Abstract
Background
Many adult females experience periodic mental and physical symptoms associated with premenstrual syndrome (PMS). Diagnosis of PMS is generally based on self-reported symptoms over several menstrual cycles, but there are concerns about its accuracy and duration. It is well known that decreased cognitive function is one of the symptoms of PMS. Near-infrared spectroscopy (NIRS) is one of the methods for imaging brain activity, similar to magnetic resonance imaging (MRI), electroencephalography (EEG), and positron emission tomography (PET). NIRS has been used to assess cognitive function decline demonstrated by decline in brain activity. However, to the best of our knowledge, there have been no report characterizing brain activity pattern in females with PMS during the luteal and follicular phases separately.
Objective
We aimed to characterize the cognitive function of females with PMS during the follicular and luteal phases using NIRS.
Methods
The level of brain activity in the prefrontal cortex was detected with NIRS while PMS women were performing cognitive tasks. NIRS detected brain activity by measuring the oxy-hemoglobin and deoxy-hemoglobin levels. Participants were females between the ages of 20 and 25 with PMS (n = 11) and without PMS (n = 11). During the participants’ follicular and luteal phases, the participants were asked to perform the cognitive task; an N-back task (0-, 1-, and 2-back tasks), which is widely used for assessing cognitive function. We also calculated the oxyhemoglobin integral value during the N-back task using the NIRS signal; this value represented the total amount of change in cerebral oxyhemoglobin and the brain activation level.
Results
The correct response rate on the 2-back task was significantly lower during both the follicular and luteal phases in females with PMS compared to that in females without PMS (P = 0.01; P = 0.02, during the follicular and luteal phases, respectively). During the luteal phase, brain activation was significantly lower in participants with PMS than in that in females without PMS (P = 0.04). In addition, during the luteal phase, the participants with PMS also had higher negative mood than those without PMS.
Conclusion
The cognitive decline during the luteal phase in participants with PMS was detected by NIRS with significant differences from participants without PMS. The difference was observed only during the luteal phase, not in the follicular phase and were related to the increase in negative mood. These results may provide an objective method for diagnosing PMS based on brain activity. We believe that the use of instruments (e.g., NIRS, MRI, EEG … etc.) to detect cognitive function decline will lead to rapid and reliable diagnosis of PMS and premenstrual dysphoric disorder.
Keywords: Premenstrual syndrome (PMS), Near-infrared spectroscopy (NIRS), Cognitive function, Oxyhemoglobin, Mood, Luteal phase
1. Introduction
Some females are vulnerable to premenstrual syndrome (PMS) during the luteal phase, which occurs after ovulation. Females affected by PMS primarily complain of mental symptoms, including irritability and emotional instability, and physical symptoms such as headache or bloating. When these mental symptoms become severe enough to impair social functioning and other aspects of life such as efficiency at work, the patient is diagnosed with premenstrual dysphoric disorder (PMDD). Definitions of PMDD and PMS are based on diagnostic and statistical manual of mental disorders, fifth edition (DSM-5). PMDD is defined as having at least five symptoms of which at least one has to be a core emotional symptom, while PMS is defined as having between one and four symptoms of which none has to be of emotional nature. According to DSM-5, the existence or non-existence of functional impairment is not used to differentiate between PMDD and PMS, but rather the number and nature of symptoms. Females with PMS and PMDD experience a reduction in their quality of life [1]. In addition, the economic loss caused by menstrual disorders in Japan is estimated to be 622.8 billion yen [2].
A definitive diagnosis of PMS is made according to the criteria set forth by the American College of Obstetricians and Gynecologists. Self-report tools such as the PMS calendars [3] and the premenstrual symptoms Screening tool [4] are also used to assess PMS. However, obtaining an accurate patient’s mental state evaluation using these tools is difficult, since these are depended on the self-report. Assessment based on the retrospective self-reports of premenstrual affective changes is known to generate a significant bias leading to false-positives [5] and is strongly influenced by beliefs about PMS [[6], [7], [8]]. Moreover, premenstrual affective self-report is listed as a diagnostic criterion of symptom assessment over at least two cycles, which makes the data collected voluminous and thus difficult to analyze. As a result, the percentage of females suffering from PMS varies widely across studies, with rates between 6.5% and 95% having been reported [9]; this makes it difficult to accurately calculate its prevalence. Some studies have been conducted to measure bio-signals such as actigraphy, which evaluates body movement [10], salivary amylase activity, cortisol concentration [11], and vital data such as heart rate or pulse waves [12,13] for an accurate and simple diagnosis of PMS. The assessment of PMS by measuring bio-signals has not yet been established, since these bio-signals are related not only to PMS but also to various physical and mental conditions.
Participants with PMDD, a severe form of PMS, have been reported to experience a decline of cognitive function and activity in the dorsolateral prefrontal cortex (DLPFC) region of the brain by functional magnetic resonance imaging (fMRI) during the luteal phase [21,22]. The DLPFC, along with ventrolateral prefrontal cortex (VLPFC), is known to regulate cognitive functions [14]. In addition, patients with depression have reduced cognitive function and brain activity in the DLPFC and amygdala [[15], [16], [17], [18]]. Cognitive function decline in major depression has been characterized by measuring the brain activity while conducting cognitive tests using a variety of brain imaging methods such as a positron emission tomography [19] functional magnetic resonance imaging (fMRI) [15,20,21], and near-infrared spectroscopy (NIRS) [22].
During the luteal phase, females with PMS also have reduced cognitive functions responsible for memory and emotional control [23]. We expected that cognitive function decline elicited by symptom of PMS can be detected as a decline in brain activity by using a brain imaging method. During the luteal phase, when the symptoms of PMS are more pronounced, patients with PMS are expected to have less brain activity than patients without PMS. In addition, since the symptoms of PMS are periodic, it is expected that the activation of the brain will be maintained at a high level even in patients with PMS during the follicular phase. These characteristic patterns of brain activity may be applied to establish the criteria for evaluating PMS. However, to the best of our knowledge, there are no reports on the measurement of cognitive function using a brain imaging method in females with PMS during the luteal and follicular phases separately.
