Abstract
Calcium (Ca) is a macronutrient and works as a modulator to mitigate oxidative stress induced by heavy metals. In this study, we investigated the role of Ca to ameliorate the Cd toxicity in Zea mays L. by modulating the growth, physio-biochemical traits, and cellular antioxidant defense system. Maize genotype Sahiwal-2002 was grown under a controlled glasshouse environment with a day/night temperature of 24 ± 4°C/14 ± 2°C in a complete randomized design with three replications and two Cd levels as (0 and 150 μM) and six regimes of Ca (0, 0.5, 1, 2.5, 5, and 10 mM). Maize seedlings exposed to Cd at 150 μM concentration showed a notable decrease in growth, biomass, anthocyanins, chlorophylls, and antioxidant enzymes activities. A higher level of Cd (150 μM) also caused an upsurge in oxidative damage observed as higher electrolyte leakage (increased membrane permeability), H2O2 production, and MDA accumulation. Supplementation of Ca notably improved growth traits, photosynthetic pigments, cellular antioxidants (APX, POD, and ascorbic acid), anthocyanins, and levels of osmolytes. The significant improvement in the osmolytes (proteins and amino acids), and enzymatic antioxidative defense system enhanced the membrane stability and mitigated the damaging effects of Cd. The present results concluded that exogenously applied Ca potentially improve growth by regulating antioxidants and enabling maize plants to withstand the Cd toxicity.
Introduction
While growing in natural environments, plants are exposed to various environmental stresses that limit yield and productivity [1]. Heavy metal pollution is spreading in cultivated lands and is causing severe environmental hazards to crop plants, human health and ecosystems [2]. Cadmium (Cd) is regarded as the most toxic heavy metal, typically when present in agricultural lands due to its higher mobility and toxicity [3]. Plants can readily absorb Cd directly through roots from the soil along with essential nutrients [4]. Like other heavy metals, Cd causes structural changes in plants and adversely affects the morphological, physiological, and biochemical mechanisms eventually leading to loss of agricultural productivity [5]. Cadmium is highly toxic to plants and imposes negative influences on growth and entire metabolism [6]. It is typically non-essential for agricultural crops as no known role is ascribed to Cd in the growth and development of crop plants [1]. Therefore, Cd even in minor concentrations disturbs photosynthesis, changes the ultrastructure of the chloroplast, increases lipid peroxidation and enhance the production of ROS that leads to oxidative damage [7–9]. The dynamics of Cd in the rhizosphere depends on uptake mechanisms, translocation, and toxicity of Cd in plants. In crop plants, the toxicity of Cd reduces uptake and translocation of nutrients and water, increases oxidative damage, disrupts plant metabolism, and inhibits plant morphology and physiology [10]. In wheat, for example, Cd exposure reduced plant growth, yield, photosynthetic efficiency, hormones, proteins and increased MDA, H2O2, soluble sugars and prolines [11]. Another direct effect of high Cd is the production of excessive ROS (H2O2, OH−, O2.-, 1O2) resulting in lipids peroxidation which ultimately reduces plant growth [12].
Many defensive mechanisms are induced in plants to counteract Cd toxicity mainly by hyper production of antioxidants (non-enzymatic or enzymatic) to control heavily produced ROS [5, 13]. These enzymatic antioxidants (like peroxidase, superoxide dismutase, ascorbate peroxidase, and catalases), and non-enzymatic antioxidants (such as α-tocopherol and glutathione) have been reported to successfully mitigate Cd-induced oxidative damage in many crop plants [12, 14]. Those plants protected by antioxidants show improved growth and yield [15]. Other reports show that heavy metals may result in hyper-accumulation of proteins as an effective strategy to mitigate Cd-induced toxicity [16]. The supplementation of Ca strengthens the anti-oxidants, reduction in lipid peroxidation, and increases proline accumulation and synthesis, clearly indicating protection against Cd stress by increasing the maintenance of systematic resistance criteria [17].
Calcium (Ca2+) is an essential macromolecule and divalent cation that performs an imperative role in membrane permeability, metabolism and signal transduction [16, 18, 19]. It is a central regulator in the physio-chemical process and regulates plant growth [20]. Exogenously applied Ca alleviates oxidative stress by chelating with target proteins (for instance calcium-binding proteins) and activating the antioxidant enzymes [21, 22]. Though, the induction of the antioxidative defense system by Ca is not yet elucidated sufficiently, some reports support that Ca is involved in the modulation of genes for antioxidant enzymes [23]. Ca mitigates Cd toxicity in plants by modifications in the morphological and physiological processes [20, 24]. For example, Ca maintains permeability of membranes by a reduction in peroxidation of lipids and solute leakage which ultimately reduces oxidative stress caused by Cd stress [25]. Calcium is involved in controlling basic functions such as photomorphogenesis, cell division, cell elongation, stress responses, and the maintenance of membrane structure and functions [24, 26]. In other reports, Ca improved growth and photosynthesis by restricting Cd translocation and accumulation, scavenging ROS, enhancing antioxidant levels, and maintaining Ca-dependent signal transduction [27, 28]. Still, the ameliorative role of Ca to alleviate heavy metals toxicity remains inconclusive and therefore it is imperative to investigate its specific roles and associated mechanisms in improving the growth of Zea mays L. seedlings.
Maize is a valuable cereal crop and provides food for humans as well as fodder for livestock. It contributes to 36% (782 Mt) of global grain production [29]. Maize seeds are enriched with energy as 100g seeds contain 365 kilocalories of energy [30]. Among worldwide production, 70–80% of maize is used as food and was ranked third in Pakistan for consumption after wheat and rice. Pakistan was ranked 18th in the production with 6130 thousand tons of maize produced annually that was cultivated at 1334 thousand hectares [31]. The requirement for maize production has significantly increased recently due to excessive usage in the wet milling industry as well as food for poultry [32]. This needs not only to increase the cultivation area but also the exploration of new promising techniques to increase crop survival and yield under stressful environments like in soils contaminated with heavy metals [33]. Maize plant is tolerant to certain levels of Cd, however, when exposure to high levels causes negative effects on different growth stages that are more severe on the emergence of the seedlings and at fourth leaf stage [34].
Cadmium contamination is gradually increasing in soils and is causing significant crop losses. Therefore, there is a dire need to devise new strategies to combat the problems associated with Cd toxicity. Considering many critical roles played by Ca in plant growth and metabolism, it was hypothesized that Ca supplementation should effectively ameliorate the adverse effects of Cd imposed on germinating seeds of maize. The research questions included probing into the toxic effects of Cd on the growth, physio-biochemical characteristics and to what extent supplemental Ca can alleviate Cd toxicity in maize. Since the information is lacking regarding the mechanisms involved in the amelioration of cadmium toxicity, this work will suggest future directions to work out the underlying molecular mechanisms involved in the mitigation of heavy metals in different plants.
Materials and methods
Plant materials
Maize seeds (Sahiwal-2002) were obtained from Maize and Millets Research Institute (MMRI), Yousaf Wala Sahiwal, Pakistan. Seeds were immersed in 30% (v/v) H2O2 for 5 min. for sterilization, washed with deionized water for 24 h, dried and stored till experimentation.
Selection of cadmium and calcium levels
A preliminary experiment was conducted with different concentrations of Cd in form of cadmium chloride (0, 50, 100, 150, 200 μM), and Ca in form of calcium nitrate (0, 0.5, 1, 2.5, 5 and 10 mM) were used to screen the optimal levels of Cd and Ca. Based on preliminary experiment’s results, the 150 μM Cd stress caused 50% growth and germination inhibition, while 5 and 10 mM Ca showed best results to improve the negative impacts of Cd.
Treatment application and experiment layout
Seeds were placed in Petri dishes lined with a double layer of Whatman # 02 filter papers. The surface of each filter paper was moistened with 15 mL of H2O and kept in dark condition 25 ± 2°C for 48 h. After germinations, six seeds were planted in plastic pots (depth; 40 cm and diameter; 35 cm) in sterilized sand with a particular particle size of 0.25 mm. The sand was soaked for 24 h in 30% (v/v) HCl solution to remove all cations and anions and then thoroughly rinsed with deionized water three times (with 24 h soaking). All pots were arranged as a CRD design with 3 replications under a controlled glasshouse environment with a day/night temperature of 24 ± 4°C/14 ± 2°C and of relative humidity 58–60% [35]. The ½-strength Hoagland’s (Hoagland 1938) nutrient solution was applied to all pots by saturating the sand at an interval of 2 days until the complete emergence of the seedlings (20 days). Once a week, the solution was completely drained by applying enough Hoagland’s nutrient solution to ensure replacing any existing solution left in sand. After complete emergence, seedlings were thinned to 4 plants in each pot. The seedlings were then treated either with Cd2+ (using CdCl2) or Ca (using Ca(NO3)2) by using analytical grades prepared in Hoagland solution. The pH of the sand medium (6.7) and nutrient solution (7.5) was adjusted with HCl or NaOH and periodically measured with a portable pH meter (Ino LAB pH/Cond 720, WTW series).
Plant sampling and measurements
Plants materials were sampled at the seedling stage (4–6 leaf stage; 30 days after seedlings emergence) to determine plant growth attributes, physio-biochemical traits, ROS, and enzymes of an antioxidants defense system. Transplants were washed with distilled water and growth attributes were recorded. Leaf samples of maize seedlings were frozen at –80°C for physio-biochemical traits and antioxidants. Sampled seedlings were dried in an oven at 70°C to achieve a constant dry weight for determination of root (RDW) and shoot (SDW) dry weight.
Growth parameters
The shoot length (SL) of plants from each treatment was measured from sand level to the topmost leaf of the plant. The roots of seedlings were carefully removed from the sand for recording root length (RL). Root (RFW) and shoot fresh weight (SFW) of seedlings were measured immediately after excision. The leaf area (LA) was estimated by measuring length and width according to Kaleem and Hameed [36]
Photosynthetic pigments measurement
Chlorophyll contents were assessed as described by Arnon [37] and carotenoids following the method of Davis [38]. For the appraisal of chlorophyll contents, 0.1 g of leaf sample was grounded in 5 mL of acetone (80%). The extract was filtered through a Whatman # 02 filter paper (GE Healthcare, UK) and absorbance was recorded through a spectrophotometer (Hitachi U-2910, Tokyo, Japan) at 645, 663, and 480 nm. The values of photosynthetic pigments were calculated by using the following formulas.
Chl. a (mg/g of leaf fresh weight) = [12.7(OD663)-2.69(OD645)] x V/1000 x W
Chl. b (mg/g of leaf fresh weight) = [22.9(OD645) - 4.68(OD663)] x V/1000 x W
Total Chl. (mg/g of leaf fresh weight) = [20.2(OD645) + 8.02 (OD663)] x V/1000 x W
Carotenoids (g/ ml of fresh leaf) = {[(OD480) +0.114 (OD663)– 0.638 (OD645)]/2500}
Where V characterizes the volume of acetone and (FW) showed the leaf fresh weight.
