Abstract
Research suggests that exposure to fine particulate air pollution (PM2.5) increases hypothalamic-pituitary-adrenal (HPA) axis activation in adults; it is unclear, however, whether PM2.5 is associated with HPA-axis functioning in psychosocial contexts, such as during the experience of social stress. One recent study of adolescents found that PM2.5 was associated with heightened autonomic reactivity to a social stress task, and that this association was strongest for adolescents with more severe internalizing symptoms. Here, we sought to replicate and extend these findings to HPA-axis stress responsivity in an independent sample of adolescent girls (N = 130). We estimated PM2.5 concentrations at each participant’s address using data from nearby air quality monitoring stations, and assessed participants’ anxiety symptoms. We measured salivary cortisol in response to a social stress task and characterized HPA-axis functioning by computing area under the curve with respect to ground (AUCg) and with respect to increase (AUCi). Controlling for demographic factors, we found that PM2.5 was associated with heightened HPA-axis stress responsivity (both AUCg and AUCi) for girls who reported more severe levels of anxiety. We did not find a main effect of PM2.5 on HPA-axis functioning. These findings suggest that anxious adolescents are particularly vulnerable to the adverse effects of PM2.5 exposure on biological sensitivity to social stress.
Keywords: Adolescence, Air pollution, Anxiety, Cortisol, Social stress
Highlights
-
•
Fine particulate air pollution (PM2.5) may affect HPA-axis responsivity to acute stress.
-
•
We estimated PM2.5 concentrations and measured anxiety and cortisol in adolescent girls.
-
•
For anxious girls, higher PM2.5 predicted greater HPA-axis responsivity to stress.
-
•
Anxious adolescents are sensitive to the effects of PM2.5 on stress biology.
1. Introduction
Ambient air pollution is one of the leading health risks worldwide [1]. Some of the adverse health effects of air pollution may be mediated through altered stress biology. In fact, continued exposure to air pollution and chronic psychosocial stress affect similar biological processes implicated in health [2], including hypothalamic-pituitary-adrenal (HPA) axis functioning [3]. For example, fine particulate air pollution (i.e., particulate matter 2.5; PM2.5 – air particles smaller than 2.5 μm in diameter) can be inhaled deeply into the lungs and absorbed into the bloodstream, leading to local and systemic inflammation which, in turn, can activate the HPA axis [3]. Smaller PM2.5 particles may also reach the brain via the olfactory nerve and bulb [4,5], potentially affecting neural regions such as the paraventricular nucleus (PVN) in the hypothalamus, which is important for the regulation of HPA-axis and autonomic stress response systems [6,7]. Indeed, experimental research with adults suggests that short-term PM2.5 exposure leads to increases in circulating glucocorticoids, markers of HPA-axis dysregulation [8]. Studies with animals have provided further experimental evidence that exposure to air pollution elevates HPA-axis activation [9]. Chronic or long-term exposure to air pollution may also lead to alterations in HPA-axis functioning, as recent epidemiological work has found evidence that exposure to long-term air pollution is associated with flatter diurnal cortisol slopes in adolescents [10]. Conversely, Hajat and colleagues [11] did not find a link between long-term measures of air pollution and diurnal cortisol in adults, but did find a moderate association between higher levels of nitrogen dioxide and higher cortisol awakening response. Taken together, long-term air pollution may lead to chronic activation of stress response systems [12]. Prior studies, however, have not considered whether air pollution exposure is associated with HPA-axis activation in response to psychosocial contexts that are implicated in health, such as social stress.
Examining air pollution and stress biology is particularly important for research focused on adolescence given that this is a period of both increased biological sensitivity to social stress [13] and heightened exposure and vulnerability to ambient air pollution [14]. To date, however, only one study has considered the association between ambient air pollution and acute responses to psychosocial stress in adolescents. Miller and colleagues [15] found that adolescents living in neighborhoods with higher concentrations of PM2.5 (based on long-term estimates of PM2.5) exhibited greater autonomic reactivity (i.e., shift to sympathetic nervous system dominance indexed by lower high-frequency heart rate variability and higher skin conductance level) in response to a social stress task. Importantly, this association was not accounted for by family- or neighborhood-level socioeconomic factors. Chronic exposure to air pollution may have systemic effects on stress biology [12]. Thus, one aim of the present study was to assess whether the association between higher PM2.5 and greater autonomic reactivity to stress in adolescents [15] would also be observed in HPA-axis reactivity to stress in an independent sample.
Although PM2.5 appears to be implicated in stress biology activation and functioning, a growing body of research suggests that some youth are more vulnerable than others to the adverse effects of PM2.5 on health [16,17,2,12]. Psychological factors, particularly those related to the experience of psychosocial stress, may moderate air pollution effects. Psychosocial stress and PM2.5 affect many of the same biological pathways that lead to HPA-axis activation, and thus may compound risk for HPA-axis dysregulation [18,12]. Indeed, prior studies focusing on other physical measures of health have found that psychosocial risk factors may exacerbate the effects of air pollution on health. For example, Dales and Cakmak [19], found that higher levels of air pollution are associated with higher blood pressure and reduced lung function in children and adolescents who reported more emotional problems. Longitudinal studies have also found evidence that long-term traffic-related air pollution exposure predicts poorer respiratory functioning and more severe asthma, primarily in children who experience more social stress [20,21]. These findings converge with experimental research with rats suggesting that those exposed to chronic stress are more vulnerable to the adverse effects of air pollution on respiratory functioning [22]. Few studies have considered the interactive effects of air pollution and psychological factors on biological response to a stressful context. Miller and colleagues found that adolescents who reported more severe internalizing symptoms appeared to be particularly vlunerable to the effects of PM2.5 on increased autonomic reactivity to social stress [15]. We do not know, however, whether the interactive effect of PM2.5 and internalizing symptoms on stress reactivity extend to stress-related HPA-axis functioning.
