Highlights
-
•
Severe dengue was common in this sudy.
-
•
In contrast to multiple prior studies, the risk of severe dengue was greater for patients with primary dengue compared to those with secondary infection.
-
•
Additional risk factors for severe dengue included age, male sex, haemoglobin S, diabetes, and hypertension. Case mapping showed that dengue cases were more concentrated in sectors located in the centre of the city and close to the health centres.
Keywords: Severe dengue, Risk factors, Primary dengue, Burkina Faso, West Africa
Abstract
Introduction
Although dengue is the most common arbovirus infection worldwide, studies of severe dengue in Africa are lacking, and risk factors for severe dengue have been insufficiently described. This study was conducted in the context of the 2016 dengue epidemic in Burkina Faso to determine the prevalence of severe dengue, identify factors associated with severe dengue, and perform mapping of dengue cases in the country's capital, Ouagadougou.
Methods
This cross-sectional study was conducted from November 2015 to January 2017. Data were collected in 15 public and private health centres, and included sociodemographic, clinical and patient outcome variables. Dengue was diagnosed using SD Bioline Dengue Duo rapid diagnostic tests. Data were analysed using Epi-Info Version 7. Logistic regression was used to identify predictors of severe dengue. P<0.05 was considered significant. Dengue case mapping was performed using Geographic Information System software (ArcGIS).
Results
Of the 811 patients who tested positive for dengue, 609 (75%) had early dengue (AgNS1 positive) and 272 (33.5%) had severe dengue. Patient age ranged from 1 to 83 years (median 30.5 years) and 393 (48.3%) were female. Renal failure (13.1%) and severe bleeding (10.6%) were the most common signs of severe dengue. Risk factors for severe dengue included age, male sex, haemoglobin S, diabetes, hypertension, and primary dengue. Dengue cases were more concentrated in sectors located in the centre of the city and close to the health centres.
Conclusion
Dengue is increasingly common in Africa and factors associated with severity should be sought systematically as soon as a patient tests positive. Additional studies are needed to determine if the factors found to be associated with severity can be used to identify patients at risk for dengue-related complications, and to provide early and specialized management to reduce morbidity and mortality related to dengue in Africa.
Introduction
Dengue is the most common arbovirus infection worldwide (Bhatt et al., 2013). It represents a significant public health problem, particularly in tropical and subtropical zones. According to the World Health Organization (WHO), dengue infects approximately 50–100 million individuals each year and has increased more than 30-fold over the last 50 years (World Health Organization, 2012). Global deaths due to dengue have increased by nearly 50%, resulting in approximately 18,500 deaths each year (GBD 2015 Mortality and Causes of Death Collaborators, 2016). Furthermore, dengue poses a substantial economic burden on individuals and governments, and is a barrier to economic development in many countries (Shepard et al., 2014; Mulligan et al., 2015).
Detection of dengue has increased substantially in Africa, from approximately 627,000 cases in 1990 to more than 5.6 million cases in 2013, of which more than 70% occurred in West Africa (Stanaway et al., 2016). Although the presence of dengue has been confirmed in more than 32 countries in Africa (Fagbami et al., 1977; Amarasinghe, 2011), severe dengue was infrequently reported in Africa until recently (Franco et al., 2011; Jaenisch et al., 2014). According to the WHO guidelines, severe dengue is defined by one or more of the following: (i) plasma leakage that may lead to shock (dengue shock) and/or fluid accumulation, with or without respiratory distress, and/or (ii) severe bleeding, and/or (iii) severe organ impairment (null_). The 2009 WHO classification is is considered more sensitive than the 1997 WHO classification, being more comprehensive and more sensitive in identifying early warning signs and severity to guide faster management of patients (Ajlan et al., 2019). To date, the precise mechanisms contributing to the pathophysiology of severe dengue are unclear 20152018. The theory of antibody dependent enhancement is widely accepted as contributing to severe disease following sequential infection with different serotypes. Dengue epidemics in Africa over the past 10 years have been particularly severe, especially in the West and Central Africa region (Franco et al., 2010; Aoussi et al., 2014; Faye et al., 2014). Due to clinical similarity to other febrile illnesses such as malaria, a lack of access to diagnostic tests, and insufficient or absent monitoring mechanisms, the burden of dengue and its socio-economic consequences are likely underestimated in this region (Bhatt et al., 2013; Eisenhut, 2013). Similarities in the clinical presentation of dengue and malaria have long contributed to the lack of recognition of dengue by health practitioners, and this results in a delayed response in many African countries (Jaenisch et al., 2014; Sharp et al., 2015).
Burkina Faso, like other countries in the region, has reported outbreaks of dengue including severe cases since 2013. However, there is evidence that the virus has been circulating for many years, and outbreaks have been reported since 1925 (Gonzalez et al., 1985; Robert et al., 1993). The most recent epidemic in 2016 was the largest, with an estimated 2526 suspected cases including 1561 probable cases and 20 deaths according to national data (Ministry of Health of Burkina Faso, Epidemiological Surveillance Service, 2016). The response to the outbreak was limited by a lack of access to rapid diagnostic tests (RDTs). RDTs were almost exclusively available in laboratories and private health structures, and were not subsidized. These factors limited their accessibility to the majority of the population. Data from the 2016 epidemic indicate that the disease caused significant household expenditures in a country where 40% live below the poverty line (Institut national de la statistique et de la démographie, 2015; Ridde et al., 2016). Although circulation of serotypes 1, 2 and 3 was confirmed in the country, serotype 2 was responsible for the majority of cases during the dengue outbreak in 2016 (Ridde et al., 2016; Tarnagda et al., 2018).
