.
Distribution of uropathogens and the renal threshold levels
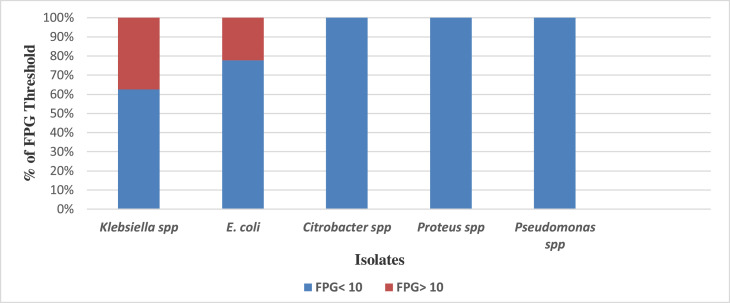
The following isolates were identified in the tested urine samples: Klebsiella spp. [55.6% (50/90)], Escherichia coli [31.1% (28/90)], Citrobacter spp. (6.7%), Proteus spp. (4.4%), and Pseudomonas spp. (2.2%). Patients with urine cultures positive for uropathogens had the following fasting blood sugar levels: 60% (30/50) of patients with Klebsiella spp. had a fasting plasma glucose (FPG) level lower than the threshold of 10 mmol/l, while 40% (20/50) of patients with Klebsiella spp. had an FPG greater than the threshold of 10 mmol/l (Figure 1). Of the patients with Escherichia coli isolates, 71.4% (20/28) had an FPG below the threshold, compared with 28.6% (8/28) of patients whose FPG values were above the threshold. However, patients with Pseudomonas spp., Proteus spp., and Citrobacter spp. isolates in their urine samples had FPG levels below the renal threshold of 10 mmol/l.
Figure 1.
Distribution of uropathogens and fasting plasma glucose (FPG) measurements in patients.
Antibiotic susceptibility patterns of uropathogens
According to sensitivity testing of uropathogens, Klebsiella spp. exhibited a relatively high level of resistance to ampicillin (88.8%), moderate resistance to trimethoprim-sulfamethoxazole (48%), nalidixic acid (48%), and ceftriaxone (40%), and low resistance to cefotaxime (28%), amoxicillin-clavulanate (24%), ciprofloxacin (24%), and gentamicin (12%) (Table 3). Escherichia coli showed similar patterns of resistance to ampicillin (85.7%), trimethoprim (42.8%), nalidixic acid (50%), cefuroxime (35.7%), cefotaxime (28.6%), amoxicillin-clavulanate (21.4%), ciprofloxacin (21.4%), and gentamicin (14.3%) (Table 3).
Table 3.
Susceptibilities and resistance profiles of uropathogens
| Antibiotic | Pattern | Klebsiella spp. (50) | E. coli (28) | Citrobacter spp. (6) | Proteus spp. (4) | Pseudomonas spp. (2) | Total No. (90) |
|---|---|---|---|---|---|---|---|
| AMP | S (%) | 6 (12) | 4(14.3) | 0(0) | 0(0) | 0(0) | 10 (11.1) |
| R (%) | 44(88) | 24(85.7) | 6(100) | 4(100) | 2(100) | 80(88.9) | |
| AMC | S (%) | 38(76) | 22(78.6) | 4(66.7) | 4(100) | 2(100) | 70(77.8) |
| R (%) | 12(24) | 6 (21.4) | 2(33.3) | 0(0) | 0(0)) | 20(22.2) | |
| CTX | S (%) | 36(72) | 20(71.4) | 4(66.7) | 4(100) | 2(100) | 66(73.3) |
| R (%) | 14(28) | 8(28.6) | 2(33.3) | 0(0) | 0(0) | 24(26.7) | |
| CTR | S (%) | 30(60) | 18(64.3) | 4(66.7) | 4(100) | 2(100) | 38(42.2) |
| R (%) | 20(40) | 10(35.7) | 2(33.3) | 0(0) | 0(0) | 32(35.6) | |
| CIP | S (%) | 38(76) | 22(78.6) | 6(100) | 4(100) | 2(100) | 72(80) |
| R (%) | 12(24) | 6(21.4) | 0(0) | 0(0) | 0(0) | 18(20) | |
| GEN | S (%) | 44(88) | 24(85.7) | 6(100) | 4(100) | 2(100) | 80(88.9) |
| R (%) | 6(12) | 4(14.3) | 0(0) | 0(0) | 0(0) | 10(11.1) | |
| NAL | S (%) | 26(52) | 14(50) | 4(66.7) | 0(0) | 0(0) | 44(48.9) |
| R (%) | 24(48) | 14(50) | 2(33.3) | 4(100) | 2(100) | 46(51.1) | |
| TRIM | S (%) | 26(52) | 16(57.1) | 4(66.7) | 4(100) | 2(100) | 52(57.8) |
| R (%) | 24(48) | 12(42.8) | 2(33.3) | 0(0) | 0(0) | 38(42.2) |
Key: AMP: ampicillin, AMC: amoxicillin-clavulanate, CTX: cefotaxime, CTR: ceftriazone, CIP: ciprofloxacin, GEN: gentamicin, NAL: nalidixic acid, TRIM: trimethoprim-sulfamethoxazole
Multidrug-resistant prevalence profiles of uropathogens
Most of the Klebsiella spp. and E. coli isolates were resistant to amoxicillin-clavulanate, cefotaxime, ceftriaxone, ciprofloxacin, and gentamicin. Klebsiella spp. exhibited a multidrug-resistant prevalence of 52% (26/50), compared with E. coli [50% (14/28)] and Citrobacter spp. [33.3% (2/6)]. The multidrug-resistant profiles of multidrug-resistant isolates were highly variable among Klebsiella spp. and E. coli (Table 4).
