Abstract
For many high-risk haematologic malignancies, such as acute myeloid leukaemia, the success of therapy relies mainly on invoking a curative antitumour immune response. This can be achieved by inducing a graft-versus-leukaemia response following allogeneic haematopoietic cell transplantation. While the contribution of T cells and natural killer cells to graft-versus-leukaemia responses is established, the contribution of B cells and antibodies is relatively unexplored. This article reviews what is known about the contribution of B cells and tumour-specific antibody responses to a successful graft-versus-leukaemia response leading to eradication of the tumour.
Key words: B lymphocytes, tumor specific antibodies, immunotherapy, allogeneic hematopoietic stem cell transplantation, graft versus leukemia response
Highlights
-
•
B cells play a role in graft-versus-leukaemia (GvL) responses after allogeneic haematopoietic cell transplantation.
-
•
Targets of GvL B-cell responses are not unique to individual patients.
-
•
Tumour antigens are shared between haematologic and non-haematologic tumours.
-
•
GvL antibodies may be employed for the development of novel immunotherapies.
Introduction
Chemotherapy is often effective in eradicating the bulk of the tumour mass of acute myeloid leukaemia (AML), but persistent complete remission of AML can only be achieved when allogeneic haematopoietic stem cell transplantation (HCT) induces a graft-versus-leukaemia (GvL) response that eliminates residual malignant cells.
The importance of allogeneic HCT for the treatment of AML and other haematologic malignancies has been acknowledged since the 1990s, when it became clear that the curative effect of allogeneic HCT relies on the induction of a donor immune response against the recipient’s tumour.1 The first successful HCT in humans had been performed decades earlier, in the late 1950s, in two girls with acute leukaemia who received bone marrow from their identical twin sisters.2 After having received supralethal, myeloablative total body irradiation in an attempt to eradicate all leukaemic cells, haematopoiesis was restored successfully. However, in both patients, acute leukaemia relapsed within weeks, suggesting that ‘evidently something more than radiation is needed to eradicate leukaemia’.2 In the following years, clinical observations supported this hypothesis. It was found that allogeneic HCT, but not autologous HCT, was associated with a reduction in disease relapse, particularly in patients with graft-versus-host disease (GvHD),1,3 suggesting that allogeneic immune responses were protective against disease relapse. In a large cohort of patients, Horowitz et al. confirmed the occurrence of GvL immunity by demonstrating that patients who received syngeneic or T-cell-depleted grafts did not have GvHD and had much higher leukaemia relapse rates.1 Additional support for the phenomenon of GvL immunity came from studies on donor lymphocyte infusions (DLIs) in patients with chronic myeloid leukaemia (CML). DLIs induced complete remission in high percentages of allogeneic HCT recipients with relapsed CML, and these remissions were persistent in many of the patients (reviewed by Kolb4).4, 5, 6, 7
Historically, in GvL, attention has predominantly been focused on T cells; this was based on mouse models which demonstrated the importance of CD4+ and CD8+ T cells to the development of GvHD and GvL responses,8 and on the observation that higher relapse rates were reported for patients who had received a ‘T-cell-depleted’ allograft.1,9, 10, 11 Ex vivo T-cell depletion of human allografts is most often achieved by targeting CD52 [alemtuzumab or (Mab)Campath ‘in the bag’] or by positive selection of CD34+ haematopoietic progenitor cells. As both of these approaches deplete B cells, natural killer (NK) cells and other immune cells, as well as T cells,12, 13, 14 relapse after transplantation with an ex vivo T-cell-depleted allograft cannot be attributed solely to the absence of T cells. The contribution of other lymphocyte subsets to the GvL effect has been demonstrated; for example, Ritz et al. were the first to describe the reactivity of NK cells against AML in allogeneic HCT recipients.15 In addition, a number of publications have demonstrated the contribution of B cells to the successful immune response against solid tumours.16, 17, 18, 19 This article provides an overview of the literature available on B cells in GvL tumour immunology, and places it in the context of potential applications of B cells and tumour-specific antibodies as novel cancer immunotherapies.
