Highlights
-
•
Differentiation of non-tuberculous mycobacteria (NTM) from Mycobacterium tuberculosis complex is important for the management of tuberculosis.
-
•
hsp65 gene amplification and sequencing provide accurate diagnosis of NTM.
-
•
Mycobacterium fortuitum is the most prevalent NTM in the Gulf of Guinea.
-
•
NTM is associated with unsuccessful treatment of patients with tuberculosis.
Keywords: NTM, hsp65 sequencing, Drug resistance, Retreatment patients, West/Central Africa
Abstract
Objective
Differentiation between non-tuberculous mycobacteria (NTM) and Mycobacterium tuberculosis complex (MTBC) is crucial for case management with the appropriate antimycobacterials. This study was undertaken in three West and Central African countries to understand NTM associated with pulmonary tuberculosis in the sub-region.
Methods
A collection of 503 isolates (158 from Cameroon, 202 from Nigeria and 143 from Ghana) obtained from solid and liquid cultures were analysed. The isolates were tested for drug susceptibility, and MTBC were confirmed using IS6110. All IS6110-negative isolates were identified by 65-kilodalton heat shock protein (hsp65) gene amplification, DNA sequencing and BLAST analysis.
Results
Overall, the prevalence of NTM was 16/503 (3.2%), distributed as 2/202 (1%) in Nigeria, 2/158 (1.3%) in Cameroon and 12/143 (8.4%) in Ghana. The main NTM isolates included 5/16 (31.3%) M. fortuitum, 2/16 (12.5%) M. intracellulare and 2/16 (12.5%) M. engbaekii. Eight (57.1%) of the 14 previously treated patients harboured NTM (odds ratio 0.21, 95% confidence interval 0.06–0.77; P=0.021). Three multi-drug-resistant strains were identified: M. engbaekii, M. fortuitum and M. intracellulare.
Conclusion
NTM were mainly found among individuals with unsuccessful treatment. This highlights the need for mycobacterial species differentiation using rapid molecular tools for appropriate case management, as most are resistant to routine first-line antimycobacterials.
Introduction
Non-tuberculous mycobacteria (NTM), recently recognized as a cause of pulmonary and extrapulmonary infection in humans, are important emerging pathogens, especially in people living with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) (Lei et al., 2019). The clinical and molecular epidemiology of prevalent pulmonary NTM disease are not as well described as those for pulmonary tuberculosis (PTB) in sub-Saharan Africa (Okoi et al., 2017) because of diagnostic challenges (van Ingen, 2013). Several difficulties associated with the use of phenotypic and molecular diagnostic methods for precise identification of NTM to species level have been linked with shared metabolic and genetic characteristics (Escobar-Escamilla et al., 2014). Moreover, it is difficult to differentiate between NTM colonization and NTM disease. Despite these challenges, accurate identification of the causative species associated with both tuberculosis (TB) and NTM is relevant to the proper management and follow-up of patients (Escobar-Escamilla et al., 2014).
In some parts of sub-Saharan Africa where PTB diagnosis is based on clinical symptoms, sputum smear microscopy and (rarely) radiological findings, NTM may be misdiagnosed and are often treated inappropriately as Mycobacterium tuberculosis complex (MTBC) (Pokam and Asuquo, 2012; Okoi et al., 2017). The clinical and microbiological criteria defined by the American Thoracic Society/Infectious Disease Society of America to diagnose pulmonary NTM disease are often not followed because of poor laboratory infrastructure. Consequently, anti-TB drugs are administered based on identification of acid-fast bacilli (AFB) (Griffith et al., 2007; Pokam and Asuquo, 2012).
The resulting treatment failure may be misconstrued as drug resistance, with the patient placed on an second-line anti-TB drug; this is ineffective given the innate resistance of NTM to conventional anti-TB drugs (Griffith et al., 2007). Thus, proper identification of NTM is required for an appropriate treatment protocol to prevent the associated morbidity and mortality that could result from such misdiagnosis. It has been proposed that several treatment regimens may be required for the management of NTM, considering that the genetic make-up of each strain determines the various drug resistance patterns encountered (Griffith, 2010).
Genotypic methods are available for identification of mycobacteria. The 65-kilodalton heat shock protein (hsp65) gene, present in all mycobacteria, has been shown to be useful for the differentiation of genetically-related species (Ringuet et al., 1999). The unique nucleotide sequences of rapidly and slowly growing mycobacteria have been identified at species level (Plikaytis et al., 1992; Steingrube et al., 1995; Devallois et al., 1997), with DNA sequencing analysis providing medically relevant sub-specific phylogenetic lineages (Ringuet et al., 1999). The distribution of distinct NTM species differs geographically (Okoi et al., 2017), but the cultivation, identification and differentiation of NTM from MTBC is not performed routinely in many TB diagnostic laboratories in sub-Saharan Africa. Therefore, this study used variability within the hsp65 gene to identity NTM species obtained from Cameroon, Ghana and Nigeria, located in the Gulf of Guinea, Africa.