Table 4.
Resistance profiles of multidrug-resistant uropathogens
| Isolates | No. of isolates | Resistant patterns |
|---|---|---|
| Klebsiella spp. | 6 | AMP+AMC+CTX+CTR+CIP+GEN+NAL+TRIM |
| 6 | AMP+AMC+CTX+CTR+CIP+NAL+TRIM | |
| 2 | AMP+CTX+CTR+CIP+NAL+TRIM | |
| 4 | AMP+CTR+NAL+TRIM | |
| 8 | AMP+CIP+NAL+TRIM | |
| Escherichia coli | 6 | AMP+AMC+CTX+CTR+CIP+GEN+NAL+TRIM |
| 4 | AMP+AMC+CTX+CTR+CIP+NAL+TRIM | |
| 2 | AMP+CTR+NAL+TRIM | |
| 2 | AMP+NAL+TRIM | |
| Citrobacter spp. | 2 | AMP+AMC+CTX+CTR+NAL+TRIM |
Key: AMP: ampicillin, AMC: amoxicillin-clavulanate, CTX: cefotaxime, CTR: ceftriazone, CIP: ciprofloxacin, GEN: gentamicin, NAL: nalidixic acid, TRIM: trimethoprim-sulfamethoxazole
Phylogenetic groups, distributions of VFs, and antibiotic resistance patterns of isolates
In total, 22 E. coli isolates were characterized for phylogenetic grouping and virulence factors. The phylogenetic distribution analyses revealed higher numbers of the E. coli isolates were group D [41% (9/22)] and group B2 [32% (7/22)] (Table 5). The distribution of VFs in each phylogenetic group was compared with those of the other phylogenetic groups combined. The results indicated that sfa-iutA-KpsTMII-KpsTMIII genes were commonly associated with group B2, while iutA-ibe were also associated with group D. The hrA gene was associated with only two group C isolates. Although group B2 accounted for most of the virulence genes, group C was more resistant to most of the tested antibiotics (Table 5).
Table 5.
Distribution of virulence factor genes and resistant patterns among different phylogenetic groups of E. coli isolates
| Phylo-groups | Total (%) | |||
|---|---|---|---|---|
| Virulence genes | B2 (n = 11) (%) | D (n = 11) (%) | C (n = 6) (%) | 28 (100%) |
| sfa (n = 2) | 1 (9.1) | 0 | 1 (16.7) | 2 (7.1%) |
| iutA (n = 5) | 2 (18.2) | 2 (18.2) | 1 (16.7) | 5 (17.9%) |
| cnf1 (n = 1) | 1 (9.1) | 0 | 0 | 1 (3.6 %) |
| papA (n = 1) | 1 (9.1) | 0 | 0 | 1 (3.6%) |
| hrA (n = 1) | 0 | 0 | 1(16.7) | 1 (3.6%) |
| ibe (n = 2) | 0 | 2 (18.2) | 0 | 2 (7.1%) |
| KpsTMII (n = 2) | 2 (18.2) | 0 | 0 | 2 (7.1%) |
| KpsMTIII (n = 4) | 3 (27.3) | 0 | 1(16.7) | 4 (14.3%) |
| Resistant patterns | ||||
| CTX (n = 6) | 2 (18.2) | 4 (36.4) | 0 | 6 (21.4%) |
| AMP+CTR+NAL+TRIM (n = 2) | 1 (9.1) | 1 (9.1) | 0 | 2 (7.1%) |
| AMP+AMC+CTX+CTR+CIP+GEN+NAL+TRIM (n = 5) | 1 (9.1) | 0 | 4 (66.7) | 5 (17.9%) |
Key: AMP: ampicillin, AMC: amoxicillin-clavulanate, CTX: cefotaxime, CTR: ceftriazone, CIP: ciprofloxacin, GEN: gentamicin, NAL: nalidixic acid, TRIM: trimethoprim-sulfamethoxazole
DISCUSSION
Urinary tract infection is a common disease associated with diabetic patients, and its diagnosis and treatment have important implications for patients’ health and healthcare costs (Alimohammadi, 2007; Orenstein and Wong, 1999; Yadav and Prakash, 2016). An overall UTI prevalence of 9.2% was found among diabetic patients in this study. This is in line with studies carried out in the USA, Romania, Ethiopia, and Canada, which reported prevalences of 8.2%, 8.4%, 10.9%, and 7.9%, respectively (Chiţă et al., 2013; Yu et al., 2014; Zhanel et al., 1995). However, in contrast to our findings, higher prevalences of 35%, 26.4%, 21%, 17.5%, 22.5%, and 22% have been reported in previous studies carried out in Kuwait, Ghana, Nepal, Ethiopia, Germany, and Uganda, respectively (Alimohammadi, 2007; Johnson and Stell, 2002; Kabiswa et al., 2018; Rabindra et al., 2013; Sakyi et al., 2013; Sewify et al., 2016; Storme et al., 2019; Yadav and Prakash 2016). The variation in prevalence across these countries may be due to varying socio-demographic and cultural differences (Kebamo et al., 2017).
