Abstract
Background
Hair cortisol concentrations (HCC) provide a biomarker for stress adaptation, which has downstream health consequences. Personality traits (e.g., neuroticism) and social processes (e.g., chronic interpersonal goals) may confer risk or buffer against dysregulated cortisol secretion. However, few studies have examined personality or interpersonal factors predicting hair cortisol, which estimates longer-term secretion and therefore provides a potential biomarker for studying trait-like psychological processes. The present study investigated effects of personality traits and daily interpersonal goals during stressors on HCC.
Method
Participants (N = 90) reported Big Five traits at baseline, recorded interpersonal (self-image and compassionate) goals pursued during their worst psychosocial stressors for 4–5 weeks (1,949 entries), then provided a hair sample to estimate cortisol secretion over the past two months.
Results
As hypothesized, neuroticism predicted higher HCC, beyond other Big Five traits (b = 7.45, SE = 3.36, p = .029). Moreover, this effect was greater for those chronically striving to promote/protect one’s self-image during psychosocial stressors (b = 14.53, SE = 4.72, p = .003), and for those low in conscientiousness (b = 14.84, SE = 4.83, p = .003). Moderate extraversion was associated with higher HCC. Striving to support others (compassionate goals) exerted no direct or interactive effect on HCC, contrary to hypotheses.
Conclusions
Results support the relevance of neuroticism and maladaptive interpersonal strivings to longer-term neuroendocrine responses, suggesting hair cortisol as a potential method for studying links of trait-like psychological and HPA processes.
Keywords: Hair cortisol, Big Five traits, Self-image goals, Compassionate goals
Highlights
-
•
Trait neuroticism uniquely predicted higher hair cortisol concentration.
-
•
Interpersonal goals during chronic social stressors amplified effects of neuroticism.
-
•
Trait conscientiousness blunted the effect of neuroticism on hair cortisol.
The hypothalamic-pituitary-adrenal (HPA) axis releases cortisol in response to stressors, shaping psychological and physiological adaptation [1]. However, chronically high—and sometimes low—cortisol poses risks for downstream health [2,3]. Cortisol measures in saliva, blood, or urine provide snapshots of HPA activity for brief periods but are less suited for studying chronic endocrine output [4]. In contrast, cortisol in hair assesses longer-term, trait-like secretion [5]. Cortisol accumulates as hair grows roughly 1 cm per month, indexing secretion over that period [6]. Thus, hair cortisol concentration (HCC) provides a potential biomarker for studying links of long-term HPA activity to individual differences in personality traits and chronic interpersonal processes, which is the aim of this study.
1. Personality traits and cortisol
Personality encompasses chronic ways of adapting to one’s environment. The Big Five [7] and Five Factor Model (FFM [8]) conceptualize personality traits as individual differences in thinking, feeling, and behavior [[7], [9]]. These overlapping models derived from factor analyses finding five higher-order factors that explain personality differences. Neuroticism reflects proneness to negative emotions, vulnerability, and self-consciousness. Conscientiousness measures self-control, organization, perseverance, and perceived competence. Extraversion assesses sociability, assertiveness, and energy. Agreeableness represents tendencies toward kindness and desire to please others. Openness reflects creativity and innovativeness. Each factor splits into multiple facets or sub-scales, but here we emphasize higher-order traits.
Personality traits capture styles of responding to situational demands and therefore may shape stress responses. Studies have linked traits and cortisol [[10], [11]], most often in saliva or sometimes plasma. However, findings have been mixed and few studies examined hair. Neuroticism, a robust risk factor for illness [12], has most often correlated with cortisol. For instance, neuroticism correlated with higher basal cortisol or area under the curve for one or more days (e.g. [[13], [14], [15], [16], [17]]), and greater cortisol response to stressors (e.g., [[18], [19], [20], [21]]). Such findings make sense given that neuroticism involves chronic perceived uncontrollability and self-consciousness [22]—parallel to the low control and social evaluation that trigger cortisol secretion in the lab [23]. However, neuroticism has also predicted lower cortisol awakening response (CAR [14,24]) or task reactivity [[25], [26], [27]], warranting consideration of curvilinear relationships with this trait [12].
Links between other traits and cortisol have similarly been inconsistent. Extraversion predicted higher resting cortisol [15] and reactivity [18,19], consistent with extraversion involving tendencies to seek and approach challenges–but also lower basal cortisol [14,24] and reactivity (e.g., [28]). Conscientiousness was linked to lower basal cortisol [14] and CAR [29], in line with this trait measuring regulation of oneself and one’s environment [30], which might buffer against cortisol secretion. However, effects of conscientiousness have also been mixed [19]. Few studies have linked agreeableness and openness to cortisol.
It remains challenging to integrate existing findings given that different methods of cortisol assessment capture distinct aspects of HPA activation and regulation. Short-term cortisol measures (e.g., saliva) entangle between- and within-person variability. HCC better estimates longer-term secretion. In one large-scale study of older adults, conscientiousness predicted lower hair cortisol [31], but no other studies have examined Big Five traits and HCC. It remains possible that HCC may provide a better means to estimate links between psychological traits and trait-like HPA activity.
2. Interpersonal goals and cortisol
Beyond traits, interpersonal goals (self-image and compassionate goals) represent processes that may increase or decrease stress, respectively [32,33]. Self-image goals involve striving to promote and protect desired self-images (i.e., being seen as competent [34]). Chronic self-image goals prospectively predicted decreased support and higher distress and belief in competition in nonclinical and clinical samples [32,33,35]. Moreover, stressors involving social evaluation (i.e., self-image) reliably trigger cortisol secretion [23]. In contrast, compassionate goals involve striving to help others, and have prospectively predicted increased support, belief in cooperation, and lower distress, and moderated effects of self-image goals [32,33].