Circulating antibodies and GVL responses
Several studies have addressed the question whether antibodies could be involved in GvL responses. Serum screening against the gene products of a CML-derived cDNA library revealed the appearance of antibodies against CML-associated antigens at the time of clinical responses to DLIs, suggesting functional involvement of these antibodies in GvL immunity. One of the targets identified was the related adhesion focal tyrosine kinase (RAFTK), an intracellular protein expressed in haematopoietic cells.7 Other targets included CML28 (hRrp46; a component of the human exosome, a multiprotein complex involved in 3’ RNA processing) and CML66 (a well-conserved protein with unknown function). CML28 and CML66 are expressed abundantly by CML blasts and CD34+ haematopoietic progenitor cells of patients with CML. Patients with relapsed CML who responded well to DLIs produced high-titre CML28-specific immunoglobulin G (IgG) antibodies.20,21 Another serologic screening of a CML cDNA library revealed antibody responses against the receptor for hyaluronan-acid-mediated motility (RHAMM) and the intracellular target M-phase phosphoprotein 11 in patients with CML, and in patients with AML, melanoma, renal cell carcinoma and other malignancies, but not in healthy volunteers or patients with autoimmune diseases.22, 23, 24 The latter indicates that the immune system is also capable of recognizing immunogenic tumour-associated antigens such as RHAMM outside of the allogeneic setting. Finally, protein microarrays were used to screen sera of allogeneic HCT recipients with GvL responses for tumour-reactive antibodies.25,26 Approximately 65% of patients with AML produced antibodies against nuclear and spindle-associated protein 1, an antigen expressed by CD34+CD90+ haematopoietic stem cells. These antibodies were not detected prior to transplantation, nor were they found in healthy volunteers.26
GvL antibody responses were also mounted against minor histocompatibility antigens (MiHAs) – polymorphic peptides that are presented on cell surfaces of haematopoietic and non-haematopoietic cells in the context of major histocompatibility complexes (MHCs). Miklos et al. demonstrated that in sex-mismatched male patients undergoing HCT, the presence of antibodies directed against particular, well-characterized MiHAs encoded by genes on the Y chromosome (H-Y antigens), such as dead box RNA helicase Y (DBY), correlated with disease-free survival in a large cohort of over 100 patients.27,28 Not only intracellular or membrane-expressed proteins or protein complexes are targeted by tumour-reactive antibodies. When allogeneic HCT recipients were vaccinated with autologous irradiated AML cells that were engineered to secrete granulocyte-macrophage colony-stimulating factor, a broad antibody response was elicited. Antibodies were directed against intracellular proteins and a number of angiogenic cytokines.29 Angiogenesis may play a role in AML pathophysiology: bone marrow microvasculature is increased in patients with AML, AML cells interact with the vascular endothelium and release angiogenic cytokines, and inhibition of these angiogenic cytokines had antileukaemic effects in experimental settings.30 The development of antibodies against angiogenic cytokines after vaccination was associated with a better outcome in allogeneic HCT recipients.29
Functional involvement of tumour-specific antibodies in GVL responses
Although the timing of appearance of these antibodies suggests a relationship with the clinical GvL responses observed in these patients, they cannot be taken to prove functional involvement in GvL immunity, particularly because most of these antibodies were targeting intracellular targets. However, two case reports provided evidence for a functional role of B cells in GvL responses. One described a patient who had received an allogeneic HCT for acute lymphoblastic leukaemia (ALL) and who relapsed after receiving treatment with the B-cell-depleting CD20 antibody rituximab for steroid-refractory chronic GvHD.31 Our group investigated the B-cell repertoire of a similar patient who had an AML relapse soon after allogeneic HCT.32 Rapid tapering of immunosuppressants resulted in strong immune responses inducing complete remission of AML, but also caused severe, steroid-refractory GvHD. Treatment with rituximab resolved GvHD, but AML relapsed soon after. B cells from this patient (of donor origin, as demonstrated by chimerism analysis) were isolated at the time of maximum tumour suppression, before rituximab therapy, and tumour-specific B-cell clones producing antibodies specifically binding to AML blasts were found. One of these antibodies induced complement-mediated lysis of AML cells, suggesting a functional role for this antibody in this patient’s GvL response.32
To further investigate whether B cells could be involved in GvL responses, we investigated the B-cell repertoire of three patients with AML who mounted potent tumour-clearing GvL responses after allogeneic HCT.33 All patients had B cells that were of HCT donor origin and which produced antibodies that recognized antigens expressed by AML cells. In all three patients, antibodies were identified that target the U5 snRNP200 complex, a large multiprotein complex that is part of the spliceosome. In non-malignant cells, the U5 snRNP200 complex is expressed in the cell nucleus and cytoplasm, but the presence of the U5 snRNP200 complex was demonstrated on the cell membrane of AML blasts in approximately 30% of cases. Strikingly, U5 snRNP200 complex antibodies were cytotoxic: they induced death of AML cells in the absence of effector cells or complement in vitro, and in NSG mice grafted with THP-1 cells in vivo.33 In addition, one patient with AMLwas found to produce antibodies against a sialylated epitope on CD43 that is expressed on myeloid but not on B and T cells. CD43sialylated (CD43s) is overexpressed on leukaemic blasts of all World Health Organization 2008 types of AML and myelodysplastic syndrome. AT1413, the antibody that recognizes CD43s, induced death of AML cells via antibody-dependent cellular cytotoxicity (ADCC) and complement-mediated lysis in vitro. In human immune system (HIS) mice grafted with human AML, treatment with AT1413 was highly efficacious in vivo.34 In addition, in patients who were treated with allogeneic HCT for relapsed multiple myeloma and who achieved complete remission after DLIs, antibodies were detected that were capable of eliminating myeloma cells.35 One of the targets was identified as B-cell maturation antigen (BCMA), a member of the tumour necrosis factor (TNF) superfamily that is expressed by mature B cells. BCMA-expressing myeloma cells were killed via ADCC when incubated with sera from patients responding to DLIs.36 The observation that patients with potent graft-versus-tumour (AML, multiple myeloma) responses generated antibodies capable of inducing death of tumour cells suggests the clinical relevance of these antibodies.
B-cell-mediated immune responses can be either dependent or independent of T cells. In a patient who cleared CML after DLI, the appearance of CD4+ T cells reactive against a polymorphic MHC-II restricted peptide derived from the enzyme protein kinase 2 beta (PTK2B; also known as RAFTK) coincided with the appearance of antibodies directed against the same target, suggesting that the B-cell response was T-cell dependent in this case.37 Similar post-DLI-coordinated T- and B-cell responses were observed against CML66 and the MiHA DBY.38,39 However, details on the phenotype of these helper T cells are lacking. Recently, it was shown that CD4+ T follicular helper (Tfh) cells, which are located in germinal centres in lymph nodes, can promote B-cell immunoglobulin secretion and maturation resulting in chronic GvHD,40 but no data on the role of Tfh cells in GvL responses are available.
It can be questioned whether sufficient numbers of helper T cells are available early after allogeneic HCT. Most AML relapses occur within 6–9 months of allogeneic HCT, suggesting that some of the successful GvL responses are generated at a time when T-cell reconstitution is still incomplete (Figure 1).
Figure 1.
Immune reconstitution and relapse of acute myeloid leukaemia (AML).