Of those diabetic patients who were positive for urinary tract infections in our study, 86.7% were female. This finding was similar to the 88.5% figure reported in Kuwait (Johnsson et al., 2013). Several other studies — in Germany, Nepal, Ethiopia, and the USA — have reported higher UTI rates in females than in male counterparts (Iranpour et al., 2013; Johnson et al., 2003; Johnson et al., 2002; Kabiswa et al., 2018). Anatomical variations in the urethra between males and females, which is shorter in females than in males, and the proximity of the urethral meatus to the anus may be responsible for an elevated UTI prevalence among females (Kebamo et al., 2017).
Our study found a higher occurrence of urinary tract infections [32 (35.6%)] in the 61–70 years age group. This finding was in agreement with studies carried out in Romania, Saudi Arabia, the UK, Netherlands, and Washington State, USA (Al-Rubeaan et al., 2013; Boyko and Lipsky, 1995; Chiţă et al., 2017; Hirji et al., 2012; Venmans et al., 2009). This supports the suggestion that, as society advances in age, the burden of urinary tract infections might be expected to escalate (Rowe and Juthani-Mehta, 2013). Moreover, the older participants were found to have other forms of comorbidity, which increased with the duration of diabetes. This finding was in line with a review report from the USA, which highlighted the impact of chronic comorbid conditions on diabetes care (Piette and Kerr, 2006).
The associated risk factors for UTI — age, duration of diabetes, previous urinary tract infection, and hypertension — were found to be statistically significant. This finding was in agreement with studies carried out in India, the Netherlands, Canada, and the USA (Boyko and Lipsky, 1995; Geerlingset et al., 2000; Janifer et al., 2009; Zhanel et al., 1995). A study by Yu et al. (2014) reported an association between advanced age, duration of diabetes, and previous UTIs with a high risk of contracting UTI (Yu et al., 2014). However, in contrast to our findings, an Ethiopian study found diabetic patients with no previous history of UTI to have higher odds of contracting a UTI (Gutema et al., 2019). It is noteworthy that the female gender did not show a significant association with urinary tract infections. However, a study carried out in Spain reported male gender as a risk factor for contracting urinary tract infection (Briongos-Figuero et al., 2012). Another study in Ethiopia found that sex was not significantly associated with bacteriuria (Nigussie and Amsalu, 2017). Furthermore, a study in Sudan found no significant association between age, duration of diabetes, or type of diabetes and the prevalence of UTI (Hamdan et al., 2015).
The predominant uropathogens identified in this study were Klebsiella spp. (55.6%), followed by Escherichia coli (31.1%) and Citrobacter spp. (6.7%). However, in contrast to our findings, most studies have reported a higher prevalence of Escherichia coli as compared with Klebsiella spp., for example in Egypt (38.6% vs 21.5%), Uganda (8.6% vs 2.85%), Ethiopia (25.6% vs 20.5%), India (41.5% vs 14.4%), Nepal (58.1 vs 21.8%), and Nigeria (42.9% vs 28.6%) (Antwi et al., 2008; Desouky et al., 2020; Ifediora et al., 2016; Sakyiet et al., 2013; Sharna et al., 2012; Yadav and Prakash, 2016). Members of the Enterobacteriaceae family of Gram-negative bacilli have been implicated as etiological agents of UTIs, and is estimated that more than 85% of the Enterobacteriaceae family are responsible for UTIs (Antwi et al., 2008). Another Ethiopian study on diabetic patients with UTI reported Escherichia coli and Klebsiella spp. to have an equal prevalence of 12.1% each (Kebamo et al., 2017). The variations in prevalence among uropathogens may be due to differences in study design and sample size. It is noteworthy that members of the Enterobacteriaceae have many factors accountable for their uro-epithelial attachment, including the colonization of phenotype receptors in the urogenital mucosa with adhesives, pili, fimbriae, and P-1 (Das et al., 2006).