However, despite relevance of interpersonal goals to processes that influence stress and HPA responses, less is known about their use in coping with stressors. Coaching individuals to pursue compassionate goals in a lab social stressor reduced HPA responses [36,37], and pursuing compassionate goals after the stressor of a college shooting (e.g., supporting traumatized peers) indirectly predicted lower posttraumatic symptoms [38], implying relevance of these goals to coping with stressors. Just as individuals can cope with stressors by strategies such as rumination (e.g. [39,40]), people may cope by self-protective or prosocial goals. Studies have not examined these goals as ways individuals can respond to stressors in daily life, a focus of this study. Furthermore, traits are theorized to interact with situational states or stressors [41,42]. Indeed, traits such as neuroticism are thought to interact with situational stressors and social processes [12]. Regularly pursuing self-image goals in the context of psychosocial stressors might amplify the effect of neuroticism on HCC, whereas striving for compassionate goals as a response to stressors might blunt that effect.
3. Current study and hypotheses
This study investigated direct and interactive effects of Big Five traits and interpersonal goals during stressors on hair cortisol in naturalistic psychosocial stressors over 4–5 weeks. Given that neuroticism is a risk factor for stress [12,30] and meta-analytic findings linking current stress exposure to higher HCC [43], we hypothesized that neuroticism would predict higher HCC. Given the formulation of conscientiousness as adaptive self-regulation and control that promotes health [30], we expected it to predict lower cortisol and moderate (buffer) effects of neuroticism. We planned exploratory tests of linear effects of extraversion, agreeableness, and openness on HCC. Also, given inconsistent past findings and the theory that both high and low cortisol in general [2] and in HCC [43] may be problematic, we also tested for curvilinear effects.
For interpersonal goals, we hypothesized that chronic self-image goals during stressors would predict higher HCC given that laboratory threats to the “social self” have elicited cortisol [23]. Based on the theory of neuroticism as a risk factor and amplifier for stress responses [12], self-image goals were likewise expected to amplify the relationship of neuroticism with HCC. Conversely, we expected compassionate goals during stressors to predict lower HCC and buffer neuroticism given direct and buffering effects of these goals in studies not specifically examining responses to stressors (e.g., [32]).
4. Method
4.1. Participants
Participants included 90 individuals (86% female) in psychology courses at a private university in the Pacific Northwest. They participated for course credit and received $15 for providing a hair sample. We included individuals who provided a hair sample and completed a minimum of three daily surveys. Participants ranged in age from 18 to 29 years (M = 19.37, SD = 1.96) and were moderately ethnically diverse (58.9% White, 20.0% Asian American, 6.7% Multiracial, 8.9% Latinx, 3.3% African American/Black, 1.1% Middle Eastern, and 1.1% Other).
4.2. Procedure and design
This study, part of a larger investigation on personality and stress, involved three phases. Following informed consent, participants completed baseline assessments of traits online (via Qualtrics). Next, they completed online/smartphone diaries about interpersonal goals in the context of daily stressors. They received surveys at noon and were asked to consider and report on the most stressful social event in the past day or two, three times weekly, for five weeks. For each stressor, they first responded to open-ended prompts and described (1) the nature of the stressor and (2) why they viewed it as stressful, to focus attention on concrete situational details. Participants were contacted with reminders to complete surveys upon missing a diary. Lastly, after five weeks of diaries, participants visited the lab to provide a hair sample. Participants also completed a short set of online health questions (to assess for factors previously shown to impact cortisol, such as oral contraceptives and recreational substance use [43]).
4.3. Measures
4.3.1. Big Five inventory
The Big Five Inventory (BFI [44]) is a 44-item measure of Big Five trait dimensions on a 1 (disagree strongly) to 5 (agree strongly) scale. Items refer to the prompt, “I see myself as someone who…,” followed by descriptors of neuroticism (e.g., Worries a lot”), extraversion (“Is outgoing, sociable”), openness to experience (e.g., “Has an active imagination”), agreeableness (“Likes to cooperate with others”), and conscientiousness (“Does a thorough job”). After reverse-scoring 16 items, items were averaged to create totals with higher scores indicating higher levels of each trait. Internal consistency was calculated in our sample for Neuroticism (0.84), Extraversion (0.89), Conscientiousness (0.85), Openness (0.74), and Agreeableness (0.63).
4.3.2. Diary ratings of interpersonal goals
We assessed interpersonal goals using six items from a larger set developed by Crocker and Canevello ([34]; study 1). Participants rated during the stressor, “… to what extent did you want or try to [engage in each goal]” on a 5-point scale from 1 (not at all) to 5 (extremely). Adapting the items to a stress context was based on studies anchoring diary assessments to stressors [39,40] and event-contingent assessment [45]. Three items assessed compassionate goals (“Be supportive of the other person,” “Avoid being selfish or self-centered,” and “Make a positive difference in the life of the other person”) and self-image goals (“Convince the other person that you were right,” “Avoid showing your weaknesses,” and “Get the other person to recognize or identify your positive qualities”) Crocker and Canevello [34] reported factor structure, reliability, and construct validity of the diary items, and Erickson and colleagues [33] demonstrated utility in a clinical sample. Given our focus on between-person individual differences in personality and HCC, we averaged diary goals to create aggregated scores. Intraclass correlation correlations (ICC = .39 for compassionate and .45 for self-image goals) suggested substantial between-person variability. Variance components analysis of multilevel data [46] suggested reliability of the aggregate scores for self-image (0.95) and compassionate goals (0.87). Positive associations of self-image goals with neuroticism and compassionate goals with openness to experience (see Table 1) replicate past interpersonal goals research that did not specifically examine stress contexts [34], suggesting preliminary evidence of reliability and validity for this methodological adaptation.
Table 1.
