Abstract
Growing evidence indicates that disturbances in the inflammatory response system can have deleterious effects on neuronal function and mental health. While the correlation between elevated peripheral inflammatory markers and psychiatric disorders are well documented, the exact molecular and neuronal mechanism underlying the connection between activated inflammation and neuropsychiatric behaviour remain elusive. Microglia activation is the key interface between neuro-inflammation and manifestation of psychiatric symptoms. Microglia are immunocompetent cells in the central nervous system (CNS) which are primarily involved in the response to inflammatory stimulation and are widely used to study neuroinflammation and test anti-inflammatory chemicals. In the brain, activated microglia play very important roles during neuroinflammation and neurodegeneration. Both stress-related disorders such as Depression and PTSD, and medical conditions such as metabolic syndrome (Mets) and type 2 diabetes (TD2) are associated with increased levels of both saturated fatty acids (SFAs) and lipopolysaccharide (LPS) in circulation. This work was aimed at determining whether SFA interacts with LPS to activate microglia, thus up-regulating neuroinflammatory processes and, if so which pathways were involved in this process. Our results showed that low-dose LPS and palmitic acid (PA) robustly stimulated the expression of proinflammatory cytokines, and the combination of PA and LPS further upregulated proinflammatory cytokines through MAPK, NFκB and AP-1 signaling pathways in the HMC3-human microglial cell line. In addition, PA stimulated ceramide production via de novo synthesis and sphingomyelin hydrolysis, and the combination of LPS and PA further increased ceramide production. HMC3 co-cultured with macrophage and lymphocyte enhanced LPS and PA induced-inflammatory response more than that in HMC3 alone. These results indicate that LPS interacts with PA to activated microglia; induced neuroinflammatory responses, upregulate proinflammatory cytokine expression via MAPK, NFκB, and AP-1 signaling pathways, and induced sphingolipid metabolism in HMC3. These observations suggest that inhibiting microglia activation and reducing LPS and PA-induced inflammatory response may be useful in the treatment of neuronal inflammatory diseases.
Keywords: Lipopolysaccharides, Palmitic acid, Sphingolipid, Microglia, Neuroinflammation
Highlights
-
•
Microglial activation plays critical role in the pathology of various psychiatric conditions.
-
•
PA interacts with LPS to active microglia induce proinflammatory cytokine and gene expression.
-
•
PA and LPS stimulate the MAPK and NFκB signaling pathway regulated IL-6 secretion in microglia.
-
•
U937 and lymphocyte enhance IL-6 secretion in microglia.
-
•
PA and LPS plus PA increase ceramide and decrease sphingomyelin productions.
1. Introduction
The role of immune system dysregulation in psychiatric disorders has attracted considerable attention over the past decades. Indeed, numerous studies have reported increased circulating inflammatory cytokines, e.g. interleukin (IL)-1, IL-6, and tumour necrosis factor (TNF), their soluble receptors, and C-reactive protein (CRP), in patients with mood, anxiety, and stress-related disorders, such as major depressive disorder (MDD) generalized anxiety disorder (GAD), panic disorder and post-traumatic stress disorder (PTSD) [[1], [2], [3], [4]]. While these disorders may have complex etiologies, it is highly possible that the heightened inflammation may be involved in the disease process and contribute to disease symptomologies. Research indicates that inflammatory signals in the body may impact the brain to drive behavioral symptoms relevant to mood and anxiety-related disorders. Access of peripheral inflammatory signals to the brain may involve trafficking of peripheral immune cells to the brain, and cytokine-induced activation of local inflammatory signaling pathways and microglia [5,6].
Microglia are primary immunocompetent cells in the central nervous system (CNS), also known as “macrophages of the CNS”, known to perform multiple roles on the maintenance of brain homeostasis. Microglias interact with other cells to support neuronal function and regulate neuroinflammation [7,8]. The linkage between peripheral immune activation and neuroinflammation has been shown in humans receiving systemic administration of endotoxin, which leads to peripheral inflammation and microglia activation in the brain [9]. Once activated, brain microglia released inflammatory mediators such as IL-6, IL-1, and TNFα. These inflammatory mediators can affect neurotransmission, mediate neurotoxicity by increasing glutamate production and inducing apoptosis, and directly influence behaviour [10,11].
It is known that the gastrointestinal microbiota is a key regulator of stress, immune response, and neuroinflammation [12,13]. Alterations in the composition of gut microbiota are associated with a variety of disease states including anxiety, depression, obesity, diabetes and inflammation. Elevated levels of circulating free fatty acids (FFAs) and increased incidence of chronic systemic inflammation are associated with obesity [14]. FFAs, in particular, saturated fatty acids (SFA), are known to upregulated proinflammatory cytokine expression [15,16]. Palmitate, the most abundant SFA in human plasma, play an important role in triggering hypothalamic inflammation through microglial activation [17,18].
Lipopolysaccharide (LPS) is the most abundant component within the cell wall of Gram-negative bacteria. It stimulates release/secretion of inflammatory cytokines such as IL-6, IL-1β, and TNFα in various cell types and leads to an inflammatory response [19]. LPS binding to Toll-like receptor 4 (TLR4) leads to the activation of NFκB and AP-1 signal transduction pathways [20]. High-fat diet increase permeability of intestinal epithelium that facilitates LPS translocation from the intestine to the bloodstream. Moreover, type 2 diabetes (TD2) is potentially associated with increased levels of both LPS and SFA in circulation [21]. However, it remains largely unknown how SFA interacts with LPS to regulate proinflammatory cytokine expression in microglia, which leads to neuroinflammation.
The brain has the highest sphingolipid content, and the changes of lipid levels can initiate pathogenic processes in neuroinflammatory diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) [22,23]. Sphingolipid metabolism involves several intermediate metabolites including sphingomyelin, ceramide, sphingosine and, sphingosine-1-phosphate (S1P). Sphingolipid metabolites are emerging as important signaling molecules that regulate cell growth, survival, immune cell trafficking and inflammation [24,25]. In the microglia, long-chain ceramide-induces proinflammatory response through activation of NFκB pathway [26]. However, it remains largely unknown how SFA interacts with LPS to regulate neuroinflammatory response in microglia.