Determination of relative membrane permeability
The fresh leaf samples were collected and washed thoroughly with 4 changes of water to eradicate any adhered electrolytes on the surface. The leaves were cut into small discs with a borer and placed in the small glass test tube containing deionized water (10 mL), The ECo was measured with the help of Cond/Salinity meter (TPS AQUA-CPA). The test tubes were incubated for 24 h at 4°C and EC1 was measured. The tubes were then wrapped with aluminium foil, autoclaved for 10 min. at 100 kPa and EC2 was recorded. The ratio of % ion leakage was computed as designated by Yang et al. [39].
Where, RMP: relative membrane permeability, EC0; Electrical conductivity before incubation, EC1; Electrical conductivity after incubation, EC2; Electrical conductivity after autoclave.
Anthocyanin contents measurement
Anthocyanin content was appraised according to the method of Giusti & Wrolstad [40]. The 0.1 g of a leaf was pulverized in trichloroacetic acid (TCA) by using a pestle and mortar. The homogenized material was transferred to test tubes and shifted to a water bath at 80°C for 20 min. Homogenized material was centrifuged at 12,000 xg for 10 min. in the absorbance was noted at 516 and 700 nm using a spectrophotometer (Hitachi U-2910, Tokyo, Japan). Acetone was run as blank and the amount of monomeric anthocyanin contents was calculated as follows.
Where, A = (A510-A700), MW = 449.2 and ε = 26900 [ε is the molar absorptivity measured the amount of cyanidin-3-glucoside pigment and DF is the dilution factor].
Oxidative stress markers (MDA and H2O2)
Lipids peroxidation (LPX) was quantified by means of malondialdehyde (MDA contents) according to the method of Heath and Packer [41]. LPX content was determined by the reaction of thiobarbituric acid-TBA with MDA. The 0.25 g leaf sample was grinded in 500 μL of TCA (0.1%) and then centrifuged at 15,000 xg. An aliquot (1 mL) was taken and mixed with 2 mL of 0.5% of TBA and 20% TCA. Test tubes containing reactants were incubated at 85°C for 20 min. and reaction was terminated in an icebox. Absorption was recorded at 532 and 600 nm by spectrophotometer (Hitachi U2910, Tokyo, Japan). All absorption ODs (at 532nm) were subtracted from 600 nm. LPX concentration was calculated by using 155 mM cm-1 as an extinction coefficient.
The Amount of H2O2 was quantified by measuring the oxidation of ferrous ions medicated by peroxidase and ferric ions react with the xylenol [42]. Leaf sample 0.5 g was grounded in 5 mL of 10 mM sodium phosphate buffer (SPB). Centrifugation of homogenized material was done at 15,000 xg. A 2 mL of aliquot was reacted with the assay reagent containing 200 mM sorbitol, 200 μM xylenol, 50 mm H2SO4, and 500 μM ammonium ferrous sulphate. The reactant material was incubated at 24°C for 1 h and absorption of yellow colour intensity of supernatant was recorded at 560 nm by using a spectrophotometer (Hitachi U-2910, Tokyo, Japan). The final concentration of H2O2 was calculated by using the coefficient of emission (0.28 mmol–1 cm–1).
Cellular antioxidants (APX and POD)
The maize seedlings, leaves were grounded in liquid nitrogen and extracted with 1 mM L–1 of 5% polyvinylpyrrolidone, and, 50 mM sodium phosphate buffer (SPB) having 1.0% w/v at pH 7.8 as homogenizing material. The extracted material was centrifuged at 15,000 xg. Enzyme crude extract was stored at 4°C for 36 h until analysis.
Ascorbate peroxidase activity (APX)
Activity of APX was quantified by oxidation of ascorbate [43]. The reaction was started by adding 10 μL of crude enzyme extract to 2 mL of assay reagent (30% H2O2, 0.5 mM C6H8O6, and sodium phosphate buffer (SPB) having pH 7.2,). After 30 s of reaction initiation, a shift in absorption was noted at 290 nm for 4 min. on a spectrophotometer (Hitachi U-2910, Tokyo, Japan). Activity of enzyme was estimated through extinction coefficient (2.8 mM cm-1), while the specific activity of the enzyme was calculated on the basis of protein contents and expressed as an mg–1 min.-1 FW.
Peroxidase activity (POD)
Peroxidase activity was appraised spectrophotometrically by using the method of Goliber [44] based on oxidisation of guaiacol in the presence of H2O2 and expressed as a Units mg-1 proteins. A 20 μL of the enzyme extract was added to the assay reagent (20 mM guaiacol, 10 mM H2O2, and 0.1 M phosphate buffer) and volume was maintained up to 3 mL. Enzyme activity was measured at 460 nm after 60 s interval through a spectrophotometer (Hitachi U-2910, Tokyo, Japan). Enzyme specific activity was expressed on the base of proteins.
Ascorbic acid determination
Ascorbic acid was determined as described by Nino and Shah [45]. Plant tissues (100 mg) were pulverized in thiobarbituric acid (TBA) and centrifuged 10,000 ×g for 10 min. An aliquot (500 μL) was taken with 500 μL of dithiocarbamate (DTC) in glass tubes. Reactants were left for ½ h at 37°C. Test tubes containing reactant material was transferred to the ice bath to terminate the reaction. After that, 2 mL of dilute H2SO4 was mixed slowly and leftover for ½ h at 37° C in an incubator. The extracted material was centrifuged at 12,000 xg. The shift in absorption was measured at 520 nm with the help of a spectrophotometer (Hitachi U-2910, Tokyo, Japan).
Total free amino acids
The free amino acid was quantified followed by Hamilton & Van-Slyke [46] method. The 0.1 g of the leaf sample was grinded and immersed in a potassium phosphate buffer (SPB) overnight. After incubation, 1 mL of plant extract was transferred to 25 mL test tubes after adding 1 mL each of 10% ninhydrin and 2% of pyridine solution. The test tubes containing reactants were placed in a boiling water bath for 1 h. The final volume of samples was made to 25 mL by using deionized H2O. Absorbance was recorded at 570 nm spectrophotometrically (Hitachi U-2910, Tokyo, Japan) and resulting absorbance was compared with the standard curve plotted for leucine.
Soluble proteins
Soluble proteins were appraised following Lowry et al. [47]. Plant sample (0.1 g) was grounded in 50 mM sodium phosphate buffer (SPB) having pH 6.8. The extracted aliquot (500 μL) was mixed in 0.3 mL of deionized H2O and 3 mL of Bio-Rad protein assay dye and vortexed for 15 s. The absorbance was measured spectrophotometrically at 750 nm (Hitachi U-2910, Tokyo, Japan). Soluble proteins were estimated by comparing the absorbance of samples with bovine serum albumin (BSA) using a standard value.
Statistical analysis
Statistical analysis and data visualization were executed by using R statistical software (R Core Team, 2021) through the R integrated development environment in R Studio (R Studio Team, 2021). Data within three replicates were subjected to an analysis of variance (ANOVA) and means values were compared by using the Tukey pairwise comparison test at (P≤ 0.05) to test the effects of Ca under Cd stress on maize seedlings. Bar plots were constructed by using the “agricolae” package of the R software. The effect of Ca and Cd treatments was assessed by using multivariate analysis (PCA by ggbiplot), correlation matrix (ggbiplot2) and heatmaps were plotted by customized code (pheatmap) by using R statistical software (R Studio Team, 2021). Response curves under cadmium and calcium stress treatments were constructed by fitting a generalized linear model (GLM) in CONACO version 5 for windows.
Results
Plant growth traits
Growth traits such as SL, RL, SFW, SDW, RFW, RDW and LA significantly (P ≤ 0.05) decreased at Cd applied at 150 μm concentration as compared to non-stressed plants (0 μM). The reduction was 75.3%, 88.3%, 77.83%, 98.6%, 91.6%, 99.86%, 68.1%, respectively. However, different levels of Ca significantly alleviated Cd toxicity and enhanced all growth traits. The increase in growth traits was more obvious in response to a higher level of Ca applied at 10 mM under Cd stress (150 μm). The percent increase was 64.6%, 28.4%, 66.2%, 23.2%, 40.3%, 46.4%, and 35.5, respectively (Table 1).
Table 1. Morphological characteristics of maize seedlings under Ca and Cd treatments.
| Cd stress (μM) | Ca treatments (mM) | SL (cm) | RL (cm) | SFW (g) | SDW (g) | RFW (g) | RDW (g) | LA (cm2) |
|---|---|---|---|---|---|---|---|---|
| 0 | 0 | 57.44±5.71d | 18.66±1.32d | 32.65±1.66d | 3.12±0.13d | 2.43±0.08d | 0.26±0.02c | 49.51±2.23d |
| 0.5 | 66.22±1.92c | 23.61±1.28d | 40.72±1.82c | 3.55±0.09d | 3.09±0.16c | 0.36±0.04c | 57.79±0.86c | |
| 1 | 66.22±1.34c | 30.94±0.80c | 47.14±0.95c | 4.46±0.15c | 3.29±0.04c | 0.45±0.03b | 60.99±4.06c | |
| 2.5 | 73.00±0.84b | 40.66±2.47b | 48.31±1.85c | 4.54±0.36c | 3.73±0.06b | 0.56±0.02b | 73.04±3.07b | |
| 5 | 93.66±2.95b | 45.61±3.11b | 59.00±1.51b | 5.87±0.22b | 4.63±0.12a | 0.62±0.02a | 75.91±3.60b | |
| 10 | 105.7±2.01a | 65.94±2.84a | 81.91±3.19a | 7.75±0.10a | 5.60±0.11a | 0.66±0.03a | 95.87±2.34a | |
| 150 | 0 | 24.64±2.38d | 11.61±2.22d | 22.17±1.74d | 1.40±0.22c | 1.35±0.17c | 0.14±0.02c | 31.96±1.06d |
| 0.5 | 52.44±3.75c | 18.95±0.80c | 28.45±1.63d | 1.61±0.03c | 1.99±0.11c | 0.16±0.01c | 39.30±0.94d | |
| 1 | 63.22±3.74b | 20.34±2.35c | 56.50±2.39c | 1.91±0.39c | 2.11±0.15b | 0.21±0.01b | 53.40±5.49c | |
| 2.5 | 72.44±2.92b | 30.60±1.45b | 73.11±2.11b | 2.81±0.28b | 2.94±0.26a | 0.23±0.01b | 54.09±5.11c | |
| 5 | 82.77±4.29a | 35.10±2.69b | 77.96±5.19b | 3.26±0.11b | 3.10±0.18a | 0.32±0.03a | 62.96±1.46b | |
| 10 | 91.55±3.83a | 51.14±3.16a | 90.64±1.52a | 4.97±0.20a | 3.11±0.09a | 0.30±0.04a | 76.99±2.57a |
Means provided with error bars; in columns different letter indicates significance (P≤0.05) between treatments
Abbreviation: Shoot length (SL); Root length (RL); Shoot fresh weight (SFW); Shoot dry weight (SDW); Root fresh weight (RFW); Root dry weight (RDW); Leaf area (LA)
Photosynthetic pigments
Under Cd stress (150 μM), a significant (P ≤ 0.05) reduction occurred in the concentration of photosynthetic pigments such as Chl a, Chl b, carotenoids (Caro), and total chlorophyll (T.Chl) of maize seedlings. The reduction was 96.4%, 98.6%, 99.8%, and 94.9% as compared to the non-stressed control (Ca-0 mM) and stressed (Cd-0 μM) seedlings of the maize. The Exogenously supplied Ca significantly increased photosynthetic pigments (Chl a, Chl b, Caro, T.Chl) both in Cd stressed and non-stressed seedlings. Calcium applied at 10 mM level was more beneficial in increasing chlorophyll and carotenoids contents of maize seedlings at 150 μM concentration of Cd. The percent increase was 286.2%, 266.0%, 215.4% and 140.8%, respectively (Table 2).