Descriptive statistics and correlations between study variables.
| Variable | M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Hair cortisol (pg/mg) | 28.71 | 23.87 | |||||||
| 2. Neuroticism | 3.13 | 0.81 | .22* | ||||||
| 3. Extraversion | 3.00 | 0.98 | .04 | -.33** | |||||
| 4. Openness | 3.45 | 0.64 | .00 | -.15 | .12 | ||||
| 5. Agreeableness | 4.03 | 0.55 | -.09 | -.18 | .03 | .20 | |||
| 6. Conscientiousness | 3.72 | 0.68 | -.08 | -.13 | .12 | .44 | .08 | ||
| 7. Self-image goals | 2.44 | 0.74 | .06 | .33*** | -.04 | .26* | -.11 | -.05 | |
| 8. Compassionate goals | 2.42 | 0.76 | -.02 | .17 | .07 | .33** | .17 | -.03 | .52*** |
Note. *p <.05, **p <.01, ***p <.001.
4.3.3. Hair cortisol
Participants were asked to provide a hair sample during the laboratory visit. A trained research assistant in sterile gloves gently twisted a strand of hair to a thickness of 2–3 mm and cut a 2–3 cm sample with sterilized scissors, capturing cortisol secretion over the past two months. Assistants cut samples as close to the scalp as possible at the posterior vertex of the skull, in line with standard recommendations and because recent growth is less vulnerable to decline related to repeated washing and hair product usage. Samples were placed in aluminum foil and stored at -20° Celsius in a University of Washington laboratory until assay. Hair samples were subsequently minced and dissolved in assay buffer to extract cortisol (protocol in [47]) prior to colorimetric assay (Salimetrics high-sensitivity cortisol assay kits). The coefficient of variation for duplicate assays was 3.61%.
4.3.4. Analysis plan
After data screening, multiple regression analyses were conducted. All predictor variables were grand-mean-centered. First, we simultaneously estimated effects of all Big Five traits in predicting HCC, examining unique effects in a conversative model. We also estimated the simultaneous effects of self-image and compassionate goals. Next, we tested for curvilinear effects. To avoid being overly conservative, we tested predictors serially, with each model testing a quadratic effect after controlling for linear effects. Lastly, we tested moderation effects for neuroticism interacting individually with conscientiousness, self-image goals, and compassionate goals. Power analyses in R via the pwr2ppl package [48] suggested that medium-sized multiple regression effects (including main, quadratic, and moderation effects) would have over 80% power.
5. Results
5.1. Preliminary analyses
Data screening (see Table 1 for descriptive statistics and correlations between variables) suggested no substantial skew or kurtosis in study variables. Participants completed an average of 13.87 diary records (SD = 1.94; total of 1,923 diaries). Among participants who met inclusion criteria (i.e., provided hair sample), no data were missing. Hair cortisol concentrations ranged 2.25–111.49 pg per milligrams of hair (M = 28.71 pg/mg, SD = 23.87), suggesting a broad range of levels from very low to relatively high concentrations.
None of the potential covariates that impact cortisol correlated with HCC, including female gender (r = .10, p = .347), BMI (r = .02, p =.875), oral contraceptives in past two months (r = .08, p = .450), smoking (r = -.07, p = .490), alcohol consumption (r = .01, p = .904), or recreational drug use in the past two months (r = -.07, p = .503). Given the lack of associations, we did not control for these variables. Only one participant reported pregnancy and lactation in the past two months, and results were similar with and without her data, so we included her data.
5.2. Core analyses
5.2.1. Unique effects of traits
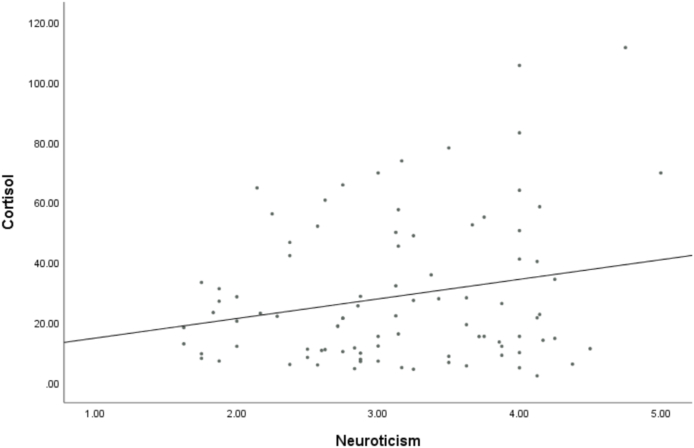
Multiple regression analyses tested for unique effects of each Big Five trait predicting hair cortisol. As hypothesized, neuroticism uniquely predicted higher HCC even after controlling for other traits (see Table 2 and Fig. 1). Contrary to hypotheses, extraversion, agreeableness, conscientiousness, and openness had no linear relationship to HCC.
Table 2.
Multiple regression testing unique effects of big five traits on hair cortisol.
| Predictor | b | SE | 95% CI | p | partial r |
|---|---|---|---|---|---|
| Neuroticism | 7.45 | 3.36 | [.76, 14.13] | .029 | .22 |
| Extraversion | 3.24 | 2.75 | [-2.22, 8.70] | .241 | .13 |
| Openness | 1.36 | 4.07 | [-6.73, 9.44] | .739 | .04 |
| Agreeableness | −2.24 | 4.73 | [-11.65, 7.18] | .638 | -.05 |
| Conscientiousness | −2.27 | 3.73 | [-9.68, 5.14] | .544 | -.07 |
Fig. 1.
Bivariate scatterplot of neuroticism and hair cortisol concentration.
5.2.2. Unique effects of interpersonal goals
Neither mean compassionate goals in daily life (b = -2.35, SE = 4.05, 95%CI [-10.40, 5.70], p = .563, pr = -.06) nor self-image goals (b = 2.96, SE = 3.91, 95%CI [-4.82, 10.74], p = .451, pr = .08) predicted unique variance in HCC, unexpectedly.