Immune reconstitution dynamics after allogeneic haematopoietic cell transplantation (HCT) differ between immune cell subsets. Innate cells such as neutrophils and natural killer (NK) cells recover within weeks. Reconstitution of numeric B cells and CD8 T cells occurs within approximately 6 months, although their subset compositions may be altered for much longer. Reconstitution of CD4 T cells, particularly naïve CD4 and naïve CD8 T cells, is slow and often incomplete. The majority of AML relapses occur in the first 6 months after allogeneic HCT, at a time when the adaptive immune system has not yet fully recovered.
Our search for AML-specific antibodies in patients with AML with potent GvL responses, as described above, uncovered many antibodies of interest, most of which were of the IgG3 isotype (and some of the IgG1 isotype).32, 33, 34 This confirms the findings of others, who also found dominance of IgG1 and IgG3 antibodies.41 IgG3 isotype antibodies may be generated without the help of T cells.42 The predominance of the IgG3 antibodies found may indicate that these antibodies were generated independent of T-cell help. The high-risk nature of AML of these patients entails that the absence of GvL immunity would have been associated with early relapse, and raises the hypothesis that these antibody responses were mounted early after allogeneic HCT, independent of T-cell help, at a time when numbers of reconstituted donor T cells were still low.
B cells beyond antibodies
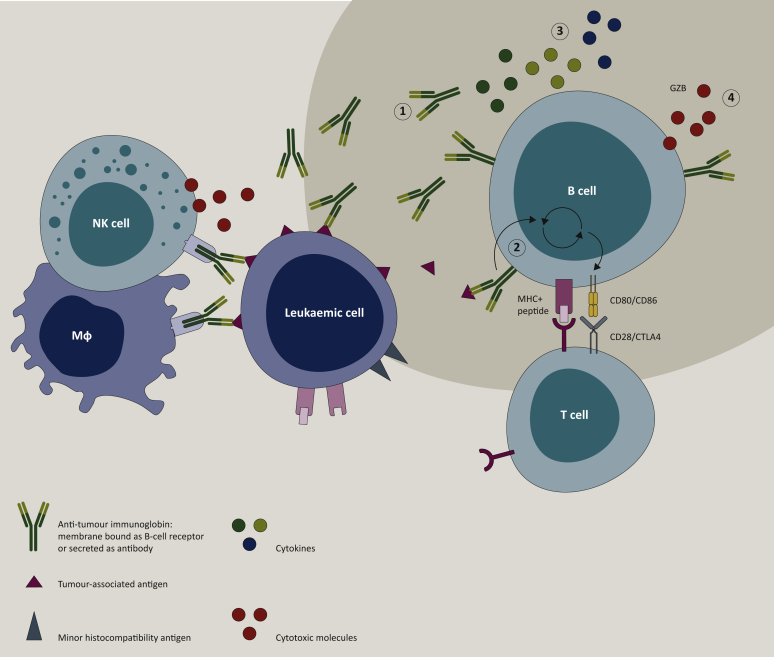
B cells can develop into plasma cells that produce antibodies, but they can exert a number of other functions that may also contribute to the immune-mediated eradication of tumour cells. B cells can function as antigen-presenting cells by presenting antigen in MHC class II after B-cell-receptor-antigen internalization and processing (Figure 2).43, 44, 45 Vaccination of mice with melanoma or lymphoma with antigen-loaded, CD40-ligand-activated B cells led to tumour-specific T-cell responses and a significant delay in tumour growth.46 One study comparing tumour-draining lymph nodes and non-tumour-draining lymph nodes of a small group of patients with solid tumours (of the bladder, colon, skin, pancreas or prostate) found an increased proportion of B-cell plasmablasts in metastatic lymph nodes. They also demonstrated the presence of CD19+ B cells with an activated phenotype, as indicated by CD86 expression, in these lymph nodes, fitting with an antigen-presenting role for B cells in this context.47
Figure 2.
B cells in graft-versus-leukaemia responses.
B cells can exert antitumour effects via a number of mechanisms. (1) Antibodies secreted by plasma cells can induce antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, complement-dependent cytotoxicity or can be directly cytotoxic. (2) B cells can act as antigen-presenting cells when cognate antigens bound to membrane-expressed immunoglobulin are internalized, processed and presented in the context of major histocompatibility complex (MHC) to T cells. (3) B cells can modulate the tumour microenvironment and antitumour immune responses via the secretion of pro-inflammatory cytokines, such as interleukin (IL)-2, tumour necrosis factor-α, IL-6, IL-12, migration inhibitory factor and interferon-γ. (4) B cells can produce cytotoxic granzyme B (GZB); Mϕ, macrophage.
In various solid tumours, B cells are one of the dominant lymphocyte populations in the tumour microenvironment. They interact with T cells (cytotoxic CD8 T cells, CD4 Tfh cells), frequently organized in tertiary lymphoid structures (TLSs), where they can differentiate into memory B cells, antibody-producing plasma cells or antigen-presenting B cells.48, 49, 50, 51, 52, 53 The presence of TLSs and active B-cell responses have been associated repeatedly with favourable outcomes, also after checkpoint inhibitor therapy.16, 17, 18, 19,54 In addition, B cells can activate or enhance local immune responses by secreting pro-inflammatory cytokines, such as interleukin (IL)-2, TNF-α, IL-6, IL-12, macrophage migration inhibitory factor and interferon-γ, to activate T cells, NK cells and macrophages.55 In addition, it has been proposed that B cells have direct cytotoxic effects via their production of granzyme B.16,56 The presence of TLSs and immune modulatory or cytotoxic donor B cells in bone marrow of allogeneic HCT recipients remains to be explored.
