Abstract
Preserving islet health and function is critical during pretransplant culture to improve islet transplantation outcome and for ex vivo modeling of diabetes for pharmaceutical drug discovery. The limited islet engraftment potential is primarily attributable to loss of extracellular matrix (ECM) support and interaction. Multipotent cells with ECM depositing competency improve islet survival during short coculture period. However, role of pancreatic stellate cells (PSCs) and their ECM support in preserving ex vivo islet physiology remains largely unknown. Here, we report novel cytoprotective effects of culture-adapted porcine PSCs and role of their ECM-mediated intercellular communication on pig, mouse and human islets ex vivo. Using direct-contact coculture system, we demonstrate that porcine PSCs preserve and significantly prolong islet viability and function from 7 ± 3 days to more than 28 ± 5 (P < .001) days in vitro. These beneficial effects of PSCs on islet health are not species-specific. Using NSC47924 to specifically inhibit 37/67 kDa laminin receptor (LR), we identified that LR-mediated intercellular communication is essential for PSCs to protect functional viability of islets in vitro. Finally, our results demonstrate that PSC co-transplantation improved function and enhanced capacity of syngeneic islets to reverse hyperglycemia in mice with preexisiting diabetes. Cumulatively, our findings unveil novel effects of culture-adapted PSCs on islet health likely mirroring in vivo niche interaction. Furthermore, islet and PSC coculture may aid in development of ex vivo diabetes modeling and also suggests that a combined islet-PSC tissue engineered implant may significantly improve islet transplantation outcome.
Keywords: extracellular matrix, direct coculture, islet transplantation, laminin receptor, pancreatic islets, pancreatic stellate cells, trophic effects, engraftment
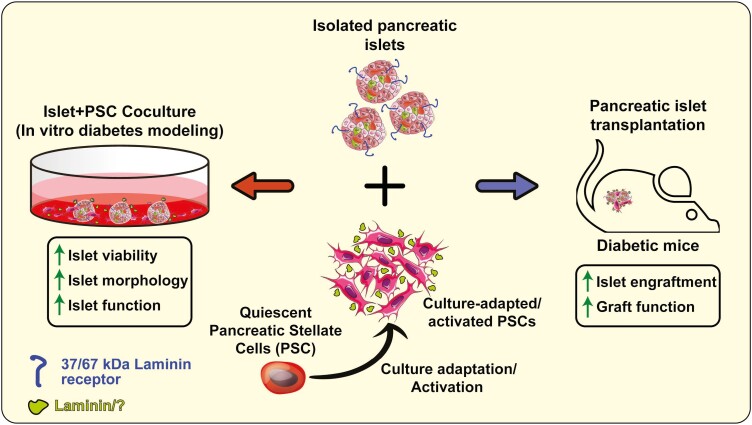
Graphical Abstract
Graphical Abstract.
Significance Statement.
Islet transplantation is the only effective therapy for autoimmune diabetes. However, it remains perfectible due to chronic loss of functional islet grafts. Post-transplant islet viability and function can be improved by reestablishing islet microenvironment. Pancreatic stellate cells (PSCs) are pancreas-resident, mesenchymal stromal cells-like cells that have not been explored for improving graft function. Here, we unveiled a novel niche role of PSCs in maintaining islet functional viability and show that PSCs improve graft function. Our findings suggest significant implication of PSCs in clinical islet transplantation and for ex vivo modeling of diabetes for pharmaceutical drug discovery.
Introduction
Maintaining pancreatic islet viability and function pre- and immediately after transplantation is critical for success of allogeneic islet transplantation therapy. Loss of islet viability during pretransplantation in vitro culture is a major hurdle in transplantation success. Islets suffer various stresses during isolation (enzymatic digestion, loss of vascular support) and in vitro culture (oxidative, hypoxic, nutrient shortage and proinflammatory cytokines).1-3 These stresses contribute to rapid onset of apoptosis and loss of islet mass peri-transplantation. This is a major hurdle in wide adoption of islet allo-transplantation therapy for Type 1 diabetes mellitus.4 Additionally, accelerated loss of islet viability and function prevents development of in vitro disease-in-a-dish models of diabetes and therefore, limits long-term studies on stress-induced beta cell failure. Such models will also enable testing of novel drug therapies targeting islet cell function and comparison of healthy vs disease donor-derived beta cells to understand the genetic component of diabetes. While significant effort has been directed toward identification and development of culture conditions to improve islet viability and function in vitro and consequently prevent islet loss to improve transplantation success rate,5-8 most of these studies have used marrow- or adipose-derived mesenchymal stromal cells (MSCs) as feeder cells. Surprisingly, very little attention has been paid to pancreatic stellate cells (PSCs)—the resident stromal cells of pancreas—for supporting islet health. Similarly, the feeder cell extracellular matrix (ECM)/islet interaction and its trophic effects also remain poorly understood.9-11
Pancreatic stellate cells are tissue endogenous mesodermal stromal cells of the pancreas. Pancreatic stellate cells constitute a small compartment (4%) in exocrine pancreas and reside in the periacinar, perivascular and periductal space of the pancreas.12,13 Due to the distinct organization of the pancreas, PSCs do not have comparable function (assisting in nutrient supply and blood flow, providing architectural support or mitigating inflammatory response) in pancreas as other stromal cells.14 Pancreatic stellate cells are primarily quiescent and contribute to the maintenance of the pancreatic peri-insular basement membrane (BM).15 Quiescent PSCs (qPSC) express several intermediate filament proteins such as desmin, glial fibrillary acidic protein (GFAP), vimentin and nestin.12,13,16 Expression of these proteins suggests diverse potential properties of PSCs including the capacity to secrete ECM. Primary PSCs isolated from healthy pancreas express desmin and GFAP, but lack α-Smooth Muscle Actin (α-SMA).16 In response to injury, damage or culture adaptation, PSCs activate and rapidly proliferate.16,17 Culture adapted PSCs (aPSC) undergo morphologic and cytologic changes (enlarged nuclei, myofibroblastic phenotype), α-SMA expression and ECM production akin to endogenous cells playing a central role in tissue fibrosis of chronic pancreatitis and supportive of pancreatic ductal adenocarcinoma (PDAC).18-20 However, fibrotic and nonfibrotic PSC activation is not equivalent.21 Culture adapted PSCs produce various ECM proteins in response to platelet-derived growth factor,22 secrete matrix metalloproteinases,23 and respond to high glucose via the renin-angiotensin system.24 Collectively, these reports strongly suggest that PSCs play an important role in BM production. However, the influence of qPSC or aPSC on BM maintenance and ECM production in homeostatic or disease conditions remains to be elucidated.
Similarities to stromal cells hint that PSCs may have comparable effect as MSCs on islet health pre- and post-transplantation.25-28 In addition, PSC-produced ECM and gradually emerging significance of ECM/islet interaction in islet survival, insulin secretion, proliferation, restoration and maintenance of islet morphology29-32 underscore the importance of ECM in islet homeostasis and BM support. Among other ECM proteins, Laminin (LM) is the primary component of both peri-islet BM33 and peripheral ECM.34 More recent findings suggest that laminins may be involved in regulation of vital cellular functions in islets.30 Despite new discoveries and growing interest in this field, islet-PSC interaction and the role of PSC-produced ECM components in intercellular communication with islets is largely unknown.
In this study, we tested the hypothesis that PSCs protect isolated islets during in vitro culture and demonstrate that direct contact coculture (IPCt) is necessary for PSCs to maintain islet viability and secretory functions. We developed an islet/PSC coculture system to evaluate the potential for PSCs to impact islet viability and function. We also identified that direct contact coculture is indispensable for PSC-promoted islet protection. Our data also unveils that laminin receptor (LR) interaction is crucial for PSC-mediated effects on islet health. Finally, we demonstrate that co-transplantation of PSCs expedites recovery of in vitro cultured islets and significantly improves islet graft function and augment transplantation outcomes.
Research Design and Methods
Islet Isolation and Culture
Juvenile porcine islets were purchased from University of California-Irvine and cultured according to previously described protocol.35 Mouse islets were isolated from male 12-16-week-old C57BL/6J (Jackson Laboratory, USA) as described previously.36 Human islets were obtained from the Clinical Islet Cell Laboratory, isolated according to previously described protocols.37 Islet preparations (75%-90% purity as assessed by dithizone staining) from 4 donors were received within 48 h of pancreas harvest from deceased donors.
Islets were cultured (37 °C, 5% CO2) in RPMI-1640 medium (Corning, USA) with 10% FBS (Gibco, USA) and 1% antibiotic--antimycotic (ThermoFisher, #15240096) for indicated duration or overnight before coculture with PSCs. Islet-PSC coculture was maintained in a 1:1 mix of complete RPMI and DMEM F-12 (RDmix) media.