Materials and methods
Study settings and patients
The study was carried out between January and December 2017 in three west and central African countries, namely Ghana, Nigeria and Cameroon and included both new and previously treated pulmonary TB patients seeking care in various hospitals. Sampling for testing in Ghana was at the Eastern region of Ghana, specifically the Eastern Regional Hospital located in the New Juaben Municipality of Koforidua - the capital of the region. The site in Nigeria was the North Central zone (Middle Belt) of the country, including the following states: Benue, Kogi, Kwara, Nasarawa, Niger, Plateau and Federal Capital Territory. The site in Cameroon was the Littoral region, including Douala, the economic capital. The study was approved by the Scientific and Technical Committee, and the Ethical Committee of the Institutional Review Board of Noguchi Memorial Institute for Medical Research (FWA 00001824, IRB 00001276, NMIMR-IRB CPN 007/16-17, IORG 0000908).
Specimen culture and drug sensitivity
sensitivityA total of 503 isolates(158 from Cameroon, 202 from Nigeria and 143 from Ghana) were obtained from culture using either LowensteinJensen medium (Nigeria and Cameroon) or Middlebrook 7H9 Broth using BACTEC MGIT 960 (Ghana). Primary identification of MTBC isolates was carried out in Nigeria and Cameroon (but not in Ghana, confirmed by Ziehl-Neelsen staining only) using the SD BIOLINE TB Ag MPT64 RAPID kit (Standard Diagnostics, Inc., Yongin, Korea) based on the manufacturer’s instructions. Drug susceptibility testing was carried out for Streptomycin (STR). Isoniazid (INH) Rifampicin (RIF) and Ethambutol (ETH) using the proportion method (Nigeria) as described previously (Pokam et al., 2019) and BACTEC MGIT 960 in Ghana using 1.0 µg/ml STR, 0.1 µg/ml INH, 1.0 µg/ml RIF, and 5.0 µg/ml ETH for low drug concentrations and 4.0 µg/ml STR, 0.4 µg/ml INH, and 7.5 µg/ml ETH for high drug concentrations. Rifampicin resistance in Cameroon was determined using Xpert MTB/RIF.
IS6110 PCR assay, hsp65 gene amplification, DNA sequencing, and blast analysis
DNA from each isolate was used for PCR amplification using the insertion sequence 6110 (IS6110) primers described earlier (Pokam et al., 2019). All IS6110-negative strains suspected to be NTM were subjected to hsp65 gene amplification using the Tb11 (5′-CAACGATGGTGTGTCCCAT-3′) and Tb12 (5′-CTTGTCGAACCGCATACCCT-3′) primers, as well as the polymerase chain reaction (PCR) and cycling condition described previously (Otchere et al., 2017). The amplified 441-bp product was confirmed on 1.5% agarose gel electrophoresis, and visualized under short-wave ultraviolet light after ethidium bromide staining.
The PCR products of the amplified hsp65 gene were shipped to Macrogen Europe (https://dna.macrogen-europe.com/eng/) for sequencing. Briefly, the procedure included removal of vector sequences, processing into gap files, and editing with gap4 of the Staden package (Staden et al., 2000). The consensus hsp65 gene sequence of each isolate was saved in FASTA format, and analysed using the National Center for Biotechnology Information nucleotide BLAST against the database of representative genomes of microbes, and MegaBLAST selecting highly similar sequences (Table S1, see online supplementary material).
Data analysis
The coded data entered into Excel (Microsoft Corp., Redmond, WA, USA) were analysed using SPSS Version 20.0 (IBM, Armonk, NY, USA) for associations between the variables using Chi-squared test for age and gender, and Fisher's exact test for treatment status and drug resistance of identified NTM. P<0.05 was considered to indicate statistical significance with 95% confidence intervals (CI).
Results
IS6110-negative isolates and hsp65 amplification results in the three study areas
Following IS6110 PCR assay of the 503 isolates, 58 (11.5%) were found to be negative. Of these, 8/158(5.1%) came from Cameroon, 9/202(4.5%) from Nigeria and 41/143(28.7%) from Ghana. Seven (12.1%) of the 58 IS6110-negative isolates could not be amplified by the mycobacterium-specific hsp65 primers, indicating that they do not belong to the genus Mycobacterium. Hence, 51 (10.1%) of the 503 isolates were positive for hsp65 amplification: 6/158(3.8%) from Cameroon, 9/202(4.5%) from Nigeria and 36/143(25.2%) from Ghana (Table 1).
