Abstract
After allogeneic hematopoietic stem cell transplantation (HSCT), donor lymphocytes may contribute to the regression of hematological malignancies and select solid tumors, a phenomenon referred to as the graft-versus-tumor effect (GVT). However, this immunologic reaction is frequently limited by either poor specificity resulting in graft-versus-host disease or the frequency of tumor-specific T cells being too low to induce a complete and sustained anti-tumor response. Over the past 2 decades, it has become clear that the driver of GVT following allogeneic HSCT is T-cell-mediated recognition of antigens presented on tumor cells. With that regard, even though the excitement for using HSCT in solid tumors has declined, clinical trials of HSCT in solid tumors provided proof of concept and valuable insights leading to the discovery of tumor antigens and the development of targeted adoptive cell therapies for cancer. In this article, we review the results of clinical trials of allogeneic HSCT in solid tumors. We focus on lessons learned from correlative studies of these trials that hold the potential for the creation of tumor-specific immunotherapies with greater efficacy and safety for the treatment of malignancies.
Keywords: adoptive cell therapy, graft-versus-tumor effect, hematopoietic stem cell transplantation, HERV-E, KIR-ligand mismatch, solid tumors
Graphical Abstract
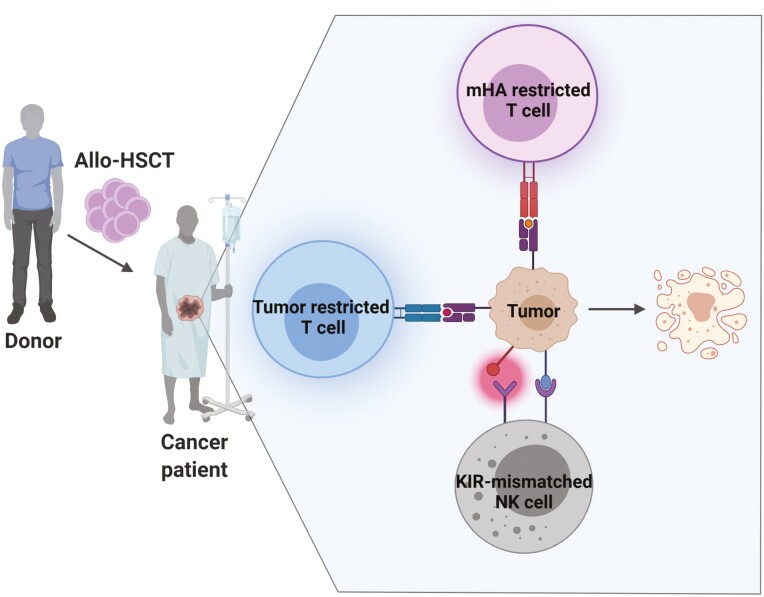
Allogeneic HSCT (Allo-HSCT) can be used to generate graft-versus-tumor effect against advanced solid tumors. The major mediators of this immunologic reaction are T lymphocytes and NK cells. HSCT: hematopoietic stem cell transplantation; mHA – minor histocompatibility antigen; KIR – killer Ig-like receptor.
Significance Statement.
The results of clinical trials using HSCT to treat solid tumors are limited compared with what is reported in hematological malignancies. However, the mechanisms of the GVT effect in solid tumors unveiled by these trials have provided an opportunity for learning how to direct different components of the immune system against cancer. This concise review discusses the results of clinical trials of HSCT in solid tumors and insights gleaned from these studies that pave the way for the development of new targeted adoptive cell therapies for cancer.
Introduction
Following the pioneering work of the Nobel laureate E. Donnall Thomas in 1950s,1 hematopoietic stem cell transplantation (HSCT) emerged as a curative intervention for patients with different hematologic, autoimmune, metabolic, and neoplastic diseases. Allogeneic HSCT showed great success in inducing durable responses in blood cancers, especially myeloid leukemias.2,3 Initially, HSCT was used as a method to rescue bone marrow function following high-dose chemotherapy. As this therapy developed, we learned that anti-tumor effects were not exclusively due to cytoreductive abilities of drugs but also to immune effects of the graft itself—the so-called graft versus tumor effect (GVT).4 Therefore, breaking immunotolerance toward the host malignancy in neoplastic diseases has now become the primary aim of allogeneic HSCT for cancer. This updated concept led to the use of lower preparatory doses of cytotoxic drugs to provide just enough immunosuppression to permit successful engraftment and expansion of donor-derived immune cells.5 Such reduced intensity or nonmyeloablative regimens showed good GVT induction against malignancies while improving the safety profile of the procedure, especially in older and severely debilitated cancer patients who could not tolerate conventional high dose regimens.4
Despite trials establishing the curative potential of the GVT effect, even the earliest studies in blood cancers identified donor T cells as mediating damage to host tissues, most prominently the skin, liver, and the intestines—a phenomenon called graft versus host disease (GVHD).1 Resorting to using better HLA-matching and T-cell depletion of the transplant, researchers significantly reduced the incidence and severity of GVHD and improved transplant outcomes.6,7 However, the occurrence of GVHD was found to be positively correlated with tumor regression, indicating that GVHD and GVT are 2 faces of the same coin.6,8 Decoding the mechanisms mediating GVHD and GVT has provided valuable insight into intricate differences between the 2, offering researchers an opportunity to develop targeted therapies boosting only GVT while abrogating GVHD. Indeed, balancing one versus the other remains one of the greatest challenges of transplantation immunotherapy.
This concise review discusses the results of allogeneic HSCT intended to induce GVT effects in solid tumors. Particular emphasis is placed on characterizing mechanisms mediating the GVT effect and the use of HSCT as a platform for tumor antigen discovery as a prelude to developing targeted cellular therapies that utilize autologous rather than allogeneic immunity.
