Abstract
Psychological stressors can lead to distress and result in autonomic arousal and activation of a stress response. Ongoing or persistent stress can disrupt the stress response feedback mechanisms and result in elevated cortisol and pro-inflammatory cytokines which can cause damage to brain regions involved in the regulation of mood and emotion. We propose that the magnitude of the stress response experienced in response to psychological stressors depends on a number of modifiable psychological processes including an individual’s level of self-compassion, dispositional mindfulness, tendency to ruminate and attentional bias. We further propose that the stress response elected by psychological stressors can be meditated by influencing these modifiable psychological processes, and that meditation practices can decrease stress and improve mood by decreasing stress reactivity on a psychological, physiological and neurobiological level. We explore this in a narrative review.
Keywords: Brain, Meditation, Stress, Autonomic nervous system
Highlights
-
•
Meditation decreases blood pressure, heart rate, cortisol and cytokine levels.
-
•
Meditation increases self-compassion, dispositional mindfulness and meta-cognition.
-
•
Meditation improves attention and memory.
-
•
Meditation results in brain changes in regions related to emotion regulation.
1. Introduction
1.1. Psychological stressors result in psychological distress
Stressors are circumstances that threaten a goal, including maintenance of physical safety (physical stressors) or psychological well-being and include psychological stressors such as those related to work, home and personal circumstances [1,2]. Distress is a negative psychological response to such threats and can include a variety of affective and cognitive states, such as anxiety, sadness, frustration [1].
1.2. Distress results in activation of the stress response
As stressors can be both real or perceived threats to homeostasis or well-being, even in the absence of a physical threat, psychological stressors and related thoughts and emotional states associated with a sense of uncertainty or distress result in the activation of a comprehensive stress response [3]. For example, Meta-Analysis shows that athletes anticipating a sporting competition commonly experience emotional and physiological responses resulting from their expectation of psychosocial and physical stress, which corresponds to increased cortisol levels [4].
Activation of a stress response is controlled by the autonomic nervous system (ANS) [3], comprising of the sympathetic nervous system (SNS) and the parasympathetic nervous system (PNS) [5]. In situations of danger, the SNS initiates a number of physiological changes known as the ‘fight-flight-freeze’ response to maximize the chances of surviving the threat [5]. The SNS also regulates pro-inflammatory cytokine production by releasing the neurotransmitter norepinephrine into peripheral tissues and organ systems, which results in the downstream up-regulation transcription of the pro-inflammatory cytokines [6].
Once the threat is gone, the PNS, the main component of which is the vagus nerve [7], activates the relaxation, or “rest and digest” response and returns the body to homeostasis. The hypothalamus activates another component of the stress response system known as the hypothalamic-pituitary-adrenal (HPA) axis, which regulates the downstream release of hormones such as the glucocorticoid steroid hormone, cortisol [8].
Cortisol has many metabolic and physiological functions, one of which is to maintain a steady supply of blood sugar, which ensures that there is sufficient energy available to cope with ongoing or prolonged stressors [9]. In response to stressors, cortisol is released for several hours. At a certain blood concentration level, cortisol binds to low-affinity glucocorticoid receptors [10]. The resulting activation of these glucocorticoid receptors terminate the stress response and this is thought to largely subserve HPA axis feedback regulation [11].
1.3. Persistent activation of the stress response results in HPA dysfunction and depressive mood
This negative feedback control mechanism ensures that the secretion of cortisol and other hormones are maintained within a relatively narrow bandwidth [8]. However, the delicate negative feedback control mechanism regulating both baseline HPA function and responsiveness to stress can be disrupted with too much exposure to stressful events, including perceived or psychological stressors [9]. While cortisol normally exerts an inhibitory effect of glucocorticoid activation on immune response gene transcription, under conditions of frequent or chronic threat, HPA axis-activation can lead to increases in inflammation as demonstrated by increases in cytokine levels (referred to as glucocorticoid resistance, or glucocorticoid insensitivity) [6]. This altered immune–endocrine interaction and increased inflammatory profile have been linked to the onset of mental disorders, such as depression [12,13]. Meta-Analysis of seven studies shows a blunted recovery following stressful events in individuals with depression, as these individuals show much higher cortisol levels following a psychologically stressful event, compared to individuals without depression, particularly among older and more severely depressed individuals [14]. Approximately 50% of individuals who have depression have flattened cortisol circadian rhythm, with evening levels being elevated [15,16].
1.4. HPA axis dysfunction is associated with damage in brain regions that regulate mood and emotion
Importantly, acute and chronic stress and subsequent excess corticosteroids can damage the brain [17], particularly in areas associated with the regulation of mood and emotion, including the hippocampus [18] and the prefrontal cortex, both of which show atrophy [19], while neurons in amygdala show growth in response to stress that corresponds with increases in anxiety [19]. Previous research clearly shows that depression is associated with an increased expression of pro-inflammatory cytokines [20]. Individuals with depression have higher circulating levels of pro-inflammatory cytokine interferon-γ (INF-γ) [21], interleukin-1β (IL-1β) [22], tumour necrosis factor-α (TNF-α) [23], and interleukin-6 (IL-6) [24]. Treatment with the cytokine interferon-α (INF-α) [25] induces depressive symptoms in humans, and symptoms are seen to reduce with the cessation of therapy [26] and are also abrogated by antidepressant treatment [27]. High plasma concentrations of acute-phase proteins and inflammatory cytokines are associated with a nonresponse to antidepressants in individuals with depression [21]. Importantly, pro-inflammatory cytokines induce depression-associated cellular apoptosis [28] in the amygdala [29] by influencing intrinsic apoptosis factors including intracellular Ca2+, glutamate excitotoxicity and free radicals [30]. Thus, heightened inflammatory cytokine levels driven by stress may contribute to depression symptoms by contributing to cell death in brain regions involved in the regulation of mood and emotion.
1.5. Meditation
Meditation is defined and characterized in a multitude of ways, stemming from different approaches and traditions throughout history, each with its own theoretical underpinnings and variety of techniques. Currently, there is no agreed-upon definition of meditation in the scientific literature [31]. For research purposes in clinical health applications, different techniques have been classified as focused attention (FA) or open monitoring (OM) (See Table 1) [32]. The most popular meditative techniques lie somewhere on a continuum between concentrative and mindful types and generally include a component of mindfulness [33]. As suggested by Awasthi [34] we have adopted an operational definition of meditation that is inclusive of traditional ontological (i.e., metaphysical) descriptions, as well as the modern neurocognitive account of the phenomena.
Table 1.
Meditation Subtypes – reproduced with permission from Ref. [32]].
| Attentional family | Constructive family | Deconstructive family |
|---|---|---|
| FA: Jhana practice (Theravada); breath counting (Zen); body awareness practices (Zen/Tibetan); Shamatha/calm abiding with support (Tibetan); mantra recitation (various traditions) | Relation orientation: loving-kindness and compassion (Theravada, Tibetan); Bodhichitta/Bodhisattva Vow (Tibetan/Zen); centering prayer (Christian); CCARE compassion cultivation training (clinical); cognitively-based compassion training-compassion component (clinical) | Object-oriented insight: mindfulness-based cognitive therapy – cognitive component (clinical); First and Second Foundations of Mindfulness (Theravada, Tibetan); Vipassana/insight (Theravada); analytical meditation (Tibetan); Koan practice (Zen) |
| OM (object-orientation): cultivation of attention (Greco-Roman philosophy); choiceless awareness (Tibetan); mindfulness-based stress reduction (clinical); dialectical behavior therapy-mindfulness component (clinical); mindfulness-based cognitive therapy-mindfulness component (clinical); acceptance and commitment therapy-mindfulness component (clinical) | Values orientation: The Six Recollections (Theravada); The Four Thoughts (Tibetan); contemplations of mortality (Theravada, Tibetan, Zen, Greco-Roman philosophy); well-being therapy (Clinical) | Subject-oriented insight: cognitive behavior therapy (Clinical); Third and Fourth Foundations of Mindfulness (Theravada, Tibetan); Mahamudra Analytical Meditation (Tibetan); Dzogchen Analytical Meditation (Tibetan); Koan practice (Zen) |
| OM (subject-orientation): Shamatha/calm abiding without support (Tibetan) | Perception orientation: development stage (Tibetan); meditation on foulness (Theravada) | Non-dual-oriented Insight: Muraqaba (Sufi); Mahamudra (Tibetan); Dzogchen (Tibetan); Shikantaza (Zen); Self-inquiry (Advaita Vedanta) |
FA=Focused attention, OM = open monitoring.
1.6. Meditation meditates maladaptive distress: an integrative model
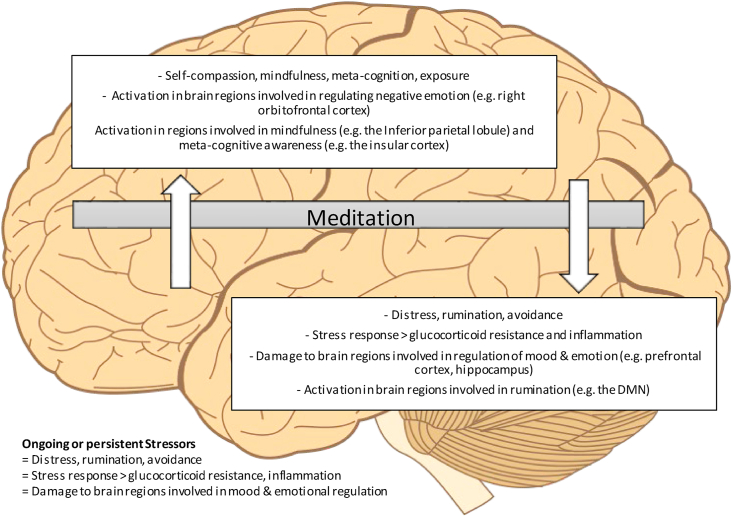
A wide body of literature shows that mediation practices are associated with improved mental health and wellbeing in diverse popuations [[35], [36], [37]]. We propose that meditation practices reduce the distress experienced resulting from psychological stressors by impacting a number of key psychological processes. By impacting these psychological process, the relationship with the stressor is altered and the associated distress is reduced. This reduction, we propose, is associated with less autonomic arousal and activation of the stress response, as well as less acute activation in brain regions associated with rumination in the longer term, less stress derived damage to brain regions important in the regulation of mood of emotion, as depicted in Fig. 1. This article adds to the existing literature as it is the first, to our knowledge, to explore the impact of mindfulness on psychological, physiological and neurobiological outcomes concurrently from the perspective of stress regulation and the impacts of this on mood, particularly depression. Studying mechanisms of action is useful in order to understand how meditation may promote mood and/or be an effective treatment of mental ill-health as well as provide a greater understanding into what populations it might be most beneficial to. In order to explore this, we aimed to narratively review meta-analytic and systematic review studies of meditation with a focus on psychological, physiological and neurobiological impacts.
Fig. 1.
Impacts of meditation on stress related psychological, physiological and neurobiological outcomes
2. Methods
We aimed to narratively review meta-analytic and systematic review studies of all forms of meditation with a focus on psychological and/or biological mechanisms linking meditation practice with mood, with a particular focus on those mechanisms involved in the stress response. This includes studies of: (1) psychological processes (self-compassion, dispositional mindfulness, rumination, exposure, meta-cognition, attention, (2) physiological processes (the autonomic nervous system - heart rate, blood pressure, cytokines, and cortisol) and, (3) neurobiological processes (brain structure and function).