5.2.3. Quadratic effects
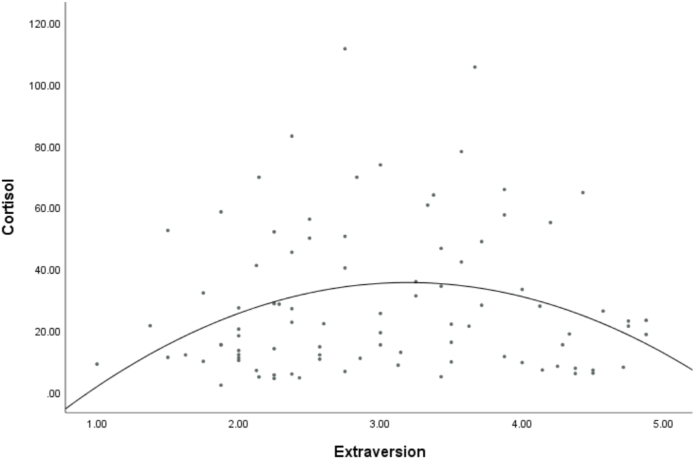
To test curvilinear effects on hair cortisol, we conducted regressions including each trait or goal as well as a squared quadratic term. Quadratic effects did not attain statistical significance for neuroticism (b = 4.10, SE = 3.52, 95%CI [-2.90, 11.09], p = .247, pr = .12). However, controlling for the non-significant linear effect (b = 1.87, SE = 2.54, 95%CI [-3.18, 6.92], p = .464, pr = .08), the quadratic effect of extraversion was significant (b = -7.03, SE = 2.69, 95%CI [-12.38, -1.68], p = .011, pr = -.27), suggesting that as levels of extraversion rose from low to moderate levels, HCC increased; however, moderate to high levels of extraversion were associated with decreasing HCC (see Fig. 2). In exploratory analyses, we found no quadratic effects for openness (b = 5.57, SE = 4.95, 95%CI [-4.28, 15.38], p = .265, pr = .12), agreeableness (b = 7.91, SE = 6.64, 95%CI [-5.29, 21.11], p = .632, pr = .05), conscientiousness (b = 1.80, SE = 3.76, 95%CI [-5.66, 9.27], p = .632, pr = .05), or chronic self-image (b = -.84, SE = 3.47, 95%CI [-7.74, 6.06], p = .810, pr = -.03) or compassionate goals (b = -2.06, SE = 3.65, 95%CI [-9.31, 5.20], p = .575, pr = -.06).
Fig. 2.
Bivariate scatterplot of extraversion and hair cortisol concentration.
5.2.4. Moderation effects
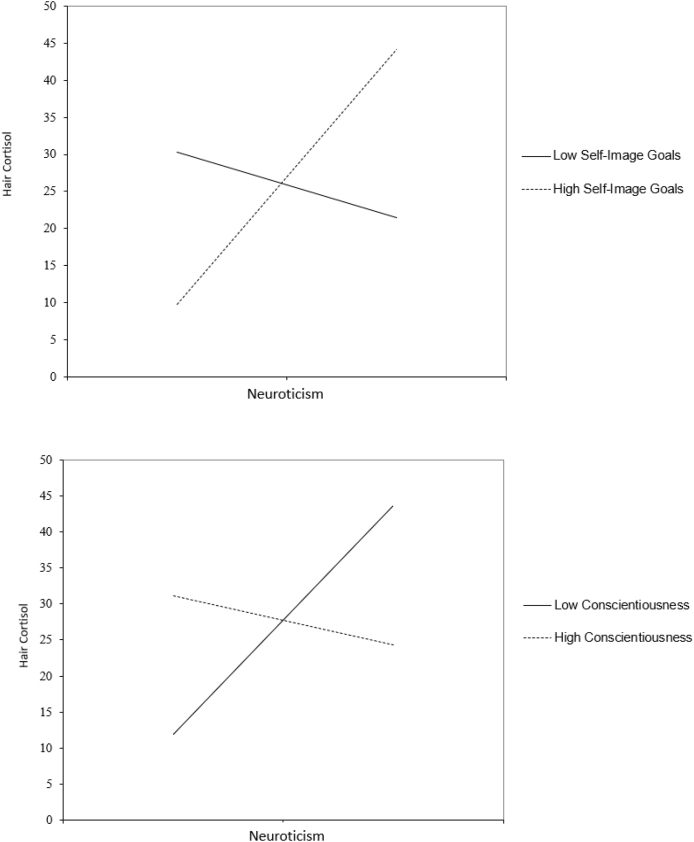
We tested the hypothesis that compassionate goals would buffer, and self-image goals would amplify, effects of neuroticism on HCC. As hypothesized, self-image goals moderated effects of neuroticism (R2 = .06; see Fig. 3 and Table 3). Simple slopes analyses showed that neuroticism most strongly predicted higher cortisol at high (+1 SD) levels of chronic self-image goals (b = 14.53, SE = 4.72, 95%CI [5.16, 23.91], p = .003). In contrast, neuroticism did not predict cortisol for individuals at low (-1 SD) levels of self-image goals (b = -1.96, SE = 4.73, 95%CI [-11.37, 7.45], p = .680). In other words, neuroticism was associated with risk for higher hair cortisol only in individuals who chronically strove to promote and defend desired self-images in stressful interaction. Contrary to hypotheses, compassionate goals did not interact with neuroticism.
Fig. 3.
Interaction of neuroticism with self-image goals and conscientiousness predicting hair cortisol.
Table 3.
Multiple regression for interaction of neuroticism and interpersonal goals predicting cortisol.
| Predictor | b | SE | 95% CI | p |
|---|---|---|---|---|
| Neuroticism | 6.37 | 3.21 | [-0.10, 12.74] | .050 |
| Self-Image Goals | 0.51 | 3.91 | [-7.27, 8.28] | .897 |
| Compassionate Goals | −2.61 | 3.88 | [-10.33, 5.12] | .503 |
| Neuroticism*Self-Image Goals | 10.78 | 4.53 | [1.78, 19.79] | .020 |
| Neuroticism*Compassionate Goals | −1.98 | 5.19 | [-12.31, 8.35] | .704 |
With regard to interactions between Big Five traits, conscientiousness moderated (blunted) the positive effect of neuroticism on hair cortisol as expected. Namely, despite no direct effect of conscientiousness (b = -.77, SE = 3.72, 95%CI [-8.18, 6.64], p = .837) and a direct effect of neuroticism (b = 7.79, SE = 3.30, 95%CI [1.23, 14.35], p = .021), conscientiousness blunted (moderated) the effect of neuroticism on cortisol (b = -10.33, SE = 4.94, 95%CI [-20.14, -.51], p = .040, See Fig. 3). Simple slopes tests showed that neuroticism predicted much higher HCC at low levels of conscientiousness (b = 14.84, SE = 4.83 95%CI [5.23, 24.46], p = .003), but did not predict HCC for participants high in conscientiousness (b = .74, SE = 4.60. 95%CI [-8.41, 9.88], p = .873).