Evidence for Graft-Versus-Tumor Effect in Solid Tumors
Because of its curative potential against hematological malignancies, researchers and clinicians applied allogeneic HSCT to exploit GVT effects against solid tumors refractory to conventional therapies. Pioneering attempts to treat solid tumors with allogeneic HSCT used myeloablative chemotherapy where direct cytotoxic effects of the ablative regimen were credited for tumor regression.9 However, this approach was followed by high transplant-related morbidity and mortality, especially in the setting of debilitated patients with metastatic tumors.10-12 With a better understanding of the mechanisms mediating GVT together with the improved safety profile, nonmyeloablative HSCT provided investigators with a safer platform to explore the potential of GVT in metastatic solid tumors.
Graft Versus Renal Cell Carcinoma
Due to its track record of being resistant to chemotherapy and sensitive to immunotherapy, renal cell carcinoma (RCC) was one of the early tumors treated with HSCT. In 2000, our team at NIH reported the first series of patients with cytokine-refractory kidney cancer treated with nonmyeloablative allogeneic HSCT: we observed a 53% response rate, including some patients who achieved durable complete responses.13 After this first report, other groups investigated different nonmyeloablative HSCT approaches to treat RCC, with objective response rates varying from 0% to 57% (Table 1). The largest series thus far comprised of 124 patients from the prospective multi-center European study that reported a response rate (complete + partial) of 29% at a median 150 (range 42-600) days post-transplant with transplant-related mortality of 16%.14
Table 1.
Selected allogeneic HSCT clinical trials in RCC patients (in chronological order).
| Authors | No of patients | TRM (%) | Response rate % (CR/PR/MR) |
Response onset day (median) |
|---|---|---|---|---|
| Childs et al13 | 19 | 12% | 53% (3/7) | 129 |
| Bregni et al78 | 7 | 14% | 57% (0/4) | 117 |
| Pedrazzoli et al79 | 7 | 29% | 0% (0/0) | N/A |
| Rini et al80 | 12 | 33% | 33% (0/4) | 180 |
| Hentschke et al19 | 10 | 30% | 30% (0/3) | NR |
| Ueno et al81 | 15 | 33% | 47% (1/2/4) | NR |
| Artz et al82 | 18 | 28% | 22% (0/4) | 182 |
| Barkholt et al14 | 124 | 16% | 29% (4/24) | 150 |
| Takahashi et al32* | 74 | 11% | 39% (7/22) | 133 |
A follow-up study of Childs et al.13
TRM, transplant-related mortality; CR, complete response; PR, partial response; MR, marginal response; NR, not reported.
These studies established for the first time the power of the allogeneic immune system to induce regression of metastatic solid tumors. However, high transplantation-related mortality and toxicity limited the widespread use of allogeneic HSCT for metastatic RCC. An additional difficulty is that the maturation of the alloimmunity may take months post-transplant,4 delaying potential GVT effects in patients with otherwise rapidly progressing disease who succumb to their tumor before an anti-tumor response can be established. Because of this limitation, and the introduction of numerous highly effective new therapies to the market, allogeneic HSCT is rarely ever used now to treat RCC patients. At present, there are no active trials using HSCT for the treatment of RCC reported on clinicaltrials.gov. Nevertheless, allogeneic HSCT served as an important platform for studying the mechanisms of the GVT effect and for identifying RCC-specific antigens. One such antigen discovered in RCC patients having tumor regression after transplant is currently used as a target in an ongoing cellular therapy clinical trial (NCT03354390).
Graft Versus Solid Tumors Other Than Renal Cell Carcinoma
Analogous to RCC, several other solid tumor types appear to be susceptible to GVT. However, reported tumor responses do not seem to be as robust and are not as thoroughly studied as reports for RCC. One of the first studies of nonmyeloablative allogeneic HSCT in breast cancer patients was conducted at National Cancer Institute.15 In order to isolate the effects of GVT from chemotherapy, the researchers in this study used T-cell-depleted allografts followed by T-cell administration at a later timepoint. Objective tumor regression attributed to allogeneic T-cell infusion was reported in 6 (37%) patients. Even though immunosuppression due to GVHD abrogated these effects, this study established the principle that breast cancer was also susceptible to GVT. Other studies in patients with breast cancer reported similar results.16,17
More modest responses were reported in patients with colorectal carcinoma (CRC). In one of the larger series of patients with metastatic CRC treated with nonmyeloablative HSCT, the authors reported overall disease control in 18/39 patients (46%) with 1 complete response (2%), 7 partial responses (18%), and 10 stable disease responses (26%).18 Additionally, Hentschke et al reported 1 complete response and 1 mixed response out of 6 patients with CRC treated with HSCT.19
In patients with refractory ovarian cancer, a multi-center retrospective study of 30 patients receiving allogeneic HSCT reported 8 (26%) responding patients, with 3 being late-responders consistent with GVT effect, while others may have responded early on to the conditioning regimen.20 The results of HSCT in patients with pancreatic cancer,21 soft-tissue sarcomas,22 and melanoma23 were also reported, however, with less encouraging outcomes.
Overall, the studies mentioned above provide proof of principle that allogeneic HSCT can be used to generate GVT against advanced solid tumors. Modest results could in part be attributed to small sample sizes and enrollment of patients in terminal stages of their disease refractory to other therapies. Further studies with designs addressing these issues could better define the potential of GVT in solid tumors and could be used to characterize the mechanisms leading to tumor regression.
Immunobiology of Effector Cells Mediating GVT in Solid Tumors Following HSCT
While the full molecular underpinnings behind GVT effects are still not completely understood, it is known that the major cell types mediating GVT effects following HSCT in patients with malignancies are T lymphocytes and natural killer (NK) cells.
The Role of Cytotoxic T Cells
Several observations expose the role of donor T cells in mediating regression of malignancies including solid tumors. First, depleting allografts of T cells before transplantation abrogates the GVT effect.24 Second, anti-tumor responses are usually delayed after non-myeloablative conditioning and are observable after mixed chimerism disappears and full donor T-cell engraftment occurs.25,26 Next, the enhanced GVT in the setting of HLA mismatch suggests that the allogeneic anti-tumor effect is directed against antigens presented in the context of HLA molecules on the surface of neoplastic cells. This observation is further supported by the correlation between acute GVHD and tumor responses.13,14 Finally, Harlin et al demonstrated the expansion of interferon alpha producing CD8+ T cells was associated with clinical tumor responses in RCC patients after HSCT.27 Even though their specificity for tumor-associated versus other antigens was not determined, this study provided evidence that donor T lymphocytes play a role in the induction of GVT effects.
