Abstract
Testosterone is one possible biomarker for depression risk among men and women. Both high and low levels of testosterone have been associated with depression, at least among men. Testosterone may be associated only with specific symptoms of depression, which might help to explain inconsistencies in previous results.
We examined the cross-sectional associations between total testosterone and the specific symptoms of depression using pooled data across three cycles of NHANES (2011–2012, 2013–2014, and 2015–2016). The sample included 4253 men and 5102 women. Testosterone was modelled as 1) a dichotomous (low testosterone cut-off <300 ng/dL for men and 15 ng/dL for women) and 2) a continuous variable using cubic splines.
In men, very low testosterone was weakly associated with problems with appetite, whereas very high testosterone was associated with sleep problems and weakly associated with tiredness. There were no consistent symptom-specific associations among women.
These findings provide only suggestive evidence for symptom-specific associations between testosterone and depression, mainly related to somatic complaints. Further data are needed to assess the reliability of these associations.
Highlights
-
•
Testosterone and depression have been associated in some past studies.
-
•
To find a possible explanation for the inconsistencies of some of the previous studies, we studied the association between testosterone and depression in men and women.
-
•
Very high testosterone was associated with sleep problems and weakly associated with tiredness among men.
-
•
Very low testosterone was weakly associated with appetite problems among men.
-
•
There was some evidence that very low testosterone may be associated with appetite problems among women.
1. Introduction
Testosterone levels may represent one important biomarker for depression risk. In men, lower testosterone has been associated with higher risk of depression [[1], [2], [3]], and this association has also been shown with the more specific measure of “free testosterone” that measures bioactive levels of testosterone not attached to sex hormone-binding globulin (SHBG; [[2], [4]]). However, some studies have suggested that both low and high levels of testosterone might be related to elevated depression risk [5]. There are fewer studies of testosterone and depression among women, with elevated depression risk being associated with low [6] or high [7,8]; testosterone levels. Two meta-analytic studies on testosterone replacement reported that testosterone therapy was accompanied by decreasing levels of depressive symptoms among men [9,10]. However, it is not clear how results from testosterone replacement populations generalize to the general population as it has been suggested that there is not consistent association among healthy men [11].
Studies of testosterone have almost always assessed depression as a single continuum, without considering possible differences between specific symptoms of depression. Studies on other risk factors of depression—inflammation [12,13] and social risk factors [14] for example—have shown that some risk factors may be more important for specific depression symptoms but not others. In a study of depression subtypes (Rodgers et al., 2015), atypical depression subtype (e.g., increased appetite, sleeping too much, mood reactivity towards positive events) was associated with lower testosterone levels among men when compared to melancholic depression (e.g., chronic sadness and anhedonia) or the control group. In addition, there is a study suggesting that testosterone was associated with anxiety disorder and social and specific phobias among men, but not depression itself [15]. Another study [16] of older men found the testosterone-regulating gene CAG-repeat length to be associated especially with suicidal ideas, anxiety, “deterioration of general well-being” and depressive mood. Also more complex associations of CAG-repeat length have been found [17]. However, there seem to be no studies comparing whether low and high levels of testosterone are differently associated with the specific symptoms of depression. Such information might be useful in better understanding the role of testosterone in the etiology of depression and clarifying why both low and high testosterone levels have been associated with elevated risk of depression.
In the present study, we used data from the repeated National Health and Nutrition Examination Studies (NHANES) to examine the cross-sectional association between testosterone and specific symptoms of depression in men and women.
2. Methods
2.1. Participants and study design
Participants were from the National Health and Nutrition Examination Surveys (NHANES) of 2011–2012, 2013–2014, and 2015–2016. The NHANES is a program of studies designed to assess the health and nutritional status of adults and children in the United States. Each year, the survey examines a nationally representative sample of about 5000 people and uses a stratified, multistage probability sampling design. Health measurements are performed in mobile examination centers and health interviews are conducted in respondents’ homes. The NHANES studies are carried out by the U.S. National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). The datasets can be freely obtained directly from their website (https://www.cdc.gov/nchs/nhanes/index.htm) or via the Inter-university Consortium for Political and Social Research (ICPSR; https://www.icpsr.umich.edu/web/ICPSR/series/39). The authors do not own the data, so the data were not archived in any additional data repositories. NHANES received approval from the National Center for Health Statistics Ethics Review Board, and all participants provided informed consent.