A single author conducted a search of the literature using a number of databases (PubMed, Web of Science, Scopus) as well as Google Scholar and a panel of expert also contributed to the literature search. The cut-off point for the literature search was June 2020. Meta-Analysis and systematic reviews were eligible for inclusion. Search terms included but were not limited to: “mindful∗,” “meditate∗”,” “psych∗” “neuro∗” “stress” “autonomic” “mental illness” “mental health” “cytokine” “endocrine.” Searches only included studies published in English. Systemtic reviews and meta-analyses were included if they examined the impact of a meditation practice on physiological, psychological or neurobiological outcomes in any population. Quality assessment of included studies was not conducted and thus all eligible articles were included. Data were coded according to the specific outcome explored (psychological processes, physiological processes, neurobiological processes).
3. Results
In total, we included 24 Meta-Analysis and/or systematic review studies assessing the impact of meditation psychological processes, the autonomic nervous system and neurobiological processes. These studies and a brief summary of their findings are presented in Table 2 and discussed in more detail in the text.
Table 2.
Studies included in the narrative review and a summary of their findings on psychological processes, autonomic and neurobiological processes assessed.
| Outcome assessed | Author | Findings | Study type |
|---|---|---|---|
| Mindfulness | [38] | Kindness-based meditation increased mindfulness (FA constructive family) | SR & MA |
| [39] | OM meditation increased mindfulness and mediated impacts of meditation on psychological outcomes | SR & MA | |
| Self-compassion | [40] | Various meditation forms increased mindfulness | SR |
| [38] | Kindness-based meditation (FA constructive family) increased self-compassion | SR & MA | |
| [41] | OM studies increased self-compassion | SR & MA | |
| [39] | OM meditation increased self-compassion and mediated effects on psychological outcomes | SR & MA | |
| Rumination | [39] | OM meditation decreased rumination and mediated impacts of meditation on psychological outcomes | SR & MA |
| [42] | OM/FA meditation-based study reduced rumination compared to treatment as usual and a waitlist control, but not compared to an active control group | SR & MA | |
| Attention | [43] | Various meditation forms enhanced executive control | SR |
| [44] | Pure mindfulness meditation more effective at impacting attention than comprehensive MBSR programs | SR & MA | |
| [45] | Programs that included FA and OM produced a significant effect on overall cognition | SR & MA | |
| [46] | FA and OM had a medium effect on attention | SR & MA | |
| Blood pressure | [47] | TM (oriented focused attention) lowered SBP and DBP | Review of SR & MAs |
| [88] | FA meditation decreased SBP, OM meditation did not. No change in DBP | SR & MA | |
| [48] | Both FA (TM) and a combination of non-transcendental meditation forms (varied) lowered ambulatory and non-ambulatory SBP and DBP | SR & MA | |
| Heart rate | [88] | OM meditation decreased HR, FA did not | SR & MA |
| HRV | [49] | Varied meditation forms analysed together did not influence HRV | SR & MA |
| [88] | OM and oriented FA meditation interventions did not influence HRV | SR & MA | |
| [50] | OM meditation interventions did not influence HRV | SR & MA | |
| Inflammatory proteins | [51] | OM reduced CRP and found a lack of replicated effects for IL-1, IL-6, IL-8, IL-10 | SR & MA |
| [88] | OM did not decrease CRP, IL-6. TNF-a decreased following combined analysis of various meditation forms | SR & MA | |
| [50] | OM did not decrease IL-6 or CRP | SR & MA | |
| [52] | OM did not influence IL-6, IL-8, TNF-α, IFN-γ, IL-10 | SR | |
| Cortisol | [53] | Varied meditation forms (mostly FA) analysed together reduced blood and salivary cortisol | SR & MA |
| [54] | OM meditation interventions reduced salivary cortisol levels in within-participants studies but not RCTs | SR | |
| [88] | Buddhist walking and Integrated Amrita Meditation decreased blood cortisol. FA meditation did not decrease blood cortisol | SR & MA | |
| [55] | Both OM and FA forms of meditation influence salivary cortisol levels | SR | |
| [56] | OM (predominately MBSR) decreased salivary cortisol | SR & MA | |
| Medial prefrontal cortex; Dorsal medial prefrontal cortex | [57] | Cortical thickening seen in BA10 of long-term meditators, irrespective of the meditation type. Various meditation forms associated with greater white matter fibre density and/or coherence in anterior/mid cingulate cortex and morphological difference in orbitofrontal cortex | SR & MA |
| [124] | FA and OM associated with activation in BA10. FA meditation associated with activation on BA9 | SR & MA | |
| [58] | MM meditations activate the prefrontal cortex and anterior cingulate cortex | SR | |
| [59] | Greater prefrontal/frontal activity associated with various meditation forms | SR | |
| Posterior cingulate cortex/retrosplenial cortex | [57] | Various meditation forms associated with structural declines in posterior cingulate cortex | SR & MA |
| [124] | FA meditation associated with deactivation in BA 30, loving-kindness/compassion meditation associated with activation in BA 23/31 | SR & MA | |
| Inferior parietal lobule | [124] | FA meditation associated with deactivation in BA 39, loving-kindness/compassion meditation associated with activation BA 2/40 | SR & MA |
| Hippocampal formation | [57] | Various meditation forms associated with structural changes in hippocampus | SR & MA |
| [58] | Vipassana meditation resulted in a thicker right hippocampus | SR | |
| The insular cortex | [57] | Various meditation forms associated with structural changes in insular cortex (e.g. cortical thickness, cortical gyrification, gray matter concentration) | SR & MA |
| [124] | FA, OM associated with activation in BA13. Mantra recitation (FA) associated with deactivation in BA13 | SR & MA | |
| [59] | Various meditation forms associated with increased insular activity | SR |
BA=Brodmanns area, CRP=C reactive protein, FA=Focused attention, DBP = Diastolic blood pressure, HR=Heart rate, HRV=Heart rate variability, IL-1 = Interleukin-1, IL-6 = Interleukin-6, IL-8 = Interleukin-8, IL-10 = = Interleukin-10, IFN-γ = interferon-gamma, IL-10 OM=Open monitoring, MA = Meta-analysis, MBSR = Mindfulness based stress reduction, MM = Mindfulness meditation, SBP=Systolic blood pressure, SR=Systemtic review, TM = Transcendental meditation, TNF-α = Tumour necrosis factor-alpha.
3.1. Meditation decreases distress experienced resulting from psychological stressors by influencing psychological processes
3.1.1. Mindfulness
Mindfulness is defined as the awareness that arises from paying attention to the present moment, non-judgmentally [60]. In their Meta-Analysis of 22 RCT’s, Galante and colleagues found that kindness-based meditation increased mindfulness compared to a no-intervention control group [38]. In their systematic review, Gu et al. also reported that in 12 RCTs and 4 quasi-experimental studies of OM meditations (MBSR and MBCT), mindfulness mediated symptoms of depression (n = 9), self-reported stress (n = 3), anxiety symptoms (n = 3), mood states (n = 3), quality of life (n = 1), and anger expression (n = 1) in diverse samples of individuals, including individuals with depressive disorders, and non-clinical samples of individuals [39]. The authors noted that evidence for mindfulness as a mediator of the impact of meditation on psychological outcomes was considered to be moderate [61]. In a Systematic Review of RCTs, pre-post studies, cross-sectional studies, cohort studies and qualitative studies, various meditation-based interventions increased mindfulness levels and improved burnout, depression, anxiety, and stress in health care professionals [40].
Mindfulness has been shown to reduce psychological distress [62]. Mindfulness training enhances self-regulation of emotion and behaviours [63] due to greater acceptance and self-awareness of unpleasant emotions and distress, rather than impulsive reaction, rumination or avoidance of these [64]. This enhanced acceptance of experiences is thought to lead to increased distress tolerance [65].
3.1.2. Self-compassion
Self-compassion is the act of extending compassion to one’s self in instances of perceived inadequacy, failure, or general suffering [66], not avoiding or disconnecting from it, and offering nonjudgmental understanding to one’s pain, inadequacies and failures [67].
In their Meta-Analysis of 22 RCT’s, Galante and colleagues found that kindness-based meditation increased self-compassion compared to a no-intervention control group [38]. These findings are consistent with the systematic review from Gu et al., which reported that in 2 RCTs and 1 quasi-experimental study, OM meditation-based studies (MBSR and MBCT) increased self-compassion and mediate its effects on symptoms on psychological outcomes, however, the authors noted that the level of evidence for self-compassion as a mediator was preliminary but inconsistent [39]. A Meta-Analysis of studies showed that self-compassion is a robust predictor of psychological health [68]. In a Systematic Review of RCTs, pre-post studies, cross-sectional studies, cohort studies and qualitative studies, various meditation-based interventions increased self-compassion levels and improved burnout, depression, anxiety, and stress in health care professionals [40]. In a Meta-Analysis of 22 OM studies (MBSR- or MBCT-based), comprising university students and community-dwelling individuals, a significant medium effect size increase in self-compassion was found compared to control conditions [41].
The psychological skills developed through meditation practices, such as self-compassion [69] have been associated with less self-reported stress, indicating that these skills change the relationships that individuals have, and reactivity to, psychological stressors. For example, in young people with elevated symptoms of depression, high self-compassion has been found to buffer against the experience of anxiety following a public speaking exercise, which is a psychological stressor [70]. Some authors have hypothesised that high levels of self-compassion may improve psychological health by decreasing avoidance and rumination as individuals with high self-compassion are less likely to avoid distressing thoughts and emotions associated with psychological stressors [71]. For example, individuals with depression self-report having less self-compassion compared to individuals who have never experienced depression [66]. This is supported by data showing that higher levels of self-compassion are associated with lower symptoms of depression, stress, increased quality of life, happiness, optimism, positive affect, wisdom, personal initiative, curiosity and exploration, agreeableness, extroversion, and conscientiousness [69].
3.1.3. Rumination
Rumination is the process of repetitively thinking about the causes, consequences, and symptoms of negative affect and it contributes to emotional distress [72].
In their systematic review Gu et al., report that in 5 RCTs and 3 quasi-experimental studies of OM meditation (MBSR and MBCT), that repetitive negative thinking, which included both rumination and worry, was found to mediate symptoms of depression (n = 5), followed by stress (n = 2), anxiety (n = 1) and global psychopathological symptoms (n = 1), mostly in individuals experiencing symptoms of depression and cancer survivors and patients, and that the level of evidence for this finding was moderate [39]. In a Meta-Analysis OM/FA meditation-based interventions (10 MBCT studies and one mindfulness yoga study), the intervention was associated with a moderate reduction in ruminative thoughts compared to treatment as usual or a waitlist control, however, no effect of the mindfulness-based intervention was seen when comparing the three OM/FA interventions with other active treatments [42].
Meditation practices have been hypothesised to decrease rumination through their ability to train and cultivate moment-to-moment awareness [73]. During meditation, attention is deliberately shifted from potentially ruminative thoughts to the immediate here-and-now experience, and individuals de-centre from their thoughts, or exercise meta-cognition as discussed below, which enables nonjudgmental acceptance of transient thoughts rather than a rumination on psychological stressors and can therefore decrease the distress associated with this rumination [74]. Current models of depression hypothesize that high avoidance of aversive stimuli contributes to the onset and maintenance of depression disorders [75]. Rumination has also been shown to mediate the negative correlation between self-compassion and symptoms of depression and anxiety amongst undergraduate students [76]. This suggests that the impact of self-compassion on unproductive repetitive thinking may be one way via which it buffers against depression [76].