Solid tumor killing mediated by CD8+ T cells relies on the paracrine delivery of effector molecules which damage tumors and induce tumor apoptosis. The cytotoxic payload of T cells includes degranulation of perforin and granzyme, induces Fas-mediated killing, and augments T-cell function through an array of activating cytokines.28 Initiation of these effector pathways is contingent upon the interaction of T-cell receptors (TCR) with their cognate antigens presented in the context of MHC Class I molecules on the surface of tumor cells.
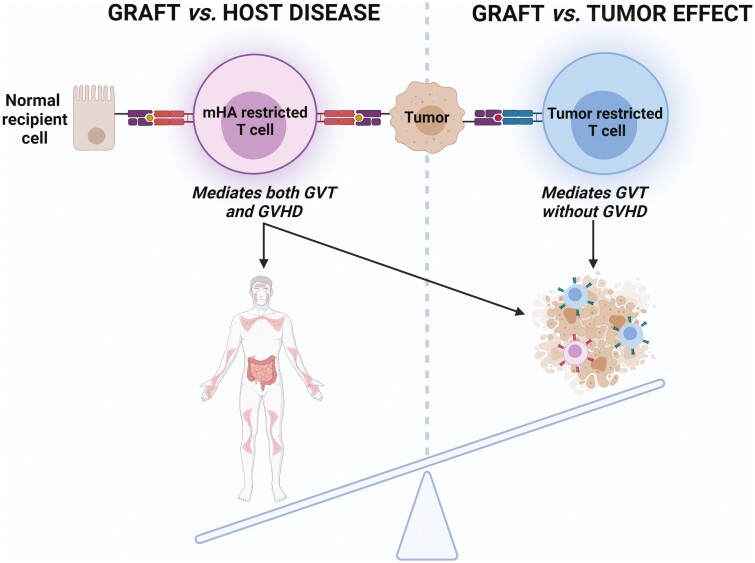
Donor T cells recognize host-restricted self-antigens classified as either minor histocompatibility antigens (mHA) or tumor-specific antigens (Fig. 1). The evidence for the existence of mHA came from observations that GVHD occurred in patients receiving a transplant from an HLA-identical sibling.29,30 These mHA are derived from genetic polymorphisms outside of the HLA locus. Because it is estimated that each person has thousands of nonsynonymous polymorphisms genome-wide which may potentially be sources of mHA, it is clear that identical HLA-matched donor-recipient pairs will still be mismatched for a large number of mHA.31 The expression distribution of mHA on normal tissues and tumor cells is important because it determines the target tissues of mHA-reactive T cells and the extent and clinical presentation of GVHD, which goes hand in hand with GVT.
Figure 1.
Donor T cells mediating GVHD and/or GVT effect. Tumor-reactive donor T cells recognize antigens presented on tumor cells in the context of HLA. These antigens are derived either from mHA or tumor-specific antigens. While mHA can be substrates of a GVT effect mediated by mHA-restricted T cells, these T cells also recognize the same mHA on normal tissues, causing GVHD. Tumor regression without objective GVHD may be achieved by T cells recognizing antigens that are specific to a tumor (tumor-restricted T cells). mHA, minor histocompatibility antigen.
Alternatively, the observation that tumor regression can occur in patients who never developed GVHD provides evidence that GVHD and GVT are not inherently linked in all patients and that donor-derived T cells might in some cases target antigens that are specific to a tumor.32,33
Unfortunately, only a few mHA or tumor-specific antigens have been identified after HSCT in solid tumors thus far, most of which are in RCC. Dorrschuck et al applied mass spectrometry to identify the target antigen of RCC-reactive T cells from cocultures of mixed lymphocytes with tumor cells from their study.34 The target antigen of the reactive T cells was an HLA-A*03:01-restricted canonical peptide encoded by the Eps-15 homology domain-containing 2 (EHD2) gene. Unfortunately, due to its ubiquitous expression in normal tissues, EHD2 and antigens derived from it are not suitable candidates for any type of tumor targeted cellular immunotherapy. This study provided a valuable lesson that even though T-cell clones may be reactive against RCC in vitro, their target antigen may be widely expressed, prohibiting its clinical use as a cell therapy target.
Tykodi et al isolated CD8+ T-cell clones from 2 patients transplanted for RCC that recognized the same mHA on RCC cells. The gene encoding this mHA was identified as C19orf48 and linked to chromosome 19q. The peptide antigen derived from this gene is HLA-A2:01-restricted, although its exact sequence requires further validation.35 Another RCC-associated mHA targeted by donor T cells was identified by Broen et al.36 This novel antigen was named ZAPHIR and is a result of a polymorphism in the splice donor site of the ZNF419 gene and is found to be HLA-B7-restricted. Both of these mHA appear to have a limited expression in normal tissues, making them potential candidates for targeted cellular therapy development.