Application of novel tumour-associated antigens for therapy
The remarkable progress in cancer immunotherapy for solid tumours, ALL and lymphomas raised hopes that immunotherapy for AML would be a matter of time. However, this has been a challenge thus far. Targets that are currently under study in AML include CD33, CD38 and CD123 (IL-3 receptor). Similar to targeting CD19 in B-ALL and B-cell lymphomas by chimeric antigen receptor (CAR)-T cells and CD19-directed bispecific T-cell engager (bTCE) therapy, or CD38 in multiple myeloma, these are all antigens that are also expressed by non-malignant haematopoietic progenitor cells (see review of Assi et al.).57
Identification of donor-derived antibodies directed against tumour-associated host antigens from patients cured after allogeneic HCT can help with indentifying novel tumour-associated antigens, independent of the context of MHC (Table 1). This seems of particular interest in AML, where downregulation of MHC appeared to be an important mechanism of escape from antileukaemic T-cell immune responses in relapse after allogeneic HCT.58 The analyses of B-cell or antibody repertoires in patients with AML who received allogeneic HCT have revealed tumour targets that may be employed for the development of tumour immunotherapies. Patient-derived AML-targeting antibodies may be explored for their capacity to enhance the effect of conventional chemotherapy, similar to the CD20 antibodies rituximab and obinutuzumab that significantly improved the prognosis of patients with B-cell non-Hodgkin lymphoma when applied in conjunction with conventional chemotherapy (R-CHOP, O-CHOP).59,60 AML-targeting antibodies can also be developed as antibody–drug conjugates or as vaccines to induce long-term antitumour T-cell responses. We have recently demonstrated the potency of the CD43s-specific antibody AT1413 in a bTCE format with AT1413 coupled to two T-cell targeting fragments.61 AT1413-bTCE was highly effective in inducing T-cell-mediated cytotoxicity of AML cells in vitro, and in HIS mice inoculated with human AML. Despite low-level expression of CD43s on non-malignant human haematopoietic progenitor cells, AT1413-bTCE treatment did not affect normal haematopoiesis.61 As described above, antibodies against the B-cell maturation antigen (BCMA) were found in patients with multiple myeloma after allogeneic HCT. A BCMA-specific antibody has been clinically developed as an antibody–drug conjugate and shows promising first results in patients, and BCMA-targeting CAR-T cells have shown clinical effect in a phase I study.62, 63, 64, 65 This proves that tumour antigens identified by carefully studying natural antitumour B-cell responses can be successfully employed clinically.
Table 1.
Tumour antigens with potential for clinical application, including the antibody targets described in the manuscript, the technology used to identify these antibodies and (pre)clinical evaluation
| Antigen | Antibody identification | Clinical evaluation | References |
|---|---|---|---|
| Targets of allogeneic B-cell responses | |||
| BCMA | cDNA serum screen of patients with MM after allogeneic HCT and DLI | Antibody–drug conjugate, CAR-T cells | 35,36,63, 64, 65 |
| CML28 CML66 | cDNA serum screen of patients with CML after allogeneic HCT and DLI | 7,20,21,85 | |
| RAFTK (PTK2B) | cDNA serum screen of patients with CML after allogeneic HCT and DLI and western blot analysis of serum specifically for PTK2B | 7,37 | |
| NuSAP1 | Protein array serum screen of patients with AML | 26 | |
| snRNP200 | Screening of immortalized donor-derived memory B cells after allogeneic HCT for AML | Tested in mouse models | 33 |
| CD43s | Screening of immortalized donor-derived memory B cells after allogeneic HCT for AML | Tested in mouse models | 34,61,68 |
| Targets of autologous B-cell responses relevant for haematologic malignancies | |||
| CD9 | Screening of immortalized patient-derived memory B cells after adoptive T-cell transfer of tumour-reactive T cells for melanoma | Preclinical evaluation | 82, 83, 84,86, 87, 88, 89 |
| MUC1 | Identified in serum of patients with many different types of tumours | Vaccine, CAR-T cells | 69, 70, 71, 72, 73, 74NCT04020575 |
AML, acute myeloid leukaemia; BCMA, B-cell maturation antigen; CAR, chimeric antigen receptor; CML, chronic myeloid leukaemia; DLI, donor lymphocyte infusion; HCT, haematopoietic cell transplantation; MM, multiple myeloma; MUC1, mucin 1; NuSAP1, nuclear and spindle-associated protein 1; RAFTK, related adhesion focal tyrosine kinase.