In this study, NIRS measurements have been applied to characterize patterns of brain activation in patients with PMS during both the follicular and luteal phases. While the use of NIRS remains controversial for various reasons [24,25], it has been approved as a method to aid the diagnosis of depression in Japan [22]. NIRS can measure changes in oxygenated hemoglobin (Oxy-Hb) levels in the prefrontal cortex (PFC) by simply attaching a measuring probe to the forehead without causing mental and physical stress to the participants. Oxy-Hb represents the amount of change in blood flow and correlates with changes in brain activity. We used NIRS to measure and assess changes in the blood flow in the PFC of females with PMS while performing a cognitive task.
2. Material and methods
2.1. Participant selections
The participants were 36 female students between the ages of 20 and 25 who were attending college in Kanagawa Prefecture. These are different participants from those in our previous study [26]. The participants' basal body temperature was measured using a basal body thermometer (MC-652-LC, OMRON Corporation, Kyoto, Japan). Each participant completed a PMS Memory (Japan Family Planning Association, Tokyo, Japan) for two menstrual cycles. Participants with regular menstrual cycles (25–38 days) based on an obstetrician-gynecologist’s diagnosis and basal body temperature across two cycles were included in the study. The participants were also required to not be expecting to change addresses or graduate during the experimental period, have no history of taking low-dose oral contraceptives for three months or longer, and have no history of gynecological disease. Cognitive function was measured in 22 participants who had biphasic basal body temperature, regular menstrual cycles, and Self-Rating Depression Scale (SDS) scores <60.; the remaining were excluded. The participants were divided into three groups according to a PMS screening method (see below).
2.2. PMS screening method
After participants' selection, the 22 participants were classified into three groups (“PMDD”, “PMS” and “non-PMS”) according to the PMDD scale [27] and gynecologist’s diagnosis based on a PMS memory.
PMDD scale was developed based on the Premenstrual Symptoms Screening Tool [4]. The scale divided participants into three groups (“PMDD”, “moderate PMS”, and “mild or no PMS”). It is difficult to discern the subtle differences between mild and non-PMS symptoms since this scale is used to distinguish PMDD and severe PMS from healthy women. The PMDD Scale consists of two subjects (Table S1). Subject I consist of questions related to the participant’s psychological and physical symptoms. Subject II consist of questions related to the interference that occurs with activities and relationships with others. Participants must answer these questions on a four-point scale ("very strong", "yes", "little", and "no"). "PMDD" was defined by the following responses.
・At least one "very strong" in items 1 to 4 for subject I.
・At least four "yes" or "very strong" in items 1 to 12 for subject I.
・At least one "very strong" in items 1 to 5 for subject II.
“Moderate PMS” was defined by the following responses.
・At least one "yes" or "very strong" in items 1 to 4 of subject I.
・At least four "yes" or "very strong" in items 1 to 12 of subject I.
・At least one "yes" or ”very strong” in items 1 to 5 for subject II.
Participants who were not categorized into "PMDD" or "moderate PMS" were grouped into "mild or no PMS".
PMS memory is a self-documentation of the participant’s daily symptoms in terms of physical, mental, and social aspects during the several menstrual cycles. When describing the symptoms, the intensity of the symptoms is listed on a scale of 1 to 3 (1 is little and 3 is strong). There is no cutoff value for the PMS memory, as it is used for self-management of PMS symptoms and as one of the diagnostic tools for PMS.
2.3. Procedure
The study procedure is illustrated in Fig. 1. Each participant was required to report the onset of menstruation and submit a daily chart of basal body temperature for two weeks after menstruation. We strictly documented the ovulation and menstruation date of each participant by the daily reports related to the basal body temperature and decided on a date to call each participant for cognitive function measurements. All measurements were conducted first in the follicular phase and then in the luteal phase. The follicular and luteal phases were defined as 5–12 days (mid to late follicular) and 21–30 days (mid to late luteal) after the start of menstruation, respectively. In this study, participants were recruited during the mid to late follicular phase as the follicular phase in order to avoid contamination of symptoms by menstruation.
Fig. 1.
Study flow diagram and number of participants
The salivary test was performed to measure progesterone and estradiol levels. PMS: premenstrual syndrome; PMDD: premenstrual dysphoric disorder; SDS: Self-rating Depression Scale; POMS: Profile of Mood States; NIRS: Near -Infrared Spectroscopy.
In the measurement of cognitive function, measurements of cerebral blood flow during the performance of the cognitive task were conducted. Before measuring the cerebral blood flow, the participants’ saliva was collected, and they were asked to complete the SDS and Profile of Mood States (POMS). The SDS is a depression severity rating scale developed by Zung [28] which is used to screen patients with depression. Fukuda created a Japanese version of the SDS and confirmed its reliability and content validity [28]. The POMS, developed by McNair, Lorr, and Droppleman [29] and revised by Heuchert [30], is a mood survey that has been confirmed to have reliability, content validity, and construct validity. POMS scores were calculated as T-scores with standard deviations.
2.4. Salivary hormone assays
Salivary estradiol and progesterone concentrations were measured using an enzyme immunoassay kit (salivary progesterone, salivary 17β-estradiol, Salimetrics LLC, U.S.A.). The participants were instructed to abstain from alcohol for 12 h, and from eating and drinking for 1 h, before saliva collection to prevent impurities and dilution of the sample. The saliva was collected with a saliva sample collection kit (Saliva Collection Aid, Salimetrics LLC, U.S.A.) using the passive drool method. The collected saliva was promptly frozen and analyzed by Kamakura Techno‒Science, Inc. (Kanagawa, Japan) on the same day. Estradiol and progesterone concentrations were measured to ensure that participants participated in both the follicular and luteal phases.
2.5. Cerebral blood flow measurement
Cerebral blood flow was measured using NIRS (OEG16-SpO2, Spectratech Inc., Tokyo, Japan) in an environment with a room temperature of 24 °C, humidity of 50–60%, illuminance of 500 lx, and no odor. NIRS probes comprised the emitter and detector units. Twelve of each type of unit were placed in a lattice-like pattern at intervals of 3 cm and measured an area of 150 mm × 60 mm. Fig. S1 illustrates the cerebral blood flow measurement procedure. The participants were seated in a chair with their feet on the ground 55 cm away from a monitor (23.8 inch) with the NIRS probes attached to the forehead. A chin rest was used to prevent head movements. The participants were asked to rest their arms on the desk and use a cushion to allow them to perform the cognitive function task in a relaxed state. They were then asked to perform the cognitive task presented on the monitor whilst the NIRS probes were attached. NIRS signals were sampled every 0.65 s.