3.1.4. Exposure
Exposure is another process that several authors have suggested may occur during mindfulness practice [77]. Prolonged observation of current thoughts and emotions, without trying to avoid or escape them, can be seen as an example of imaginal exposure, which should encourage the extinction of fear responses and avoidance behaviors previously elicited by these stimuli [78,79]. We were unable to locate any systematic reviews or Meta-Analysis assessing the impact of meditation on exposure, however consistent with this theory is pre-post data, showing that participation in the MBSR program, which includes mindfulness meditation, is associated with increases in exposure, which corresponded with decreasing depression and anxiety symptoms and stress in adults with chronic pain, anxiety, personal, illness- and employment-related stress [80].
This may occur because individuals with higher self-compassion are less likely to ruminate on distressing thoughts, to self-criticise, to avoid distressing thoughts. Indeed, previous work involving individuals with depression shows that rumination and avoidance mediate the relationship between self-compassion and depressive symptoms, suggesting that less self-compassionate people tend to function in a more avoiding manner [66]. This is supported by earlier findings showing that increases in self-compassion mediate the effect of MBSR on decreasing worry and fear of emotion, after controlling for changes in mindfulness [81]. Interventions that lessen experiential avoidance produce measurable reductions in fear-based pathology [82]. The work of Ford and colleagues shows that the act of avoiding thoughts and emotions can result in psychological stress [83]. In a series of studies involving university students and community-dwelling individuals, including a longitudinal study, Ford et al. reported that individuals who accepted their mental experiences experienced fewer negative emotional responses to stress, both within a laboratory setting and in daily life [83]. These authors highlighted that negative emotions and thoughts are common and that some people tend to accept their emotions and thoughts, while others tend to judge them as inappropriate or bad. Authors propose that the act of accepting or judging emotions or thoughts can impact longer-term psychological outcomes [83].
3.1.5. Metacognition
Metacognitive awareness is the ability to decenter from thoughts and emotions, and view them as passing mental events rather than identify with them or believe thoughts to be accurate representations of reality [84]. High levels of metacognition appear to protect against depression relapse [85]. High metacognitive awareness has been hypothesised to lead to reductions in rumination [84]. We could not identify any Meta-Analysis or systemic reviews assessing the impact of meditation on meta-cognition, however, one RCT’s showed that, in patients in remission or recovery from major depression, the mindfulness program, MBSR increased metacognition [86].
3.1.6. Attention
Meditation practices appear to benefit attention capacity, though the mechanisms are unclear. Comprehensive meta-analyses of FA and OM based meditation studies in diverse populations reveal medium positive effects across a range of attentional measures (concentration/attention, sustained attention, orienting, alerting, conflict monitoring, executive processing, behavioural inhibition) [46]. Some attention-training programs even appear to bolster the amygdala-prefrontal connectivity that is important for emotional regulation and downregulation of survival responses [87].
A Meta-Analysis of 39 various mindfulness meditation programs delivered to non-clinical populations of adults shows that pure mindfulness meditation interventions appear to be more effective at impacting attention than comprehensive MBSR programs that incorporate meditation among other strategies [44]. In a MA or RCTs in adults, cognitive processes underpinned by attention, such as working memory and inhibitory control, appear to benefit from a combination of MBSR and other mindfulness-based training programs [43]. A Meta-Analysis of both FA and OM based studies in healthy adults showed that programs that included FA and OM elements produced a significant but small effect on overall cognition, but no significant effect was found for FA and there was not a sufficient number of OM studies to conduct Meta-Analysis. The authors concluded that even single-session mindfulness inductions that direct attentional awareness confers short-term benefits to higher-order capacities, such as decision-making [45]. Increased operational and methodological consistency across studies is needed to better understand how meditation improves attention.
3.2. Meditation decreases autonomic arousal
We propose that meditation influences both psychological processes that decrease distress experienced in relation to stressors, and the corresponding autonomic arousal generally produced in response to stressors. These include increases in heart rate, respiration and blood pressure, and the release of blood sugar (glucose) into the bloodstream, [8]. This is explored below.
3.2.1. Blood pressure
There is strong evidence for the beneficial effects of meditation on blood pressure (BP), as shown from multiple Meta-Analyses of randomised controlled trials (RCTs). One Meta-Analysis shows that concentrative or focused attention forms of meditation decreased resting SBP by 5.55 mm of mercury (mmHg) at post-intervention, in 3 studies comprising of 72 individuals. Transcendental Meditation (TM) was similarly seen to significantly decrease resting systolic blood pressure (SBP) at post intervention by 8.97 mmHg, compared to the time/attention control group, in 3 studies comprising of 151 individuals. Open monitoring meditations, however, were not seen to decrease resting SBP at post-intervention, in 5 studies comprising of 359 individuals, from diverse populations [88]. Additionally, none of the meditation subtypes was found to decrease resting diastolic BP at post-intervention [88]. However, a limitation is that many of the included studies did not include a follow-up period. Therefore the longevity of the observed effects is unknown.
This is consistent with findings from a review of eight systematic reviews and meta-analyses showing a clear trend of TM in lowering SBP by ~4 mm Hg and DBP by ~2 mm Hg, which is comparable with other lifestyle interventions such as weight loss, diet, and exercise [47]. Another recent Meta-Analysis of RCTs showed that TM reduced ambulatory SBP by 2.49 mmHg and DBP by 4.26 mmHg. This was smaller than the decrease seen following a combined analysis of contemplative meditation forms (n = 7 studies) which reduced ambulatory SBP by −3.77 mmHg but DBP by −2.18 mmHg [48].
3.2.2. Heart rate
In a Meta-Analysis of 5 RCTs comprising of 140 individuals from diverse populations, OM meditation decreased resting heart rate by 3.11 beats per minute (bpm) at post-intervention, compared to a time/attention control group [88]. This is slightly smaller than the effects of aerobic exercise, which has been reported to reduce resting heart rate by 5 bpm in Meta-Analysis [89]. Focused attentions forms of meditation however were not seen to significantly decrease resting HR at post-intervention, in three RCTs comprising 72 individuals [88]. It appears that there may be differences between meditation subtypes in terms of autonomic regulation and perhaps stress reactivity, however, the longevity of the observed effects is unknown as few studies have assessed the long term impacts of meditation on heart rate.
3.2.3. Meditation and heart rate variability
Heart rate variability (HRV) is the variation in the time interval between heart beats. HRV reflects the flexibility of the cardiovascular system to cope with physical/psychological challenges and is measured by the variation in the beat-to-beat interval [90]. Higher HRV is associated with better emotional regulation [90].
A previous Meta-Analysis identifies only two RCTs assessing the impact of OM meditation on HRV, and only one RCT assessing the impact of self-transcending meditation (can be considered FA as mantra based according to Table 1) on HRV. These few studies show that meditation does not influence HRV compared to a time/attention control group [88]. This is consistent with the findings of a more recent Meta-Analysis including nineteen Mindfulness-Based Interventions (MBIs) RCTs (including MBSR), which found that MBIs were not efficacious in increasing vagally-mediated resting-state HRV relative to control conditions [49], however, this Meta-Analysis combined different types of MBIs in the analysis including loving-kindness meditation, mindful awareness in body-oriented therapy, mindful awareness practices and MBSR, and TM among others, so the impact of different meditation types on HRV is still unknown. Finally, in a Meta-Analysis of two RCTs and one pilot assessing MBSR (OM, object orientation) pooled estimates did not reach statistical significance [50]. However, these authors noted that the scarcity of studies assessing the effects of the standardized MBIs on HRV is a limitation, demonstrating that further research is required to understand the possible impact, if any, of meditation on HRV.
3.2.4. Inflammatory proteins
Inflammatory proteins are produced by immune cells and serve as extracellular communicators during immune system activity and can promote inflammation. Cytokines are small cell signalling protein molecules that mediate and regulate immunity and inflammation [20].
Limited evidence suggests that meditation may influence cytokine levels. In a Meta-Analysis comparing the impact of meditation and a time/attention control group, five RCTs comprising of 298 participants, found that OM forms of meditation resulted in a small effect size decrease c-reactive protein (CRP), the production of which is induced by pro-inflammatory cytokines [91]. However, the difference was not statistically significant [88]. Similarly, in four RCTs comprising 98 participants, IL-6 was not seen to change following OM meditation [88]. There was however significant variation between these four studies with regards to the type of time/attention control intervention delivered and the intervention duration. Furthermore, two of the studies included in the analysis measured CRP and IL-6 levels at 2 and 10 months post intervention, while the remaining two studies assessed CRP and IL-6 levels directly at post intervention [88], indicating that significant variability exists in the methodology used to assess the impact of meditation on inflammation.
In three RCTs comprising 100 participants, in a combined analysis of two MBSR interventions and one meditation retreat, TNF-α was found to decrease by 0.21 pg/mL following the meditation program. However, when the two MBSR interventions were assessed alone, there was no difference between meditation and the time attention/control group [88]. These findings are consistent with a recent Meta-Analysis of four RCTs assessing mindfulness-based interventions (OM, object orientation) which showed no impact of meditation on IL-6 or CRP [50]. In a Systematic Review of RCTs, non-RCTs, and open trials with a pre-post analysis and delivered to healthy populations and cancer patients, combined analysis of MBSR, MBCT, mindfulness meditation, mindfulness training and mindful awareness practices, (can be considered predominantly OM object-orientation) had no effect on cytokines (IL-6 measured in three studies, IL-8 in two studies, TNF-α in two studies, and IFN-γ and IL-10 in one study) but were found to increase the levels of the neuropeptide insulin-like growth factor 1 [52]. Contrary to the above findings, a Meta-Analysis of RCTs using OM meditation interventions (predominantly MBSR) found that OM reduced CRP but there were no effects for IL-1, IL-6, IL-8, IL-10 [51].
The above-reported Meta-Analysis highlight that few well-controlled trials have assessed the impact of meditation on cytokines and that significant variably is present in methodology in existing studies. Therefore, it would be valuable for future trials to further explore the impact of meditation on CRP, IL-6 and TNF-α, to explore if these effects are dependent upon the delivery format, duration, and dosage of the meditation practice.
Many studies indicate that various stressors, including psychological stressors alone, can induce pro-inflammatory (interleukin-1 [IL-1], interleukin-6 [IL-6]) and tumour necrosis factor-alpha [TNF-α] cytokine secretion [[92], [93], [94]]. A bi-directional feedback loop between cytokines and cortisol is hypothesised to be central to the appropriate functioning of the HPA axis while maintaining homeostasis of the immune system [95]. For example, the stress-induced release of certain inflammatory cytokines such as IL-6, TNF-α, and interleukin (IL-10), activate the HPA axis and cause the release of cortisol [95,96]. The increased cortisol induced negative-feedback then suppress the further release of cytokines [95,97,98]. However, glucocorticoid receptor abnormalities resulting from excessive cortisol can reduce the immune system’s capacity to respond to cortisol and subsequently lower inflammation. This results in concurrently sustained levels of cortisol and cytokine release [97,98].
