Abstract
T cells engineered to express chimeric antigen receptors (CARs) specific for CD19 have yielded remarkable clinical outcomes in patients with refractory B-cell malignancies. The first CARs to be approved by the US Food and Drug Administration and the European Medicines Agency are CD19 CARs that comprise either CD28/CD3ζ or 4-1BB/CD3ζ dual-signalling domains. While their efficacy and safety profiles in patients with B-cell malignancies are comparable overall, the functional properties these two CAR designs impart upon engineered T cells differ significantly. Remarkably, alternative costimulatory domains have not, to date, superseded these foundational designs. Rather, recent CAR advances have focused on perfecting the original CD28- and 4-1BB-based CD19 CARs by calibrating strength of activation, pre-empting T-cell exhaustion and increasing the functional persistence of CAR T cells. This article reviews the essential biological properties of these first-in-class prototypes and their recent evolution.
Key words: cancer immunotherapy, CAR therapy, CD19, CD28, 4-1BB, chimeric antigen receptors, immune-oncology
Highlights
-
•
CD19 chimeric antigen receptor (CAR) therapy has shown remarkable success against B-cell malignancies.
-
•
The prototypic CD19 CARs comprise either CD28/CD3ζ or 4-1BB/CD3ζ signalling domains.
-
•
Both CD19 CARs yield similar efficacy but impart distinct T-cell functionalities.
-
•
Novel CAR designs aim to enhance the persistence or effector potency of T cells.
-
•
Genome editing averts variegated CAR expression and sustains T-cell function.
Introduction
Chimeric antigen receptors (CARs) are synthetic receptors that reprogram the specificity, phenotype and functions of immune cells.1 Early fusion receptors that combined an extracellular antigen-binding moiety derived from an antibody with a T-cell activating domain,2,3 later referred to as ‘first-generation CARs’,4 redirected the cytolytic activity of T-cell lines or hybridomas but could not sustain the function of primary T cells.5 The integration of activating and costimulatory functions within a single receptor for antigen, yielding ‘second-generation CARs’,4 enabled human peripheral blood T cells to not only lyse their targets but expand upon repeated exposure to antigen.6 Focusing on CD19 as a promising CAR target,7 second-generation CARs encoding either CD28 or 4-1BB costimulatory domains6,8 were approved by the US Food and Drug Administration (FDA) in 2017 and the European Medicines Agency in 2018 for the treatment of relapsed/refractory non-Hodgkin lymphoma and acute lymphoblastic leukaemia (ALL).9 Several reviews that address the clinical results of CAR therapy trials targeting CD19 using engineered T cells or natural killer (NK) cells are available.10, 11, 12, 13, 14, 15 There are currently over 500 CAR trials worldwide registered at clinicaltrials.gov, with more than 230 targeting CD19.16 Second-generation CARs using either CD28 or 4-1BB costimulatory domains account for approximately 80% of the investigated CAR designs.16
In the context of B-cell malignancies, 28ζ and BBζ CARs targeting CD19 (hereafter referred to as ‘1928ζ’ and ‘19BBζ’) have achieved overall comparable therapeutic efficacy.1,9, 10, 11, 12,14,17 Due to rapidly increasing clinical experience, the major challenges of CD19 CAR therapy, irrespective of the costimulatory component, have become evident, including a significant relapse rate despite remarkable initial response to therapy and treatment-associated toxicities.14,15,18,19 Tumour relapses have been associated with insufficient T-cell persistence, dysfunctional T-cell states, and antigen escape with absent or low-level antigen expression.19, 20, 21, 22 The two most severe toxicities caused by CAR T-cell therapy include cytokine release syndrome (CRS), which is characterized by elevated fever, hypotension and hypoxia associated with abundant release of pro-inflammatory cytokines, and immune effector cell-associated neurotoxicity syndrome (ICANS), which has been associated with endothelial dysfunction, increased permeability of the blood–brain barrier and microglial activation.18,23,24
Preclinical and clinical studies have revealed important differences in the properties imparted by 28ζ and BBζ CARs on T cells. The detailed investigation of their respective features, together with an ever-progressing understanding of intrinsic and extrinsic determinants that promote T-cell-mediated tumour immunity, instruct the development of novel CAR T-cell designs at a rapid pace. This review will summarize current knowledge on the foundational 28ζ and BBζ CARs themselves and their respective evolution. Complementary efforts to enhance CAR T cells, including improvements in the manufacturing process, the selection of T-cell subsets and CD4/CD8 ratios, combinatorial targeting and logic gating strategies, armoured CARs and safety switches, are beyond the scope of this review.
CD28 and 4-1BB receptors in natural T-cell responses
The ligation of the physiological T-cell receptor (TCR) with cognate peptide–major histocompatibility complexes initiates T-cell activation, but naïve T cells require costimulation to prevent T-cell unresponsiveness and mount an effective response.25, 26, 27 The immunoglobulin superfamily member CD28 and the tumour necrosis factor receptor superfamily member 4-1BB are the two main costimulatory receptors to have been originally co-opted within second-generation CAR T cells.27,28