Tumour-associated antigens are shared between solid and haematologic cancers
Application of tumour-selective antibodies do not have to be restricted to a specific tumour cell type, as illustrated by daratumumab. This antibody, which targets CD38, was first developed as a therapy for multiple myeloma in which CD38 is overexpressed. Daratumumab is now being evaluated for the treatment of AML and ALL in phase II clinical trials (NCT03067571 and NCT03384654, clinicaltrials.gov). Interestingly, tumour-associated antigens can also be shared between liquid and solid malignancies. CD43, expressed highly on haematopoietic cells, has also been shown to be aberrantly expressed in solid tumours, such as colon and breast cancer.66,67 This suggests that CD43 can also be targeted in solid tumours. We have demonstrated that AT1413 can be used to target CD43s-expressing melanoma cells (De Jong et al., manuscript in preparation).68 Another example is the transmembrane glycoprotein mucin 1 (MUC1), which, like CD43, is overexpressed by and differentially glycosylated in a wide range of solid and haematologic tumours.69 Antibodies against MUC1 have been detected in the serum of patients with cancer,70, 71, 72 and various clinical trials testing vaccines against MUC1 in patients with multiple myeloma and solid tumours have been performed, albeit with varying degrees of success.73,74 Currently, CAR-T cells directed against MUC1 are being tested in a phase I clinical trial in patients with metastatic breast cancer (NCT04020575). A third example of a tumour-associated antigen expressed on haematologic as well as solid malignancies is CD9. CD9 is a member of the tetraspanin protein family, with broad but not ubiquitous tissue expression (skin, gut epithelium, lung, fibroblasts, bile ducts, neuronal tissue, endothelium, adrenal cortex).75,76 Tetraspanins have a myriad of functions, and play a role in metastasis and tumour progression.77 CD9 is overexpressed in precursor B-ALL, AML, glioblastoma, gastric carcinoma and breast cancer.78, 79, 80, 81, 82 A CD9 antibody-producing B cell was isolated from the B cells of a patient that was cured of stage IV metastatic melanoma after adoptive T-cell therapy (abstract EACR2018).83 Multiple CD9-targeting antibodies have been developed, inhibiting growth or irradicating tumours in mice, indicating a potential for CD9 as a therapeutic target (Table 1).82,84
Concluding remarks
Many studies are now pointing to a significant contribution of B cells and antibodies in successful GvL immunity. Moreover, recent studies indicate that tumour-infiltrating B cells are also beneficial for survival in solid tumours. Studying the role of B cells in tumour immunity contributes to a more comprehensive understanding of antitumour immunity, and will lead to the identification of novel tumour antigens that can be employed to develop novel immunotherapies to treat cancer.
Acknowledgments
Funding
GdJ is financially supported by the Dutch Cancer Society (KWF 2014-6557). MDH is supported by a VIDI grant (NWO ZonMW 91715362) and a LSBR fellowship (1438F).
Disclosure
HSP has ownership interest in AIMM Therapeutics.
References
- 1.Horowitz M.M., Gale R.P., Sondel P.M., et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 2.Thomas E.D., Sahler O.D., Ferrebee J.W., Sahler O.D., Ferrebee J.W. Supralethal whole body irradiation and isologous marrow transplantation in man. J Clin Invest. 1959;38:1709–1716. doi: 10.1172/JCI103949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiden P.L., Flournoy N., Thomas E.D., et al. Antileukemic effect of graft-versus-host disease in Human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 4.Kolb H.-J. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 5.Kolb H.J., Schattenberg A., Goldman J.M., et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 6.Sehn L.H., Alyea E.P., Weller E., et al. Comparative outcomes of T-cell-depleted and non-T-cell-depleted allogeneic bone marrow transplantation for chronic myelogenous leukemia: impact of donor lymphocyte infusion. J Clin Oncol. 1999;17:561–568. doi: 10.1200/JCO.1999.17.2.561. [DOI] [PubMed] [Google Scholar]
- 7.Wu C.J., Yang X.F., McLaughlin S., et al. Detection of a potent humoral response associated with immune-induced remission of chronic myelogenous leukemia. J Clin Invest. 2000;106:705–714. doi: 10.1172/JCI10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler D.H., Gress R.E. Th2 and Tc2 cells in the regulation of GVHD, GVL, and Graft rejection: considerations for the allogeneic transplantation therapy of leukemia and lymphoma. Leuk Lymphoma. 2000;38:221–234. doi: 10.3109/10428190009087014. [DOI] [PubMed] [Google Scholar]
- 9.Marmont A.M., Horowitz M.M., Gale R.P., et al. T-cell depletion of HLA-identical transplants in leukemia. Blood. 1991;78:2120–2130. [PubMed] [Google Scholar]
- 10.Wagner J.E., Thompson J.S., Carter S.L., Kernan N.A. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-centre, randomised phase II–III trial. Lancet. 2005;366:733–741. doi: 10.1016/S0140-6736(05)66996-6. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A., Reljic T., Hamadani M., Mohty M., Kharfan-Dabaja M.A. Antithymocyte globulin for graft-versus-host disease prophylaxis: an updated systematic review and meta-analysis. Bone Marrow Transplant. 2019;54:1094–1106. doi: 10.1038/s41409-018-0393-0. [DOI] [PubMed] [Google Scholar]
- 12.Williams R.J., Clarke E., Blair A., et al. Impact on T-cell depletion and CD34+ cell recovery using humanised CD52 monoclonal antibody (CAMPATH-1H) in BM and PSBC collections; comparison with CAMPATH-1M and CAMPATH-1G. Cytotherapy. 2000;2:5–14. doi: 10.1080/146532400539008. [DOI] [PubMed] [Google Scholar]
- 13.Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. 2007;21:1387–1394. doi: 10.1038/sj.leu.2404683. [DOI] [PubMed] [Google Scholar]