From our group’s work,32 the observation that transplanted donor T cells could shrink tumors and induce long-term disease-free survival in a subset of patients who did not develop signs of GVHD has guided translational research aimed at dissecting the exact mechanisms mediating this specific regression of metastatic RCC. In this regard, we isolated a T-cell clone with specific reactivity against clear cell RCC from a patient who showed complete tumor regression without evidence of GVHD following allogeneic HSCT. This T-cell clone was found to target an HLA-A11-restricted peptide derived from a human endogenous retrovirus type E, named CT-RCC HERV-E. Remarkably, this HERV-E was found to be expressed only in clear cell RCC and not in other tumors or normal tissues. We then further identified VHL mutation as the primary driver of CT-RCC HERV-E expression, accounting for almost universal CT-RCC HERV-E expression in ccRCC, making it an ideal target for cellular therapy.37 Using CT-RCC HERV-E-reactive T-cell clone, we sequenced its TCR and showed that T cells transduced with this TCR acquire potent and selective cytotoxicity against ccRCC in vitro (manuscript in preparation). Our team has since initiated a clinical trial at the NIH Clinical Center testing infusions of autologous T cells transduced with a HERV-E TCR (NCT03354390) in patients with metastatic ccRCC.38 To our best knowledge, this is the only solid tumor-specific antigen identified following HSCT that has led to the development of a subsequent clinical trial of targeted tumor immunotherapy. The major limitation of this approach is the HLA-A11 restriction, as HLA-A11 is one of the less common HLA types in most population groups.39 Our group subsequently identified 3 HLA-A2 restricted peptides predicted to be products of CT-RCC HERV-E envelope which were used to generate peptide-reactive CD8+ T cells that recognized clear cell RCC cells expressing CT-RCC HERV-E in an HLA-A2-restricted fashion.40 The characterization of these antigens and their potential use as targets for T-cell-based therapy is still under investigation. The discovery of the entire repertoire of CT-RCC HERV-E-derived peptides presented by the most common HLA Class I types is an active research topic in our group.41
Similar to studies in RCC, target antigens-mediating GVT after allogeneic HSCT in CRC were also reported42; however, their precise validation and further clinical development have not yet been pursued.
While tumor cells directly present antigens that can be recognized by the allogeneic immune system, they often lack factors such as co-stimulatory molecules which are necessary to prime an effective and potent anti-tumor T-cell response. Antigen-presenting cells (APCs) such as dendritic cells (DCs) are critical for priming and amplifying a GVT effect mediated by CD8+ T cells.43 Through cross-presentation of tumor antigens, provision of costimulatory signals, and activating cytokines, host DCs are now known to play an important role in orchestrating allogeneic cytotoxic T-cell responses.43 A variety of different strategies have been explored to modulate the effects of DCs to augment GVT, mainly by enhancing the cross-presentation process and/or by blocking inhibitory receptors.44-46
The Role of NK Cells
The role of NK cells in mediating GVT after HSCT to treat solid tumors is not as well studied as in hematological malignancies. Therefore, the insight into mechanisms behind NK cell contribution to GVT is primarily derived from studies in blood cancers.
Several observations expose the role of NK cells in mediating GVT following HSCT. First, the GVT effect was observed even in transplants using T-cell-depleted grafts.47,48 These grafts contained NK cells which are the first population of lymphocytes to be reconstituted following allogeneic HSCT.49 Second, in haploidentical HSCT, engrafted donor-derived stem cells regenerate the donor’s repertoire of NK cells.50 These alloreactive NK cells were found to enhance the GVT effect following HSCT in addition to T cells.51,52
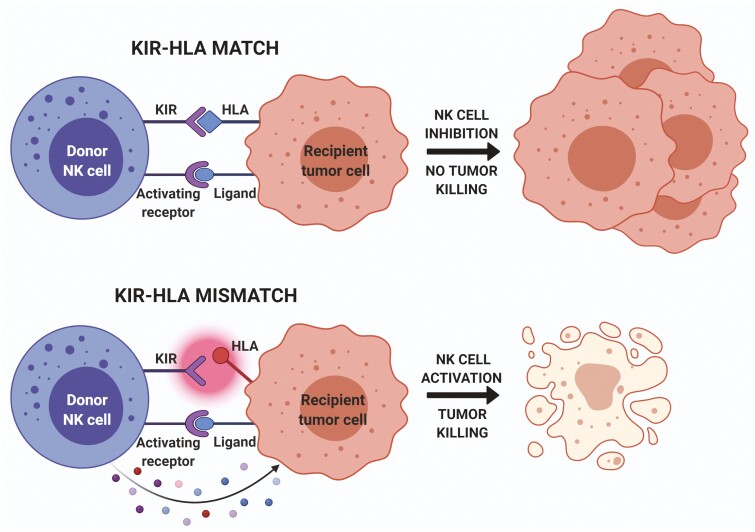
One of the major mechanisms tumors use to evade the immune system is the downregulation of HLA Class I molecules. By doing so, tumor cells become essentially invisible to T cells, which require peptide antigens to be presented to them in the context of HLA. In this context, the body’s next line of tumor defense is NK cells. Indeed, susceptibility to NK-cell-mediated attack increases with decreasing surface expression of HLA class I molecules on target cells, showing that the presence of HLA molecules protects cells against NK-cell-mediated lysis. Additionally, human NK cells can discriminate between different allelic forms of HLA molecules via clonally distributed receptors named killer cell Ig-like receptors (KIRs).53 The ligands for KIRs are HLA-A, -B, or -C molecules, and different KIR isoforms recognize different HLA types. Indeed, it was reported that a mismatch between KIRs expressed by donor NK cells and HLA Class I ligands expressed on tumor cells of a recipient is the major mechanism of NK-cell-mediated GVT effect.50
Since KIRs and HLA loci are housed on different chromosomes and segregate independently, it is not uncommon to inherit an inhibitory KIR gene but not its corresponding ligand, or vice versa. Therefore, it is likely that even in HLA-matched transplants, there will be a KIR mismatch.54 KIR-HLA mismatches under the right circumstances can lead to NK cell activation, which may improve transplant success by enhancing the ability of NK cells to mediate a GVT effect in hematological malignancies (Fig. 2). Such KIR mismatched NK cells can even exist in the context of autologous transplants.
Figure 2.
KIR-HLA mismatch mediating GVT effect by donor NK cells. When donor NK KIRs and recipient HLAs are matched, NK cells are inhibited from further action despite activation signals (top). In the case of KIR-HLA mismatch, KIRs do not successfully engage with the HLA ligand; activation signals prevail and lead to NK cell activation. Activated NK cells then release cytotoxic contents that eliminate tumor cells bearing these KIR-ligand mismatched HLA molecules (bottom). KIR, killer Ig-like receptor; KIR-L, killer Ig-like receptor ligand.