The frequency of severe dengue varies according to study, geographical site and classification system used. Approximately 500,000 cases of dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS) occur each year (World Health Organization, 2017). According to the 2009 WHO criteria, severe dengue is characterized by severe plasma leakage, leading to shock and fluid accumulation with respiratory distress; severe bleeding; and organ failure, including hepatic, renal, neurologic and cardiovascular impairment (World Health Organization, 2009). Risk factors for progression to DSS or DHF are poorly understood, particularly among adults, and include age, secondary infection, chronic disease and the presence of organ dysfunction (Guzman and Harris, 2015). Previous studies from Asia have reported a number of factors associated with the severity of dengue, including age; female sex; presence of neurological, digestive, liver or renal function disorders; and hypoalbuminaemia (Anders et al., 2011; Toledo et al., 2016). Infection with DENV-2, thrombocytopenia, activated partial thromboplastin time, secondary infection, elevated fibrinogen, diabetes and asthma have been associated with progression to DHF or DSS (Tee et al., 2009; Amâncio et al., 2015; Toledo et al., 2016; Pang et al., 2017). However, data from Africa are lacking (Amarasinghe, 2011). The present study aimed to identify the factors associated with severe dengue in Burkina Faso, and to establish a map of severe cases in the capital, Ouagadougou.
Methods
Type of study and selection of private health facilities
This cross-sectional study took place over 15 months, from 1 November 2015 to 31 January 2017, and captured the period of the 2016 dengue epidemic. In Burkina Faso, the health system is organized into three levels that provide primary, secondary and tertiary health care: the first level includes health districts [health and social promotion centres and medical centres with surgical antenna (CMAs)], the second level includes regional hospitals, and the third level is represented by teaching hospitals (CHUs). The goal of this pyramid system is to decentralize access to healthcare services. The city of Ouagadougou has three CHUs, four CMAs and 240 private facilities to serve an estimated population of 1,934,397 inhabitants. This study took place at 15 sites located in Ouagadougou, including three tertiary-level health centres or reference sites (Yalgado Ouedraogo, Tengandogo and Charles de Gaulle paediatric teaching hospitals), four secondary-level health centres and eight private health facilities. The private health facilities selected for this study were those that had access to Dengue Duo RDTs (SD BIOLINE (Santa Clara, USA)). The RDTs were not subsidized and their cost, ranging from 10,000 FCFA to 15,000 FCFA per unit (approximately €15–25), was entirely the responsibility of the patient.
Criteria for inclusion of patients
The patients included in this study were those who presented with fever (>37.5°C) and/or other signs suggestive of malaria or dengue (such as headache, retro-orbital pain, myalgia and arthralgia, anorexia, abdominal pain and nausea, rash), and for whom the Dengue Duo RDT AgNS1 and/or immunoglobulin M (IgM) was positive, regardless of the date of symptom onset. The presence of isolated immunoglobulin G (IgG), in the absence of titration techniques, was considered as serological evidence of prior dengue infection, and these patients were excluded from the study (Figure 1).
Figure 1.
Flow chart for patient inclusion.
Collection of data and variables studied
Data were collected from a review of hospital medical records and laboratory data. Data were collected on standardized questionnaires that included sociodemographic characteristics (age, sex, level of education, residence, occupation), medical history [medications, history of dengue or other haemorrhagic fever, pregnancy, human immunodeficiency virus (HIV) infection, hepatitis, asthma, sickle cell disease, hypertension, diabetes, alcohol or tobacco use], and complications or death of the patient.
Diagnostic testing
The test used was the SD BIOLINE Dengue Duo kit which is a rapid immunochromatographic test that detects the dengue virus NS1 antigen (NS1 Ag) and anti-dengue IgG/IgM antibodies in serum, plasma or whole blood. Dengue polymerase chain reaction assays are minimally accessible and were not used for the majority of dengue cases in this study. In 2016, the Pasteur Institute of Dakar reported that serotype 2 was responsible for the 2016 outbreak in Ouagadougou (Ministry of Health of Burkina Faso, Epidemiological Surveillance Service, 2016). The diagnosis of malaria was made using malaria RDTs (histidin rich protein 2) and/or blood smears.
Operational definitions
Primary dengue cases: Patients for whom dengue IgG was negative, and IgM and/or NS1 Ag was positive.
Severe dengue: The presence of one or more of the severity criteria established by the 2009 WHO dengue classification system. The following specific criteria were used:
-
•
Plasma leakage: defined by haemoconcentration with an increase in haematocrit of >5%.
-
•
Severe bleeding: defined as diffuse and/or abundant haemorrhage requiring blood transfusion.
-
•
Organ dysfunction: defined by transaminitis [alanine transaminase (ALT) or aspartate transaminase (AST) >1000], encephalopathy (as measured by the Glasgow Score) and renal failure (serum creatinine >120 µmol/L). Extreme values observed during the patient's management (highest for AST, ALT and serum creatinine; and lowest for platelets) were considered.
-
•
Hypovolaemic shock: plasma leakage with shock or respiratory distress.
-
•
Severe thrombocytopenia: defined by a platelet count <20 × 103 per μL.