6. Discussion
Few studies have investigated links between personality and hair cortisol, but the present study supports the idea that trait-like psychological phenomena (personality dispositions and chronic goals during stressors) may have downstream effects on long-term HPA activity over several months. Specifically, even after controlling for other traits, neuroticism demonstrated specificity in predicting higher HCC; this association was amplified in individuals who endorsed chronically responding to stressors with self-image goals but was blunted in individuals high in conscientiousness. These effects supported hypotheses and provided novel evidence linking psychological phenomena to HCC.
The relationship of neuroticism to HCC is a novel finding. It may fit with studies in which neuroticism correlated with higher basal cortisol or total secretion in saliva or plasma prior to or outside of lab stressors (e.g. [[13], [14], [15], [16], [17]]), and greater secretion following stressors (e.g., [[18], [19], [20], [21]]). However, because various cortisol measurement methods index different aspects of the HPA system, findings from one method (e.g., saliva) may not generalize to HCC. Thus, it is useful to find that a trait involving chronically interpreting experiences as stressful was associated with higher “chronic” (over two months) cortisol secretion. It remains an open question what pathways or mechanisms might account for this link. Neuroticism is thought to measure a neurobehavioral threat-detection system responsive to cues of uncontrollability [22], uncertainty, and punishment [30]. Given that perceived lack of control has been established as a key psychological input for cortisol release [23,49], chronic perceptions of low control might underlie our findings. Alternative pathways might include paying disproportionate attention to threats or proneness to generating new stressors. Future studies should test these possibilities directly.
Two moderation effects of neuroticism might also speak to mechanisms. As hypothesized, conscientiousness blunted the positive association with HCC; the neuroticism-HCC link disappeared in high-conscientiousness individuals. Conscientiousness reflects a suite of self-regulation abilities including self-control [30], so chronic perceptions of self-control might translate into HPA modulation akin to effects of perceived control [23]. Trait resilience, which features ability to persist or persevere over time, correlated negatively with hair cortisol [50]. Alternatively, conscientious individuals’ self-control might simply lead them to select or create lower-stress environments, consistent with their preventive health behavior and avoidance of risky situations [30]. This buffering role for the trait was consistent with the only extant Big Five/FFM study of hair cortisol [31], which reported negative correlation between conscientiousness and HCC. In the other hypothesized moderation, chronic self-image goals pursued during naturalistic stressors amplified the effect of neuroticism on HCC. Neuroticism predicted HCC more strongly in individuals who reported regularly trying to promote/defend desired self-images (e.g., being intelligent) in the context of stressors. Although self-image goals by themselves did not predict cortisol, their interaction with neuroticism fits with lab studies finding perceived evaluation of the “social self” as a key psychological mechanism for cortisol responses [23]. In those studies, social evaluation was a key feature of the experimental environment; our findings show that one’s internal sense of striving to protect self-image during naturalistic stressors may be important for HPA activity for stressors beyond the lab. However, establishing mechanisms goes beyond the present study.
Compassionate goals did not predict HCC or blunt effects of neuroticism, contrary to hypotheses and previous studies linking compassionate goals experimentally to lower cortisol response to stressors [36,37]. Sampling method (e.g., hair versus plasma), design (naturalistic versus lab stressors), and population (unselected young adults versus healthy controls) might account for null findings. Although past research detected effects of compassionate goals pursued following stressors [36,38], not enough is known about how measurement in stressor-contexts might change the goals’ effects. Perhaps stress-buffering properties of compassionate goals are stronger when pursued in non-stressor situations. Nonetheless, links of compassionate goals to adaptive social and mental health outcomes [33,34] warrant further research on their stress-buffering properties and whether these effects are stronger in non-stressor versus stressor contexts.
Timing may also be important for understanding HCC, how it differs from other cortisol measures, and past mixed findings. Some studies have reported negative associations between neuroticism and CAR [24,51] or response to stress tasks [[25], [26], [27]]. Moreover, one study found lower HCC in a small sample of individuals with generalized anxiety disorder (which correlates with neuroticism), relative to healthy controls [52]. Similarly, despite some studies linking anxiety disorders to high cortisol secretion, effects have been mixed [2], and disorders characterized by longer-term chronic stress or trauma (e.g., posttraumatic stress disorder; borderline personality disorder) appear to involve low cortisol output [3,49,53]. Meta-analytic findings point to the timing and chronicity of stress or trauma as influencing high versus low cortisol output. During shorter-term stress, larger cortisol responses reflect healthy adaptation of the HPA axis, marshalling mind and body to cope; however, repeated regular secretion associated with current, ongoing stressors may lead to high cortisol [43,49]. Eventually, however, chronically high cortisol may lead to downregulation of the HPA axis and blunted reactivity to stressors (e.g., both lower basal levels and lower response curves; [54]). However, although these ideas suggested a possible curvilinear association linking both low and high HCC to high neuroticism, we detected no quadratic effect. The present sample, given younger age, may have included individuals prone to anxiety more than chronic stress or trauma exposure that has been linked to HPA downregulation. Alternatively, future research might differentiate effects of neuroticism, acute stressors, and developmental trauma on HCC, given that developmental trauma may be associated with high distress but low HPA responses to stressors [55].