As previously mentioned, data on NK cells contributing to GVT effects following HSCT in solid tumors are limited. In vitro studies have shown compelling evidence that KIR incompatible NK cells mount more potent cytotoxicity against RCC and melanoma than KIR-matched or autologous NK cells.55 Similarly, alloreactive NK cells following allogeneic HSCT in a murine model of RCC decreased GVHD and mediated the GVT effects resulting in prolonged animal survival.56
Only a few clinical studies address the relevance of the KIR mismatch on the GVT effect following HSCT in solid tumors. A retrospective analysis of an RCC patient cohort not expressing HLA-Bw4 (ligand for KIR3DL1) showed an improved response when they received a transplant from a donor who expressed KIR3DL1.57 Leung et al report that autologous KIR-mismatched NK cells lead to a lower risk of disease relapse in different solid tumors such as neuroblastoma and Ewing sarcoma.58 Similar results were reported in a cohort of patients with high-risk neuroblastoma undergoing autologous HSCT where patients not expressing HLA-C1 (the ligand for KIR2DL2/KIR2DL3) had the most favorable outcomes.59 In a small series of pediatric patients with metastatic solid tumors treated with HSCT, Perez-Martinez et al described one complete remission and one partial response out of 3 treated cases.60 Both responding patients demonstrated KIR mismatch, while a complete KIR match was found to occur in the non-responding patient. These studies suggest a role for KIR-HLA mismatching in inducing and maintaining GVT after HSCT in solid tumors.61 Currently, there is only one active clinical trial reported on clinicaltrials.gov, which utilizes haploidentical HSCT to evaluate the contribution of NK cells in mediating GVT effects in Ewing sarcoma, Neuroblastoma, and Rhabdomyosarcoma (NCT02100891).
Further studies are needed to fully define the role of NK cells in mediating GVT effects following HSCT in solid tumors. However, proof of principle from the above-described studies paves the road for exploring adoptive transfer NK cell therapies analogous to those described for T cells.
Toward Adoptive Cellular Therapies
Clinical trials from the first decade of the 2000s established the susceptibility of various solid tumors to GVT. Due to modest results in most solid cancers, toxicities, and treatment-related mortality, allogeneic HSCT has largely fallen out of favor as an immunotherapeutic approach to treating solid tumors. However, proof of concept has been established from these studies with important correlative work identifying several novel tumor antigens targeted by engrafting donor T cells that may be excellent targets for future autologous adoptive cellular transfer (ACT) therapies.
ACT strategies involve the collection of the patients’ or donors’ T cells, modifying and/or expanding them in a laboratory to achieve desired properties and cell numbers, and reinfusing them back to the patient. The 3 most commonly delivered cell types are tumor-infiltrating lymphocytes (TIL), TCR-engineered T cells, and chimeric antigen receptor (CAR) T cells. Despite successes with treating hematological malignancies, ACT for solid tumors has encountered several unique obstacles. One of the most important roadblocks is the antigenic heterogeneity of solid tumors and the inability to use a “one-antigen-fits-all” strategy. Additionally, many identified solid tumor antigens are also expressed in normal tissues, which could lead to on-target off-tumor toxicity.62 Therefore, as was illustrated by early studies of the delicate balance between GVT and GVHD, careful selection of target antigens is the crucial determinant to predict the success of ACT, and currently represents the major bottleneck in the development of ACT for solid tumors.
Researchers today have different strategies available in their toolbox to help identify tumor-specific antigens. An example of an innovative, rapidly developing, and highly promising method for high-throughput antigen discovery is the immunopeptidomics-based platform.63 This mass spectrometry-based method is currently the only direct method for identifying intact tumor antigens as they are presented on cellular surfaces. However, antigen presentation is necessary but not sufficient to determine immunogenicity. Therefore, further refinement of analytical instruments and sophisticated analysis algorithms are needed to assist in the development of quick and high-throughput strategies that identify tumor-restricted antigens which could serve as the target for future, more efficacious cellular-based therapies for solid tumors.
Specificity for tumor antigens is a basic requirement of a cellular product intended for ACT. However, adoptively transferred cells must also overcome a number of post-infusion challenges to successfully eradicate solid tumors. Unlike blood cancers, solid tumors maintain a microenvironment that supports tumor survival and growth while simultaneously inhibiting immune attack.64 In this immunologically hostile environment, upregulation of inhibitory receptors on tumors and APCs effectively hinder anti-tumor surveillance.65 Reverting tolerogenic mechanisms by blocking interactions of inhibitory receptors on T cells with their ligands has proven to be a remarkably effective therapy in the clinic for a variety of different malignancies. Therefore, combining therapeutic inhibition of PD-1, PD-L1, CTLA-4, or other emerging inhibitory molecules could potentially bolster the efficacy of adoptively transferred tumor-reactive T cells.66
Another emerging factor found to significantly affect the success of ACT is the gut microbiome. The gut microbiome repertoire educates immune cells and shapes immune surveillance.67 Metabolites secreted by different bacterial species have the potential to modify T-cell differentiation and function, including adoptively transferred cells.68 Additionally, the gut microbiota has been found to impact the efficacy of immune checkpoint inhibitors (ICI) in different solid tumors.69-72 Manipulating intestinal flora to modulate the response to ACT or ICI therapy is an active area of clinical research. For example, fecal microbiota transfer performed in conjunction with therapeutic PD-1 blockade led to improved clinical outcomes in a subset of PD-1-refractory melanoma patients, overcoming tumor resistance to ICIs.70
To address the above-mentioned challenges and to modulate processes that were found to affect ACT success, the ACT armamentarium has now expanded beyond first-generation TILs, to TCR-engineered T cells, CARs, and/or NK cells. Bispecific killer cell engagers (BiKEs),73 CAR-T cells co-expressing necessary cytokines (TRUCKs),74 TCRs engineered into γδ T cells to avoid TCR chain mispairing with endogenous αβ TCRs,75 introduction of homing receptors to ensure adequate cell trafficking to tumor sites,76 and gene-edited NK cells that boost the efficacy of targeted therapies77 are some of the current strategies being explored to overcome post-infusion obstacles in an effort to augment the potency of ACT in eradicating refractory malignancies.