Cell Culture with Laminin Proteins and Integrin/Laminin-receptor Blockade
Nontreated six-well plates (Nunc, Thermo Fisher, USA) were coated overnight at 4 °C with sterile LM-111 (#L2020, Sigma Aldrich, USA) or LM-511 (BioLamina AG, Sweden) at 2.5 μg/cm2. Islets were cultured in laminin-coated or noncoated plates in RPMI medium with or without laminin (10 μg/mL).
For integrin or LR blockade, PSC-islet cocultures were pretreated for 15 minutes in RDmix medium with 1% FBS at room temperature and then incubated in 10% FBS with RGDS peptide (all integrins, Tocris #3498, 100 μM) or NSC47924 (37/67kDa LR inhibitor, Focus Biomolecules, # 10-1562-0005, 20 μM).
Isolation and Characterization of PSC and MSC Populations
Pancreatic stellate cells were isolated from adult male Yorkshire pig pancreas obtained from the UW Swine Research Center, Arlington, WI using a modified protocol. Briefly, pancreatic tissue from duodenal lobe was resected and minced in cold GBSS solution followed by enzymatic digestion as described previously.38 Following digestion, PSCs were enriched by density gradient centrifugation (OptiPrep, #D1556, SigmaAldrich, USA) as described previously.12 PSCs were cultured in DMEM-F12 medium (Corning, USA) supplemented with 10% FBS and 1% AA. Mouse bone marrow MSCs were obtained by flushing the femurs and tibiae of 3-6-month old C57BL/6J mice in aseptic conditions and cultured as described previously.39 Human adipose MSCs were collected from deidentified, consenting individuals upon UW-Madison Institutional Review Board approval (IRB# 2016-1545).
Coculture of Pancreatic Islets with PSC
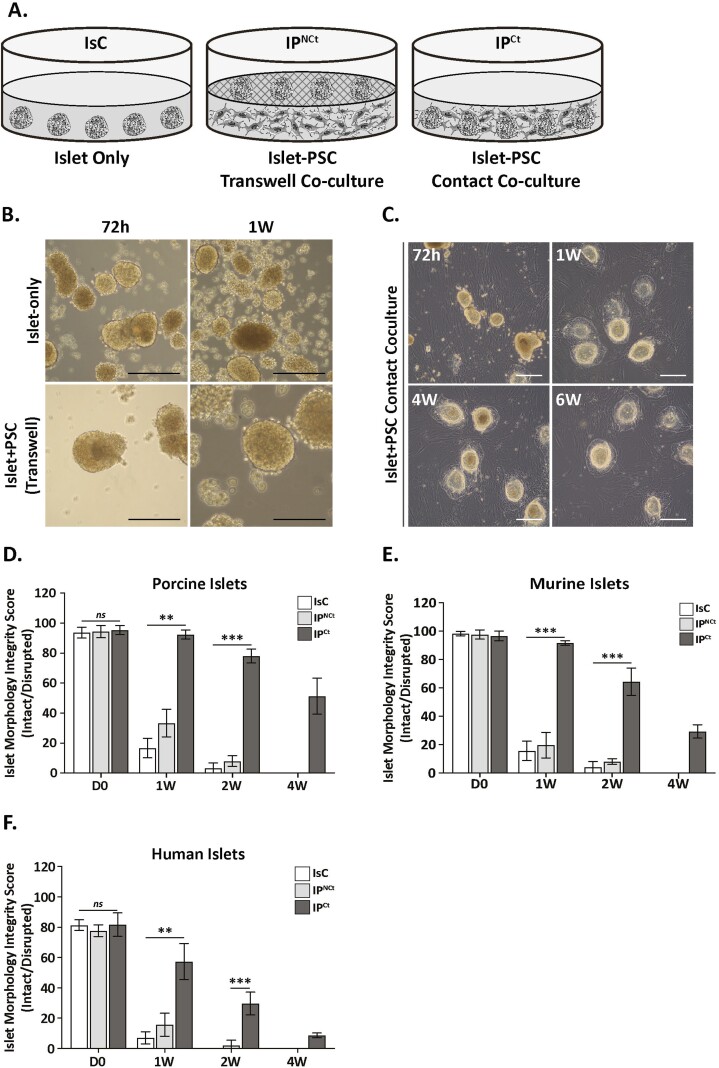
Pancreatic stellate cells (passage 2/3) or MSC (passage 6/7) cells were plated at a density of 5 × 103/cm2 on 12-well culture plates and cultured for 16-24 h prior to coculture with islets. Pancreatic islets (50 islets) were cultured overnight and then placed on top of the PSC or MSC culture, with or without Transwell inserts (Corning Inc., New York, USA). Pancreatic stellate cells were cocultured with islets in 3 configurations: islets only (Fig. 1A, IsC); islets in indirect contact with PSCs (IPNCt); and islets cultured directly with PSCs (IPCt) and assayed at indicated time points.
Figure 1.
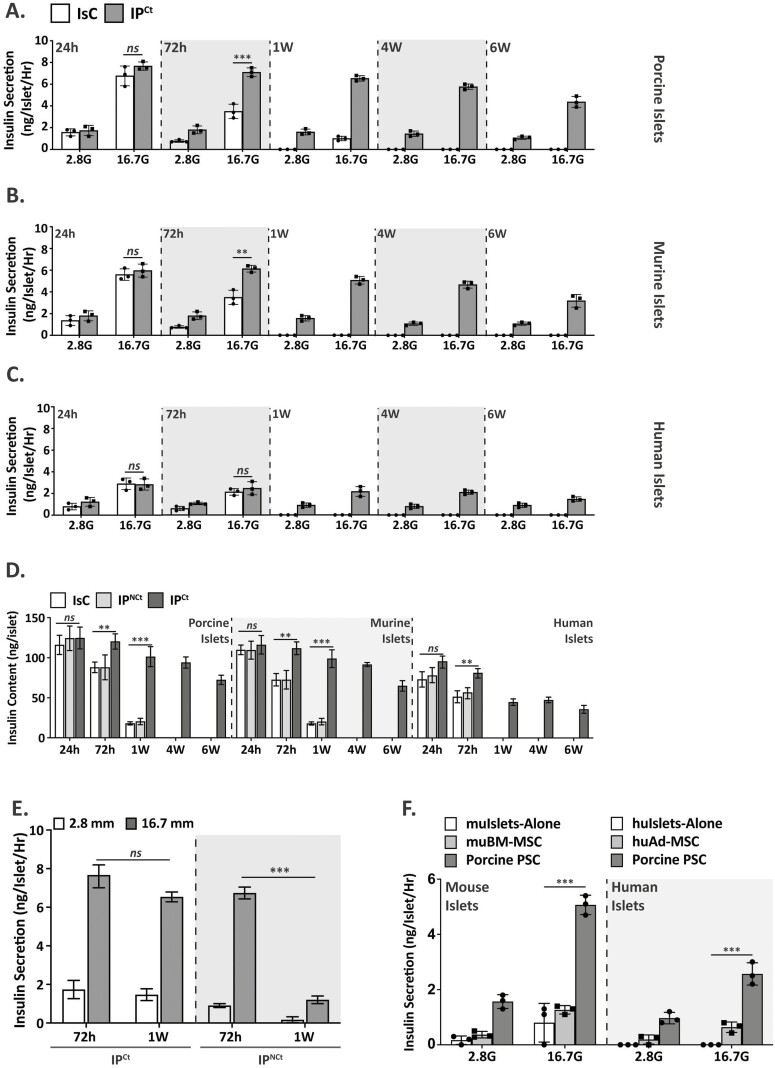
Pancreatic stellate cells maintain islet morphology integrity in direct contact coculture. (A). Schematic of different coculture configurations of islets with PSCs. (Left to right), 3 experimental groups were established to examine the effects of PSC on islet viability and function: IsC (islet-only control), IPNCt (islet-PSC indirect contact coculture) and IPCt (islet-PSC direct coculture). In both indirect (IPNCt) and direct (IPCt) culture systems, porcine PSCs were plated and cultured in monolayer and islets were added either directly or separated from PSCs in transwell inserts, allowing sharing of soluble factors. Bright-field images of porcine islets cultured alone or in transwell coculture with PSC at 72 h or 1 week (B) and in direct coculture with PSCs at 72 h, 1 wk, 4 wk, and 6 wk (C) (scale bar = 100 µm). Direct coculture with PSCs preserved porcine, murine and human islet morphology up to 6 weeks compared to islets cultured alone or in transwell system. (D)-(F) Morphologic integrity score represents percent of islets with intact morphology (well-rounded border and unfragmented) cultured alone (IsC, white bars), indirect (IPNCt, gray bars) or direct coculture (IPCt, dark bars). Morphology was preserved in vitro significantly longer in porcine, mouse and human islets only in IPCt group. Islets were imaged weekly and counted (optical magnification 10X). Data are presented as the mean ± SEM (n = 3, 50 islets per test group, **P < .01, ***P < .001 vs. IPCt).
