Abstract
We investigated the mechanism of resistance to demethylation inhibitors (DMI) in Penicillium digitatum by isolating the CYP51 gene, which encodes the target enzyme (P45014DM) of DMI, from three DMI-resistant and three DMI-sensitive strains. The structural genes of all six strains were identical, but in the promoter region, a unique 126-bp sequence was tandemly repeated five times in the DMI-resistant strains and was present only once in the DMI-sensitive strains. Constitutive expression of CYP51 in the resistant strains was about 100-fold higher than that in the sensitive strains. We introduced CYP51, including the promoter region, from a DMI-resistant strain into a DMI-sensitive strain, which rendered the transformants DMI resistant and increased CYP51 expression. We also found that if the number of copies of the repeat was reduced to two, resistance and CYP51 expression also decreased. These results indicate that the 126-bp unit acts as a transcriptional enhancer and that a tandem repeat of the unit enhances CYP51 expression, resulting in DMI resistance. This is a new fungicide resistance mechanism for filamentous fungi.
Demethylation inhibitors (DMIs) are widely used as fungicides in agriculture and medicine. In the 1970s, the development of resistance to DMI fungicides under practical conditions was thought to be unlikely (9, 16). However, in practice, DMI-resistant strains occur widely in several important plant and animal pathogens, such as Erysiphe graminis, Sphaerotheca fuliginea, Pyrenophora teres (12), and Candida albicans (11), causing acute problems in crop production and in the treatment of candidiasis of AIDS patients. Determining the mechanism of resistance is, therefore, quite important.
We previously showed that an ATP-binding cassette (ABC) transporter gene, PMR1, is involved in DMI resistance in Penicillium digitatum by disrupting the PMR1 gene and concomitantly increasing sensitivity to DMI fungicides (21). PMR1 expression is strongly induced by fungicide treatment in both DMI-sensitive and DMI-resistant strains, but the constitutive expression level of PMR1 in the resistant strain was relatively higher than that in the sensitive strain (21). ABC transporter-mediated resistance to toxicants in yeast and human cells is a consequence of increased constitutive expression of the ABC transporter gene (3, 7), so we thought that the higher level of constitutive expression of the PMR1 gene could be responsible for the higher DMI resistance of the resistant strains. However, introduction of the PMR1 coding region under the control of a strong constitutive promoter, PgpdA, into a DMI-sensitive strain had no observable effect on DMI resistance (H. Hamamoto, O. Nawata, K. Hasegawa, R. Nakaune, Y. J. Lee, Y. Makizumi, K. Akutsu, and T. Hibi, submitted for publication), suggesting that the constitutive expression level of PMR1 is not the determinative factor for DMI resistance. The coding sequences of this gene from three DMI-sensitive and three DMI-resistant strains were also identical. These results suggested that another factor was responsible for the higher DMI resistance (Hamamoto et al., submitted).
Possible resistance mechanisms include mutations of CYP51, the gene encoding cytochrome P450 sterol 14α-demethylase (P45014DM), the target enzyme of DMIs (29), or increased expression of CYP51 (11, 12). Our initial objective in this study was to determine if DMI resistance is attributable to these mechanisms associated with CYP51. We found that a tandem repeat of a unique 126-bp sequence in the region upstream of the CYP51 gene contributes to the overexpression of the gene and results in higher DMI resistance. To our knowledge, this is the first report of the acquisition of fungicide resistance triggered by a transcriptional enhancer in filamentous fungi.
MATERIALS AND METHODS
Fungal strains.
The three DMI-sensitive strains (PD5, DF1, and U1) and the three DMI-resistant strains (LC2, M1, and I1) of P. digitatum were field isolates of different origins (21). The effective concentrations inhibiting radial growth by 50% (EC50s), and the MICs of the DMI fungicide triflumizole were 0.01 to 0.08 and 1 μg/ml, respectively, for the sensitive strains and 1.3 to 2.8 and >100 μg/ml, respectively, for the resistant strains (21). LC2M, a spontaneous mutant with diminished resistance, was isolated from the resistant strain LC2. The EC50 and MIC of triflumizole for LC2M were 0.5 and 10 μg/ml, respectively. We previously sequenced PMR1 (GenBank accession number AB010442), a gene encoding a toxicant-extruding ABC transporter, in LC2 and LC2M, and we observed no difference between the nucleotide sequences in the two strains (data not shown). All of the strains were stored as frozen spore suspensions at −80°C. These strains were deposited in the Institute for Fermentation (IFO), Osaka, Japan. IFO accession numbers are 33112 through 33118 for PD5, DF1, U1, LC2, M1, I1, and LC2M, respectively.
Oligonucleotide primers.