We also conducted exploratory analyses for the remaining traits. The trait of extraversion predicted unique variance in hair cortisol concentrations in a nonlinear pattern. Increasing extraversion predicted increasing HCC up to a point, but above moderate levels of extraversion, higher levels of this trait predicted lower HCC. Whereas neuroticism represents a threat-detection system, trait extraversion indexes a neurobehavioral approach system that is sensitive to reward cues and motivates goal pursuit [30]. Positive correlation of extraversion with HCC for participants with low-to-moderate extraversion might reflect higher approach behavior and assertiveness, with concomitant HPA mobilization to face challenges. Further extraversion above moderate levels may confer adaptive benefits, as extraversion encompasses assertiveness, social confidence, and resilience (i.e., functionally the opposite of the low perceived control and evaluation sensitivity that elicit cortisol secretion). Our curvilinear effect might also be taken as consistent with studies reporting either negative or positive associations of extraversion to cortisol [14,15,18,19,24,28]; however, none of those studies examined HCC, so the present finding is novel but warrants replication. Lastly, the lack of findings for openness to experience and agreeableness was not surprising given that those traits have less commonly demonstrated HPA links. Additionally, openness captures information-processing style and does not unambiguously predict psychopathology [30]. One might theorize that agreeableness would predict lower cortisol given that it shapes social affiliation and bonding. However, affiliation also involves vulnerability to people-pleasing and conflict avoidance, suggesting mixed implications for well-being. Future investigation of Big Five traits at the level of facets may help to clarify which subcomponents have relevance for HPA responses.
7. Limitations and conclusion
Several study limitations warrant mention. First, although the study included a larger range of participants than many HCC studies, even larger samples would increase power for quadratic and moderation hypotheses; replication is also warranted for those types of effects, which were likely underpowered. Second, HCC provides an estimate of chronic cortisol secretion more than other common measures, but truly long-term assessment of cortisol may require longer hair samples to assess secretion over longer time periods (e.g., 4–6 months). Third, although hair cortisol provides a meaningful estimate of between-person differences in HPA activity, it does not estimate within-person variability, which remains important given evidence that within-person diurnal patterns (e.g., flatter curves) can be dysregulated. Fourth, future studies should include greater age and gender diversity given our predominantly young, female sample. Lastly, although HCC was not related to covariates we assessed (e.g., smoking, BMI), other potential confounds such as hair washing and treatment should be considered. Given the history of difficulty replicating links between psychosocial variables and biomarkers such as cortisol, as well as normal sampling variability, the possibility of capitalization on chance warrants future replication.
Few studies have examined personality and hair cortisol, and the present study, despite limitations, provides evidence of the relevance of Big Five traits to HCC. Other contributions include investigation of nonlinear and interactive effects, underscoring the need to better understand the conditions under which personality traits serve as risk versus resilience factors, and under which high versus low cortisol secretion might indicate dysregulation. Because hair provides a longer-term measure of secretion that is not redundant with other cortisol measures, further HCC investigation may yield greater understanding of the pathways between trait-like psychological phenomena and biological stress responses.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
This research was supported by a Faculty Research Grant to the first author from Seattle Pacific University. The authors have no conflicts of interest to report.
References
- 1.Heaney J. Encyclopedia of Behavioral Medicine; 2013. Hypothalamic-pituitary-adrenal Axis. New York, NY: Springer. [DOI] [Google Scholar]
- 2.Bandelow B., Baldwin D., Abelli M., Bolea-Alamanac B., Bourin M., Chamberlain S.R., Riederer P. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J. Biol. Psychiatr. 2017;18:162–214. doi: 10.1080/15622975.2016.1190867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharpley C.F., McFarlane J.R., Slominski A. Stress-linked cortisol concentrations in hair: what we know and what we need to know. Rev. Neurosci. 2011;23(1):111–121. doi: 10.1515/RNS.2011.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sauvé B., Koren G., Walsh G., Tokmakejian S., van Uum S.H.M. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clinical & Investigative Medicine. 2007;30(5):E183–E191. doi: 10.25011/cim.v30i5.2894. https://doi-org.ezproxy.spu.edu/10.25011/cim.v30i5.2894 [DOI] [PubMed] [Google Scholar]
- 5.Short S.J., Stalder T., Marceau K., Entringer S., Moog N.K., Shirtcliff E.A., Buss C. Correspondence between hair cortisol concentrations and 30-day integrated daily salivary and weekly urinary cortisol measures. Psychoneuroendocrinology. 2016;71:12–18. doi: 10.1016/j.psyneuen.2016.05.007. https://doi-org.ezproxy.spu.edu/10.1016/j.psyneuen.2016.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer J., Novak M. Minireview: hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology. 2012;153(9):4120–4127. doi: 10.1210/en.2012-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg L.R. The development of markers for the big-five factor structure. Psychol. Assess. 1992;4(1):26–42. 1040-3590/92/$3.00. [Google Scholar]
- 8.McCrae R.R., Costa P.T. Validation of the five-factor model of personality across instruments and observers. J. Pers. Soc. Psychol. 1987;52(1):81–90. doi: 10.1037/0022-3514.52.1.81. [DOI] [PubMed] [Google Scholar]