We pooled data across three cycles of NHANES (2011–2012, 2013–2014, and 2015–2016). First, we identified participants aged 18 years and older with data on testosterone (7805 men and 8273 women). Participants with a testosterone level above the normal range (more than 1100 ng per deciliter (ng/dL) for men and 70 ng/dL for women) [18,19] were excluded (47 men and 186 women). Participants with missing information on depressive symptoms (3372 men and 2825 women), or any covariate (133 men and 160 women) were also excluded, resulting in a final sample size of 9355 (4253 men and 5102 women).
2.2. Measures
Depressive symptoms were assessed using the Patient Health Questionnaire (PHQ-9) [20]. The nine items of the questionnaire query how often the participant had been bothered by specific depression symptoms during the last 2 weeks, each self-rated on a 4-point response scale (0 = Not at all, 1 = Several days, 2 = More than half the days, 3 = Nearly every day). Corresponding to the DSM-IV criteria of depression, PHQ items measure cognitive and emotional symptoms (“have little interest in doing things” (little interest); “feeling down, depressed or hopeless” (feeling down); “feeling bad about yourself or that you are a failure” (feeling bad); “trouble concentrating”; “thoughts that you would be better off dead” (suicidality)) and somatic symptoms (“trouble falling or staying asleep, or sleeping too much” (sleep problems); “feeling tired or having little energy” (feeling tired); “poor appetite or overeating” (appetite problems); “moving or speaking slowly or being more restless than usual” (psychomotor change)). We first calculated a depressive symptom sum score (range 0–27) that reflected the severity of depression. Next, we dichotomized the items so that values 0 and 1 were coded as no symptom, and values 2 and 3 were coded as endorsing the symptom.
Total testosterone levels were measured in serum using isotope dilution liquid chromatography tandem mass spectrometry by the National Center for Environmental Health, Centers for Disease Control and Prevention (https://www.icpsr.umich.edu/web/ICPSR/series/39). Total testosterone was measured with LC-MS/MS [21]; NHANES web page https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/TST_G.htm). Testosterone was isolated from 100 μL serum by 2 serial liquid–liquid extraction steps and quantified with [13C] stable isotope–labelled testosterone as the internal standard. Imprecision over 2 years was <4.8%, and the limit of detection was 0.3 ng/dL (0.01 nmol/L). Vials were stored under appropriate frozen (−20 °C) conditions until they were shipped testing. More detailed descriptions of the specimen collection and laboratory protocols are provided in the NHANES Laboratory Procedures Manual (https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/manuals/2011-12_Laboratory_Procedures_Manual.pdf).
Covariates included age, body mass index (BMI), alcohol use (no heavy use/heavy use at least once during the previous 12 months), smoking (yes/no), and physical activity (moderate or vigorous, no vigorous but at least moderate, or vigorous). For women, information about being pregnant (yes/no) was also included in the analysis.
2.3. Statistical analysis
Modelling was performed in R Statistical Software (version 3.6.1) using the svyglm function [22]. The complete code is freely available on GitHub (https://github.com/Gluschkoff/tst-depr). Statistical significance for all tests was set at p < .05, and all tests were two-tailed. Given the exploratory nature of the study, nonsignificant associations with p values < .10 are also noted.
Linear regression models were fitted to examine the sex-specific associations of total testosterone with depression sum score and logistic regression for associations with specific symptoms of depression. We first examined the associations of low total testosterone. For this analysis, total testosterone levels were dichotomized into low and normal categories (cut-off <300 ng/dL for men and <15 ng/dL for women) [18,19,23]. To accommodate for more complex, potential non-linear associations, we next used restricted (also known as natural) cubic splines to model the distribution of total testosterone (see Ref. [24].) A spline is constructed of piecewise polynomials which pass through a set of data values (i.e., knots). The spline included two boundary knots (the lowest and highest values of testosterone) and two internal knots that were placed at the 33rd and 66th percentiles of total testosterone (316.83 and 456.73 for men, 14.75 and 24.30 for women). Statistical significance of the overall effect of total testosterone was tested with a Wald test.
After fitting unadjusted models, we adjusted for the effects of age, BMI, alcohol use, smoking, physical activity (men and women), and pregnancy status (women). As a sensitivity analysis, we repeated all the analysis in the subsets of men and women who screened positive for at least mild depression (PHQ9 sum score > 4). We also assessed the sensitivity of our results to an alternative coding strategy where we used log-transformed testosterone values instead of raw concentrations.