Analysis of Islet Morphology and Viability
Morphologic examinations of cultured islets were conducted by using a Zeiss AX10 inverted microscope equipped with a Zeiss Axiocam 305 color camera. A morphologic score was assigned on the basis of shape (irregular vs. spherical) and integrity (fragmented vs. compact). Each islet was scored semi-quantitatively and assigned a score of 0 (extensive fragmentation, highly irregular shape with extensive blebbing on surface), 25 (significant fragmentation and roughness on surface), 50 (noticeable fragmentation and some loss of surface sphericity), 75 (minor fragmentation and negligible surface roughness) to 100 (no fragmentation, normal spherical shape) by 2 blinded individuals. This method was modified from a previously described scoring system.25
Islet viability was assessed using fluorescein diacetate (FDA, green) for live cells and propidium iodide (PI, red) to identify dead cells. A solution of FDA and PI (both at 0.1 mg/mL) was added directly to culture wells and incubated for 5 minutes before examination under a confocal microscope (Nikon A1R; Nikon, USA). Islet viability was calculated as percentage of FDA-positive and PI-negative islets at indicated times.
Quantitative Real-Time PCR
Total RNA was prepared using RNeasy total RNA preparation kit (Qiagen, USA). Complementary DNA was synthesized using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). Quantitative real-time PCR was performed using Taq PCR Master Mix (Qiagen, USA) and detected using the Bio-Rad PCR Detection System (Bio-Rad, USA). Primers were designed using IDT PrimerQuest primer design tool (See Supplementary Table S1). PCR was performed at 95 °C for 20 s, followed by 40 cycles of 95 °C for 1 s and 60 °C for 20 s. Results are expressed as fold change relative to the control sample (freshly isolated PSCs) for copy numbers of each mRNA. The values of the target mRNA were normalized to GAPDH mRNA.
Immunocytochemical Staining and Confocal Imaging
Functional islets in all groups were detected using insulin immunocytochemistry. IsC or IPCt islets were cultured on glass coverslips (Electron Microscopy Sciences 72290-02 and 72291-01) for immunofluorescence staining. Cells were fixed in 4% paraformaldehyde for 20 minutes, washed with PBS containing 0.3% Triton X-100 followed by incubation with a rabbit anti-insulin antibody (1:1000; Immunostar, USA), mouse anti-α-SMA antibody (1:500; Novus Biologicals, USA), rabbit anti-GFAP antibody (1:500; Proteintech, USA), rabbit anti-glucagon antibody (1:400; Cell Signaling Technology, USA) or rabbit anti-Pdx antibody (1:500; Cell Signaling Technology, USA). Nuclei were counterstained with ProLong Diamond Antifade Mountant (#P36970, ThermoFisher, USA).
Western Blot
Protein samples for western blotting were isolated from culture-adapted PSCs by homogenization with lysis buffer (#9803, CST, USA). The samples were boiled in laemmli buffer (#161-0737, Bio-Rad, USA) for 5 min and were resolved on a 4%-12% gradient SDS-PAGE gel and blotted to PVDF membrane. Following overnight incubation with primary antibodies against α-SMA (1:1000; #NBP2-33006, Novus Biologicals, USA), GFAP (1:1000; #60190-1-Ig, Proteintech, USA) and beta-actin (1:1000, # NB600-503, Novus Biologicals, USA) and detection by HRP-conjugated IgG. Bands were visualized using an Azure 300 chemiluminescent imaging system (Azure Biosystems, USA).
Islet Function In Vitro
Islet function was evaluated by measuring glucose-stimulated insulin secretion (GSIS) from groups (50) of size-matched islets (cultured with or without PSC/MSC, in 48-well plates) in response to glucose challenge. Islets were washed with KRB buffer, followed by pre-incubation in glucose-free KRB buffer for 30 minutes. Static insulin secretion was measured by incubating islets in media with basal (2.8 mM or 2.8 G) or stimulatory (16.7 mM or 16.7 G) glucose for 2 h each. The supernatant was collected for insulin assay. Intracellular insulin content was determined by harvesting islets, rinsing with PBS, resuspending in 300 μL acid ethanol and homogenizing by ultrasonic disruption of the cell membrane. Insulin was measured using a porcine, mouse or human insulin ELISA kit (Mercodia, Uppsala, Sweden) according to the manufacturer’s protocol. Secretion in islets was normalized to the number of islets.
Islet Function In Vivo
Animal studies were performed in accordance with University of Wisconsin-Madison Institutional Animal Care and Use Committee under approved protocols.
Mice were randomly designated for STZ treatment and transplantation groups. Mouse number per group was selected to allow for statistical significance (n = 9 and 15). Surgical procedures and follow-up studies were performed by unblinded individuals. Male 8-week-old C57B6/j mice were purchased from The Jackson Laboratory, rendered diabetic with STZ injection (45 mg/kg; R&D systems) for 5 days, with diabetes confirmed after 7 days. Diabetic mice were randomly divided into 3 groups, that received PSCs only (n = 8), islets only (n = 10) or islet + PSC (n = 10).
Anaesthetized mice were transplanted with ~150 hand-picked, size-matched islets (in vitro cultured for 72 h), mixed with 1 × 106 culture-adapted PSCs (passage 3 or 4) or normal saline under the kidney capsule. Blood glucose was measured with a Contour Blood Glucose Monitoring System (Bayer). Glucose tolerance and in vivo GSIS assays were performed by fasting mice for 4 h and injecting with glucose (2 g/kg). Serum insulin were quantified using ELISA kits (#10-1247-01) following manufacturer’s instructions (Mercodia, Uppsala, Sweden).
Statistical Analysis
Differences between treatments were assessed initially using 2-way analysis of variance with post hoc testing using Bonferroni or unpaired Student’s t test, as appropriate, and considered significant when p < .05. The number of biological and/or technical replicates are indicated in the figure legends.
Results
Culture-adapted PSCs Preserve Islet Morphology and Prolong Survival in Direct-contact Coculture
We started our study by determining the identity of culture adapted porcine PSCs. We assessed PSC expression of signature genes desmin, GFAP, CD133, vimetin, nestin and α-SMA. Analysis of gene expression in freshly isolated PSCs (P0) and culture-adapted PSCs (at different passages P1, P2, and P3) by quantitative PCR (qPCR) revealed that expression of desmin, GFAP and CD133 was repressed (~0.5, ~0.5 and ~0.3-fold respectively), while vimentin, nestin and a-SMA expression was significantly induced by ~4, ~2 and ~4000-fold, respectively (Supplementary Fig. 1A). Immunofluorescence staining and Western blot analysis of culture-activated PSCs confirmed their robust expression of myofibroblast marker α-SMA as well as detectable expression of GFAP (Supplementary Fig. 1B, C). Next, we determined the effects of PSC coculture on islets. Isolated porcine or mouse islets were cultured with porcine PSCs in 3 different configurations to identify the most suitable coculture system (Fig. 1A). Control porcine islets were cultured alone (IsC) in the standard culture conditions. In IPNCt configurations porcine islets were cultured in a Transwell insert to prevent islet-PSC contact. In IPCt configuration islets were cultured with PSCs in direct contact. IsC islets started showing signs of fragmentation after 72 h of culture and exhibited widespread disaggregation of islets in 7 days of in vitro culture (Fig. 1B). In IPNCt configuration, although islets did not demonstrate disrupted morphology as early as in IsC configuration, within 1 week of in vitro culture, a majority of large islets fragmented and only small IPNCt islets remained intact. In contrast, morphologic integrity of IPCt islets was preserved up to 4 weeks without demonstrating substantial fragmentation or disaggregation (Fig. 1C, D-F) and started to decline after ~4 weeks in culture. Morphologic integrity scores (MIS) for porcine, murine and human islets show that islets in IsC or IPNCt configurations suffered a significantly higher loss of morphology (>90%, MIS <10) within 2 weeks and were virtually absent by 4 weeks (MIS = 0). In contrast, the majority of islets (>80%) cocultured with PSC retained their morphologic integrity (MIS > 85) at 2 weeks with a sustained morphology (MIS ~40) at 4 weeks for porcine and mouse islets (p < .001) (Fig. 1D and E). The effect of direct PSC coculture was equally pronounced on human islets (MIS 60 vs. 20 in IPCt vs IPNCt or IsC configurations); however, MIS of human islets was always lower compared to porcine or murine islets. While 60% of porcine and 30% of murine islets remained intact up to 4 weeks in coculture with porcine PSCs, human islets suffered fragmentation and only 10% remained intact after the same culture duration (Fig. 1D-F).