3.2.5. Cortisol
In individuals considered at risk for elevated cortisol levels, such as due to illness or disease, Meta-Analysis showed various meditation interventions, but mostly FA based, decreased blood cortisol in individuals with a somatic illness. However, salivary cortisol was only seen to decrease in the sub-set of individuals living in stressful life situations [53]. In a Systematic Review of six OM meditation-based intervention studies, significant reductions in salivary cortisol levels were observed among within-participants studies but not in randomised controlled trial designs comprising of diverse populations [54]. In a Meta-Analysis of four MBSR and one MBCT RCTs, cortisol was seen to decrease in healthy adults following the meditation-based intervention, with no difference between active and passive control groups in diverse populations [56]. Intervention duration had a significant effect; interventions longer than 1200 min seem to be most effective at reducing cortisol levels, and the meditation was effective for men compared to women [56].
This is consistent with our previous Meta-Analysis work comparing the impact of meditation and a time/attention control group, that shows from two studies of Buddhist walking meditation and one study of Integrated Amrita Meditation, that meditation decreased blood cortisol, (comprising 102 individuals from diverse populations). This Meta-Analysis also showed that autonomic self-transcending forms of meditation, which can be considered an object-oriented focused attention meditation form, did not decrease blood cortisol levels, in three RCTs comprising 72 individuals [88]. This is consistent with our earlier systematic review showing that different forms of meditation appear to impact cortisol levels to a differing degree [55]. This systematic review included RCTs of interventions that included meditation practice and measured cortisol in conjunction with a measure of anxious symptomology and showed that mindfulness meditation, MBSR, and MBCT, and to a lesser degree Integrative-Mind-Body-Training and mindful breathing/diaphragmatic exercises, are all associated with decreases in cortisol levels, indicating that both OM and FA forms of meditation reduce cortisol levels (see Table 1). The findings from this systematic review also preliminarily indicated that meditation practices comprising of more hours of meditation practice per week may be more effective than those with fewer hours, in terms of regulating the autonomic nervous system and stress response, for example, mindfulness bases stress reduction was seen to decrease salivary cortisol when practiced by healthy adults for at least 2.5 h a week for eight weeks, and when participants were encouraged to practice at home for at least a further 315 min/week [99], but not to decrease salivary cortisol levels when practiced for 50 min/week over 12 weeks in healthy school children [100]. However, the impact of medication practices on cortisol levels likely varies depending on the particular population studied and the particular meditation practice, and the precise duration and frequency of meditation practice required to elicit such changes is still unknown. Furthermore, a focus on breathing was found to be a key component in each of the reviewed meditation interventions that was associated with biological changes including decreases in cortisol levels, such as paying attention to the sensation of breath moving through the nostrils and the rise and fall of the abdomen in mindfulness meditation, or focusing on breathing slowly via contraction of the pharynx in integrative-Mind-Body-Training, compared to some of the other forms of meditation studied, which instead focused largely on mantra, visualisations, or muscle relaxation, and were less frequently found to influence the autonomic outcomes studied. Indeed, breathing is one of the actions of the ANS that individuals can control, and activation of the PNS and homeostatic state is characterized by calm, relaxed breathing [101].
3.3. Meditation and changes in brain structure and function related to mood
Repeatedly high levels of cortisol resulting from persistent or repeated activation of the stress response can result in glucocorticoid receptor resistance, by impairing the function of glucocorticoid receptors [102]. This occurs in brain regions such as the prefrontal cortex (as well as the paraventricular nucleus) and hippocampus [103] which disrupts the glucocorticoid feedback control of the HPA axis [104,105]. Accordingly, baseline glucocorticoid release is also seen to increase following recurrent or persistent stress [106]. There is also evidence that high ongoing amounts of cortisol lead to damage and remodeling in the hippocampus and prefrontal cortex, as well as the amygdala [107]. The prefrontal cortex is important for executive function, working memory and self-regulatory behaviours [108], while the hippocampus is involved in the regulation of mood [107] and the amygdala is involved in the regulation of emotional and behavioural responses [109]. Changes in the structure and function of these regions are observed in individuals with mood disorders such as depression [107], and illustrates, at least in part, why persistent activation of amygdala-prefrontal survival responses is associated with the onset of a number of psychiatric disorders such as depression [110,111], which are charactered by an increase inflammatory state [12].
On the other hand, meditation practices have been shown to be associated with functional and structural changes in brain regions that are involved in the modifiable psychological process discussed above. The default mode network (DMN) for example is involved in emotional regulation and hypothesised to be important in terms of the impact of meditation on mood [112]. Alterations in DMN activity have been associated with a number of mental disorders, including depression [113]. For example, a number of studies have found an increase in DMN resting-state connectivity in individuals with depression, and that this is associated with higher levels of maladaptive rumination, indicating that increased levels of DMN activity underlie dysfunctional emotional processing in depression [114]. These are discussed below.
3.3.1. Default mode network
The DMN is a large-scale network of interacting brain regions that are highly correlated with each other and appear relatively distinct from other brain networks. This brain network shows higher activity during the resting state, compared to when performing tasks, in a specific constellation of brain regions, including the posterior cingulate cortex and adjacent precuneus, medial prefrontal cortex, medial and inferior temporal lobes, and inferior parietal lobe [113,115]. The default mode network is most commonly shown to be active when the brain is at wakeful rest, such as during daydreaming and mind-wandering [115]. Rumination is specifically correlated with the DMN core regions and the dorsal medial prefrontal cortex subsystem [116].
As previously reviewed, there is compelling evidence that mindfulness practices reduce DMN activity [117]. The DMN is shown to be deactivated during goal-directed tasks [113,115] and accordingly, various forms of meditation have been associated with reduced activity in the DMN [[117], [118], [119]]. This may be because meditation practices require metacognition and attentional focus as individuals observe their thoughts, bring attention back to the present moment as well as focus attention on a particular point, such as breath, a mantra, or sensory experience. Attention and cognitively demanding activities such as meditation, activate the task-positive brain network (TPN) [120], which include the dorsal and ventral frontoparietal attentional networks [121] and previous work shows activation of the TPN during task execution suppresses DMN activity [122]. Indeed, in healthy individuals, DMN activity correlates negatively with the goal-oriented TPN [123].
This paper will now further explore the role of mindfulness practices on the three major subdivisions of the DMN: the ventral medial prefrontal cortex; the dorsal medial prefrontal cortex; and the posterior cingulate cortex and adjacent precuneus plus the lateral parietal cortex (approximately Brodmann area 39).
3.3.2. Medial prefrontal cortex; dorsal medial prefrontal cortex
In their two meta-analyses, Fox and colleagues found the various meditation practices are associated with both changes in brain morphology/structural changes and functional changes in the medial prefrontal cortex [57,124]. Open monitoring and loving-kindness/compassion meditation (a relation orientation focused attention form of meditation, see Table 1) are associated with activation in the rostrolateral prefrontal cortex (BA10), which extend into the dorsolateral prefrontal cortex (BA9) for loving-kindness/compassion meditation [124]. Increased cortical thickness is seen in the rostrolateral prefrontal cortex (BA10) of long-term meditators, irrespective of the meditation type practiced, indicating that morphological changes in rostrolateral prefrontal cortex may be generalizable beyond any particular meditative practice [57]. Focused attention meditation and OM meditation are associated with activation in the anterior/midcingulate cortex (BA 24 and 32 respectively), both of which are part of the medial prefrontal cortex [125]. Fox and colleagues also reported dependable morphological difference in the anterior/midcingulate cortex following different meditation subtypes, specifically, in two studies, greater white matter fibre density and/or coherence were found by means of fractional anisotropy both in novice and long term meditators, and in a third study increased cortical thickness was seen in long term meditators [57].
The right orbitofrontal cortex (OFC), which is sometimes referred to as ventromedial prefrontal cortex (vmPFC), is involved in the regulation of negative emotion and self-monitoring [126]. Specifically, it is involved in down-regulating and reappraising negative emotional states [127]. It is also hypothesised that meditative training may lead to greater reliance on the OFC to guide behavior, instead of relying on static stimulus-reward associations from the past [57,128]. Fox et al. found a sizable (240 mm3) meta-analytic clusters of morphological difference in the OFC [57].
These findings are consistent with those of an earlier Systematic Review reporting that mindfulness meditation practice (can be considered OM) activate the prefrontal cortex and the anterior cingulate cortex [58] as well as a more recent Systematic Review by Magalhaes et al., which showed greater prefrontal/frontal activity associated with various meditation forms [59].
The results of the previous systematic review indicate that these structural changes indicate that mindfulness can lead to heightened self-monitoring, as well as a better ability to regulate negative emotion [58,86]. The ability to regulate negative emotion may contribute to the decrease in stress resulting from distressing thoughts and emotions.
3.3.3. Posterior cingulate cortex/retrosplenial cortex
The posterior cingulate cortex (PCC)/retrosplenial cortex (RSP) is a core region in the DMN. It may serve as the main “hub” of the DMN [125] and is associated with episodic memory retrieval [129]. In their Meta-Analysis, Fox and colleagues found the various meditation practices are associated with both structural and functional changes in the posterior cingulate cortex/retrosplenial cortex. Focused attention meditation is associated with deactivation in the posterior cingulate cortex (BA 30), while loving-kindness/compassion meditation was associated with activation near the parieto-occipital sulcus (BA 23/31) [124]. In their earlier Meta-Analysis, Fox et al. report structural differences in the posterior cingulate cortex of meditators vs non-meditators, which they interpreted as structural declines or decreases in meditation practitioners, perhaps indicating a weakening of default mode network function [57]. Given that DMN activity is linked to mind wandering and spontaneous thought [130], decreased activation in the PCC/RSP may indicate a blunting of DMN activity perhaps reflecting reduced mind wandering in meditators, as indicated by previous work comparing long-term meditators with non-meditators [131].
3.3.4. Inferior parietal lobule
In their meta-analyses, Fox and colleagues found that focused attention meditation is associated with deactivation in the inferior parietal lobule (IPL) (BA 39) while loving-kindness/compassion meditation is associated with activations in the left inferior parietal lobule (BA 2/40) [124], suggesting decreased processing of external sensory inputs [124]. Along with the PCC, the IPL is involved in mind-wandering [132], episodic memory retrieval, and the simulation of future events [125]. Fox and colleagues speculated that deactivations in the IPL might indicate that focused attention meditation can decrease spontaneous thoughts about past and future events as well as reflecting on the meaning and implications of suggested events [124], which may influence the experience of distress by decreasing the frequency of worrying thoughts about the future and increase dispositional mindfulness. Previous research states the IPL is one brain region altered with extended daily focused attention to moment-to-moment experience and therefore may represent the neural underpinnings of self-reference in the psychological present [133,134]. The IPL has been associated with experiences stepping outside of themselves, affording a detached, or meta-cognitive perspective [135].
3.3.5. Hippocampal formation
In their Meta-Analysis, Fox and colleagues found structural brain differences in the hippocampus in meditators compared to non-meditators, including increases in gray matter concentration and gray matter volume [57]. In their systematic review, Chiesa and Serretti concluded that Vipassana meditation (FA object orientation, see Table 1) resulted in a thicker right hippocampus, based on the work of Holzel et al., which compared the regional gray matter concentration of meditators and non-meditators [58].