Table 2. Physiological traits of maize seedlings under various levels of Ca and Cd treatments.
| Cd stress (μM) | Ca levels (mM) | Chl a (mg g-1 FW) | Chl b (mg g-1 FW) | Caro. (mg g-1 FW) | T. Chl (mg g-1 FW) | APX (Units mg-1 Pro) | POD (Units mg-1 Pro) |
|---|---|---|---|---|---|---|---|
| 0 | 0 | 9.76±0.25c | 2.34±0.09c | 0.35±0.01d | 10.69±0.26c | 0.92±0.05c | 0.55±0.11c |
| 0.5 | 12.98±0.54c | 2.84±0.23c | 0.43±0.01c | 11.43±0.75c | 1.02±0.04c | 0.60±0.12c | |
| 1 | 16.56±0.84b | 4.44±0.15b | 0.48±0.02c | 14.02±0.64b | 1.98±0.02b | 0.73±0.04b | |
| 2.5 | 18.32±0.62a | 4.57±0.19b | 0.51±0.02b | 14.93±0.83b | 1.93±0.02b | 0.78±0.03b | |
| 5 | 18.60±0.38a | 5.48±0.18a | 0.52±0.03b | 16.00±1.06a | 1.98±0.06b | 0.87±0.07a | |
| 10 | 19.26±0.76a | 6.49±0.10a | 0.70±0.02a | 16.02±0.25a | 2.17±0.03a | 0.91±0.06a | |
| 150 | 0 | 3.63±0.22d | 1.41±0.03c | 0.13±0.01c | 5.02±0.23d | 1.27±0.03c | 0.45±0.09d |
| 0.5 | 5.33±0.19c | 2.85±0.12c | 0.17±0.01c | 6.35±0.46c | 1.39±0.19b | 0.72±0.02c | |
| 1 | 6.50±0.82c | 3.08±0.08b | 0.22±0.01b | 7.25±0.45c | 2.01±0.04b | 0.85±0.06b | |
| 2.5 | 10.72±0.47b | 3.47±0.14b | 0.27±0.01b | 8.22±0.22b | 2.17±0.03a | 0.82±0.06b | |
| 5 | 12.42±1.11b | 4.13±0.13a | 0.32±0.03a | 9.18±0.37b | 2.10±0.02a | 1.10±0.01a | |
| 10 | 14.02±0.53a | 5.16±0.20a | 0.41±0.03a | 12.09±0.52a | 2.22±0.01a | 1.12±0.06a |
Means provided with error bars; in columns different letter indicates significance (P≤0.05) between treatments
Abbreviations: Chlorophyll a (Chl a); Chlorophyll b (Chl b); Carotenoids (Caro); Total chlorophyll (T. Chl); Ascorbate per oxidase (APX); Peroxidase (POD); Protein (Pro)
Antioxidative enzyme activities
Mean values for antioxidant activity was higher in Cd stressed (150 μM) as compared to non-stressed plants (0 μM). However, the activity of APX significantly enhanced as levels of Ca increased both in non-stressed (0 μM) and stressed plants (150 μM). The maximum activity of the APX (74.8%) and POD (148.9%) was recorded at 10 mM Ca concentration as compared to its control. However, significant reduction was recorded in stressed seedlings (150 μM) as compared to non-stressed (0 μM) control plants under no supplementation of the Ca as 98.8% and 99.5%, respectively (Table 2).
Anthocyanin and relative membrane permeability
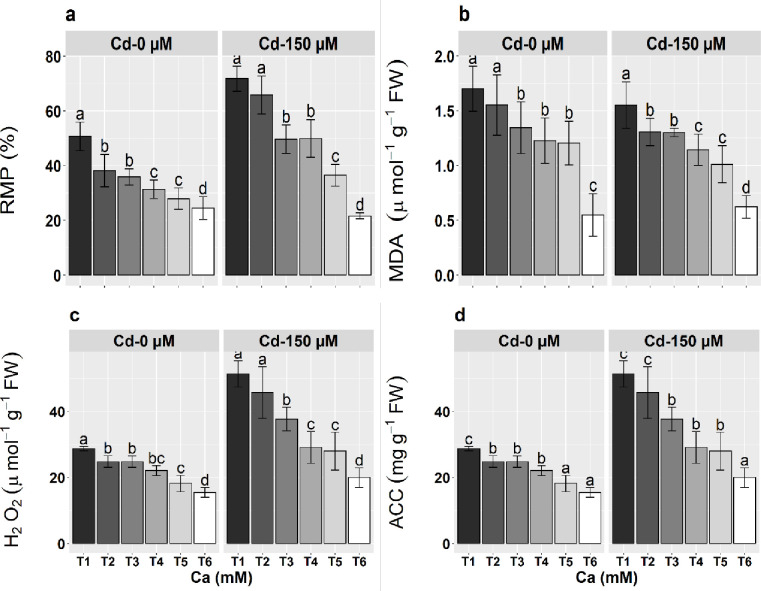
Under Cd stress, maximum RMP values were observed which indicate a high level of electrolyte leakage due to membrane damage. A significant (P ≤ 0.05) reduction (69.9%) was observed as the level of Ca increased (Fig 1A). Cadmium applied at 150 μm level and without any Ca supplementation had the most toxic effects as the highest electrolyte leakage was observed at this treatment level. Anthocyanin contents (ACC) under both treatments of Cd significantly increased as levels of Ca increased. Maximum increase (133.4%) in ACC were noticed in stressed plants (150 μM) at 10 mM Ca concentration (Fig 1D).
Fig 1. Effect of calcium (Ca; T1-0 mM, T2-0.5 mM, T3-1 mM, T4-2.5 mM, T5-5 mM, T6-10 mM) and cadmium (Cd) treatments on the a) relative membrane permeability (RMP), b) melanoaldehyde contents, c) H2O2, and d) anthocyanine contents (ACC) of maize seedlings.
Means ± SE provided with error bars; different letter indicates significance (P≤0.05) between Ca and Cd treatments.
Lipid peroxidation (LPX) and ROS
The accumulation of H2O2 and MDA significantly increased in maize seedlings under Cd stress. However, the elevated levels of Ca significantly reduced the generation of H2O2 and LPX. The LPX in terms of MDA contents significantly decreased as the level of Ca increased in the growth medium of the stressed seedlings. The maximum decrease (59.9%) was observed under 10 mM concentration of Ca. The 10 mM concentration of Ca also cause reduction (61.2%) in the generations of H2O2 in Cd stressed seedlings (Fig 1C).
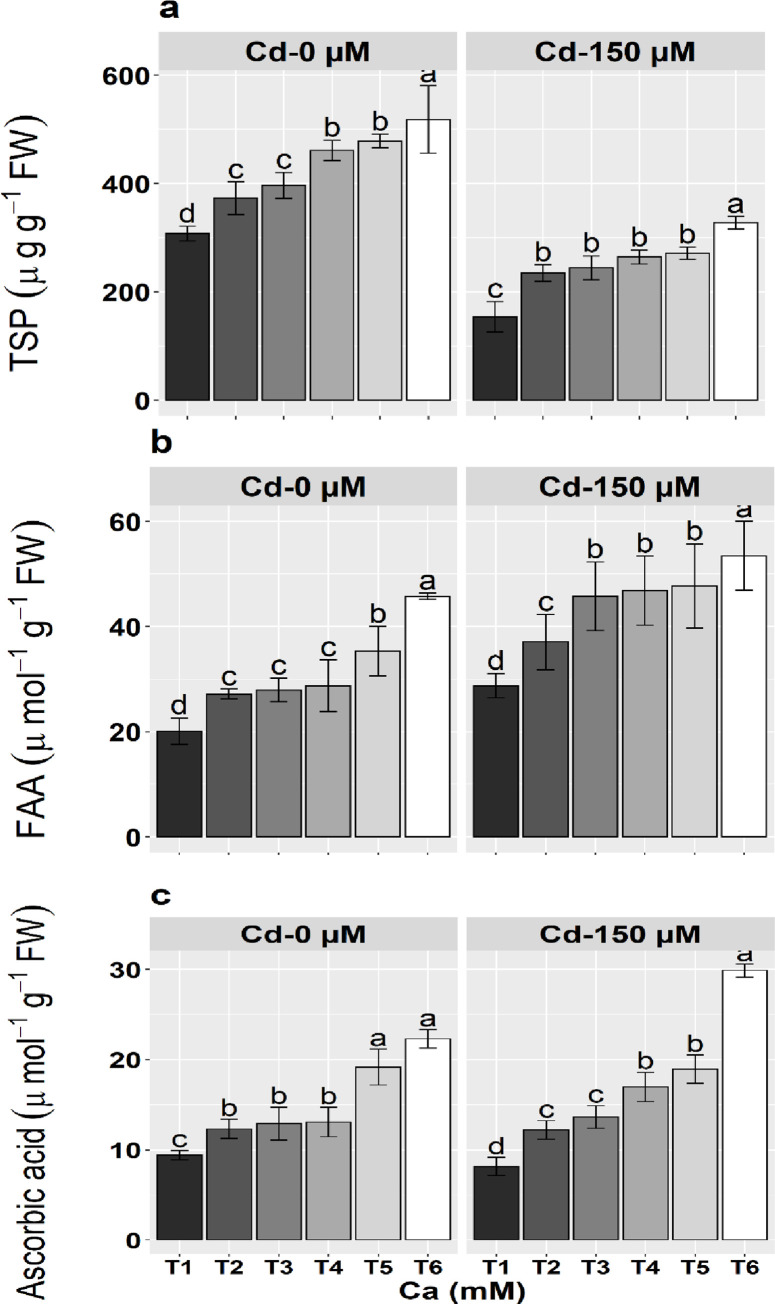
Organic osmolytes
Organic osmolyte (proteins and amino acids) production was significantly increased in maize seedlings in stressed and non-stressed maize seedlings. In stressed plants, the increase was 133.2% and 66.6%, respectively. However, soluble proteins were significantly higher in non-stressed maize seedlings as the level of Ca increased. In Cd stressed seedlings (150 μM), the concentration of soluble proteins significantly increased and the maximum was observed under 10 mM Ca concentration (Fig 2A). Applications of Ca substantially increased the concentration of amino acids in both stressed and non-stressed seedlings and almost parallel results were observed as noted for soluble proteins (Fig 2B).