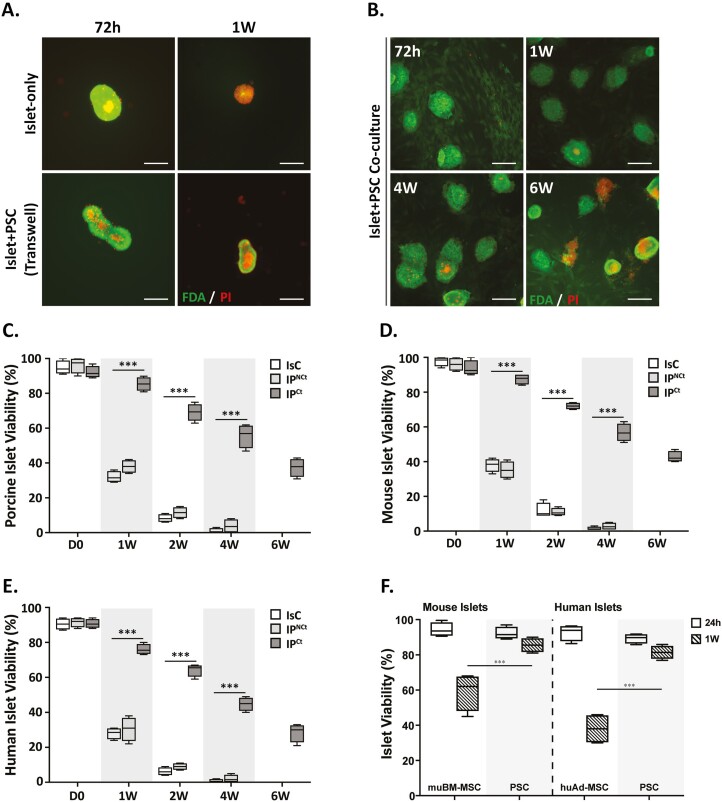
Next, we assessed the effect of PSC coculture on islet viability by Fluorescein diacetate and PI staining. Conversion of FDA into a green fluorescent metabolite is esterase-dependent, therefore, FDA can only label viable cells in normal physiologic state. In contrast, PI, a DNA-intercalating dye, can only pass though membranes of dead or dying cells. Viable cells fluoresced bright green whereas nonviable cells were marked by the presence of punctate bright red stains. Porcine islets cultured in IsC and IPNCt configurations started demonstrating nonviable cells as early as 3 days in culture. Initially, the nonviable cells appeared more at the center of the islet, but within a week, distributed evenly in entire islet (Fig. 2A). However, islets cocultured with PSCs (IPCt), demonstrated very few nonviable cells per islet for up to 4 weeks in coculture (Fig. 2B). Based on FDA/PI staining from 4 independent experiments, we calculated viable and nonviable islet proportions. Weekly viable islet assessment at indicated intervals showed that in IPCt coculture configuration 85.5% (± 3.9 SD) of porcine islets survived at 1 week, decreasing to 69.25% (± 4.9 SD) at 2 weeks and then slowly decreasing to 37.5% (± 5.2 SD) at 6 weeks. In contrast, islet viability in IsC and IPNCt configurations quickly reduced to 32% (± 3.2 SD) and 38% (± 3.6 SD) at 1 week, respectively, and decreased to <1% and 4% in 4 weeks (Fig. 2C). By the endpoint of the study (6 weeks), none of the islets in IsC and IPNCt groups survived. Interestingly, porcine PSC coculture also extended murine and human islets viability (Fig. 2D-E), suggesting that beneficial effects of coculture are not species-specific. Interestingly, respective MSCs (bone marrow derived from mouse and adipose-derived from human donors) failed to preserve islet viability as effectively as PSCs for mouse and human islets (Fig. 2F).Together, these data demonstrate that porcine PSCs preserved islet morphology and prolonged islet survival and these effects are not restricted to syngeneic interactions. Additionally, morphology and survival data established that IPNCt coculture failed to improve islet health or function compared to IsC islets, therefore, subsequent experiments to further define islet-PSC interaction focused on IsC and IPCt groups only.
Figure 2.
Direct contact coculture of PSCs significantly prolongs islet viability in vitro. (A, B) Viability assessment by fluorescent imaging of porcine islets stained with FDA (green) and PI (red) after culture for 72 h, 1 wk, 4 wk and 6 wk in different configurations. Diffused green stain indicates live cells and bright red spots show dead cells (scale bar = 100 μm). Quantification of viable porcine islets (C), murine islets (D) and human islets (E) in IsC (white bars), IPNCt (gray bars) or IPCt configurations (dark bars) show significantly higher islet viability in IPCt group (n = 4, ***P < .0001 vs. IPCt). (F) Islet viability measured by FDA/PI staining after 24 h and 1 week of coculture with bone marrow-derived mouse MSCs (mouse islets) or adipose-derived human MSCs (human islets) compared with porcine PSCs (***P < .001 PSC vs. mouse BM-MSCs or human adipose-MSC at 1 week). Data are presented as mean ± SEM.
Pancreatic Stellate Cells Maintain Islet Cell Insulin, Glucagon and Pdx1 Expression in Long-term IPCt Coculture System
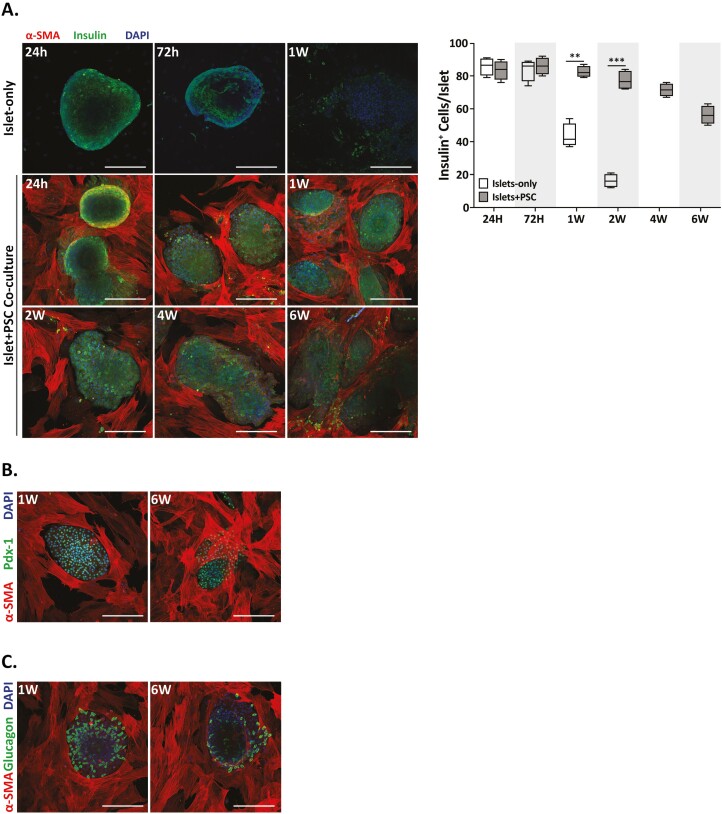
Next, we determined the effect of culture-adapted PSC on the expression of hallmark genes that determine endocrine phenotype of islet cells. We detected insulin in porcine islets in IsC and IPCt configuration. However, more than half of the cells in IsC islets lost insulin expression within 1 week of culture and by 4 weeks there were no insulin-positive cells remaining. In contrast, PSC coculture preserved insulin expression in 70% of islets for 4 weeks in culture and 55% by sixth week in culture (Fig. 3A). Similarly, IPCt islets stained positive for Pdx-1 and Glucagon after 1 week and 6 week of coculture (Fig. 3B, C). These data clearly demonstrate that culture adapted PSCs support islet health by preserving islet cell endocrine phenotype for significantly longer duration of in vitro culture.
Figure 3.
Activated PSCs preserve islet cell insulin, glucagon and Pdx1 expression in long-term in vitro culture in IPCt configuration. Confocal images (20×, scale bar = 100 μm) of islets cultured with or without PSCs. (A) Insulin immunofluorescence (green) and α-SMA expression (red) mark islet beta cells and aPSCs, respectively. Pancreatic stellate cell coculture preserves islet insulin expression significantly longer (6 wk) compared to islets cultured alone (1 wk). Insulin and DAPI expression was used to calculate number of beta cells per islet (A, right panel). Pancreatic stellate cell coculture (dark bars) protected beta cell insulin expression ~4× longer than islets cultured alone (white bars). Islet cell expression (B). Pdx-1 (green) and (C). Glucagon (green) demonstrate preserved islet cell phenotype of all endocrine and α-cells, respectively, when cocultured with PSCs (α-SMA+). All data are presented as the mean ± SEM (n = 4, **P < .001, ***P < .0001 vs. islet only).