In the present work, we investigated the effects of a low-dose LPS and PA on microglia cell line (HMC3) and the signaling and molecular mechanisms involved in pro-inflammatory response. Addition of LPS and PA to microglia cells led to significantly induced secretion of proinflammatory mediators. In addition, when HMC3 were co-cultured with human microphage or lymphocyte further increased inflammatory cytokine secretion. Those findings strongly indicate that HMC3 is a valuable experimental model of neuroinflammatory signaling in the CNS, and is a suitable candidate to examine possible mechanisms to elicit anti-inflammatory responses and to effectively develop drug therapy for neuroinflammation.
2. Materials and methods
2.1. Primary cell culture and treatment
The human microglia clone 3 cell line (HMC3) was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). HMC3 cells were cultured in Eagle’s Minimum Essential Media (EMEM, ATCC)) supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO, USA) and 100 units/ml (U/ml) penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA), and incubated in a humidified atmosphere (5% CO2) at 37 °C.
For cell treatment, E. Coli LPS and Palmitic acid (PA, Sigma, St. Louis, MO, USA) were used. PA used in this study was bovine serum albumin-free. PA was dissolved in 0.1 N NaOH and 70% ethanol at concentration of 50 mM. The 12-well Corning Transwell plates (Fisher, Waltham, MA, USA) that have 2 compartments separated by a polycarbonate membrane with 0.4 μm pores were used for co-culture of HMC3, human lymphocytes, or human macrophage cell line U937. HMC3 was placed in the lower compartment, and U937 cells or human lymphocyte were grown (1 × 106 cells/well) in the upper compartment. U937 cells were purchased from ATCC. The cells were cultured in a 5% CO2 atmosphere in Roswell Park Memorial Institute (RPMI) 1640 medium (GIBCO, Invitrogen Corp, Carlsbad, CA, USA) containing 10% FBS. Human lymphocytes were isolated from blood obtained from healthy donors and treated in the same medium as that used for U937 cells. Lymphocytes were activated with Dynabeads™ Human T-Activator CD3/CD28 for T Cell Expansion and Activation (Thermo Fisher, Waltham, MA, USA) and IL-2 supplement. The blood donation for lymphocyte isolation was approved by university Institutional Review Board.
2.2. Cell Proliferation Assay
Cell viability and proliferation were determined using a MTT (3-(4, 5 dimethylthiazolyl-2-yl)-2, 5-diphenyltetrazolium bromide) Cell Proliferation Assay kit (ThermoFisher, Waltham, MA, USA) by following the instruction provided by the manufacturer.
2.3. Enzyme-linked immunosorbent assay
Cytokines were quantified in the medium using sandwich enzyme linked immunosorbent assay (ELISA) kits according to the protocol provided by the manufacturer (Biolegend, San Diego, CA, USA).
2.4. Flow cytometric staining and analysis (FACS)
Fluorescence-conjugated mouse anti-human antibodies and kits were all purchased from BD Biosciences (San Diego, CA). Anti-CD11b-APC, anti-CD86-PE-Cy7, anti-CD206-PE, anti-TLR4-PE, anti-CCR7-APC, anti-CD163-PerCP-Cy5.5, anti-Arg1-PE and anti-TNF- PerCP-Cy5.5 were used to determine the cell surface microglial marker expressions. Following cell fixation and permeabilization with Cytofix/Cytoperm kit, anti-anti-CD68-Alexa Fluor 647 antibody was used to determine the intracellular microglial marker expression. The stained cells were phenotypically analyzed using a FACSCanto flow cytometer (BD Biosciences).
Cytokine and chemokine profiles in culture medium were detected using BD cytometric bead array (CBA) human Th1/Th2/Th17 and chemokine kit, according to the manufacturer’s instructions (BD Biosciences). Samples were acquired and measured on the BD FACSCanto flow cytometer and analyzed by FCAP Array software to generate results. All samples were examined in duplicate.
2.5. RNA isolation and quantitative real-time PCR
Total RNA was isolated from cells using RNeasy minikit (Qiagen, Santa Clarita, CA, USA). First-strand complementary DNA (cDNA) was synthesized with the iScript™ cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA) using 15 μl of reaction mixture containing 1 μg of total RNA, 4 μl of 5 × iScript reaction mixture, and 1 μl of iScript reverse transcriptase. The complete reaction was cycled for 5 min at 25 °C, 30 min at 42 °C and 5 min at 85 °C using a PTC-200 DNA Engine (MJ Research, Waltham, MA, USA). The sequences of the real-time PCR are presented in Supplementary Table S1. Primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA, USA). RT-qPCR was repeated three times per sample using a real-time PCR system (CFX96, Bio-Rad). The expression levels of all mRNAs were normalized to the mRNA level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) followed by quantitative analyses according to the relative quantitation Ct (2−ΔΔCt) method.
2.6. PCR array
Human TLR signal pathway PCR array (Qiagen, Santa Clarita, CA, USA) was used to profile gene expression according to the instructions from the manufacturer.
2.7. Luciferase assay
Cells were seeded into 96-well plates and co-transfected with a mixture of NFκB and AP-1 luciferase reporter (Qiagen, Santa Clarita, CA, USA) using Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA). Cells were lysed 12 and 24 h after incubation with LPS, PA and LPS plus PA. Luciferase activity was determined using the Dual-Luciferase Reporter Assay System (Promega, Madison, MI, USA) according to the manufacturer’s instructions.
2.8. Lipidomics
HMCs were collected, fortified with internal standards, extracted with ethyl acetate/isopropyl alcohol/water (60:30:10, v/v/v), evaporated to dryness, and reconstituted in 100 μl of methanol. Simultaneous ESI/MS/MS analyses of sphingoid bases, sphingoid base 1-phosphates, ceramides, and sphingomyelins (SM) were performed on a Thermo Finnigan TSQ 7000 triple quadrupole mass spectrometer operating in a multiple reaction monitoring positive ionization mode. The phosphate contents of the lipid extracts were used to normalize the MS measurements of sphingolipids. The phosphate contents of the lipid extracts were measured with a standard curve analysis and a colorimetric assay of ashed phosphate.