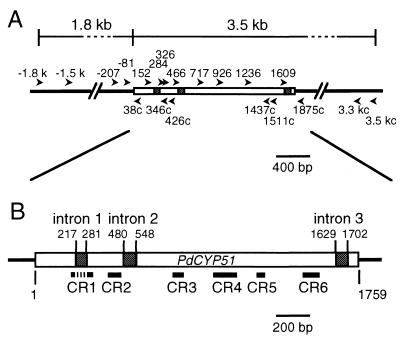
The oligonucleotide primers we designed for use in PCR and nucleotide sequencing are listed in Table 1. The positions of these primers within and around the coding region of the P. digitatum CYP51 (PdCYP51) gene are shown in Fig. 1A.
TABLE 1.
Sequences of the primers used in this study
| Primer | Sequence |
|---|---|
| Pri −1.8k | 5′ CGAGCAACCAGTCTACCCGAAT 3′ |
| Pri −1.5k | 5′ TCGGCGCACATTCTCTACAACC 3′ |
| Pri −207 | 5′ TAGCTCCAAAACAAATCGTCTGCC 3′ |
| Pri −81 | 5′ CAAAACCCTAACTGTGTGGCCTCA 3′ |
| Pri 152 | 5′ TCATTGGGAGTACAGTCGCCTATG 3′ |
| Pri 284 | 5′ CGGCGACATCTT(T/C)TGTCTNCCA(C/G)CNCC(A/G)AA 3′ |
| Pri 326 | 5′ CGTATATCTCGGCGTTGAAGGAAA 3′ |
| Pri 466 | 5′ TGGAGCAAAAGAAGGTACAACTCC 3′ |
| Pri 717 | 5′ CTCCAAGGCGAAGAAGTACGATCA 3′ |
| Pri 926 | 5′ CAACGGCAGGACAGACCCAAAG 3′ |
| Pri 1230 | 5′ CGTCTCCACTCTTCCATTCACAC 3′ |
| Pri 1609 | 5′ TGCCAGACACGGATTACTCGGT 3′ |
| Pri 38c | 5′ CACTTGATCTGCCCTGTTACCA 3′ |
| Pri 346c | 5′ CCTTCAACGCCGAGATATACG 3′ |
| Pri 426c | 5′ CGAAGACGGGGGTTGTAAGCT 3′ |
| Pri 1437c | 5′ CCGTATCAGAAGAATCCTCGGC 3′ |
| Pri 1511c | 5′ AGCG(A/G)TGTCTNCCA(C/G)CNCC(A/G)AA 3′ |
| Pri 1875c | 5′ CTCGCGTGAACCTATGATCGTC 3′ |
| Pri 3.3kc | 5′ AAGACCGATCATACCCTGGCTC 3′ |
| Pri 3.5kc | 5′ GTCAACACCCAACGAAAAGGCC 3′ |
| oligo(dT)Sph | 5′ AACTGGAAGAATTGCATGCAGGAATTTTTTTTTTTTTTTTTT 3′ |
| TSP | 5′ TGGAAGAATTGCATGCAGGAA 3′ |
FIG. 1.
(A) Positions of the primers used for PCR, nucleotide sequencing, and transformation experiments. Numeral components of primer names are shown along boxes and lines, which represent the PdCYP51 ORF and surrounding sequences, respectively. (B) Schematic representation of the structure of the PdCYP51 gene of strain PD5. Open boxes, ORF; shaded boxes, introns. Six regions encoding highly conserved CR domains are indicated below.
Cloning and sequencing of the putative PdCYP51 gene.
Based on the conserved amino acid sequences encoded by the CYP51 genes of Penicillium italicum (25), Ustilago maydis (10), Saccharomyces cerevisiae (14), and C. albicans (17), we designed a set of degenerate primers, Pri 284 and Pri 1511c, to PCR amplify a genomic DNA fragment encoding part of the PdCYP51 gene. The PCR conditions were as follows: 3 min at 94°C followed by 30 cycles each of 30 s at 94°C, 1 min at 60°C, and 2 min at 72°C, using a GeneAmp 2400 or 9700 thermal cycler (Perkin-Elmer [PE] Applied Biosystems, Foster City, Calif.) with Ex Taq polymerase (Takara, Tokyo, Japan). The amplified 1.2-kb fragment was cloned into pGEM-T (Promega, Madison, Wis.) and sequenced by following the instructions in the pGEM-T and pGEM-T Easy Vector Systems technical manual (Promega). The sequence obtained was used to design four inverse PCR (IPCR) primers: Pri 346c, Pri 426c, Pri 926, and Pri 1230. Genomic DNA of PD5 was digested with SalI or XhoI and was self-ligated in order to be subjected to IPCR (22). The IPCR conditions were as follows: 3 min at 94°C followed by 30 cycles each of 30 s at 94°C, 30 s at 55°C, and 3 min at 72°C. The 1.2-kb IPCR products were sequenced directly and contained putative initiation and termination codons. Based on these sequences, we designed a set of primers, Pri −207 and Pri 1875c, to amplify the entire CYP51 gene. The PCR conditions were as follows: 5 min at 94°C followed by 25 cycles each of 30 s at 94°C, 30 s at 56°C, and 2.5 min at 72°C. The amplified 2.1-kb fragment was passed through a SUPREC-02 ultrafilter (Takara) to remove PCR primers. The concentrate was applied to dye terminator labeling reactions by using a dRhodamine Terminator Cycle Sequencing FS Ready Reaction Kit (PE Applied Biosystems) for direct sequencing (Fig. 1A) on an ABI PRISM 377 HM or an ABI PRISM 310 (PE Applied Biosystems) DNA sequencer.