Statistical analyses
Descriptive analyses were performed to determine mean, standard deviation, median, percentage and frequency. Data for all patients were analysed and then stratified according to age >15 years (defined as adults) or ≤15 years (defined as children). Adults with severe dengue were compared with adults who did not have any severity criteria to identify factors associated with severe dengue. Chi-squared test or Fisher's exact test were used to assess statistical significance, as appropriate.
Univariate and multi-variate logistic regression were used to identify predictors of severe dengue. Univariate analysis was used to select variables to include in the multi-variate analysis. Variables associated with severe dengue with P<0.20 on univariate analysis were included in the multi-variate analysis. The first model includes all of these variables. The second model comes from a procedure of descending selection of variables. The results are presented as odds ratios (OR), including 95% confidence intervals (CI) and P-values. P<0.05 was considered significant.
Data were analysed using Epi-Info Version 7.
Mapping of dengue cases
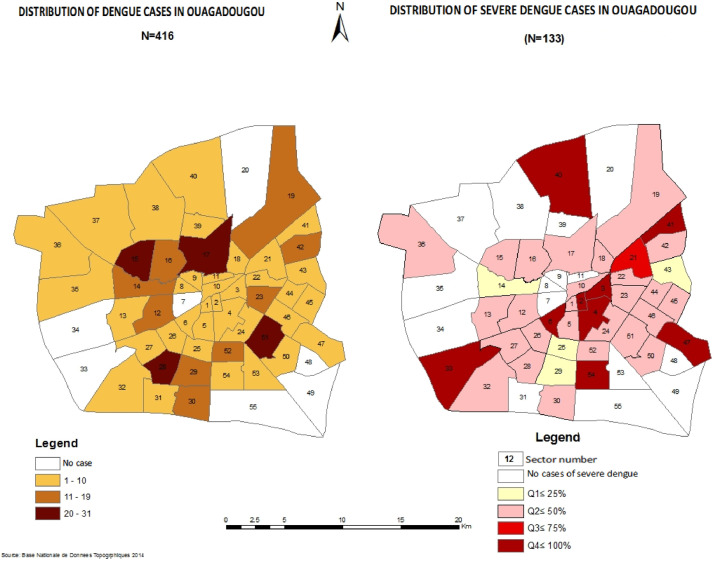
Dengue case mapping was performed using Geographic Information System software (ArcGIS). The base maps used are from the national database of topographic data 2014. These maps represent the administrative divisions of the cities of Burkina Faso, and the districts of the city of Ouagadougou. These data were edited and updated to include data collected in the cities of Burkina Faso, and sectors and districts of Ouagadougou. Mapping was performed such that areas where there was an absence of dengue cases were represented in white, and areas with increasing numbers of dengue cases were represented in shades of brown.
Ethical considerations
The study protocol was submitted to the Regional Health Directorate of the Centre Regional Director of Health of Ouagadougou, and the work was authorized through decision number 2015/159/MS. The study protocol was not submitted to the ethics committee for health research because the clinical evaluation was performed as part of the standard management of dengue. Hospitalization was determined by the severity of disease. A grant from the State of Burkina Faso and its technical and financial partners was provided in October 2016, which allowed for free treatment of those hospitalized with severe dengue in tertiary-level structures. Data were anonymized on the collection forms to ensure confidentiality.
Results
This cross-sectional study included 811 patients with dengue over the course of 15 months, of which 609 (75%) were AgNS1 positive on RDT. The peak of the 2016 epidemic was observed in November 2016, representing 38.5% of all patients included in the study. Over half (n=472, 58.8%) of patients initially presented to a private facility. The majority of patients (97.8%) were hospitalized in the same facility where they presented, and only 2.2% were treated as outpatients.
Sociodemographic characteristics of patients and mapping severe dengue prevalence zones
Patient age ranged from 1 to 83 years (median 30.5 years). The distribution by age group was 85 patients (11.5%) aged ≤15 years, 662 patients (80.8%) aged 16–60 years, and 64 patients (7.8%) aged >60 years. There was a slight male predominance (418 patients, 51.5% of cases) and the sex ratio was 1.06. Secondary school education or higher was noted for 464 patients (57.2%). In terms of profession, 263 (35.4%) participants were employed in the public or private sectors, 180 (24.20%) were employed in the informal sector, 251 (33.8%) were students, and 49 (7.8%) were not employed professionally (housewife, unemployed) (Table 1).
Table 1.
Prevalence of clinical characteristics, including comorbidities and treatments.
| Sociodemographic characteristic | n | % |
|---|---|---|
| Age (years) | ||
| 1–15 | 85 | 11.4 |
| 16–60 | 662 | 80.8 |
| ≥60 | 64 | 7.8 |
| Sex | ||
| Male | 418 | 51.7 |
| Level of education | ||
| No instructions | 51 | 6.3 |
| Primary | 296 | 36.5 |
| Secondary or more | 464 | 57.2 |
| Professional activity | ||
| Unemployed | 49 | 6.6 |
| Pupils/students | 251 | 33.8 |
| Workers in the informal sector | 180 | 24.2 |
| Public or private sector employees | 263 | 35.4 |
| Comorbidities, Clinical characteristics, and Treatments | ||
| Hypertension | 71/811 | 8.8 |
| History of diabetes | 30/811 | 3.7 |
| Presence of haemoglobin S | 26/811 | 3.2 |
| History of asthma | 20/811 | 2.5 |
| Receiving dialysis | 10/346 | 2.9 |
| Pregnant | 24/408 | 5.9 |
| Hepatitis C (HCV Ab positive) | 2/811 | 0.3 |
| Hepatitis B (Hbs Ag positive) | 13/811 | 1.6 |
| Malaria (blood smear or RDT positive) | 183/811 | 22.6 |
| Medication (self-prescribed) | 274/696 | 39.4 |
| Antimalarial | 178/274 | 65.0 |
| Traditional medicine | 14/261 | 5.5 |
| Medication (prescription) | ||
| Antimalarial | 267/374 | 71.4 |
| Antibiotic | 73/292 | 25.0 |
| Anti-inflammatory | 40/291 | 13.8 |
When mapping severe dengue prevalence zones by sector in Ouagadougou, the sector number was reported for 416 patients. All cases from areas 2, 3, 4, 6, 33, 40, 41, 47, 54 had severe dengue. Cases in areas 8, 9, 11, 31, 37, 38, 39 and 53 were not affected by severe dengue. The mapping of severe cases is shown in Figure 2.