Fig 2. Effect of calcium (Ca; T1-0 mM, T2-0.5 mM, T3-1 m M, T4-2.5 m M, T5-5 m M, T6-10 mM) and cadmium (Cd) treatments on the a) toatl soluble protiens, b) amino acids and c) ascorbic acid contents of maize seedlings.
Means ± SE provided with error bars; different letter indicates significance (P≤0.05) between Ca and Cd treatments.
Ascorbic acid contents measurement
Ascorbic acid contents were substantially improved as Ca levels increased in maize seedlings under normal and stress conditions. Maximum values of ascorbic contents were observed under 10 mM concentration of Ca in both stressed and non-stressed conditions (Fig 2C). The concentration of ascorbic acid was significantly increased (197.0%) in stressed plants at the higher concentration of the Ca in the soil medium.
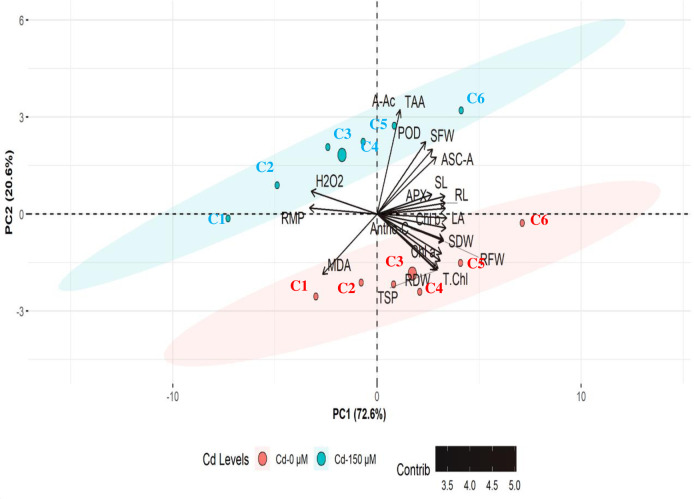
Principal component analysis (PCAs)
PCAs results demonstrated high variations in the effects of Cd and Ca treatments among different growth and physio-biochemical traits of maize seedlings (Fig 3). The first and second PCAs explained 75.8% and 17.2% (total 93%) variation among treatments and seedlings characteristics. The major contributors to the 150 μM Cd level were amino acids (A-Ac), peroxidase (POD), H2O2, and RMP with high positive eigenvalues. The activity of antioxidative enzymes (POD, APX, ASC-A), photosynthetic pigments (Chl a), and growth traits significantly increased under Cd stress (150 μM) The SFW, TAA, and A-Ac excelled in strong association with a higher concentration of Ca (C5-C6). Under lower levels of Ca i.e. C1 and C2, the ROS and RMP increased under Cd stress. The major principal components to control plants (0 μM Cd) were RFW, RDW, anthocyanin contents, Chl b, T. Chl, carotenoids, TSP, and MDA with negative eigenvalues (Fig 3). Higher concentration of Ca (C6) closely related to growth attributes as LA, SDW, RFW, while C5 contributed to T.Chl, C3 with TSP, C2 and C1 excelled concerning MDA. The variable Antho-C, LA, SDW, RFW, Chl a and T.Chl had shown positive loading of eigenvalues toward the PCA1. and LPX with negative eigenvalues (Fig 3). The Cd stress significantly increased the level of reactive oxygen species, while supplemented Ca significantly increased the antioxidative enzymes activity and growth parameters (Fig 3).
Fig 3. PCA biplot for growth and physio-biochemical traits under Cd and calcium (C1-0 mM, C2-0.5 mM, C3-1 mM, C4-2.5 mM, C5-5 mM, C6-10 mM) treatments.
Abbreviations are given at start of manuscript.
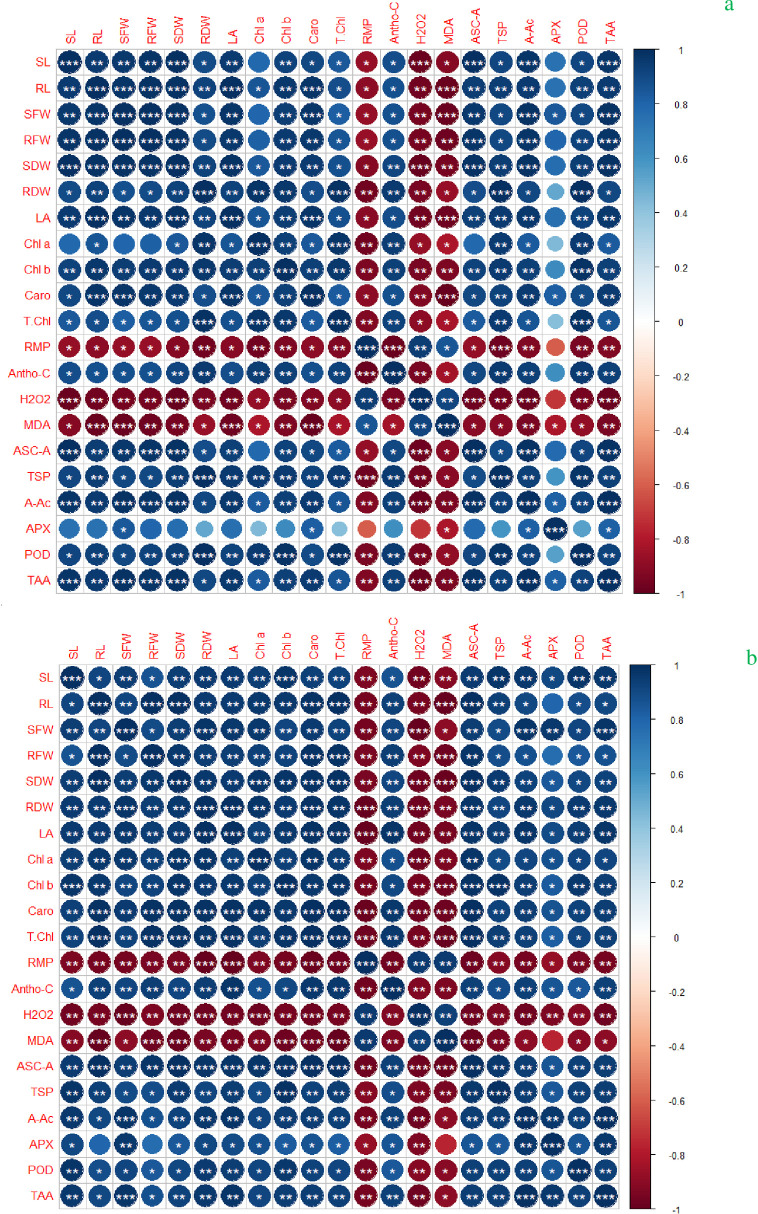
Correlation matrix
In control plants, anthocyanin contents (Antho-C) was positively correlated with RFW, RDW, SL, LA, Caro, Chl b and TSP. The RMP, H2O2, and RMP were negatively correlated with RFW, RDW, Chl a, b, RL, LA, A-AC and APX (Fig 4A). Under Cd stress, a highly positive correlation was assessed between POD, SFW, and ASC.A, APX, Chl a, SL, and RL. However, a strong negative correlation was assessed between H2O2, RMP, and antioxidant enzymes under Cd-150 μM stress (Fig 4B).
Fig 4.
Correlation among morphological and physio-biochemical traits of maize seedlings under (a) under control and (b) Cd stress condition. Abbreviations are given at start of manuscript.
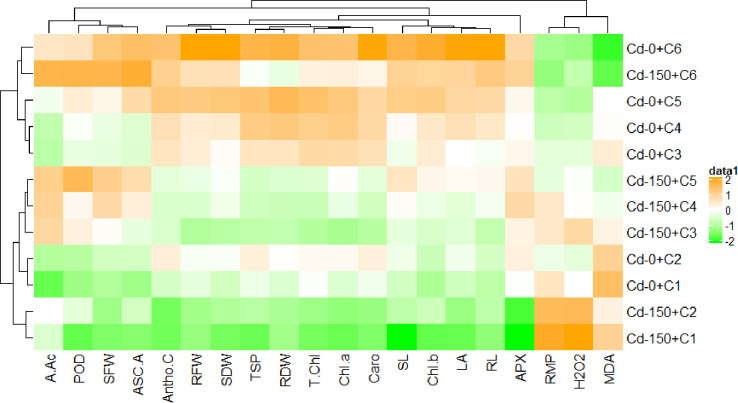
Clustered heatmap
A clustered heatmap was constructed to evaluate the effect of Cd and Ca treatments on the different traits as shown in Fig 5. Under higher concentrations of Ca (10 mM), RMP, H2O2 and MDA showed a significant reduction in response to 0 and 150 μM concentrations of Cd indicating a parallel response in both treatments. A noteworthy influence of 10 mM level of Ca in non-stressed seedlings (0 μM Cd) was recorded with a greater increase in growth traits (RFW, RDW, SFW, SDW, SL, RL, LA), chlorophyll (Chl a & b, T. Chl), organic osmolytes (TSP), anthocyanin contents (Antho. C) and ascorbic acid (ASC.A). All these traits were tightly grouped together and indicated high performance of 10 mM level of Ca under non-stressed conditions. In Cd stressed (150 μM) seedlings, 10 mM level of Ca contributed to a significant increase in amino acids (A.AC), peroxidase (POD), ascorbic acid (ASC.A) and shoot fresh weight (SFW). Shoot length (SL), root length (RL), leaf area (LA), the activity of ascorbate peroxidase (APX) and chlorophyll showed a strong and clear similarity and strongly clustered together. Antioxidants (APX and POD), ascorbic acid (ASC.A), anthocyanin contents (Antho. C) reduce the RMP, H2O2 and MDA and are clustered together in the same group. At the highest level of Ca (10 mM), clustering and similarity indicated a high performance and a possible relationship between different traits under stress treatments.
Fig 5. Clustered heatmap representing the effect of Cd and Ca (C1- 0 mM, C2- 0.5 mM, C3- 1 mM, C4- 2.5 mM, C5- 5 mM, C6- 10 mM) treatments on different studied traits.
Abbreviations are given at start of manuscript.