Reverse transcription (RT)-PCR was carried out as follows. Total RNA was isolated from mycelia of strain PD5 grown in potato dextrose broth medium and subjected to RT with a 42-base oligo(dT)Sph anchor primer binding to poly(A) (Table 1) using GibcoBRL Superscript (Life Technologies, Rockville, Md.) according to the manufacturer's instructions. The product of RT was first subjected to PCR with Pri −207 and a 21-base TSP primer (Table 1) corresponding to the oligo(dT)Sph anchor primer. The PCR conditions were as follows: 3 min at 94°C followed by 40 cycles each of 30 s at 94°C, 1 min at 55°C, and 1.5 min at 72°C. The second PCR was carried out with Pri −207 and Pri 1875c, and the product of the second PCR was used for sequencing. The conditions for the second PCR were as follows: 3 min at 94°C followed by 25 cycles each of 30 s at 94°C, 1 min at 58°C, and 1 min at 72°C.
Southern and Northern blot analysis.
Extraction of genomic DNA and total RNA, Southern and Northern blotting, hybridization, and signal detection using the ECL system (Amersham Pharmacia Biotech UK, Bucks, England) were performed as described previously (21). The PCR product amplified with Pri −207 and Pri 1875c was passed through a SUPREC-02 ultrafilter (Takara) and used as a probe to detect the PdCYP51 gene in Southern and Northern blotting. For blotting control, a cDNA fragment containing the actin gene (actA) of P. digitatum was used. To examine the induction of gene expression by a DMI fungicide, the fungus was treated with 50 μg of triflumizole/ml for 10 min in a liquid culture medium. All the expression assays were carried out at least twice.
Cotransformation and toxicant sensitivity assay.
To obtain the sequences upstream and downstream of PdCYP51, the IPCR products were cloned into pGEM-T and sequenced. Based on the sequence data, we designed four primers, Pri −1.8 k, Pri −1.5 k, Pri 3.3 kc, and Pri 3.5 kc (Table 1; Fig. 1A). Pri −1.8k and Pri 3.5 kc were used to amplify the putative promoter and coding region of the PdCYP51 gene. Fragments of 5.3 kbp (PdCYP51-P) and 5.8 kbp (PdCYP51-L) were amplified from strains PD5 and LC2, respectively. These clones were introduced into the DMI-sensitive strain PD5 by cotransformation with plasmid pBF101, which carries a blasticidin S resistance cassette (15). Transformation was performed by the polyethylene glycol method as described by Itoh et al. (13). For selection of blasticidin S-resistant transformants, 100 μg of blasticidin S/ml was used. To isolate the DMI-resistant transformants, conidia of blasticidin S-resistant lines were inoculated onto potato dextrose agar (PDA) plates containing 0, 0.01, 0.1, 1.0, or 10.0 μg of triflumizole/ml, and germination and growth were examined after incubation for 3 days at 25°C. For determination of EC50s and MICs of DMIs and other toxicants, the transformants were purified by subculture of single spores, and EC50s and MICs were determined as described previously (21). Experiments for each toxicant were carried out at least three times. Toxicants tested included four DMIs (triflumizole, fenarimol, bitertanol, and pyrifenox), one antibiotic (cycloheximide), and two mutagens (acriflavine and 4-nitroquinoline-N-oxide [4NQO]).
Nucleotide sequence accession numbers.
The sequences of PdCYP51 from PD5 and LC2 are available under GenBank accession numbers AB030178 and AB030179, respectively.
RESULTS
Sequence and structure of the PdCYP51 gene of strain PD5.
Southern blot analysis suggested that CYP51 is present as a single-copy gene in the genome of P. digitatum strain PD5 (data not shown). The PCR fragment amplified from genomic DNA of P. digitatum strain PD5 with primers Pri −207 and Pri 1875c was 2,082 bp long and had one putative open reading frame (ORF) (nucleotides 1 to 1759) divided into four exons by three introns (nucleotides 217 to 281, 480 to 548, and 1629 to 1702). The positions of the introns were the same as those reported for P. italicum, and the excision of these introns was confirmed by sequencing the RT-PCR product derived from the mRNA of this gene. The deduced amino acid sequence of CYP51 had 87% similarity to that of P. italicum and more than 40% similarity to CYP51 of S. cerevisiae and C. albicans. The P. digitatum sequence contained six regions, CR1 through CR6 (encoded by nucleotides 214 to 302 [containing intron 217 to 281], 405 to 455, 759 to 791, 981 to 1082, 1215 to 1235, and 1482 to 1541, respectively) (Fig. 1B), known to be highly conserved among CYP51 family proteins (1). We named this gene PdCYP51.