Figure 2.
Mapping of dengue and prevalence of severe dengue by city sector, Ouagadougou.
Comorbidities, clinical characteristics and treatments
Seventy-one patients (8.8%) had a history of hypertension (Table 1). Dengue occurred in 24 pregnant women (2.9%), nine of whom (36%) were in their third trimester of pregnancy. Two patients (0.25%) had known HIV infection, and five of 108 (3.1%) patients were screened for HIV infection during the dengue episode.
According to WHO classification, of the 811 patients with dengue (adults and children), 272 patients (33.5%) presented without warning signs, 539 patients (66.5%) presented with warning signs, and 245 patients (30.2%) presented with severe dengue. More than half of the patients had warning signs; the main signs were vomiting [n=366 (45%)], asthenia [n=274 (33.8)], abdominal pain [n=260 (32%)], non-severe thrombocytopenia [n=197 (24.3)] and mucosal bleeding [n=111 (13.7%)].
Malaria was diagnosed in 183 patients (22.6%) by RDTs and/or blood smears. Self-medication was reported by 274 patients (39.4%), mainly antimalarials (71.4%). Traditional medicines were used by 5.5% of patients, most commonly papaya leaves, eucalyptus leaves or other tinctures of unspecified origin (Table 1).
Severe dengue
In this study, 272 patients (33.5%) had severe dengue. Among 35 patients (4.3%) with primary dengue, 17 patients (48.6%) had severe dengue.
Among the criteria for severe dengue, renal failure and severe bleeding were the most common., Renal failure occurred in 106 patients (13.1%); severe bleeding occurred in 86 patients (10.6%); plasma leakage occurred in 56 patients (6.9%); hypovolaemic shock occurred in 11 patients (1.4%); encephalopathy occurred in 52 patients (6.4%); and hepatic dysfunction occurred in 43 patients (5.3%), including 13 patients (1.7%) with ALT>1000 and 29 patients (3.6%) with AST >1000. Thirty-seven patients (5.1%) had severe thrombocytopenia. Twenty-four patients (9.8%) in the study died.
Factors associated with severe dengue
Among the 85 patients who were aged ≤15 years, 10 (11.8%) had severe dengue, and 20 cases of malaria (23.5%) were diagnosed, including three patients with both malaria and severe dengue. Adults had a significantly higher risk of developing severe dengue compared with children aged ≤15 years (OR 3.50, 95% CI 1.77–6.92; P=0.0002).
In univariate analysis among adults, male sex (OR 2.39, 95% CI 1.72–3.34), the presence of haemoglobin S (OR 3.16, 95% CI 1.41–7.09), arterial hypertension (OR 2.56, 95% CI 1.56– 4.21), diabetes (OR 2.48, 95% CI 1.19–5.16), primary dengue (OR 2.35, 955 CI 1.18–4.65) and use of non-steroidal anti-inflammatory drugs (OR 1.90, 95% CI 1.01–3.67) were associated with severe dengue..
In multi-variate analysis among adults, male sex (OR 2.71, 95% CI 1.91–3.84), the presence of haemoglobin S (OR 3.73, 95% CI 1.59–8.77), arterial hypertension (OR 2.78, 95% CI 1.53– 5.02), diabetes (OR 2.62, 95% CI 1.15–5.97) and primary dengue (OR 2.62, 95% CI 1.25– 5.48) were independently associated with severe dengue (Table 2).
Table 2.
Factors associated with severe dengue among adults.