In an independent sample of adolescent girls, we aimed to replicate and extend prior findings from Miller et al. [15] to HPA-axis reactivity to stress. Specifically, we examined (a) whether long-term estimates of PM2.5 are associated with HPA-axis responses to an acute psychosocial stressor; and (b) whether symptoms of anxiety moderate this association. Based on recent work implicating PM2.5 in HPA-axis activation and autonomic reactivity to stress [[8], [15]], we expected that PM2.5 concentrations would be positively associated with HPA-axis responsivity. Based on the growing evidence suggesting that psychosocial risk factors, including internalizing symptoms [15], may amplify the association between air pollution and pediatric health, we expected that the association between PM2.5 and HPA-axis responsivity would be significantly stronger in girls with higher levels of anxiety.
2. Methods
2.1. Participants
Participants were 130 adolescent girls from the San Francisco Bay Area (mean age = 12.27 years, SD = 1.41) who were part of a larger study (N = 200) on familial risk for depression in adolescent girls [[23], [24], [25]]. Half of the girls were at risk for depression due to having a mother with a history of recurrent depression; the other half of the sample had mothers with no history of depression. All girls had no current or past diagnosis of psychiatric disorder. Girls with complete data were included as participants in the current analyses. Participants were not significantly different from girls who provided incomplete data in terms of age, pubertal development, family income, or neighborhood poverty (all p > .471). This study was approved by the Stanford University Institutional Review Board and all families signed consent and assent forms to participate.
2.2. Measures
2.2.1. PM2.5
Long-term exposure to ambient PM2.5 was estimated using air quality monitoring station data made publicly available on the US Environmental Protection Agency’s Air Quality System online database (https://www.epa.gov.aqs). Inverse distance-squared weighting was used to estimate PM2.5 concentrations (μg/m3) at each participant’s home address using data from monitoring stations within 30 miles [26]. These estimates were based on annual average PM2.5 concentrations from the calendar year prior to visiting the laboratory, which included data collected from 2002 to 2010.
2.2.2. Symptoms of anxiety
Miller et al. [15] used the anxious/depressed subscale of the Youth Self-Report [27] to assess the moderating role of internalizing symptoms on the association between PM2.5 and autonomic reactivity. In the current study we did not administer the YSR to participants. Instead, girls reported on their anxiety using the Multidimensional Anxiety Scale for Children (MASC; [28]. The MASC included 39 items that were rated on a 4-point scale ranging from 0 (“never true about me”) to 3 (“often true about me”). Item responses were summed to form an index of overall severity of anxiety symptoms (Cronbach’s α in this sample was .91).
2.2.3. Stress task and cortisol collection
We measured salivary cortisol in response to a social stress task. As described in Gotlib et al. [23]; participants were instructed to refrain from eating or drinking 1 h before arriving at the laboratory. Following a 30-min rest period, participants were given instructions about and participated in the stress task. Participants first completed a 3-min serial subtraction task with an experimenter present. The experimenter interrupted participants when they made a mistake and instructed them to start over. After the serial subtraction task, participants completed a 12-min semi-structured interview designed to induce stress in adolescents by having them talk about stressful situations [29]. After completing the stressor task, participants watched a 30-min neutral video intended to serve as a recovery period.
As described in Gotlib et al. [23]; participants used Sarstedt Salivettes (Sarstedt, Numbrecht, Germany) to provide saliva samples immediately before the stressor, and at 15, 30, and 45 min after the onset of the stressor. Saliva samples were stored in a freezer chest until they could be transferred to and stored in a −20 °C freezer until radioimmunoassay. Cortisol levels were assayed by luminescence immunoassay reagents using a commercial kit from Immuno-Biological Laboratories (Hamburg, Germany), and the assay sensitivity was set at 0.015 mg dl−1. Samples were assayed together in large batches to control for interassay error, and control samples were included to evaluate variability. Average Intraassay coefficients of variation of three saliva pools of the low, medium, and high controls were 2.8%, 10.5%, and 4.8%, respectively. Average interassay coefficients of the variation of the low, medium, and high controls were 10.9%, 10.5%, and 5.5%, respectively.
We calculated area under the curve with respect to ground (AUCg) and area under the curve with respect to increase (AUCi) [30] to capture total cortisol production and stressor-specific change in cortisol, respectively. AUCg represents both basal cortisol and cortisol reactivity to the stress task (sample 1 cortisol plus changes in cortisol from sample 1 to sample 4). Higher and lower AUCg values should be interpreted as higher and lower total cortisol output, respectively. AUCi represents reactive increases or decreases in cortisol to the stress task (changes in cortisol from sample 1 to sample 4 minus sample 1). More positive AUCi values should be interpreted as greater increases in cortisol levels in response to the stress task, whereas more negative AUCi values should be interpreted as decreasing levels of cortisol. Visual observation of the distributions of the cortisol data suggested that AUCg values were positively skewed, whereas AUCi values were more normally distributed. Extreme outlier values for AUCg and AUCi were defined as those values greater or less than the mean by 3 times the interquartile range. This is considered to be a better method for detecting outliers than is using standard deviations, which can miss outliers due to extreme values changing the sample mean and increasing the sample variance [31]. AUCg and AUCi outlier values were winsorized to the next most extreme value before analyses. This winsorization method maximizes power and accuracy by minimizing missing data points while effectively reducing the influence of outlier values [32].