Sequence comparison of PdCYP51 genes in seven strains.
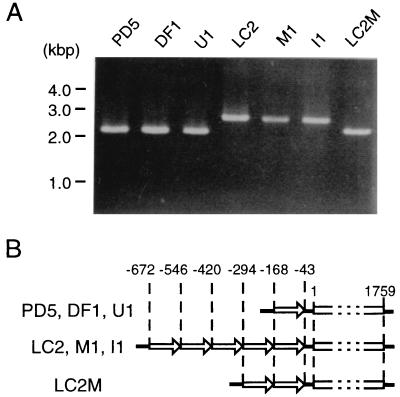
Each of the seven P. digitatum strains studied had a single copy of the PdCYP51 gene in its genome as indicated by Southern blot analysis (data not shown). The PCR products amplified with Pri −207 and Pri 1875c were separated by agarose gel electrophoresis, and fragments of 2.1, 2.7, and 2.3 kb were observed (Fig. 2A). When sequenced, the PdCYP51 structural genes in all of the strains were identical; however, there were apparent differences in the promoter region. Each of the three resistant strains had a tandem repeat of five copies of a unique 126-bp sequence, 5′ GGATCATTTTTGCTCCGGCTGGTGTGACATCTGGGGATGGCCTGACCTGATGATTAATCGTCAATCCTTCCCTCCTGATTTGTCTTACAAAACCCTAACTGTGTGGCCTCATACTTCCGATTCCAG 3′, in the upstream promoter region between nucleotides −672 and −43. In all three sensitive strains this sequence was present only once, while in LC2M, the number of copies was two (Fig. 2B). This sequence was identical in every repeat and was repeated in tandem without any intervening residues. Except for the difference in the number of copies, the sequences of the upstream region amplified with Pri −207 and Pri 1875c from all seven strains were identical. A BLAST and FASTA database search based on the sequence of the repeat unit identified no striking similarities to known sequences except for a similar sequence (80% homology) observed in the region of nucleotides −173 to −47 in the promoter region of the CYP51 gene of P. italicum (25). There is no apparent TATA box or CCAAT box in the region upstream of PdCYP51.
FIG. 2.
Comparison of genomic PCR products derived from three DMI-sensitive strains (PD5, DF1, and U1), three DMI-resistant strains (LC2, M1, and I1), and the mutant strain LC2M, amplified with Pri −207 and Pri 1875c. (A) Ethidium bromide-stained image of the PCR products separated in a 0.7% agarose gel. (B) Schematic representation of the sequences of the PCR products derived from the seven strains. Each open arrow represents one unit of the tandem repeat. Open box, ORF of PdCYP51.
Expression of the PdCYP51 gene.
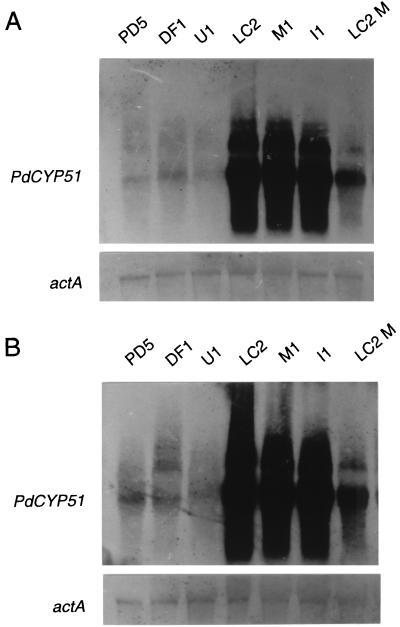
The levels of accumulation of PdCYP51 mRNA (about 2.0 kb) in all three DMI-resistant strains were much higher than those in the DMI-sensitive strains (Fig. 3; autoradiograms were slightly overexposed to show the bands of the sensitive strains). In analyses with a shorter exposure time or diluted samples (data not shown), the levels of PdCYP51 mRNA in the resistant strains were shown to be approximately 100-fold higher than those in the sensitive strains. In the autoradiograms, a second, larger band (about 3.5 kb) was observed, the nature of which is unknown. The level of PdCYP51 mRNA in LC2M was somewhat lower than that in LC2 but higher than those in the sensitive strains. Treatment with the DMI fungicide triflumizole had little effect on the expression level of this gene. These results suggest that the 126-bp sequence in the promoter region of PdCYP51 may be a transcriptional enhancer and that additional copies increase the expression of PdCYP51 and result in higher DMI resistance.