2.6. Design of N-back task
In the n-back task, letters of the alphabet are presented in a random order, and the participants have to respond when the presented letter matches the letter that appeared “N” letters earlier. We used almost the same N-back task procedure as used in the previous study [26,31]. The task consists of nine trials: three trials of 0-back task, three trials of 1-back task, and three trials of 2-back task. Although the order in which each trial was presented was randomized, all participants performed the 0-back tasks, 1-back tasks, and 2-back tasks three times each. In the single task, a sentence informing the type of task (0-back, 1-back, or 2 back) was displayed for 3 s at t = 0 s, a symbol “+” was displayed for 9 s at t = 3 s, and then the presentation of the first letter began at t = 12 s. One of the twelve letters (B, D, G, P, Q, T, b, d, g, p, q, t) was presented randomly 32 times at intervals of 1.0 s for 0.5 s each, and the display of final letter began at t = 58.5 s. Therefore, the time required for one trial was 60 s. The symbol “+” were presented for 9 s between each trial, and the total time required to perform the N-back task was 10 min. In the case of 0-back task, the participants were presented a single target character and click on it when it appears. The single target character that participants should detect was displayed with the sentence informing the type of task. In this study, the characters were “p” in the first trial, “t” in the second trial, and “D” in the third trial for the luteal phase, while the characters were “g” in the first trial, “V” in the second trial, and “P” in the third trial for the follicular phase. The overall progress of the N-back task and the participants' responses were managed using the presentation software (Neurobehavioral Systems, Inc., Berkeley, CA, USA).
2.7. NIRS data analysis
The NIRS probes placed over the temporal area (ch 1–3, ch 14–16) adhered poorly to the forehead because the participants were primarily females in their early 20s and tended to have small faces. Thus, only the NIRS data obtained from ch4–ch13 at the probes were used. Oxy-Hb and deoxy-Hb values were obtained 5 s before the start of each session of the N-back task (t = -5 s) to the end of task presentation (t = 60) using ch4-ch13 of the NIRS probe. In each channel, the mean values of oxy-Hb and doxy-Hb for 5 s before the task presentation were calculated and these values were used as the baseline for that trial. The amount of change in oxy-Hb and doxy-Hb (△oxy-Hb and △doxy-Hb) was determined by subtracting the baseline from the values of the oxy-Hb and doxy-Hb data. The values of △oxy-Hb and △doxy-Hb obtained from each channel of the NIRS probe were averaged over three trials of the same n-back in one task. Finally, the mean values of △oxy-Hb and △doxy-Hb in 0-,1-, or 2-back tasks were averaged over ch4-7 as typical for the left hemisphere and ch10-ch13 as typical for the right hemisphere. Using these values, we calculated the integral and centroid values [32,33]. Integral values indicate the level of brain activity while centroid values represent the speed for brain activation [33].
2.8. Data analysis
In this study due to the small number of participants, SDS scores, POMS scores, and the correct answer rate for the N-back task were compared between the PMS and non-PMS groups using the Mann-Whitney U test.
2.9. Ethical considerations
Participants were given a written and verbal explanation of the study and they signed written informed consent. The study was conducted in accordance with the Declaration of Helsinki. The research was approved by the ethics committee of the University of Tsukuba Faculty of Medicine (approval no. 1389-1) and the Kanagawa Institute of Technology (approval no. 20191011-01).
3. Results
3.1. Participant characteristics
The number of participants is summarized in Fig. 1. Twenty-two participants with regular menstrual cycles were selected out of the 36 participants. Participants were classified into three groups by the PMDD scale scores: zero in “PMDD”, eleven in “moderate PMS”, and eleven in “mild- or non-PMS”. The 11 participants classified as “mild- or non-PMS” were assessed as non-PMS based on the gynecologist’s diagnosis from the participants' records of PMS memory. The 11 participants in the “moderate PMS” group were all given an assessment of “PMS” by the gynecologist. The basic properties of the PMS and non-PMS groups are shown in Table 1. There were no significant differences in age, body mass index, or menstrual cycle length between the two groups (P = 0.17, 0.70, and 0.65, respectively).
Table 1.
Properties of PMS group and non-PMS group.
| PMS groupa) |
Non-PMS groupb) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | SE | Mdn | Interquartile range | Mean | SD | SE | Mdn | Interquartile range | pc) | |
| Age, year | 20.8 | 0.8 | 0.3 | 21.0 | 21.0–21.0 | 20.9 | 0.8 | 0.3 | 21.0 | 21.0–21.0 | .17 |
| BMI, kg m−2 | 21.28 | 2.2 | 0.7 | 22.0 | 19.0–20.0 | 20.2 | 1.5 | 0.5 | 20.0 | 19.0–20.0 | .70 |
| Menstrual cycle, day | 30.0 | 3.4 | 0.9 | 30.0 | 30.0–31.0 | 30.0 | 4.8 | 1.3 | 30.0 | 28.0–32.0 | .65 |
n = 11.
n = 11.
Mann-Whitney U test. BMI: body mass index.
3.2. Concentration of salivary sex hormone in the follicular and luteal phases
The salivary sex hormone concentrations were measured to confirm the participants’ menstrual cycle phase at the time of cognitive function measurement. The results are presented in Table 2. The median (Mdn) salivary progesterone concentration was significantly higher in the luteal phase (P = 0.01) than in the follicular phase with values of Mdn = 108.0 pg mL-1 (interquartile range, 42.4–135.4) in the follicular phase and Mdn = 147 pg mL-1 (interquartile range, 102.7–215.1) in the luteal phase. The median salivary estradiol concentration did not differ significantly between the two phases (P = 0.88) with values of Mdn = 1.29 pg mL-1 (interquartile range, 1.1–1.5) in the follicular phase and Mdn = 1.50 pg mL-1 (interquartile range, 1.3–1.8) in the luteal phase.
Table 2.