The hippocampus contributes to the regulation of emotion [136] including emotional reactions and social emotions [137]. Several mental disorders such as major depression [138] and PTSD [139] are associated with decreased density or volume of the hippocampus [140]. The hippocampus however is the region where remodeling of synapses and the generation new neurons have been observed [141]. Volume loss in this region seems to be reversible [142]. Indeed, treatment with selective serotonin reuptake inhibitors (SSRIs) has been found to lead to an increase in hippocampal volume [143]. It has been suggested that some of the behavioural effects of antidepressant treatment might depend on neurogenesis in the hippocampus [144]. Some authors have suggested that structural changes in the hippocampus area following mindfulness practice may reflect improved function in regulating emotional responding [57,145] and that structural hippocampal increases relate to meditation’s potential amelioration of clinical conditions such as depression [58].
3.3.6. The insular cortex
The insular cortex is the only brain region that Fox and colleagues found to be consistently recruited or activated irrespective of the meditation form, with small/non-significant activations from focused attention in BA13, and deactivation from mantra recitation, and larger (significant) activations resulting from OM and loving-kindness meditations [146]. With regards to structural differences, in their Meta-Analysis, Fox et al. [57] propose that the various structural differences they found in the insular cortex (cortical thickness, cortical gyrification, gray matter concentration) are tied to body-centred meditations. This is because the studies that reported insula differences involved explicit focus on the body, including paying attention to body posture, respiration, temperature, and other sensations. These findings support those reported by Treves et al., in their Meta-Analysis which showed that mindfulness meditation practice was associated with greater body awareness accuracy, as measured by visceral, somatosensory and proprioceptive signals in seven RCTs [147]. Finally, these findings are consistent with those reported in a Systematic Review by Magalhaes et al., which reported increased insular activity in meditators [59].
The insula cortex has many functions, including involvement in empathy [148], metacognition [149], and interoception, which is the awareness and monitoring of internal bodily states, including respiration or heart rate [150]. Given that many forms of meditation involve some degree of monitoring of the body and basic metacognitive monitoring, it is understandable that many meditation forms are associated with activation of the insular cortex.
Dysfunction of interoception is increasingly recognized as an important component of a number of different mental disorders, and dysfunction of the insular cortex has been shown to be involved in a range of mental illnesses, including mood disorders [151,152]. Many mental disorders are associated with altered attention to the body or bodily sensations. For example, in major depressive disorder, a large body of evidence shows that individuals have altered interoception and decreased activity in the insula cortex [151,152]. Therefore, it has been hypothesised that greater activity in the insular cortex may play a role in the emotional and clinical benefits associated with meditation practices [57,146].
3.4. The importance of individual differances
One outstanding question relates to the impact of individual differances on the efficacy of the variety of meditation practices for mood. For example, some breathing techniques may be uncomfortable or even distressing for some individuals [153]. Therefore it might be more beneficial to focus attention on another sensory domain, such as sound or repeating a mantra. Indeed, previous work has shown that HRV varies significantly between participants in response to a single exposure to a) classical music b) white noise c) a guided relaxation/meditation exercise and d) a paced breathing exercise [154]. This study involved 100 healthy individuals, and 45% of participants showed the greatest response in HRV to classical music, 36% to white noise, 13% to a guided relaxation/meditation exercise and 6% to a paced breathing exercise. Interestingly, there was little correlation between the activity eliciting the largest change in HRV and self-reported well-being following the task [154]. While this review did not compare different forms of mindfulness practices, it did compare different attention activities, some of which include aspects of mindfulness (such as paying attention to breathing). Indeed, meditation can be seen as an attentional activity, and visa versa. The finding that individual variations in HRV exist in response to single exposures to various attention activities, suggest that it is possible that individuals will respond uniquely to different types of mindfulness practices.
Consistent with this conclusion is a more recent study that compared partiality towards meditation practices, based on anchors from different sensory modalities, in 82 healthy individuals. Individuals reported that their motivation to engage in meditation practices differed based on the attentional anchor (breath vs image vs mantra). Interestingly, the favoured anchor changed at some point for 77% of the participants, after engaging with each form of meditation. Additionally, over half (56%) of the participants reported preferring a different anchor by the end of the study, compared to their initial meditation-naive preference. Moreover, the attentional anchor was not found to predict changes in HR and HRV response. However, it should be noted that the technology used to monitor cardiac response in this particular study was somewhat unreliable [155]. There is limited research exploring the importance of individual differences with regard to meditation practices. Therefore, future research should be conducted to better understand individual response differences to meditation and how these might impact stress reactivity and mental health outcomes.
3.5. Strengths and limitations
The strengths of this review are that it is the first to examine the effects of meditation on physiological, psychological and neurobiological outcomes collectively, in the context of mood outcomes. Therefore, we have provided a comprehensive synthesis demonstrating the meditation impacts multiple processes, however, further research should explore the potential interaction between these processes as it is possible that effects may be synergistic rather than in silos. To best capture the current state of research in this field, studies were not included or excluded based on quality assessment. Furthermore, this review does not provide an exhaustive list of all of the psychological, physiological and neurobiological changes associated with meditation, but rather focuses of those we hypothesis to be particularly relevant for stress regulation. A limitation of the current research is that we have not conducted a quality assessment of included studies and therefore the methodological rigour of the discussed studies is unknown. In future research, it would be of value to include a formal assessment of the quality of reviewed research. While we included systematic reviews and Meta-Analysis, some of the included Meta-Analysis [57,146] included primary studies based on long-term practitioners, and studies that involved a small number of participants, and therefore the level of evidence provided in these studies is limited [156] and should be considered as preliminary, indicating a need for further research into the impact of meditation on structural and functional brain changes.
4. Discussion and future directions
This narrative literature adds to the existing body of research by identifying how meditation practices impact stress reactivity from a psychological, physiological and neurobiological perspective concurrently and therefore demonstrates that the positive impacts of meditation on mood likely result from influencing stress reactivity and stress resilience, via multiple and interacting pathways. Based on the evidence reviewed, we suggest that meditation-based practices can decrease stress reactivity, resulting in overall improved health and wellbeing [157]. Meditation practices are shown to influence many psychological processes that can influence an individual’s psychological response and relationship with stressors [158], including self-compassion [159], rumination [160], exposure [161], metacognition [85] and attention [46]. Such practices influence physiological markers of stress reactivity, including changes in blood pressure, heart rate, cortisol or cytokine levels, in diverse populations of adults [55,88,162,163]. Acute and chronic stress and subsequent excess corticosteroids can damage the brain [17], particularly in areas associated with the regulation of mood and emotion, including the hippocampus [18] and the prefrontal cortex [19]. On the other hand, meditation practice is associated with structural and functional changes in brain regions involved in the regulation of mood, emotion and relevant modifiable psychological processes such as rumination and meta-cognition. Meditation is associated with changes in stress-related measures such as cortisol, and possibly some inflammatory proteins, however, further research is required to determine if these effects are robust. We speculate that the various psychological, physiological and neurobiological effects discussed in the current study influence one another in a bi-directional manner.
There are still a number of other outstanding questions that are relevant for future research. For example, there are potentially important differences between various forms of meditation, various populations, the lengths of training and other factors. At present, there are vastly different amounts of evidence supporting different forms of meditation for different clinical conditions (e.g., the evidence for MBCT as a depression relapse prevention strategy is strong [39,164,165], while the evidence for MBCT as treatment of bipolar disorder is less robust [166]). Therefore, future research should further examine the difference between various forms of meditations in different populations to better understand differences in the impact of various forms of meditation on mood outcomes in different populations.
It is also possible that meditation practices may be detrimental in specific circumstances and for some people. For example, case reports exist of one or a few individuals describing severe symptoms induced by several forms of meditation (Transcendental, Zen, Mindfulness), often occurring in the context of intensive retreats. However, these incidents have not been reported following evidence-based mindfulness interventions [167]. Even in the absence of severe symptoms, meditation can occasionally result in subjective distress and functional impairment [168]. This highlights the need for teachers to understand the theoretical and mechanistic foundations of meditation practices, so that they can explain why they might be relevant, what difficulties might arise, and how these can be managed [169].
Ethics approval and consent to participate
No ethics approval was required for this work.
Consent for publication
Not applicable.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding
None.
Author contribution
MP conceived the current study and research question and designed the study. Data collection and analysis was completed by all authors and all authors contributed to writing the publication. The corresponding author (MP) has access to data in the study and had final responsibility for the decision to submit for publication. All authors have read and approved the manuscript.
CRediT authorship contribution statement
Michaela C. Pascoe: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Michael de Manincor: Conceptualization, Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. Jana Tseberja: contribued to data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. Mats Hallgren: contribued to data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. Peter A. Baldwin: contribued to data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. Alexandra G. Parker: Conceptualization, Supervision, project adminstration, Writing – original draft, Writing – review & editing.
Declaration of competing interest
None to declare.
Acknowledgements
None.