A high level of resistance to various classes of antibiotic was found among uropathogenic isolates. Forty-six per cent of the isolates isolated were multidrug-resistant (MDR), as defined by resistance to at least three or more antimicrobial agents (Magiorakos et al., 2012). Higher prevalences of MDR isolates have been reported in Nepal (67.2%), Turkey (68.7%), and Ethiopia (93.9%) (Alimohammadi, 2007; Gulay et al., 2019; Nigussie and Amsalu, 2017). The emergence of multidrug-resistant bacterial strains may be due to the indiscriminate administration of multiple courses of antibiotic therapy for asymptomatic or mild symptomatic UTI. Patients with chronic diseases such as diabetes are most likely to harbor multidrug-resistant bacteria during UTI, including extended-spectrum β-lactamase Enterobacteriaceae and fluoroquinolone-resistant uropathogens (Desouky et al., 2020). Klebsiella spp. and Escherichia coli exhibited resistance rates to ampicillin of 88% and 85.7% respectively. This was comparable with studies in Ethiopia (100% and 100%), Nigeria (100% and 100%), and India (88.9% and 86.3%) (Aswani et al., 2014; Ifediora et al., 2016; Kebamo et al., 2017). However, in contrast to our findings, lower levels of resistance to ampicillin (53.5% and 72.13%) in Klebsiella spp. and Escherichia coli have been reported in Egypt (Desouky et al., 2020). The very high level of resistance to ampicillin may be due to the fact that ampicillin is an old β-lactam antibiotic with minimal side-effects has been abused through incessant misuse. With regard to the β-lactam inhibitors, Klebsiella spp. and Escherichia coli showed resistances of 24% and 21.4% to amoxicillin-clavulanate. This findings is consistent with studies in Kuwait (24% and 20%), Turkey (12%), Ethiopia (12.5% and 30%), and Egypt (43.2% and 9.9%) (Desouky et al., 2020; Gutema et al., 2019; Gulay et al., 2019; Sewify et al., 2016). In contrast to our findings, higher levels of resistance to amoxicillin-clavulanate have been reported for Klebsiella spp. and Escherichia coli in Nepal (89.7% and 90.1%) and India (82%) (Nigussie and Amsalu, 2017; Sewify et al., 2016). These differences in resistance levels may be due to varying dependency on antibiotic usage among diabetic patients in various countries.
Klebsiella spp. and Escherichia coli uropathogens demonstrated resistance levels of of 28% and 28.6% to cefotaxime, which is in the third-generation cephalosporin class of antibiotics. This was consistent with studies in Egypt (19.7% and 28.7%) and Kuwait (25% and 28%) (Desouky et al., 2020; Sewify et al., 2016). In contrast, a higher levels of Klebsiella spp. and Escherichia coli resistance to cefotaxime have been reported in India (61.1% and 55.6%) (Aswani et al., 2014). This was comparable to a study from India (51.7% and 50%, respectively) (Aswani et al., 2014). However, in contrast to our findings, a study in Nigeria reported no resistance to ceftriaxone for both pathogens (Ifediora et al., 2016). The high levels of resistance observed among uropathogens in some regions could be the result of high levels of extended-spectrum β-lactamases (ESBLs) induced by selective pressure from broad-spectrum antimicrobial therapy (We et al., 2014).
Our study found high levels of resistance to ciprofloxacin by Klebsiella spp. and Escherichia coli (24% and 11.4%, respectively). This is comparable to levels reported in studies in Kuwait (34% and 34%), Nepal (32.7%), and Ethiopia (37.5% and 30%) (Desouky et al., 2020; Sewify et al., 2016; Yadav and Prakash, 2016). In contrast to our study findings, resistance levels for Klebsiella spp. and Escherichia coli to ciprofloxacin of 0% and 0% were reported in a study in Nigeria (Ifediora et al., 2016). Opintan et al. (2015) study on antimicrobial resistance, reported a resistance prevalence of > 50% to third-generation cephalosporins and fluoroquinolones throughout Ghana. In Ghana, the standard treatment guidelines recommend the use of ciprofloxacin for treating urinary tract and bloodstream infections (MOH, 2010) and this may be one of the reason for the relatively low resistance to ciprofloxacin detected in this study.
Resistance levels to gentamicin — an aminoglycoside class of antibiotic — in Klebsiella spp. and Escherichia coli were found to be 12% and 14.3%, respectively. This findings is in agreement with levels of 20% and 12.5% reported in Ethiopia (Gutema et al., 2019). In contrast to our findings, levels of 0% and 0% have been reported in Nigeria (Ifediora et al., 2016). However, higher levels of resistance to gentamicin have been reported in India (31.8% and 31.5%), Nepal (40.9%), and Ethiopia (72.1%) (Aswani et al., 2014; Nigussie and Amsalu, 2017; Yadav and Prakash, 2016). Aminoglycosides, such as gentamicin, are often used as a combination therapy, and the low usage of this antibiotic may have contributed to the low resistance levels observed in our findings.
With regard to to trimethoprim-sulfamethoxazole antibiotic, our study found resistance levels of 48% and 42.8% for Klebsiella spp. and Escherichia coli. This finding was consistent with levels of 47% and 48% reported in Kuwait (Sewify et al., 2016). An even higher level of resistance of 81.8% was reported in a study in Turkey (Gulay et al., 2019). The levels of resistance observed for trimethoprim-sulfamethoxazole in our study are quite concerning, and may be due to the inappropriate use of these antibiotic.
Our study also reports for the first time the prevalences of phylogenetic groups and their virulence factors in 22 E. coli isolates from diabetic patients in Ghana. The majority of the virulence genes were found to be more diverse in phylogenetic group B2 isolates, and the detection of toxin gene cnf1 may participate in the dissemination of E. coli strains (Wiles et al., 2008). Furthermore, the association of toxin gene cnf1 with group B2 supports the identity of this group as a more virulent strain. The rapid surge in the population of resistant E. coli among hospitalized patients is of public health concern (Lee et al., 2015). Therefore, the timely detection of antibacterial-resistant E. coli strains, phylogenetic groupings, and virulence factor genes is of utmost priority in order to monitor resistance phenotypes as well as to provide physicians with appropriate treatment guidelines (Aghemwenhio et al., 2017). It has been reported that E. coli of the phylogenetic groups A and B1 are commensals, while groups B2 and D are more pathogenic and have been associated with extra-intestinal infections (Clermont et al., 2000). The majority of E. coli in this study belonged to phylogenetic group B2, and these contained most of the virulence genes. This finding was consistent with studies from different geographical regions that have documented higher prevalences of group B2 (Bashir et al., 2012; Iranpour et al., 2015; Miranda-Estrada et al., 2017). However, in contrast to our findings, a previous study has reported a higher prevalence of group A (Kabiswa et al., 2018). The prevalences and distributions of E. coli phylogenetic groups have been recorded to be largely associated with the health status of the patients, patients’ genetic factors, dietary factors, antibiotic stewardship, and geographical conditions (Derakhshandeh et al., 2013).