Concentrations of salivary progesterone and estradiol in samples obtained from participants at FP and LP.
| FP |
LP |
|||||||
|---|---|---|---|---|---|---|---|---|
| n = 22 |
n = 22 |
|||||||
| Mean(SE) | Mdn | Interquartile range | Mean (SE) | Mdn | Interquartile range | ES | pa) | |
| Progesterone/pmolL-1 | 95.8(14.9) | 79.7 | 65.5–105.6 | 187.1 (22.4) | 165.3 | 126.8–274.4 | 0.57 | .01 |
| Estradiol/pmolL-1 | 2.0(0.2) | 2.0 | 1.3-2.2 | 1.9 (0.1) | 1.9 | 1.3-2.1 | 0.03 | .88 |
Wilcoxon signed rank test. FP: follicular phase; LP: luteal phase; ES:effect size.
3.3. SDS and POMS scores
The SDS and POMS scores in the luteal and follicular phases are shown in Fig. 2, Fig. 3, respectively. In both phases, the SDS scores were higher in the PMS group than in the non-PMS group, but the difference was not significant (P = 0.07 and 0.08, respectively).
Fig. 2.
Comparison of SDS Scores in PMS group and Non-PMS group
The data are shown as the mean and error bars are standard error. FP, follicular phase; LP, luteal phase; PMS, premenstrual syndrome. A statistical significance test was carried out by Mann-Whitney U test.
Fig. 3.
Comparison of scores for seven subscales and total mood disturbance in the POMS
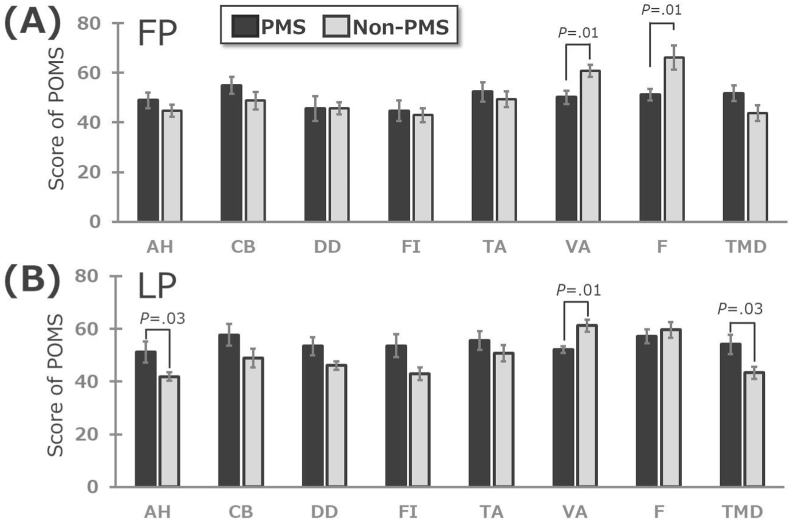
The POMS questionnaire was administered in the luteal phase and follicular phase between the PMS and the Non- PMS groups (Mann-Whitney U test). The data are shown as the mean and error bars are standard error. (A) is follicular phase, (B) is luteal phase. Only p-values < 0.05 are shown. FP, follicular phase; LP, luteal phase; PMS, premenstrual syndrome; AH, Anger-hostility; CB, Confusion-bewilderment; DD, Depression-dejection; FI, Fatigue-inertia; TA, Tension-anxiety; VA, Vigor-activity; F, Friendliness; TMD, Total mood disturbance.
In the follicular phase, the scores for two POMS subscales vigor-activity (VA) and friendliness (F) were significantly lower in the PMS group than in the non-PMS group (P = 0.01 for both). In the luteal phase, scores for the POMS subscales of anger-hostility (AH) and total mood disturbance (TMD) were significantly higher in the PMS group than in the non-PMS group (P = 0.03 and 0.01, respectively), while the scores for VA were significantly lower in the PMS group than in the non-PMS group (P = 0.01).
3.4. N-back task correct response rate
The correct response rates for the N-back task in the follicular and luteal phases are shown in Fig. 4. The correct response rate for the 2-back task was significantly lower in the PMS group than in the non-PMS group in both the follicular and luteal phases (P = 0.01, 0.02, respectively).
Fig. 4.
Total correct response rate on the N-back task
Comparison of total correct response rate on the n-back task in the PMS and non- PMS groups at FP and LP (Mann-Whitney U test). The data are shown as the mean and error bars are standard error. FP, follicular phase; LP, luteal phase; PMS, premenstrual syndrome.
3.5. Changes in the frontal lobe blood flow during the N-back task
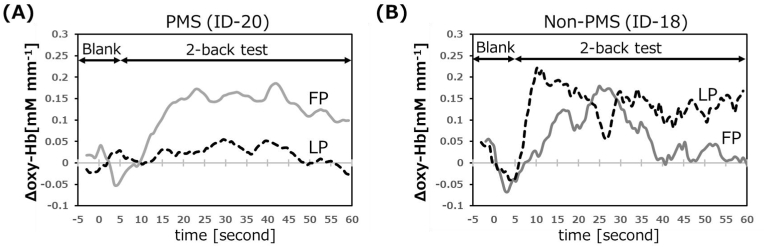
The typical results of the Δoxy-Hb (amount of change in oxy-Hb from baseline) measurement from ch10–13 during the 2-back task are shown in Fig. 5. In the follicular phase, the Δoxy-Hb level in the PFC of the PMS participants gradually increased with the start of the 2-back task, and plateaued 30 s after presenting the task. As an increase in oxy-Hb indicates an increase in brain activity, this demonstrates that the PFC was activated by the 2-back task. Meanwhile, in the luteal phase, PFC activation of PMS participant was not found, as no increase in oxy-Hb was observed during the 2-back task. This suggests a decrease in cognitive function during the luteal phase in this PMS participant. In contrast, the expected brain activity associated with the task in the non-PMS participant was observed in both the luteal and follicular phases. The non-PMS participant’s result is consistent with 2-back task NIRS measurements of adult females in previous studies [34], demonstrating the validity of our NIRS measurements.
Fig. 5.
Typical time course of Δoxy-Hb in CH10-13 during performing 2-back task
Δoxy-Hb represents amount of change of oxy-Hb from the reference value. FP, follicular phase; LP, luteal phase; PMS, premenstrual syndrome.