Pancreatic stellate cells Coculture Preserves Islet Insulin Secretory Function
The effects of PSC coculture on beta cell function are summarized in Fig. 4. GSIS experiments measured beta cell function at basal (2.8G) and stimulatory (16.7G) glucose concentrations in IsC vs IPCt islets cultured in vitro for different durations (24 h, 72 h, 1wk, 4wk and 6wk). Figure 4A-C show that PSC coculture preserved beta cell function significantly longer than control islets. Porcine, murine and human islets responded to stimulatory glucose concentration for up to 6 weeks of culture without showing a significant decrease at intermediate time points. In contrast, control islets lost glucose responsiveness dramatically by the end of first week. Total insulin content in IsC, IPNCt and IPCt islets was comparable at 24 h; however, both IsC and IPNCt islets lost insulin content rapidly and significantly at 1 week (or longer) of in vitro culture (Fig. 4D) compared to IPCt islets. Next, we compared the effect of direct (IPCt) versus indirect (IPNCt) coculture on islet function. During 1 week of culture, IPCt islets remained responsive to stimulatory glucose concentrations, whereas IPNCt islets secreted significantly reduced insulin (6.5 ng/islet/h vs. 1.2 ng/islet/h) (Fig. 4E). Furthermore, we interrogated whether the protective effects of PSC are comparable to that of marrow- or adipose-derived MSCs. To determine this, we cocultured mouse or human islets with mouse bone marrow-derived MSCs or human adipose-MSCs respectively. GSIS data shows that in comparison to porcine PSCs, MSC-cocultured mouse or human islets lost function and secreted significantly less insulin at stimulatory concentrations (1.3 ng/islet/h vs. 5.1 ng/islet/h for mouse islets and 0.6 ng/islet/h vs. 2.6 ng/islet/h for human islets, Fig. 4F). Cumulatively, our data show that PSC coculture significantly preserves beta cell function and exceeds the beneficial effects of marrow or adipose-derived MSCs on islet function and viability.
Figure 4.
Direct contact PSC coculture protects islet function (A). Islets were cultured alone (IsC, white bars) for 24 h, 72 h and 1 wk or cocultured with PSCs (IPCt, gray bars) for 24 h, 72 h, 1 wk, 4 wk, or 6 wk prior to insulin secretion assay. Data are presented as secreted insulin (ng)/islet/h at low (2.8G) and high (16.7G) glucose concentration for porcine (A), mouse (B) and human (C) islets (n = 3). (D) Comparison of porcine, murine and human islet insulin content in different configurations at indicated time points. (E) Comparison of GSIS by islets in IPCt versus IPNCt at basal (2.8G) and high (16.7G) glucose concentration showed preserved function at 1 wk in IPCt islets vs IPNCt islets. (F) Compared to PSCs, syngeneic MSCs (mouse bone marrow-derived or human adipose-derived, white bars) fail to preserve islet function after 1 wk in coculture and did not show significant improvement over islets cultured alone. Data are presented as mean ± SEM. (n = 3, *P < .05, **P < .01, ***P < .001 vs. respective controls).
37/67 kDa LR Inhibitor Abrogates Protective Effects of PSC
Endogenous PSCs likely contribute to regulation of in vivo islet survival and function.40 Interestingly, both qPSC and aPSC are capable of establishing major ECM components of the islet BM41 suggesting that PSCs, via their ECM, may have more elaborate involvement in regulation of islet physiology.
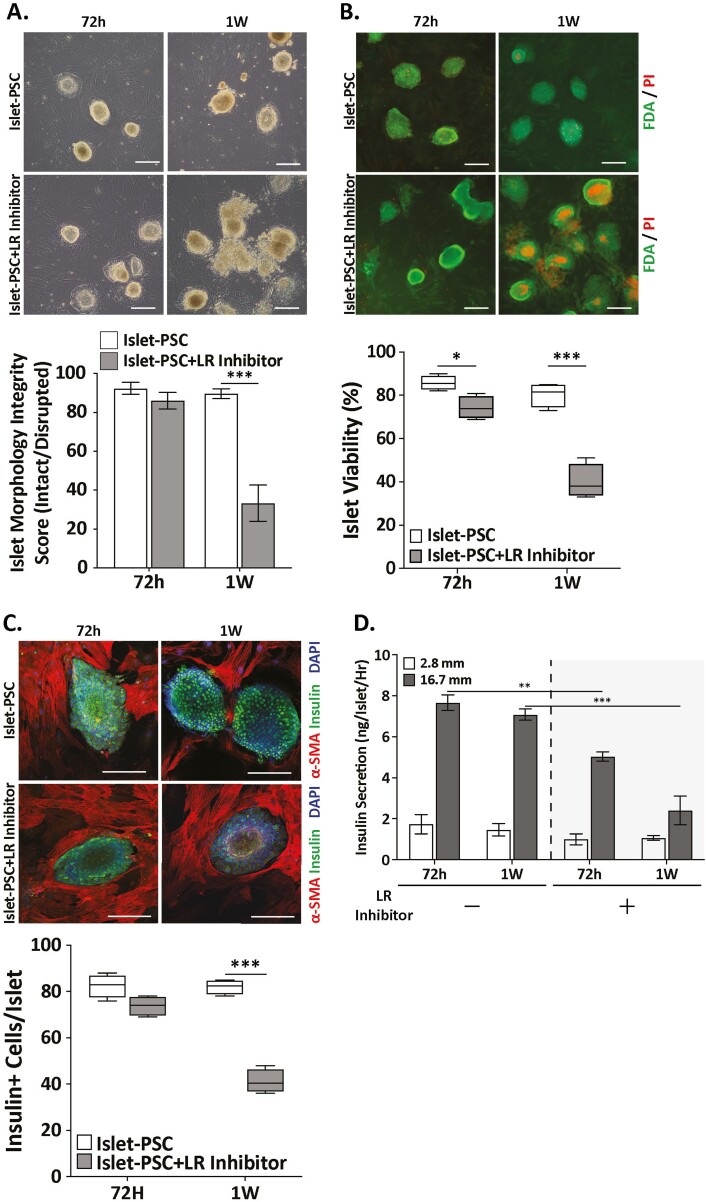
A primary constituent islet BM, laminins are extracellular molecules that bind to both integrin (IR) and nonintegrin receptors. We asked whether laminins are the mediators of PSC-mediated protection of islet viability and function and hypothesized that blocking integrin or 37/67 kDa laminin receptor (LR) will abrogate the beneficial effect of PSC coculture on islets. Interestingly, IR blockade with 100 μM RGDS pan-IR inhibitor peptide did not influence islet survival or function (Supplementary Fig. S2). However, inhibition of 37/67 kDa LR with a highly specific LR inhibitor showed a profound effect on islet health in PSC coculture. Laminin receptor inhibitor (20 μM) was added to the PSC monolayer before addition of islets. In the presence of LR inhibitor, porcine islets showed fragmentation within 1 week compared to vehicle-treated islet-PSC controls (Fig. 5A). At 1 week, morphological integrity of LR inhibitor-treated islets reduced to <40% while ~90% of vehicle-treated control islets remained morphologically intact. Similarly, LR inhibitor treatment caused significant loss in islet viability (~40% vs. ~85% in control) during 1 week of coculture (Fig. 5B). A concomitant loss in islet beta cell insulin expression was also observed in response to LR inhibition. Compared to ~80% insulin-positive beta cells in vehicle-treated control islets, only ~50% of cells expressed insulin in treated islets (Fig. 5C). Last, we also assessed effect of LR inhibitor on insulin secretory function of cocultured islets. While change in insulin secretion at basal glucose concentration (2.8G) was not significantly different, LR inhibitor-treated islets demonstrated an early (at 72 h) and significant reduction (~50%) in insulin secretion at 16.7 G (Fig. 5D). It is important to note here that the loss of insulin expression or reduced secretory function of islets may have resulted from rapid loss of islet morphology and viability and therefore, can be interpreted as a secondary effect of LR inhibition.
Figure 5.
Laminin-receptor interaction is required for PSC-promoted prolonged islet viability and function. (A) Bright-field images (10×, scale bar = 100 μm)of islet-PSC coculture in presence of laminin-receptor (LR) inhibitor NSC47924 for 72 h and 1 wk. The box-plot compares morphological integrity of islets in contact coculture with PSCs in presence or absence of LR-inhibitor. (B) FDA (green) and PI (red) staining (10×, scale bar = 100 μm) demonstrate diminished viability in LR-inhibitor-treated islet (gray bars) compared to vehicle-treated islets (white bars) in IPCt PSC coculture. (C) Representative confocal images (20×, scale bar = 100 μm) showing effects of LR-inhibitor treatment on insulin expression (green) in islets cocultured with PSCs. The box-plot on the right contrasts beta cell insulin expression in response to LR inhibition (gray bars) versus control (white bars) in islets cocultured with PSCs for 72 h or 1 wk. (D) Detrimental effect of inhibited LR interaction on islet function. Data compares GSIS at basal (2.8 mM) and high (16.7 mM) glucose concentrations from islets cocultured with PSCs for 72 h and 1 wk without or with LR-inhibitor. Images in panels A-C Data are presented as mean ± SEM (n = 3, *P < .01, **P < .001 vs. control).