Following NHANES analytic and reporting guidelines, all analyses were conducted using survey procedures to account for the complex survey design (including oversampling), survey nonresponse, and post-stratification. The weighted sample is representative of the US Census civilian noninstitutionalized population. No sampling biases are expected.
3. Results
Combining data from the NHANES 2011–2016 surveys gave us an unweighted sample size of 9355 individuals (weighted = 134 million). Table 1 shows the sex-stratified demographic characteristics of the sample and descriptive statistics.
Table 1.
Sample characteristics and descriptive statistics.
| Variable | Men (n = 4253) |
Women (n = 5102) |
p for difference |
|---|---|---|---|
| Mean (SD) or % | Mean (SD) or % | ||
| Age (range 18–80) | 45.51 (16.95) | 47.88 (17.71) | <0.001 |
| BMI (range 13.60–82.10) | 29.04 (6.38) | 29.73 (7.81) | <0.001 |
| Smoker (“yes") | 19% | 16% | 0.003 |
| Heavy alcohol use (“yes") | 18% | 9% | <0.001 |
| Physical activity | <0.001 | ||
| No moderate or vigorous | 45% | 49% | |
| No vigorous but at least moderate | 45% | 45% | |
| Vigorous | 10% | 6% | |
| Pregnant (“yes") | NA | 1% | |
| Depression sum score | 4.11 (4.10) | 4.86 (4.53) | <0.001 |
| Depressive symptoms (“yes") | |||
| Little interest | 10% | 12% | 0.030 |
| Feeling down | 8% | 10% | 0.035 |
| Sleep problems | 21% | 23% | 0.078 |
| Feeling tired | 20% | 26% | <0.001 |
| Appetite | 10% | 15% | <0.001 |
| Feeling bad | 6% | 9% | <0.001 |
| Trouble concentrating | 8% | 9% | 0.009 |
| Psychomotor change | 5% | 5% | 0.156 |
| Suicidality | 2% | 2% | 0.968 |
| Testosterone, total (ng/dL)† | 404.89 (170.16) | 21.79 (12.28) | <0.001 |
| Low total testosterone | 29% | 33% |
Note. † range 1.23–1096.30 for men, 0.25–70 for women.
3.1. Results for low total testosterone
In the unadjusted models for men, there was no association between low total testosterone and depression sum score (β = 0.03, 95% CI -0.04 to 0.11) or the specific symptoms, except for the symptom of feeling tired (OR = 1.27, 95% CI 1.04 to 1.54). After adjusting for age, alcohol use, smoking, physical activity, and BMI, the association with feeling tired attenuated (OR = 1.18, 95% CI 0.98 to 1.43). In the unadjusted models for women, there was no association between low total testosterone and depression sum score (β = 0.02, 95% CI -0.04 to 0.09) or the specific symptoms. However, in the adjusted model, low testosterone was associated with higher depression sum score (β = 0.07, 95% CI 0.01 to 0.14). The adjusted symptom-specific associations in men and women are shown in Supplementary Fig. 1 in Appendix.
3.2. Results using splines to model testosterone
In the unadjusted models for men, there was no association between total testosterone and depression sum score (p = .104). As for the unadjusted symptom-specific associations, testosterone was associated with sleep problems (p = .032), feeling tired (p = .019) and appetite problems (p = .042). After adjusting for age, alcohol use, smoking, physical activity, and BMI, the association remained significant for sleep problems (p = .032), but no longer for feeling tired (p = .077) or appetite problems (p = .055).
In the unadjusted models for women, there were no associations between total testosterone and depression sum score (p = .699) or individual depressive symptoms. After adjusting for age, alcohol use, smoking, physical activity, BMI, and pregnancy, testosterone was associated with appetite problems (p = .034).