The integral values obtained from the NIRS probes for ch4–7 and ch10-13 are shown in Fig. 6. There were no differences in the integral values obtained from ch4-7 between the PMS and non-PMS groups for the 0-back, 1-back, or 2-back tasks in the follicular phase or the luteal phase. There were no significant differences in the integral values obtained from ch10-13 between the PMS and non-PMS groups for the 0-back, 1-back, and 2-back tasks in the follicular phase, too. However, the integral values in the PMS group were significantly smaller than those in the non-PMS group for the 1-back and 2-back tasks in the luteal phase (P = 0.02 and 0.04, respectively).
Fig. 6.
The integral values of oxy-Hb during the N-back task
Comparison of the integral value of oxy-Hb during the n-back task in the PMS and Non-PMS groups at FP and LP (Mann-Whitney U test). The data are shown as the mean and error bars are standard error. FP, follicular phase; LP, luteal phase; PMS, premenstrual syndrome.
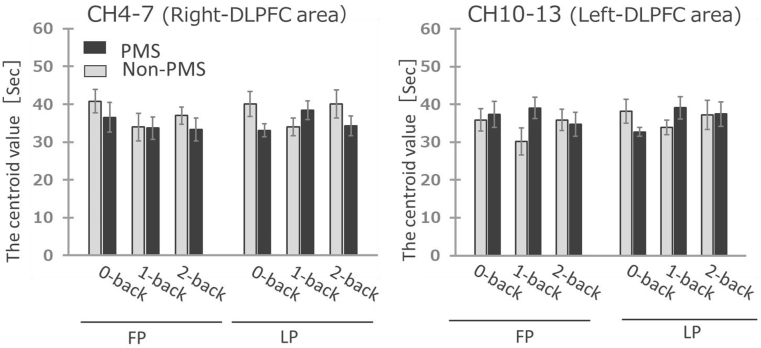
Centroid values obtained from NIRS-probes for ch4–7 and ch10–13 are shown in Fig. 7, respectively. There were no differences in centroid values between the PMS and non-PMS groups for the 0-back, 1-back, and 2-back tasks for either ch4–7 or ch10–13.
Fig. 7.
The centroid values of oxy-Hb during the N-back task
Comparison of the centroid values of oxy-Hb during the n-back task in the PMS and Non-PMS groups at FP and LP (Mann-Whitney U test). The data are shown as the mean and error bar is standard error. FP, follicular phase; LP, luteal phase; PMS, premenstrual syndrome.
4. Discussion
4.1. Basic properties and concentrations of sex hormones (estradiol and progesterone) of the 22 participants
The 22 participants had a body mass index of 20.9 ± 0.4 kg m-2, which is consistent with survey results from the Ministry of Health, Labor and Welfare in Japan. This indicates that the research participants were typical Japanese women in their early 20s. Menstrual cycles increase in length from age 15 to the early 20s, peaking at age 23 (30.7 days) [35]. Participants had an average menstrual cycle of 30.8 ± 0.6 days which matches the cycle length for women in their early 20s.
The mean salivary estradiol concentration among all participants was consistent between the follicular and luteal phases (Table 2). In healthy women, salivary estradiol concentration peaks in the late follicular phase (approximately 10 days after the start of menstruation) and begin to increase again in the late luteal phase (20 days after the start of menstruation or later) [36]. Serum estradiol is also known to exhibit a bimodal pattern; it reaches a maximum approximately 24 h before ovulation, then declines, but rises again, reaching a slightly lower peak in the middle of the luteal phase [36]. For this study, the follicular phase was defined as 5 to 12 days (corresponding to mid to late follicular phase) after the start of menstruation and the luteal phase was defined as 21 to 30 days after the start of menstruation (corresponding to mid to late luteal phase). These periods are consistent with the time at which the estradiol level increases. The fact that salivary estradiol concentration was at the same level in the luteal and follicular phases indicates that participants in the current study were recruited during the appropriate menstrual cycle corresponding to the follicular and luteal phases.
The salivary progesterone concentration was significantly higher in the luteal phase than in the follicular phase (Table 2). Serum progesterone concentrations are known to reach their highest levels during the luteal phase. It has been reported that salivary progesterone concentrations follow the same pattern as serum progesterone levels [36,37]. Therefore, our salivary progesterone concentration results were consistent with those of previous studies. As described above, salivary estradiol and progesterone concentrations vary according to the menstrual cycle. Therefore, the participants were convened in the follicular and luteal phases.
4.2. Characterization of the PMS group by the SDS and POMS score
Participants with PMS had greater TMD scores in the luteal phase compared with those without PMS (Fig. 3). This means that the PMS group had increased negative mood in the luteal phase. This is because participants with PMS are prone to psychological symptoms, such as mental and emotional instability during the luteal phase [38]. During both the luteal and follicular phase, the PMS group also had lower scores than the non-PMS group for the VA- and F- subscales, which indicated a positive mood (Fig. 3). A previous study of Chinese participants found no difference in the mean VA score between participants with and without PMS [39]. Moreover, it has been reported that individuals with PMS experienced increased positive emotion in the follicular phase, while there were no differences in mood between both phases in those without PMS [40]. Participants in the current PMS group could have a slightly lower positive mood and higher negative mood due to their lower depression status, their SDS score being slightly greater than 40 (Fig. 2). As a result, VA scores of participants in the PMS group were lower than those of the non-PMS group. In addition, the TMD score of PMS group during the luteal phase was higher, consequently, the PMS symptoms could be superimposed on symptoms of minimal depression. In this study, we identified the characteristic mood changes of PMS group which was a decrease in positive mood during the follicular phase and an increase in negative mood during the luteal phase although the mood characters could be overestimated by the effects of symptoms caused by minimal depression.