- 9.Widiger T.A., Crego C. The Five Factor Model of personality structure: an update. World Psychiatr. 2019;18(3):271–272. doi: 10.1002/wps.20658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill E.M., Billington R., Krägeloh C. The cortisol awakening response and the big five personality dimensions. Pers. Indiv. Differ. 2013;55(5):600–605. doi: 10.1016/j.paid.2013.05.010. [DOI] [Google Scholar]
- 11.Oswald L., Zandi P., Nestadt G., Potash J., Kalaydjian A., Wand G. Relationship between cortisol responses to stress and personality. Neuropsychopharmacology. 2006;31(7):1583–1591. doi: 10.1038/sj.npp.1301012. Official Publication Of The American College Of Neuropsychopharmacology. [DOI] [PubMed] [Google Scholar]
- 12.Lahey B.B. Public health significance of neuroticism. Am. Psychol. 2009;64(4):241–256. doi: 10.1037/a0015309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Banda G., Servera M., Chellew K., Meisel V., Fornes J., Cardo E., Perez G., Riesco M., Doctor R.M. Prosocial personality traits and adaptation to stress. SBP (Soc. Behav. Pers.): Int. J. 2011;39(10):1337–1348. https://doi-org.ezproxy.spu.edu/10.2224/sbp.2011.39.10.1337 [Google Scholar]
- 14.Laceulle O.M., Nederhof E., van Aken M.A.G., Ormel J. Adolescent personality: associations with basal, awakening, and stress-induced cortisol responses: adolescent personality and cortisol. J. Pers. 2015;83(3):262–273. doi: 10.1111/jopy.12101. [DOI] [PubMed] [Google Scholar]
- 15.Miller G.E., Cohen S., Rabin B.S., Skoner D.P., Doyle W.J. Personality and tonic cardiovascular, neuroendocrine, and immune parameters. Brain Behav. Immun. 1999;13(2):109–123. doi: 10.1006/brbi.1998.0545. [DOI] [PubMed] [Google Scholar]
- 16.Nater U.M., Hoppmann C., Klumb P.L. Neuroticism and conscientiousness are associated with cortisol diurnal profiles in adults—role of positive and negative affect. Psychoneuroendocrinology. 2010;35(10):1573–1577. doi: 10.1016/j.psyneuen.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Parent-Lamarche A., Marchand A. The moderating role of personality traits in the relationship between work and salivary cortisol: a cross-sectional study of 401 employees in 34 Canadian companies. BMC Psychology. 2015;3(45) doi: 10.1186/s40359-015-0102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agrigoroaei S., Polito M., Lee A., Kranz-Graham E., Seeman T., Lachman & M.E. Cortisol response to challenge involving low controllability: the role of control beliefs and age. Biol. Psychol. 2013;93(1):138–142. doi: 10.1016/j.biopsycho.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apalkova Y., Butovskaya M.L., Shackelford T.K., Fink B. Personality, aggression, sensation seeking, and hormonal responses to challenge in Russian alpinists and special operation forces. Pers. Indiv. Differ. 2020;169:110238. doi: 10.1016/j.paid.2020.110238. [DOI] [Google Scholar]
- 20.Wirtz P.H., Elsenbruch S., Emini L., Rüdisüli K., Groessbauer S., Ehlert U. Perfectionism and the cortisol response to psychosocial stress in men. Psychosom. Med. 2007;69(3):249–255. doi: 10.1097/PSY.0b013e318042589e. [DOI] [PubMed] [Google Scholar]
- 21.Zobel A., Barkow K., Schulze‐Rauschenbach S., Widdern O.V., Metten M., Pfeiffer U., Schnell S., Wagner M., Maier W. High neuroticism and depressive temperament are associated with dysfunctional regulation of the hypothalamic–pituitary–adrenocortical system in healthy volunteers. Acta Psychiatr. Scand. 2004;109(5):392–399. doi: 10.1111/j.1600-0447.2004.00313.x. [DOI] [PubMed] [Google Scholar]
- 22.Barlow D.H., Ellard K.K., Sauer-Zavala S., Bullis J.R., Carl J.R. The origins of neuroticism. Perspect. Psychol. Sci. 2014;9(5):481–496. doi: 10.1177/1745691614544528. [DOI] [PubMed] [Google Scholar]
- 23.Dickerson S.S., Kemeny M.E. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130(3):355. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 24.Ouanes S., Castelao E., von Gunten A., Vidal P.M., Preisig M., Popp J. Personality, cortisol, and cognition in non-demented elderly subjects: results from a population-based study. Front. Aging Neurosci. 2017;9 doi: 10.3389/fnagi.2017.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bibbey A., Carroll D., Roseboom T.J., Phillips A.C., de Rooij S.R. Personality and physiological reactions to acute psychological stress. Int. J. Psychophysiol. 2013;90(1):28–36. doi: 10.1016/j.ijpsycho.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Coyle D.K., Howard S., Bibbey A., Gallagher S., Whittaker A.C., Creaven A.M. Personality, cardiovascular, and cortisol reactions to acute psychological stress in the Midlife in the United States (MIDUS) study. Int. J. Psychophysiol. 2020;148:67–74. doi: 10.1097/PSY.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 27.Phillips A.C., Carroll D., Burns V.E., Drayson M. Neuroticism, cortisol reactivity, and antibody response to vaccination. Psychophysiology. 2005;42(2):232–238. doi: 10.1111/j.1469-8986.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 28.Evans B.E., Stam J., Huizink A.C., Willemen A.M., Westenberg P.M., Branje S., Meeus W., Koot H.M., van Lier P.A.C. Neuroticism and extraversion in relation to physiological stress reactivity during adolescence. Biol. Psychol. 2016;117:67–79. doi: 10.1016/j.biopsycho.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Bogg T., Slatcher R.B. Activity mediates conscientiousness’ relationship to diurnal cortisol slope in a national sample. Health Psychol. 2015;34(12):1195–1199. doi: 10.1037/hea0000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeYoung C.G. Cybernetic big five theory. J. Res. Pers. 2014;56:33–58. doi: 10.1016/j.jrp.2014.07.004. [DOI] [Google Scholar]
