Abstract
Oxytocin is known for its stress-reducing effects and has been associated with autonomic nervous system measures (ANS) involved in the stress response, such as heart rate variability (HRV). The current study examined the effects of intranasal oxytocin on HRV among women (oxytocin N = 87, placebo N = 86) during rest. Results show that oxytocin reduced RMSSD and low frequency (LF)-HRV, but only in women with positive childhood rearing experiences, and not in women with negative childhood experiences. These findings suggest that oxytocin plays a role in ANS regulation and that childhood rearing experiences may influence oxytocin effects on this stress regulating system.
Keywords: Oxytocin, Heart rate variability, Autonomic nervous system, Childhood rearing experiences
Highlights
-
•
Oxytocin reduces resting-state HRV in women without the history of a negative childhood rearing style.
-
•
Oxytocin reduces RMSSD and low frequency HRV during rest.
-
•
Oxytocin effects on HRV are absent after negative childhood experiences.
1. Introduction
Autonomic nervous system (ANS) changes in response to stress are regulated by neuroendocrine factors. More specifically, previous research points to a role of oxytocin, a neuropeptide important for social behaviour that may underlie previously reported social influences on psychophysiological measures relevant to cardiovascular health [1]. Intranasal oxytocin (inOT) administration experiments point to a causal role of oxytocin in stress reduction and show that ANS measures relevant to the stress response, such as heart rate variability (HRV), are influenced by inOT [2]. InOT increases HRV during stress but has also been shown to affect HRV during rest; thus, without potential biases related to responses to external demands [3]. More specifically, two previous studies showed increasing effects of oxytocin on resting levels of HRV in predominantly male samples consistent with higher parasympathetic nervous system (PNS) activity [1,4]. However, inOT effects on HRV may be different in women because of sex differences and associated hormonal influences on cardiac HRV patterns [5,6]. Therefore, the first objective of this study is to examine the effect of inOT on HRV among women during a resting condition.
InOT effects may not only differ by sex [2]; a vast amount of literature has demonstrated that the effects are also moderated by negative childhood rearing experiences [7]. More specifically, studies indicate that prosocial and stress-reducing effects of oxytocin are only present in individuals with positive childhood rearing experiences, but absent in individuals with negative childhood rearing experiences, possibly due to epigenetic changes resulting in reductions in oxytocin receptor expression and a dysregulated oxytocin system [7,8]. For example, one previous study showed that oxytocin enhanced functional connectivity within complex brain networks during rest, but not in women with negative childhood rearing experiences [9]. However, it remains unclear whether oxytocin effects on HRV in rest are influenced by childhood rearing experiences. The second objective of this study is therefore to examine the role of childhood rearing experiences in shaping oxytocin effects on HRV in rest among women.
2. Materials and methods
2.1. Participants
The current study is part of a project examining prosocial oxytocin effects [10] which is approved by the Medical Ethics Committee Brabant and registered in the Dutch Trial Registry (NTR6513). Participants were female undergraduates enrolled at Tilburg University, the Netherlands and gave informed consent. A sample of 200 female students was invited to participate in the study, but 20 participants dropped out due to cancelled participation or no data on primary outcomes (see supplement), resulting in a final sample consisting of 180 participants. All participants were nulliparous, 57.8% used hormonal contraceptives. Exclusion criteria involved drug/alcohol abuse, nasal problems, use of medication (except contraception), psychiatric, cardiovascular/neurological disorders, hypertension, or pregnancy. As oxytocin levels vary during the menstruation cycle [11] participants were preferably invited to the lab in the luteal phase.
2.2. Procedure
Participants were randomly assigned to the oxytocin (N = 90) or a placebo group (N = 90). A random half of both groups (placebo N = 45, oxytocin N = 45) was instructed to bring a female friend with them to the laboratory session for the purpose of another experiment in the overall project. Participants were instructed to abstain from alcohol during the 24 h before the study, and from caffeine and smoking on the data collection day. Of all participants 88.7% participated in the luteal phase (see supplement).
Participants completed questionnaires on childhood experiences one week prior to the session. At the start of the experiment, participants took 6 puffs of nasal spray containing oxytocin (24 IU total) or a placebo under supervision of the experimenter (double blind administration). Afterwards, participants performed a stop-distance task for the purpose of another study in the overall project. State anxiety was measured using the Spielberger Trait State Anxiety Inventory ~35 min after administration. Resting-state HRV was measured 40 min after intranasal administration by ECG electrodes. The participant’s friend was not present in the room during the resting-state measure.