4.3. Characterization of the PMS group by the correct answer rate in the N-back task
The PMS group had significantly lower correct answer rates than the non-PMS group for the 2-back task in the follicular and luteal phases (Fig. 4). This indicates that females with PMS performed worse than those without PMS during a difficult cognitive task. Several studies have previously administered the N-back task to females with PMS and PMDD. These studies found that females with PMDD give significantly fewer correct answers in the 2-back and 3-back tasks compared to healthy individuals in the luteal phase [41], and that females with PMS gave more incorrect answers in the 2-back task in the follicular phase compared to those without PMS [42]. Our results are consistent with those of previous studies. Cognitive function could be affected by progesterone levels [42]. However, some studies have reported that there is no association between progesterone levels and cognitive function [44]. We assessed the effect of the concentration of salivary progesterone on the performance of N-back task. The concentration of salivary progesterone of PMS and Non-PMS groups in the luteal phase were not significantly different (198.5 ± 25.1 pmol L-1 and 178.3 ± 37.3 pmol L-1, respectively) (see Table S2). During the follicular phase, there was no significant difference in the salivary progesterone levels in the PMS group compared to the non-PMS group. Therefore, the lower correct rate to 2-back task in the PMS group could be attributed to the decline of cognitive function, which was one the symptoms of PMS rather than to the effects of progesterone. On the other hand, the decrease in correct rate to the 2-back task in the PMS group was observed not only in the luteal phase but also in the follicular phase. The lack of periodicity in the correct response rate decline in the PMS group may be due to the superimposed symptoms of minimal depression on the PMS symptoms.
4.4. Changes in frontal lobe blood flow during the cognitive task
In the non-PMS, ΔOxy-Hb increased during the presentation of the 2-back task, and activation was observed in the left PFC (Fig. 5). Meanwhile, in the luteal phase, cerebral blood flow among females with PMS was largely consistent across the task presentation period, and no brain activation was observed. The N-back task evaluates working memory. Working memory comprises an important part of cognitive functions [45] and is regulated by the VLPFC and DLPFC [46,47]. For example, studies have found that blood flow in the PFC increases when performing the N-back task [48,49]. In other words, the amount of change in PFC blood flow when completing a cognitive task is known to be an objective indicator of cognitive task performance. A previous study found that patients with PMDD had lower DLPFC blood flow than healthy participants during an N-back task [50]. Our results suggest that females with PMS, which is less severe than PMDD, have lower cerebral blood flow during the N-back task in the luteal phase.
4.5. The relationship between cognitive task performance, cerebral blood flow characteristics, and mood among females with PMS
In this study, individuals with PMS had higher SDS scores, increased negative mood, and poorer cognitive task performance. Furthermore, during the luteal phase, decreased blood flow in the PFC, which indicates poorer cognitive task performance, was detected. The concentration of salivary progesterone showed no significant difference between the PMS and non-PMS groups. These results suggest that the increased negative mood of PMS participants during the luteal phase may be related to the decreased blood flow in the PFC, the region that controls working memory. The relationship between the negative mood and decline of working memory has been reported previously. Using NIRS data collected from adult males, Sato et al. found that heightened negative mood is accompanied by reduced working memory [51]. Our results are consistent with those of previous studies, although there is a gender difference: female in this study and male in the previous study.
SDS scores in the PMS group were above 40 in both the follicular and luteal phases, indicating that participants in the PMS group could have symptoms due to minimal depression. The lower positive mood, the lower correct response rate to the 2-back task in the PMS group could be caused not only by the PMS symptoms but also by the symptoms of the minimal depression, since these phenotypes were observed in both the follicular and luteal phases. In the case of NIRS measurement, the brain activity in the PMS group decreased only during the luteal phase, while during the follicular phase, the ΔOxy-Hb in the PMS group increased with the performance of the 2-back task, as also in the non-PMS group. In this study, during the follicular phase, we obtained different results between the NIRS measurements and the correct rate to 2-back task. This may be due to the presence of minimal depressive symptoms in the PMS group. The results obtained by NIRS reflects the intension to try to perform the tasks regardless of the correctness of the answer. In the follicular phase, the performance to 2-back task could have been inhibited by the symptoms of minimal depression while maintaining the intensions to perform the N-back task. Whereas, in the luteal phase, PMS participants could have been deprived of the intension to perform the task by both symptoms of PMS and minimal depression. However, it is difficult to clarify whether the cause of the symptoms during the luteal phase originated from PMS or minimal depression. This study is primitive in that the number of participants is small. In the future, we would like to conduct a large-scale study using the same framework to elucidate the cognitive decline caused the symptoms of PMS. Recently, various attempts have been made to redefine depression and other mental illnesses using brain activity measured by functional brain imaging [52]. Similarly, we believe that it is possible to redefine PMS by combining conventional tools such as SDS and POMS scores with tools that utilize bio-signals such as cerebral blood flow characteristics.
4.6. Limitations
In our study, the number of participants and implementations were reduced in the longitudinal study, and the sample size used for analysis was small. Therefore, caution should be exercised in interpreting the results. In the future, we would like to increase the number of subjects further and clarify the characteristics of PMS in more detail. The effect of the order of measurement for each phase cannot be ignored. In addition, we need to check the reverse order.
5. Conclusion
Females with PMS had lower correct response rates during a cognitive task in the follicular and luteal phases compared to those without PMS. NIRS results showed that the PFC activity in the PMS group decreased during the luteal phase, while during the follicular phase, the PFC activity was similar to that of the non-PMS group. These results suggest associations between the cognitive task performance, reduced PFC blood flow, and TMD during the luteal phase.
Data code and availability
The data and the code used for this project will be made available upon request.
Funding
This work was supported by JSPS KAKENHI Grant Number 18K12168.