2.9. Statistical analysis
All experiments were performed independently at least three times. Differences between conditions were quantitatively analyzed using GraphPad statistical software (GraphPad Software, Inc. La Jolla, CA, USA). The oneway analysis of variance (ANOVA) was used to analyze data from multiple groups. For data with normal distribution, Student’s t-test was used for comparison of means between two experimental groups. p values were regarded as statistically significant if it was less than 0.05, and significant differences are depicted as follows: ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
3. Results
3.1. PA potentiates LPS induced proinflammatory cytokine and gene expression
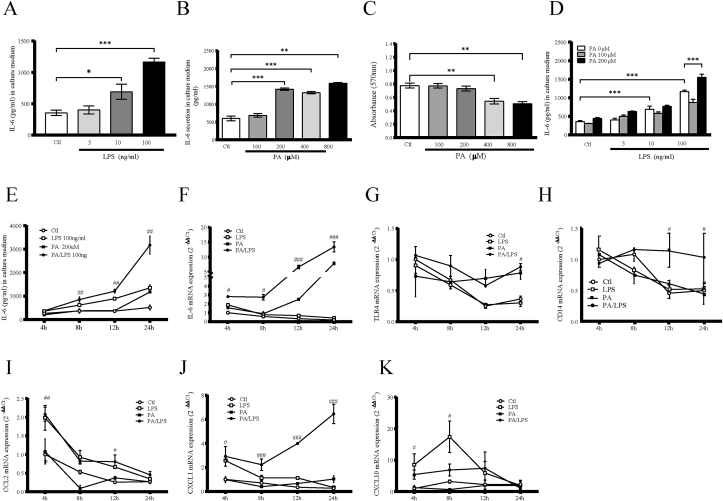
HMC3 microglial cell line was used as a model of human microglia. In the present study, we aimed to investigate the synergistic effect of LPS and PA on the expression of inflammatory cytokines in HMC3. Results showed that LPS (Fig. 1A) and PA (Fig. 1B) stimulated IL-6 secretion from HMC3 in a concentration-dependent manner. To determine the cytotoxic effect of PA on HMC3, we evaluated the effect of increasing PA concentration on cell viability using the MTT assay. Incubation with 0–200 μM PA for 24 h did not affect the viability of HMC3, but exposure to 400–800 μM PA significantly decreased the cell viability (Fig. 1C). This indicate that cell viability tended to be suppressed when the PA concentration was above 400 μM. LPS (100 ng/ml) and PA (200 μM) exerted the highest secretion of IL-6 secretion by HMC3 (Fig. 1D) without risk of affecting cell viability. Therefore, these concentrations were used in the experiments examining the synergy of these two compounds.
Fig. 1.
Effect of LPS, PA or LPS plus PA on IL-6 secretion in HMC3. The effect of LPS (A) and (B) PA on IL-6 secretion. HMC3 were treated with different concentrations of LPS (0–100 ng/ml) and PA (0–800 μM) for 24 h. After the treatment, IL-6 level in culture medium was quantified using ELISA. (C) MTT assay was performed. (D) The effect of LPS plus PA on IL-6 secretion. HMC3 were treated with 100 or 200 μM of PA in the absence or presence of 5, 10 or 100 ng/ml of LPS for 24 h and IL-6 in culture medium was then quantified. Values are expressed as means ± SEM. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001 vs control respectively. Time course of proinflammatory genes expression. HMC3 were treated with 100 ng/ml LPS, 200 μM of PA, or LPS plus PA respectively, and the cells were harvested at 4, 8, 12, and 24 h. (E) IL-6 in culture medium was quantified using ELISA. Total RNA was isolated from cells and IL-6 (F), TLR4 (G), CD14 (H), CCL2 (I), CXCL1 (J) and CXCL10 (K) mRNA was quantified using real-time PCR. mRNA levels were normalized against the levels of GAPDH mRNA respectively. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001 vs control respectively.
We performed a time course study to determine the effect of LPS and PA on the expression of IL-6 gene expression and protein secretion (Fig. 1E and F). PA had nearly no effect on IL-6 secretion before 12 h, LPS was the more potent stimulator of IL-6 secretion, and the combination of LPS and PA further increased IL-6 secretion and was higher than the release of IL-6 by cells stimulated by LPS alone (Fig. 1E). LPS induced IL-6 mRNA expression with a peak increase at 4 h, LPS plus PA showed more impact on IL-6 mRNA expression than LPS and PA alone (Fig. 1F). To compare the inflammatory response elicited by either LPS or PA and by the combination of LPS and PA, a PCR array analysis for several inflammatory genes was performed (Table 1). Several genes such as CCR2, CXCL1, CXCL2, CXCL10, IL-6 were significantly more upregulated by LPS/PA than LPS or PA alone, by contrast, some genes, such as CCL5, CD14, TLR4 were downregulated as observed when compared to their 4 h-level of expression which was used as control group. To confirm the findings from PCR array, gene expression was quantified using real-time PCR. LPS and PA treatment significantly decreased TLR4 mRNA (Fig. 1G) and its co-receptor CD14 mRNA (Fig. 1H) levels after 12 and 24 h, but LPS plus PA had a synergistic effect on gene expression after 24 h. The expressions of TLR4 were confirmed by flow cytometry experiments after exposure to LPS, PA and LPS plus PA for 24 h. PA and PA plus LPS had strongly increased TLR4 expression (Fig. S1). In contrast, LPS, PA and LPS plus PA treatment significantly decreased CCL2 mRNA level (Fig. 1I). CXCL1 mRNA level were increased by LPS plus PA stimulation (Fig. 1J). LPS significantly increased CXCL10 mRNA expression after 4 and 8 h, PA had no effect on CXCL10 (Fig. 1K) and CXCL1 mRNA expression (Fig. 1J).
Table 1.
Gene expression Fold Up and Down-regulation (vs. 4 h Control group).