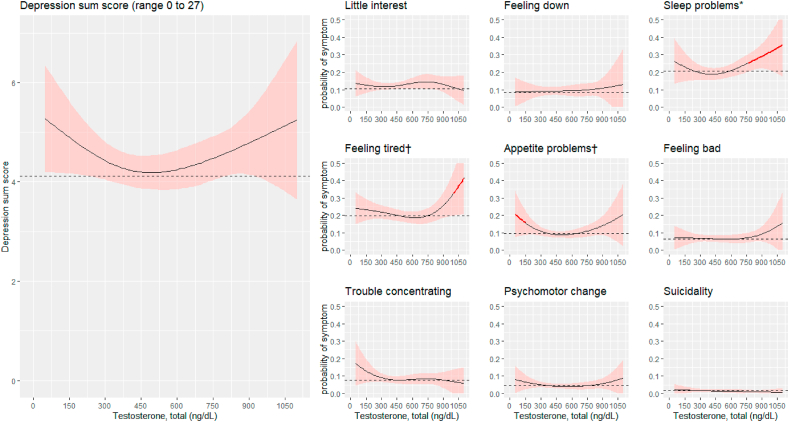
The results from the splines analysis were further inspected by examining the model-predicted probabilities of symptom endorsement at different levels of testosterone. Although the overall non-linear effects of testosterone were not significant for most of the symptoms, extreme levels of testosterone appeared be associated with a higher probability of symptom endorsement for some specific symptoms (see Fig. 1, Fig. 2). An examination of the predicted probabilities for men showed that, if compared to a total testosterone level of 400 ng/dL (which roughly corresponds to the average and median values of testosterone in men), testosterone level of 150 ng/dL or lower was associated with a higher probability of appetite problems (corresponding to an odd ratio (OR) 1.81 at 150 ng/dL), a level of 760 ng/dL or higher with a higher probability of sleep problems (OR 1.44 at 760 ng/dL), a level of 1000 ng/dL or higher with a higher probability of feeling tired (OR 1.82 at 1000 ng/dL, all p values < .05).
Fig. 1.
Predicted depression sum scores and predicted probabilities of specific symptoms of depression across different levels of testosterone in men (n = 4251). The predictions were obtained from cubic spline regression models with sampling weights, adjusting for age, BMI, alcohol use, smoking, and physical activity. The predicted values reflect a situation where the covariates are set at their mean or mode. The dashed lines indicate the predicted mean of depression sum score and the predicted probability (i.e., prevalence) for each depressive symptom. The red line color marks total testosterone levels at which the probability of the symptom is significantly higher than at the average level of total testosterone (p < .05). The shaded light red regions represent a 95% confidence interval band. ∗p < .05; †p < .10 for the association between total testosterone and the depressive symptom (Wald test). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
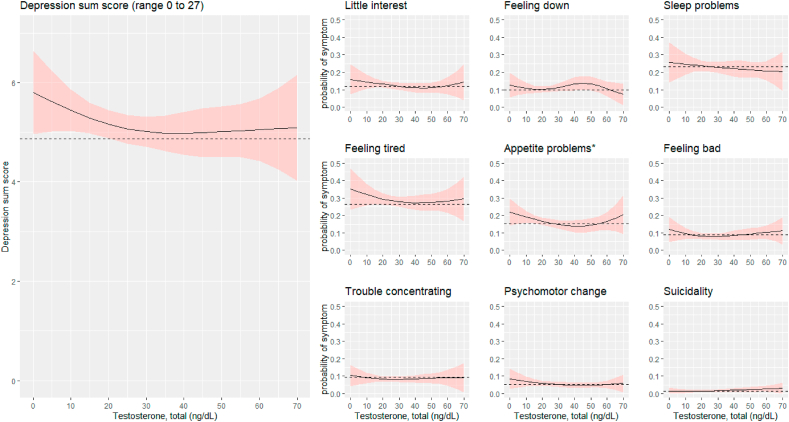
Fig. 2.
Predicted depression sum scores and predicted probabilities of specific symptoms of depression across different levels of testosterone in women (n = 5102). The predictions were obtained from cubic spline regression models with sampling weights, adjusting for age, BMI, alcohol use, smoking, physical activity, and pregnancy. The predicted values reflect a situation where the covariates are set at their mean or mode. The dashed lines indicate the predicted mean of depression sum score and the predicted probability (i.e., prevalence) for each depressive symptom. The shaded light red regions represent a 95% confidence interval band. ∗p < .05 for the association between total testosterone and depression or individual depressive symptom (Wald test).
In women, the probability of endorsing a specific symptom was not higher at the extreme levels of testosterone compared to a total testosterone level of 20 ng/dL, which roughly corresponds to the average and median values of testosterone in women.