2.2.4. Covariates
Previous analyses with this sample indicated that daughters of mothers with a history of recurrent depression have heightened cortisol reactivity to stress [23]. We included depression risk status as a binary covariate in regression analyses. Other covariates included age, pubertal status, family income, neighborhood-level poverty, and time of day of the first cortisol measurement (seconds since midnight). Pubertal status was assessed using self-reported Tanner stage [33]. Families reported on annual family income before taxes using a 6-point scale ranging from 0 (“less than $10,000”) to 5 (“More than $100,000”). Poverty rate at the neighborhood-level was assessed using data indicating the percent of residents within a given census tract with income less than two times the federal poverty level. The California Office of Environmental Health and Hazard Assessment (OEHHA; https://oehha.ca.gov/calenviroscreen) converted these data into percentiles representing the amount of poverty relative to other census tracts in California.
2.3. Statistical analysis
We conducted two linear regression analyses (one with AUCg and one with AUCi as the dependent variables) to examine whether PM2.5 was associated with cortisol response to stress after controlling for the covariates described above, and whether anxiety symptoms moderated this association. Taken together, each regression model included depression risk status (i.e., maternal history of recurrent depression), pubertal stage, age, time of day of the stressor task (i.e., time of first cortisol assessment), family income, neighborhood poverty, anxiety symptoms, PM2.5, and the interaction between PM2.5 and anxiety as predictors of cortisol response (either AUCg or AUCi). All continuous predictors and covariates were centered prior to forming a interaction term that was the product of PM2.5 and anxiety. Significant interactions were probed by examining the effect of PM2.5 on cortisol response at 1 SD above and below the mean of anxiety symptoms (i.e., high and low anxiety, respectively) adjusting for other covariates [34]. We included False Discovery Rate (FDR) corrected p-values within each regression model to provide more conservative estimates of statistical significance corrected for multiple comparisons [35].
3. Results
Participants (N = 130) were predominantly White (68%) and upper-middle class (mean annual family income = US $75,000-$100,000, range from US <$10,000 to >$100,000). Descriptive statistics are presented in Table 1. Zero-order correlations among predictor variables and covariates are presented in Table 2. Maternal history of recurrent depression was associated with lower family income (r = −0.35, p < .001) and higher neighborhood poverty (r = 0.22, p = .011). Adolescents from wealthier families resided in neighborhoods with less poverty (r = −0.26, p = .003). There were no statistically significant associations among socioeconomic variables, PM2.5, and cortisol metrics (all p > .098).
Table 1.
Descriptive statistics.
| M or % | SD | Range | |
|---|---|---|---|
| Maternal history of recurrent depression (risk = 1) | 50% | ||
| Pubertal stage (Tanner stage) | 3.16 | 1.03 | 1.00–5.00 |
| Age (years) | 12.27 | 1.41 | 9.87–14.88 |
| Time of day of stressor task | 13:07 | 2:53 | 8:19–19:25 |
| Family income | 4.08 | 1.31 | 0.00–5.00 |
| Neighborhood poverty rate (percentile) | 17.75 | 18.17 | 0.03–88.46 |
| Anxiety | 37.78 | 15.10 | 8.00–71.00 |
| PM2.5 (μg/m3) | 10.46 | 1.38 | 6.52–14.20 |
| AUCg | 0.65 | 0.51 | 0.07–2.66 |
| AUCi | 0.01 | 0.39 | −0.95–1.05 |
Note. AUCg = area under the curve with respect to ground; AUCi = area under the curve with respect to increase; PM2.5 = particulate matter <2.5 μm. We present the descriptive statistics for time of day of stressor task in hours:minutes for the sake of clarity, but the metric of this variable is seconds since midnight. The family income mean of 4.08 corresponds to $75,000 to $100,000 per year, and the range of 0.00–5.00 corresponds to less than $10,000 per year to more than $100,000 per year.
Table 2.
Zero-order correlations.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
|
.01 | |||||||||
|
-.02 | .06 | ||||||||
|
-.03 | -.11 | .04 | |||||||
|
-.35∗∗∗ | -.14 | .01 | -.03 | ||||||
|
.22∗ | -.01 | .17∗ | .23∗∗ | -.26∗∗ | |||||
|
-.10 | .11 | -.02 | -.05 | .12 | -.10 | ||||
|
.00 | .04 | .11 | -.20∗ | .13 | .07 | .07 | |||
|
.14 | .09 | -.07 | -.21∗ | .07 | -.15 | .10 | .08 | ||
|
.09 | .06 | -.05 | .09 | .05 | -.01 | .03 | .06 | .35∗∗∗ |
Note. ∗∗∗p < .001, ∗∗p < .01, ∗p < .05. AUCg = area under the curve with respect to ground; AUCi = area under the curve with respect to increase; PM2.5 = particulate matter <2.5 μm.