| Symbol | 4 h |
12 h |
|||||
|---|---|---|---|---|---|---|---|
| LPS | PA | LPS/PA | Ctl | LPS | PA | LPS/PA | |
| CCL2 | 2.09 | −1.25 | 1.07 | −3.67 | −1.41 | −2.83 | −1.25 |
| CCL22 | 1.90 | 1.62 | 1.51 | 2.51 | 1.87 | 2.37 | 3.17 |
| CCL24 | −1.78 | −1.85 | −1.02 | 2.76 | 2.05 | 2.61 | 2.41 |
| CCL3 | 1.37 | 1.39 | 1.21 | 2.72 | 2.02 | 2.57 | 2.95 |
| CCR1 | 1.43 | −1.26 | 1.90 | 2.49 | 1.85 | 2.59 | 2.17 |
| CCR2 | 1.87 | 2.22 | 2.21 | 2.36 | 2.16 | 1.80 | 22.82 |
| CXCL1 | 3.08 | −1.66 | 2.49 | −1.79 | 2.08 | −1.59 | 6.89 |
| CXCL10 | 2.06 | −1.66 | 1.63 | 1.27 | 1.09 | 1.20 | 3.88 |
| CXCL2 | 2.52 | −1.66 | 2.26 | −1.93 | 1.81 | 1.02 | 5.98 |
| IL15 | −1.05 | 1.42 | 1.46 | 3.57 | 2.20 | 3.74 | 3.42 |
| IL6 | 1.63 | 1.45 | 2.38 | −1.51 | −1.17 | 3.80 | 7.49 |
| CXCL8 | 1.89 | −2.00 | 1.82 | −2.73 | 1.17 | −1.48 | 4.42 |
| TNFSF14 | 0.55 | 1.08 | 1.37 | 4.21 | 3.13 | 3.98 | 3.68 |
| C3 | −1.72 | −1.87 | −2.37 | −2.25 | −1.92 | −1.48 | −2.58 |
| CCL2 | 2.09 | −1.25 | 1.07 | −3.67 | −1.41 | −2.83 | −1.25 |
| CCL11 | 1.18 | −1.24 | 1.02 | −1.79 | −1.19 | −3.46 | −2.32 |
| CCL5 | −1.55 | −1.50 | 1.09 | −4.18 | −4.76 | −4.42 | −4.79 |
| CD14 | −1.49 | −2.59 | −1.77 | −2.54 | −2.56 | −1.87 | −1.36 |
| CRP | −1.16 | −4.34 | −1.52 | −1.23 | −1.03 | 1.28 | 1.67 |
| CXCL6 | −1.18 | −2.26 | −2.21 | −1.11 | −1.74 | 1.02 | 2.45 |
| IL10RB | 1.09 | 1.14 | 1.09 | −2.06 | −3.58 | −3.01 | −4.39 |
| IL17A | −1.38 | −2.73 | −1.45 | −1.23 | −2.19 | −1.25 | 1.17 |
| TLR4 | −1.27 | 1.17 | 1.08 | −1.47 | −2.22 | −1.21 | 1.05 |
| TLR5 | −4.44 | −2.56 | −1.77 | −1.84 | −2.48 | −1.95 | −2.11 |
| TLR7 | −1.14 | 1.49 | 1.26 | −1.87 | −3.35 | −2.14 | −2.27 |
| GAPDH | −1.07 | −1.33 | −1.17 | −1.89 | −1.03 | −1.02 | −1.21 |
HMC3 were treated with 100 ng/ml of LPS, 200 μM of PA or LPS plus PA for 4 and 12 h. RNA was isolated and subjected to PCR array as described in the Methods. Bold numbers indicated fold changes (>2.0 or < -2.0) of gene expression compare with 4 h control group.
3.2. Effect of toll-like receptor ligands in LPS-Stimulated IL-6 expression
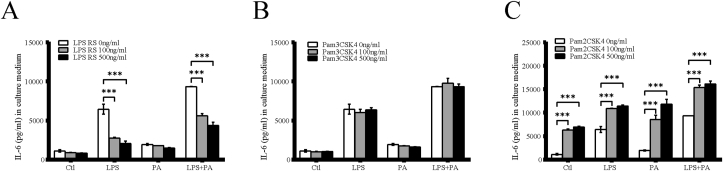
It has been shown that LPS binding to TLR4 will initiate an inflammatory response. LPS is usually used to activate TLR4 while Pam3CSK4 and Pam2CSK4 are used to activate TLR1/2 and TLR2/6, respectively. To investigate whether the release of IL-6 depends on engagement of TLR1, TLR2, and TLR4 on HMC3, we incubated the cells with 100 ng/ml of LPS and 200 μM PA alone, or a combination of LPS and PA (same concentration) in the absence or presence of 100, or 500 ng/ml LPS RS, Pam3CSK4 or Pam2CSK4. While PA had no effect on IL-6 secretion, LPS strongly increased IL-6 secretion and the combination of LPS and PA led to further increase of IL-6. Interestingly, LPS-RS attenuated the stimulatory effect of LPS or LPS plus PA on IL-6 secretion (Fig. 2A), indicating that LPS-RS is a potent TLR4 antagonist. In addition, we observed that Pam2CSK4 strongly enhanced LPS, PA and LPS plus PA-induced IL-6 secretion (Fig. 2B), whereas HMC3 are not responsive to TLR1/2 agonist, Pam3CSK4 (Fig. 2C). In conclusion, co-activation of TLR4 and TLR2/6 coordinates an additive augmentation of IL-6 secretion in HMC3.
Fig. 2.
IL-6 secretion by Toll-like receptor ligands. HMC3 were treated with 100 ng/ml of LPS, 200 μM of PA and LPS plus PA in the absence or presence of LPS RS (A), Pam3CSK4 (B) and Pam2CSK4 (C) for 24 h and IL-6 in culture medium was then quantified. Values are expressed as means ± SEM. ∗∗∗p < 0.001.
3.3. The MAPK and NFκB signaling pathway regulated IL-6 secretion stimulated by LPS, PA and LPS plus PA
To examine if changes in the inflammatory response stimulated by LPS, PA and LPS plus PA, cell culture medium was collected using the BD CBA kit to measure inflammatory cytokine and chemokine levels. As seen in Supplementary Fig. S2, LPS plus PA significantly increased the levels of IL-4, IL-2, IL-17A, IL-10, IFNγ, TNFα and IL-8 (Figs. S2A–F). LPS treatment strongly increased IL-8, MCP-1, RANTES and IP-10 secretion (Figs. S2G–J) and LPS plus PA also increased the level of IL-8. In contrast, PA treatment significantly inhibited MCP-1 secretion (Fig. S2H).
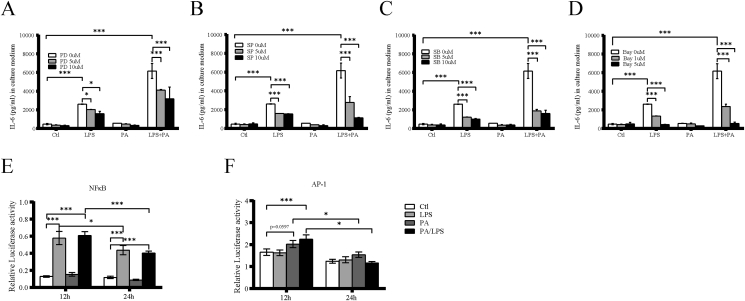
It is known that the MAPK and NFκB signaling are involved in proinflammatory gene expression. In this study, we analyzed whether MAPK and NFκB pathways are involved in the LPS and PA induced IL-6 expression using pharmacological inhibitors. The results showed that the ERK pathway inhibitor PD98059 (Fig. 3A), JNK pathway inhibitor SP600126 (Fig. 3B), p38 MAPK pathway inhibitor SB203580 (Fig. 3C), and NFκB pathway inhibitor Bay11-7082 (Fig. 3D) blocked the stimulatory effect of LPS and LPS plus PA on IL-6 secretion in a dose-dependent manner. These inhibitors did not affect IL-6 secretion induced by PA alone.