| Characteristic | n=726 | Severe dengue | OR non-adjusted | P-value | OR adjusted | P-value |
|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||
| Sex | ||||||
| Female | 356 (49,0) | 73 (20.6) | Ref. | Ref. | ||
| Male | 370 (51,1) | 142 (38.4) | 2.39 (1.72–3.34) | <0.0001 | 2.71 (1.91–3.84) | <0.0001 |
| Arterial hypertension | ||||||
| No | 655 (90.2) | 180 (27.5) | Ref. | Ref. | ||
| Yes | 71 (9.8) | 35 (49.3) | 2.56 (1.56–4.21) | 0.0001 | 2.78 (1.53–5.02) | 0.0007 |
| Haemoglobin S | ||||||
| No | 701 (96.6) | 201 (28.7) | Ref. | Ref. | ||
| Yes | 25 (3.4) | 14 (56.0) | 3.16 (1.41–7.09) | 0.003 | 3.73 (1.59–8.77) | 0.002 |
| Diabetes | ||||||
| No | 696 (95.9) | 200 (28.7) | Ref. | Ref. | ||
| Yes | 30 (4.1) | 15 (50.0) | 2.48 (1.19–5.16) | 0.01 | 2.62 (1.15–5.97) | 0.02 |
| Primary dengue | ||||||
| No | 691 (95.2) | 198 (28.7) | Ref. | Ref. | ||
| Yes | 35 (4.8) | 17 (48.6) | 2.35 (1.18–4.65) | 0.01 | 2.62 (1.25–5.48) | 0.01 |
| Use of non-steroidal anti-inflammatory drugs | ||||||
| No | 687 (94.6) | 198 (28.8) | Ref. | Ref. | ||
| Yes | 39 (5.4) | 17 (43.6) | 1.90 (1.01–3.67) | 0.04 | 1.67 (0.82–3.38) | 0.15 |
| Age (years) | ||||||
| 16–30 | 325 (44.8) | 97 (29.9) | Ref. | Ref. | ||
| 31–60 | 337 (46.4) | 95 (28.2) | 0.92 (0.65–1.29) | 0.63 | 0.79 (0.55–1.13) | 0.20 |
| 61–90 | 64 (8.8) | 23 (35.9) | 1.31 (0.75–2.31) | 0.33 | 0.79 (0.39–1.56) | 0.5 |
| Malaria | ||||||
| No | 563 (77.6) | 167 (29.6) | Ref. | Ref. | ||
| Yes | 163 (22.4) | 48 (29.5) | 0.98 (0.67–1.45) | 0.95 | 1.03 (0.68–1.55) | 0.86 |
| Asthma | ||||||
| No | 711 (97.9) | 211 (29.7) | Ref. | Ref. | ||
| Yes | 15 (2.1) | 4 (26.7) | 0.86 (0.27–2.73) | 0.8 | 0.69 (0.20–2.33) | 0.55 |
OR, odds ratio.
Discussion
In this study, we found a high prevalence of severe dengue, identified factors associated with severe dengue, and conducted dengue case mapping in Ouagadougou, Burkina Faso. Among 811 patients with RDT-positive dengue, 245 (30.2%) presented with severe dengue and 24 (9.8%) of the severe cases died.
The range of reported prevalence rates for severe dengue in previous studies may be due to differences in the criteria used to define severe dengue (Gan et al., 2013). Most published studies require the presence of either DHF or DSS as criteria for severe dengue. In contrast, the 2009 WHO classification used in this study includes organ failure, which could have contributed to the high prevalence of severe dengue (Horstick et al., 2015). This classification is more inclusive than the 1997 WHO classification which included dengue, febrile DHF and DSS alone (Deen et al., 2006). The system was revised because it underestimated the number of cases of severe dengue by not including organ dysfunction (Guzman and Harris, 2015; Horstick et al., 2015). It is also possible that the high prevalence of severe dengue reported in this study may be due to improved access to diagnostic testing and higher rates of case reporting. Alternatively, the high prevalence of severe dengue in this study may be an underestimate resulting from limited access to RDTs during the peak of the epidemic, and ongoing confusion of dengue with malaria.
Criteria for severe dengue
Severe bleeding was the second most common complication in this study, observed in 10.6% of patients. In previous studies of dengue outbreaks, approximately 23% of patients had DHF (Guo et al., 2017). As well as being among the most common complications observed in severe dengue, DHF is also the leading cause of hospitalization and death among patients with dengue (World Health Organization, 2017; Bodinayake et al., 2018). Of note, according to the WHO 2009 dengue classification, severe bleeding is defined according to the discretion of the clinician, which may explain the variability in reported frequency across studies. The risk of death in patients with DHF is further exacerbated by comorbidities including severe hepatitis or myocarditis (20152020).
The proportion of patients with renal failure (13%) was high in this study. Renal failure during severe dengue is under-reported (Vachvanichsanong et al., 2016), and its frequency in patients with dengue differs according to the definition and evaluation method used (Uchino et al., 2006). A Taiwanese study found that 4% of dengue patients had renal failure, and reported an association between renal failure and mortality (Kuo et al., 2008). Mallhi et al. (2016) reported a prevalence rate for renal failure of 14%, which was also associated with increased mortality. The mechanisms leading to renal insufficiency in patients with dengue are likely related to hypoperfusion due to plasma leakage, haemorrhage and severe sepsis (Vachvanichsanong et al., 2016). Early identification of renal insufficiency should be a priority in patients with dengue to allow for timely intervention.
Factors associated with severe dengue
In this study, risk factors for severe dengue included age, male sex, primary dengue, haemoglobin S, diabetes and hypertension. Adults were at greater risk of severe dengue compared with children (age ≤15 years). This is consistent with previous studies which reported that adults are at greater risk of severe dengue and death compared with children under 15 years of age (Tantawichien, 2012). Other studies have reported a higher risk of severe dengue and death in elderly subjects compared with younger subjects (Liew et al., 2016; Lin et al., 2017).
This study also found an association between haemoglobin S and severe dengue. This relationship has been described previously, largely based on case studies. A proposed mechanism is that monocyte activation during sickle cell disease leads to endothelial cell activation by cytokines, leading to more frequent microvascular occlusions and apoptosis of endothelial cells (Limonta et al., 2009). The apoptosis of endothelial cells likely contributes to plasma leakage (Limonta et al., 2007). Another plausible explanation is that patients with chronic anaemia due to sickle cell disease are susceptible to milder degrees of haemorrhage, and therefore progress more rapidly to severe dengue. The relationship between haemoglobin S and severe dengue should be evaluated further in future studies.