Funding
This research was supported by the Intramural Research Program of the NIH, Cellular and Molecular Therapeutics Branch and Hematology Branch of the NHLBI, the Commissioned Corps of the United States Public Health Service, and O’Neill-Rancic Renal Cell Cancer Research Fellowship Fund. All figures were created with BioRender.com.
Conflict of Interest
The authors declare no potential conflicts of interest.
Author Contributions
S.B.: conception, manuscript writing, final approval of manuscript. R.W.C.: conception, financial support, manuscript writing, final approval of manuscript.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Thomas ED, Lochte HL, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491-496. [DOI] [PubMed] [Google Scholar]
- 2. Baron F, Storb R.. Allogeneic hematopoietic cell transplantation as treatment for hematological malignancies: a review. Springer Semin Immunopathol. 2004;26:71-94. [DOI] [PubMed] [Google Scholar]
- 3. Pasquini MC, Wang ZS.. Current use and outcome of hematopoietic stem cell transplantation: part I-CIBMTR summary slides, 2007. CIBMTR Newsl. 2007;13:5-9. [Google Scholar]
- 4. Lundqvist A, Srivastava S, Childs R.. Solid tumors in adults. Hematop. Stem Cell Transplant. Clin. Pract., Churchill Livingstone, 2009:137-1–45.. [Google Scholar]
- 5. Storb RF, Champlin R, Riddell SR, et al. Non-myeloablative transplants for malignant disease. Hematol Am Soc Hematol Educ Program. 2001;375:391. [DOI] [PubMed] [Google Scholar]
- 6. Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555-562. [PubMed] [Google Scholar]
- 7. Al-Jurf M, Aranha F, Annasetti C, et al. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23:5074-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stern M, de Wreede LC, Brand R, et al. Sensitivity of hematological malignancies to graft-versus-host effects: an EBMT megafile analysis. Leukemia. 2014;28:2235-2240. [DOI] [PubMed] [Google Scholar]
- 9. Eibl B, Schwaighofer H, Nachbaur D, et al. Evidence for a graft-versus-tumor effect in a patient treated with marrow ablative chemotherapy and allogeneic bone marrow transplantation for breast cancer. Blood. 1996;88:1501-1508. [PubMed] [Google Scholar]
- 10. Nash RA, Storb R.. Graft-versus-host effect after allogeneic hematopoietic stem cell transplantation: GVHD and GVL. Curr Opin Immunol. 1996;8:674-680. [DOI] [PubMed] [Google Scholar]
- 11. Majolino I, Saglio G, Scimè R, et al. High incidence of chronic GVHD after primary allogeneic peripheral blood stem cell transplantation in patients with hematologic malignancies. Bone Marrow Transplant. 1996;17:555-560. [PubMed] [Google Scholar]
- 12. Parr MD, Messino MJ, McIntyre W.. Allogeneic bone marrow transplantation: procedures and complications. Am J Hosp Pharm. 1991;48:127-137. [PubMed] [Google Scholar]
- 13. Childs R, Chernoff A, Contentin N, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000;343:750-758. [DOI] [PubMed] [Google Scholar]
- 14. Barkholt L, Bregni M, Remberger M, et al. Allogeneic haematopoietic stem cell transplantation for metastatic renal carcinoma in Europe. Ann Oncol. 2006;17:1134-1140. [DOI] [PubMed] [Google Scholar]
- 15. Bishop MR, Fowler DH, Marchigiani D, et al. Allogeneic lymphocytes induce tumor regression of advanced metastatic breast cancer. J Clin Oncol. 2004;22:3886-3892. [DOI] [PubMed] [Google Scholar]
- 16. Carella AM, Beltrami G, Corsetti MT, et al. Reduced intensity conditioning for allograft after cytoreductive autograft in metastatic breast cancer. Lancet. 2005;366:318-320. [DOI] [PubMed] [Google Scholar]
- 17. Ueno NT, Rizzo JD, Demirer T, et al. Allogeneic hematopoietic cell transplantation for metastatic breast cancer. Bone Marrow Transplant. 2008;41:537-545. [DOI] [PubMed] [Google Scholar]
- 18. Aglietta M, Barkholt L, Schianca FC, et al. Reduced-intensity allogeneic hematopoietic stem cell transplantation in metastatic colorectal cancer as a novel adoptive cell therapy approach. The European group for blood and marrow transplantation experience. Biol Blood Marrow Transplant. 2009;15:326-335. [DOI] [PubMed] [Google Scholar]
- 19. Hentschke P, Barkholt L, Uzunel M, et al. Low-intensity conditioning and hematopoietic stem cell transplantation in patients with renal and colon carcinoma. Bone Marrow Transplant. 2003;31:253-261. [DOI] [PubMed] [Google Scholar]
- 20. Bay JO, Cabrespine-Faugeras A, Tabrizi R, et al. Allogeneic hematopoietic stem cell transplantation in ovarian cancer - the EBMT experience. Int J Cancer. 2010;127:1446-1452. [DOI] [PubMed] [Google Scholar]
- 21. Takahashi T, Omuro Y, Matsumoto G, et al. Nonmyeloablative allogeneic stem cell transplantation for patients with unresectable pancreatic cancer. Pancreas. 2004;28:e65-e69. [DOI] [PubMed] [Google Scholar]
- 22. Secondino S, Carrabba MG, Pedrazzoli P, et al. Reduced intensity stem cell transplantation for advanced soft tissue sarcomas in adults: a retrospective analysis of the European group for blood and marrow transplantation. Haematologica. 2007;92:418-420. [DOI] [PubMed] [Google Scholar]
- 23. Kurokawa T, Fischer K, Bertz H, et al. In vitro and in vivo characterization of graft-versus-tumor responses in melanoma patients after allogeneic peripheral blood stem cell transplantation. Int J Cancer. 2002;101:52-60. [DOI] [PubMed] [Google Scholar]
- 24. Dang N, Lin Y, Rutgeerts O, et al. Solid tumor-induced immune regulation alters the GvHD/GvT paradigm after allogenic bone marrow transplantation. Cancer Res. 2019;79:2709-2721. [DOI] [PubMed] [Google Scholar]
- 25. Childs R, Clave E, Contentin N, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94:3234-3241. [PubMed] [Google Scholar]