- 14.Clatworthy M.R. Targeting B cells and antibody in transplantation. Am J Transplant. 2011;11:1359–1367. doi: 10.1111/j.1600-6143.2011.03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hercend T., Takvorian T., Nowill A., et al. Characterization of natural killer cells with antileukemia activity following allogeneic bone marrow transplantation. Blood. 1986;67:722–728. [PubMed] [Google Scholar]
- 16.Sharonov G.V., Serebrovskaya E.O., Yuzhakova D.V., Britanova O.V., Chudakov D.M. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol. 2020;20:294–307. doi: 10.1038/s41577-019-0257-x. [DOI] [PubMed] [Google Scholar]
- 17.Sautès-Fridman C., Petitprez F., Calderaro J., Fridman W.H. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19:307–325. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 18.Cabrita R., Lauss M., Sanna A., et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577:561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 19.Helmink B.A., Reddy S.M., Gao J., et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577:549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X., Wu C.J., Chen L., et al. CML28 is a broadly immunogenic antigen, which is overexpressed in tumor cells. Cancer Res. 2002;62:5517–5522. [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C.J., Biernacki M., Kutok J.L., et al. Graft-versus-leukemia target antigens in chronic myelogenous leukemia are expressed on myeloid progenitor cells. Clin Cancer Res. 2005;11:4504–4512. doi: 10.1158/1078-0432.CCR-05-0036. [DOI] [PubMed] [Google Scholar]
- 22.Greiner J., Ringhoffer M., Taniguchi M. Receptor for hyaluronan acid-mediated motility (RHAMM) Is a new immunogenic leukemia-associated antigen in acute and chronic myeloid leukemia. Exp Hematol. 2002;30:1029–1035. doi: 10.1016/s0301-472x(02)00874-3. [DOI] [PubMed] [Google Scholar]
- 23.Greiner J., Ringhoffer M., Taniguchi M., et al. Characterization of several leukemia-associated antigens inducing humoral immune responses in acute and chronic myeloid leukemia. Int J Cancer. 2003;106:224–231. doi: 10.1002/ijc.11200. [DOI] [PubMed] [Google Scholar]
- 24.Siegel S., Wirth S., Schweizer M., Schmitz N., Zeis M. M-phase phosphoprotein 11 is a highly immunogenic tumor antigen in patients with acute myeloid leukemia. Acta Haematol. 2012;127:193–197. doi: 10.1159/000335133. [DOI] [PubMed] [Google Scholar]
- 25.Marina O., Hainz U., Biernacki M.A., et al. Serologic markers of effective tumor immunity against chronic lymphocytic leukemia include nonmutated B-cell antigens. Cancer Res. 2010;70:1344–1355. doi: 10.1158/0008-5472.CAN-09-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wadia P.P., Coram M., Armstrong R.J., Mindrinos M., Butte A.J., Miklos D.B. Antibodies specifically target AML antigen NuSAP1 after allogeneic bone marrow transplantation. Blood. 2010;115:2077–2087. doi: 10.1182/blood-2009-03-211375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miklos D.B., Kim H.T., Zorn E., et al. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103:353–359. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miklos D.B., Kim H.T., Miller K.H., et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piesche M., Ho V.T., Kim H., et al. Angiogenic cytokines are antibody targets during graft-versus-leukemia reactions. Clin Cancer Res. 2015;21:1010–1018. doi: 10.1158/1078-0432.CCR-14-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reikvam H., Hatfield K.J., Fredly H., Nepstad I., Mosevoll K.A., Bruserud Ø. The angioregulatory cytokine network in human acute myeloid leukemia – from leukemogenesis via remission induction to stem cell transplantation. Eur Cytokine Netw. 2012;23:140–153. doi: 10.1684/ecn.2012.0322. [DOI] [PubMed] [Google Scholar]
- 31.Deneberg S., Lerner R., Ljungman P., Ringden O., Hägglund H. Relapse of preB-ALL after rituximab treatment for chronic graft versus host disease. Implications for its use? Med Oncol. 2007;24:354–356. doi: 10.1007/s12032-007-0002-3. [DOI] [PubMed] [Google Scholar]
- 32.Gillissen M.A., De Jong G., Levie S.E., et al. AML relapse after rituximab treatment for GvHD: Crucial role for B cells in GvL responses. Bone Marrow Transplant. 2016;51:1245–1248. doi: 10.1038/bmt.2016.90. [DOI] [PubMed] [Google Scholar]
- 33.Gillissen M.A., Kedde M., De Jong G., et al. AML-specific cytotoxic antibodies in patients with durable graft-versus-leukemia responses. Blood. 2018;131:131–143. doi: 10.1182/blood-2017-02-768762. [DOI] [PubMed] [Google Scholar]
- 34.Gillissen M.A., de Jong G., Kedde M., et al. Patient-derived antibody recognizes a unique CD43 epitope expressed on all AML and has antileukemia activity in mice. Blood Adv. 2017;1:1551–1564. doi: 10.1182/bloodadvances.2017008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellucci R., Wu C.J., Chiaretti S., et al. Complete response to donor lymphocyte infusion in multiple myeloma is associated with antibody responses to highly expressed antigens. Blood. 2004;103:656–663. doi: 10.1182/blood-2003-07-2559. [DOI] [PubMed] [Google Scholar]