Although the pathophysiology of shock due to dengue is multi-factorial, DSS occurs most commonly during secondary infection with a different serotype (Guzman and Harris, 2015). This is in contrast to the findings of the present study, where the risk of severe dengue was greater for patients with primary dengue. Cases of severe dengue among patients with primary dengue have been reported previously. In a study conducted in Malaysia, a higher risk of DHF was observed in patients with primary dengue but only in those aged >30 years (Tee et al., 2009). Similarly, Wichmann et al. (2004) reported a link between primary dengue and DHF in children.
In this study, both hypertension and diabetes were associated with severe dengue. The presence of comorbidities such as cardiovascular disease, diabetes and asthma has been associated with a higher risk of severe dengue in previous studies (Pang et al., 2017; 20152019). The link between hypertension and severe dengue has been reported previously, as well as a link between hypertension and death in patients with dengue in Asia (Htun et al., 2015; Pang et al., 2017). The pathophysiological mechanisms by which hypertension contributes to severe organ dysfunction in patients with dengue and progression to severe disease are insufficiently documented and may be related to an immune disorder. In addition, as individuals with hypertension often suffer from chronic organic damage, infection with dengue may lead to complications more quickly.
Dengue mapping
At least 95% of dengue cases in Burkina Faso were concentrated in the large cities of Ouagadougou and Bobo Dioulasso (Fournet et al., 2016; Tarnagda et al., 2018). Other studies in Africa have found a predominance of epidemics in urban areas (Were, 2012; Demanou et al., 2014). This may be explained by socio-environmental factors, particularly the density of the population resulting from the increasing migration of rural populations to urban areas, the rapid geographical expansion of large cities, and insufficient vector control measures. In this study, dengue cases were more concentrated in sectors located in the centre of the city and close to the health centres. Cases of severe dengue specifically were more concentrated around the health facilities investigated. Limited access to RDTs may have led to underdiagnosis of dengue and severe dengue in peripheral areas. Additional studies could contribute to the utilizationof dengue mapping to strengthen vector control measures.
Bias and limitations
This was a cross-sectional study and data were collected at the end of patient care. A cohort study involving standardized monitoring of patients would have contributed to a more precise understanding of the prevalence and predictors of severe dengue.
In addition, one of the major limitations of this study was the selection bias resulting from limited access to RDTs.
Exclusion of patients with isolated IgG may have contributed to underestimation of the number of dengue cases. Nevertheless, this large-scale study provided an opportunity to investigate factors associated with severe dengue in West Africa.
Conclusions
Studies to determine the risk factors for severe dengue in Africa are minimal, and are largely absent from West Africa. In this study conducted in Burkina Faso, West Africa, factors associated with severe dengue were identified. Additional studies are needed to determine if these factors can be used systematically to identify patients at risk for dengue-related complications, and to provide early and specialized management to reduce morbidity and mortality related to dengue in Africa.
Conflict of interest statement
None declared.
Acknowledgments
Acknowledgements
The authors wish to thank the Regional Director of Health of Ouagadougou for authorizing this study, the persons in charge of the study sites (public and private health structures in Ouagadougou), and all those who took part in the study.
Funding
None.
Ethical approval
This article is a retrospective study based on data collected from patients’ clinical records authorized by the Regional Director of Health of Ouagadougou.
Author contributions
KAS, EAD, BIM and ID designed the study, wrote the research protocol, assembled and analysed the data, and wrote the manuscript. BB, AG, CJK, GS and IY collected data. JZ, AP and ZT provided the bibliography. NMM and NAB helped write the manuscript and provided the bibliography. KS, SMO, ROT and SM directed the study, and undertook critical reading and final correction of the article.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2021.09.010.
Appendix. Supplementary materials
References