CD28 engagement by its ligands CD80 or CD86 expressed in antigen-presenting cells (APCs) initiates signal transduction events that affect a variety of T-cell processes.28,29 CD28 costimulation augments signals generated by TCR ligation, lowers the threshold for naïve T-cell activation,25,26 and recruits the PI3K-AKT pathway and the transcription factors NF-kB, NFAT and AP1 to regulate cell proliferation, survival, effector function and differentiation.26,27 In addition, CD28 signalling upregulates the expression of several cytokines and chemokines, most significantly interleukin-2 (Il-2); increases glucose uptake upon T-cell activation; and induces epigenetic changes that are required for commitment to cell growth, cell-cycle entry and differentiation.25, 26, 27,29 Lack of CD28 costimulation is associated with decreased T-cell proliferation and differentiation, inhibition of germinal centre formation and repressed cytokine expression, coinciding with reduced responses to a wide spectrum of immune challenges in CD28-deficient mice.26,30
4-1BB (CD137) is induced transiently by TCR and CD28 signalling in CD4+ and CD8+ T cells and binds to its ligand 4-1BBL (CD137L) expressed in APCs and activated T cells.28 4-1BB ligation transmits signalling via recruitment of TNF-receptor-associated factor (TRAF) proteins, leading to activation of the PI3K/AKT, stress-activated protein kinase/JNK, p38 MAPK, ERK1/2 and NF-kB pathways.28,31 4-1BB-mediated signalling promotes mitochondrial function and biogenesis in T cells; augments T-cell survival; and enhances cytokine production, proliferation and memory formation.28,32 Mice deficient in 4-1BB show diminished CD8+ T-cell responses with decreased cytolytic activity and cytokine secretion, as well as a reduced CD8+ memory pool.28,33
Essential functional characteristics of 28ζ and BBζ CAR T cells
The combinatorial structure of CARs (Figure 1) determines unique spatial and temporal constraints that warrant close examination of the properties these synthetic molecules impart on T-cell subsets. 28ζ CAR T cells have been shown to possess potent effector functions, associated with robust cytokine secretion and effector differentiation following antigen encounter and relatively rapid tumour elimination.28,34, 35, 36, 37, 38, 39 In comparison, BBζ CAR T cells show a slower effector response but greater T-cell persistence.34,40,41 In accordance with the metabolic features of their costimulatory receptor of origin,32,42,43 the high effector functions of 28ζ CAR T cells are associated with increased aerobic glycolysis, while BBζ CAR T cells show greater mitochondrial biogenesis and oxidative metabolism.41 1928ζ CAR T cells mediate more rapid tumour eradication in several aggressive leukaemia and lymphoma mouse models, including ‘stress test conditions’ based on the administration of low T-cell doses, compared to 19BBζ.21,34,38,39,44 However, 19BBζ CAR T cells accumulate to increased numbers over time, compensating through their durability for their slighter cytolytic capabilities on a single cell basis.21,34 These findings in mouse models are corroborated in clinical trials that report earlier clearance of 1928ζ45, 46, 47 CAR T cells relative to 19BBζ CAR T cells. The latter can be detected in some patients for months and even years.15,48, 49, 50
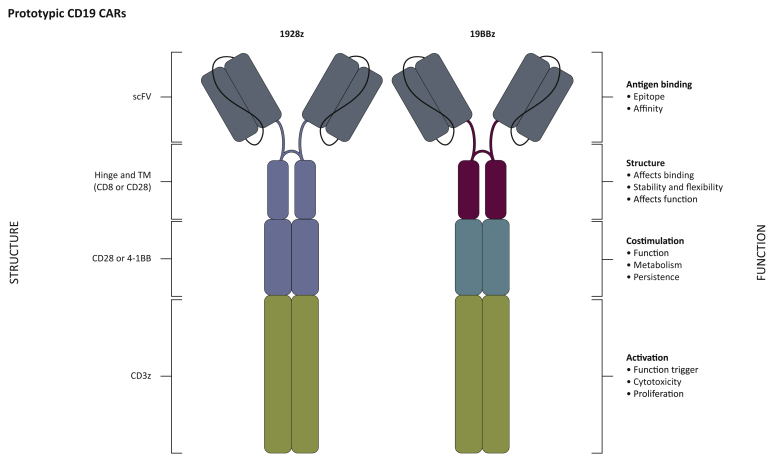
Figure 1.
Structure–function of US Food and Drug Administration (FDA)-approved CD19 chimeric antigen receptors (CARs).
Second-generation CARs incorporate an extracellular antigen-binding moiety (scFv) linked to hinge (H) and transmembrane (TM) domains, a costimulatory and a T-cell-activating element. Each structural domain contributes to the overall CAR functionality. Current FDA-approved CAR T cells comprise an anti-CD19 single-chain variable fragment (scFv) fused to CD28 H/TM (blue), intracellular CD28 (blue) and CD3ζ signalling domains [‘1928ζ’ (axicabtagene ciloleucel, Yescarta)] or an anti-CD19 scFv linked to CD8 H/TM (magenta), 4-1BB costimulatory (turquoise) and CD3ζ domains [‘19BBζ’ CAR T cells (tisagenlecleucel, Kymriah)].
Although side-by-side comparative studies are lacking in patients, the capability of both CAR constructs to achieve efficient tumour control and similar response rates overall, in spite of the distinct kinetics of tumour eradication and T-cell accumulation, is reflected in multiple clinical trials reported to date.14,15 While CAR T-cell persistence is an important correlate of long-term remission in 19BBζ-treated patients, such persistence does not seem to be an essential prerequisite for durable responses induced by 1928ζ.36,48,50, 51, 52 The lesser correlation between CAR T-cell persistence and survival in 1928ζ-treated patients indicates that this CAR design can induce sustained remissions on the strength of rapid responses without obligate persistence.36,45,46 Thus, persistence becomes an increasingly important requirement for T cells with more modest intrinsic effector potential. The contribution of T-cell persistence should also be evaluated with caution, not only through the mere presence of CAR T cells, but by assessing the functionality of those persisting T cells, such as their capability for memory formation, rapid re-expansion and protection against tumour rechallenge or prevention of endogenous relapse. Some poor responses to 19BBζ CAR T-cell therapy have been linked to impaired T-cell states in preclinical and clinical studies, highlighting the importance of evaluating functional persistence, not mere T-cell survival.21,53,54
T-cell exhaustion and tonic signalling in CAR T cells
T-cell exhaustion is a functional state characterized by diminished effector function, hypo-responsiveness to antigen, and an altered transcriptional and epigenetic profile.55, 56, 57 Exhausted T cells typically co-express multiple inhibitory receptors, such as PD1, TIM3, LAG3, CTLA4 and/or TIGIT, and exhibit a reduced Tbet/EOMES ratio.56,58, 59, 60 CAR T-cell dysfunction due to T-cell exhaustion is an important limitation of both CD28ζ and BBζ CAR T cells. CAR-induced exhaustion should be distinguished from a pre-existing condition in patient T cells present upon their collection or reduced functionality secondary to suboptimal culture conditions,50,52,61,62 all of which may contribute to impaired T-cell quality.