Fig. 3.
Involvement of MAPK and NFκB signaling pathway in IL-6 secretion stimulated by LPS, PA or LPS plus PA. HMC3 were treated with 100 ng/ml of LPS, 200 μM of PA or LPS plus PA in the absence or presence of (A) 5 or 10 μM SB-203580 (SB), an inhibitor for the p38 MAPK pathway, (B) 5 or 10 μM SP-600125 (SP), an inhibitor for the JNK pathway, (C) 5 or 10 μM PD 98059 (PD), an inhibitor for the ERK pathway, (D) 1 or 5 μM of Bay11-7082, inhibitor for NFκB pathway, for 24 h. After the treatment, IL-6 level in culture medium was quantified. HMC3 were transfected with the NFκB (E) and AP-1(F) luciferase reporter and stimulated with LPS, PA and LPS plus PA for 12 and 24 h and then relative luciferase activity was analyzed. Firefly luciferase was used as reporter, and renilla luciferase was used as a control. ∗p < 0.05, ∗∗∗p < 0.001.
NFκB and AP-1 activity response to LPS, PA and LPS plus PA was examined after NFκB and AP-1 luciferase vector transfection. LPS and LPS plus PA significantly increased NFκB luciferase activities approximately 4.5–5.0 fold and 3.5–4.0 fold after 12 and 24 h stimulation, respectively (Fig. 3E). AP-1 luciferase activities were induced by PA and LPS plus PA at 12 h, then decreased at 24 h. Surprisingly, LPS failed to increase AP-1 activation (Fig. 3F). These results indicate that the both MAPK and NFκB signaling pathways were involved in IL-6 upregulation induced by LPS, PA and LPS plus PA.
3.4. U937 and lymphocyte enhance IL-6 secretion in microglia
M1 and M2 polarization states are defined by specific phenotypic and secretory patterns. To determine whether the inflammatory effect of LPS and PA is associated with regulation of the expression of M1/M2 phenotype specific surface maker, CD68 and CD11b, the M1 maker, CD86, and M2 marker, CD206 was analyzed by flow cytometry in LPS and PA stimulated HMC3. CD68 expression was confirmed on HMC3 (Supplementary Fig. S3A). Furthermore, higher expression of CD86+CD206- was detected in response to PA (32.4%) and LPS plus PA (34.7%) stimulation compared to LPS (29.5%) control group (28.8%) (Supplementary Figs. S3B–E). The expression of CD206+CD86−expression was observed in response to PA and LPS plus PA. We also compared the other M1 (TNF and CCR7) and M2 (AGR1 and CD163) surface markers by flow cytometry. Our results showed PA and PA plus LPS increased M1 or M2 surface marker expression compared to LPS (Supplementary Fig. S4). These data demonstrate that HMC3 can be polarized to M1 states by stimulation with PA and LPS plus PA.
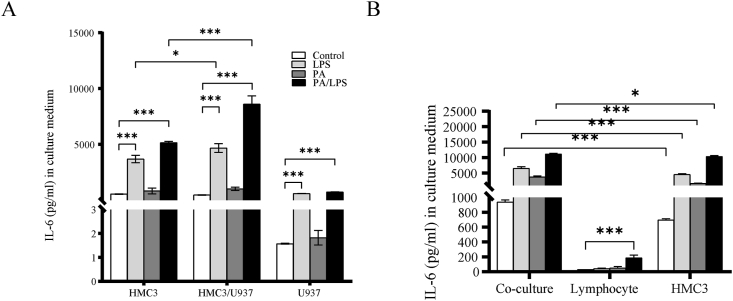
Neuroinflammation is a complex integration of the responses of all cells within the CNS including microglia, neurons, and infiltrating leukocytes. To investigate whether microglia interacts with macrophage or lymphocyte through released agents that enhance neuroinflammatory response, we treated U937 cells or health human lymphocyte with LPS, PA or LPS plus PA, and co-culture with HMC3 for 24 h. Quantification of IL-6 in culture medium showed that IL-6 secretion by the co-culture of HMC3 with U937 (Fig. 4A) or lymphocyte (Fig. 4B) was markedly increased when compared with IL-6 secretion by HMC3, U937 or lymphocyte when incubated alone. This data strongly suggests that soluble factors released by U937 or lymphocyte enhanced inflammatory response induced by LPS, PA and LPS plus PA on microglia cells.
Fig. 4.
Augmentation of LPS, PA and LPS plus PA stimulated IL-6 production by co-culture of microglia with U937 and human lymphocyte. HMC3 and U937 (A) or human lymphocyte (B) were cultured independently or together (co-culture) in the absence or presence of 100 ng/ml of LPS, 200 μM of PA, and LPS plus PA for 24 h. After the treatment, IL-6 level in culture medium was quantified using ELISA. Values are expressed as means ± SEM. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001.
3.5. PA and LPS plus PA increase ceramide and decrease sphingomyelin productions
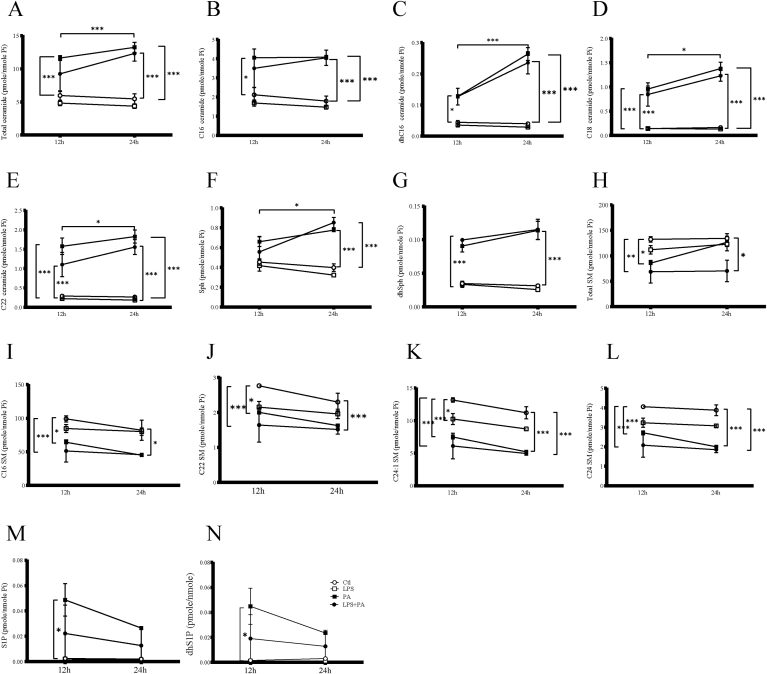
Since it has been reported that PA increases ceramide de novo synthesis by increasing the cellular level of palmitoyl-CoA, we examined the effect of PA and LPS on Ceramide production. Results showed that incubation of HMC3 cells with PA led to an increased ceramide production and that both ceramide as well as sphingosine and dihydrosphingosine production were further increased in cells incubated with a combination of LPS and PA, both at 12 and 24 h (Fig. 5A–G). Results also showed that a higher production of total dihydroceramide C16-, C18-, and C22- ceramide and sphingosine was observed at 24 h than at 12 h.