References
- 1.Kemeny M.E. The psychobiology of stress. Curr. Dir. Psychol. Sci. 2003;12:124–129. [Google Scholar]
- 2.Lazarus R.S., Folkman S. Springer publishing company; 1984. Stress, Appraisal, and Coping. [Google Scholar]
- 3.Charmandari E., Tsigos C., Chrousos G. Endocrinology of the stress response. Annu. Rev. Physiol. 2005;67:259–284. doi: 10.1146/annurev.physiol.67.040403.120816. [DOI] [PubMed] [Google Scholar]
- 4.van Paridon K.N., Timmis M.A., Nevison C.M., Bristow M. The anticipatory stress response to sport competition; a systematic review with meta-analysis of cortisol reactivity. BMJ Open Sport & Exercise Med. 2017;3 doi: 10.1136/bmjsem-2017-000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nesse R.M., Bhatnagar S., Ellis B. Series Academic Press; 2016. Evolutionary Origins and Functions of the Stress Response System, Stress: Concepts, Cognition, Emotion, and Behavior Handbook of Stress; pp. 95–101. [Google Scholar]
- 6.Slavich G.M., Irwin M.R. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull. 2014;140:774. doi: 10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olshansky B., Sabbah H.N., Hauptman P.J., Colucci W.S. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation. 2008;118:863–871. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- 8.Herman J.P., McKlveen J.M., Ghosal S., Kopp B., Wulsin A., Makinson R., Scheimann J., Myers B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comp. Physiol. 2016;6:603–621. doi: 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiller-Sturmhofel S., Bartke A. The endocrine system: an overview. Alcohol Health Res. World. 1998;22:153–164. [PMC free article] [PubMed] [Google Scholar]
- 10.De Kloet E.R., Vreugdenhil E., Oitzl M.S., Joels M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 11.Myers B., McKlveen J.M., Herman J.P. Neural regulation of the stress response: the many faces of feedback. Cell. Mol. Neurobiol. 2012 doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dantzer R. Depression and inflammation: an intricate relationship. Biol. Psychiatr. 2012;71:4–5. doi: 10.1016/j.biopsych.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Pascoe M.C., Crewther S.G., Carey L.M., Crewther D.P. Inflammation and depression: why poststroke depression may be the norm and not the exception. Int. J. Stroke. 2011;6:128–135. doi: 10.1111/j.1747-4949.2010.00565.x. [DOI] [PubMed] [Google Scholar]
- 14.Burke H.M., Davis M.C., Otte C., Mohr D.C. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30:846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Hallonquist J., Goldberg M., Brandes J. Affective disorders and circadian rhythms. Can. J. Psychiatr. 1986;31:259–272. doi: 10.1177/070674378603100315. [DOI] [PubMed] [Google Scholar]
- 16.Sachar E.J. Evidence for neuroendocrine abnormalities in the major mental illnesses. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1975;54:347–358. [PubMed] [Google Scholar]
- 17.Echouffo-Tcheugui J.B., Conner S.C., Himali J.J., Maillard P., DeCarli C.S., Beiser A.S., Vasan R.S., Seshadri S. Circulating cortisol and cognitive and structural brain measures: the Framingham Heart Study. Neurology. 2018;91:e1961–e1970. doi: 10.1212/WNL.0000000000006549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herbert J., Goodyer I., Grossman A., Hastings M., De Kloet E., Lightman S., Lupien S., Roozendaal B., Seckl J. Do corticosteroids damage the brain? J. Neuroendocrinol. 2006;18:393–411. doi: 10.1111/j.1365-2826.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- 19.McEwen B.S. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54:20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Salim S., Chugh G., Asghar M. Inflammation in anxiety. Adv. Protein Chem. Struct. Biol. 2012;88:1–25. doi: 10.1016/B978-0-12-398314-5.00001-5. [DOI] [PubMed] [Google Scholar]
- 21.Maes M. The cytokine hypothesis of depression: inflammation, oxidative & nitrosative stress (IO&NS) and leaky gut as new targets for adjunctive treatments in depression. Neuroendocrinol. Lett. 2008;29:287–291. [PubMed] [Google Scholar]
- 22.Thomas A.J., Davis S., Morris C., Jackson E., Harrison R., O’Brien J.T. Increase in interleukin-1 beta in late-life depression. Am. J. Psychiatr. 2005;162:175–177. doi: 10.1176/appi.ajp.162.1.175. [DOI] [PubMed] [Google Scholar]
- 23.Tuglu C., Kara S.H., Caliyurt O., Vardar E., Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology (Berlin) 2003;170:429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- 24.Alesci S., Martinez P.E., Kelkar S., Ilias I., Ronsaville D.S., Listwak S.J., Ayala A.R., Licinio J., Gold H.K., Kling M.A., Chrousos G.P., Gold P.W. Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J. Clin. Endocrinol. Metab. 2005;90:2522–2530. doi: 10.1210/jc.2004-1667. [DOI] [PubMed] [Google Scholar]
- 25.Capuron L., Miller A.H. Cytokines and psychopathology: lessons from interferon-alpha. Biol. Psychiatr. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 26.Capuron L., Dantzer R. Cytokines and depression: the need for a new paradigm. Brain Behav. Immun. 2003;17(Suppl 1):S119–S124. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 27.Musselman D.L., Lawson D.H., Gumnick J.F., Manatunga A.K., Penna S., Goodkin R.S., Greiner K., Nemeroff C.B., Miller A.H. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N. Engl. J. Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 28.Sagoo P., Chan G., Larkin D.F., George A.J. Inflammatory cytokines induce apoptosis of corneal endothelium through nitric oxide. Invest. Ophthalmol. Vis. Sci. 2004;45:3964–3973. doi: 10.1167/iovs.04-0439. [DOI] [PubMed] [Google Scholar]
- 29.Wann B.P., Boucher M., Kaloustian S., Nim S., Godbout R., Rousseau G. Apoptosis detected in the amygdala following myocardial infarction in the rat. Biol. Psychiatr. 2006;59:430–433. doi: 10.1016/j.biopsych.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 30.Mergenthaler P., Dirnagl U., Meisel A. Pathophysiology of stroke: lessons from animal models. Metab. Brain Dis. 2004;19:151–167. doi: 10.1023/b:mebr.0000043966.46964.e6. [DOI] [PubMed] [Google Scholar]
- 31.Bond K., Ospina M.B., Hooton N., Bialy L., Dryden D.M., Buscemi N., Shannahoff-Khalsa D., Dusek J., Carlson L.E. Defining a complex intervention: the development of demarcation criteria for “meditation”. Psychol. Religion Spirituality. 2009;1:129. [Google Scholar]
- 32.Dahl C.J., Lutz A., Davidson R.J. Reconstructing and deconstructing the self: cognitive mechanisms in meditation practice. Trends Cognit. Sci. 2015;19:515–523. doi: 10.1016/j.tics.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiesa A., Malinowski P. Mindfulness-based approaches: are they all the same? J. Clin. Psychol. 2011;67:404–424. doi: 10.1002/jclp.20776. [DOI] [PubMed] [Google Scholar]
- 34.Awasthi B. Issues and perspectives in meditation research: in search for a definition. Front. Psychol. 2012;3:613. doi: 10.3389/fpsyg.2012.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldberg S.B., Riordan K.M., Sun S., Davidson R.J. The empirical status of mindfulness-based interventions: a systematic review of 44 meta-analyses of randomized controlled trials. Perspect. Psychol. Sci. 2021 doi: 10.1177/1745691620968771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofmann S.G., Grossman P., Hinton D.E. Loving-kindness and compassion meditation: potential for psychological interventions. Clin. Psychol. Rev. 2011;31:1126–1132. doi: 10.1016/j.cpr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khoury B., Lecomte T., Fortin G., Masse M., Therien P., Bouchard V., Chapleau M.A., Paquin K., Hofmann S.G. Mindfulness-based therapy: a comprehensive meta-analysis. Clin. Psychol. Rev. 2013;33:763–771. doi: 10.1016/j.cpr.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Galante J., Galante I., Bekkers M.-J., Gallacher J. Effect of kindness-based meditation on health and well-being: a systematic review and meta-analysis. J. Consult. Clin. Psychol. 2014;82:1101. doi: 10.1037/a0037249. [DOI] [PubMed] [Google Scholar]
- 39.Gu J., Strauss C., Bond R., Cavanagh K. How do mindfulness-based cognitive therapy and mindfulness-based stress reduction improve mental health and wellbeing? A systematic review and meta-analysis of mediation studies. Clin. Psychol. Rev. 2015;37:1–12. doi: 10.1016/j.cpr.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Conversano C., Ciacchini R., Orrù G., Di Giuseppe M., Gemignani A., Poli A. Mindfulness, compassion, and self-compassion among health care professionals: what’s new? A systematic review. Front. Psychol. 2020;11:1683. doi: 10.3389/fpsyg.2020.01683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golden H.L., Vosper J., Kingston J., Ellett L. Mindfulness; 2020. The Impact of Mindfulness-Based Programmes on Self-Compassion in Nonclinical Populations: a Systematic Review and Meta-Analysis; pp. 1–24. [Google Scholar]
- 42.Perestelo-Perez L., Barraca J., Penate W., Rivero-Santana A., Alvarez-Perez Y. Mindfulness-based interventions for the treatment of depressive rumination: systematic review and meta-analysis. Int. J. Clin. Health Psychol. 2017;17:282–295. doi: 10.1016/j.ijchp.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cásedas L., Pirruccio V., Vadillo M.A., Lupiáñez J. Does mindfulness meditation training enhance executive control? A systematic review and meta-analysis of randomized controlled trials in adults. Mindfulness. 2020;11:411–424. [Google Scholar]
- 44.Eberth J., Sedlmeier P. The effects of mindfulness meditation: a meta-analysis. Mindfulness. 2012;3:174–189. [Google Scholar]
- 45.Gill L.-N., Renault R., Campbell E., Rainville P., Khoury B. Mindfulness induction and cognition: a systematic review and meta-analysis. Conscious. Cognit. 2020;84:102991. doi: 10.1016/j.concog.2020.102991. [DOI] [PubMed] [Google Scholar]
- 46.Sedlmeier P., Eberth J., Schwarz M., Zimmermann D., Haarig F., Jaeger S., Kunze S. The psychological effects of meditation: a meta-analysis. Psychol. Bull. 2012;138:1139. doi: 10.1037/a0028168. [DOI] [PubMed] [Google Scholar]
- 47.Ooi S.L., Giovino M., Pak S.C. Transcendental meditation for lowering blood pressure: an overview of systematic reviews and meta-analyses. Compl. Ther. Med. 2017;34:26–34. doi: 10.1016/j.ctim.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 48.Shi L., Zhang D., Wang L., Zhuang J., Cook R., Chen L. Meditation and blood pressure: a meta-analysis of randomized clinical trials. J. Hypertens. 2017;35:696–706. doi: 10.1097/HJH.0000000000001217. [DOI] [PubMed] [Google Scholar]
- 49.Brown L., Rando A.A., Eichel K., Van Dam N.T., Celano C.M., Huffman J.C., Morris M.E. The effects of mindfulness and meditation on vagally-mediated heart rate variability: a meta-analysis. Psychosom. Med. 2021 doi: 10.1097/PSY.0000000000000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rådmark L., Sidorchuk A., Osika W., Niemi M. A systematic review and meta-analysis of the impact of mindfulness based interventions on heart rate variability and inflammatory markers. J. Clin. Med. 2019;8:1638. doi: 10.3390/jcm8101638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Black D.S., Slavich G.M. Mindfulness meditation and the immune system: a systematic review of randomized controlled trials. Ann. N. Y. Acad. Sci. 2016;1373:13. doi: 10.1111/nyas.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanada K., Díez M.A., Valero M.S., Pérez-Yus M.C., Demarzo M.M., Montero-Marín J., García-Toro M., García-Campayo J. Effects of mindfulness-based interventions on biomarkers in healthy and cancer populations: a systematic review. BMC Compl. Alternative Med. 2017;17:1–13. doi: 10.1186/s12906-017-1638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koncz A., Demetrovics Z., Takacs Z.K. Meditation interventions efficiently reduce cortisol levels of at-risk samples: a meta-analysis. Health Psychol. Rev. 2020:1–29. doi: 10.1080/17437199.2020.1760727. [DOI] [PubMed] [Google Scholar]
- 54.O’Leary K., O’Neill S., Dockray S. A systematic review of the effects of mindfulness interventions on cortisol. J. Health Psychol. 2016;21:2108–2121. doi: 10.1177/1359105315569095. [DOI] [PubMed] [Google Scholar]
- 55.Pascoe M.C., Crewther S.G. SMGroup; 2016. A Systematic Review of Randomised Control Trials Examining the Effects of Mindfulness on Stress and Anxious Symptomatology, Anxiety Disorder. [Google Scholar]