3.3. Sensitivity analysis
As for of the associations of low testosterone, the results with subsets of men and women who screened positive for at least mild depression were similar as in the main analysis in terms of direction and (non-)significance (see Supplementary Fig. 2 for symptom-specific associations). The only exception was that the (weak) association between low testosterone and depression sum score which was observed in women in the main analysis was not supported in the sensitivity analysis. As for the analysis using splines (Supplementary Figs. 3 and 4), the sensitivity analysis supported the results of the main analysis with regard to the association between higher testosterone and sleep problems in men. This association was, however, non-significant, likely due to wider confidence intervals attributable to the smaller sample size. In contrast to the main analysis, higher testosterone was associated with suicidality in women (p = .024).
As a final step, we assessed the sensitivity of our results to an alternative coding strategy where we used log-transformed testosterone values instead of raw concentrations. In men, the results were similar to those obtained with raw testosterone data with the exception that instead of high testosterone, low testosterone values seemed to drive testosterone’s (weak) association with feeling tired. In women, a greater number of significant associations were found when using log-transformed values of testosterone. Log-transformed testosterone was associated with sleep problems (p = .038) and suicidality (p = .025) in the unadjusted models, and with appetite problems (p = .033), feeling bad (p = .029), and suicidality (p = .016) in the adjusted models. The associations were driven by either low (sleep problems and appetite problems) or high levels of testosterone (feeling bad and suicidality).
4. Discussion
In these nationally representative data from U.S. NHANES cohort studies, we observed no consistent association of testosterone with a depression sum score in either men or women. In men, very high testosterone was associated with sleep problems and tiredness, whereas very low testosterone was related to problems with appetite. However, these associations were weak. While testosterone was associated with appetite problems also in women, the association was significant only after adjustments, suggesting an unreliable association. The analyses using cut-off points for low testosterone, on the other hand, provided no consistent results for neither sex.
Several studies have reported that lower total testosterone [[1], [2], [3]] and lower free testosterone [2,4] are associated with higher risk of depression in men. In addition, two meta-analytic studies also found that testosterone therapy was accompanied by decreasing levels of depressive symptoms in men [9,10]. Our findings related to appetite problems would be in line with these findings, although the present results did not support an association between low testosterone and higher level of overall depressive symptoms.
While many studies have focused on low testosterone as a risk factor for depression, there is considerable evidence suggesting that higher levels of testosterone may also be associated with elevated depression risk among men [5]. In fact, one review suggested that high levels of testosterone have been associated with depression even more consistently than low levels [25]. In one study, change in testosterone levels, rather than the level, was inversely associated with depression [26]. The current associations of higher testosterone with sleep problems and fatigue would be in agreement with this line of research. Very high testosterone appears to be associated with “antisocial behavior, risk behavior, unemployment, low paying jobs and being unmarried” [5], which may, in turn, be risk factors for depression. Testosterone is not associated with depression-related temperament traits [27].
Adjusted for BMI and other covariates, very low testosterone in women was related to appetite problems. Previous cross-sectional studies among women have found that both low [6] and high [7,8] levels of testosterone may be associated with depression. According to another study, testosterone and free testosterone increased among women who had received antidepressant treatment [28], which might imply a reverse direction of the causation. There is also some evidence for the association from cross-sectional studies. Being overweight has previously been associated with both high testosterone and high levels of depressive symptoms, and free testosterone has been suggested to mediate the association of overweight/obesity with depressive symptoms among women [7]. Both activational and organisational effects of testosterone are likely to contribute to the sex difference in depression [29].
There are some limitations regarding the present study that should be noted. One major limitation is the cross-sectional design of the NHANES which precludes inferences of temporal or causal relationships between testosterone and depressive symptoms. Second, given the exploratory nature of the analysis, the potential for type 1 errors should be noted and the results should be viewed as preliminary. Finally, the PHQ-9 items measure only a subset of the symptoms of depression [30]. Apart from the nine symptoms of the PHQ, other depressive symptoms were not included in this study which may be counted as a limitation. We believe, however that the PHQ gives a reliable estimate of depression and separate depressive symptoms. The sample of the study is representative of the general population. Thus, sampling bias is not expected.