Table 1.
Sixty-five kilodalton heat shock protein (hsp65) gene amplification results of insertion sequence 6110 (IS6110)-negative isolates in three West and Central African countries.
| Country of origin | No. of isolates |
||
|---|---|---|---|
| Isolates under study | IS6110 negative (%) | hsp65 amplification positive (%) | |
| Cameroon | 158 | 8 (5.1) | 6 (3.8) |
| Nigeria | 202 | 9 (4.5) | 9 (4.5) |
| Ghana | 143 | 41 (28.7) | 36 (25.2) |
| Total | 503 | 58 (11.5) | 51 (10.1) |
Distribution of MTBC, NTM and Non-Mycobacterial (NM) Species
The distribution of the 51 sequenced isolates is shown in Table 2. Thirty-five (68.6%) were MTBC [33 (64.7%) M. tuberculosis and 2 (3.9%) M. africanum], 14 (27.5%) were NTM and 2(3.9%) non mycobacteria (one (1.9%) each of Nocardia veterana and Streptomyces sp.) following sequencing and BLASTn. Among the 51 sequenced isolates, 5 (9.8%) were M. fortuitum strain/subsp. fortuitum, 2 (3.9%) M. intracellulare, 2 (3.9%) M. engbaekii, 1 (1.9%) each of M. colombiense, M. gordonae, M. avium, M. paraense, M. peregrinum.
Table 2.
Strain distribution of non-tuberculous mycobacteria (NTM) and non-mycobacteria (NM) species in the Gulf of Guinea.
| Strains | Frequency (%) |
|---|---|
| MTBC (n=35) | |
| Mycobacterium tuberculosis sensu stricto | 33 (64.7) |
| Mycobacterium africanum | 2 (3.9) |
| NTM (n=14) | |
| Mycobacterium avium | 1 (1.9) |
| Mycobacterium colombiense | 1 (1.9) |
| Mycobacterium engbaekii | 2 (3.9) |
| Mycobacterium fortuitum subsp. fortuitum | 5 (9.8) |
| Mycobacterium gordonae | 1 (1.9) |
| Mycobacterium intracellulare strain | 2 (3.9) |
| Mycobacterium paraense | 1 (1.9) |
| Mycobacterium peregrinum | 1 (1.9) |
| NM (n=2) | |
| Nocardia veteran | 1 (1.9) |
| Streptomyces sp. | 1 (1.9) |
| Total | 51 |
MTBC, Mycobacterium tuberculosis complex.
Distribution and prevalence of hsp65-sequenced isolates in the three countries
The distribution of NTM in each country showed that of the 51 hsp65-sequenced isolates, 2/9 (22.2%) were obtained in Nigeria, 2/6 (33.3%) in Cameroon and 10/36 (27.8%) in Ghana. Thus, the overall prevalence of NTM was 2/202 (1.0%) in Nigeria, 2/158 (1.3%) in Cameroon and 10/143 (7.0%) in Ghana. Two NM species were also obtained from the Ghanaian isolates, giving a prevalence of 2/143 (1.4%) (Table 3). Further analysis included the two NM species among the Ghanian NTM, for an overall prevalence of 12/143 (8.4%).
Table 3.
Distribution and prevalence of non-tuberculous mycobacteria (NTM) and non-mycobacteria (NM) in each studied country.
| Isolates/country | Nigeria | Cameroon | Ghana | Total |
|---|---|---|---|---|
| MTBC |
M. tuberculosis (n=6) M. africanum (n=1) |
M. tuberculosis (n=4) |
M. tuberculosis (n=23) M. africanum (n=1) |
35 |
| NTM |
M. engbaekii (n=1) M. intracellulare (n=1) |
M. colombiense (n=1) M. peregrinum (n=1) |
M. avium (n=1) M. engbaekii (n=1) M. fortuitum (n=5) M. gordonae (n=1) M. intracellulare (n=1) M. paraense (n=1) |
14 |
| NM |
Nocardia veterana (n=1)a Streptomyces sp. (n=1)a |
2 | ||
| Total | 9 | 6 | 36 | 51 |
| Overall prevalence (NTM/NM) | 2/202 (1%) | 2/158 (1.3%) | 12/143 (8.4%) | 16/503 (3.2%) |
MTBC, Mycobacterium tuberculosis complex.
Both included in the prevalence calculation of NTM and further analysis.