Fig. 5.
Effect of LPS, PA and LPS plus PA on ceramide de novo synthesis and Sphingomyelin (SM) hydrolysis. The effect of LPS, PA or LPS plus PA on cellular ceramide level. HMC3 were treated with 100 ng/ml of LPS, 200 μM of palmitate or LPS plus PA for 12 or 24 h and lipidomic analysis was conducted to quantify the cellular total (A), C16- (B), dhC16- (C), C18- (D), C22- (E) ceramide, total SM (F), C16-SM (G), C22-SM (H), C24:1-SM (I), C24-SM (J), Sph (K), dhSph (L), S1P (M), and S1P (N) levels. The data presented are mean ± SEM of duplicate samples. ○:Ctl,□:LPS, ■:PA,●: LPS/PA. Values are expressed as means ± SEM. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001.
While ceramide de novo synthesis is an important pathway to generate ceramide, sphingomyelin hydrolysis is another pathway to produce ceramide. To determine whether sphingomyelin hydrolysis in response to LPS and PA, or LPS plus PA contributes to ceramide production, we quantified cellular sphingomyelin (SM) using lipidomics. Results showed that PA and LPS plus PA decreased total, C16-, C22-, C24:1- and C24-SM at 12 and 24 h (Fig. 5H–J). Results also showed that PA and LPS plus PA increased S1P and dihydroS1P production at 12 h. Although, the contents of S1P and dihydroS1P decreased at 24 h than that at 12 h, statistic significant differences were not reached (Fig. 5M–N). LPS had no significant effect on ceramide and SM production (Fig. 5). Taken together, these data demonstrated that PA increased ceramide production by stimulating ceramide de novo synthesis and sphingomyelin hydrolysis, but the novo synthesis ceramide was further increased when the cells were incubated by a combination of LPS and PA.
4. Discussion
Microglia is the first line of immune defense in the CNS, playing a role in many neuroinflammatory and neurodegenerative diseases including multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, retinal degeneration diseases, anxiety, depression, PTSD, and others [[27], [28], [29]]. During inflammatory processes, the activated microglia, polarized into M1 type in response to LPS, releases pro-inflammatory factors and inducing neuroinflammatory responses [30,31]. In the present study, we have shown that LPS and PA alone or in combination activated HMC3 leading not only to upregulated proinflammatory cytokines, chemokines, and gene expression but also to an increase in ceramide production.
To explore the mechanisms underlying the interaction between LPS and PA, we examined the effect of LPS, PA and the combination of LPS and PA on the inflammatory response of HMC3 and the signaling pathways involved. Our results showed that stimulation of a microglia cell line with LPS or PA significantly increased both IL-6 gene and protein expression. When the same cell line was stimulated simultaneously by LPS and PA, the IL-6, CXCL1, CXCL2, and CXCL10 mRNA expression as well as IL-6, IL-2, IL-4, IL-17A, IL10, IFNγ, TNFα, and IL-8 secretion were significantly increased.
Microglia have been shown to express TLR4 [32]. LPS binding to TLR4 is known to activate NFκB, inducing increased secretion of proinflammatory cytokines and therefore leading to neuronal damage. Notably, in the present study, we found that LPS-induced upregulation of inflammatory cytokines due to LPS binding to TLR4 receptors was mediated by both NFκB and MAPK pathway, which are well known as important regulators of immune response. Our study showed however that only LPS not PA was able to upregulate IL-6 expression through the NFκB and AP-1 pathways, therefore leading us to conclude that PA-induced inflammatory observed in the microglia cell line was mediated by a pathway other than MAPK and NFκB.
SFAs, mainly palmitate, are known to strongly contribute to neuronal inflammation. Cholesterol and fatty acids are the most impartment components of the brain cell membrane, helping to maintain neuronal plasticity [33,34] and sphingolipids specifically ceramides are well known for their role in activating inflammation [35]. We anticipate that the increased production of ceramides elicited by PA stimulation of HMC3 is likely the mechanism involved in a robust and sustained PA-induced upregulation of IL-6.
Activated brain microglia release inflammatory mediators such as nitric oxide, IL-6, IL-1, TNFα which impact neurotransmission, mediate neurotoxicity by increasing glutamate production and inducing neuronal apoptosis, neuroendocrine function, neural plasticity; and directly influence behaviour [10,11,[36], [37], [38], [39]]. Levels of IL-6 have shown to be increased on neurodegenerative conditions, which is likely resulted from activated microglia [40]. A variety of brain cell types can secrete and respond to IL-6 in response to peripheral inflammatory disorders and systemic inflammation has been shown to impact the CNS [[41], [42], [43]].
The interaction between activated microglia and lymphocytes that elicits the release of several proinflammatory cytokines has been described [44,45]. In conclusion, the above studies support the possible impact of systemic inflammation on neuro-immune interactions. Our co-cultured studies, examining whether the microglia can interact with mediators released by lymphocytes and macrophages, did clearly show an enhanced IL-6 secretion, supporting previous work by other investigators.
Upregulation of proinflammatory cytokines, in particular IL-6, TNFα, IFNγ, IL-1b, ROS, NO, and other immunomodulatory factors are associated to the M1 microglia phenotype which is known to be associated with enhanced neuroinflammation and neurodegeneration [19,46,47]. Other studies have also shown that over-activated microglia releasing large concentrations of proinflammatory cytokines that will lead to neuronal death and increased accumulation of toxic proteins [48].