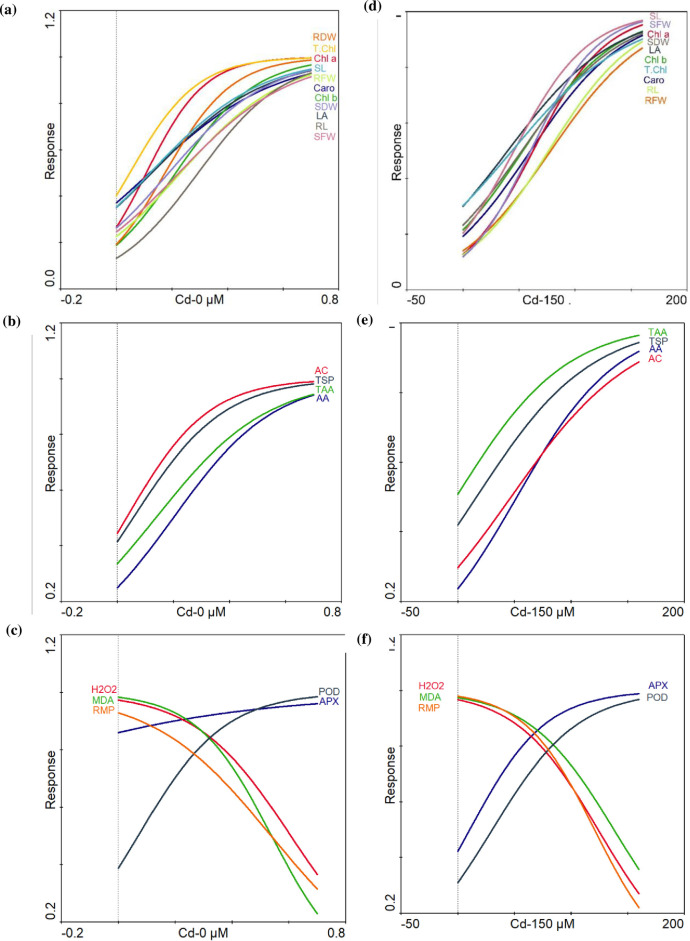
Response of different traits under stressed and non-stressed conditions
In non-stressed conditions (0 μM), a conspicuous positive response was observed for the growth traits (RL, SL, SFW, SDW, and LA) and chlorophyll (Chl a, Chl b and T. Chl) as Ca levels increased (Fig 6A). Organic osmolytes (TAA, TSP), anthocyanin contents (AC) and ascorbic acid (ASc-A) showed a sharp positive response with increasing Ca regimes (Fig 6B). H2O2, MDA and RMP exhibit a strong negative response with an increase in Ca levels, however, APX and POD exhibit an increasing pattern in curve with elevated Ca gradients (Fig 6C). In Cd stressed conditions (150 μM), growth traits (RL, SL, SFW, and SDW, LA) and chlorophyll (Chl a, Chl b and T.Chl) displayed a strong positive response and in response to Ca levels (Fig 6D). The concentration of TAA, TSP, AC and TAA was the maximum with a positive response (Fig 6E). A strong positive response was noted in the activity of APX and POD along with increasing Ca levels. In contrast, a strong negative response was assessed for H2O2, MDA and RMP with an increase in Ca regimes (Fig 6F).
Fig 6. Generalized linear model showing response curve of traits under Cd and Ca treatments.
Cd- 0 μM stress: (a) growth and chlorophyll (b) organic osmolytes, ascorbic acid and anthocyanin contents (c) hydrogen peroxide, relative membrane permeability, lipid peroxidation and antioxidants. Cd- 150 μM stress; (d) growth and chlorophyll (e) organic osmolytes, ascorbic acid and anthocyanin contents (f) hydrogen peroxide, relative membrane permeability, lipid peroxidation and antioxidants. Abbreviations are given at start of manuscript.
Discussion
Calcium plays an essential role in the mitigation of abiotic stresses and protection from drastic impacts [24, 48–50]. It interacts with proteins like calmodulin to up-regulate gene expression and regulate the movement of metal ions across membranes [51]. The present work demonstrated that Ca significantly alleviated the toxic effect of Cd in maize by improving all growth traits. Furthermore, the alleviation of Cd toxicity was more obvious at higher treatment levels of Ca in stressed and non-stress plants. Previous studies revealed that Ca applications regulate the uptake of heavy metal ions as it competes for transporter sites on plasma membrane [24]. Supplemented Ca2+ reduced Cd toxicity by enhancing growth traits as reported in other crops like in mustard [24] and rice [52]. Additionally, Ca reduced the toxic effect of nickel in rice seedlings [53] Calcium is essential for plants and is involved in the various physiological processes, cell division, and photosynthesis and interacts with intracellular signal transduction. Due to chemical similarity with Cd, Ca mediate various Cd-mediated physiological and metabolic processes [24]. Recent works elaborated that Ca use as an exogenous supplement to prevent the noxious impacts of the Cd [28].
Reduction in growth traits under Cd toxicity is directly linked to the reduction of photosynthetic contents. As anticipated, the photosynthetic pigments significantly declined under 150 μM Cd treatment level. However, higher levels of Ca significantly improved carotenoids, total Chlorophyll (Chl), Chl a, b pigments in maize seedlings under Cd stress (Table 2). Previously, the interactive effect between Ca and heavy metal was reported in some studies where exogenously applied Ca significantly prevented the damaging effects of Cd on photosynthetic pigments [53, 54]. Calcium is also obligatory required to activate the oxidation of H2O oxidation, maintain photochemical efficiency of PSII, and restore photosynthesis by aggravating the concentrations of the photosynthetic pigments [55, 56]. Calcium is a divalent cation and shares many parallel physical properties (like pH) with divalent heavy metals like Cd, Ni, and Co [57]. Therefore, exogenously applied Ca ions through the rooting medium can successfully restrict the uptake of Cd metal ions through competition for uptake and transport in plants [57]. In current work, the enhanced amount of photosynthetic pigments in Cd treated maize seedlings seemed to be a direct effect of enhanced activities of anti-oxidative enzymes, and other protective molecules that reduced membrane damage [58].
The improvement in the antioxidant defense system enables plants to alleviate heavy metals toxicity [53]. In the current study, APX and POD activities significantly improved in Ca treated plants under Cd toxicity. These results suggest that applied Ca effectively alleviate Cd-induced oxidative stress [59]. Under heavy metal stress, Ca activates diverse protein kinases and strengthens the antioxidant defense system [20, 60]. Tolerant plants had evolved an efficient antioxidant system to balance the concentration of reactive oxygen species [28, 61]. Enzymes like APX and POD also take part in the detoxification of free radicles and lead to sequestering of H2O2 [62]. APX is mainly localized in chloroplast, apoplast, cytosol, mitochondria, and peroxisome and POD in cell walls, cytosol, and vacuoles, Both APX and POD are mainly implicated to scavenging the H2O2 [61]. Their efficiency is enhanced during Cd stresses and that greatly imparts stress tolerance and modulates the physiological process in maize seedlings in this study [63, 64]. Exogenous application of Ca remarkably decreases the intracellular level of Cd by activation of antioxidant defense mechanism to a level capable of suppressing the generation of ROS. This suppression is corroborated by the enhancement of APX, SOD, POD and CAT to efficiently scavenge the toxic ROS [33]. Activation of these antioxidants attributed to photosynthetic efficiency and perception of stress signals by elevation of cytosolic Ca as an early signal event, known as Ca signature. This Ca signature is detected by Ca sensors and then a downstream signal is transduced that subsequently enhances the defense mechanisms [65].
Plants exposed to metal stress showed alterations in cell membrane permeability (RMP) and consequently, the cell loses membranes integrity [66]. Cell membrane integrity is considered as a tool to regulate ionic movements and use as a selection criterion to quantify damage magnitude. In current results, the relative RMP markedly increased under Cd stress. However, the RMP significantly was markedly reduced by the Ca treatments that alleviated the damaging consequences of Cd. In plants exposed to Cd stress, relative membrane permeability (RMP) substantially increased and caused membrane impairments [67]. Under Cd stress, supplemented Ca decrease the electrolyte leakage that showing the defensive role of Ca to increase the membrane stability [68]. Calcium mainly stabilizes the membrane integrity and also controls the movement of divalent cations and prevent solute leakage by reducing peroxidation of lipids [52, 69]. In addition, Ca is known to maintain the membrane integrity that is concomitant with Ca-chelators to target the ROS and reduce the lipid peroxidation [70].
The scavenging of excessive ROS is a vital process by regulates the regular operation of a cellular system. Excessive ROS can be rid of by inbuilt antioxidants defense system attributed to enzymes viz, MDHAR, DHAR, GR, APX, POX, CAT, SOD and non-enzymes DHA, AsA, GSSG and GSH [33].
The excessive accumulation of both MDA and H2O2 under metal stresses damages biomolecules by excessive lipid peroxidation, degrades membranes, decreases photosynthesis and hampered the activity of other essential enzymes [61]. Plants enhance the antioxidant system to deplete the ROS which ultimately reduces oxidative stress generated by high metal concentrations [71]. The Ca applications as observed in this study, improved the activities of various antioxidants (enzymatic or non-enzymatic) and reduced the level of H2O2 and lipid peroxidation [20, 72]. Previous studies authenticate the pivotal role of Ca to prevent the accumulation of cellular Cd and improve ROS-scavenging capacities that led to the reduction of ROS in plants [33]. Calcium also up-regulates genes that are responsible to encode the antioxidant under oxidative stress [73]. In the present work, the level of ROS increased under Cd stress, however, the addition of Ca considerably reduced the production of ROS in maize seedlings (Fig 1).
Anthocyanin belongs to flavonoids and naturally occurs in water-soluble plant pigments [74]. In plants, anthocyanin plays a pivotal physiological role as scavenges free radicles, increases the organic osmolytes and photosynthetic efficiency [75, 76]. The biosynthesis of anthocyanin is regulated by environmental and developmental signals [77, 78]. The excessive accumulation of anthocyanin is regarded as defense mechanism [79]. The anthocyanin contents remarkably increased in the present study which was more pronounced in the highest levels of Ca (Fig 1) that is are parallel to many previous findings [60, 80]. A high level of anthocyanin regulates heavy metal transport toward the vacuole and sequestration [81]. Exogenously applied calcium is reported to reduce Cd toxicity by stimulating the synthesis of glutathione-S-transferase (GST) enzyme to increase anthocyanin contents that in turn ameliorates the oxidative stress by scavenging the free radicals [81].