- 26. Imamura M, Tsutsumi Y, Miura Y, et al. Immune reconstitution and tolerance after allogeneic hematopoietic stem cell transplantation. Hematology. 2003;8:19-26. [DOI] [PubMed] [Google Scholar]
- 27. Harlin H, Artz AS, Mahowald M, et al. Clinical responses following nonmyeloablative allogeneic stem cell transplantation for renal cell carcinoma are associated with expansion of CD8+ IFN-γ-producing T cells. Bone Marrow Transplant. 2004;33:491-497. [DOI] [PubMed] [Google Scholar]
- 28. Golstein P, Griffiths GM.. An early history of T cell-mediated cytotoxicity. Nat Rev Immunol. 2018;18:527-535. [DOI] [PubMed] [Google Scholar]
- 29. Goulmy E, Schipper R, Pool J, et al. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N Engl J Med. 2009;334:281-285. [DOI] [PubMed] [Google Scholar]
- 30. Santos N, Rodríguez-Romanos R, Nieto JB, et al. UGT2B17 minor histocompatibility mismatch and clinical outcome after HLA-identical sibling donor stem cell transplantation. Bone Marrow Transplant. 2016;51:79-82. [DOI] [PubMed] [Google Scholar]
- 31. Ng PC, Levy S, Huang J, et al. Genetic variation in an individual human exome. PLoS Genet. 2008;4:e1000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takahashi Y, Harashima N, Kajigaya S, et al. Regression of human kidney cancer following allogeneic stem cell transplantation is associated with recognition of an HERV-E antigen by T cells. J Clin Invest. 2008;118:1099-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tykodi SS, Warren EH, Thompson JA, et al. Allogeneic hematopoietic cell transplantation for metastatic renal cell carcinoma after nonmyeloablative conditioning: toxicity, clinical response, and immunological response to minor histocompatibility antigens. Clin Cancer Res. 2004;10:7799-7811. [DOI] [PubMed] [Google Scholar]
- 34. Dörrschuck A, Schmidt A, Schnürer E, et al. CD8+ cytotoxic T lymphocytes isolated from allogeneic healthy donors recognize HLA class Ia/Ib-associated renal carcinoma antigens with ubiquitous or restricted tissue expression. Blood. 2004;104:2591-2599. [DOI] [PubMed] [Google Scholar]
- 35. Tykodi SS, Fujii N, Vigneron N, et al. C19orf48 encodes a minor histocompatibility antigen recognized by CD8 + cytotoxic T cells from renal cell carcinoma patients. Clin Cancer Res. 2008;14:5260-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Broen K, Levenga H, Vos J, et al. A polymorphism in the splice donor site of ZNF419 results in the novel renal cell carcinoma-associated minor histocompatibility antigen ZAPHIR. PLoS One. 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cherkasova E, Malinzak E, Rao S, et al. Inactivation of the von Hippel-Lindau tumor suppressor leads to selective expression of a human endogenous retrovirus in kidney cancer. Oncogene. 2011;30:4697-4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nadal R, Cherkasova E, Barisic S, et al. A phase I study of HERV-E TCR transduced autologous T cells (HERV-E TCR T Cells) in patients (pts) with metastatic clear cell renal cell carcinoma (mccRCC). Ann Oncol. 2018;29(suppl 8):Abstract 2956. [Google Scholar]
- 39. González-Galarza FF, Takeshita LYC, Santos EJM, et al. Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res. 2015;43:D784-D788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cherkasova E, Scrivani C, Doh S, et al. Detection of an immunogenic HERV-E envelope with selective expression in clear cell kidney cancer. Cancer Res. 2016;76:2177-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barisic S, Cherkasova E, Chen Y, et al. Targeted mass spectrometry provides direct evidence of a human endogenous retrovirus type E (HERV-E) antigen presented on the surface of clear cell renal cell carcinoma cells. Cancer Res. 2021;81(suppl 13):Abstract 1891. [Google Scholar]
- 42. Carnevale-Schianca F, Cignetti A, Capaldi A, et al. Allogeneic nonmyeloablative hematopoietic cell transplantation in metastatic colon cancer: tumor-specific T cells directed to a tumor-associated antigen are generated in vivo during GVHD. Blood. 2006;107:3795-3803. [DOI] [PubMed] [Google Scholar]
- 43. Toubai T, Mathewson N, Reddy P.. The role of dendritic cells in graft-versus-tumor effect. Front Immunol. 2014;5:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Toubai T, Sun Y, Luker G, et al. Host-derived CD81 dendritic cells are required for induction of optimal graft-versus-tumor responses after experimental allogeneic bone marrow transplantation. Blood. 2013;121:4231-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schulz O, Diebold SS, Chen M, et al. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 2005;433:887-892. [DOI] [PubMed] [Google Scholar]
- 46. Diamond MS, Kinder M, Matsushita H, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu J, Venstrom JM, Liu XR, et al. Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function after T cell-depleted allogeneic hematopoietic cell transplantation. Blood. 2009;113:3875-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. De Angelis C, Mancusi A, Ruggeri L, et al. Expansion of CD56-negative, CD16-positive, KIR-expressing natural killer cells after T cell-depleted haploidentical hematopoietic stem cell transplantation. Acta Haematol. 2011;126:13-20. [DOI] [PubMed] [Google Scholar]
- 49. Ullah MA, Hill GR, Tey SK.. Functional reconstitution of natural killer cells in allogeneic hematopoietic stem cell transplantation. Front Immunol. 2016;7:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333-339. [PubMed] [Google Scholar]
- 51. Locatelli F, Pende D, Falco M, et al. NK cells mediate a crucial graft-versus-leukemia effect in haploidentical-HSCT to cure high-risk acute leukemia. Trends Immunol. 2018;39:577-590. [DOI] [PubMed] [Google Scholar]