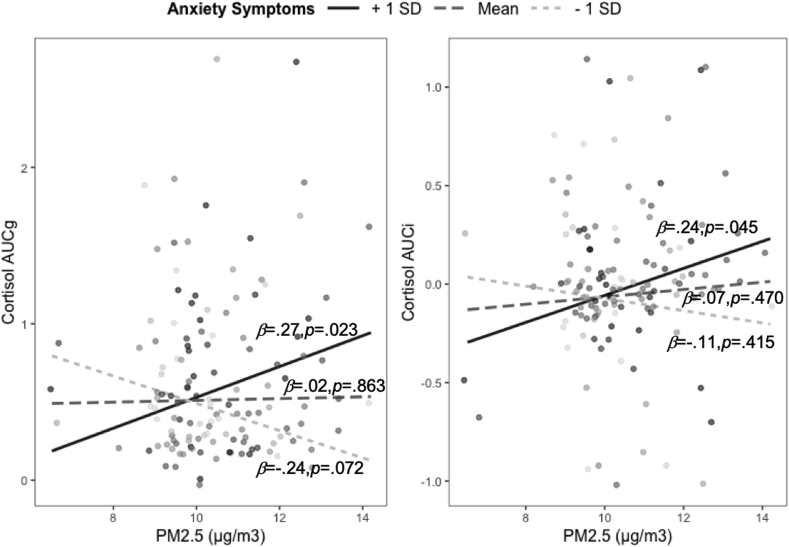
The full, adjusted regression models testing PM2.5 by anxiety interaction effects on AUCg and AUCi covariates are presented in Table 3. The interaction of PM2.5 and anxiety symptoms significantly predicted both AUCg (β = 0.26, p = .004, 95% CI = 0.08-0.42) and AUCi (β = 0.18, p = .049, 95% CI = 0.00-0.35). Fig. 1 presents the simple slopes of the interaction effects from the regression models adjusted for covariates (anxiety is grouped for visualization purposes). PM2.5 (μg/m3) was positively associated with AUCg and AUCi only for girls who reported more severe anxiety symptoms (i.e., 1 SD above the mean; β = 0.27, p = .023, 95% CI = 0.04-0.50 and β = 0.24, p = .045, 95% CI = 0.01-0.48 for AUCg and AUCi, respectively), but not for girls who reported anxiety symptoms at the sample mean (β = 0.02, p = .863, 95% CI = −0.16–0.19 and β = 0.07, p = .470, 95% CI = −0.12–0.25 for AUCg and AUCi, respectively), or for girls who reported less severe anxiety symptoms (i.e., 1 SD below the mean; β = −0.24, p = .072, 95% CI = −0.49–0.02, and β = −0.11, p = .415, 95% CI = −0.38–0.16 for AUCg and AUCi, respectively). As has been documented previously, daughters of mothers with a history of depression also exhibited greater HPA-axis responsivity as assessed by AUCg; no other predictor or covariate was statistically significantly associated with AUCg or AUCi. Variance inflation factor values for all predictor variables were <1.28, suggesting that our analyses did not have issues related to multicollinearity. Analyses that include neighborhood education and minority status as additional covariates did not change the findings (see Supplement).
Table 3.
Regression models predicting HPA-Axis responsivity to stress.
| Cortisol AUCg |
Cortisol AUCi |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
β |
p |
FDR p |
95% Confidence Interval for β |
β |
p |
FDR p |
95% Confidence Interval for β |
|||
| Lower | Upper | Lower | Upper | |||||||
| Intercept | <.001 | .269 | ||||||||
| Maternal history of recurrent depression (risk = 1) | .26 | .007 | .032 | .07 | .44 | .16 | .094 | .375 | -.03 | .35 |
| Pubertal stage (Tanner stage) | .12 | .157 | .266 | -.05 | .30 | -.14 | .125 | .375 | -.32 | .04 |
| Age (years) | -.07 | .433 | .487 | -.24 | .10 | -.05 | .579 | .640 | -.23 | .13 |
| Time of day of stressor task | -.16 | .080 | .240 | -.33 | .02 | .11 | .250 | .563 | -.08 | .29 |
| Family income | .13 | .182 | .266 | -.06 | .31 | .07 | .511 | .640 | -.13 | .26 |
| Neighborhood poverty rate (percentile) | -.12 | .207 | .266 | -.30 | .07 | -.05 | .640 | .640 | -.24 | .15 |
| Anxiety | .12 | .164 | .266 | -.05 | .29 | .08 | .383 | .640 | -.10 | .26 |
| PM2.5 (μg/m3) | .02 | .863 | .863 | -.16 | .19 | .07 | .470 | .640 | -.12 | .25 |
| PM2.5 X Anxiety | .26 | .004 | .032 | .08 | .42 | .18 | .049 | .375 | .00 | .35 |
Note. AUCg = area under the curve with respect to ground; AUCi = area under the curve with respect to increase; PM2.5 = particulate matter <2.5 μm.
Fig. 1.
Interactions between anxiety symptoms and PM2.5 in predicting HPA-axis responsivity to stress.
Note. AUCg = area under the curve with respect to ground; AUCi = area under the curve with respect to increase; PM2.5 = particulate matter <2.5 μm. Anxiety is only grouped for visualization.