Author contributions
Makiko Aoki, Masato Suzuki and Hisayo Okayama were involved in study design and data interpretation. Satoshi Suzuki and Hidenobu Takao were involved in the data analysis. All authors critically revised the report, commented on drafts of the manuscript, and approved the final report.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the participants who took part in the basal body temperature measurement and NIRS measurement for data collection, and all the participants involved in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpnec.2022.100117.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Craner J., Sigmon S., Martinson A., McGillicuddy M. Perceptions of health and somatic sensations in women reporting premenstrual syndrome and premenstrual dysphoric disorder. J. Nerv. Ment. Dis. 2013;201:780–785. doi: 10.1097/NMD.0b013e3182a213f1. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka E., Momoeda M., Osuga Y., Rossi B., Nomoto K., Hayakawa M., et al. Burden of menstrual symptoms in Japanese women: results from a survey-based study. J. Med. Econ. 2013;16:1255–1266. doi: 10.3111/13696998.2013.830974. [DOI] [PubMed] [Google Scholar]
- 3.Feuerstein M., Shaw W.S. Measurement properties of the calendar of premenstrual experience in patients with premenstrual syndrome. J. Reprod. Med. 2002;47:279–289. [PubMed] [Google Scholar]
- 4.Steiner M., Macdougall M., Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health. 2003;6:203–209. doi: 10.1007/s00737-003-0018-4. [DOI] [PubMed] [Google Scholar]
- 5.Eisenlohr-Moul T.A., Girdler S.S., Schmalenberger K.M., Dawson D.N., Surana P., Johnson J.L., et al. Toward the reliable diagnosis of dsm-5 premenstrual dysphoric disorder: the carolina premenstrual assessment scoring system (C-PASS) Am. J. Psychiatr. 2017;174:51–59. doi: 10.1176/appi.ajp.2016.15121510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen L.S., Soares C.N., Otto M.W., Sweeney B.H., Liberman R.F., Harlow B.L. Prevalence and predictors of premenstrual dysphoric disorder (PMDD) in older premenopausal women: the Harvard Study of Moods and Cycles. J. Affect. Disord. 2002;70:125–132. doi: 10.1016/s0165-0327(01)00458-x. [DOI] [PubMed] [Google Scholar]
- 7.Hart W.G., Coleman G.J., Russell J.W. Psychiatric screening in the premenstrual syndrome. Med. J. Aust. 1987;146:518–522. doi: 10.5694/j.1326-5377.1987.tb120391.x. [DOI] [PubMed] [Google Scholar]
- 8.Marvan M.L., Cortes-Iniestra S. Women's beliefs about the prevalence of premenstrual syndrome and biases in recall of premenstrual changes. Health Psychol. 2001;20:276–280. doi: 10.1037//0278-6133.20.4.276. [DOI] [PubMed] [Google Scholar]
- 9.Sattar K. Epidemiology of premenstrual syndrome, a systematic review and meta-analysis study. J. Clin. Diagn. Res. 2014;8:106–109. doi: 10.7860/JCDR/2014/8024.4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baek J., Choi-Kwon S. Secondary data analysis on the factors influencing premenstrual symptoms of shift work nurses: focused on the sleep and occupational stress. Korean Soc Nurs Sci. 2020;50:631–640. doi: 10.4040/jkan.19230. [DOI] [PubMed] [Google Scholar]
- 11.Montero-López E., Santos-Ruiz A., García-Ríos M.C., Rodríguez-Blázquez M., Rogers H.L., Peralta-Ramírez M.I. The relationship between the menstrual cycle and cortisol secretion: daily and stress-invoked cortisol patterns. Int. J. Psychophysiol. 2018;131:67–72. doi: 10.1016/j.ijpsycho.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Barba-Moreno L., Cupeiro R., Romero-Parra N., Janse de Jonge X., Peinado A. Cardiorespiratory responses to endurance exercise over the menstrual cycle and with oral contraceptive use. J. Strength Condit Res. 2022;36(2):392–399. doi: 10.1519/JSC.0000000000003447. [DOI] [PubMed] [Google Scholar]
- 13.Tada Y., Yoshizaki T., Tomata Y., Yokoyama Y., Sunami A., Hida A., et al. The impact of menstrual cycle phases on cardiac autonomic nervous system activity: an observational study considering lifestyle (diet, physical activity, and sleep) among female college students. J. Nutr. Sci. Vitaminol. 2017;63:249–255. doi: 10.3177/jnsv.63.249. [DOI] [PubMed] [Google Scholar]
- 14.Rypma B., Prabhakaran V., Desmond J.E., Glover G.H., Gabrieli J.D.E. Load-dependent roles of frontal brain regions in the maintenance of working memory. Neuroimage. 1999;9:216–226. doi: 10.1006/nimg.1998.0404. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald P.B., Srithiran A., Benitez J., Daskalakis Z.Z., Oxley T.J., Kulkarni J., et al. An fMRI study of prefrontal brain activation during multiple tasks in patients with major depressive disorder. Hum. Brain Mapp. 2008;29:490–501. doi: 10.1002/hbm.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen N., Ghahremani D.G., Rapkin A.J., Berman S.M., Liang L., London E.D. Brain activation during emotion regulation in women with premenstrual dysphoric disorder. Psychol. Med. 2018;48:1795–1802. doi: 10.1017/S0033291717003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gotlib I.H., Krasnoperova E., Neubauer Yue D., Joormann J. Attentional Biases for negative interpersonal stimuli in clinical depression. J. Abnorm. Psychol. 2004;113(1965):127–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- 18.Hasler G., Drevets W.C., Manji H.K., Charney D.S. Discovering endophenotypes for major depression. Neuropsychopharmacology. (New York, N.Y.) 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 19.Tashiro M., Kano M., Fukudo S., Yanai K. Development of neuroimaging research on human emotion. Nihon yakurigaku zasshi. 2005;125:88–96. doi: 10.1254/fpj.125.88. [DOI] [PubMed] [Google Scholar]
- 20.Baller E.B., Wei S., Kohn P.D., Rubinow D.R., Alarcón G., Schmidt P.J., et al. Abnormalities of dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am. J. Psychiatr. 2013;170:305–314. doi: 10.1176/appi.ajp.2012.12030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen N., Ghahremani D.G., Rapkin A.J., Berman S.M., Liang L., London E.D. Brain activation during emotion regulation in women with premenstrual dysphoric disorder. Psychol. Med. 2018;48:1795–1802. doi: 10.1017/S0033291717003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takizawa R., Fukuda M., Kawasaki S., Kasai K., Mimura M., Pu S., et al. Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage. 2014;85:498–507. doi: 10.1016/j.neuroimage.2013.05.126. [DOI] [PubMed] [Google Scholar]