Increased expression of exhaustion markers appears earlier in infused 1928ζ CAR T cells compared to 19BBζ.21,63 This phenotypic difference has been attributed to the strong activation mediated by CD28ζ CARs, driving rapid T-cell differentiation and high effector function preceding T-cell contraction.34,35 T-cell dysfunction also occurs in 19BBζ CAR T cells and has been associated with poor clinical responses and tumour escape in murine models.21,53 Progressive functional impairment of 19BBζ CAR T cells with concomitant transcriptional and epigenetic reprogramming is further observed upon persistent antigen stimulation by death-receptor-dysregulated leukaemia cells.54
While T-cell exhaustion may arise following repeated exposure to antigen,56 CARs can produce antigen-independent or tonic signalling which promotes T-cell expansion64 and also induces premature T-cell dysfunction, impairing antitumour activity.55,63 T-cell dysfunction occurring in the absence of cognate antigen is a general phenomenon observed with different CAR designs. In some settings, it may occur faster in 28ζ CAR T cells,63 but tonic signalling does not only depend on the costimulatory moiety of the CAR. Other contributing factors involve CAR expression levels, vector type and copy number, promotor strength, as well as other CAR structural components including the scFv (e.g. prone to spontaneous oligomerization), the CD3ζ chain or the selection of hinge/transmembrane (H/TM) domains.55,63, 64, 65, 66, 67, 68, 69 For example, T-cell exhaustion triggered by tonic signalling was observed in CAR T cells targeting the disialoganglioside GD2, but was not detected in CD19 CAR T cells, independent of 4-1BB or CD28 costimulation.55,63 Other studies have observed antigen-independent signalling in 19BBζ and 1928ζ CAR T cells,38,40,70,71 albeit with differences in the underlying signalling pathways.40 Tonic CAR-derived 4-1BB signalling may be amplified in gammaretroviral vectors through continuous activation of the NF-kB pathway, which enhances LTR promoter activity and, in turn, amplifies CAR expression and tonic signalling.71 Attenuated CAR expression can mitigate these effects and augment therapeutic potency.71 Importantly, controlling transcription of the CARs by means of genome editing can abate tonic signalling and T-cell exhaustion, resulting in improved antitumour efficacy.66
Tumour relapse and antigen sensitivity
Similar to other targeted immunotherapies such as monoclonal or bispecific T-cell-engager antibodies, antigen modulation on tumour cells can impede therapeutic efficacy of CAR T cells.19,22,72,73 Tumour escape with variants expressing low levels of antigen is one important cause of primary and/or acquired resistance to CAR therapy.19,21,44,74 Although some studies demonstrate the potential of CAR T cells to target tumour cells with very low antigen density in vitro,75,76 it has become evident that antigen expression below a certain threshold impedes in vivo antitumour efficacy of both 1928ζ and 19BBζ in vivo.21,44 Several studies indicate that the costimulatory composition of CARs contributes to determining CAR sensitivity to low levels of target antigen.21,44,77 CAR T cells incorporating CD28 generally have higher activity against tumour cells with low antigen levels compared to BBζ-based CARs, which are more vulnerable to antigen downregulation.21,38,44
The differential antigen sensitivity of 1928ζ and 19BBζ CAR T cells was demonstrated in an experimental relapse model using the B-ALL cell line NALM6, in which antigen reduction resulted from trogocytic transfer of the CD19 antigen from the malignant cells to T cells, thereby reducing the antigen density on the remaining target cells.21 1928ζ CAR T cells showed improved control of relapses compared with 19BBζ, resulting in enhanced survival of mice rescued with 1928ζ CAR T cells.21 The higher antigen sensitivity threshold of 19BBζ CAR T cells was confirmed in models using stable CD19 antigen expression, in which 1928ζ CAR T cells outperformed 19BBζ CAR T cells at low antigen levels and significantly prolonged survival of treated mice.21,44 1928ζ CAR T cells killed efficiently, proliferated robustly and produced IL-2 in response to antigen-low leukaemia cells, in contrast to 19BBζ CAR T cells which exhibited lesser responses.44 The observed differences were linked to a more rapid tumour lysis in 1928ζ T-cell-target cell conjugates and to increased signalling strength observed with 1928ζ CAR T cells.21,37,44 The lower antigen expression levels required for effective antitumour activity of CD28ζ-based CARs were further linked to their increased basal CD3ζ phosphorylation owing to LCK recruitment into the CAR synapse, imprinting 1928ζ CAR T cells to a higher magnitude of activity in response to low antigen stimulation, whereas CAR-CD3ζ phosphorylation in BBζ-based CARs has been shown to be negatively regulated by the THEMIS–SHP1 complex.38
Together, these findings establish a link between the CAR's costimulatory component and its target sensitivity (i.e. the target antigen density required for effective tumour elimination). This observation points to the importance of monitoring antigen density in tumour escape specimens, and clearly delineating antigen-negative versus antigen-low relapses.
Signalling of 1928ζ and 19BBζ CARs
Mass spectrometry analyses have revealed that protein phosphorylation events activated by the stimulation of CD28ζ- and 4-1BBζ-based CAR T cells are highly similar.37,78 Major differences in T-cell fates observed between CD28ζ- and 4-1BBζ-based CAR T cells were linked to changes in the kinetics and magnitude of protein phosphorylation after activation rather than to specific/unique signalling pathways triggered by each costimulatory element.37 The faster and more intense phosphoprotein signals induced by CD28 compared with 4-1BB costimulation were associated with an effector-like T-cell phenotype,37 and the elevated signal intensity in CD28ζ-based CARs was partly related to a greater constitutive association of LCK with this domain.37 Expanding on this finding, a recent study has demonstrated that CD28 and 4-1BB regulate the equilibrium of kinases and phosphatases differentially within the CAR synapse, thereby determining the magnitude of CAR T-cell activation.38 LCK has been shown to promote basal CD3ζ phosphorylation in the synapse of CD28ζ-based CAR T cells, resulting in increased antigen-dependent T-cell activation, while the THEMIS-SHP1 phosphatase complex attenuates these effects in CAR T cells expressing 4-1BBζ38 by dephosphorylating the CD3ζ endodomain and diminishing T-cell activation.
Pharmacological inhibition of the PI3K/AKT axis limits glycolytic metabolism and T-cell differentiation in 1928ζ CAR T cells, pointing towards an important role of this signalling pathway in CD28ζ-based CAR T cells.36,40,79 NF-kB has been identified as a critical signalling pathway for 19BBζ CAR T cells, and the functionality of 4-1BB within CARs is in part mediated by specific TRAF proteins to activate NF-kB.36,71 Moreover, specific interrogation of the non-canonical (nc) NF-kB pathway has indicated that 19BBζ CAR T cells exhibit constitutive and higher ligand-induced ncNF-kB signalling compared with 1928ζ CAR T cells.40 ncNF-kB signalling was required to promote cell survival in 19BBζ CAR T cells and was associated with reduced abundance of the most pro-apoptotic isoforms of Bim.40 Much still remains to be elucidated with regards to 28ζ and BBζ CAR signalling.