- Ajlan BA, Alafif MM, Alawi MM, Akbar NA, Aldigs EK, Madani TA. Assessment of the new World Health Organization's dengue classification for predicting severity of illness and level of healthcare required. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amâncio FF, Heringer TP, de Oliveira C da CHB, Fassy LB, de Carvalho FB, Oliveira DP, et al. Clinical profiles and factors associated with death in adults with dengue admitted to intensive care units, Minas Gerais, Brazil. PloS One. 2015;10 doi: 10.1371/journal.pone.0129046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarasinghe A. Dengue virus infection in Africa. Emerg Infect Dis. 2011;17 doi: 10.3201/eid1708.101515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders KL, Nguyet NM, Chau NVV, Hung NT, Thuy TT, Lien LB, et al. Epidemiological factors associated with dengue shock syndrome and mortality in hospitalized dengue patients in Ho Chi Minh City, Vietnam. Am J Trop Med Hyg. 2011;84:127–134. doi: 10.4269/ajtmh.2011.10-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoussi EBF, Ehui E, Kassi NA, Kouakou G, Nouhou Y, Adjogoua EV, et al. Seven native cases of dengue in Abidjan, Ivory Coast. Med Mal Infect. 2014;44:433–436. doi: 10.1016/j.medmal.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodinayake CK, Tillekeratne LG, Nagahawatte A, Devasiri V, Kodikara Arachchi W, Strouse JJ, et al. Evaluation of the WHO 2009 classification for diagnosis of acute dengue in a large cohort of adults and children in Sri Lanka during a dengue-1 epidemic. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deen JL, Harris E, Wills B, Balmaseda A, Hammond SN, Rocha C, et al. The WHO dengue classification and case definitions: time for a reassessment. Lancet. 2006;368:170–173. doi: 10.1016/S0140-6736(06)69006-5. [DOI] [PubMed] [Google Scholar]
- Demanou M, Pouillot R, Grandadam M, Boisier P, Kamgang B, Hervé JP, et al. Evidence of dengue virus transmission and factors associated with the presence of anti-dengue virus antibodies in humans in three major towns in Cameroon. PLoS Negl Trop Dis. 2014;8:e2950. doi: 10.1371/journal.pntd.0002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhut M. Diagnosis of viral hemorrhagic fevers in travelers returning from West Africa. J Travel Med. 2013;20:63. doi: 10.1111/j.1708-8305.2012.00664_1.x. [DOI] [PubMed] [Google Scholar]
- Fagbami AH, Monath TP, Fabiyi A. Dengue virus infections in Nigeria: a survey for antibodies in monkeys and humans. Trans R Soc Trop Med Hyg. 1977;71:60–65. doi: 10.1016/0035-9203(77)90210-3. [DOI] [PubMed] [Google Scholar]
- Faye O, Ba Y, Faye O, Talla C, Diallo D, Chen R, et al. Urban epidemic of dengue virus serotype 3 infection, Senegal, 2009. Emerg Infect Dis. 2014;20:456–459. doi: 10.3201/eid2003.121885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournet F, Rican S, Vaillant Z, Roudot A, Meunier-Nikiema A, Kassié D, et al. The influence of urbanization modes on the spatial circulation of flaviviruses within Ouagadougou (Burkina Faso) Int J Environ Res Public Health. 2016;13:1226. doi: 10.3390/ijerph13121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco L, Di Caro A, Carletti F, Vapalahti O, Renaudat C, Zeller H, et al. Recent expansion of dengue virus serotype 3 in West Africa. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2010:15. [PubMed] [Google Scholar]
- Franco L, Palacios G, Martinez JA, Vázquez A, Savji N, De Ory F, et al. First report of sylvatic DENV-2-associated dengue hemorrhagic fever in West Africa. PLoS Negl Trop Dis. 2011;5:e1251. doi: 10.1371/journal.pntd.0001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan VC, Lye DC, Thein TL, Dimatatac F, Tan AS, Leo Y-S. Implications of discordance in World Health Organization 1997 and 2009 dengue classifications in adult dengue. PloS One. 2013;8:e60946. doi: 10.1371/journal.pone.0060946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JP, Du Saussay C, Gautun JC, McCormick JB, Mouchet J. [Dengue in Burkina Faso (ex-Upper Volta): seasonal epidemics in the urban area of Ouagadougou] Bull Soc Pathol Exot Filiales. 1985;78:7–14. [PubMed] [Google Scholar]
- Guo C, Zhou Z, Wen Z, Liu Y, Zeng C, Xiao D, et al. Global epidemiology of dengue outbreaks in 1990–2015: a systematic review and meta-analysis. Front Cell Infect Microbiol. 2017;7:317. doi: 10.3389/fcimb.2017.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman MG, Dengue Harris E. Lancet. 2015;385:453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- Horstick O, Martinez E, Guzman MG, Martin JLS, Ranzinger SR. WHO dengue case classification 2009 and its usefulness in practice: an expert consensus in the Americas. Pathog Glob Health. 2015;109:19–25. doi: 10.1179/2047773215Y.0000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htun NSN, Odermatt P, Eze IC, Boillat-Blanco N, D'Acremont V, Probst-Hensch N. Is diabetes a risk factor for a severe clinical presentation of dengue? Review and meta-analysis. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institut national de la statistique et de la démographie 2015.Institut national de la statistique et de la démographie . INSD; Burkina Faso: 2015. Annuaire statistique 2014. [Google Scholar]
- Jaenisch T, Junghanss T, Wills B, Brady OJ, Eckerle I, Farlow A, et al. Dengue expansion in Africa – not recognized or not happening? Emerg Infect Dis. 2014;20 doi: 10.3201/eid2010.140487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M-C, Lu P-L, Chang J-M, Lin M-Y, Tsai J-J, Chen Y-H, et al. Impact of renal failure on the outcome of dengue viral infection. Clin J Am Soc Nephrol. 2008;3:1350–1356. doi: 10.2215/CJN.00020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew SM, Khoo EM, Ho BK, Lee YK, Omar M, Ayadurai V, et al. Dengue in Malaysia: factors associated with dengue mortality from a national registry. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limonta D, Capó V, Torres G, Pérez AB, Guzmán MG. Apoptosis in tissues from fatal dengue shock syndrome. J Clin Virol. 2007;40:50–54. doi: 10.1016/j.jcv.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Limonta D, González D, Capó V, Torres G, Pérez AB, Rosario D, et al. Fatal severe dengue and cell death in sickle cell disease during the 2001–2002 Havana dengue epidemic. Int J Infect Dis. 2009;13:e77–e78. doi: 10.1016/j.ijid.2008.06.028. [DOI] [PubMed] [Google Scholar]