CONCLUSION
Our study revealed a UTI prevalence of 9.2% among diabetic patients, with Klebsiella spp. and Escherichia coli being the predominant uropathogens identified. Female patients within the age range 61–70 years were the most infected. The associated risk factors for urinary tract infections in this study were age, duration of diabetes, and previous history of UTI. The uropathogens exhibited a high level of resistance to ampicillin, ceftriaxone, trimethoprim-sulfamethoxazole, nalidixic acid, and amoxicillin-clavulanate. Most of the E. coli isolates identified in this study belonged to the phylogenetic groups B2 and D, and they contained a range of virulence factor genes.
Declaration of Competing Interests
The authors declare that they have no competing interests.
Acknowledgments
Ethical approval and consent to participate
This study was approved by the Ethics Committee of the College of Health Sciences, the University of Ghana (Ethics Identification Number: CHS-ET/M.2-PS.9/2018-2019). Participation was voluntary, and written consent was obtained from each participant, following the Ethics Committee's guidelines.
Consent to publish
Not applicable
Availability of data and materials
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors’ contributions
FOA and FSC conceived, designed, and performed the study, and drafted the manuscript. DAM and MNQ performed the experiments and drafted some sections of the manuscript. SBD and OTM assisted with experimental analysis, and interpreted the data. All authors were involved in the drafting of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to express their gratitude to all the staff of the National Diabetes Management and Research Centre (NDMRC) in the Korle-Bu Teaching Hospital (KBTH), and all the diabetic patients for their cooperation and support during the various aspects of the study. Special thanks go to Dr James R. Johnson, Adam L. Stell and Brian Johnston of the University of Minnesota Department of Medicine and Infectious Diseases for providing the positive controls for the virulence factors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2021.10.007.
Contributor Information
Akua Obeng Forson, Email: asobeng@ug.edu.gh, obeng.akua@yahoo.com.
Francis Samuel Codjoe, Email: fscodjoe@chs.edu.gh.
Appendix. Supplementary materials
REFERENCES
- Aghemwenhio IS, Timilehin AA, Alpheus GA. Susceptibility of beta-haemolytic Escherichia coli to commonly used antibiotics in selected hospitals in Delta State, Southern Nigeria. Arc Clin Microbiol. 2017;8(2):36. [Google Scholar]
- Alimohammadi H.N.F. Study of urinary tract infection in diabetic and non-diabetic patients and antibiotic sensitivity pattern of isolated organisms. 17th European Congress of Clinical Microbiology and Infectious Diseases ICC; Munich, Germany; 2007. pp. 234–239. [Google Scholar]
- Al-Rubeaan A.K., Moharran O., Al-Naqeb D., Hassan A., Rafiullah M.R.M. Prevalence of urinary tract infection and risk factors among Saudi patient with diabetes. World J Urol. 2013;31:573–578. doi: 10.1007/s00345-012-0934-x. [DOI] [PubMed] [Google Scholar]
- Aswani S.M., Chandrashekar U.K., Shivashankara K.N., Pruthvi B.C. Clinical profile of urinary tract infections in diabetics and non-diabetics. Australasian Medical Journal. 2014;7(1):29–34. doi: 10.4066/AMJ.2014.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antwi S., Bates I., Baffoe-Bonnie B., Critchley J. Urine dipstick as a screening test for urinary tract infection. Annals of Tropical Paediatrics: International Child Health. 2008;28(2):117–122. doi: 10.1179/146532808X302134. [DOI] [PubMed] [Google Scholar]
- Bashir S, Haque A, Sarwar Y, Ali A, Anwar MI. Virulence profile of different phylogenetic groups of locally isolated community acquired uropathogenic E. coli from Faisalabad region of Pakistan. Ann Clin Microbiol Antimicrob. 2012;11:23. doi: 10.1186/1476-0711-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briongos-Figuero L.S., Gomez-Traveso T., Bachiller-Luque P., et al. Epidemiology, risk factors and comorbidity for urinary tract infections caused by extended-spectrum beta-lactamase (ESBL)-producing Enterobacteria. International Journal of Clinical Practice. 2012;66(9):891–896. doi: 10.1111/j.1742-1241.2012.02991.x. [DOI] [PubMed] [Google Scholar]
- Bissong M.E.A., Fon P., Tabe-Besong F., et al. Asymptomatic bacteriuria in diabetic mellitus patients in southwest Cameroun. Afr Health Sci. 2013;13(3):661–666. doi: 10.4314/ahs.v13i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen-Bidois M., Clermont O., Bonacorsi S., Terki M., Brahimi N., Loukil C., et al. Phylogenetic analysis and prevalence of urosepsis strains of Escherichia coli bearing pathogenicity island-like domains. Infect Immun. 2002;70:3216–3226. doi: 10.1128/IAI.70.6.3216-3226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko E.J., Lipsky B.A. In: Diabetes in America. 2nd ed. National Diabetes Data Group, editor. Harris; Bethesda, MA: 1995. Infection in diabetes; pp. 485–499. editor. [Google Scholar]
- Chiţă T., Timar B., Muntean D., Bădiţoiu L., Horhat F., Hogea E., Moldovan R., Timar R, Licker M. Urinary tract infections in Romanian patients with diabetes: prevalence, etiology, and risk factors. Therapeutics and Clinical Risk Management. 2017;13:1–7. doi: 10.2147/TCRM.S123226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiţă T, Licker M, Sima A, Vlad A, Timar B, Sabo P, et al. Prevalence of urinary tract infections in diabetic patients. Romanian Journal of Diabetes Nutrition & Metabolic Diseases. 2013;20(2l):99–105. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; twenty-fifth informational supplement. CLSI document M100-S25 Clinical and Laboratory Standards Institute, Wayne, Pennsylvania, USA. 2015.