- 36.Bellucci R., Alyea E.P., Chiaretti S., et al. Graft-versus-tumor response in patients with multiple myeloma is associated with antibody response to BCMA, a plasma-cell membrane receptor. Blood. 2005;105:3945–3950. doi: 10.1182/blood-2004-11-4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kremer A.N., van der Griendt J.C., van der Meijden E.D., et al. Development of a coordinated allo T cell and auto B cell response against autosomal PTK2B after allogeneic hematopoietic stem cell transplantation. Haematologica. 2014;99:365–369. doi: 10.3324/haematol.2013.086652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W., Choi J., Zeng W., et al. Graft-versus-leukemia antigen CML66 elicits coordinated B-cell and T-cell immunity after donor lymphocyte infusion. Clin Cancer Res. 2010;16:2729–2739. doi: 10.1158/1078-0432.CCR-10-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zorn E., Miklos D.B., Floyd B.H., et al. Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation. J Exp Med. 2004;199:1133–1142. doi: 10.1084/jem.20031560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forcade E., Kim H.T., Cutler C., et al. Circulating T follicular helper cells with increased function during chronic graft-versus-host disease. Blood. 2016;127:2489–2497. doi: 10.1182/blood-2015-12-688895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luetkens T., Kobold S., Cao Y., et al. Functional autoantibodies against SSX - 2 and NY - ESO - 1 in multiple myeloma patients after allogeneic stem cell transplantation. Cancer Immunol Immunother. 2014;63:1151–1162. doi: 10.1007/s00262-014-1588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markowitz J.S., Rogers P.R., Grusby M.J., Parker D.C., Glimcher L.H. B lymphocyte development and activation independent of MHC class II expression. J Immunol. 1993;150:1223–1233. [PubMed] [Google Scholar]
- 43.Lanzavecchia A. Antigen-specfic interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 44.Barroso M., Tucker H., Drake L., Nichol K., Drake J.R. Antigen-B cell receptor complexes associate with intracellular major histocompatibility complex (MHC) class II molecules. J Biol Chem. 2015;290:27101–27112. doi: 10.1074/jbc.M115.649582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubtsov A.V., Rubtsova K., Kappler J.W., Jacobelli J., Friedman R.S., Marrack P. CD11c-expressing B cells are located at the T cell/B cell border in spleen and are potent APCs. J Immunol. 2015;195:71–79. doi: 10.4049/jimmunol.1500055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wennhold K., Weber T.M., Klein-Gonzalez N., et al. CD40-activated B cells induce anti-tumor immunity in vivo. Oncotarget. 2017;8:27740–27753. doi: 10.18632/oncotarget.7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zirakzadeh A.A., Marits P., Sherif A., Winqvist O. Multiplex B cell characterization in blood, lymph nodes, and tumors from patients with malignancies. J Immunol. 2013;190:5847–5855. doi: 10.4049/jimmunol.1203279. [DOI] [PubMed] [Google Scholar]
- 48.Bindea G., Mlecnik B., Tosolini M., et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 49.Milne K., Köbel M., Kalloger S.E., et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4:e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nielsen J.S., Sahota R.A., Milne K., et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27 - memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18:3281–3292. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 51.Kroeger D.R., Milne K., Nelson B.H. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-cell responses, and superior prognosis in ovarian cancer. Clin Cancer Res. 2016;22:3005–3015. doi: 10.1158/1078-0432.CCR-15-2762. [DOI] [PubMed] [Google Scholar]
- 52.Garaud S., Buisseret L., Solinas C., et al. Tumor-infiltrating B cells signal functional humoral immune responses in breast cancer. JCI Insight. 2019;5:e129641. doi: 10.1172/jci.insight.129641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pitzalis C., Jones G.W., Bombardieri M., Jones S.A. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14:447–462. doi: 10.1038/nri3700. [DOI] [PubMed] [Google Scholar]
- 54.Petitprez F., de Reyniès A., Keung E.Z., et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577:556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 55.Shen P., Fillatreau S. Antibody-independent functions of B cells : a focus on cytokines. Nat Rev Immunol. 2015;15:441–451. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]
- 56.Hagn M., Jahrsdörfer B. Why do human B cells secrete granzyme B? Insights into a novel B-cell differentiation pathway. Oncoimmunology. 2012;1:1368–1375. doi: 10.4161/onci.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Assi R., Kantarjian H., Ravandi F., Daver N. Immune therapies in acute myeloid leukemia: a focus on monoclonal antibodies and immune checkpoint inhibitors. Curr Opin Hematol. 2018;25:136–145. doi: 10.1097/MOH.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 58.Christopher M.J., Petti A.A., Rettig M.P., et al. Immune escape of relapsed AML cells after allogeneic transplantation. N Engl J Med. 2018;379:2330–2341. doi: 10.1056/NEJMoa1808777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pfreundschuh M., Trümper L., Österborg A., et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 60.Radford J., Davies A., Cartron G., et al. Obinutuzumab (GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma: results of the GAUDI study (BO21000) Blood. 2013;122:1137–1143. doi: 10.1182/blood-2013-01-481341. [DOI] [PubMed] [Google Scholar]
- 61.Bartels L., De Jong G., Gillissen M.A., et al. A chemo-enzymatically linked bispecific antibody retargets T cells to a sialylated epitope on CD43 in acute myeloid leukemia. Cancer Res. 2019;79:3372–3382. doi: 10.1158/0008-5472.CAN-18-0189. [DOI] [PubMed] [Google Scholar]
- 62.Tai Y.T., Mayes P.A., Acharya C., et al. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood. 2014;123:3128–3138. doi: 10.1182/blood-2013-10-535088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trudel S., Lendvai N., Popat R., et al. Antibody – drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: an update on safety and effi cacy from dose expansion phase I study. Blood Cancer J. 2019;9:37. doi: 10.1038/s41408-019-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raje N., Berdeja J., Lin Y., et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380:1726–1737. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agostino M.D., Raje N. Anti-BCMA CAR T-cell therapy in multiple myeloma: can we do better? Leukemia. 2020;34:21–34. doi: 10.1038/s41375-019-0669-4. [DOI] [PubMed] [Google Scholar]