- 31.Steptoe A., Easterlin E., Kirschbaum C. Conscientiousness, hair cortisol concentration, and health behaviour in older men and women. Psychoneuroendocrinology. 2017;86:122–127. doi: 10.1016/j.psyneuen.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Crocker J., Canevello A., Breines J.G., Flynn H. Interpersonal goals and change in anxiety and dysphoria in first-semester college students. J. Pers. Soc. Psychol. 2010;98(6):1009. doi: 10.1037/a0019400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erickson T.M., Granillo M.T., Crocker J., Abelson J.L., Reas H.E., Quach C.M. Compassionate and self-image goals as interpersonal maintenance factors in clinical depression and anxiety. J. Clin. Psychol. 2018;74(4):608–625. doi: 10.1002/jclp.22524. [DOI] [PubMed] [Google Scholar]
- 34.Crocker J., Canevello A. Creating and undermining social support in communal relationships: the role of compassionate and self-image goals. J. Pers. Soc. Psychol. 2008;95(3):555–575. doi: 10.1037/0022-3514.95.3.555. [DOI] [PubMed] [Google Scholar]
- 35.Canevello A., Crocker J. Interpersonal goals, others’ regard for the self, and self-esteem: the paradoxical consequences of self-image and compassionate goals: goals, others’ regard, and self-esteem. Eur. J. Soc. Psychol. 2011;41(4):422–434. doi: 10.1002/ejsp.808. [DOI] [Google Scholar]
- 36.Abelson J., Erickson T., Mayer S., Crocker J., Briggs H., Lopez-Duran N., Liberzon I. Brief cognitive intervention can modulate neuroendocrine stress responses to the Trier Social Stress Test: buffering effects of a compassionate goal orientation. Psychoneuroendocrinology. 2014;44:60–70. doi: 10.1016/j.psyneuen.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erickson T.M., Mayer S.E., Lopez-Duran N.L., Scarsella G.M., McGuire A.P., Crocker J., Abelson J.L. Mediators of compassionate goal intervention effects on human neuroendocrine responses to the Trier Social Stress Test. Stress. 2017;20(6):533–540. doi: 10.1080/10253890.2017.1368489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stebbins O.L., Tingey J.L., Verdi E.K., Erickson T.M., McGuire A.P. Compassionate goals predict social support and PTSD symptoms following a university shooting: a moderated mediation analysis. J. Soc. Clin. Psychol. 2019;38(4):277–300. doi: 10.1521/jscp.2019.38.4.277. [DOI] [Google Scholar]
- 39.Aldrich J.T., Lisitsa E., Chun S.K., Mezulis A.H. Examining the relationship between daily co-rumination and rumination in response to negative events among adolescents using ecological momentary assessment. J. Soc. Clin. Psychol. 2019;38:704–719. doi: 10.1521/jscp.2019.38.7.704. [DOI] [Google Scholar]
- 40.Nicolai K.A., Laney T., Mezulis A.H. Different stressors, different strategies, different outcomes: how domain-specific stress responses differentially predict depressive symptoms among adolescents. J. Youth Adolesc. 2013;42(8):1183–1193. doi: 10.1007/s10964-012-9866-4. [DOI] [PubMed] [Google Scholar]
- 41.Fleeson W., Jayawickreme E. Whole trait theory. J. Res. Pers. 2015;56:82–92. doi: 10.1016/j.jrp.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furr R.M., Funder D.C. In: Handbook of Personality: Theory and Research. John O.P., Robins R.W., editors. Guilford; 2018. Persons, situations, and person-situation interactions; pp. 1–42. [Google Scholar]
- 43.Stalder T., Steudte-Schmiedgen S., Alexander N., Klucken T., Vater A., Wichmann S., Kirschbaum C., Miller R. Stress-related and basic determinants of hair cortisol in humans: a meta-analysis. Psychoneuroendocrinology. 2017;77:261–274. doi: 10.1016/j.psyneuen.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 44.John O.P., Srivastava S. In: Pervin L.A., John O.P., editors. vol. 2. Guilford Press; New York: 1999. The Big-Five trait taxonomy: history, measurement, and theoretical perspectives; pp. 102–138. (Handbook of Personality: Theory and Research). [Google Scholar]
- 45.Moskowitz D.S., Sadikaj G. In: Mehl M.R., Conner T.S., editors. The Guilford Press; 2012. Event-contingent recording; pp. 160–175. (Handbook of Research Methods for Studying Daily Life). [Google Scholar]
- 46.Shrout P.E., Lane S.P. In: Mehl M.R., Conner T.S., editors. 2012. Psychometrics; pp. 302–320. Handbook of research methods for studying daily life. The Guilford Press. [Google Scholar]
- 47.Meyer J., Novak M., Hamel A., Rosenberg K. Extraction and analysis of cortisol from human and monkey hair. JoVE: JoVE. 2014;(83) doi: 10.3791/50882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aberson C. 2020. pwr2ppl. R Package Version 0.1.2.https://CRAN.R-project.org/package=pwr2ppl [Google Scholar]
- 49.Miller G.E., Chen E., Zhou E.S. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol. Bull. 2007;133(1):25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- 50.García-León M.Á., Pérez-Mármol J.M., Gonzalez-Pérez R., García-Ríos M. del C., Peralta-Ramírez M.I. Relationship between resilience and stress: perceived stress, stressful life events, HPA axis response during a stressful task and hair cortisol. Physiol. Behav. 2019;202:87–93. doi: 10.1016/j.physbeh.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 51.LeBlanc J., Ducharme M.B. Influence of personality traits on plasma levels of cortisol and cholesterol. Physiol. Behav. 2005;84(5):677–680. doi: 10.1016/j.physbeh.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 52.Steudte S., Stalder T., Dettenborn L., Klumbies E., Foley P., Beesdo-Baum K., Kirschbaum C. Decreased hair cortisol concentrations in generalised anxiety disorder. Psychiatr. Res. 2011;186(2-3):310–314. doi: 10.1016/j.psychres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Thomas N., Gurvich C., Hudaib A.R., Gavrilidis E., Kulkarni J. Systematic review and meta-analysis of basal cortisol levels in Borderline Personality Disorder compared to non-psychiatric controls. Psychoneuroendocrinology. 2019;102:149–157. doi: 10.1016/j.psyneuen.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Phillips A.C., Ginty A.T., Hughes B.M. The other side of the coin: blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. Int. J. Psychophysiol. 2013;90(1):1–7. doi: 10.1016/j.ijpsycho.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 55.Kuhlman K.R., Abelson J.L., Mayer S.E., Rajaram N., Briggs H., Young E. Childhood maltreatment and within-person associations between cortisol and affective experience. Stress. 2021:1–11. doi: 10.1080/10253890.2021.1928069. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]