Demography and association with NTM/NM
Among the 51 amplified isolates, 18 (35.3%) and 33 (64.7%) were obtained from female and male participants, respectively, aged between 20 and 89 years (mean age 45.14 years). There was no association between gender [9/33 (27.3%) males vs 7/18 (38.9%) females] of the 16 NTM/NM compared with MTBC [odds ratio (OR) 0.59, 95% CI 0.17–1.99; P=0.393]. Similarly, the distribution of NTM and MTBC across the age groups was not significant (P=0.259), as shown in Table 4. However, the age ranges of 35–44 years and 44–54 years were represented with 6/13 (46.2%) and 5/10 (50%) of the 16 NTM/NM isolates, respectively.
Table 4.
Association of age, gender, treatment status and drug resistance with non-tuberculous mycobacteria (NTM) in the three countries.
| Variables | NTM/NM (%) | MTBC (%) | Total | Odds ratio (95% CI) | P-value |
|---|---|---|---|---|---|
| Gender | 0.393 | ||||
| Male | 9 (27.3) | 24 (72.7) | 33 | 0.59 (0.17–1.99) | |
| Female | 7 (38.9) | 11 (61.1) | 18 | ||
| Total | 16 | 35 | 51 | ||
| Age (years) | 0.259 | ||||
| <25 | 1 (33.3) | 2 (66.7) | 3 | 1.1 (0.09–13.09) | 0.686 |
| 25–34 | 1 (8.3) | 11 (91.7) | 12 | 0.15 (0.02–1.24) | 0.075 |
| 35–44 | 6 (46.2) | 7 (53.8) | 13 | 2.4 (0.65–8.88) | 0.298 |
| 45–54 | 5 (50) | 5 (50) | 10 | 2.73 (0.66–11.27) | 0.253 |
| 55–64 | 2 (28.6) | 5 (71.4) | 7 | 0.86 (0.15–4.97) | 0.618 |
| >64 | 1 (16.7) | 5 (83.3) | 6 | 0.4 (0.04–3.74) | 0.651 |
| Total | 16 | 35 | 51 | ||
| Treatment status | 0.021 | ||||
| ND | 8 (21.6) | 29 (78.4) | 37 | 0.21 (0.06–0.77) | |
| PT/TMDR | 8 (57.1) | 6 (42.9) | 14 | ||
| Total | 16 | 35 | 51 | ||
| Drug resistance | |||||
| STR | 0.999 | ||||
| S | 4 (28.6) | 10 (71.4) | 14 | 1.47 (0.26–8.23) | |
| R | 3 (21.4) | 11 (78.6) | 14 | ||
| Total | 7 | 21 | 28 | ||
| INH | 0.184 | ||||
| S | 1 (8.3) | 11 (91.7) | 12 | 0.15 (0.02–1.49) | |
| R | 6 (37.5) | 10 (62.5) | 16 | ||
| Total | 7 | 21 | 28 | ||
| RIF | 0.391 | ||||
| S | 4 (18.2) | 18 (81.8) | 22 | 0.39 (0.08–2.00) | |
| R | 4 (36.4) | 7 (63.6) | 11 | ||
| Total | 8 | 25 | 33a | ||
| ETH | 0. 999 | ||||
| S | 5 (22.7) | 17 (77.2) | 22 | 0.59 (0.08–4.21) | |
| R | 2 (33.3) | 4 (66.7) | 6 | ||
| Total | 7 | 21 | 28 |
NM, non-mycobacteria; MTBC, Mycobacterium tuberculosis complex; ND, newly diagnosed; PT/TMDR, previously treated or treated as multi-drug-resistant strains; CI, confidence interval; STR, streptomycin; INH, isoniazid; RIF, rifampicin; ETH, ethambutol; S, sensitive; R, resistant.
Rifampicin sensitivity alone, carried out using GeneXpert in Cameroon.
Association of treatment status and drug resistance with NTM
Of the 51 identified isolates, 37 (72.5%) were newly diagnosed and 14 (27.5%) were either previously treated patients or multi-drug resistant. Eight of 14 (57.1%) of the 16 patients with NTM/NM were previously treated patients (OR 0.21, 95% CI 0.06–0.77; P=0.021) compared with those with MTBC infection. On the other hand, drug resistance between the two groups NTM/NM and MTBC was not associated with STR (OR, 1.47, 95%CI, 0.26–8.23, p=0.999), INH (OR, 0.15 95%CI, 0.02–1.49, p=0.184), RIF (OR, 0.39, 95%CI, 0.08–2.00, p=0.391) or ETH (OR, 0.59, 95%CI, 0.08–4.21, p=0.999). However, INH [6/16 (37.5%)] and RIF [4/11 (36.4%)] showed the highest drug resistance among the NTM/NM isolates compared to MTBC (Table 4).