5. Conclusions
The study provided only suggestive evidence for associations between testosterone and specific symptoms of depression, mostly related to somatic complaints. Additional data are needed to test the reliability of these associations.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The project was funded by Academy of Finland (project 311578).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpnec.2021.100044.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Barrett-Connor E., von Mühlen D.G., Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the rancho bernardo study. J. Clin. Endocrinol. Metab. 1999;84(2):573–577. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]
- 2.McIntyre R.S., Mancini D., Eisfeld B.S., Soczynska J.K., Grupp L., Konarski J.Z., Kennedy S.H. Calculated bioavailable testosterone levels and depression in middle-aged men. Psychoneuroendocrinology. 2006;31(9):1029–1035. doi: 10.1016/j.psyneuen.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Shores M.M., Sloan K.L., Matsumoto A.M., Moceri V.M., Felker B., Kivlahan D.R. Increased incidence of diagnosed depressive illness in HypogonadalOlder men. Arch. Gen. Psychiatr. 2004;61(2):162–167. doi: 10.1001/archpsyc.61.2.162. [DOI] [PubMed] [Google Scholar]
- 4.Joshi D., Van Schoor N.M., De Ronde W., Schaap L.A., Comijs H.C., Beekman A.T.F., Lips P. Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clin. Endocrinol. 2010;72(2):232–240. doi: 10.1111/j.1365-2265.2009.03641.x. [DOI] [PubMed] [Google Scholar]
- 5.Booth A., Johnson D.R., Granger D.A. Testosterone and men’s depression: the role of social behavior. J. Health Soc. Behav. 1999;40(2):130–140. doi: 10.2307/2676369. [DOI] [PubMed] [Google Scholar]
- 6.Giltay E.J., Enter D., Zitman F.G., Penninx B.W.J.H., van Pelt J., Spinhoven P., Roelofs K. Salivary testosterone: associations with depression, anxiety disorders, and antidepressant use in a large cohort study. J. Psychosom. Res. 2012;72(3):205–213. doi: 10.1016/j.jpsychores.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Stanikova D., Zsido R.G., Luck T., Pabst A., Enzenbach C., Bae Y.J.…Sacher J. Testosterone imbalance may link depression and increased body weight in premenopausal women. Transl. Psychiatry. 2019;9(1) doi: 10.1038/s41398-019-0487-5. 160-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber B., Lewicka S., Deuschle M., Colla M., Heuser I. Testosterone, androstenedione and dihydrotestosterone concentrations are elevated in female patients with major depression. Psychoneuroendocrinology. 2000;25(8):765–771. doi: 10.1016/S0306-4530(00)00023-8. [DOI] [PubMed] [Google Scholar]
- 9.Walther A., Breidenstein J., Miller R. Association of testosterone treatment with alleviation of depressive symptoms in men: a systematic review and meta-analysis. JAMA Psychiatry. 2019;76(1):31–40. doi: 10.1001/jamapsychiatry.2018.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarrouf F.A., Artz S., Griffith J., Sirbu C., Kommor M. Testosterone and depression: systematic review and meta-analysis. J. Psychiatr. Pract. 2009;15(4):289–305. doi: 10.1097/01.pra.0000358315.88931.fc. [DOI] [PubMed] [Google Scholar]
- 11.Amiaz R., Seidman S.N. Testosterone and depression in men. Curr. Opin. Endocrinol. Diabetes Obes. 2008;15(3):278–283. doi: 10.1097/MED.0b013e3282fc27eb. [DOI] [PubMed] [Google Scholar]
- 12.Jokela M., Virtanen M., Batty G.D., Kivimäki M. Inflammation and specific symptoms of depression. JAMA Psychiatry. 2016;73(1):87–88. doi: 10.1001/jamapsychiatry.2015.1977. [DOI] [PubMed] [Google Scholar]
- 13.Fried E.I., von Stockert S., Haslbeck J.M.B., Lamers F., Schoevers R.A., Penninx B.W.J.H. Using network analysis to examine links between individual depressive symptoms, inflammatory markers, and covariates. Psychol. Med. 2019:1–9. doi: 10.1017/S0033291719002770. [DOI] [PubMed] [Google Scholar]