FIG. 3.
Northern blot analysis of expression of the PdCYP51 gene. (A) Expression without fungicide treatment. (B) Expression after triflumizole (50 μg/ml) treatment for 10 min. The RNA on the membrane was rehybridized with an actin gene (actA)-specific probe to check for equal loading of RNA, and the pattern is shown below.
Transformation of a DMI-sensitive strain with a PdCYP51 allele from a DMI-resistant strain.
PdCYP51-P (a 5.3-kb fragment with a promoter with one repeat from PD5) or PdCYP51-L (a 5.8-kb fragment with a promoter with five repeats from LC2) was transformed into the DMI-sensitive strain PD5. Cloning of the 5.8-kb fragment in the plasmid vector sometimes resulted in deletion of the tandem repeats, so we introduced the PCR product directly into the fungus by cotransformation with pBF101, which encodes blasticidin S resistance. We first obtained 23, 21, and 12 blasticidin S-resistant transformants following transformation with PdCYP51-P plus pBF101, PdCYP51-L plus pBF101, and pBF101 alone, respectively. Conidia from the nontransformed parental strain and the 35 transformants obtained by the introduction of PdCYP51-P plus pBF101 or pBF101 alone did not germinate on PDA plus 1 μg of triflumizole/ml. One transformant generated by the introduction of PdCYP51-P plus pBF101, designated PD5(PdCYP51-P)-21, germinated and grew on PDA plus 0.1 μg of triflumizole/ml and had integrated one copy of PdCYP51-P (data not shown). Conidia from 3 transformants obtained by the introduction of PdCYP51-L plus pBF101 did germinate on PDA plus 10 μg of triflumizole/ml, whereas the other 18 transformants did not germinate on PDA plus 1.0 μg of triflumizole/ml and were shown by PCR analysis to harbor no PdCYP51-L gene (data not shown). Two of the transformants acquiring increased DMI resistance, PD5(PdCYP51-L)-7 and PD5(PdCYP51-L)-15, had one and five copies of the foreign PdCYP51-L, respectively (data not shown).
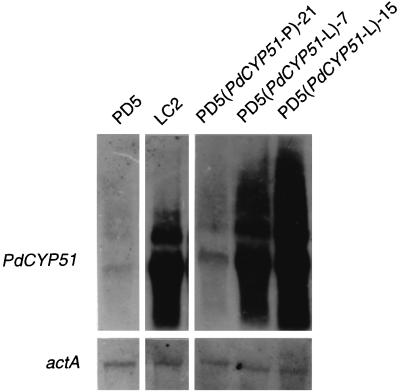
PdCYP51 mRNA levels were increased in PD5(PdCYP51-L)-7 and PD5(PdCYP51-L)-15 to equal that in LC2, but only a slight increase was observed in PD5(PdCYP51-P)-21 (Fig. 4). PD5(PdCYP51-L)-7 and PD5(PdCYP51-L)-15 showed significant increases in DMI resistance (Table 2), and in the cases of triflumizole, fenarimol, and bitertanol, they were almost as resistant as LC2, whereas PD5(PdCYP51-P)-21 showed only a slight increase in resistance. On the other hand, the sensitivities of these transformants to other toxicants remained unchanged. These results suggest that the 126-bp tandem repeat in the promoter enhances expression of PdCYP51 and increases DMI resistance in P. digitatum.
FIG. 4.
Northern blot analysis of constitutive expression of the PdCYP51 gene in the transformed PD5 lines PD5(PdCYP51-P)-21, PD5(PdCYP51-L)-7, and PD5(PdCYP51-L)-15. The RNA on the membrane was rehybridized with an actin gene (actA)-specific probe to check for equal loading of RNA, and the pattern is shown below.
TABLE 2.