- Clermont O, Christenson JK, Denamur E, Gordon DM. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo groups. Environ Microbiol Rep. 2013;5:58–65. doi: 10.1111/1758-2229.12019. [DOI] [PubMed] [Google Scholar]
- Clermont O., Bonacorsi S., Bingen E. Rapid and simple determination of the Escherichiacoli phylogenetic group. Applied and Environmental Microbiology. 2000;66:4555–4558. doi: 10.1128/aem.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel W, W. 7th edition. John Wiley & Sons; New York: 1999. Biostatistics: A foundation for analysis in the health sciences. [Google Scholar]
- Das R.N., Chandrashekhar T.S., Joshi H.S., Gurung M., Shrestha N., Shivananda P.G. Frequency and susceptibility profile of pathogens causing urinary tract infections at a tertiary care hospital in western Nepal. Singapore Medical Journal. 2006;47(4):281. [PubMed] [Google Scholar]
- Derakhshandeh A., Firouzi R., Moatamedifar M, et al. Phylogenetic analysis of Escherichia coli strains isolated from human samples. Molecular Biology Research Communications. 2013;2(4):143–149. [Google Scholar]
- Desouky D.E., Gabr Hala M., El-Helbawy M, Hathout H.M. Urinary tract infection: prevalence, risk factors, bacterial etiologies and antimicrobial resistance profile among Egyptian diabetic patients. European Journal of Medical and Health Sciences. 2020;2:4. [Google Scholar]
- Donkor E., Darkwah S., Akpalu A. Post-Stroke Bacteriuria: a longitudinal Study among stroke outpatients and inpatients at the Korle-Bu teaching Hospitalin Ghana. Med Sci. 2017;5(2):11. doi: 10.3390/medsci5020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers C., Janssen T., Kiessling S., Philipp H.C., Wieler L.H. Rapid detection of virulence-associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction. Avian Dis. 2005;49:269–273. doi: 10.1637/7293-102604R. [DOI] [PubMed] [Google Scholar]
- Firoozeh F, Saffari M, Neamati F, Zibaei M. Detection of virulence genes in Escherichia coli isolated from patients with cystitis and pyelonephritis. Int J Infect Dis. 2014;29:219–222. doi: 10.1016/j.ijid.2014.03.1393. [DOI] [PubMed] [Google Scholar]
- Foxman B.e. Epidemiology of urinary tract infection. Nature Reviews Urology. 2010;7(12):653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- Geerlings S.E., Stolk R.P., Camps M.J., et al. Asymptomatic bacteriuria may be considered a complication in women with diabetes. Diabetes Care. 2000;23(6):744–749. doi: 10.2337/diacare.23.6.744. [DOI] [PubMed] [Google Scholar]
- Gutema T., Weldegebreal F., Marami D, Teklemariam Z. Prevalence, antimicrobial susceptibility pattern, and associated factors of urinary tract infections among adult diabetic patients at Metu Karl Heinz Referral Hospital, Southwest Ethiopia. International Journal of Microbiology. 2019:7. doi: 10.1155/2018/7591259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulay S.B., Filiz E.H, Sule C., Okan S.B., Kursad A.O., Melek E.E. Urinary tract infection in diabetes: susceptible organisms and antibiogram patterns in an outpatient clinic of a tertiary health care center. Medicine Science. 2019;8(4):881–886. [Google Scholar]
- Hall V., Thomsen R.W., Henriksen O., Lohse N. Diabetes in Sub-Saharan Africa 1999–2011: epidemiology and public health implications. A systematic review. BMC Public Health. 2011;11(1):564. doi: 10.1186/1471-2458-11-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan Z.H., Eman K., Amar M.A., Onab S.H., Sarah O.S., Ishag A. Urinary tract infections and antimicrobial sensitivity among diabetic patients at Khartoum, Sudan. Annals of Clinical Microbiology and Antimicrobials. 2015;14(26):1–6. doi: 10.1186/s12941-015-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirji I., Guo Z., Andersson S.W., Hammar N., Gomez-Caminero A. Incidence of urinary tract infection among patients with type 2 diabetes in the UK General Practice Research Database (GPRD) Journal of Diabetes Complications. 2012;26(6):513–516. doi: 10.1016/j.jdiacomp.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Huang S.H., Wass C., Fu Q., Prasadarao N.V., Stins M., Kim K.S. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect Immun. 1995;63:4470–4475. doi: 10.1128/iai.63.11.4470-4475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifediora A.C., Obeagu E.I., Akahara I.C., Eguzouwa U.P. Prevalence of urinary tract infections in diabetics patients attending Umuahia Health Care Facilities. Journal of Bio Innovation. 2016;5(1):68–82. [Google Scholar]