Heavy metal stress causes determinal changes in cellular structures and causes osmotic stress [82]. Plants mitigate osmotic stress by accumulating the lower or higher weight osmolytes that do not hinder the functioning of important metabolites [83]. During Cd stress, plants employed several protective strategies to reduce the noxious impacts of Cd stress [17]. Osmolytes primarily reduce water potential and ensure the water balance [84], protects subcellular structures, and reduce oxidative damage [85]. Plants accumulate the organic osmotica to maintain the tissue water contents and upregulate the working capabilities of antioxidants during stressful conditions [86, 87]. Amino acids act as organic osmolytes and participate in osmotic adjustments, stabilize proteins in membranes [88], ion homeostasis [47], scavenges the ROS and neutralize the redox potential during oxidative stress caused by noxious heavy metals [88]. Calcium is an indispensable element for plant osmotic adjustments and increase the levels of free amino acids under heavy metal stresses [89]. In the present studies, the seedlings showed more accumulation of osmolytes under the application of Ca (Fig 2). Ascorbic acids (ASc) are non-enzymatic antioxidant enzymes, which act as a cofactor for many important enzymes and accumulate in leaves [63]. Ascorbic acid plays a crucial role in protecting the cellular metabolism from oxidative damage by acting as a reductant [90]. It serves a defensive role during oxidative stress and reduces the H2O2 and detoxifies the free radicals [91]. In the present study, ascorbic acid in maize seedlings was significantly enhanced by the addition of Ca (Fig 2). Foliar applications of AsA potentially alleviate the Cd toxicity in maize by modulating the physio-chemical attributes, boosting the activities of antioxidant enzymes, and improving the photosynthetic process, and concentrations of organic osmolytes [92].
Calcium, a ubiquitous messenger, is well known to regulate metabolic processes, act as a transducer, regulate photosynthesis and balanced the level of essential nutrients [93]. A slight change in an intracellular Ca concentration can modulate a large array of fundamental biological processes such as growth, physiology and biochemical process under heavy metal stress [94]. In the present work, Ca modulated various fundamental processes such as growth, physiological and biochemical processes in maize seedlings which ultimately enhanced Cd tolerance. Supplementation of Ca to plants actively participates in heavy metals tolerance mechanisms [95]. Exogenously applied Ca2+ enhanced activities of cellular antioxidants such as APX, POD which were helpful to restrict the production of ROS to prevent oxidative damage [24]. Several studies confirm the importance of the Ca2+ by induction of Cd tolerance since physio-biochemical characters of Ca2+ are quite similar to that of Cd2+, these similarities result in the replacement of Cd2+ with Ca2+ metal. Thus, Ca2+ uptake by receptors/ channels can be enhanced which increases Ca2+ storage viability in plants under Cd stress because of the similarity in ionic radii of both metals [19, 96]. Calcium (Ca) also works as a second messenger in plants, which underpins the abiotic stress-induced damage. However, the sequence of action of these signalling molecules against cadmium (Cd)-induced cellular oxidative damage remains unrevealed [33]. In this prospect, more work can be done to understand the exact mechanism of the Ca induced mitigation of Cd stress by enhancing the growth, physiochemical and genetic approaches.
Conclusions
In conclusion, Cd-induced oxidative stress caused negative influences on the growth and physio-biochemical traits of plants. In response to Cd stress, plants got triggered their defense mechanisms, nonetheless at the same time, Cd stress increased the level of stress markers (MDA, H2O2) and plants are unable to handle Cd-induced cellular impairment as witnessed by elevation in RMP and reduction in anthocyanin contents (ACC). Plants showed a low level of organic osmolytes (TSP, Amino acids) under Cd stress. Exposure to Cd caused a reduction in growth traits (SL, RL, SFW, SDW, RFW, RDW), a reduction in activities of antioxidants (APX, POD), and markedly declined the photosynthetic pigments (Chl a, Chl b and T.Chl). However, supplementation of Ca markedly reduced the oxidative stress by elevating the level of antioxidants (APX, POD), and non-antioxidants (ascorbic acid) to scavenge the ROS. Exogenously applied Ca ameliorated the oxidative stress by increasing the level of organic osmolytes as total soluble proteins, and free amino acids to maintain the integrity of cellular membranes and cell osmotica. Maintenance of organic osmotica causes an increase in antioxidant enzymes that ultimately suppressed ROS and enhanced the accumulation of organic osmolytes. Collective responses are reflected in the form of improved growth and more photosynthetic pigments. Therefore, Ca supplementation under Cd stress, enabled the maize to counter determinal effects of Cd-induced damages. In future prospect, the biosynthetic pathways involved in the up-regulation of antioxidants and organic osmolytes should be further investigated.
Acknowledgments
This manuscript was part of first author Muhammad Kaleem MSc Thesis research work and submitted to Government College University, Faisalabad (GCUF). The authors acknowledge the support provided by the GCUF for planning, execution and analysis of this research work.
Abbreviations
- MDA
Malondialdehyde
- RMP
Relative membrane permeability
- H2O2
Hydrogen peroxide
- RFW
Root fresh weight
- RL
Root length
- T. Chl
Total chlorophyll
- SDW
Shoot dry weight
- ASC.A
Ascorbic acid
- Caro
Carotenoids
- Antho-C, ACC
Anthocyanin contents
- Chl b
Chlorophyll b
- Chl a
Chlorophyll a
- TSP
Total soluble proteins
- LA
Leaf area
- RDW
Root dry weight
- POD
Peroxidase
- SFW
Shoot fresh weight
- APX
Ascorbate peroxidase
- A-Ac
Amino Acids
- SPB
Sodium phosphate buffer
Data Availability
All relevant data are within the manuscript.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Huybrechts M, Cuypers A, Deckers J, Iven V, Vandionant S, Jozefczak M, et al. Cadmium and plant development: An agony from seed to seed. Int J Mol Sci. 2019;20(16):3971. doi: 10.3390/ijms20163971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Vijver MG, Peijnenburg WJ. Impacts of major cations (K+, Na+, Ca 2+, Mg 2+) and protons on toxicity predictions of nickel and cadmium to lettuce (Lactuca sativa L.) using exposure models. Ecotoxicology. 2014;23(3):385–95. doi: 10.1007/s10646-014-1202-1 [DOI] [PubMed] [Google Scholar]
- 3.Goix S, Lévêque T, Xiong T-T, Schreck E, Baeza-Squiban A, Geret F, et al. Environmental and health impacts of fine and ultrafine metallic particles: assessment of threat scores. Environ Res. 2014;133:185–94. doi: 10.1016/j.envres.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 4.Nogueirol RC, Monteiro FA, Gratão PL, da Silva BKdA, Azevedo RA. Cadmium application in tomato: nutritional imbalance and oxidative stress. Water Air Soil Pollut. 2016;227(6):1–20. 10.1007/s11270-016-2895-y [DOI] [Google Scholar]
- 5.Kováčik J, Babula P, Hedbavny J, Švec P. Manganese-induced oxidative stress in two ontogenetic stages of chamomile and amelioration by nitric oxide. Plant Sci. 2014;215:1–10. doi: 10.1016/j.plantsci.2013.10.015 [DOI] [PubMed] [Google Scholar]
- 6.Semida WM, Hemida KA, Rady MM. Sequenced ascorbate-proline-glutathione seed treatment elevates cadmium tolerance in cucumber transplants. Ecotoxicol Environ Saf. 2018;154:171–9. doi: 10.1016/j.ecoenv.2018.02.036 [DOI] [PubMed] [Google Scholar]
- 7.Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, et al. Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot. 2012;83:33–46. 10.1016/j.envexpbot.2012.04.006 [DOI] [Google Scholar]
- 8.Dourado M, Franco M, Peters L, Martins P, Souza L, Piotto F, et al. Antioxidant enzymes activities of Burkholderia spp. Strains-oxidative responses to Ni toxicity. Environmental Science and Pollution Research. 2015;22(24):19922–32. doi: 10.1007/s11356-015-5204-1 [DOI] [PubMed] [Google Scholar]
- 9.Gratão PL, Monteiro CC, Tezotto T, Carvalho RF, Alves LR, Peters LP, et al. Cadmium stress antioxidant responses and root-to-shoot communication in grafted tomato plants. Biometals. 2015;28(5):803–16. doi: 10.1007/s10534-015-9867-3 [DOI] [PubMed] [Google Scholar]
- 10.Haider FU, Liqun C, Coulter JA, Cheema SA, Wu J, Zhang R, et al. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol Environ Saf. 2021;211:111887. doi: 10.1016/j.ecoenv.2020.111887 [DOI] [PubMed] [Google Scholar]
- 11.Alzahrani Y, Rady MM. Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol Environ Saf. 2019;182:109378. doi: 10.1016/j.ecoenv.2019.109378 [DOI] [PubMed] [Google Scholar]
- 12.Maleki M, Ghorbanpour M, Kariman K. Physiological and antioxidative responses of medicinal plants exposed to heavy metals stress. Plant Gene. 2017;11:247–54. 10.1016/j.plgene.2017.04.006 [DOI] [Google Scholar]
- 13.Asgher M, Khan NA, Khan MIR, Fatma M, Masood A. Ethylene production is associated with alleviation of cadmium-induced oxidative stress by sulfur in mustard types differing in ethylene sensitivity. Ecotoxicol Environ Saf. 2014;106:54–61. doi: 10.1016/j.ecoenv.2014.04.017 [DOI] [PubMed] [Google Scholar]
- 14.Mishra B, Sangwan RS, Mishra S, Jadaun JS, Sabir F, Sangwan NS. Effect of cadmium stress on inductive enzymatic and nonenzymatic responses of ROS and sugar metabolism in multiple shoot cultures of Ashwagandha (Withania somnifera Dunal). Protoplasma. 2014;251(5):1031–45. doi: 10.1007/s00709-014-0613-4 [DOI] [PubMed] [Google Scholar]
- 15.Rady MM, Hemida KA. Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol Environ Saf. 2015;119:178–85. doi: 10.1016/j.ecoenv.2015.05.008 [DOI] [PubMed] [Google Scholar]