- 56.Sanada K., Montero-Marin J., Alda Diez M., Salas-Valero M., Pérez-Yus M.C., Morillo H., Demarzo M.M., García-Toro M., García-Campayo J. Effects of mindfulness-based interventions on salivary cortisol in healthy adults: a meta-analytical review. Front. Physiol. 2016;7:471. doi: 10.3389/fphys.2016.00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fox K.C., Nijeboer S., Dixon M.L., Floman J.L., Ellamil M., Rumak S.P., Sedlmeier P., Christoff K. Is meditation associated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation practitioners. Neurosci. Biobehav. Rev. 2014;43:48–73. doi: 10.1016/j.neubiorev.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 58.Chiesa A., Serretti A. A systematic review of neurobiological and clinical features of mindfulness meditations. Psychol. Med. 2010;40:1239–1252. doi: 10.1017/S0033291709991747. [DOI] [PubMed] [Google Scholar]
- 59.Magalhaes A.A., Oliveira L., Pereira M.G., Menezes C.B. Does meditation alter brain responses to negative stimuli? A systematic review. Front. Hum. Neurosci. 2018;12:448. doi: 10.3389/fnhum.2018.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kabat-Zinn J. Mindfulness-based interventions in context: past, present, and future. Clin. Psychol. Sci. Pract. 2003;10:144–156. [Google Scholar]
- 61.Hoogendoorn W.E., van Poppel M.N., Bongers P.M., Koes B.W., Bouter L.M. Systematic review of psychosocial factors at work and private life as risk factors for back pain. Spine. 2000;25:2114–2125. doi: 10.1097/00007632-200008150-00017. [DOI] [PubMed] [Google Scholar]
- 62.Parto M., Besharat M.A. Mindfulness, psychological well-being and psychological distress in adolescents: assessing the mediating variables and mechanisms of autonomy and self-regulation. Procedia-Soc. Behav. Sci. 2011;30:578–582. [Google Scholar]
- 63.Jimenez S.S., Niles B.L., Park C.L. A mindfulness model of affect regulation and depressive symptoms: positive emotions, mood regulation expectancies, and self-acceptance as regulatory mechanisms. Pers. Indiv. Differ. 2010;49:645–650. [Google Scholar]
- 64.Baer R.A. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clin. Psychol. Sci. Pract. 2003;10:125–143. [Google Scholar]
- 65.Carpenter J.K., Sanford J., Hofmann S.G. The effect of a brief mindfulness training on distress tolerance and stress reactivity. Behav. Ther. 2019;50:630–645. doi: 10.1016/j.beth.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krieger T., Altenstein D., Baettig I., Doerig N., Holtforth M.G. Self-compassion in depression: associations with depressive symptoms, rumination, and avoidance in depressed outpatients. Behav. Ther. 2013;44:501–513. doi: 10.1016/j.beth.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 67.Neff K. Self-compassion: an alternative conceptualization of a healthy attitude toward oneself. Self Ident. 2003;2:85–101. [Google Scholar]
- 68.MacBeth A., Gumley A. Exploring compassion: a meta-analysis of the association between self-compassion and psychopathology. Clin. Psychol. Rev. 2012;32:545–552. doi: 10.1016/j.cpr.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 69.Neff K.D., Rude S.S., Kirkpatrick K.L. An examination of self-compassion in relation to positive psychological functioning and personality traits. J. Res. Pers. 2007;41:908–916. [Google Scholar]
- 70.Bluth K., Roberson P.N., Gaylord S.A., Faurot K.R., Grewen K.M., Arzon S., Girdler S.S. Does self-compassion protect adolescents from stress? J. Child Fam. Stud. 2016;25:1098–1109. doi: 10.1007/s10826-015-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Svendsen J.L., Kvernenes K.V., Wiker A.S., Dundas I. Mechanisms of mindfulness: rumination and self-compassion. Nord. Psychol. 2017;69:71–82. [Google Scholar]
- 72.Calmes C.A., Roberts J.E. Repetitive thought and emotional distress: rumination and worry as prospective predictors of depressive and anxious symptomatology. Cognit. Ther. Res. 2007;31:343–356. [Google Scholar]
- 73.Jain S., Shapiro S.L., Swanick S., Roesch S.C., Mills P.J., Bell I., Schwartz G.E. A randomized controlled trial of mindfulness meditation versus relaxation training: effects on distress, positive states of mind, rumination, and distraction. Ann. Behav. Med. 2007;33:11–21. doi: 10.1207/s15324796abm3301_2. [DOI] [PubMed] [Google Scholar]
- 74.Wolkin J.R. Cultivating multiple aspects of attention through mindfulness meditation accounts for psychological well-being through decreased rumination. Psychol. Res. Behav. Manag. 2015;8:171. doi: 10.2147/PRBM.S31458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trew J.L. Exploring the roles of approach and avoidance in depression: an integrative model. Clin. Psychol. Rev. 2011;31:1156–1168. doi: 10.1016/j.cpr.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 76.Raes F. Rumination and worry as mediators of the relationship between self-compassion and depression and anxiety. Pers. Indiv. Differ. 2010;48:757–761. [Google Scholar]
- 77.Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen. Hosp. Psychiatr. 1982;4:33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- 78.Linehan M.M. 1993. Cognitive-behavioral Treatment of Borderline Personality Disorder. [Google Scholar]
- 79.Linehan M.M. Guilford Press; 1993. Skills Training Manual for Treating Borderline Personality Disorder. [Google Scholar]
- 80.Carmody J., Baer R.A., E L.B.L., Olendzki N. An empirical study of the mechanisms of mindfulness in a mindfulness-based stress reduction program. J. Clin. Psychol. 2009;65:613–626. doi: 10.1002/jclp.20579. [DOI] [PubMed] [Google Scholar]
- 81.Keng S.L., Smoski M.J., Robins C.J., Ekblad A.G., Brantley J.G. Mechanisms of change in mindfulness-based stress reduction: self-compassion and mindfulness as mediators of intervention outcomes. J. Cognit. Psychother. 2012;26:270–280. [Google Scholar]
- 82.Eustis E.H., Cardona N., Nauphal M., Sauer-Zavala S., Rosellini A.J., Farchione T.J., Barlow D.H. Experiential avoidance as a mechanism of change across cognitive-behavioral therapy in a sample of participants with heterogeneous anxiety disorders. Cognit. Ther. Res. 2020;44:275–286. [Google Scholar]
- 83.Ford B.Q., Lam P., John O.P., Mauss I.B. The psychological health benefits of accepting negative emotions and thoughts: laboratory, diary, and longitudinal evidence. J. Pers. Soc. Psychol. 2018;115:1075. doi: 10.1037/pspp0000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sheppard L.C., Teasdale J.D. Dysfunctional thinking in major depressive disorder: a deficit in metacognitive monitoring? J. Abnorm. Psychol. 2000;109:768–776. doi: 10.1037//0021-843x.109.4.768. [DOI] [PubMed] [Google Scholar]
- 85.Fresco D.M., Segal Z.V., Buis T., Kennedy S. Relationship of posttreatment decentering and cognitive reactivity to relapse in major depression. J. Consult. Clin. Psychol. 2007;75:447–455. doi: 10.1037/0022-006X.75.3.447. [DOI] [PubMed] [Google Scholar]
- 86.Teasdale J.D., Moore R.G., Hayhurst H., Pope M., Williams S., Segal Z.V. Metacognitive awareness and prevention of relapse in depression: empirical evidence. J. Consult. Clin. Psychol. 2002;70:275–287. doi: 10.1037//0022-006x.70.2.275. [DOI] [PubMed] [Google Scholar]
- 87.Cohen N., Ochsner K.N. From surviving to thriving in the face of threats: the emerging science of emotion regulation training. Curr. Opin. Behav. Sci. 2018;24:143–155. doi: 10.1016/j.cobeha.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pascoe M.C., Thompson D.R., Jenkins Z.M., Ski C.F. Mindfulness mediates the physiological markers of stress: systematic review and meta-analysis. J. Psychiatr. Res. 2017;95:156–178. doi: 10.1016/j.jpsychires.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 89.Kelley G.A., Kelley K.A., Tran Z.V. Aerobic exercise and resting blood pressure: a meta-analytic review of randomized, controlled trials. Prev. Cardiol. 2001;4:73–80. doi: 10.1111/j.1520-037x.2001.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perna G., Riva A., Defillo A., Sangiorgio E., Nobile M., Caldirola D. Heart rate variability: can it serve as a marker of mental health resilience?: special section on “translational and neuroscience studies in affective disorders” section editor, maria nobile MD, PhD. J. Affect. Disord. 2020;263:754–761. doi: 10.1016/j.jad.2019.10.017. [DOI] [PubMed] [Google Scholar]
- 91.Eklund C.M. Proinflammatory cytokines in CRP baseline regulation. Adv. Clin. Chem. 2009;48:111–136. doi: 10.1016/s0065-2423(09)48005-3. [DOI] [PubMed] [Google Scholar]
- 92.Dhabhar F.S., Miller A.H., McEwen B.S., Spencer R.L. Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J. Immunol. 1996;157:1638–1644. [PubMed] [Google Scholar]
- 93.Lemay L.G., Vander A.J., Kluger M.J. The effects of psychological stress on plasma interleukin-6 activity in rats. Physiol. Behav. 1990;47:957–961. doi: 10.1016/0031-9384(90)90024-x. [DOI] [PubMed] [Google Scholar]
- 94.Ruzek M.C., Miller A.H., Opal S.M., Pearce B.D., Biron C.A. Characterization of early cytokine responses and an interleukin (IL)-6–dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J. Exp. Med. 1997;185:1185–1192. doi: 10.1084/jem.185.7.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Turnbull A.V., Rivier C.L. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol. Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 96.Steensberg A., Fischer C.P., Keller C., Møller K., Pedersen B.K. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am. J. Physiol. Endocrinol. Metab. 2003;285:E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- 97.Chrousos G.P. The hypothalamic–pituitary–adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 1995;332:1351–1363. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 98.Elenkov I.J., Chrousos G.P. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol. Metabol. 1999;10:359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 99.Rosenkranz M.A., Davidson R.J., Maccoon D.G., Sheridan J.F., Kalin N.H., Lutz A. A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behav. Immun. 2013;27:174–184. doi: 10.1016/j.bbi.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sibinga E.M.S., Perry-Parrish C., Chung S.E., Johnson S.B., Smith M., Ellen J.M. School-based mindfulness instruction for urban male youth: a small randomized controlled trial. Prev. Med. 2013;57:799–801. doi: 10.1016/j.ypmed.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 101.Jänig W. In: Human Physiology. Schmidt R., Thews G., editors. Springer Berlin Heidelberg; 1989. Autonomic nervous system; pp. 333–370. [Google Scholar]
- 102.Silverman M.N., Sternberg E.M. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cohen S., Janicki-Deverts D., Doyle W.J., Miller G.E., Frank E., Rabin B.S., Turner R.B. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gómez F., Lahmame A., de Kloet R., Armario A. Hypothalamic-pituitary-adrenal response to chronic stress in five inbred rat strains: differential responses are mainly located at the adrenocortical level. Neuroendocrinology. 1996;63:327–337. doi: 10.1159/000126973. [DOI] [PubMed] [Google Scholar]
- 105.Herman J.P., Watson S.J., Spencer R.L. Defense of adrenocorticosteroid receptor expression in rat hippocampus: effects of stress and strain. Endocrinology. 1999;140:3981–3991. doi: 10.1210/endo.140.9.6962. [DOI] [PubMed] [Google Scholar]
- 106.Gray M., Bingham B., Viau V. A comparison of two repeated restraint stress paradigms on hypothalamic-pituitary-adrenal axis habituation, gonadal status and central neuropeptide expression in adult male rats. J. Neuroendocrinol. 2010;22:92–101. doi: 10.1111/j.1365-2826.2009.01941.x. [DOI] [PubMed] [Google Scholar]
- 107.McEwen B.S., Nasca C., Gray J.D. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41:3. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McEwen B.S., Morrison J.H. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.LeDoux J. The amygdala. Curr. Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 110.Canteras N.S. Hypothalamic survival circuits related to social and predatory defenses and their interactions with metabolic control, reproductive behaviors and memory systems. Curr. Opin. Behav. Sci. 2018;24:7–13. [Google Scholar]