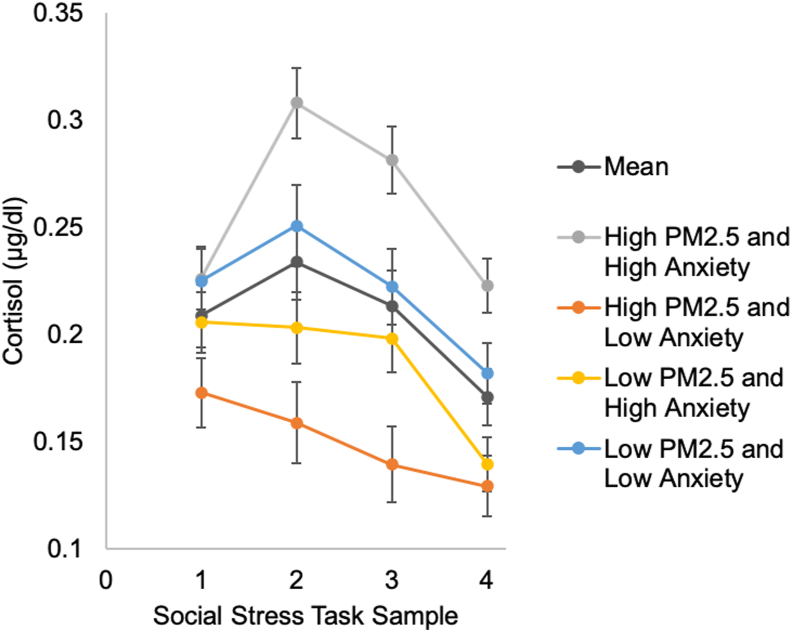
In follow-up analyses, we investigated whether cortisol levels at specific samples were accounting for the positive associations between PM2.5 and AUC measures in girls who reported more severe anxiety symptoms. Prior to analyses, we used the same methods to detect and winsorize extreme outliers for cortisol levels that we used for AUC measures. In four linear regression models, we replaced the AUC measures with the cortisol values for sample 1 (before task), sample 2 (after 15 min), sample 3 (after 30 min), and sample 4 (after 45 min) as the dependent variables. Adjusting for covariates, the interaction between PM2.5 and anxiety was significantly associated with cortisol levels at sample 2 (β = 0.34, p = .005), sample 3 (β = 31, p = .011), and sample 4 (β = 0.32, p = .009). Probing these interaction effects showed that PM2.5 was positively associated with cortisol levels only for girls who reported more severe anxiety symptoms (i.e., 1 SD above the mean; β = 0.27, p = .023, β = 0.22, p = .058, and β = 0.28, p = .018 for sample 2, 3, and 4, respectively), but not for girls who reported anxiety symptoms at the sample mean (all p > .567), or for girls who reported less severe anxiety symptoms (i.e., 1 SD below the mean; all p > .078). The interaction between PM2.5 and anxiety was not significantly associated with cortisol at sample 1 (β = 0.11, p = .219). Fig. 2 presents the average cortisol response and the estimated response (based on regression model results) associated with high PM2.5 (1 SD above the mean) in more anxious girls.
Fig. 2.
Cortisol response to stress on average and at different levels of PM2.5 and anxiety.
Note. PM2.5 = particulate matter <2.5 μm. Error bars represent standard errors.
4. Discussion
Investigators have suggested that exposure to PM2.5 adversely affects health, in part by altering stress biology [2,3]. The majority of relevant studies, however, assessed physiology during resting states [8,10], rather than in psychosocial contexts that have been implicated in health, such as social stress [[36], [37]]. Studying adolescents, Miller et al. [15], found a link between PM2.5 and heightened autonomic reactivity to a social stress task, which was stronger for adolescents who reported more severe internalizing symptoms. The current study partially replicates these findings, providing preliminary evidence that anxious adolescent girls are vulnerable to the effects of PM2.5 on HPA-axis responsivity to stress. This finding was not accounted for by our family- and neighborhood-level measures of socioeconomic status. Contrary to our hypotheses, however, we did not find an overall association between PM2.5 and HPA-axis responsivity.
Psychological experiences and traits, such as anxiety, may exacerbate the biological effects of exposure to physical pollutants. Indeed, previous work suggests that youth who experience higher levels of psychosocial stress and mental health difficulties combined with higher levels of air pollution are at greater risk for physical health problems [19,21]. Our current findings and those from Miller et al. [15] extend prior studies by demonstrating that mental health difficulties may amplify the effects of PM2.5 on biological sensitivity to psychosocial stress contexts. The mechanisms underlying this vulnerability are still unclear. One possibility is related to proneness to hyperarousal states and a proinflammatory phenotype that may be typical of individuals prone to internalizing symptoms [38]. For anxious adolescents, hyperarousal states characterized by increased cardiorespiratory activity may increase the amount of PM2.5 that enters the body in much the same way physical activity increases inhalation and deposition of PM2.5 [39]; further, increased inflammatory reactivity to PM2.5 may heighten responsivity in other stress systems, such as the HPA-axis. Administration of proinflammatory cytokines to animals has been shown to stimulate the HPA-axis [40].
We should note, however, that the interaction effect was present only in analyses predicting AUCg, not AUCi, when using thresholds for statistical significance corrected for multiple comparisons. In anxious girls, long-term PM2.5 appears to be associated more robustly with total cortisol output during and after the stress task (AUCg) than with cortisol increases or decreases over time (AUCi). Compared to AUCi, a measure of HPA-axis sensitivity to the stress task, the AUCg measure may be more closely related to, or be a product of, the chronic activation and general dysregulation of the HPA-axis that may result from living in areas with higher levels of PM2.5 [12].
We did not find evidence in this sample of the hypothesized overall association between PM2.5 and stress reactivity that Miller et al. [15] found for autonomic measures in an independent sample of adolescents. In addition, the previously observed effect of PM2.5 on autonomic reactivity to stress at high levels of internalizing symptoms [15] was roughly twice as strong as the effect that we observed in this study for PM2.5 and HPA-axis reactivity to stress in more anxious girls. One explanation for these inconsistencies may be that PM2.5 is implicated more strongly in autonomic than in HPA-axis functioning. Inhaled air pollutants change vagal afferent activity in the autonomic nervous system [3]. Parasympathetic neurons in the nucleus of the solitary tract (NTS) project to and can regulate corticotropin-releasing hormone (CRH) neurons in the PVN of the hypothalamus [41]. This is one path by which air pollution may alter hypothalamic functioning and activation of the HPA-axis [3], but the autonomic nervous system is more proximal to the immediate physiological changes produced by inhaled pollutants. Furthermore, autonomic activity supports rapid changes in mobilization of bodily resources [42], and has been found to precede and predict cortisol release [43]. One interpretation is that the autonomic nervous system plays a role in regulating the sensitivity of the HPA-axis system to moderate levels of PM2.5, such as those observed in this study. Future studies of air pollution studies should include multiple measures of stress biology to test this possibility in adolescents and examine whether intersystem coordination is a mechanism that underlies increased vulnerability to the adverse effects of PM2.5 in adolescents who are experiencing mental health difficulties.