These data warranted further investigation on the sufficiency of laminins for maintenance of islet health or function in vitro. To address this, porcine islets were cultured either in medium supplemented with 10 μg/mL laminins (LM-111 or LM-511) or plated on surface coated with LM-111 or LM-511. We found that both laminins were insufficient to support islet health. Islets lost morphology and started dispersing into a heterogenous monolayer of cells within 72 h (Supplementary Fig. S3A, B). Furthermore, LM111 or LM511 supplementation of media did not affect porcine islet GSIS (Supplementary Fig. S3C). We also investigated effect of LR-inhibitor on islets and PSCs. Our data demonstrate that at physiologically relevant concentrations LR-inhibitor has no detrimental effects on islet function (Supplementary Fig. S3D) or PSCs viability (Supplementary Fig. S4A, B). These data strongly suggest that inhibition of LR abrogates the beneficial effects of PSC coculture and highlight that LR-mediated interaction between islets and PSCs is necessary yet requires complementation with unidentified factors for full effect.
Co-transplantation of PSCs Improves Islet Graft Function and Plasma Insulin Levels
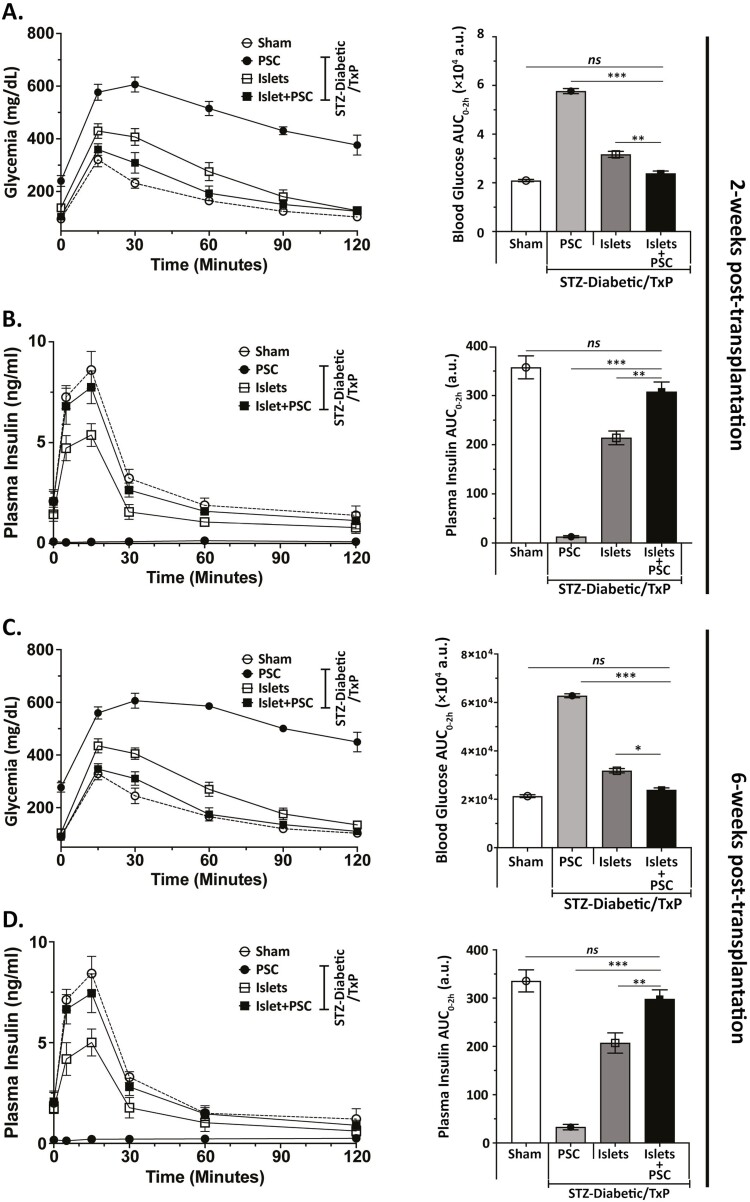
Our in vitro data unveiled a spectrum of cytoprotective effects of PSCs on islets. Based on these, we hypothesized that PSC co-transplantation can potentially improve islet graft function. To test this hypothesis, we transplanted syngeneic islets alone or with 1 × 106 murine PSCs into the renal subcapsular space of C57BL6/J mice with streptozotocin (STZ)-induced diabetes. A marginal mass (~150) of hand-picked size-matched islets were cultured for 72 h before transplantation to simulate the obligate culture period in clinical islet transplant setting. Figure 6 summarizes the outcomes of islet transplantation with or without PSCs. Average blood glucose level for the islet + PSC group was slightly, but significantly lower than islet-alone group in the first 2 weeks (data not shown). This observation suggested a possible in vivo protection of islet function by PSCs and prompted us to perform oral glucose tolerance test (oGTT) on the graft recipients. Both groups (islets alone and islets + PSCs) showed reversal of hyperglycemia by day 14 post-transplant. However, glucose levels (both blood concentration and oGTT AUC) in islet + PSC group were significantly lower than islet-alone group at time points between 10 and 90 minutes (Fig. 6A). Interestingly, glycemia was indistinguishable between islet + PSC and nondiabetic (sham treated) groups of mice. Corresponding plasma insulin levels in islet + PSC group were significantly higher than islet-alone group (Fig. 6B). An oGTT at 6 weeks post-transplant mirrored the previous data at 2 weeks and suggested that under glucose metabolic stress, co-transplanted PSCs stably improved glycemia and insulin secretory function by islet grafts (Fig. 6C, D). Summarized data from post-transplant blood glucose measurements in transplant recipient and sham groups demonstrates an improved kinetics of glycemic recovery in islet + PSC co-transplant group compared to only islet-recipient group (Supplementary Fig. S5A). To corroborate our in vivo findings, we also validated these effects (preserved viability and function) in vitro. Our data suggest that murine PSCs are equally competent in protecting islet viability and function in vitro (Supplementary Fig. S5B, C).
Figure 6.
Co-transplantation of culture-adapted mouse PSC improved function of islet grafts. Blood glucose and plasma insulin measurements in STZ-diabetic mice treatment after transplantation with islets or islet + PSCs. Four groups were studied: vehicle treated nondiabetic mice without a transplant (No STZ, No Txp; n = 5; dotted line), diabetic mice transplanted with PSCs (STZ, Txp; 1 × 106 cells; n = 7; filled circles), diabetic mice transplanted with islets alone (STZ, Txp; 150 islets; n = 10; open rectangles) and diabetic mice transplanted with islet + PSCs (STZ, Txp; 150 islets and 1 × 106 PSCs; n = 10; closed rectangles). Oral glucose tolerance test (oGTT) was performed at 2 and 6 weeks after transplantation. Fasting blood glucose concentrations were measured before glucose bolus of 2 g/kg d-glucose and at 15, 30, 60, 90, and 120 min post-bolus. Glucose levels in islet + PSC group were significantly lower than those in and islets-alone of PSC-only control groups at the time-points from 10 to 90 min at 2 weeks (A), and 6 weeks (C) after transplant. Right panels show glucose AUC at 2 and 6 weeks after transplantation. (B and D): Plasma insulin levels and insulin AUC after oGTT at 2 and 6 weeks after transplantation. Data are presented as mean ± SEM. (*P < .05, **P < .01, ***P < .001).
Discussion
Accumulating evidence suggests that tissue resident stromal cells and culture-adapted MSCs benefit islet health and function in vitro and in vivo. These beneficial effects could be a crucial determinant of transplantation success which depends more on islet quality than mass.42 While anti-inflammatory and immunosuppressant effects of marrow-derived MSCs are well documented and are shown to improve islet beta cell function in vitro and graft survival in vivo,25,26,43 relatively very little is known about the effects of PSCs on islet health. The little information available on the homeostatic roles of PSCs via ECM-islet interaction suggests that PSCs may be effective in supporting islet health in vitro and in vivo.11 This raises the possibility of using PSCs to preserve islet quality by in vitro coculture and consequently to improve islet engraftment, function and outcome after transplantation. Similar to islets, PSCs are also derived from pancreatic tissue. Mesodermal progenitors which give rise to culture adapted MSCs display functional features specific to their tissue of origin and niche-like functionalities as has been demonstrated for MSCs derived from liver, bowel and marrow.44 Hence, we hypothesized that PSCs would deploy islet adapted niche properties which can effectively preserve islet quality through their ECM components and can maintain islet viability and function pre- and post-transplant.
To test this hypothesis, we compared different coculture configurations and optimized a direct cell-to-cell contact PSC/islet coculture system (IPCt) and show that PSCs preserve islet health only in (IPCt) configuration. Furthermore, we also observed that PSCs preserved islet health in vitro up to 6 weeks, exceeding the beneficial effects of marrow-derived MSCs or any other cell type previously studied in a similar context. In contrast to intraspecific interactions, very little is known about inter-species islet-feeder cell interaction.45 Interestingly, our data presents the first evidence that porcine PSCs can protect morphology and functional viability of islets effectively in xenogeneic and syngeneic settings during extended in vitro coculture. Cross-species conserved characteristics and previously unknown competence of PSCs in supporting islet health suggests that human donor-derived allogeneic PSCs may also effectively preserve human islet function and viability, and therefore, may be co-transplanted with islet in clinical settings to improve outcome of transplantation therapy.