Strategies to improve CD28ζ CARs
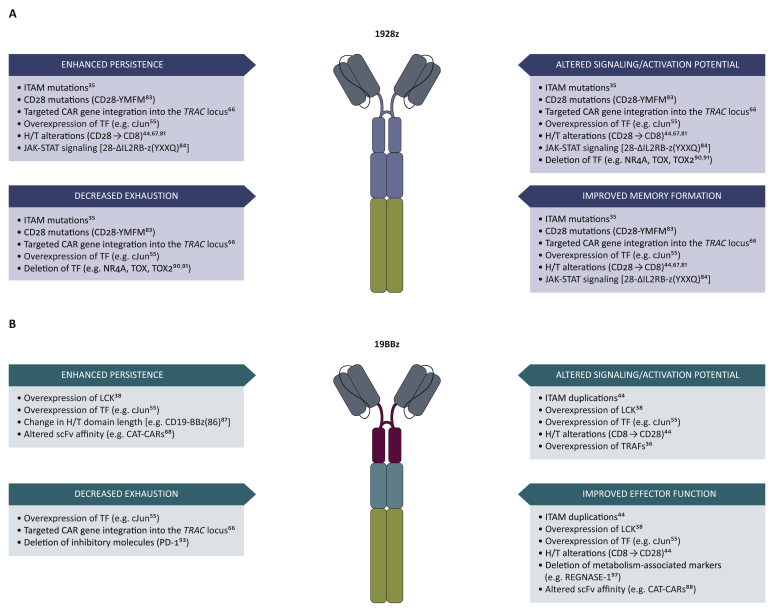
One approach to increase the therapeutic efficacy of 1928ζ CAR T cells is to extend their functional persistence (Figure 2). Given the redundancy of CD28 and CD3ζ signalling, we hypothesized that signalling strength may be excessive and thus calibrated down the CAR activation potential by mutating immune-tyrosine-based activation (ITAM) motifs in the CD3ζ chain. 1928ζ CAR T cells bearing only two functional ITAMs in membrane-proximal position, termed ‘1928ζ1XX’, outperformed conventional 1928ζ CAR T cells under stress test conditions in a murine leukaemia model in vivo.35 In contrast to the short-lived 1928ζ CAR T cells, which may differentiate rapidly and acquire an exhausted phenotype, 1928ζ1XX CAR T cells show increased persistence in a functional state, resulting in long-term T-cell memory and resistance to multiple tumour rechallenges.35 28ζ-1XX CAR T cells will soon be evaluated in the clinic.
Figure 2.
Engineering strategies to improve 1928ζ and 19BBζ chimeric antigen receptors (CARs).
(a) Approaches to overcome limitations of CD28ζ-based CAR T cells. (b) Strategies to improve functionality of BBz-based CAR T cells. Recent developments are focused on tuning strength of activation, reversing exhaustion, enhancing functional persistence, and combining features of both CAR prototypes by enhancing memory formation in 1928ζ CAR T cells and enhancing the effector function of 19BBζ CAR T cells.
Studies focusing on the length and composition of the H/TM domain44,67,68,80 have shown that CD19 CARs with a CD8∝ H/TM domain display reduced cytokine secretion, decreased T-cell activation and less susceptibility to activation-induced cell death compared with CARs incorporating CD28 H/TM domains, albeit displaying inferior antitumour activity in disseminated leukaemia models.44,67 In a phase 1 clinical trial in patients with B-cell lymphoma, CD8α(H/TM)-28ζ CAR T-cell therapy showed comparable antitumour efficacy as the commercial FMC63-28ζ CAR, but suggested increased CAR T-cell persistence and lower incidence of neurologic toxicities.81 Longer patient follow-up is needed to determine if CD8α(H/TM)-28ζ CAR T cells retain high therapeutic efficacy.
Other approaches have aimed to alter the signalling of 28ζ CARs by mutating the CD28 signalling domain.82,83 Modifications in the CD28 PYAPP motif to disrupt LCK binding decreased IL-2 secretion upon CAR engagement, without impairing proliferation and cytotoxicity.82 Another single amino acid mutation in the YMNM motif of CD28, turning it into the YMFM motif of ICOS, a CD28-like costimulatory receptor, mediated a transcriptional signature associated with reduced T-cell differentiation and exhaustion, resulting in enhanced CAR T-cell persistence and antitumour activity.83
CARs comprising a truncated cytoplasmic IL-2Rβ and a STAT3 binding (YXXQ) motif together with CD28 and CD3ζ domains [28-ΔIL2RB-ζ(YXXQ)] enable antigen-dependent activation of the JAK-STAT pathway.84 28-ΔIL-2RB-ζ(YXXQ) CAR T cells show delayed T-cell differentiation, increased proliferation and cytokine polyfunctionality compared with 1928ζ or 19BBζ CAR T cells.84 The enhanced persistence and augmented cytokine secretion of engineered T cells with increased JAK-STAT signalling will require cautious evaluation of potential toxicities.
Strategies to improve BBζ CARs
While reducing the CAR activation potential by mutating ITAMs in the CD3ζ signalling domain has been shown to be a successful strategy to extend the persistence of 1928ζ CAR T cells,35 4-1BBζ CAR T cells may, on the contrary, benefit from signalling augmentation to boost their performance. Inclusion of additional ITAMs to augment activating strength can indeed increase the functionality of less potent 19BBζ CAR T cells (Figure 2). Incorporating two copies of the CD3ζ chain in 19BBζζ CAR T cells, yielding 12 ITAMs per CAR, enhanced antigen sensitivity, increased IL-2 secretion and improved antitumour activity in response to target cells with low CD19 density relative to 19BBζ CAR T cells, but still with inferior antitumour activity compared with 1928ζ CAR T cells.44 LCK overexpression in 19BBζ CAR T cells was associated with increased basal phosphorylation of CAR-CD3ζ and also resulted in improved antitumour activity compared with 19BBζ CAR T cells, without diminishing the potential for increased persistence.38 NF-kB signalling, which plays an essential role in 4-1BB costimulation, also promotes increased CAR T-cell proliferation. 19BBζ CAR T cells with mutated TRAF binding domains display reduced NF-kB signalling and attenuated T-cell function. Conversely, modulation of NFkB signalling through overexpression of TRAF proteins can enhance antitumour T-cell activity. Thus, 19BBζ CAR T cells that overexpress TRAF2 or TRAF3 display increased viability, proliferation and cytotoxicity function in vitro. However, 19BBζ CAR T cells with excess TRAF2 did not reach superior antitumour activity in vivo compared with 19BBζ CAR T cells.36 The optimization of TRAF recruitment may thus have the potential to enhance CAR T-cell function but requires further investigation.36 Blockade of TGF-β signalling enhances 4-1BB-mediated T-cell function as demonstrated by improved antitumour activity induced by a chimeric TGF-βR2-41BB receptor.85 On the other hand, 28ζ CAR T cells are intrinsically resistant to TGF-ß repression relative to BBζ CARs.86
Similar to 28ζ CARs,67,81 alterations in the H/TM region can impact T-cell effector function in 19BBζ CAR T cells.44 Substitution of a CD8 H/TM domain with the CD28 H/TM region from 1928ζ CARs6 resulted in increased cytokine production and enhanced antitumour activity of 19BBζ CAR T cells, especially at low CD19 expression levels.44 In a recent phase I clinical trial with 19BBζ CAR T cells, in which the length of the CD8α H/TM domain was modified to potentially reduce toxicities,87 19-BBζ(86) variant CAR T cells comprising an 86-amino-acid fragment from human CD8α with a longer extracellular domain and a longer intracellular sequence compared with the 19BBζ(71) prototype, reduced CRS and neurological toxicities after treatment with 19BBζ(86) CAR T cells while therapeutic responses were effective.87 Further studies are needed to ascertain that therapeutic efficacy induced by 19BBζ(86) is not impaired.