NTM distribution by country, drug resistance and previous treatment status
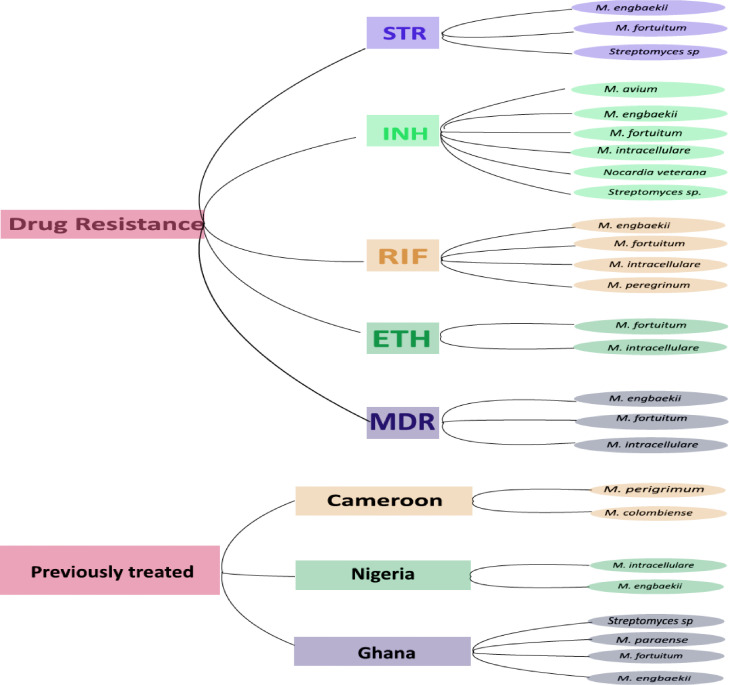
Three NTM were resistant to STR, six were resistant to INH, four were resistant to RIF and two were resistant to ETH. There were three MDR strains: M. engbaekii, M. fortuitum subsp. fortuitum and M. intracellulare. The M. fortuitum subsp. fortuitum MDR strain was found to be equally resistant to STR and ETH, while the M. intracellulare MDR strain was equally resistant to ETH but not STR.
Among the patients treated previously, M. perigrimum and M. colombiense strains were found in Cameroon; M. engbaekii and M. intracellulare were found in Nigeria; and M. fortuitum, M. paraense, M. engbaekii and Streptomyces sp. were found in Ghana (Figure 1).
Figure 1.
Distribution of non-tuberculous mycobacteria/non-mycobacteria isolates by country, drug resistance and previous treatment status. STR, streptomycin; INH, isoniazid; RIF, rifampicin; ETH, ethambutol; MDR, multi-drug resistant.
Discussion
NTM are an important cause of pulmonary diseases worldwide, and are being isolated increasingly. They are often mistakenly treated as MTBC in countries devoid of laboratory competence for species differentiation (Pokam and Asuquo, 2012).
This study showed an overall prevalence of NTM of 3.2% in three countries in the Central and West African region. The prevalence of NTM was lowest in Nigeria at 1%; previous research reported prevalence of 4.1% in a South-South state (Pokam and Asuquo, 2012), and 15% across the country (Aliyu et al., 2013). As the present study included a collection of strains cultured previously from PTB patients, some isolates may have been excluded following primary identification in the laboratory using MTB protein 64 (mpt64) antigen, differentiating NTM from MTBC through immunochromatographic methods. Nevertheless, 1% escaped this prior screening, probably as a result of the polymorphisms of the mpt64 gene in MTBC which lead to changes in the antigen produced and alterations in related functions (Jiang et al., 2013). Mutation within the mpt64 gene has been shown to lead to incomplete protein production as a result of deletion of the C-terminal region of the protein (Hirano et al., 2004). The similar prevalence of NTM of 1.3% observed in Cameroon can be explained by the fact that isolates in both Cameroon and Nigeria were cultured using Lowenstein–Jensen medium with prior exclusion of some NTM (as rapid growers), whereas in Ghana, with NTM prevalence of 8.4%, BACTEC MGIT liquid was used and no isolates were excluded prior to sequencing.
Information about NTM epidemiology in Cameroon is scarce, although a study reported these organisms in cattle from four abattoirs across the country and hypothetically linked their transmission to humans (Egbe et al., 2016). Egbe et al. reported M. fortuitum, M. gordonae, M. mucogenicum, M. phlei and M. scrofulaceum, whereas the present study reported M. colombiense and M. peregrinum. Although the area studied by Egbe et al. was different from that in the present study, the high interaction between animals, the environment and humans has been shown to pose a risk of NTM transmission from animals to humans in Africa (Katale et al., 2014). M. colombiense, a new species belonging to the M. avium complex (MAC), has been isolated in children in France (Vuorenmaa et al., 2009) and Spain (Esparcia et al., 2008). Underestimation of emerging MAC species has been highlighted due to the lack of modern molecular techniques for their identification (Vuorenmaa et al., 2009).