2.3. Heart rate variability
HRV measures were derived from continuous ECG recordings obtained during 5 min while participants looked at a presentation of landscape photographs. Data were obtained using the Biopac-MP150 system with ECG100C-module, MEC110C-amplifier, and three hydrogel-ECG electrodes. Data were processed with AcqKnowledge,v4.4 (see supplement). Time domain-root mean square of successive RR interval differences (RMSSD); frequency domain-HF power (0.15–0.40 Hz), LF power (0.04–0.15 Hz), and heart rate (HR) were derived. RMSSD, LF-and HF-HRV were log-transformed because of skewed distributions. Seven participants were excluded because of poor quality ECG data.
2.4. Childhood rearing experiences
Two childhood rearing experiences were measured; maternal use of love withdrawal and traumatic childhood experiences. Maternal use of love withdrawal is an insensitive disciplinary strategy that involves withholding love and affection when a child misbehaves or fails at a task and has been associated with negative child outcomes. It was measured by an 11-item questionnaire which combines 7 items of the Withdrawal of Relations subscale of the Children’s Report of Parental Behavior Inventory [12] and 4 -items from the Parental Discipline Questionnaire [13]. Participants rated how well each statement described their mother on a 5-point scale, with higher scores indicating negative childhood rearing experiences. Previous studies have shown that experienced maternal use of love withdrawal moderate the effects of oxytocin in samples similar to the current study (healthy female students) [9]. Cronbach’s α was 0.83.
Traumatic childhood experiences was measured with the self-reported 28-item Childhood Trauma Questionnaire Short Form (CTQ-SF, [[14], [15]]) assessing experiences of physical abuse, emotional abuse, sexual abuse, physical neglect, and emotional neglect. Each item is rated on a 5-point Likert scale ranging from never true to very often true. Higher scores were indicative of negative childhood rearing experiences. Cronbach’s α was 0.73. The CTQ was log-transformed because of skewness. Love withdrawal and CTQ scores were significantly correlated, r = 0.36, p < .001.
Statistical analysis To test whether inOT affected HRV, analyses of variance (ANCOVA) were performed. Treatment group (oxytocin/placebo) and the control variables; presence of a friend, use of hormonal contraceptives, and menstrual phase were entered as between-subject factors, and age, and state anxiety as covariates. To examine whether childhood rearing experiences moderate the effect of inOT on HRV, analyses were repeated with interaction terms treatment condition∗love withdrawal/childhood trauma (continuous and separately) and their main effect. Analyses were performed for RMSSD, HF-HRV, LF-HRV, and HR separately. Sensitivity analyses were performed without the inclusion of control variables. The Benjamini Hochberg procedure was applied to p values resulting from the analyses with autonomic arousal (4 repeated tests: RMSSD, HF-HRV, LF-HRV, and HR) in order to correct significance levels for Type I error.
3. Results
3.1. Sample characteristics
A total of 173 women participated (age M = 20.17, SD = 1.89). Negative childhood rearing experiences were similar in both treatment groups (love withdrawal: M = 2.26; SD = 0.74; (t(171) = -1.28, p = 0.203), CTQ: M = 1.34; SD = 0.30; (t(171) = -1.17, p = 0.868)).
3.2. Treatment effect
The ANCOVA did not show significant oxytocin effects on HR (F(1,166) = 0.02, p = .882), RMSSD (F(1,166) = 1.83, p = .179), HF-HRV (F(1,166) = 0.58, p = .448), or LF-HRV (F(1,166) = 0.00, p = .955).
3.3. Moderation by childhood rearing experiences
The ANCOVA examining the role of love withdrawal showed a significant treatment effect (F(1,163) = 6.20, p = .014) and interaction effect (treatment∗love withdrawal, F(1,163) = 4.46, p = .036) on RMSSD during rest (Table 1). Similarly, a significant treatment effect of inOT (F(1,163) = 5.07, p = .026) and a significant love withdrawal × treatment interaction (F(1,163) = 5.22, p = .024) was found in the analyses with LF-HRV. Results remained significant after Benjamini Hochberg correction. No main effects or interactions were found in the analyses with HR and HF-HRV (ps > .183).
Table 1.