- 16.El-Beltagi HS, Mohamed HI. Alleviation of cadmium toxicity in Pisum sativum L. seedlings by calcium chloride. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2013;41(1):157–68. 10.15835/nbha4118910 [DOI] [Google Scholar]
- 17.Hashem A, Abd_Allah EF, Alqarawi AA, Malik JA, Wirth S, Egamberdieva D. Role of calcium in AMF-mediated alleviation of the adverse impacts of cadmium stress in Bassia indica [Wight] AJ Scott. Saudi J Biol Sci. 2019;26(4):828–38. 10.1016/j.sjbs.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vigani G, Costa A. Harnessing the new emerging imaging technologies to uncover the role of Ca2+ signalling in plant nutrient homeostasis. Plant Cell Environ. 2019;42(10):2885–901. doi: 10.1111/pce.13611 [DOI] [PubMed] [Google Scholar]
- 19.Malik Z, Afzal S, Danish M, Abbasi GH, Bukhari SAH, Khan MI, et al. Role of nitric oxide and calcium signaling in abiotic stress tolerance in plants. Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives. 2020:563–81. 10.1002/9781119552154.ch28 [DOI] [Google Scholar]
- 20.Siddiqui MH, Al-Whaibi MH, Sakran AM, Basalah MO, Ali HM. Effect of calcium and potassium on antioxidant system of Vicia faba L. under cadmium stress. Int J Mol Sci. 2012;13(6):6604–19. doi: 10.3390/ijms13066604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song J, Chen M, Feng G, Jia Y, Wang B, Zhang F. Effect of salinity on growth, ion accumulation and the roles of ions in osmotic adjustment of two populations of Suaeda salsa. Plant and Soil. 2009;314(1–2):133–41. 10.1007/s11104-008-9712-3 [DOI] [Google Scholar]
- 22.Grzybowska EA. Calcium-binding proteins with disordered structure and their role in secretion, storage, and cellular signaling. Biomolecules. 2018;8(2):42. 10.3390/biom8020042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niu L, Liao W. Hydrogen peroxide signaling in plant development and abiotic responses: crosstalk with nitric oxide and calcium. Frontiers in Plant Science. 2016;7:230. doi: 10.3389/fpls.2016.00230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad P, Sarwat M, Bhat NA, Wani MR, Kazi AG, Tran L-SP. Alleviation of cadmium toxicity in Brassica juncea L.(Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS One. 2015;10(1). e0114571. doi: 10.1371/journal.pone.0114571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lwalaba JLW, Zvobgo G, Fu L, Zhang X, Mwamba TM, Muhammad N, et al. Alleviating effects of calcium on cobalt toxicity in two barley genotypes differing in cobalt tolerance. Ecotoxicol Environ Saf. 2017;139:488–95. doi: 10.1016/j.ecoenv.2017.02.019 [DOI] [PubMed] [Google Scholar]
- 26.Ahanger MA, Tyagi SR, Wani MR, Ahmad P. Drought tolerance: role of organic osmolytes, growth regulators, and mineral nutrients. Physiological mechanisms and adaptation strategies in plants under changing environment: Springer; 2014. p. 25–55. 10.1007/978-1-4614-8591-9_2 [DOI] [Google Scholar]
- 27.Srivastava RK, Pandey P, Rajpoot R, Rani A, Gautam A, Dubey R. Exogenous application of calcium and silica alleviates cadmium toxicity by suppressing oxidative damage in rice seedlings. Protoplasma. 2015;252(4):959–75. doi: 10.1007/s00709-014-0731-z [DOI] [PubMed] [Google Scholar]
- 28.Huang D, Gong X, Liu Y, Zeng G, Lai C, Bashir H, et al. Effects of calcium at toxic concentrations of cadmium in plants. Planta. 2017;245(5):863–73. doi: 10.1007/s00425-017-2664-1 [DOI] [PubMed] [Google Scholar]
- 29.Zeng G, Wan J, Huang D, Hu L, Huang C, Cheng M, et al. Precipitation, adsorption and rhizosphere effect: the mechanisms for phosphate-induced Pb immobilization in soils-a review. J Hazard Mater. 2017;339:354–67. doi: 10.1016/j.jhazmat.2017.05.038 [DOI] [PubMed] [Google Scholar]
- 30.Nuss ET, Tanumihardjo SA. Maize: a paramount staple crop in the context of global nutrition. Comprehensive reviews in food science and food safety. 2010;9(4):417–36. doi: 10.1111/j.1541-4337.2010.00117.x [DOI] [PubMed] [Google Scholar]
- 31.Rani P, Chakraborty M, Sah RP. Identification and genetic estimation of nutritional parameters of QPM hybrids suitable for animal feed purpose. Range Management and Agroforestry. 2015; 36(2):175–82. [Google Scholar]
- 32.Boomsma CR, Vyn TJ. Maize drought tolerance: potential improvements through arbuscular mycorrhizal symbiosis? Field Crops Research. 2008;108(1):14–31. 10.1016/j.fcr.2008.03.002 [DOI] [Google Scholar]
- 33.Khan MN, Siddiqui MH, AlSolami MA, Alamri S, Hu Y, Ali HM, et al. Crosstalk of hydrogen sulfide and nitric oxide requires calcium to mitigate impaired photosynthesis under cadmium stress by activating defense mechanisms in Vigna radiata. Plant Physiol Biochem. 2020;156:278–90. doi: 10.1016/j.plaphy.2020.09.017 [DOI] [PubMed] [Google Scholar]
- 34.Rizwan M, Ali S, Qayyum MF, Ok YS, Zia-ur-Rehman M, Abbas Z, et al. Use of maize (Zea mays L.) for phytomanagement of Cd-contaminated soils: a critical review. Environ Geochem Health. 2017;39(2):259–77. doi: 10.1007/s10653-016-9826-0 [DOI] [PubMed] [Google Scholar]
- 35.Rady MM, Elrys AS, El-Maati MFA, Desoky E-SM. Interplaying roles of silicon and proline effectively improve salt and cadmium stress tolerance in Phaseolus vulgaris plant. Plant Physiol Biochem. 2019;139:558–68. doi: 10.1016/j.plaphy.2019.04.025 [DOI] [PubMed] [Google Scholar]
- 36.Kaleem M, Hameed M. Functional traits for salinity tolerance in differently adapted populations of Fimbristylis complanata (Retz.). International Journal of Phytoremediation. 2021; 23(12):1319–132. doi: 10.1080/15226514.2021.1895718 [DOI] [PubMed] [Google Scholar]
- 37.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1. doi: 10.1104/pp.24.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carotenoids Davies B. Chemistry and biochemistry of plant pigments. Academic Press, London. 1976:38–165. [Google Scholar]
- 39.Yang G, Rhodes D, Joly RJ. Effects of high temperature on membrane stability and chlorophyll fluorescence in glycinebetaine-deficient and glycinebetaine-containing maize lines. Funct Plant Biol. 1996;23(4):437–43. 10.1071/PP9960437 [DOI] [Google Scholar]
- 40.Giusti MM, Wrolstad RE. Current protocols in food analytical chemistry. Current protocols in food analytical chemistry, I’ pp F. 2001;1:F1. 10.1002/0471142913.faf0102s00 [DOI] [Google Scholar]
- 41.Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125(1):189–98. doi: 10.1016/0003-9861(68)90654-1 [DOI] [PubMed] [Google Scholar]
- 42.Bellincampi D, Dipierro N, Salvi G, Cervone F, De Lorenzo G. Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol. 2000;122(4):1379–86. doi: 10.1104/pp.122.4.1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen G-X, Asada K. Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 1989;30(7):987–98. 10.1093/oxfordjournals.pcp.a077844 [DOI] [Google Scholar]
- 44.Goliber TE. Gravitational stress and lignification in aerial vs. submerged shoots of Hippuris vulgaris. Physiol Plant. 1989;75(3):355–61. 10.1111/j.1399-3054.1989.tb04638.x [DOI] [Google Scholar]
- 45.Nino H, Shah W. Vitamins In: Fundamentals of Clinical Chemistry. Tietz, NW. WB Saunders, Philadelphia; 1986. [Google Scholar]
- 46.Hamilton P, Van Slyke D. Amino acid determination with ninhydrin. J biol Chem. 1943;150(1):231–50. 10.1016/S0021-9258(18)51268-0 [DOI] [Google Scholar]
- 47.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. 10.1016/S0021-9258(19)52451-6 [DOI] [PubMed] [Google Scholar]
- 48.Liang Y, Wong J, Wei L. Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil. Chemosphere. 2005;58(4):475–83. doi: 10.1016/j.chemosphere.2004.09.034 [DOI] [PubMed] [Google Scholar]
- 49.Rady MM. Effects of seed pre-treatment with a calcium paste on the growth, phytohormone content, and enzyme activities in bean (Phaseolus vulgaris L.) seedlings grown under high NaCl stress. The Journal of Horticultural Science and Biotechnology. 2012;87(3):217–22. 10.1080/14620316.2012.11512855 [DOI] [Google Scholar]
- 50.Tahjib-Ul-Arif M, Roy PR, Sohag AAM, Afrin S, Rady MM, Hossain MA. Exogenous calcium supplementation improves salinity tolerance in BRRI dhan28; a salt-susceptible high-yielding Oryza sativa cultivar. Journal of Crop Science and Biotechnology. 2018;21(4):383–94. 10.1007/s12892-018-0098-0 [DOI] [Google Scholar]
- 51.Niu L, Yu J, Liao W, Yu J, Zhang M, Dawuda MM. Calcium and calmodulin are involved in nitric oxide-induced adventitious rooting of cucumber under simulated osmotic stress. Front Plant Sci. 2017;8:1684. doi: 10.3389/fpls.2017.01684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farzadfar S, Zarinkamar F, Modarres-Sanavy SAM, Hojati M. Exogenously applied calcium alleviates cadmium toxicity in Matricaria chamomilla L. plants. Enviro Sci Pollut Res. 2013;20(3):1413–22. doi: 10.1007/s11356-012-1181-9 [DOI] [PubMed] [Google Scholar]
- 53.Aziz H, Sabir M, Ahmad HR, Aziz T, Zia‐ur‐Rehman M, Hakeem KR, et al. Alleviating effect of calcium on nickel toxicity in rice. CLEAN–Soil, Air, Water. 2015;43(6):901–9. 10.1002/clen.201400085 [DOI] [Google Scholar]
- 54.Wu Y, Hendershot WH. The effect of calcium and pH on nickel accumulation in and rhizotoxicity to pea (Pisum sativum L.) root–empirical relationships and modeling. Environmental Pollut. 2010;158(5):1850–6. 10.1016/j.envpol.2009.10.046 [DOI] [PubMed] [Google Scholar]
- 55.Andosch A, Affenzeller MJ, Lütz C, Lütz-Meindl U. A freshwater green alga under cadmium stress: ameliorating calcium effects on ultrastructure and photosynthesis in the unicellular model Micrasterias. J Plant Physiol. 2012;169(15):1489–500. doi: 10.1016/j.jplph.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 56.Hochmal AK, Schulze S, Trompelt K, Hippler M. Calcium-dependent regulation of photosynthesis. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 2015;1847(9):993–1003. doi: 10.1016/j.bbabio.2015.02.010 [DOI] [PubMed] [Google Scholar]