Culture-adapted porcine PSC expression of canonical endothelial-mesenchymal transition (EMT) markers, α-SMA and other ECM proteins suggests that they undergo EMT. However, culture adapted/activated stellate cells also regain their quiescence after in vivo transplantation.46 Although their fibrogenic role is well understood, relevance of nonfibrogenic role of activated PSCs in homeostatic maintenance has begun to be appreciated only recently.47 Our data demonstrate that culture adapted PSCs not only support islet health but also preserve function without altering morphology, cytoarchitecture or insulin content of islets (Figs. 3 and 4). In coculture with PSCs, porcine, murine and human islets responded accordingly to stimulatory glucose concentration which is the most important physiological initiator of insulin secretion in mammalian species. While PSC-mediated protection preserved human islet function, it was more variable and not as robust compared to porcine or murine islets. This can be attributed to the inherent variability in glucose-response of human islets due to the difference in donor health status, age, BMI and cold-ischemia time.48 Despite the donor-influenced variability, we observed the beneficial effects of PSC coculture on human islets similar to those on porcine or murine islets. This emphasizes the potential benefits of including PSCs into clinical islet transplantation therapy protocols. Based on the evidence from our study, we envision that human donor-derived allogeneic PSCs may also be expanded and banked to provide an off-the-shelf source optimal for a translational setting.
Islet-ECM interactions are known to be essential for diverse ECM-induced effects such as beta cell survival, growth and function.43,49 Despite a known significant overlap between ECM composition of peri-islet BM and culture adapted PSCs, the current body of evidence on the role of qPSCs or aPSCs in ECM production for BM and homeostatic support of islets is very limited. A growing body of evidence has emphasized the importance of ECM-islet interaction and more specifically, role of laminin in this interaction.10,11,41 Therefore, we supposed that laminin and its receptors may underlie the cytoprotective effects of PSCs. In this work, we discovered that 37/67 kDa LR is required for all beneficial effects of PSCs on islet health. This LR is identified as the first non-integrin receptor that binds laminin (the only known ligand for this LR).50 This receptor has been implicated in several diverse functions including viability and growth.50 Despite its functional repertoire and published reports linking PSCs/stromal cells, laminin and islets together, there is a complete lack of information about the role of this axis in maintenance of islet health in vitro. Our study is first to report that inhibition of LR-mediated interaction abrogates the PSC-mediated protection of islet viability and function. However, canonical laminins (LM-111 and LM-511) alone are not sufficient to preserve islet health. The effects of this LR-inhibition and insufficiency of laminins alone to support islet health suggest other possible LR-interaction with secreted factors and/or ECM proteins effecting distinct downstream regulatory pathways. Further studies are needed to reveal the full repertoire of ligands for this LR and to elucidate their role in regulation of islet physiology.
Stromal cell co-transplantation has been proved to improve survival and function of islet grafts in diabetic animals.51 Although PSC coculture significantly extended islet function and viability in vitro, in this study, we chose to culture islets alone prior to transplant to simulate clinical transplant setting as closely as possible. Whereas PSCs alone failed to influence glucose metabolism, islets-alone and islet + PSC both reversed hyperglycemia. This suggests that the effect of PSCs on transplantation outcome is not due to PSCs themselves but rather to their impact on islet grafts. Most importantly, islet + PSC co-transplantation achieved glucose homeostasis more effectively and maintained significantly superior islet function compared to islets-alone even under high glucose metabolic stress (oGTT) throughout the duration of the study. Cumulatively, these data show that PSCs protect the function of islet grafts during the early stage after transplantation and consequently improve engraftment and transplantation outcome.
Conclusion
In summary, we demonstrate that PSCs protect islet viability and insulin secretory function ex vitro in direct coculture and improve graft function post-transplant. We show that the in vitro effects require interaction of PSC-produced ECM components with islets. Our study unveils that 37/67 kDa LR is an important mediator of the downstream effects of islet-PSC interaction. This study identifies a novel potential of PSCs that may have significant implications in clinical islet transplantation and offers a novel strategy of engineering islet-PSC implants to improve transplantation outcome. In addition, the ability to maintain functional murine or human islets in culture for expanded duration would greatly expand the potential for ex vivo modeling of diabetes for pharmaceutical drug discovery.
Supplementary Material
Acknowledgment
This work was supported by NIH/NIDDK award R01DK109508 to JG and in part by NIH/NIDDK award R01DK110324 to DBD and US Department of Veterans Affairs Merit Award I01BX001880 to DBD. This work does not represent the views of the Department of Veterans Affairs or the US government. We extend our deepest gratitude to the courageous families who generously donated their loved one’s organs and tissues for biomedical research. Such important research like this would not be possible without this selfless gift of hope. Thanks to our partner organ procurement organizations, Kentucky Organ Donor Affiliates (KODA) and LifeCenter, Ohio, for supporting these special families.
Contributor Information
Pradyut K Paul, Department of Medicine, University of Wisconsin-Madison, Madison, WI, USA.
Rahul Das, Department of Medicine, University of Wisconsin-Madison, Madison, WI, USA.
Travis J Drow, Department of Biochemistry, University of Wisconsin-Madison, Madison, WI, USA.
Arnaldo H de Souza, Department of Medicine, Division of Endocrinology, Diabetes and Metabolism, University of Wisconsin-Madison, Madison, WI, USA.
Appakalai N Balamurugan, Clinical Islet Cell Laboratory, Center for Clinical and Translational Research, Abigail Wexner Research Institute, Nationwide Children’s Hospital, Department of Pediatrics, College of Medicine, The Ohio State University, Columbus, OH, USA.
Dawn Belt Davis, Department of Medicine, Division of Endocrinology, Diabetes and Metabolism, University of Wisconsin-Madison, Madison, WI, USA; William S. Middleton Memorial Veterans Hospital, Madison, WI, USA.
Jacques Galipeau, Department of Medicine, University of Wisconsin-Madison, Madison, WI, USA.
Conflict of Interest
The authors declare no conflict of interests.
Author Contributions
P.K.P., J.G.: conceptualization, methodology, investigation, visualization, funding acquisition, project administration, supervision, writing (original draft), writing (review, editing). R.D.: methodology, investigation, visualization, writing (review, editing). A.H.D-S.: methodology, writing (review, editing). A.N.B.: methodology, human islet isolation. D.B.D.: methodology. T.J.D.: investigation, writing (review, editing). J.G.: funding acquisition, project administration, supervision, writing—original draft, writing (review, editing).
Data Availability
All data and material used in this study and analysis will be available upon request to corresponding author and may be subject to material transfer agreements (MTAs).