Other efforts to improve 4-1BBζ-based CAR T cells have focused on CAR binding affinity. 19BBζ CAR T cells incorporating a lower-affinity scFv with a faster off-rate (CAT CARs) than the FMC63 high-affinity binder used in FDA-approved CD19 CAR T cells were evaluated in paediatric patients and young adults with high-risk B-ALL.88 Initial responses and survival rates were comparable to historical CD19 CAR trials in ALL, but associated with enhanced expansion and persistence of CAR T cells without severe CRS or ICANS. These lesser toxicities have to be interpreted with caution due to low tumour burden before treatment, which is generally associated with reduced risk for severe CRS.89 Here too, the efficacy and durability of responses will have to be assessed with longer follow-up.
General approaches to enhance both 28ζ and BBζ CAR T cells
There are other potential improvements to CAR function that apply to both 28ζ and BBζ CARs (Figure 3).
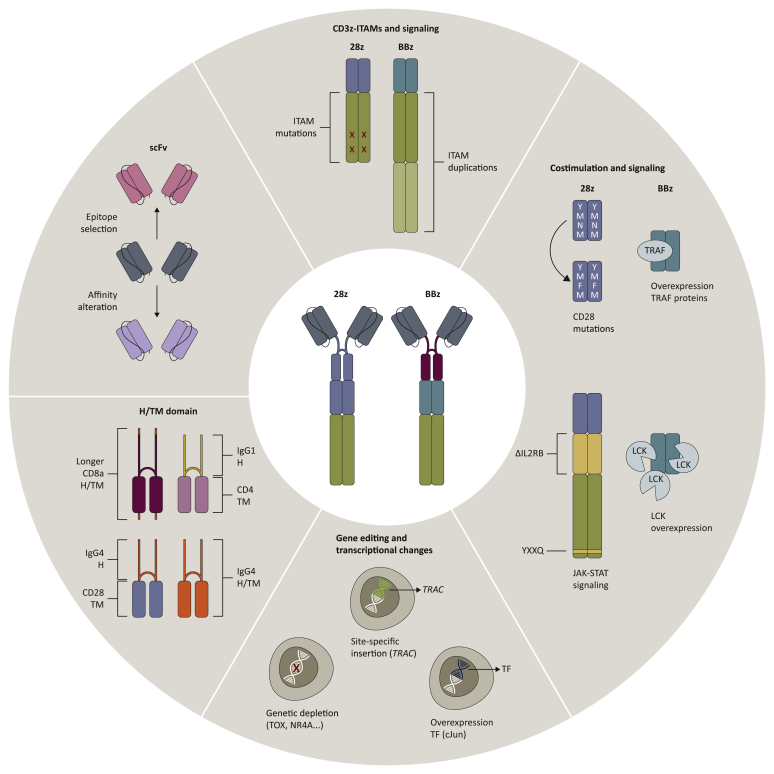
Figure 3.
Structural evolution of 1928ζ and 19BBζ chimeric antigen receptor (CAR) T cells.
Current strategies to enhance therapeutic potency of prototype CD19 CAR T cells (centre) involve alterations in each structural component of the CAR design, including scFv, hinge and transmembrane (H/T) domains, and intracellular signalling moieties (costimulation and CD3ζ chain). Novel approaches to tailor CAR T-cell properties further include changes in downstream signalling, transcriptional alterations and gene-editing strategies.
The genomic integration site of the CAR cDNA plays a central role in determining the phenotype and function of CAR T cells. Controlled CAR expression through targeted insertion of the 1928ζ CAR cDNA in the TRAC locus using CRISPR/Cas9 results in uniform CAR expression levels and distinct patterns of antigen-induced CAR internalization and re-expression in comparison to retrovirally engineered CAR T cells. TRAC-CAR T cells exhibit reduced tonic signalling, delayed T-cell exhaustion, differentiation and enhanced antitumour activity relative to conventionally engineered CAR T cells.66 Similarly, TRAC-19BBζ CAR T cells yield constant CAR expression and achieve superior tumour eradication in vivo.66
Transcriptional and epigenetic alterations associated with T-cell exhaustion include epigenomic dysregulation of AP-1 transcription factor binding motifs and increased expression of the bZIP and IRF transcription factors, which have been implicated in mediating T-cell dysfunction.55 Overexpression of the activating AP-1 family transcription factor c-Jun in CD28ζ- or 4-1BBζ-based CAR T cells establishes a more propitious balance between activating and inhibitory AP-1 complexes.55
To prevent T-cell exhaustion in CD28ζ-based CARs, further efforts have involved genetic targeting of transcription factors driving T-cell exhaustion,90,91 such as the high-mobility group-box transcription factors TOX and TOX2; and the members of the NR4A family of nuclear receptor transcription factors Nr4a1, Nr4a2 and Nr4a3. 1928ζ CAR T cells lacking all three Nr4a transcription factors promoted improved tumour regression and prolonged survival of CD19+ tumour-bearing mice relative to 1928ζ wild-type CAR T cells in murine models. The enhanced effector function of Nr4a triple knockout CAR T cells was associated with increased cytokine production, downregulation of inhibitory receptors, and strong enrichment in accessible chromatin for binding motifs of transcription factors involved in effector function.91 1928ζ CAR T cells double deficient in TOX and TOX2 showed reduced expression of T-cell exhaustion markers, higher cytokine secretion, and displayed transcriptional and epigenetic alterations associated with T-cell activation and effector function, resulting in enhanced antitumour effects in vivo.90,91
CRISPR-Cas9-mediated disruption of the inhibitory molecules PD-1 or LAG3 has been investigated as a strategy to prevent T-cell dysfunction in 19BBζ CAR T cells,92,93 and deletion of the Pdcd1 (PD-1) locus in 19BBζ CAR T cells resulted in enhanced in vivo antitumour activity against PD-L1+ tumours.93 Further gene-editing approaches that are currently evaluated involve multiplex genome editing targeting TCR, β2-microglobulin and Fas, PD-1 and CTLA-4 or other molecules to promote efficacy and more universal applicability of CD19 CAR T cells.94, 95, 96 Deletion of REGNASE-1 in CD8+ 19BBζ CAR T cells reprogrammed T cells in the tumour microenvironment to long-lived effector cells by enhancing mitochondrial metabolism and effector responses.97 Previous findings further demonstrated improved functionality of PTPN2-deleted CAR T cells in solid tumour models.98 The ablation of the histone methyltransferase Suv39h1 was shown to enhance long-term memory and functionality in murine CD8+ T cells.99 These latter findings open novel perspectives for epigenetic reprogramming of CAR T cells.