EC50s and MICs of DMIs and other toxicants for the transformants into which PdCYP51 was introduced
| Toxicant | Result with the indicated strain (μg/ml)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PD5
|
PD5 (PdCYP51-P)-21
|
PD5 (PdCYP51-L)-7
|
PD5 (PdCYP51-L)-15
|
LC2
|
||||||
| EC50 | MIC | EC50 | MIC | EC50 | MIC | EC50 | MIC | EC50 | MIC | |
| Triflumizole | 0.05 ± 0.02 | 0.5 | 0.15 ± 0.02 | 5 | 1.6 ± 0.9 | >100 | 1.6 ± 0.8 | >100 | 2.5 ± 0.7 | >100 |
| Fenarimol | 0.11 ± 0.03 | 1 | 0.25 ± 0.12 | 10 | 0.64 ± 0.14 | 100 | 0.95 ± 0.18 | >100 | 1.9 ± 0.3 | >100 |
| Bitertanol | 0.14 ± 0.04 | 5 | 0.35 ± 0.03 | 10 | 1.2 ± 0.3 | >100 | 1.6 ± 0.2 | >100 | 2.6 ± 1.6 | >100 |
| Pyrifenox | 0.03 ± 0.00 | 5 | 0.28 ± 0.17 | 10 | 1.5 ± 0.3 | 100 | 3.1 ± 1.6 | >100 | 42 ± 12 | >100 |
| Cycloheximide | 0.11 ± 0.02 | 1 | 0.11 ± 0.03 | 1 | 0.08 ± 0.03 | 1 | 0.07 ± 0.02 | 1 | 0.21 ± 0.07 | 5 |
| 4NQO | 0.37 ± 0.02 | 5 | 0.33 ± 0.17 | 5 | 0.35 ± 0.16 | 5 | 0.29 ± 0.16 | 5 | 0.75 ± 0.52 | 10 |
| Acriflavine | 0.58 ± 0.24 | 5 | 0.60 ± 0.08 | 5 | 0.52 ± 0.08 | 5 | 0.77 ± 0.17 | 5 | 2.8 ± 1.2 | >100 |
DISCUSSION
DMI resistance in fungi could be acquired either as a result of mutations in the CYP51 gene or as a result of an increased expression level of this gene (11, 12). In some Candida species that cause oral candidiasis in AIDS patients, the molecular mechanism of DMI resistance has been intensively studied, and point mutations in CYP51 were associated with increased DMI resistance (2, 18, 23, 27). Also, in the phytopathogenic fungi P. italicum, Uncinula necator, and E. graminis, changes in the amino acid sequence of CYP51 are associated with DMI resistance (4, 5, 6).
Changes in the expression level of CYP51 might contribute to the gradual development of DMI resistance (8, 20, 26). In Candida glabrata, increased expression of CYP51 could occur due to an increase in CYP51 copy number resulting from a chromosome duplication (20). Such a mechanism for toxicant resistance is also known in mammalian systems as gene amplification (24). However, the contribution of increased expression of CYP51 to DMI resistance in field isolates of phytopathogenic fungi is unknown.
With respect to the P. digitatum CYP51 gene, PdCYP51, the protein-coding regions of the six alleles we cloned were identical. More specifically, none of the three amino acid residues, Tyr126, Ile313 and Arg457, substitution at which has been reported to be associated with DMI resistance (2, 4, 5, 6, 18, 23, 27), were resistant-type residues. Thus, changes in the amino acid sequence of the CYP51 protein were not responsible for the differences we observed in DMI resistance. We did find a unique 126-bp sequence that was tandemly repeated five times in the DMI-resistant strains. We have further checked the presence of the tandem repeat in another seven DMI-resistant strains and nine DMI-sensitive strains of P. digitatum by PCR with primers Pri −207 and 38c, and we have confirmed that all resistant strains tested had a tandem repeat of five copies of a 126-bp sequence, while all sensitive strains had only one copy (H. Hamamoto et al., unpublished data). This 126-bp sequence may act as a transcriptional enhancer, with the tandem repeat increasing the level of expression of PdCYP51 and thereby conferring DMI resistance. Also, the results of the transformation of PD5 with PdCYP51 suggest that the copy number of PdCYP51 may have a small effect on the level of expression of this gene and DMI resistance. This report is the first to show that overexpression of CYP51 contributes to DMI resistance in phytopathogenic fungi.
Previously, we showed that PMR1, a gene encoding an ABC transporter involved in toxicant extrusion, plays a role in DMI resistance in P. digitatum and that a mutant in which PMR1 was disrupted exhibited a loss of DMI resistance (21). Considering these findings on PMR1 together with the present results on CYP51, we hypothesize that DMI sensitivity depends on the ratio of toxicant molecules to target enzyme molecules inside the fungal cell. The number of toxicant molecules inside the cell is controlled by PMR1, which extrudes to the outside of the cell the intruding toxicant and/or the endogenous toxic oxysterols accumulated by the action of DMI. The number of target enzyme molecules is dependent on the level of expression of CYP51. Therefore, when PMR1 and CYP51 are expressed at ordinary levels, the fungi are DMI sensitive. Also, when PMR1 is disrupted, the fungi are DMI sensitive, even when CYP51 is overexpressed (21). When PMR1 is expressed at an ordinary level and CYP51 is overexpressed, the fungi exhibit resistance, as shown in this study. It is also possible that the fungi will exhibit resistance if PMR1 is overexpressed at an extraordinary level and CYP51 is expressed at an ordinary level, although the strains used in the present study could not be used to test this hypothesis.