Long-chain saturated fatty acid are known to increase membrane fluidity and flexibility of the cell membrane therefore altering receptor expression, disrupting receptor signaling and leading to changes in lipid raft composition which contribute to polarization of the microglia towards M1 phenotype and increases secretion of proinflammatory cytokine [49]. We examined the effect of HMC3 polarization by LPS, and PA, and our results showed PA and PA combination with LPS upregulated M1 marker CD86 expression. Lipidomics data showed that PA and PA plus LPS stimulated ceramide production by increasing both ceramide do novo synthesis and SM hydrolysis. Our previous study showed PA-increased ceramide production by activating NFκB activity human retinal microvascular endothelia cells [50]. Thus, our findings indicate that the increase in ceramide and SM production by PA and LPS plus PA may be through NFκB and AP-1 transcriptional activity. LPS and PA induced sphingolipid may play a critical role in the proinflammatory response via modulating microglia M1/M2 polarization.
5. Conclusions
Our present study was focused on the role of low-dose LPS and PA in microglial polarization, in the up-regulation of proinflammatory cytokine expression, and in the responsible underlying mechanisms. HMC3 proved to be a useful experimental model to investigate the physiopathology of microglia cells, including the interactions between the microglia and other cell types in CNS during neuroinflammation and neurodegeneration. Our results strongly suggest preventing systemic inflammation and the release of proinflammatory mediators by monocyte-derived macrophages and lymphocytes may contribute to decrease microglia activation and the subsequent CNS inflammatory process.
Ethics statement
This study was approved by the IRB of the Medical University of South Carolina, which is the IRB of record for both the Ralph H. Johnson VA Medical Center and the Medical University of South Carolina.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Dr. Rita Young for her initial design of this study. All work was supported by the Clinical Sciences Research and Development Program of the Department of Veterans Affairs (I01CX000851) (Zhewu Wang).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpnec.2021.100048.
Contributor Information
Zhongyang Lu, Email: luz@musc.edu.
Zhewu Wang, Email: wanzh@musc.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Felger J.C., Lotrich F.E. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haroon E., Raison C.L., Miller A.H. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maes M. Major depression and activation of the inflammatory response system. Adv. Exp. Med. Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- 4.Michopoulos V., Powers A., Gillespie C.F., Ressler K.J., Jovanovic T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology. 2017;42:254–270. doi: 10.1038/npp.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Mello C., Le T., Swain M.G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welser-Alves J.V., Milner R. Microglia are the major source of TNF-alpha and TGF-beta1 in postnatal glial cultures; regulation by cytokines, lipopolysaccharide, and vitronectin. Neurochem. Int. 2013;63:47–53. doi: 10.1016/j.neuint.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graeber M.B., Streit W.J. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- 8.Nimmerjahn A., Kirchhoff F., Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 9.Sandiego C.M., Gallezot J.D., Pittman B., Nabulsi N., Lim K., Lin S.F., Matuskey D., Lee J.Y., O’Connor K.C., Huang Y., Carson R.E., Hannestad J., Cosgrove K.P. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc. Natl. Acad. Sci. U. S. A. 2015;112:12468–12473. doi: 10.1073/pnas.1511003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaur C., Sivakumar V., Zou Z., Ling E.A. Microglia-derived proinflammatory cytokines tumor necrosis factor-alpha and interleukin-1beta induce Purkinje neuronal apoptosis via their receptors in hypoxic neonatal rat brain. Brain Struct. Funct. 2014;219:151–170. doi: 10.1007/s00429-012-0491-5. [DOI] [PubMed] [Google Scholar]
- 11.Ye L., Huang Y., Zhao L., Li Y., Sun L., Zhou Y., Qian G., Zheng J.C. IL-1beta and TNF-alpha induce neurotoxicity through glutamate production: a potential role for neuronal glutaminase. J. Neurochem. 2013;125:897–908. doi: 10.1111/jnc.12263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Aidy S., Dinan T.G., Cryan J.F. Immune modulation of the brain-gut-microbe axis. Front. Microbiol. 2014;5:146. doi: 10.3389/fmicb.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulders W.H., McMahen C., Robertson D. Effects of chronic furosemide on central neural hyperactivity and cochlear thresholds after cochlear trauma in Guinea pig. Front. Neurol. 2014;5:146. doi: 10.3389/fneur.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karelis A.D., Faraj M., Bastard J.P., St-Pierre D.H., Brochu M., Prud’homme D., Rabasa-Lhoret R. The metabolically healthy but obese individual presents a favorable inflammation profile. J. Clin. Endocrinol. Metab. 2005;90:4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 15.Ralston J.C., Metherel A.H., Stark K.D., Mutch D.M. SCD1 mediates the influence of exogenous saturated and monounsaturated fatty acids in adipocytes: effects on cellular stress, inflammatory markers and fatty acid elongation. J. Nutr. Biochem. 2016;27:241–248. doi: 10.1016/j.jnutbio.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Valdearcos M., Xu A.W., Koliwad S.K. Hypothalamic inflammation in the control of metabolic function. Annu. Rev. Physiol. 2015;77:131–160. doi: 10.1146/annurev-physiol-021014-071656. [DOI] [PubMed] [Google Scholar]