- 57.Tian S, Lu L, Zhang J, Wang K, Brown P, He Z, et al. Calcium protects roots of Sedum alfredii H. against cadmium-induced oxidative stress. Chemosphere. 2011;84(1):63–9. doi: 10.1016/j.chemosphere.2011.02.054 [DOI] [PubMed] [Google Scholar]
- 58.Zouari M, Ahmed CB, Elloumi N, Bellassoued K, Delmail D, Labrousse P, et al. Impact of proline application on cadmium accumulation, mineral nutrition and enzymatic antioxidant defense system of Olea europaea L. cv Chemlali exposed to cadmium stress. Ecotoxicol Environ Saf. 2016;128:195–205. doi: 10.1016/j.ecoenv.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 59.Rodríguez-Serrano M, Romero-Puertas MC, Pazmino DM, Testillano PS, Risueño MC, Luis A, et al. Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol. 2009;150(1):229–43. doi: 10.1104/pp.108.131524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sudha G, Ravishankar GA. The role of calcium channels in anthocyanin production in callus cultures of Daucus carota. Plant Growth Regul. 2003;40(2):163–9. 10.1023/A:1024298602617 [DOI] [Google Scholar]
- 61.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Sci. 2002;7(9):405–10. doi: 10.1016/s1360-1385(02)02312-9 [DOI] [PubMed] [Google Scholar]
- 62.Sharma SS, Dietz K-J. The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress. J Exp Bot. 2006;57(4):711–26. doi: 10.1093/jxb/erj073 [DOI] [PubMed] [Google Scholar]
- 63.Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol. 2010;30(3):161–75. doi: 10.3109/07388550903524243 [DOI] [PubMed] [Google Scholar]
- 64.Siddiqui MH, Al-Whaibi MH, Basalah MO. Interactive effect of calcium and gibberellin on nickel tolerance in relation to antioxidant systems in Triticum aestivum L. Protoplasma. 2011;248(3):503–11. doi: 10.1007/s00709-010-0197-6 [DOI] [PubMed] [Google Scholar]
- 65.Yang Z, Wang C, Xue Y, Liu X, Chen S, Song C, et al. Calcium-activated 14-3-3 proteins as a molecular switch in salt stress tolerance. Nature Commun. 2019;10(1):1–12. doi: 10.1038/s41467-019-09181-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Lexy R, Kasai K, Clark N, Fujiwara T, Sozzani R, Gallagher KL. Exposure to heavy metal stress triggers changes in plasmodesmatal permeability via deposition and breakdown of callose. J Exp Bot. 2018;69(15):3715–28. doi: 10.1093/jxb/ery171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mishra S, Bharagava RN, More N, Yadav A, Zainith S, Mani S, et al. Heavy metal contamination: an alarming threat to environment and human health. Environmental biotechnology: For sustainable future: Springer; 2019. p. 103–25. 10.1007/978-981-10-7284-0_5 [DOI] [Google Scholar]
- 68.Javed MT, Akram MS, Tanwir K, Chaudhary HJ, Ali Q, Stoltz E, et al. Cadmium spiked soil modulates root organic acids exudation and ionic contents of two differentially Cd tolerant maize (Zea mays L.) cultivars. Ecotoxicol Environ Saf. 2017;141:216–25. doi: 10.1016/j.ecoenv.2017.03.027 [DOI] [PubMed] [Google Scholar]
- 69.Antosiewicz DM, Hennig J. Overexpression of LCT1 in tobacco enhances the protective action of calcium against cadmium toxicity. Environ Pollut. 2004;129(2):237–45. doi: 10.1016/j.envpol.2003.10.025 [DOI] [PubMed] [Google Scholar]
- 70.Palma JM, Mateos RM, López-Jaramillo J, Rodríguez-Ruiz M, González-Gordo S, Lechuga-Sancho AM, et al. Plant catalases as NO and H2S targets. Redox Bio. 2020;34:101525. 10.1016/j.redox.2020.101525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karuppanapandian T, Manoharan K. Uptake and translocation of tri-and hexa-valent chromium and their effects on black gram (Vigna mungo L. Hepper cv. Co4) roots. J Plant Biol. 2008;51(3):192. 10.1007/BF03030698 [DOI] [Google Scholar]
- 72.Candan N, Tarhan L. Effects of calcium, stress on contents of chlorophyll and carotenoid, LPO levels, and antioxidant enzyme activities in Mentha. J Plant Nutr. 2005;28(1):127–39. 10.1081/PLN-200042192 [DOI] [Google Scholar]
- 73.Jiang Y, Huang B. Effects of calcium on antioxidant activities and water relations associated with heat tolerance in two cool‐season grasses. J Exp Bot. 2001;52(355):341–9. 10.1093/jexbot/52.355.341 [DOI] [PubMed] [Google Scholar]
- 74.Jaakola L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trend Plant Sci. 2013;18(9):477–83. doi: 10.1016/j.tplants.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 75.Glover BJ, Martin C. Anthocyanins. Curr Biol. 2012;22(5):R147–R50. doi: 10.1016/j.cub.2012.01.021 [DOI] [PubMed] [Google Scholar]
- 76.Zhang X, Wei J, Huang Y, Shen W, Chen X, Lu C, et al. Increased Cytosolic Calcium Contributes to Hydrogen-Rich Water-Promoted Anthocyanin Biosynthesis Under UV-A Irradiation in Radish Sprouts Hypocotyls. Fron Plant Sci. 2018;9. doi: 10.3389/fpls.2018.01020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lotkowska ME, Tohge T, Fernie AR, Xue G-P, Balazadeh S, Mueller-Roeber B. The Arabidopsis transcription factor MYB112 promotes anthocyanin formation during salinity and under high light stress. Plant Physiol. 2015;169(3):1862–80. doi: 10.1104/pp.15.00605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xie Y, Chen P, Yan Y, Bao C, Li X, Wang L, et al. An atypical R2R3 MYB transcription factor increases cold hardiness by CBF‐dependent and CBF‐independent pathways in apple. New Phytol. 2018;218(1):201–18. doi: 10.1111/nph.14952 [DOI] [PubMed] [Google Scholar]
- 79.Xu Z, Mahmood K, Rothstein SJ. ROS induces anthocyanin production via late biosynthetic genes and anthocyanin deficiency confers the hypersensitivity to ROS-generating stresses in Arabidopsis. Plant Cell Physiol. 2017;58(8):1364–77. doi: 10.1093/pcp/pcx073 [DOI] [PubMed] [Google Scholar]
- 80.Yamdech R, Aramwit P, Kanokpanont S, editors. Stability of Anthocyanin in Mulberry Fruits Extract Adsorbed on Calcium Alginate Beads. International Conference Chulalongkorn University, Bangkok Thailand; 2012. [Google Scholar]
- 81.Amiri J, Entesari S, Delavar K, Saadatmand M, Rafie NA. The effect of silicon on cadmium stress in Echium amoenum. World Acad Sci Eng Technol. 2012;62:242–5. [Google Scholar]
- 82.Rucińska-Sobkowiak R. Water relations in plants subjected to heavy metal stresses. Acta Physiol Plant. 2016;38(11):1–13. 10.1007/s11738-016-2277-5 [DOI] [Google Scholar]
- 83.Rhodes D, Nadolska-Orczyk A, Rich P. Salinity, osmolytes and compatible solutes. Salinity: Environment-plants-molecules: Springer; 2002. p. 181–204. 10.1007/0-306-48155-3_9 [DOI] [Google Scholar]
- 84.Wang H-Y, Huang Y-C, Chen S-F, Yeh K-W. Molecular cloning, characterization and gene expression of a water deficiency and chilling induced proteinase inhibitor I gene family from sweet potato (Ipomoea batatas Lam.) leaves. Plant Sci. 2003;165(1):191–203. 10.1016/S0168-9452(03)00158-4 [DOI] [Google Scholar]
- 85.Slama I, Ghnaya T, Savouré A, Abdelly C. Combined effects of long-term salinity and soil drying on growth, water relations, nutrient status and proline accumulation in Sesuvium portulacastrum. Comptes Rendus Biolog. 2008;331(6):442–51. doi: 10.1016/j.crvi.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 86.EF AA, Abeer H, Alqarawi A, Hend AA. Alleviation of adverse impact of cadmium stress in sunflower (Helianthus annuus L.) by arbuscular mycorrhizal fungi’. Pak J Bot. 2015;47(2):785–95. [Google Scholar]
- 87.Gleeson D, Lelu-Walter M-A, Parkinson M. Overproduction of proline in transgenic hybrid larch (Larix x leptoeuropaea (Dengler)) cultures renders them tolerant to cold, salt and frost. Mol Breed. 2005;15(1):21–9. 10.1007/s11032-004-1363-3 [DOI] [Google Scholar]
- 88.Lee G, Carrow RN, Duncan RR, Eiteman MA, Rieger MW. Synthesis of organic osmolytes and salt tolerance mechanisms in Paspalum vaginatum. Environ Exp Bot. 2008;63(1–3):19–27. 10.1016/j.envexpbot.2007.10.009 [DOI] [Google Scholar]
- 89.Nedjimi B. Heavy metal tolerance in two Algerian saltbushes: A review on plant responses to cadmium and role of calcium in its mitigation. In: Hasanuzzaman M., Fujita M., Oku H., Nahar K., Hawrylak-Nowak B. (eds) Plant Nutrients and Abiotic Stress Tolerance. Springer, Singapore. 10.1007/978-981-10-9044-8_9 [DOI] [Google Scholar]
- 90.Alamri SA, Siddiqui MH, Al-Khaishany MY, Nasir Khan M, Ali HM, Alaraidh IA, et al. Ascorbic acid improves the tolerance of wheat plants to lead toxicity. J Plant Interact. 2018;13(1):409–19. 10.1080/17429145.2018.1491067 [DOI] [Google Scholar]
- 91.Türkan I, Bor M, Özdemir F, Koca H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci. 2005;168(1):223–31. 10.1016/j.plantsci.2004.07.032 [DOI] [Google Scholar]
- 92.Zhang K, Wang G, Bao M, Wang L, Xie X. Exogenous application of ascorbic acid mitigates cadmium toxicity and uptake in Maize (Zea mays L.). Enviro Sci Pollut Res. 2019;26(19):19261–71. 10.1007/s11356-019-05265-0 [DOI] [PubMed] [Google Scholar]
- 93.Siddiqui MH, Alamri S, Khan MN, Corpas FJ, Al-Amri AA, Alsubaie QD, et al. Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J Hazard Mater. 2020;398:122882. doi: 10.1016/j.jhazmat.2020.122882 [DOI] [PubMed] [Google Scholar]
- 94.Lindberg S, Kader MA, Yemelyanov V. Calcium signalling in plant cells under environmental stress. In: Ahmad P., Prasad M. (eds) Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change. Springer, New York, NY. 10.1007/978-1-4614-0815-4_15 [DOI] [Google Scholar]
- 95.Valivand M, Amooaghaie R, Ahadi A. Seed priming with H2S and Ca2+ trigger signal memory that induces cross-adaptation against nickel stress in zucchini seedlings. Plant Physiol Biochem. 2019;143:286–98. doi: 10.1016/j.plaphy.2019.09.016 [DOI] [PubMed] [Google Scholar]
- 96.Choong G, Liu Y, Templeton DM. Interplay of calcium and cadmium in mediating cadmium toxicity. Chem Biol Interact. 2014;211:54–65. doi: 10.1016/j.cbi.2014.01.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.