Inconsistencies between the current findings and those of Miller et al. [15] may also be due to study differences, such as assessing community versus at-risk samples, including boys and girls versus girls only, using different approaches in the assessment of air pollution, using similar but not identical social stress tasks, and considering self-reported anxiety versus self-reported internalizing symptoms more broadly. Conversely, these differences also reflect a strength of this research, as a similar interaction effect was observed across these methodological variations. Nonetheless, further research is needed to determine whether PM2.5 is associated with general heightened reactivity to stress, or whether this association is more specific to certain stress biology systems.
We should note several limitations of this study. First, we estimated PM2.5 concentrations at residential addresses based on data from nearby monitoring sites; we did not measure individual PM2.5 exposure directly. As a related point, we did not account for geographic factors that affect air pollution (e.g., proximity to roads, wind patterns), which may have resulted in misclassification of PM2.5 estimates. Second, although we controlled for variables that have been posited to be correlated with ambient air pollution, we cannot definitively rule out all confounds for our observed associations between PM2.5 and HPA-axis responsivity in anxious girls. It is possible that our findings are due to unmeasured factors that may be associated with air pollution and stress biology, such as noise exposure, urbanicity, or marginalization not captured by family income or census tract data. Third, our sample included only adolescent girls; future research must be conducted to examine the generalizability of these findings to other populations. Fourth, all participants resided in the same general area (i.e., within driving distance of the Bay Area in California), which may have limited variability in PM2.5 concentrations in our sample and contributed to the lack of an overall association between PM2.5 and cortisol. It is also possible that higher levels of air pollution, such as those observed in some prior epidemiological studies focused on PM2.5 in more polluted regions, are required to detect the association between PM2.5 and HPA-axis response to stress. For example, Khamirchi and colleagues [44] studied the implications of long-term average PM2.5 levels that were roughly four times as high as the average PM2.5 in our study. It is unclear whether the findings documented in this study generalize to adolescents living in communities outside of the Bay Area that are characterized by higher (or lower) levels of long-term PM2.5. Our sample size may have also limited our ability to detect statistically significant effects. Larger samples are necessary to have sufficient power to detect interaction effects. In addition, prior epidemiological research suggests that PM2.5 is associated with elevated anxiety [45], but we did not observe this relation in our study. Future studies with greater statistical power, and perhaps more variability in PM2.5, should be conducted to replicate our findings. Finally, we considered anxiety symptoms as a continuous variable rather than diagnosis of anxiety disorders based on clinical cutoffs. Although this may limit the clinical significance of these findings, it is important to note that dimensional approaches can be more useful for capturing the range of individual differences in anxiety symptom number and severity, and may align more closely with the latent structure of variability in anxiety [46,47].
In summary, we found that PM2.5 is associated with heightened HPA-axis responsivity to social stress in anxious adolescent girls; we did not find that PM2.5 is associated directly with HPA-axis responsivity. Thus, our findings partially replicate those of [15]; but importantly are the first to provide evidence for an association between PM2.5 and HPA-axis activation specific to psychosocial stress in more anxious girls. Taken together with the recent findings reported by Miller et al., adolescents who experience more severe internalizing symptoms may be particularly vulnerable to the adverse effects of PM2.5 on biological sensitivity to stress.
Sources of funding
This research was supported by the National Institutes of Mental Health (R01MH074849 and R37MH101495 to IHG, T32MH019908 to Allan L. Reiss (funding JGM), and F32MH096385 to KK), the Brain and Behavior Research Foundation (Young Investigator Award 20814 to KK), Michael Smith Foundation for Health Research (Scholar Award to JL), and the Canadian Institutes of Health Research (389703 to JL).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the National Institutes of Mental Health (R01MH074849 and R37MH101495 to IHG, T32MH019908 to Allan L. Reiss (funding JGM), and F32MH096385 to KK), the Brain and Behavior Research Foundation (Young Investigator Award 20814 to KK), Michael Smith Foundation for Health Research (Scholar Award to JL), and the Canadian Institutes of Health Research (389703 to JL).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpnec.2020.100015.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cohen A.J., Brauer M., Burnett R., Anderson H.R., Frostad J., Estep K., Balakrishnan K., Brunekreef B., Dandona L., Dandona R., Feigin V., Freedman G., Hubbell B., Jobling A., Kan H., Knibbs L., Liu Y., Martin R., Morawska L.…Forouzanfar M.H. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389(10082):1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olvera Alvarez H.A., Kubzansky L.D., Campen M.J., Slavich G.M. Early life stress, air pollution, inflammation, and disease: an integrative review and immunologic model of social-environmental adversity and lifespan health. Neurosci. Biobehav. Rev. 2018;92:226–242. doi: 10.1016/j.neubiorev.