References
- 1. Goto M, Yoshikawa Y, Matsuo K, et al. Optimization of a prominent oxygen-permeable device for pancreatic islets. Transplant Proc. 2008;40(2):411-412. 10.1016/j.transproceed.2008.01.056. [DOI] [PubMed] [Google Scholar]
- 2. Kin T, Senior P, O’Gorman D, et al. Risk factors for islet loss during culture prior to transplantation. Transpl Int. 2008;21(11):1029-1035. https://onlinelibrary.wiley.com/doi/10.1111/j.1432-2277.2008.00719.x. [DOI] [PubMed] [Google Scholar]
- 3. Ricordi C, Fraker C, Szust J, et al. Improved human islet isolation outcome from marginal donors following addition of oxygenated perfluorocarbon to the cold-storage solution. Transplantation. 2003;75(9):1524-1527. 10.1097/01.TP.0000058813.95063.7A. [DOI] [PubMed] [Google Scholar]
- 4. Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. 2012;35(7):1436-1445. 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chao KC, Chao KF, Chen CF, et al. A novel human stem cell coculture system that maintains the survival and function of culture islet-like cell clusters. Cell Transplant. 2008;17(6):657-664. https://journals.sagepub.com/doi/10.3727/096368908786092801. [DOI] [PubMed] [Google Scholar]
- 6. Miki A, Narushima M, Okitsu T, et al. Maintenance of mouse, rat, and pig pancreatic islet functions by coculture with human islet-derived fibroblasts. Cell Transplant. 2006;15(4):325-334. [PubMed] [Google Scholar]
- 7. Solari MG, Srinivasan S, Boumaza I, et al. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J Autoimmun. 2009;32(2):116-124. [DOI] [PubMed] [Google Scholar]
- 8. Luo L, Badiavas E, Luo JZ, et al. Allogeneic bone marrow supports human islet beta cell survival and function over six months. Biochem Biophys Res Commun. 2007;361(4):859-864. 10.1016/j.bbrc.2007.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hadavi E, Leijten J, Engelse M, et al. Microwell scaffolds using collagen-IV and laminin-111 lead to improved insulin secretion of human islets. Tissue Eng Part C Methods. 2019;25(2):71-81. 10.1089/ten.TEC.2018.0336. [DOI] [PubMed] [Google Scholar]
- 10. Llacua LA, Faas MM, de Vos P.. Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia. 2018;61(6):1261-1272. 10.1007/s00125-017-4524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stendahl JC, Kaufman DB, Stupp SI.. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant. 2009;18(1):1-12. https://journals.sagepub.com/doi/10.3727/096368909788237195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Apte MV, Haber PS, Applegate TL, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43(1):128-133. 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bachem MG, Schneider E, Gross H, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115(2):421-432. [DOI] [PubMed] [Google Scholar]
- 14. Vizoso FJ, Eiro N, Costa L, et al. Mesenchymal stem cells in homeostasis and systemic diseases: hypothesis, evidences, and therapeutic opportunities. Int J Mol Sci. 2019;20(15):3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Means AL. Pancreatic stellate cells: small cells with a big role in tissue homeostasis. Lab Invest. 2013;93(1):4-7. [DOI] [PubMed] [Google Scholar]
- 16. Nielsen MFB, Mortensen MB, Detlefsen S.. Identification of markers for quiescent pancreatic stellate cells in the normal human pancreas. Histochem Cell Biol. 2017;148(4):359-380. [DOI] [PubMed] [Google Scholar]
- 17. Apte MV, Pirola RC, Wilson JS.. Pancreatic stellate cells: a starring role in normal and diseased pancreas. Front Physiol. 2012;3:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Apte MV, Wilson JS.. Mechanisms of pancreatic fibrosis. Dig Dis. 2004;22(3):273-279. https://www.karger.com/Article/Abstract/82799# [DOI] [PubMed] [Google Scholar]
- 19. Casini A, Galli A, Pignalosa P, et al. Collagen type I synthesized by pancreatic periacinar stellate cells (PSC) co-localizes with lipid peroxidation-derived aldehydes in chronic alcoholic pancreatitis. J Pathol. 2000;192(1):81-89. [DOI] [PubMed] [Google Scholar]
- 20. Jaster R. Molecular regulation of pancreatic stellate cell function. Mol Cancer. 2004;3:26. 10.1186/1476-4598-3-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pandol SJ. Are we studying the correct state of the stellate cell to elucidate mechanisms of chronic pancreatitis?. Gut. 2005;54(6):744-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang X, Abiatari I, Kong B, et al. Pancreatic islet and stellate cells are the main sources of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 in pancreatic cancer. Pancreatology. 2009;9(1-2):165-172. [DOI] [PubMed] [Google Scholar]
- 23. Phillips PA, McCarroll JA, Park S, et al. Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover. Gut. 2003;52(2):275-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ko SH, Hong OK, Kim JW, et al. High glucose increases extracellular matrix production in pancreatic stellate cells by activating the renin-angiotensin system. J Cell Biochem. 2006;98(2):343-355. [DOI] [PubMed] [Google Scholar]
- 25. Jung EJ, Kim SC, Wee YM, et al. Bone marrow-derived mesenchymal stromal cells support rat pancreatic islet survival and insulin secretory function in vitro. Cytotherapy. 2011;13(1):19-29. [DOI] [PubMed] [Google Scholar]
- 26. Rackham CL, Dhadda PK, Chagastelles PC, et al. Pre-culturing islets with mesenchymal stromal cells using a direct contact configuration is beneficial for transplantation outcome in diabetic mice. Cytotherapy. 2013;15(4):449-459. [DOI] [PubMed] [Google Scholar]
- 27. Kerby A, Jones ES, Jones PM, et al. Co-transplantation of islets with mesenchymal stem cells in microcapsules demonstrates graft outcome can be improved in an isolated-graft model of islet transplantation in mice. Cytotherapy. 2013;15(2):192-200. [DOI] [PubMed] [Google Scholar]
- 28. Hayward JA, Ellis CE, Seeberger K, et al. Cotransplantation of mesenchymal stem cells with neonatal porcine islets improve graft function in diabetic mice. Diabetes. 2017;66(5):1312-1321. [DOI] [PubMed] [Google Scholar]
- 29. Navarro-Alvarez N, Rivas-Carrillo JD, Soto-Gutierrez A, et al. Reestablishment of microenvironment is necessary to maintain in vitro and in vivo human islet function. Cell Transplant. 2008;17(1-2):111-119. [DOI] [PubMed] [Google Scholar]
- 30. Sigmundsson K, Ojala JRM, Ohman MK, et al. Culturing functional pancreatic islets on alpha5-laminins and curative transplantation to diabetic mice. Matrix Biol. 2018;70:5-19. 10.1016/j.matbio.2018.03.018 [DOI] [PubMed] [Google Scholar]
- 31. Weber LM, Hayda KN, Anseth KS.. Cell-matrix interactions improve beta-cell survival and insulin secretion in three-dimensional culture. Tissue Eng Part A. 2008;14(12):1959-1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xiaohui T, Wujun X, Xiaoming D, et al. Small intestinal submucosa improves islet survival and function in vitro culture. Transplant Proc. 2006;38(5):1552-1558. [DOI] [PubMed] [Google Scholar]
- 33. Jiang FX, Cram DS, DeAizpurua HJ, et al. Laminin-1 promotes differentiation of fetal mouse pancreatic beta-cells. Diabetes. 1999;48(4):722-730. [DOI] [PubMed] [Google Scholar]
- 34. Virtanen I, Banerjee M, Palgi J, et al. Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia. 2008;51(7):1181-1191. [DOI] [PubMed] [Google Scholar]
- 35. Vanderschelden R, Sathialingam M, Alexander M, et al. Cost and scalability analysis of porcine islet isolation for islet transplantation: comparison of juvenile, neonatal and adult pigs. Cell Transplant. 2019;28(7):967-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Souza AH, Santos LRB, Roma LP, et al. NADPH oxidase-2 does not contribute to beta-cell glucotoxicity in cultured pancreatic islets from C57BL/6J mice. Mol Cell Endocrinol. 2017;439:354-362. [DOI] [PubMed] [Google Scholar]
- 37. Loganathan G, Subhashree V, Narayanan S, et al. Improved recovery of human islets from young donor pancreases utilizing increased protease dose to collagenase for digesting peri-islet extracellular matrix. Am J Transplant. 2019;19(3):831-843. [DOI] [PubMed] [Google Scholar]
- 38. Vonlaufen A, Phillips PA, Yang L, et al. Isolation of quiescent human pancreatic stellate cells: a promising in vitro tool for studies of human pancreatic stellate cell biology. Pancreatology. 2010;10(4):434-443. [DOI] [PubMed] [Google Scholar]
- 39. Tropel P, Noel D, Platet N, et al. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004;295(2):395-406. [DOI] [PubMed] [Google Scholar]
- 40. Zang G, Sandberg M, Carlsson PO, et al. Activated pancreatic stellate cells can impair pancreatic islet function in mice. UPS J Med Sci. 2015;120(3):169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hammar E, Parnaud G, Bosco D, et al. Extracellular matrix protects pancreatic beta-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes. 2004;53(8):2034-2041. [DOI] [PubMed] [Google Scholar]
- 42. Robertson RP. Islet transplantation as a treatment for diabetes—a work in progress. N Engl J Med. 2004;350(7):694-705. [DOI] [PubMed] [Google Scholar]
- 43. Arzouni AA, Vargas-Seymour A, Rackham CL, et al. Mesenchymal stromal cells improve human islet function through released products and extracellular matrix. Clin Sci (Lond). 2017;131(23):2835-2845. [DOI] [PubMed] [Google Scholar]
- 44. Chamberlain G, Fox J, Ashton B, et al. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739-2749. [DOI] [PubMed] [Google Scholar]
- 45. Holt RIG, Cockram CS, Flyvbjerg A, et al. Textbook of Diabetes. John Wiley & Sons, 2017. [Google Scholar]
- 46. Apte MV, Pirola RC, Wilson JS.. Stellate Cells in Health and Disease [in English]. Stellate Cells in Health and Disease. In: Gandhi CR, Pinzani M, eds. Chapter 16 - Pancreatic Stellate Cells. Academic Press, 2015:271-306. 10.1016/B978-0-12-800134-9.00016-6 [DOI] [Google Scholar]
- 47. Vonlaufen A, Joshi S, Qu C, et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 2008;68(7):2085-2093. [DOI] [PubMed] [Google Scholar]
- 48. Lakey JR, Warnock GL, Rajotte RV, et al. Variables in organ donors that affect the recovery of human islets of Langerhans. Transplantation. 1996;61(7):1047-1053. [DOI] [PubMed] [Google Scholar]
- 49. Rackham CL, Jones PM.. Potential of mesenchymal stromal cells for improving islet transplantation outcomes. Curr Opin Pharmacol. 2018;43:34-39. [DOI] [PubMed] [Google Scholar]
- 50. Malinoff HL, Wicha MS.. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983;96(5):1475-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Qu Z, Lou Q, Cooper DKC, et al. Potential roles of mesenchymal stromal cells in islet allo- and xenotransplantation for type 1 diabetes mellitus. Xenotransplantation. 2021;28(3):e12678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and material used in this study and analysis will be available upon request to corresponding author and may be subject to material transfer agreements (MTAs).