- 23.Slyepchenko A., Lokuge S., Nicholls B., Steiner M., Hall G.B.C., Soares C.N., et al. Subtle persistent working memory and selective attention deficits in women with premenstrual syndrome. Psychiatr. Res. 2017;249:354–362. doi: 10.1016/j.psychres.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi T., Takikawa Y., Kawagoe R., Shibuya S., Iwano T., Kitazawa S. Influence of skin blood flow on near-infrared spectroscopy signals measured on the forehead during a verbal fluency task. Neuroimage. 2011;57:991–1002. doi: 10.1016/j.neuroimage.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Cyranoski D. Neuroscience: thought experiment. Nature (London) 2011;469:148–149. doi: 10.1038/469148a. [DOI] [PubMed] [Google Scholar]
- 26.Aoki M., Suzuki M., Okayama H. Assessing n-back task performance of menstrual adult women: comparison with and without premenstrual syndrome. J Nurs Sci Eng. 2020;8:47–57. [Google Scholar]
- 27.Miyaoka Y., Akimoto Y., Ueda K., Kamo T. The reliability and validity of the newly developed PMDD scale. J Jp Soc Psychosom Obstet Gynecol. 2009;14:194–201. [Google Scholar]
- 28.Zung W.W. A self-rating depression score. Arch. Gen. Psychiatr. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 29.McNair D.M., Lorr M., Droppleman L.F. Educational and Industrial Testing Services; San Diego, CA: 1971. Profile of Mood States (POMS) Manual. [Google Scholar]
- 30.Yokoyama K. second ed. Kanekosyobou; Tokyo, Japan: 2015. Profile of Mood States. Japanese Version. [Google Scholar]
- 31.Harvey P., Fossati P., Pochon J., Levy R., LeBastard G., Lehéricy S., et al. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. Neuroimage. 2005;26:860–869. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 32.Kameyama M., Fukuda M., Yamagishi Y., Sato T., Uehara T., Ito M., et al. Frontal lobe function in bipolar disorder: a multichannel near-infrared spectroscopy study. Neuroimage. 2006;29:172–184. doi: 10.1016/j.neuroimage.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 33.Suto T., Fukuda M., Ito M., Uehara T., Mikuni M. Multichannel near-infrared spectroscopy in depression and schizophrenia: cognitive brain activation study. Biol. Psychiatr. 2004;55(1969):501–511. doi: 10.1016/j.biopsych.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Sun J., Liu F., Wang H., Yang A., Gao C., Li Z., et al. Connectivity properties in the prefrontal cortex during working memory: a near-infrared spectroscopy study. J. Biomed. Opt. 2019;24:1–7. doi: 10.1117/1.JBO.24.5.051410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tatsumi T., Sampei M., Saito K., Honda Y., Okazaki Y., Arata N., et al. Age-dependent and seasonal changes in menstrual cycle length and body temperature based on big data. Obstet. Gynecol. 2020;136(4):666–674. doi: 10.1097/AOG.0000000000003910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Celec P., Ostanikova D., Skoknova M., Hodosy J., Putz Z., Kudela M. Salivary sex hormones during the menstrual cycle. Endocr. J. 2009;56:521–523. doi: 10.1507/endocrj.k09e-020. [DOI] [PubMed] [Google Scholar]
- 37.Walker R.F., Read G.F., Riad-Fahmy D. Radioimmunoassay of progesterone in saliva: application to the assessment of ovarian function. Clin. Chem. 1979;25:2030–2033. [PubMed] [Google Scholar]
- 38.Miyaoka Y., Akimoto Y., Ueda K., Ujiie Y., Kametani M., Uchiide Y., et al. Fulfillment of the premenstrual dysphoric disorder criteria confirmed using a self-rating questionnaire among Japanese women with depressive disorders. Biopsychosoc. Med. 2011;5:5. doi: 10.1186/1751-0759-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hou L., Chang L., Chen L., Zhou R. Reduced reward responsiveness in women with moderate - to - severe premenstrual syndrome: evidence from a probabilistic reward task. Front. Psychiatr. 2020;11:28. doi: 10.3389/fpsyt.2020.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metcalf M.G., Livesey J.H. Distribution of positive moods in women with the premenstrual syndrome and in normal women. J. Psychosom. Res. 1995;39:609–618. doi: 10.1016/0022-3999(94)00167-7. [DOI] [PubMed] [Google Scholar]
- 41.Yen J., Chang S., Long C., Tang T., Chen C., Yen C. Working memory deficit in premenstrual dysphoric disorder and its associations with difficulty in concentrating and irritability. Compr. Psychiatr. 2012;53:540–545. doi: 10.1016/j.comppsych.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 42.Slyepchenko A., Lokuge S., Nicholls B., Steiner M., Hall G.B.C., Soares C.N., et al. Subtle persistent working memory and selective attention deficits in women with premenstrual syndrome. Psychiatr. Res. 2017;249:354–362. doi: 10.1016/j.psychres.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 44.Hjelmervik H., Hausmann M., Osnes B., Westerhausen R., Specht K. Resting states are resting traits – an fMRI study of sex differences and menstrual cycle effects in resting state cognitive control networks. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacola L.M., Willard V.W., Ashford J.M., Ogg R.J., Scoggins M.A., Jones M.M., et al. Clinical utility of the N-back task in functional neuroimaging studies of working memory. J. Clin. Exp. Neuropsychol. 2014;36:875–886. doi: 10.1080/13803395.2014.953039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fishburn F.A., Norr M.E., Medvedev A.V., Vaidya C.J. Sensitivity of fNIRS to cognitive state and load. Front. Hum. Neurosci. 2014;8:76. doi: 10.3389/fnhum.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Owen Kathryn Adrian M., McMillan Angela M., Laird Ed Bullmore R. N‐back working memory paradigm: a meta‐analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marquand A., Mourão-Miranda J., Brammer M., Cleare A., Fu C. Neuroanatomy of verbal working memory as a diagnostic biomarker for depression. Neuroreport. 2008;19:1507–1511. doi: 10.1097/WNR.0b013e328310425e. [DOI] [PubMed] [Google Scholar]
- 50.Baller E.B., Wei S., Kohn P.D., Rubinow D.R., Alarcón G., Schmidt P.J., et al. Abnormalities of Dorsolateral prefrontal function in women with premenstrual dysphoric disorder: a multimodal neuroimaging study. Am. J. Psychiatr. 2013;170:305–314. doi: 10.1176/appi.ajp.2012.12030385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sato H., Dresler T., Haeussinger F.B., Fallgatter A.J., Ehlis A. Replication of the correlation between natural mood states and working memory-related prefrontal activity measured by near-infrared spectroscopy in a German sample. Front. Hum. Neurosci. 2014;8:37. doi: 10.3389/fnhum.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drysdale A.T., Grosenick L., Downar J., Dunlop K., Mansouri F., Meng Y., et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 2016;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.