Clinical experience with gene-edited T cells is still very limited. Cautious interrogation of efficacy and safety risks, changes in the manufacturing process, and identification of host factors influencing therapeutic potency are required for their successful clinical translation.
Conclusions
The incorporation of costimulatory molecules in the design of synthetic receptors for antigen paved the way for the success of CD19 CAR therapy and the advent of ‘living drugs’. 1928ζ and 19BBζ are the most frequently investigated CAR T-cell constructs worldwide. Their unprecedented success has laid the foundation for a global increase in CAR T-cell clinical trials. While overall comparable efficacy has been reported for 1928ζ and 19BBζ CARs across different B-cell malignancies, the combination of activating and costimulatory signals can be finely tuned to modulate CAR T-cell effector functions and persistence. A deepening understanding of how CARs work will enable the design of better and safer therapies. It is noteworthy that while many other natural costimulatory signalling domains are available and easy to incorporate into a CAR structure, the most profound recent insights into CAR T-cell biology come from detailed studies of the functional properties of the canonical 28ζ and BBζ CAR T cells – in vitro, in xenogeneic murine models and in clinical trial correlative studies. The development of tumour models that recapitulate the human diseases, including patient-derived xenograft models and human-derived organoids, should further accelerate the testing and comparison of different CAR T-cell products. Complementing these analyses and the rapid development of novel CAR designs, the use of genome editing,66,95,100 still limited in clinical practice, is poised to further advance the design of CAR T cells for a wide range of medical applications.
Acknowledgments
Funding
This work was supported by the Memorial Sloan Kettering Cancer Center Support Grant [P30 CA008748]. J.F. was supported by the Care-for-Rare Foundation and the German Research Foundation.
Disclosure
M.S. reports research funding from Fate Therapeutics, Takeda Pharmaceuticals and Atara Biotherapeutics. M.S. serves on the Scientific Advisory Board of St Jude Children Research Hospital. M.S. and J.F. hold several patents on CAR technologies.
References
- 1.Sadelain M., Riviere I., Riddell S. Therapeutic T cell engineering. Nature. 2017;545(7655):423–431. doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eshhar Z., Waks T., Gross G., Schindler D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90(2):720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brocker T., Peter A., Traunecker A., Karjalainen K. New simplified molecular design for functional T cell receptor. Eur J Immunol. 1993;23(7):1435–1439. doi: 10.1002/eji.1830230705. [DOI] [PubMed] [Google Scholar]
- 4.Sadelain M., Brentjens R., Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21(2):215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gong M.C., Latouche J.B., Krause A., Heston W.D., Bander N.H., Sadelain M. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1999;1(2):123–127. doi: 10.1038/sj.neo.7900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maher J., Brentjens R.J., Gunset G., Riviere I., Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20(1):70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 7.Brentjens R.J., Latouche J.B., Santos E., et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9(3):279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 8.Imai C., Mihara K., Andreansky M., et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18(4):676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 9.June C.H., Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brudno J.N., Kochenderfer J.N. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol. 2018;15(1):31–46. doi: 10.1038/nrclinonc.2017.128. [DOI] [PubMed] [Google Scholar]
- 11.Salter A.I., Pont M.J., Riddell S.R. Chimeric antigen receptor-modified T cells: CD19 and the road beyond. Blood. 2018;131(24):2621–2629. doi: 10.1182/blood-2018-01-785840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain M.D., Davila M.L. Concise review: emerging principles from the clinical application of chimeric antigen receptor T cell therapies for B cell malignancies. Stem Cells. 2018;36(1):36–44. doi: 10.1002/stem.2715. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka J., Miller J.S. Recent progress in and challenges in cellular therapy using NK cells for hematological malignancies. Blood Rev. 2020:100678. doi: 10.1016/j.blre.2020.100678. [DOI] [PubMed] [Google Scholar]
- 14.Frigault M.J., Maus M.V. State of the art in CAR T cell therapy for CD19+ B cell malignancies. J Clin Invest. 2020;130(4):1586–1594. doi: 10.1172/JCI129208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Majzner R.G., Mackall C.L. Clinical lessons learned from the first leg of the CAR T cell journey. Nat Med. 2019;25(9):1341–1355. doi: 10.1038/s41591-019-0564-6. [DOI] [PubMed] [Google Scholar]
- 16.MacKay M., Afshinnekoo E., Rub J., et al. The therapeutic landscape for cells engineered with chimeric antigen receptors. Nat Biotechnol. 2020;38(2):233–244. doi: 10.1038/s41587-019-0329-2. [DOI] [PubMed] [Google Scholar]
- 17.Guedan S., Calderon H., Posey A.D., Jr., Maus M.V. Engineering and design of chimeric antigen receptors. Mol Ther Methods Clin Dev. 2019;12:145–156. doi: 10.1016/j.omtm.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santomasso B., Bachier C., Westin J., Rezvani K., Shpall E.J. The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book. 2019;39:433–444. doi: 10.1200/EDBK_238691. [DOI] [PubMed] [Google Scholar]
- 19.Shah N.N., Fry T.J. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16(6):372–385. doi: 10.1038/s41571-019-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majzner R.G., Mackall C.L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8(10):1219–1226. doi: 10.1158/2159-8290.CD-18-0442. [DOI] [PubMed] [Google Scholar]
- 21.Hamieh M., Dobrin A., Cabriolu A., et al. CAR T cell trogocytosis and cooperative killing regulate tumour antigen escape. Nature. 2019;568(7750):112–116. doi: 10.1038/s41586-019-1054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh N., Orlando E., Xu J., et al. Mechanisms of resistance to CAR T cell therapies. Semin Cancer Biol. 2020;65:91–98. doi: 10.1016/j.semcancer.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gust J., Hay K.A., Hanafi L.A., et al. Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giavridis T., van der Stegen S.J.C., Eyquem J., Hamieh M., Piersigilli A., Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24(6):731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boomer J.S., Green J.M. An enigmatic tail of CD28 signaling. Cold Spring Harb Perspect Biol. 2010;2(8):a002436. doi: 10.1101/cshperspect.a002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Acuto O., Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat Rev Immunol. 2003;3(12):939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 27.Chen L., Flies D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Stegen S.J., Hamieh M., Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov. 2015;14(7):499–509. doi: 10.1038/nrd4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esensten J.H., Helou Y.A., Chopra G., Weiss A., Bluestone J.A. CD28 costimulation: from mechanism to therapy. Immunity. 