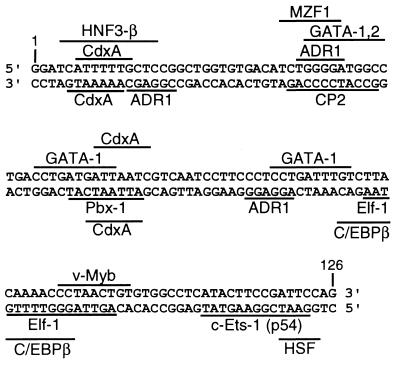
The mechanism by which the tandem repeat unit enhances the expression of PdCYP51 remains to be determined. The 126-bp sequence contains four sites for known fungal transcriptional binding factors (ADR1 and HSF) and 15 sites for vertebrate binding factors [HNF3-β, CdxA, MZF1, GATA-1, GATA-2, Pbx-1, Elf-1, c/EBPβ, v-Myb, and c-Ets-1(p54)] (Fig. 5). Similar clusters of transcription factor binding sites also occur in TATA-less promoter sequences (19, 28, 30), so the 126-bp sequence might function as both a transcriptional enhancer and a promoter.
FIG. 5.
Transcription factor binding sites within the 126-bp sequence found by means of a computer search on the GenomeNet server using the MOTIF program (The Supercomputer Laboratory, The Institute for Chemical Research, Kyoto University [http://www.genome.ad.jp/]). The sites found in the upper strand are shown above the sequence, and the sites in the lower strand are shown below the sequence.
We do not know if the resistant strains were derived from sensitive strains by mutation while under selection for fungicide resistance. We did not detect any additional repeats in DMI-sensitive strains that had been subcultured on PDA containing low concentrations of triflumizole for 6 months. From LC2M, we know that a resistant strain can lose some resistance by loss of some of the copies of the repeated sequence. At present, we cannot explain how the copy number of the enhancer sequence increases.
This transcriptional enhancer-based mechanism is a new fungicide resistance mechanism for filamentous fungi. Whether the mechanism also occurs in other filamentous fungi or plays a role in resistance to toxicants other than DMIs remains to be determined. Finally, we think that the strong enhancer activity of the tandem repeats might be a useful tool to increase gene expression in other applied microbiology and biotechnology contexts.
ACKNOWLEDGMENTS
This study was supported by the program for promotion of basic research activities for innovative biosciences of the Bio-oriented Technology Research Advancement Institution (BRAIN).
REFERENCES
- 1.Aoyama Y, Noshiro M, Gotoh O, Imaoka S, Funae Y, Kurosawa N, Horiuchi T, Yoshida Y. Sterol 14-demethylase P450 (P45014DM) is one of the most ancient and conserved P450 species. J Biochem. 1996;119:926–933. doi: 10.1093/oxfordjournals.jbchem.a021331. [DOI] [PubMed] [Google Scholar]
- 2.Asai K, Tsuchimori N, Okonogi K, Perfect J R, Gotoh O, Yoshida Y. Formation of azole-resistant Candida albicans by mutation of sterol 14-demethylase P450. Antimicrob Agents Chemother. 1999;43:1163–1169. doi: 10.1128/aac.43.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balzi E, Goffeau A. Yeast multidrug resistance: the PDR network. J Bioenerg Biomembr. 1995;27:71–76. doi: 10.1007/BF02110333. [DOI] [PubMed] [Google Scholar]
- 4.Délye C, Bousset L, Corio-Costet M-F. PCR cloning and detection of point mutations in the eburicol 14α-demethylase (CYP51) gene from Erysiphe graminis f. sp. hordei, a “recalcitrant” fungus. Curr Genet. 1998;34:399–403. doi: 10.1007/s002940050413. [DOI] [PubMed] [Google Scholar]
- 5.Délye C, Laigret F, Corio-Costet M-F. A mutation in the 14α-demethylase gene of Uncinula necator that correlates with resistance to a sterol biosynthesis inhibitor. Appl Environ Microbiol. 1997;63:2996–2970. doi: 10.1128/aem.63.8.2966-2970.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Waard M A. Molecular genetics of resistance in fungi to azole fungicides. ACS Symp Ser. 1996;645:62–71. [Google Scholar]
- 7.Endicott J A, Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- 8.Franz R, Kelly S L, Lamb D C, Kelly D E, Ruhnke M, Morschhäuser J. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob Agents Chemother. 1998;42:3065–3072. doi: 10.1128/aac.42.12.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs A, Drandarevski C A. The likelihood of development of resistance to systemic fungicides which inhibit ergosterol biosynthesis. Neth J Plant Pathol. 1976;82:85–87. [Google Scholar]
- 10.Hargreaves J A, Keon J P R. Isolation of an Ustilago maydis ERG11 gene and its expression in a mutant deficient in sterol 14α-demethylase activity. FEMS Microbiol Lett. 1996;139:203–207. doi: 10.1111/j.1574-6968.1996.tb08203.x. [DOI] [PubMed] [Google Scholar]