- 52. Giebel S, Locatelli F, Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814-819. [DOI] [PubMed] [Google Scholar]
- 53. Ruggeri L, Mancusi A, Burchielli E, et al. Natural killer cell alloreactivity and haplo-identical hematopoietic transplantation. Cytotherapy. 2006;8:554-558. [DOI] [PubMed] [Google Scholar]
- 54. Shilling HG, Young N, Guethlein LA, et al. Genetic control of human NK cell repertoire. J Immunol. 2002;169:239-247. [DOI] [PubMed] [Google Scholar]
- 55. Igarashi T, Wynberg J, Srinivasan R, et al. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood. 2004;104:170-177. [DOI] [PubMed] [Google Scholar]
- 56. Lundqvist A, McCoy JP, Samsel L, et al. Reduction of GVHD and enhanced antitumor effects after adoptive infusion of alloreactive Ly49-mismatched NK cells from MHC-matched donors. Blood. 2007;109:3603-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Srinivasan R, Carrington M, Suffredini D, et al. Impact of KIR and HLA genotypes on outcome in nonmyeloablative hematopoietic cell transplantation (HCT) using HLA matched related donors. Blood. 2006;108:323-323. [Google Scholar]
- 58. Leung W, Handgretinger R, Iyengar R, et al. Inhibitory KIR-HLA receptor-ligand mismatch in autologous haematopoietic stem cell transplantation for solid tumour and lymphoma. Br J Cancer. 2007;97:539-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Venstrom JM, Zheng J, Noor N, et al. KIR and HLA genotypes are associated with disease progression and survival following autologous hematopoietic stem cell transplantation for high-risk neuroblastoma. Clin Cancer Res. 2009;15:7330-7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pérez-Martínez A, Leung W, Muñoz E, et al. KIR-HLA receptor-ligand mismatch associated with a graft-versus-tumor effect in haploidentical stem cell transplantation for pediatric metastatic solid tumors. Pediatr Blood Cancer. 2009;53:120-124. [DOI] [PubMed] [Google Scholar]
- 61. Hsu K, Keever-Taylor C, Wilton A, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chong C, Coukos G, Bassani-Sternberg M.. Identification of tumor antigens with immunopeptidomics. Nat Biotechnol. 2021;1:14. [DOI] [PubMed] [Google Scholar]
- 64. Gowrishankar K, Birtwistle L, Micklethwaite K.. Manipulating the tumor microenvironment by adoptive cell transfer of CAR T-cells. Mamm Genome. 2018;29:739-756. [DOI] [PubMed] [Google Scholar]
- 65. Waldman AD, Fritz JM, Lenardo MJ.. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kverneland AH, Pedersen M, Wulff Westergaard MC, et al. Adoptive cell therapy in combination with checkpoint inhibitors in ovarian cancer. Oncotarget. 2020;11:2092-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zheng D, Liwinski T, Elinav E.. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Uribe-Herranz M, Bittinger K, Rafail S, et al. Gut microbiota modulates adoptive cell therapy via CD8α dendritic cells and IL-12. JCI Insight. 2018;3(4):e94952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Huang C, Li M, Liu B, et al. Relating gut microbiome and its modulating factors to immunotherapy in solid tumors: a systematic review. Front Oncol. 2021;11:760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371:595-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reusing SB, Vallera DA, Manser AR, et al. CD16xCD33 Bispecific Killer Cell Engager (BiKE) as potential immunotherapeutic in pediatric patients with AML and biphenotypic ALL. Cancer Immunol Immunother. 2021;70:3701-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Abid MB, Shah NN, Maatman TC, et al. Gut microbiome and CAR-T therapy. Exp Hematol Oncol. 2019;8:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gustafsson K, Herrmann T, Dieli F.. Editorial: understanding gamma delta T cell multifunctionality - towards immunotherapeutic applications. Front Immunol. 2020;11:921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Levy ER, Clara JA, Reger RN, et al. RNA-seq analysis reveals ccr5 as a key target for CRISPR gene editing to regulate in vivo NK cell trafficking. Cancers (Basel). 2021;13:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Clara JA, Levy ER, Reger R, et al. High-affinity CD16 integration into a CRISPR/Cas9-edited CD38 locus augments CD38-directed antitumor activity of primary human natural killer cells. J ImmunoTher Cancer. 2022;10:e003804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bregni M, Dodero A, Peccatori J, et al. Nonmyeloablative conditioning followed by hematopoietic cell allografting and donor lymphocyte infusions for patients with metastatic renal and breast cancer. Blood. 2002;99:4234-4236. [DOI] [PubMed] [Google Scholar]
- 79. Pedrazzoli P, Da Prada GA, Giorgiani G, et al. Allogeneic blood stem cell transplantation after a reduced-intensity, preparative regimen: a pilot study in patients with refractory malignancies. Cancer. 2002;94:2409-2415. [DOI] [PubMed] [Google Scholar]
- 80. Rini BI, Zimmerman T, Stadler WM, et al. Allogeneic stem-cell transplantation of renal cell cancer after nonmyeloablative chemotherapy: feasibility, engraftment, and clinical results. J Clin Oncol. 2002;20:2017-2024. [DOI] [PubMed] [Google Scholar]
- 81. Ueno NT, Cheng YC, Rondón G, et al. Rapid induction of complete donor chimerism by the use of a reduced-intensity conditioning regimen composed of fludarabine and melphalan in allogeneic stem cell transplantation for metastatic solid tumors. Blood. 2003;102:3829-3836. [DOI] [PubMed] [Google Scholar]
- 82. Artz AS, Van Besien K, Zimmerman T, et al. Long-term follow-up of nonmyeloablative allogeneic stem cell transplantation for renal cell carcinoma: The University of Chicago experience. Bone Marrow Transplant. 2005;35:253-260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.