- 14.Jokela M., García-Velázquez R., Airaksinen J., Gluschkoff K., Kivimäki M., Rosenström T. Chronic diseases and social risk factors in relation to specific symptoms of depression: evidence from the U.S. national health and nutrition examination surveys. J. Affect. Disord. 2019;251:242–247. doi: 10.1016/j.jad.2019.03.074. [DOI] [PubMed] [Google Scholar]
- 15.Smeeth D.M., Dima D., Jones L., Jones I., Craddock N., Owen M.J.…Powell T.R. Polygenic risk for circulating reproductive hormone levels and their influence on hippocampal volume and depression susceptibility. Psychoneuroendocrinology. 2019;106:24–292. doi: 10.1016/j.psyneuen.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Härkönen K., Huhtaniemi I., Mäkinen J., Hübler D., Irjala K., Koskenvuo M.…Pöllänen P. The polymorphic androgen receptor gene CAG repeat, pituitary–testicular function and andropausal symptoms in ageing men. Int. J. Androl. 2003;26(3):187–194. doi: 10.1046/j.1365-2605.2003.00415.x. [DOI] [PubMed] [Google Scholar]
- 17.Seidman S.N., Araujo A.B., Roose S.P., McKinlay J.B. Testosterone level, androgen receptor polymorphism, and depressive symptoms in middle-aged men. Biol. Psychiatr. 2001;50(5):371–376. doi: 10.1016/S0006-3223(01)01148-9. [DOI] [PubMed] [Google Scholar]
- 18.NHANES laboratory procedures manual 2018. https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/TST_H_MET_Total_Estradiol_and_Total_Testosterone.pdf
- 19.University of Rochester Medical Center Total testosterone. Health encyclopedia. https://www.urmc.rochester.edu/encyclopedia/content.aspx?contenttypeid=167&contentid=testosterone_total
- 20.Kroenke K., Spitzer R.L., Williams J.B.W. The PHQ-9. J. Gen. Intern. Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vesper H.W., Wang Y., Vidal M., Botelho J.C., Caudill S.P. Serum total testosterone concentrations in the US household population from the NHANES 2011–2012 study population. Clin. Chem. 2015;61(12):1495–1504. doi: 10.1373/clinchem.2015.245969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lumley T. Analysis of complex survey samples. J. Stat. Software. 2004 doi: 10.18637/jss.v009.i08. [DOI] [Google Scholar]
- 23.Mulhall J.P., Trost L.W., Brannigan R.E., Kurtz E.G., Redmon J.B., Chiles K.A.…Platz E.A. Evaluation and management of testosterone deficiency: AUA guideline. J. Urol. 2018;200(2):423–432. doi: 10.1016/j.juro.2018.03.115. [DOI] [PubMed] [Google Scholar]
- 24.Shepherd B.E., Rebeiro P.F. Assessing and interpreting the association between continuous covariates and outcomes in observational studies of HIV using splines. J. Acquir. Immune Defic. Syndr. 2017;74(3):e60–e63. doi: 10.1097/QAI.0000000000001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson J.M., Nachtigall L.B., Stern T.A. The effect of testosterone levels on mood in men: a review. Psychosomatics. 2013;54(6):509–514. doi: 10.1016/j.psym.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Kische H., Pieper L., Venz J., Klotsche J., März W., Koch-Gromus U.…Haring R. Longitudinal change instead of baseline testosterone predicts depressive symptoms. Psychoneuroendocrinology. 2018;89:7–12. doi: 10.1016/j.psyneuen.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Määttänen I., Jokela M., Hintsa T., Firtser S., Kähönen M., Jula A.…Keltikangas-Järvinen L. Testosterone and temperament traits in men: longitudinal analysis. Psychoneuroendocrinology. 2013;38(10):2243–2248. doi: 10.1016/j.psyneuen.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Kumsar Ş., Kumsar N.A., Sağlam H.S., Köse O., Budak S., Adsan Ö. Testosterone levels and sexual function disorders in depressive female patients: effects of antidepressant treatment. J. Sex. Med. 2014;11(2):529–535. doi: 10.1111/jsm.12394. [DOI] [PubMed] [Google Scholar]
- 29.McHenry J., Carrier N., Hull E., Kabbaj M. Sex differences in anxiety and depression: role of testosterone. Front. Neuroendocrinol. 2014;35(1):42–57. doi: 10.1016/j.yfrne.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fried E.I. The 52 symptoms of major depression: lack of content overlap among seven common depression scales. J. Affect. Disord. 2017;208:191–197. doi: 10.1016/j.jad.2016.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.