- Lin RJ, Lee TH, Leo YS. Dengue in the elderly: a review. Expert Rev Anti Infect Ther. 2017;15:729–735. doi: 10.1080/14787210.2017.1358610. [DOI] [PubMed] [Google Scholar]
- Mallhi TH, Khan AH, Sarriff A, Adnan AS, Khan YH, Jummaat F. Defining acute kidney injury in dengue viral infection by conventional and novel classification systems (AKIN and RIFLE): a comparative analysis. Postgrad Med J. 2016;92:78–86. doi: 10.1136/postgradmedj-2015-133582. [DOI] [PubMed] [Google Scholar]
- Ministry of Health of Burkina Faso . Ministry of Health of Burkina Faso; Ouagadougou: 2016. Epidemiological Surveillance Service. Outbreak of dengue cases in Burkina Faso: epidemiological situation of dengue fever: Situat Rep N ° 052. [Google Scholar]
- Mulligan K, Dixon J, Joanna Sinn C-L, Elliott SJ. Is dengue a disease of poverty? A systematic review. Pathog Glob Health. 2015;109:10–18. doi: 10.1179/2047773214Y.0000000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J, Hsu JP, Yeo TW, Leo YS, Lye DC. Diabetes, cardiac disorders and asthma as risk factors for severe organ involvement among adult dengue patients: a matched case–control study. Sci Rep. 2017;7:39872. doi: 10.1038/srep39872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridde V, Agier I, Bonnet E, Carabali M, Dabiré KR, Fournet F, et al. Presence of three dengue serotypes in Ouagadougou (Burkina Faso): research and public health implications. Infect Dis Poverty. 2016;5:23. doi: 10.1186/s40249-016-0120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V, Lhuillier M, Meunier D, Sarthou JL, Monteny N, Digoutte JP, et al. [Yellow fever virus, dengue 2 and other arboviruses isolated from mosquitos, in Burkina Faso, from 1983 to 1986. Entomological and epidemiological considerations] Bull Soc Pathol Exot. 1993;86:90–100. [PubMed] [Google Scholar]
- Sharp TM, Moreira R, Soares MJ, Miguel da Costa L, Mann J, DeLorey M, et al. Underrecognition of dengue during 2013 epidemic in Luanda. Angola. Emerg Infect Dis. 2015;21:1311–1316. doi: 10.3201/eid2108.150368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard DS, Undurraga EA, Betancourt-Cravioto M, Guzmán MG, Halstead SB, Harris E, et al. Approaches to refining estimates of global burden and economics of dengue. PLoS Negl Trop Dis. 2014;8:e3306. doi: 10.1371/journal.pntd.0003306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanaway JD, Shepard DS, Undurraga EA, Halasa YA, Coffeng LE, Brady OJ, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:712–723. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantawichien T. Dengue fever and dengue haemorrhagic fever in adolescents and adults. Paediatr Int Child Health. 2012;32(Suppl. 1):22–27. doi: 10.1179/2046904712Z.00000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnagda Z, Cissé A, Bicaba BW, Diagbouga S, Sagna T, Ilboudo AK, et al. Dengue fever in Burkina Faso, 2016. Emerg Infect Dis. 2018;24:170–172. doi: 10.3201/eid2401.170973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee HP, How SH, Jamalludin AR, Safhan MNF, Sapian MM, Kuan YC, et al. Risk factors associated with development of dengue haemorrhagic fever or dengue shock syndrome in adults in Hospital Tengku Ampuan Afzan Kuantan. Med J Malaysia. 2009;64:316–320. [PubMed] [Google Scholar]
- Toledo J, George L, Martinez E, Lazaro A, Han WW, Coelho GE, et al. Relevance of non-communicable comorbidities for the development of the severe forms of dengue: a systematic literature review. PLoS Negl Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0004284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–1917. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- Vachvanichsanong P, Thisyakorn U, Thisyakorn C. Dengue hemorrhagic fever and the kidney. Arch Virol. 2016;161:771–778. doi: 10.1007/s00705-015-2727-1. [DOI] [PubMed] [Google Scholar]
- Were F. The dengue situation in Africa. Paediatr Int Child Health. 2012;32(Suppl. 1):18–21. doi: 10.1179/2046904712Z.00000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann O, Hongsiriwon S, Bowonwatanuwong C, Chotivanich K, Sukthana Y, Pukrittayakamee S. Risk factors and clinical features associated with severe dengue infection in adults and children during the 2001 epidemic in Chonburi, Thailand. Trop Med Int Health. 2004;9:1022–1029. doi: 10.1111/j.1365-3156.2004.01295.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO; Geneva: 2009. Dengue: guidelines for diagnosis, treatment, prevention and control. [PubMed] [Google Scholar]
- World Health Organization . Global strategy for dengue prevention and control. WHO; Geneva:: 2012. , 2012–2020. [Google Scholar]
- World Health Organization . WHO; Geneva: 2017. Dengue and severe dengue. [Google Scholar]
- Aguilar-Briseño José A, et al. Understanding immunopathology of severe dengue: lessons learnt from sepsis. Current Opinion in Virology, 2020;43:41–49. doi: 10.1016/j.coviro.2020.07.010. [DOI] [PubMed] [Google Scholar]
- Rathore Abhay PS, et al. Risk factors and biomarkers of severe dengue. Current Opinion in Virology, 2020;43:1–8. doi: 10.1016/j.coviro.2020.06.008. [DOI] [PubMed] [Google Scholar]
- Chang K, et al. Differences in Mortality and Clinical Manifestations of Dengue Hemorrhagic Fever in Taiwan in Different Years: A Comparison for Cases in 2014 and 2015 Epidemics. Am. J. Trop. Med. Hyg. 2017 doi: 10.4269/ajtmh.16-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.