- 11.Hitchcock C A. Resistance of Candida albicans to azole antifungal agents. Biochem Soc Trans. 1993;21:1039–1047. doi: 10.1042/bst0211039. [DOI] [PubMed] [Google Scholar]
- 12.Hollomon D W. Resistance to azole fungicides in the field. Biochem Soc Trans. 1993;21:1047–1051. doi: 10.1042/bst0211047. [DOI] [PubMed] [Google Scholar]
- 13.Itoh Y, Johnson R, Scott B. Integrative transformation of the mycotoxin-producing fungus Penicillium paxilli. Curr Genet. 1994;25:508–513. doi: 10.1007/BF00351670. [DOI] [PubMed] [Google Scholar]
- 14.Kalb V F, Woods C W, Turi T G, Dey C R, Sutter T R, Loper J C. Primary structure of the P450 lanosterol demethylase gene from Saccharomyces cerevisiae. DNA. 1987;6:529–537. doi: 10.1089/dna.1987.6.529. [DOI] [PubMed] [Google Scholar]
- 15.Kimura M, Kamakura T, Tao Q Z, Kaneko I, Yamaguchi I. Cloning of the blasticidin S deaminase gene (BSD) from Aspergillus terreus and its use as a selectable marker for Schizosaccharomyces pombe and Pyricularia oryzae. Mol Gen Genet. 1994;242:121–129. doi: 10.1007/BF00391004. [DOI] [PubMed] [Google Scholar]
- 16.Köller W, Scheinpflug H. Fungal resistance to sterol biosynthesis inhibitors: a new challenge. Plant Dis. 1987;71:1066–1074. [Google Scholar]
- 17.Lai M H, Kirsch D R. Nucleotide sequence of cytochrome P450L1A1 (lanosterol 14α-demethylase) from Candida albicans. Nucleic Acids Res. 1989;17:804. doi: 10.1093/nar/17.2.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamb D C, Kelly D E, Schunck W-H, Shyadehi A Z, Akhtar M, Lowe D J, Baldwin B C, Kelly S L. The mutation T315A in Candida albicans sterol 14α-demethylase causes reduced enzyme activity and fluconazole resistance through reduced affinity. J Biol Chem. 1997;272:5682–5688. doi: 10.1074/jbc.272.9.5682. [DOI] [PubMed] [Google Scholar]
- 19.Luckhart S, Rosenberg R. Gene structure and polymorphism of an invertebrate nitric oxide synthase gene. Gene. 1999;232:25–34. doi: 10.1016/s0378-1119(99)00121-3. [DOI] [PubMed] [Google Scholar]
- 20.Marichal P, Bossche H V, Odds F C, Nobels G, Warnock D W, Timmerman V, van Broeckhoven C, Fay S, Mose-Larsen P. Molecular biological characterization of an azole-resistant Candida glabrata isolate. Antimicrob Agents Chemother. 1997;41:2229–2237. doi: 10.1128/aac.41.10.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakaune R, Adachi K, Nawata O, Tomiyama M, Akutsu K, Hibi T. A novel ATP-binding cassette transporter involved in multidrug resistance in the phytopathogenic fungus Penicillium digitatum. Appl Environ Microbiol. 1998;64:3983–3988. doi: 10.1128/aem.64.10.3983-3988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochman H, Gerber A S, Hartl D L. Genetic application of an inverse polymerase chain reaction. Genetics. 1988;12:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanglard D, Ischer F, Koymans L, Bille J. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother. 1998;42:241–253. doi: 10.1128/aac.42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schimke R T. Gene amplification in cultured animal cells. Cell. 1984;37:705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- 25.van Nistelrooy J G M, van den Brink J M, van Kan J A L, van Gorcom R F M, de Waard M A. Isolation and molecular characterization of the gene encoding eburicol 14α-demethylase (CYP51) from Penicillium italicum. Mol Gen Genet. 1996;250:725–733. doi: 10.1007/BF02172984. [DOI] [PubMed] [Google Scholar]
- 26.White T C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White T C. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14α-demethylase in Candida albicans. Antimicrob Agents Chemother. 1997;41:1488–1494. doi: 10.1128/aac.41.7.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wrehlke C, Wiedemeyer W-R, Schmitt-Wrede H-P, Mincheva A, Lichter P, Wunderlich F. Genomic organization of mouse gene zfp162. DNA Cell Biol. 1999;18:419–428. doi: 10.1089/104454999315303. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida Y, Aoyama Y. Sterol 14α-demethylase and its inhibition: structural considerations on interaction of azole antifungal agents with lanosterol 14α-demethylase (P-45014DM) of yeast. Biochem Soc Trans. 1991;19:778–782. doi: 10.1042/bst0190778. [DOI] [PubMed] [Google Scholar]
- 30.Zeng G, Gao L, Yu R K. Isolation and functional analysis of the promoter of the rat CMP-NeuAc:GM3 α2,8 sialyltransferase gene. Biochim Biophys Acta. 1998;1397:126–136. doi: 10.1016/s0167-4781(98)00030-x. [DOI] [PubMed] [Google Scholar]