2016;44(5):973–988. doi: 10.1016/j.immuni.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shahinian A., Pfeffer K., Lee K.P., et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261(5121):609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 31.Ward-Kavanagh L.K., Lin W.W., Sedy J.R., Ware C.F. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity. 2016;44(5):1005–1019. doi: 10.1016/j.immuni.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menk A.V., Scharping N.E., Rivadeneira D.B., et al. 4-1BB costimulation induces T cell mitochondrial function and biogenesis enabling cancer immunotherapeutic responses. J Exp Med. 2018;215(4):1091–1100. doi: 10.1084/jem.20171068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon B.S., Hurtado J.C., Lee Z.H., et al. Immune responses in 4-1BB (CD137)-deficient mice. J Immunol. 2002;168(11):5483–5490. doi: 10.4049/jimmunol.168.11.5483. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Z., Condomines M., van der Stegen S.J.C., et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell. 2015;28(4):415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feucht J., Sun J., Eyquem J., et al. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat Med. 2019;25(1):82–88. doi: 10.1038/s41591-018-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li G., Boucher J.C., Kotani H., et al. 4-1BB enhancement of CAR T function requires NF-kappaB and TRAFs. JCI Insight. 2018;3(18) doi: 10.1172/jci.insight.121322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salter A.I., Ivey R.G., Kennedy J.J., et al. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci Signal. 2018;11(544) doi: 10.1126/scisignal.aat6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun C., Shou P., Du H., et al. THEMIS-SHP1 recruitment by 4-1BB tunes LCK-mediated priming of chimeric antigen receptor-redirected T cells. Cancer Cell. 2020;37(2):216–225.e6. doi: 10.1016/j.ccell.2019.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng Z., Wei R., Ma Q., et al. In vivo expansion and antitumor activity of coinfused CD28- and 4-1BB-engineered CAR-T cells in patients with B Cell leukemia. Mol Ther. 2018;26(4):976–985. doi: 10.1016/j.ymthe.2018.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philipson B.I., O'Connor R.S., May M.J., June C.H., Albelda S.M., Milone M.C. 4-1BB costimulation promotes CAR T cell survival through noncanonical NF-kappaB signaling. Sci Signal. 2020;13(625) doi: 10.1126/scisignal.aay8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawalekar O.U., O'Connor R.S., Fraietta J.A., et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity. 2016;44(2):380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 42.Frauwirth K.A., Riley J.L., Harris M.H., et al. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16(6):769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 43.Jacobs S.R., Herman C.E., Maciver N.J., et al. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180(7):4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Majzner R.G., Rietberg S.P., Sotillo E., et al. Tuning the antigen density requirement for CAR T cell activity. Cancer Discov. 2020;10(5):702–723. doi: 10.1158/2159-8290.CD-19-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park J.H., Riviere I., Gonen M., et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378(5):449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davila M.L., Riviere I., Wang X., et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maude S.L., Frey N., Shaw P.A., et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maude S.L., Laetsch T.W., Buechner J., et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fraietta J.A., Lacey S.F., Orlando E.J., et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mueller K.T., Waldron E., Grupp S.A., et al. Clinical pharmacology of tisagenlecleucel in B-cell acute lymphoblastic leukemia. Clin Cancer Res. 2018;24(24):6175–6184. doi: 10.1158/1078-0432.CCR-18-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finney O.C., Brakke H.M., Rawlings-Rhea S., et al. CD19 CAR T cell product and disease attributes predict leukemia remission durability. J Clin Invest. 2019;129(5):2123–2132. doi: 10.1172/JCI125423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuster S.J., Svoboda J., Chong E.A., et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377(26):2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh N., Lee Y.G., Shestova O., et al. Impaired death receptor signaling in leukemia causes antigen-independent resistance by inducing CAR T-cell dysfunction. Cancer Discov. 2020;10(4):552–567. doi: 10.1158/2159-8290.CD-19-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lynn R.C., Weber E.W., Sotillo E., et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature. 2019;576(7786):293–300. doi: 10.1038/s41586-019-1805-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blank C.U., Haining W.N., Held W., et al. Defining ‘T cell exhaustion’. Nat Rev Immunol. 2019;19(11):665–674. doi: 10.1038/s41577-019-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wherry E.J. T cell exhaustion. Nat Immunol. 2011;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 58.Pauken K.E., Wherry E.J. SnapShot: T cell exhaustion. Cell. 2015;163(4):1038–1038.e1. doi: 10.1016/j.cell.2015.10.054. [DOI] [PubMed] [Google Scholar]
- 59.Paley M.A., Kroy D.C., Odorizzi P.M., et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338(6111):1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Philip M., Schietinger A. Heterogeneity and fate choice: T cell exhaustion in cancer and chronic infections. Curr Opin Immunol. 2019;58:98–103. doi: 10.1016/j.coi.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghassemi S., Nunez-Cruz S., O'Connor R.S., et al. Reducing ex vivo culture improves the antileukemic activity of chimeric antigen receptor (CAR) T cells. Cancer Immunol Res. 2018;6(9):1100–1109. doi: 10.1158/2326-6066.CIR-17-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stock S., Schmitt M., Sellner L. Optimizing manufacturing protocols of chimeric antigen receptor T cells for improved anticancer immunotherapy. Int J Mol Sci. 2019;20(24) doi: 10.3390/ijms20246223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Long A.H., Haso W.M., Shern J.F., et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21(6):581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frigault M.J., Lee J., Basil M.C., et al. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol Res. 2015;3(4):356–367. doi: 10.1158/2326-6066.CIR-14-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ajina A., Maher J. Strategies to address chimeric antigen receptor tonic signaling. Mol Cancer Ther. 2018;17(9):1795–1815. doi: 10.1158/1535-7163.MCT-17-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eyquem J., Mansilla-Soto J., Giavridis T., et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543(7643):113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alabanza L., Pegues M., Geldres C., et al. Function of novel anti-CD19 chimeric antigen receptors with human variable regions is affected by hinge and transmembrane domains. Mol Ther. 2017;25(11):2452–2465. doi: 10.1016/j.ymthe.