- 66.Fernandez-Rodriguez J., Andersson C.X., Laos S., et al. The leukocyte antigen CD43 is expressed in different cell lines of nonhematopoietic origin. Tumor Biol. 2002;23:193–201. doi: 10.1159/000067252. [DOI] [PubMed] [Google Scholar]
- 67.Batdorf B.H., Kroft S.H., Hosking P.R., Harrington A.M., Mackinnon A.C., Olteanu H. Evaluation of CD43 expression in non-hematopoietic malignancies. Ann Diagn Pathol. 2017;29:23–27. doi: 10.1016/j.anndiagpath.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 68.De Jong G., Bartels L., Kedde M., et al. AT1413 antibody derived from a cured AML patient recognizes a unique CD43 epitope shared by AML, MDS and melanoma and has in vivo activity as unmodified anitbody and as bispecific T cell engager. HemaSphere. 2019;3:437–438. [Google Scholar]
- 69.von Mensdorff-Pouilly S., Moreno M., Verheijen R.H.M. Natural and induced humoral responses to MUC1. Cancers (Basel) 2011;3:3073–3103. doi: 10.3390/cancers3033073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von Mensdorff-Pouilly S., Verstraeten A.A., Kenemans P., et al. Survival in early breast cancer patients is favorably influenced by a natural humoral immune response to polymorphic epithelial mucin. J Clin Oncol. 2000;18:574–583. doi: 10.1200/JCO.2000.18.3.574. [DOI] [PubMed] [Google Scholar]
- 71.Hamanakai Y., Suehiro Y., Fukui M., Shikichi K., Imai K., Hinoda Y. Circulating anti-MUC1 IGG antibodies as a favorable prognostic factor for pancreatic cancer. Int J Cancer. 2003;103:97–100. doi: 10.1002/ijc.10801. [DOI] [PubMed] [Google Scholar]
- 72.Pedersen J.W., Gentry-Maharaj A., Nøstdal A., et al. Cancer-associated autoantibodies to MUC1 and MUC4 - A blinded case-control study of colorectal cancer in UK collaborative trial of ovarian cancer screening. Int J Cancer. 2014;134:2180–2188. doi: 10.1002/ijc.28538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nath S., Mukherjee P. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med. 2014;20:332–342. doi: 10.1016/j.molmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pillai K., Pourgholami M.H., Chua T.C., Morris D.L. MUC1 as a potential target in anticancer therapies. Am J Clin Oncol. 2015;38:108–118. doi: 10.1097/COC.0b013e31828f5a07. [DOI] [PubMed] [Google Scholar]
- 75.Sincock P.M., Mayrhofer G., Ashman L.K. Localization of the transmembrane 4 superfamily (TM4SF) member PETA-3 (CD151) in normal human tissues: comparison with CD9, CD63, and α5β1 integrin. J Histochem Cytochem. 1997;45:515–525. doi: 10.1177/002215549704500404. [DOI] [PubMed] [Google Scholar]
- 76.Boucheix C., Rubinstein E. Tetraspanins. Cell Mol Life Sci. 2001;58:1189–1205. doi: 10.1007/PL00000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hemler M.E. Tetraspanin proteins promote multiple cancer stages. Nat Rev Cancer. 2014;14:49–60. doi: 10.1038/nrc3640. [DOI] [PubMed] [Google Scholar]
- 78.Podergajs N., Motaln H., Rajčević U., et al. Transmembrane protein CD9 is glioblastoma biomarker, relevant for maintenance of glioblastoma stem cells. Oncotarget. 2016;7:593–609. doi: 10.18632/oncotarget.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liang P., Miao M., Liu Z., et al. CD9 expression indicates a poor outcome in acute lymphoblastic leukemia. Cancer Biomarkers. 2018;21:781–786. doi: 10.3233/CBM-170422. [DOI] [PubMed] [Google Scholar]
- 80.Baek J., Jang N., Choi J.E., Kim J.R., Bae Y.K. CD9 expression in tumor cells is associated with poor prognosis in patients with invasive lobular carcinoma. J Breast Cancer. 2019;22:77–85. doi: 10.4048/jbc.2019.22.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Touzet L., Dumezy F., Roumier C., et al. CD9 in acute myeloid leukemia: Prognostic role and usefulness to target leukemic stem cells. Cancer Med. 2019;8:1279–1288. doi: 10.1002/cam4.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakamoto T., Murayama Y., Oritani K., et al. A novel therapeutic strategy with anti-CD9 antibody in gastric cancers. J Gastroenterol. 2009;44:889–896. doi: 10.1007/s00535-009-0081-3. [DOI] [PubMed] [Google Scholar]
- 83.Schotte R., Villaudy J., Verdegaal E., et al. A patient derived antibody targeting the tetraspanin CD9 inhibits tumour progression and metastasis. ESMO Open. 2018;3:A6–A7. [Google Scholar]
- 84.Menendez J., Jin L., Poeppl A., et al. Anti-CD9 antibody, AR40A746.2.3, inhibits tumor growth in pancreatic and breast cancer models and recognizes CD9 on CD34+CD38- leukemic cancer stem cells. Cancer Res. 2008;68:3993. [Google Scholar]
- 85.Yang X.-F., Wu C.J., McLaughlin S., et al. CML66, a broadly immunogenic tumor antigen, elicits a humoral immune response associated with remission of chronic myelogenous leukemia. Proc Natl Acad Sci U S A. 2001;98:7492–7497. doi: 10.1073/pnas.131590998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verdegaal E.M.E., Visser M., Ramwadhdoebé T.H., et al. Successful treatment of metastatic melanoma by adoptive transfer of blood-derived polyclonal tumor-specific CD4+ and CD8+ T cells in combination with low-dose interferon-alpha. Cancer Immunol Immunother. 2011;60:953–963. doi: 10.1007/s00262-011-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spits H., Van Helden H.P.M.W., Schotte R., et al. 2017. Therapeutic anti-CD9 antibody (Patent number WO2017119811) [Google Scholar]
- 88.Leung K.T., Zhang C., Chan K.Y.Y., et al. CD9 blockade suppresses disease progression of high-risk pediatric B-cell precursor acute lymphoblastic leukemia and enhances chemosensitivity. Leukemia. 2020;34:709–720. doi: 10.1038/s41375-019-0593-7. [DOI] [PubMed] [Google Scholar]
- 89.De Jong G., Levie S.E., Schotte R., et al. AT1412, a patient-derived antibody in development for the treatment of CD9 positive precursor B-acute lymphoblastic leukemia. Blood. 2019;134:4461. [Google Scholar]