M. peregrinum has been isolated from sputum samples in the Eastern region of China (Shao et al., 2015). It reportedly represents up to 19% of isolates from human respiratory sites in the USA, despite the fact that its clinical significance is unknown, probably due to the lack of large evaluation studies (Wallace et al., 2005). M. peregrinum type I isolates have shown approximately 97.1% identity with M. fortuitum. M. fortuitum, a rapidly growing mycobacteria, was the most commonly isolated (9.8%) NTM in this study, and all isolates were found in Ghana, contrary to a previous study in Ghana that did not isolate any M. fortuitum (Otchere et al., 2017). Instead, Otchere et al. found that M. intracellulare (30.2%) was the most prevalent NTM in Ghana, whereas it was much less prevalent in the present study. This is surprising considering the ubiquitous nature of M. fortuitum, which has been widely recovered from humans as well as animals (da Costa et al., 2013; Katale et al., 2014). Other NM species (Streptomyces sp. and N. veterana) were isolated in Ghana, and further evaluation is required to determine their importance in TB-like infection. An earlier study showed that some isolates from sputum-smear-positive patients considered to be MTB were not members of the genus Mycobacterium, but resembled Nocardia spp. (Pokam and Asuquo, 2012). Thus, there is a need for newer molecular techniques to unfold these hidden and unrecognized infections in TB patients, especially in sub-saharan Africa.
In contrast to Ghana and Cameroon with limited data on the distribution of NTM, several studies in Nigeria have reported the prevalence of NTM in different parts of the country (Pokam and Asuquo, 2012; Aliyu et al., 2013; Cadmus et al., 2016). Aliyu et al. (2013) and other studies have associated the Harmattan season with the occurrence of NTM cases. M. intracellulare reported in this study has been described previously, and the absence of M. abscessus and M. fortuitum, which are commonly found in Nigeria (Pokam and Asuquo, 2012; Aliyu et al., 2013), may be due to the low prevalence reported previously as a result of prior elimination of NTM in the present study. In this study, the identification of a new species – M. engbaekii – highlights the diversity and large spectrum of NTM that can be encountered in Nigeria.
Drug resistance was observed among NTM isolated in this study, with up to 85.7% resistance to INH, 42.9% resistance to STR and 50% resistance to RIF. It has been shown that most NTM do not respond to standard anti-TB drugs (Viveiros et al., 2003). In support of the present study, Otchere et al. (2017) reported high resistance to INH and RIF in Ghana, suggesting the need for proper species identification and drug sensitivity evaluation in patients. MAC infections are difficult to treat due to the intrinsic multi-drug resistance of the organism. The present study showed that virtually all the species were resistant to one or more anti-TB drugs, and M. engbaekii, M. fortiutum and M. intracellulare were MDR. Resistance of M. intracellulare to INH and RIF has been reported previously in Ghana (Otchere et al., 2017), highlighting that NTM may be MDR (Shahraki et al., 2015).
This study also showed that NTM (57.1%) were significantly associated with previous treatment or MDR-TB. With the exception of M. avium, M. gordonae, M. paraense and N. veterana in Ghana, all the species isolated in each country were MDR. In Nigeria, Cadmus et al. (2016) noted, in line with the present study, that 40% of NTM were recovered from patients diagnosed as new TB cases, and 33% of patients were diagnosed as relapsed TB, buttressing the public health implications of TB misdiagnosis in NTM-infected patients. This appears to be recurrent in the country, as Pokam and Asuquo (2012) also noted the consequences of directly observed treatment in AFB-positive individuals without further proof of the organisms involved in TB-like symptoms.
Approximately 69% of patients infected with NTM in this study were aged between 35 and 54 years, with more males (56%) than females (44%). This is contrary to a study undertaken in Brazil, which found that more females harboured NTM compared with males (72.4% vs 37.4%). The study found that the average age of NTM patients was 52 years, which was significantly higher than that of TB patients (39 years). The difference can be explained by the younger age of the African population, which may be connected to the implications of poverty and malnutrition among younger age groups, and the consequent effect on the burden of infectious diseases such as NTM, as in the present study. In contrast, in developed countries, elderly individuals respond to infections less favourably than young individuals, mainly as a result of immunosenescence (da Costa et al., 2013).