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watanabe N., Bajgain P., Sukumaran S., et al. Fine-tuning the CAR spacer improves T-cell potency. Oncoimmunology. 2016;5(12):e1253656. doi: 10.1080/2162402X.2016.1253656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng W., O'Hear C.E., Alli R., et al. PI3K orchestration of the in vivo persistence of chimeric antigen receptor-modified T cells. Leukemia. 2018;32(5):1157–1167. doi: 10.1038/s41375-017-0008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Milone M.C., Fish J.D., Carpenito C., et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17(8):1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomes-Silva D., Mukherjee M., Srinivasan M., et al. Tonic 4-1BB costimulation in chimeric antigen receptors impedes T cell survival and is vector-dependent. Cell Rep. 2017;21(1):17–26. doi: 10.1016/j.celrep.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mejstrikova E., Hrusak O., Borowitz M.J., et al. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017;7(12):659. doi: 10.1038/s41408-017-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhojwani D., Sposto R., Shah N.N., et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia. 2019;33(4):884–892. doi: 10.1038/s41375-018-0265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fry T.J., Shah N.N., Orentas R.J., et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nerreter T., Letschert S., Gotz R., et al. Super-resolution microscopy reveals ultra-low CD19 expression on myeloma cells that triggers elimination by CD19 CAR-T. Nat Commun. 2019;10(1):3137. doi: 10.1038/s41467-019-10948-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watanabe K., Terakura S., Martens A.C., et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 zeta chimeric antigen receptor-modified effector CD8+ T cells. J Immunol. 2015;194(3):911–920. doi: 10.4049/jimmunol.1402346. [DOI] [PubMed] [Google Scholar]
- 77.Priceman S.J., Gerdts E.A., Tilakawardane D., et al. Co-stimulatory signaling determines tumor antigen sensitivity and persistence of CAR T cells targeting PSCA+ metastatic prostate cancer. Oncoimmunology. 2018;7(2):e1380764. doi: 10.1080/2162402X.2017.1380764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramello M.C., Benzaid I., Kuenzi B.M., et al. An immunoproteomic approach to characterize the CAR interactome and signalosome. Sci Signal. 2019;12(568) doi: 10.1126/scisignal.aap9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klebanoff C.A., Crompton J.G., Leonardi A.J., et al. Inhibition of AKT signaling uncouples T cell differentiation from expansion for receptor-engineered adoptive immunotherapy. JCI Insight. 2017;2(23) doi: 10.1172/jci.insight.95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hudecek M., Sommermeyer D., Kosasih P.L., et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2015;3(2):125–135. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brudno J.N., Lam N., Vanasse D., et al. Safety and feasibility of anti-CD19 CAR T cells with fully human binding domains in patients with B-cell lymphoma. Nat Med. 2020;26(2):270–280. doi: 10.1038/s41591-019-0737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kofler D.M., Chmielewski M., Rappl G., et al. CD28 costimulation impairs the efficacy of a redirected t-cell antitumor attack in the presence of regulatory t cells which can be overcome by preventing Lck activation. Mol Ther. 2011;19(4):760–767. doi: 10.1038/mt.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guedan S., Madar A., Casado-Medrano V., et al. Single residue in CD28-costimulated CAR T cells limits long-term persistence and antitumor durability. J Clin Invest. 2020;130(6):3087–3097. doi: 10.1172/JCI133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kagoya Y., Tanaka S., Guo T., et al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat Med. 2018;24(3):352–359. doi: 10.1038/nm.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roth T.L., Li P.J., Blaeschke F., et al. Pooled knockin targeting for genome engineering of cellular immunotherapies. Cell. 2020;181(3):728–744.e1. doi: 10.1016/j.cell.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Golumba-Nagy V., Kuehle J., Hombach A.A., Abken H. CD28-zeta CAR T cells resist TGF-beta repression through IL-2 signaling, which can be mimicked by an engineered IL-7 autocrine loop. Mol Ther. 2018;26(9):2218–2230. doi: 10.1016/j.ymthe.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ying Z., Huang X.F., Xiang X., et al. A safe and potent anti-CD19 CAR T cell therapy. Nat Med. 2019;25(6):947–953. doi: 10.1038/s41591-019-0421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ghorashian S., Kramer A.M., Onuoha S., et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med. 2019;25(9):1408–1414. doi: 10.1038/s41591-019-0549-5. [DOI] [PubMed] [Google Scholar]
- 89.Brentjens R.J., Davila M.L., Riviere I., et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seo H., Chen J., Gonzalez-Avalos E., et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8(+) T cell exhaustion. Proc Natl Acad Sci U S A. 2019;116(25):12410–12415. doi: 10.1073/pnas.1905675116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen J., Lopez-Moyado I.F., Seo H., et al. NR4A transcription factors limit CAR T cell function in solid tumours. Nature. 2019;567(7749):530–534. doi: 10.1038/s41586-019-0985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y., Zhang X., Cheng C., et al. CRISPR-Cas9 mediated LAG-3 disruption in CAR-T cells. Front Med. 2017;11(4):554–562. doi: 10.1007/s11684-017-0543-6. [DOI] [PubMed] [Google Scholar]
- 93.Rupp L.J., Schumann K., Roybal K.T., et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep. 2017;7(1):737. doi: 10.1038/s41598-017-00462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ren J., Zhang X., Liu X., et al. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget. 2017;8(10):17002–17011. doi: 10.18632/oncotarget.15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qasim W., Zhan H., Samarasinghe S., et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9(374) doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 96.Poirot L., Philip B., Schiffer-Mannioui C., et al. Multiplex genome-edited T-cell manufacturing platform for “off-the-shelf” adoptive T-cell immunotherapies. Cancer Res. 2015;75(18):3853–3864. doi: 10.1158/0008-5472.CAN-14-3321. [DOI] [PubMed] [Google Scholar]
- 97.Wei J., Long L., Zheng W., et al. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature. 2019;576(7787):471–476. doi: 10.1038/s41586-019-1821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wiede F., Lu K.H., Du X., et al. PTPN2 phosphatase deletion in T cells promotes anti-tumour immunity and CAR T-cell efficacy in solid tumours. EMBO J. 2020;39(2):e103637. doi: 10.15252/embj.2019103637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pace L., Goudot C., Zueva E., et al. The epigenetic control of stemness in CD8(+) T cell fate commitment. Science. 2018;359(6372):177–186. doi: 10.1126/science.aah6499. [DOI] [PubMed] [Google Scholar]
- 100.Stadtmauer E.A., Fraietta J.A., Davis M.M., et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367(6481) doi: 10.1126/science.aba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]