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snow S.J., Henriquez A.R., Costa D.L., Kodavanti U.P. Neuroendocrine regulation of air pollution health effects: emerging insights. Toxicol. Sci. 2018;164(1):9–20. doi: 10.1093/toxsci/kfy129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Angiulli A. Severe urban outdoor air pollution and children’s structural and functional brain development, from evidence to precautionary strategic action. Frontiers in Public Health. 2018;6 doi: 10.3389/fpubh.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maher B.A., Ahmed I.A.M., Karloukovski V., MacLaren D.A., Foulds P.G., Allsop D., Mann D.M.A., Torres-Jardón R., Calderon-Garciduenas L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2016;113(39):10797–10801. doi: 10.1073/pnas.1605941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sirivelu M.P., MohanKumar S.M.J., Wagner J.G., Harkema J.R., MohanKumar P.S. Activation of the stress axis and neurochemical alterations in specific brain areas by concentrated ambient particle exposure with concomitant allergic airway disease. Environ. Health Perspect. 2006;114(6):870–874. doi: 10.1289/ehp.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ying Z., Xu X., Bai Y., Zhong J., Chen M., Liang Y., Zhao J., Liu D., Morishita M., Sun Q., Spino C., Brook R.D., Harkema J.R., Rajagopalan S. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ. Health Perspect. 2014;122(1):79–86. doi: 10.1289/ehp.1307151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H., Cai J., Chen R., Zhao Z., Ying Z., Wang L., Chen J., Hao K., Kinney P.L., Chen H., Kan H. Particulate matter exposure and stress hormone levels: a randomized, double-blind, crossover trial of air purification. Circulation. 2017;136(7):618–627. doi: 10.1161/CIRCULATIONAHA.116.026796. [DOI] [PubMed] [Google Scholar]
- 9.Thomson E.M., Vladisavljevic D., Mohottalage S., Kumarathasan P., Vincent R. Mapping acute systemic effects of inhaled particulate matter and ozone: multiorgan gene expression and glucocorticoid activity. Toxicol. Sci.: An Official Journal of the Society of Toxicology. 2013;135(1):169–181. doi: 10.1093/toxsci/kft137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wing S.E., Bandoli G., Telesca D., Su J.G., Ritz B. Chronic exposure to inhaled, traffic-related nitrogen dioxide and a blunted cortisol response in adolescents. Environ. Res. 2018;163:201–207. doi: 10.1016/j.envres.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajat A., Hazlehurst M.F., Golden S.H., Merkin S.S., Seeman T., Szpiro A.A., Kaufman J.D., Roux A.D. The cross-sectional and longitudinal association between air pollution and salivary cortisol: evidence from the Multi-Ethnic Study of Atherosclerosis. Environ. Int. 2019;131:105062. doi: 10.1016/j.envint.2019.105062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson E.M. Air pollution, stress, and allostatic load: linking systemic and central nervous system impacts. J. Alzheim. Dis.: JAD. 2019;69(3):597–614. doi: 10.3233/JAD-190015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sumter S.R., Bokhorst C.L., Miers A.C., Van Pelt J., Westenberg P.M. Age and puberty differences in stress responses during a public speaking task: do adolescents grow more sensitive to social evaluation? Psychoneuroendocrinology. 2010;35(10):1510–1516. doi: 10.1016/j.psyneuen.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Sacks J.D., Stanek L.W., Luben T.J., Johns D.O., Buckley B.J., Brown J.S., Ross M. Particulate matter-induced health effects: who is susceptible? Environ. Health Perspect. 2011;119(4):446–454. doi: 10.1289/ehp.1002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller J.G., Gillette J.S., Manczak E.M., Kircanski K., Gotlib I.H. Fine particle air pollution and physiological reactivity to social stress in adolescence: the moderating role of anxiety and depression. Psychosom. Med. 2019;81(7):641–648. doi: 10.1097/PSY.0000000000000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen E., Schreier H.M.C., Strunk R.C., Brauer M. Chronic traffic-related air pollution and stress interact to predict biologic and clinical outcomes in asthma. Environ. Health Perspect. 2008;116(7):970–975. doi: 10.1289/ehp.11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clougherty J.E., Kubzansky L.D. A framework for examining social stress and susceptibility to air pollution in respiratory health. Environ. Health Perspect. 2009;117(9):1351–1358. doi: 10.1289/ehp.0900612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McEwen B.S., Tucker P. Critical biological pathways for chronic psychosocial stress and research opportunities to advance the consideration of stress in chemical risk assessment. Am. J. Publ. Health. 2011;101(Suppl 1):S131–S139. doi: 10.2105/AJPH.2011.300270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dales R.E., Cakmak S. Does mental health status influence susceptibility to the physiologic effects of air pollution? A population based study of Canadian children. PloS One. 2016;11(12) doi: 10.1371/journal.pone.0168931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clougherty J.E., Levy J.I., Kubzansky L.D., Ryan P.B., Suglia S.F., Canner M.J., Wright R.J. Synergistic effects of traffic-related air pollution and exposure to violence on urban asthma etiology. Environ. Health Perspect. 2007;115(8):1140–1146. doi: 10.1289/ehp.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rancière F., Bougas N., Viola M., Momas I. Early exposure to traffic-related air pollution, respiratory symptoms at 4 Years of age, and potential effect modification by parental allergy, stressful family events, and sex: a prospective follow-up study of the PARIS birth cohort. Environ. Health Perspect. 2017;125(4):737–745. doi: 10.1289/EHP239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clougherty J.E., Rossi C.A., Lawrence J., Long M.S., Diaz E.A., Lim R.H., McEwen B., Koutrakis P., Godleski J.J. Chronic social stress and susceptibility to concentrated ambient fine particles in rats. Environ. Health Perspect. 