Study limitations
The results of this study should be interpreted with caution as the HIV status of the patients was not included (HIV results were only available for Nigeria, and hence too few individuals were identified for inclusion in this analysis). Nevertheless, of the nine Nigerian patients included in this study, two were HIV positive, one of whom harboured M. intracellulare. Repeated isolation of NTM is required for definitive diagnosis of the isolate in a patient.
Conclusion
This study highlights the importance of hsp65 sequencing in the identification of NTM in the Gulf of Guinea to prevent the burden associated with management of presumed relapse or MDR patients. This may help to prevent the far-reaching consequences for patients and the development of drug resistance in this sub-region of Africa, where the means to effectively combat the emergence and threat of MDR across the continent is limited. A full evaluation of the NTM species circulating in this part of the world is needed urgently. The need for rapid and correct identification of the causative Mycobacterium spp. cannot be overemphasized considering that each infection is unique in terms of choice of drugs and duration of therapy. To achieve this, laboratory capacity needs to be increased, especially in the Gulf of Guinea where persistence of this gap will continue to lead to morbidity and mortality of overburdened patients that could have been avoided.
Conflict of interest statement
None declared.
Acknowledgments
Acknowledgements
This work is dedicated to Prof. Lovett Lawson (one of the authors), who died before submission of this paper, who provided isolates from Nigeria used in this study and contributed tremendously to TB research in his country. May his soul rest in peace.
Funding
This work was supported by the Bill and Melinda Gates Foundation to BDTP under the Postdoctoral and Postgraduate Training in Infectious Diseases Research awarded to the Noguchi Memorial Institute for Medical Research (Global Health Grant No. OPP52155). The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author contributions
BDTP, DYM and AEA conceived and designed the experiment. BDTP, LL, SO, DA and PMT performed the laboratory experiments. BDTP, PWG and NYY analysed the data. BDTP, PWG and ICD drafted the manuscript and wrote the paper. DYM and AEA substantially revised the manuscript. All the authors read and approved the final version.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2022.05.003.
Appendix. Supplementary materials
References
- Aliyu G, El-Kamary SS, Abimiku A, Brown C, Tracy K, Hungerford L, et al. Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PLoS One. 2013;8:e63170. doi: 10.1371/journal.pone.0063170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadmus SI, Diarra B, Traore B, Maiga M, Siddiqui S, Tounkara A, et al. Nontuberculous mycobacteria isolated from tuberculosis suspects in Ibadan. Nigeria. J Pathogens. 2016;2016 doi: 10.1155/2016/6547363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Costa ARF, Falkinham JO, III, Lopes ML, Barretto AR, Felicio JS, Sales LHM, et al. Occurrence of nontuberculous mycobacterial pulmonary infection in an endemic area of tuberculosis. PLoS Negl Trop Dis. 2013;7:e2340. doi: 10.1371/journal.pntd.0002340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devallois A, Goh KS, Rastogi N. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of the hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J Clin Microbiol. 1997;35:2969–2973. doi: 10.1128/jcm.35.11.2969-2973.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egbe NF, Muwonge A, Ndip L, Kelly RF, Sander M, Tanya V, et al. Abattoir-based estimates of mycobacterial infections in Cameroon. Sci Rep. 2016;6:24320. doi: 10.1038/srep24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Escamilla N, Ramírez-González JE, González-Villa M, Torres-Mazadiego P, Mandujano-Martínez A, Barrón-Rivera C, et al. Hsp65 phylogenetic assay for molecular diagnosis of nontuberculous mycobacteria isolated in Mexico. Arch Med Res. 2014;45:90–97. doi: 10.1016/j.arcmed.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Esparcia O, Navarro F, Quer M, Coll P. Lymphadenopathy caused by Mycobacterium colombiense. J Clin Microbiol. 2008;46:1885–1887. doi: 10.1128/JCM.01441-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- Griffith DE. Nontuberculous mycobacterial lung disease. Curr Opin Infect Dis. 2010;23:185–190. doi: 10.1097/QCO.0b013e328336ead6. [DOI] [PubMed] [Google Scholar]