- 111.Iwata M., Ota K.T., Duman R.S. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav. Immun. 2013;31:105–114. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tang Y.Y., Holzel B.K., Posner M.I. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015;16:213–225. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- 113.Broyd S.J., Demanuele C., Debener S., Helps S.K., James C.J., Sonuga-Barke E.J. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci. Biobehav. Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 114.Hamilton J.P., Furman D.J., Chang C., Thomason M.E., Dennis E., Gotlib I.H. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol. Psychiatr. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Greicius M.D., Supekar K., Menon V., Dougherty R.F. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebr. Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou H.-X., Chen X., Shen Y.-Q., Li L., Chen N.-X., Zhu Z.-C., Castellanos F.X., Yan C.-G. Rumination and the default mode network: meta-analysis of brain imaging studies and implications for depression. Neuroimage. 2020;206:116287. doi: 10.1016/j.neuroimage.2019.116287. [DOI] [PubMed] [Google Scholar]
- 117.Ramirez-Barrantes R., Arancibia M., Stojanova J., Aspe-Sanchez M., Cordova C., Henriquez-Ch R.A. Default mode network, meditation, and age-associated brain changes: what can we learn from the impact of mental training on well-being as a psychotherapeutic approach? Neural Plast. 2019;2019:7067592. doi: 10.1155/2019/7067592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garrison K.A., Zeffiro T.A., Scheinost D., Constable R.T., Brewer J.A. Meditation leads to reduced default mode network activity beyond an active task. Cognit. Affect Behav. Neurosci. 2015;15:712–720. doi: 10.3758/s13415-015-0358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Simon R., Engstrom M. The default mode network as a biomarker for monitoring the therapeutic effects of meditation. Front. Psychol. 2015;6:776. doi: 10.3389/fpsyg.2015.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Corbetta M., Patel G., Shulman G.L. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leech R., Kamourieh S., Beckmann C.F., Sharp D.J. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J. Neurosci. 2011;31:3217–3224. doi: 10.1523/JNEUROSCI.5626-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. Unit. States Am. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fox K.C.R., Dixon M.L., Nijeboer S., Girn M., Floman J.L., Lifshitz M., Ellamil M., Sedlmeier P., Christoff K. Functional neuroanatomy of meditation: a review and meta-analysis of 78 functional neuroimaging investigations. Neurosci. Biobehav. Rev. 2016;65:208–228. doi: 10.1016/j.neubiorev.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 125.Buckner R.L., Andrews-Hanna J.R., Schacter D.L. 2008. The Brain’s Default Network: Anatomy, Function, and Relevance to Disease. [DOI] [PubMed] [Google Scholar]
- 126.Lebreton M., Jorge S., Michel V., Thirion B., Pessiglione M. An automatic valuation system in the human brain: evidence from functional neuroimaging. Neuron. 2009;64:431–439. doi: 10.1016/j.neuron.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 127.Hansel A., von Kanel R. The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity? Biopsychosoc. Med. 2008;2:21. doi: 10.1186/1751-0759-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wallis J.D. Orbitofrontal cortex and its contribution to decision-making. Annu. Rev. Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- 129.Lou H.C., Luber B., Crupain M., Keenan J.P., Nowak M., Kjaer T.W., Sackeim H.A., Lisanby S.H. Parietal cortex and representation of the mental self. Proc. Natl. Acad. Sci. Unit. States Am. 2004;101:6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fox K.C., Nijeboer S., Solomonova E., Domhoff G.W., Christoff K. Dreaming as mind wandering: evidence from functional neuroimaging and first-person content reports. Front. Hum. Neurosci. 2013;7:412. doi: 10.3389/fnhum.2013.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kang D.-H., Jo H.J., Jung W.H., Kim S.H., Jung Y.-H., Choi C.-H., Lee U.S., An S.C., Jang J.H., Kwon J.S. The effect of meditation on brain structure: cortical thickness mapping and diffusion tensor imaging. Soc. Cognit. Affect Neurosci. 2013;8:27–33. doi: 10.1093/scan/nss056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fox K.C., Spreng R.N., Ellamil M., Andrews-Hanna J.R., Christoff K. The wandering brain: meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage. 2015;111:611–621. doi: 10.1016/j.neuroimage.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 133.Farb N.A., Segal Z.V., Mayberg H., Bean J., McKeon D., Fatima Z., Anderson A.K. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Soc. Cognit. Affect Neurosci. 2007;2:313–322. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lazar S.W., Kerr C.E., Wasserman R.H., Gray J.R., Greve D.N., Treadway M.T., McGarvey M., Quinn B.T., Dusek J.A., Benson H. Meditation experience is associated with increased cortical thickness. Neuroreport. 2005;16:1893. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Blanke O., Ortigue S., Landis T., Seeck M. Stimulating illusory own-body perceptions. Nature. 2002;419:269–270. doi: 10.1038/419269a. [DOI] [PubMed] [Google Scholar]
- 136.Milad M.R., Wright C.I., Orr S.P., Pitman R.K., Quirk G.J., Rauch S.L. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatr. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 137.Immordino-Yang M.H., Singh V. Hippocampal contributions to the processing of social emotions. Hum. Brain Mapp. 2013;34:945–955. doi: 10.1002/hbm.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sheline Y.I. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol. Psychiatr. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- 139.Kasai K., Yamasue H., Gilbertson M.W., Shenton M.E., Rauch S.L., Pitman R.K. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol. Psychiatr. 2008;63:550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Masi G., Brovedani P. The Hippocampus, neurotrophic factors and depression possible implications for the pharmacotherapy of depression. CNS Drugs. 2011;25:913–931. doi: 10.2165/11595900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 141.Gage F.H. Neurogenesis in the adult brain. J. Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jacobs B.L., van Praag H., Gage F.H. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol. Psychiatr. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- 143.Vermetten E., Vythilingam M., Southwick S.M., Charney D.S., Bremner J.D. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol. Psychiatr. 2003;54:693–702. doi: 10.1016/s0006-3223(03)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., Belzung C., Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 145.Hölzel B.K., Carmody J., Vangel M., Congleton C., Yerramsetti S.M., Gard T., Lazar S.W. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. Neuroimaging. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fox K.C., Dixon M.L., Nijeboer S., Girn M., Floman J.L., Lifshitz M., Ellamil M., Sedlmeier P., Christoff K. Functional neuroanatomy of meditation: a review and meta-analysis of 78 functional neuroimaging investigations. Neurosci. Biobehav. Rev. 2016;65:208–228. doi: 10.1016/j.neubiorev.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 147.Treves I.N., Tello L.Y., Davidson R.J., Goldberg S.B. The relationship between mindfulness and objective measures of body awareness: a meta-analysis. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-53978-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lamm C., Decety J., Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 149.Fleming S.M., Dolan R.J. The neural basis of metacognitive ability. Phil. Trans. Biol. Sci. 2012;367:1338–1349. doi: 10.1098/rstb.2011.0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Critchley H.D., Wiens S., Rotshtein P., Öhman A., Dolan R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 151.Avery J.A., Drevets W.C., Moseman S.E., Bodurka J., Barcalow J.C., Simmons W.K. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biol. Psychiatr. 2014;76:258–266. doi: 10.1016/j.biopsych.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sliz D., Hayley S. Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging research. Front. Hum. Neurosci. 2012;6:323. doi: 10.3389/fnhum.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Meuret A.E., Wilhelm F.H., Ritz T., Roth W.T. Breathing training for treating panic disorder. Useful intervention or impediment? Behav. Modif. 2003;27:731–754. doi: 10.1177/0145445503256324. [DOI] [PubMed] [Google Scholar]
- 154.Cvejic E., Lynar E.C., Chung Y.M., Vollmer-Conna U. One size does not fit all: individual differences in cardiac autonomic and subjective responses to brief relaxation activities. Int. J. Cardiol. 2016;223:265–267. doi: 10.1016/j.ijcard.2016.08.252. [DOI] [PubMed] [Google Scholar]
- 155.Anderson T., Farb N.A.S. Personalising practice using preferences for meditation anchor modality. Front. Psychol. 2018;9 doi: 10.3389/fpsyg.2018.02521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ackley B., Ladwig G., Swan B., Tucker S. Mosby Elsevier; 2008. Evidence Based Nursing Care Guidelines. Medical Surgical Interventions. syf 15. [Google Scholar]
- 157.Riley K.E., Park C.L. How does yoga reduce stress? A systematic review of mechanisms of change and guide to future inquiry. Health Psychol. Rev. 2015:1–30. doi: 10.1080/17437199.2014.981778. [DOI] [PubMed] [Google Scholar]
- 158.Keng S.L., Smoski M.J., Robins C.J. Effects of mindfulness on psychological health: a review of empirical studies. Clin. Psychol. Rev. 2011;31:1041–1056. doi: 10.1016/j.cpr.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Campos D., Cebolla A., Quero S., Breton-Lopez J., Botella C., Soler J., Garcia-Campayo J., Demarzo M., Banos R.M. Meditation and happiness: mindfulness and self-compassion may mediate the meditation-happiness relationship. Pers. Indiv. Differ. 2016;93:80–85. [Google Scholar]
- 160.Labelle L.E., Campbell T.S., Carlson L.E. Mindfulness-based stress reduction in oncology: evaluating mindfulness and rumination as mediators of change in depressive symptoms. Mindfulness. 2010;1:28–40. [Google Scholar]
- 161.Baer R.A., Lykins E.L.B., Peters J.R. Mindfulness and self-compassion as predictors of psychological wellbeing in long-term meditators and matched nonmeditators. J. Posit. Psychol. 2012;7:230–238. [Google Scholar]
- 162.Pascoe M.C., Bauer I.E. A systematic review of randomised control trials on the effects of yoga on stress measures and mood. J. Psychiatr. Res. 2015;68:270–282. doi: 10.1016/j.jpsychires.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 163.Pascoe M.C., Thompson D.R., Ski C.F. Yoga, mindfulness-based stress reduction and stress-related physiological measures: a meta-analysis. Psychoneuroendocrinology. 2017;86:152–168. doi: 10.1016/j.psyneuen.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 164.Godfrin K.A., Van Heeringen C. The effects of mindfulness-based cognitive therapy on recurrence of depressive episodes, mental health and quality of life: a randomized controlled study. Behav. Res. Ther. 2010;48:738–746. doi: 10.1016/j.brat.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 165.Piet J., Hougaard E. The effect of mindfulness-based cognitive therapy for prevention of relapse in recurrent major depressive disorder: a systematic review and meta-analysis. Clin. Psychol. Rev. 2011;31:1032–1040. doi: 10.1016/j.cpr.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 166.Williams J.M.G., Alatiq Y., Crane C., Barnhofer T., Fennell M.J., Duggan D., Hepburn S., Goodwin G. Mindfulness-based cognitive therapy (MBCT) in bipolar disorder: preliminary evaluation of immediate effects on between-episode functioning. J. Affect. Disord. 2008;107:275–279. doi: 10.1016/j.jad.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Baer R., Crane C., Miller E., Kuyken W. Doing no harm in mindfulness-based programs: conceptual issues and empirical findings. Clin. Psychol. Rev. 2019;71:101–114. doi: 10.1016/j.cpr.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lindahl J.R., Fisher N.E., Cooper D.J., Rosen R.K., Britton W.B. The varieties of contemplative experience: a mixed-methods study of meditation-related challenges in Western Buddhists. PloS One. 2017;12 doi: 10.1371/journal.pone.0176239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Santorelli S. Center for Mindfulness in Medicine, Health Care & Society; 2014. Mindfulness-Based Stress Reduction (MBSR): Standards of Practice: University of Massachusetts Medical School. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.