2010;118(6):769–775. doi: 10.1289/ehp.0901631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gotlib I.H., LeMoult J., Colich N.L., Foland-Ross L.C., Hallmayer J., Joormann J., Lin J., Wolkowitz O.M. Telomere length and cortisol reactivity in children of depressed mothers. Mol. Psychiatr. 2015;20(5):615–620. doi: 10.1038/mp.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeMoult J., Joormann J., Kircanski K., Gotlib I.H. Attentional bias training in girls at risk for depression. J. Child Psychol. Psychiatry Allied Discip. 2016;57(11):1326–1333. doi: 10.1111/jcpp.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeMoult J., Ordaz S.J., Kircanski K., Singh M.K., Gotlib I.H. Predicting first onset of depression in young girls: interaction of diurnal cortisol and negative life events. J. Abnorm. Psychol. 2015;124(4):850–859. doi: 10.1037/abn0000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padula A.M., Tager I.B., Carmichael S.L., Hammond S.K., Lurmann F., Shaw G.M. The association of ambient air pollution and traffic exposures with selected congenital anomalies in the San Joaquin Valley of California. Am. J. Epidemiol. 2013;177(10):1074–1085. doi: 10.1093/aje/kws367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Achenbach T.M., Rescorla L.A. University of Vermont, Research Center for Chlidren, Youth, & Families; Burlington, VT: 2001. Manual for the ASEBA School-Age Forms & Profiles. [Google Scholar]
- 28.March J.S., Parker J.D., Sullivan K., Stallings P., Conners C.K. The multidimensional anxiety scale for children (MASC): factor structure, reliability, and validity. J. Am. Acad. Child Adolesc. Psychiatr. 1997;36(4):554–565. doi: 10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Ewart C.K., Jorgensen R.S., Suchday S., Chen E., Matthews K.A. Measuring stress resilience and coping in vulnerable youth: the social competence interview. Psychol. Assess. 2002;14(3):339–352. doi: 10.1037/1040-3590.14.3.339. [DOI] [PubMed] [Google Scholar]
- 30.Pruessner J.C., Kirschbaum C., Meinlschmid G., Hellhammer D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 31.Wilcox R. Modern statistics for the social and behavioral sciences: A practical introduction. CRC Press; Boca Raton, FL: 2011. [Google Scholar]
- 32.Erceg-Hurn D.M., Mirosevich V.M. Modern robust statistical methods: an easy way to maximize the accuracy and power of your research. Am. Psychol. 2008;63(7):591–601. doi: 10.1037/0003-066X.63.7.591. [DOI] [PubMed] [Google Scholar]
- 33.Morris N.M., Udry J.R. Validation of a self-administered instrument to assess stage of adolescent development. J. Youth Adolesc. 1980;9(3):271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 34.Aiken L.S., West S.G. Sage Publications, Inc; 1991. Multiple Regression: Testing and Interpreting Interactions; p. xi. 212. [Google Scholar]
- 35.Benjamini Y., Hochberg Y. Controlling the False Discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. B. 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 36.Juster R-P., McEwen B.S., Lupien S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 37.McEwen B.S., Bowles N.P., Gray J.D., Hill M.N., Hunter R.G., Karatsoreos I.N., Nasca C. Mechanisms of stress in the brain. Nature Neurosci. 2015;18(10):1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Donovan A., Slavich G.M., Epel E.S., Neylan T.C. Exaggerated neurobiological sensitivity to threat as a mechanism linking anxiety with increased risk for diseases of aging. Neurosci. Biobehav. Rev. 2013;37(1):96–108. doi: 10.1016/j.neubiorev.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daigle C.C., Chalupa D.C., Gibb F.R., Morrow P.E., Oberdörster G., Utell M.J., Frampton M.W. Ultrafine particle deposition in humans during rest and exercise. Inhal. Toxicol. 2003;15(6):539–552. doi: 10.1080/08958370304468. [DOI] [PubMed] [Google Scholar]
- 40.Dunn A.J. Cytokine activation of the HPA Axis. Ann. N. Y. Acad. Sci. 2000;917(1):608–617. doi: 10.1111/j.1749-6632.2000.tb05426.x. [DOI] [PubMed] [Google Scholar]
- 41.Herman J.P. Regulation of hypothalamo-pituitary-adrenocortical responses to stressors by the nucleus of the solitary tract/dorsal vagal complex. Cell. Mol. Neurobiol. 2018;38(1):25–35. doi: 10.1007/s10571-017-0543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porges S.W. The polyvagal perspective. Biol. Psychol. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Engert V., Vogel S., Efanov S.I., Duchesne A., Corbo V., Ali N., Pruessner J.C. Investigation into the cross-correlation of salivary cortisol and alpha-amylase responses to psychological stress. Psychoneuroendocrinology. 2011;36(9):1294–1302. doi: 10.1016/j.psyneuen.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 44.Khamirchi R., Moslem A., Agah J., Pozo Ó.J., Miri M., Dadvand P. Maternal exposure to air pollution during pregnancy and cortisol level in cord blood. Sci. Total Environ. 2020;713 doi: 10.1016/j.scitotenv.2020.136622. [DOI] [PubMed] [Google Scholar]
- 45.Power M.C., Kioumourtzoglou M.-A., Hart J.E., Okereke O.I., Laden F., Weisskopf M.G. The relation between past exposure to fine particulate air pollution and prevalent anxiety: observational cohort study. BMJ. 2015;350 doi: 10.1136/bmj.h1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haslam N., Holland E., Kuppens P. Categories versus dimensions in personality and psychopathology: a quantitative review of taxometric research. Psychol. Med. 2012;42(5):903–920. doi: 10.1017/S0033291711001966. [DOI] [PubMed] [Google Scholar]
- 47.Hudziak J.J., Achenbach T.M., Althoff R.R., Pine D.S. A dimensional approach to developmental psychopathology. Int. J. Methods Psychiatr. Res. 2007;16(Suppl 1):S16–S23. doi: 10.1002/mpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.