- Iranpour D., Hassanpour M., Ansari H., Tajbakhsh S., Khamisipour G., Najafi A. Phylogenetic groups of Escherichia coli strains from patients with urinary tract infection in Iran based on the new Clermont phylotyping method. BioMed Research International. 2015:1–7. doi: 10.1155/2015/846219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janifer J., Geethalakshmi S., Satyavani K., Viswanathan V. Prevalence of lower urinary tract infection in South Indian type 2 diabetic subjects. Indian Journal of Nephrology. 2009;19(3):107–111. doi: 10.4103/0971-4065.57107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J R. Stell A L,Extended virulencce genotype of Escherichia coli strains from patients with urosepsis in relationship to phylogeny and host compromise. The J Infect Dis. 2000;181(1):261–272. doi: 10.1086/315217. [DOI] [PubMed] [Google Scholar]
- Johnsson K.M., Ptaszynska A., Schmitz B., Sugg J., Parikh S.J., List J.F. Urinary tract infections in patients with diabetes treated with dapagliflozin. Journal of Diabetes and its Complications. 2013;27(5):473–478. doi: 10.1016/j.jdiacomp.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Johnson TJ, Wannemuehler Y, Johnson SJ, Stell AL, Doetkott C, Johnson JR, Kim KS, Spanjaard L, Nolan LK. Comparison of extraintestinal pathogenic Escherichia coli strains from human and avian sources reveals a mixed subset representing potential zoonotic pathogens. Appl Environ Microbiol. 2008;74(22):7043–7050. doi: 10.1128/AEM.01395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR, Gajewski A, Lesse AJ, Russo TA. Extraintestinal pathogenic Escherichia coli as a cause of invasive non urinary infections. J Clin Microbiol. 2003;41(12):5798–5800. doi: 10.1128/JCM.41.12.5798-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR, Stell AL. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis. 2002;181(1):261–272. doi: 10.1086/315217. [DOI] [PubMed] [Google Scholar]
- Kabiswa W., Nanteza A., Tumwine G., Majalija S. Phylogenetic groups and antimicrobial susceptibility patterns of Escherichia coli from healthy chicken in eastern and central Uganda. Journal of Veterinary Medicine. 2018:1–6. doi: 10.1155/2018/9126467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebamo S., Regea D.R., Deressa A., Mohammed G. Urinary tract infection: bacterial etiologies, drug resistance profile and associated risk factors among diabetic patients attending NRH. American Journal of Current Microbiology. 2017;5 [Google Scholar]
- KonemannAllen E.S., Janda W. Koneman’s Color Atlas and Textbook of Diagnostic Microbiology. Sixth edition. Lippincott Williams& Wilkins; Philadelphia, USA: 2006. [Google Scholar]
- Lee JH, Subhadra B, Son YJ, Kim DH, Park HS, Kim JM, et al. Phylogenetic group distributions, virulence factors and antimicrobial resistance properties of uropathogenic Escherichia coli strains isolated from patients with urinary tract infections in South Korea. Lett Appl Microbiol. 2015;62(1):84–90. doi: 10.1111/lam.12517. [DOI] [PubMed] [Google Scholar]
- Malekzadegan Y, Khashei R, Sedigh Ebrahim-Saraie H, Jahanabadi Z. Distribution of virulence genes and their association with antimicrobial resistance among uropathogenic Escherichia coli isolates from Iranian patients. BMC Infect Dis. 2018;18(1):572. doi: 10.1186/s12879-018-3467-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- Mgbakogu RA, Eledo BO. Studies on urinary tract infection among diabetic patients in some eastern state of Nigeria. Advances in Life Sciences and Technology. 2015;34 [Google Scholar]
- Mogaka M.V.M. Prevalence of urinary tract infections among type 2 diabetic patients in Kissi Teaching Hospital and Referral Hospital, Kenya. Journal of Medical Sciences. 2020;8(8):1–4. [Google Scholar]
- MOH . 6th ed. 2010. Ministry of Health, Ghana. Standard treatment guidelines. Available from: http://who.int/medicinedoc. [Google Scholar]
- Miranda-Estrada LI, Ruíz-Rosas M, Molina-López J, Parra-Rojas I, González-Villalobos E, Castro-Alarcón N. Relationship between virulence factors, resistance to antibiotics and phylogenetic groups of uropathogenic Escherichia coli in two locations in Mexico. Enferm Infecc Microbiol Clin. 2017;35(7):426–433. doi: 10.1016/j.eimc.2016.02.021. [DOI] [PubMed] [Google Scholar]