- Hirano K, Aono A, Takahashi M, Abe C. Mutations including IS6110 insertion in the gene encoding the MPB64 protein of Capilia TB-negative Mycobacterium tuberculosis isolates. J Clin Microbiol. 2004;42:390–392. doi: 10.1128/JCM.42.1.390-392.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liu H, Wang H, Dou X, Zhao X, Bai Y, et al. Polymorphism of antigen MPT64 in Mycobacterium tuberculosis strains. J Clin Microbiol. 2013;51:1558–1562. doi: 10.1128/JCM.02955-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katale BZ, Mbugi EV, Botha L, Keyyu JD, Kendall S, Dockrell HM, et al. Species diversity of non-tuberculosis-like mycobacteria isolated from humans, livestock and wildlife in the Serengeti ecosystem, Tanzania. BMC Infect Dis. 2014;14:616. doi: 10.1186/s12879-014-0616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z, Cuidie M, Tongyang X, Machao L, Haican L, Xiuqin Z, et al. New single gene differential biomarker for Mycobacterium tuberculosis complex and non-tuberculosis mycobacteria. Front Microbiol. 2019;10:1887. doi: 10.3389/fmicb.2019.01887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoi C, Anderson STB, Antonio M, Mulwa SN, Gehre F, Adetifa IMO. Non-tuberculous mycobacteria isolated from pulmonary samples in sub-Saharan Africa – a systematic review and meta analyses. Sci Rep. 2017;7:12002. doi: 10.1038/s41598-017-12175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otchere ID, Asante-Poku A, Osei-Wusu S, Aboagye SY. Yeboah-Manu D. Isolation and characterization of nontuberculous mycobacteria from patients with pulmonary tuberculosis in Ghana. Int J Mycobacteriol. 2017;6:70–75. doi: 10.4103/2212-5531.201895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikaytis BB, Plikaytis BD, Yakrus A, Butler WR, Woodley CL, Silcox VA, et al. Differentiation of slowly growing Mycobacterium species, including Mycobacterium tuberculosis, by gene amplification and restriction fragment length polymorphism analysis. J Clin Microbiol. 1992;30:1815–1822. doi: 10.1128/jcm.30.7.1815-1822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokam TB, Asuquo AE. Acid-fast bacilli other than mycobacteria in tuberculosis patients receiving directly observed therapy short course in Cross River State, Nigeria. Tuberculosis Res Treat. 2012;2012:1–4. doi: 10.1155/2012/301056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokam TDB, Yeboah-Manu D, Lawson L, Guemdjom PW, Okonu R, Madukaji RL, et al. Molecular analysis of Mycobacterium tuberculosis isolated in the North Central zone of Nigeria. J Epidemiol Global Health. 2019;9:259–265. doi: 10.2991/jegh.k.191015.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringuet H, Akoua-Koffi C, Honore S, Varnerot A, Vincent V, Berche P, et al. hsp65 sequencing for identification of rapidly growing mycobacteria. J Clin Microbiol. 1999;37:852–857. doi: 10.1128/jcm.37.3.852-857.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahraki AH, Heidarieh P, Bostanabad SZ, Khosravi AD, Hashemzadeh M, Khandan S, et al. Multidrug-resistant tuberculosis may be nontuberculous mycobacteria. Eur J Intern Med. 2015;26:279–284. doi: 10.1016/j.ejim.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Chen C, Song H, Li G, Liu Q, Li Y, et al. The epidemiology and geographic distribution of nontuberculous mycobacteria clinical isolates from sputum samples in the eastern region of China. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R, Beal KF, Bonfield JK. The Staden package, 1998. Methods Mol Biol. 2000;132:115–130. doi: 10.1385/1-59259-192-2:115. [DOI] [PubMed] [Google Scholar]
- Steingrube VA, Gibson JL, Brown BA, Zhang Y, Wilson RW, Rajagonpalan M, et al. PCR amplification and restriction endonuclease analysis of a 65-kilodalton heat shock protein gene sequence for taxonomic separation of rapidly growing mycobacteria. J Clin Microbiol. 1995;33:149–153. doi: 10.1128/jcm.33.1.149-153.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ingen J. Diagnosis of nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:103–109. doi: 10.1055/s-0033-1333569. [DOI] [PubMed] [Google Scholar]
- Viveiros M, Leandro C, Amaral L. Mycobacterial efflux pumps and chemotherapeutic implications. Int J Antimicrob Agents. 2003;22:274–278. doi: 10.1016/s0924-8579(03)00208-5. [DOI] [PubMed] [Google Scholar]
- Vuorenmaa K, Ben Salah I, Barlogis V, Chambost H, Drancourt M. Mycobacterium colombiense and pseudotuberculous lymphadenopathy. Emerg Infect Dis. 2009;15:619–620. doi: 10.3201/eid1504.081436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RJ, Jr, Brown-Elliott BA, Brown J, Steigerwalt AG, Hall L, Woods G, et al. Polyphasic characterization reveals that the human pathogen Mycobacterium peregrinum type II belongs to the bovine pathogen species Mycobacterium senegalense. J Clin Microbiol. 2005;43:5925–5935. doi: 10.1128/JCM.43.12.5925-5935.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.