- 17.Valdearcos M., Douglass J.D., Robblee M.M., Dorfman M.D., Stifler D.R., Bennett M.L., Gerritse I., Fasnacht R., Barres B.A., Thaler J.P., Koliwad S.K. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metabol. 2017;26:185–197. doi: 10.1016/j.cmet.2017.05.015. e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valdearcos M., Robblee M.M., Benjamin D.I., Nomura D.K., Xu A.W., Koliwad S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014;9:2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweet M.J., Hume D.A. Endotoxin signal transduction in macrophages. J. Leukoc. Biol. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 20.Akira S. Toll-like receptor signaling. J. Biol. Chem. 2003;278:38105–38108. doi: 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- 21.Gomes J.M.G., Costa J.A., Alfenas R.C.G. Metabolic endotoxemia and diabetes mellitus: a systematic review. Metabolism. 2017;68:133–144. doi: 10.1016/j.metabol.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Assi E., Cazzato D., De Palma C., Perrotta C., Clementi E., Cervia D. Sphingolipids and brain resident macrophages in neuroinflammation: an emerging aspect of nervous system pathology. Clin. Dev. Immunol. 2013;2013:309302. doi: 10.1155/2013/309302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosicek M., Hecimovic S. Phospholipids and Alzheimer’s disease: alterations, mechanisms and potential biomarkers. Int. J. Mol. Sci. 2013;14:1310–1322. doi: 10.3390/ijms14011310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hannun Y.A., Obeid L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018;19:175–191. doi: 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merrill A.H., Jr. Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 2011;111:6387–6422. doi: 10.1021/cr2002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung J.S., Shin K.O., Lee Y.M., Shin J.A., Park E.M., Jeong J., Kim D.H., Choi J.W., Kim H.S. Anti-inflammatory mechanism of exogenous C2 ceramide in lipopolysaccharide-stimulated microglia. Biochim. Biophys. Acta. 2013;1831:1016–1026. doi: 10.1016/j.bbalip.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Aldana B.I. Microglia-specific metabolic changes in neurodegeneration. J. Mol. Biol. 2019;431:1830–1842. doi: 10.1016/j.jmb.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Colonna M., Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf S.A., Boddeke H.W., Kettenmann H. Microglia in physiology and disease. Annu. Rev. Physiol. 2017;79:619–643. doi: 10.1146/annurev-physiol-022516-034406. [DOI] [PubMed] [Google Scholar]
- 30.Orihuela R., McPherson C.A., Harry G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016;173:649–665. doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker D.G., Lue L.F. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimer’s Res. Ther. 2015;7:56. doi: 10.1186/s13195-015-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanke M.L., Kielian T. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin. Sci. (Lond.) 2011;121:367–387. doi: 10.1042/CS20110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartmann T., Kuchenbecker J., Grimm M.O. Alzheimer’s disease: the lipid connection. J. Neurochem. 2007;103(Suppl 1):159–170. doi: 10.1111/j.1471-4159.2007.04715.x. [DOI] [PubMed] [Google Scholar]
- 34.Pfrieger F.W. Cholesterol homeostasis and function in neurons of the central nervous system. Cell. Mol. Life Sci. 2003;60:1158–1171. doi: 10.1007/s00018-003-3018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Wit N.M., den Hoedt S., Martinez-Martinez P., Rozemuller A.J., Mulder M.T., de Vries H.E. Astrocytic ceramide as possible indicator of neuroinflammation. J. Neuroinflammation. 2019;16:48. doi: 10.1186/s12974-019-1436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee Y.Y., Lee E.J., Park J.S., Jang S.E., Kim D.H., Kim H.S. Anti-inflammatory and antioxidant mechanism of tangeretin in activated microglia. J. Neuroimmune Pharmacol. 2016;11:294–305. doi: 10.1007/s11481-016-9657-x. [DOI] [PubMed] [Google Scholar]
- 37.Qian J., Zhu L., Li Q., Belevych N., Chen Q., Zhao F., Herness S., Quan N. Interleukin-1R3 mediates interleukin-1-induced potassium current increase through fast activation of Akt kinase. Proc. Natl. Acad. Sci. U. S. A. 2012;109:12189–12194. doi: 10.1073/pnas.1205207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reshef R., Kreisel T., Beroukhim Kay D., Yirmiya R. Microglia and their CX3CR1 signaling are involved in hippocampal- but not olfactory bulb-related memory and neurogenesis. Brain Behav. Immun. 2014;41:239–250. doi: 10.1016/j.bbi.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 39.Takeuchi H., Jin S., Wang J., Zhang G., Kawanokuchi J., Kuno R., Sonobe Y., Mizuno T., Suzumura A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J. Biol. Chem. 2006;281:21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- 40.Gruol D.L. IL-6 regulation of synaptic function in the CNS. Neuropharmacology. 2015;96:42–54. doi: 10.1016/j.neuropharm.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones K.A., Thomsen C. The role of the innate immune system in psychiatric disorders. Mol. Cell. Neurosci. 2013;53:52–62. doi: 10.1016/j.mcn.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Smid G.E., van Zuiden M., Geuze E., Kavelaars A., Heijnen C.J., Vermetten E. Cytokine production as a putative biological mechanism underlying stress sensitization in high combat exposed soldiers. Psychoneuroendocrinology. 2015;51:534–546. doi: 10.1016/j.psyneuen.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Wohleb E.S., McKim D.B., Shea D.T., Powell N.D., Tarr A.J., Sheridan J.F., Godbout J.P. Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol. Psychiatr. 2014;75:970–981. doi: 10.1016/j.biopsych.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui Z.Q., Liu B.L., Wu Q.L., Cai Y., Fan W.J., Zhang M.C., Ding W.L., Zhang B., Kang J.M., Yan H. Could intrathymic injection of myelin basic protein suppress inflammatory response after Co-culture of T lymphocytes and BV-2 microglia cells? Chin. Med. J. (Engl.) 2016;129:831–837. doi: 10.4103/0366-6999.178955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janke A.D., Yong V.W. Impact of IVIg on the interaction between activated T cells and microglia. Neurol. Res. 2006;28:270–274. doi: 10.1179/016164106X98143. [DOI] [PubMed] [Google Scholar]
- 46.Tracy L.M., Bergqvist F., Ivanova E.V., Jacobsen K.T., Iverfeldt K. Exposure to the saturated free fatty acid palmitate alters BV-2 microglia inflammatory response. J. Mol. Neurosci. 2013;51:805–812. doi: 10.1007/s12031-013-0068-7. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z., Liu D., Wang F., Liu S., Zhao S., Ling E.A., Hao A. Saturated fatty acids activate microglia via Toll-like receptor 4/NF-kappaB signalling. Br. J. Nutr. 2012;107:229–241. doi: 10.1017/S0007114511002868. [DOI] [PubMed] [Google Scholar]
- 48.Lopez-Valdes H.E., Martinez-Coria H. The role of neuroinflammation in age-related dementias. Rev. Invest. Clin. 2016;68:40–48. [PubMed] [Google Scholar]
- 49.Laye S., Nadjar A., Joffre C., Bazinet R.P. Anti-inflammatory effects of omega-3 fatty acids in the brain: physiological mechanisms and relevance to pharmacology. Pharmacol. Rev. 2018;70:12–38. doi: 10.1124/pr.117.014092. [DOI] [PubMed] [Google Scholar]
- 50.Lu Z., Li Y., Ru J.H., Lopes-Virella M.F., Lyons T.J., Huang Y. Interaction of palmitate and LPS regulates cytokine expression and apoptosis through sphingolipids in human retinal microvascular endothelial cells. Exp. Eye Res. 2019;178:61–71. doi: 10.1016/j.exer.2018.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.