- Nabaigwa B., Mwambi B., John O.J., Oyet C. Common uropathogens among diabetic patients with urinary tract infection at Jinja Regional Referral Hospital, Uganda. African Journal of Laboratory Medicine. 2017;7(1):a621. doi: 10.4102/ajlm.v7i1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitzan O., Elias M., Chazan B., Saliba W. Urinary tract infections in patients with type 2 diabetes mellitus: review of prevalence, diagnosis, and management. Diabetes Metab Syndr Obes. 2015;8:129–136. doi: 10.2147/DMSO.S51792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opintan J.A., Newman M.J., Arhin R.E., Sampane-Donkor E.S., Gyansa-Lutterodt M., Mills-Pappoe W. Laboratory-based nationwide surveillance of antimicrobial resistance in Ghana. Infection and Drug Resistance. 2015;8:379–389. doi: 10.2147/IDR.S88725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orenstein R., Wong E.S. Urinary tract infections in adults. American Family Physician. 1999;59:1225–1234. [PubMed] [Google Scholar]
- Piette J.D, Kerr E.A. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725–731. doi: 10.2337/diacare.29.03.06.dc05-2078. [DOI] [PubMed] [Google Scholar]
- Rowe T.A., Juthani-Mehta M. Urinary tract infection in older adults. Aging Health. 2013;9(6) doi: 10.2217/ahe.13.38. 10.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakyi S.A., Ephraim R.K.D., Adebisi B.O., Yeboah J.O., Osei-Berchie G. Asymptomatic bacteriuria among type 2 diabetics in the Sekondi-Takoradi Metropolis, Ghana. Journal of Medical Sciences. 2013;13:290–295. [Google Scholar]
- Sewify M., Shinu N., Samia W., Mohamed M., Asma A., Kazem B., Faisal A., Ali T. Prevalence of urinary tract infection and antimicrobial susceptibility among diabetic patients with controlled and uncontrolled glycemia in Kuwait. Journal of Diabetes Research. 2016:7. doi: 10.1155/2016/6573215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharna V., Gupta V., Mittal M. Prevalence of uropathogens in diabetic patients and their antimicrobial susceptibility pattern. National Journal of Laboratory Medicine. 2012;1(1):26–28. [Google Scholar]
- Storme O., Saucedo J.T., Garcia-Mora A., Dahesa-Davila M., Naber K.G. Risk factors and predisposing condition for urinary tract infection. Therapeutic Advances in Urology. 2019;11 doi: 10.1177/1756287218814382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs S., Cserhati I., Csapo G., Balazs V. The relation between diabetes mellitus and infections of the unirary tract. A clinical, qualitative and quantitative bacteriological study based upon 300 diabetics and 200 controls. The American Journal of the Medical Sciences. 1960;240:186–191. [PubMed] [Google Scholar]
- Venmans L., Hak E., Gorter K.J., Rutten G. Incidence and Antibiotic Prescription Rates for Common Infections in Patience with Diabetes in Primary Care Over years 1995–2003. International Journal of Infectious Diseases. 2009;13(6):e344–e351. doi: 10.1016/j.ijid.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Walsh C., Collyns T. Pathophysiology of urinary tract infections. Surgery (Oxford) 2017;35(6):293–298. [Google Scholar]
- We I., Adeniyi B.A., Soge O.O. Prevalence of multidrug resistant Acinetobacter baumannii in eight tertiary hospitals in southwestern Nigeria. New York Science Journal. 2014;7(11):86–93. [Google Scholar]
- Wiles T.J., Kulesus R.R., Mulvey M.A. Origins and virulence mechanisms of s Escherichia coli. Exp Mol Pathol. 2008;85:11–19. doi: 10.1016/j.yexmp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, K and Prakash, S. Antimicrobial resistance pattern of uropathogens causing urinary tract infection (UTI) among diabetics. 2016;1:07–15
- Yamamoto S., Terai A., Yuri K., Kurazono H., Takeda Y., Yoshida O. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol Med Microbiol. 1995;12:85–90. doi: 10.1111/j.1574-695X.1995.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Yeshitela B., Gebre-Selassie S., Feleke Y. Asymptomatic bacteriuria and symptomatic urinary tract infections (UTI) in patients with diabetes mellitus in Tikur Anbessa Specialized University Hospital. Addis Ababa, Ethiopia. Ethiopian Medical Journal. 2012;50(3):239–249. [PubMed] [Google Scholar]
- Yu S., Fu A.Z., Qiu Y. Disease burden of urinary tract infections among type 2 diabetes mellitus patients in the US. Journal of Diabetes Complications. 2014;28(5):621–626. doi: 10.1016/j.jdiacomp.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Zhanel G.G, Nicolle L.E, Harding G.K.M. Prevalence of asymptomatic bacteriuria and associated host factors in women with diabetes mellitus. The Manitoba Diabetic Urinary Infection Study Group. Clinical Infectious Diseases. 1995;21:316–322. doi: 10.1093/clinids/21.2.316. [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen X, Zhu X, Yang W, Dong L, Xu X, et al. Prevalence of virulence factors and antimicrobial resistance of uropathogenic Escherichia coli in Jiangsu province (China) Urology. 2009;74:702–707. doi: 10.1016/j.urology.2009.01.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.