Adjusted mean and standard deviations of RMSSD, HF-HRV, LF-HRV and HR for individuals in the oxytocin and placebo group.
| RMSSD |
HF (log ms2) |
LF (log ms2) |
BPM |
||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SE | M | SE | M | SE | M | SE | ||
| Model A | Placebo | 70.93 | 4.44 | 2.21 | 0.05 | 2.15 | 0.04 | 72.16 | 1.05 |
| Oxytocin | 63.45 | 4.41 | 2.15 | 0.05 | 2.13 | 0.04 | 72.72 | 1.05 | |
| Model B | Placebo | 71.93 | 4.45 | 2.21 | 0.05 | 2.15 | 0.04 | 72.18 | 1.05 |
| Oxytocin | 64.37 | 4.42 | 2.16 | 0.05 | 2.15 | 0.04 | 72.59 | 1.04 | |
Note: Model A: adjusted for love withdrawal, treatment condition∗love withdrawal, age, menstrual cycle, presence of friend, hormonal contraceptives, anxiety. Model B: adjusted for childhood trauma, treatment condition∗childhood trauma, age, menstrual cycle, presence of friend, hormonal contraceptives, anxiety.
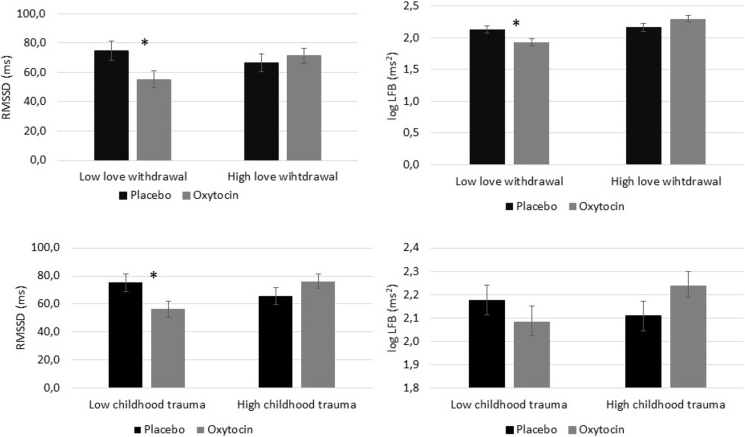
Results of the ANCOVA examining the role of childhood trauma also showed a significant effect of oxytocin on RMSSD (F(1,163) = 5.00, p = .027), but no effect on LF-HRV (F(1,163) = 2.58, p = .110). In addition, there was a significant childhood trauma∗treatment interaction for RMSSD (F(1,163) = 3.93, p = .049) and for LF-HRV (F(1,163) = 4.20, p = .042), but no significant interactions for HR and HF-HRV (p’s > 0.278). Results remained significant after Benjamini Hochberg correction. Stratified analyses dichotomizing childhood rearing experiences using low or high scores on love withdrawal and childhood trauma using median splits (median: love withdrawal = 2.27, childhood trauma = 1.24) were performed in order to interpret the interactions (Fig. 1). Planned contrasts showed that oxytocin significantly reduced RMSSD and LF-HRV only in individuals with low levels of love withdrawal (RMSSD: t(169) = 2.32, p = .022, LF-HRV t(55) = 2.39, p = .018) and reduced RMSSD in individuals with low levels of childhood trauma (t(169) = 2.43, p = .016). No significant effects on LF-HRV were found in individuals with low childhood trauma (t(169) = 1.16, p = .247).
Fig. 1.
Mean RMSSD and LF-HRV for individuals in the oxytocin and placebo group with negative or positive childhood rearing experiences (i.e. higher or lower levels of love withdrawal or childhood trauma). The range subsamples was N = 35–53, ∗p < .05.
Analyses were repeated without controlling for the inclusion of presence of a friend, use of hormonal contraceptives, menstrual phase, age, and state anxiety. Analyses with RMSSD showed associations in the same direction, that is, a similar significant interaction between treatment and love withdrawal (F(1,169) = 4.41, p = .037) and a trend for the interaction term with childhood trauma (F(1,169) = 3.05, p = .083). However, the interactions between treatment and childhood trauma or love withdrawal were not significant in the uncorrected analyses with LF-HRV (ps > .18).
4. Discussion
InOT significantly reduced RMSSD among healthy women without the history of a negative childhood rearing style during rest. Additionally, negative childhood rearing experiences moderated the effects of oxytocin on HRV, such that oxytocin reduced RMSSD and LF-HRV among those without a history of negative childhood rearing style, whereas oxytocin did not affect RMSSD and LF-HRV among participants who experienced positive or negative childhood rearing experiences. No significant effects were seen for oxytocin on HR and HF-HRV, regardless of childhood rearing experiences.
A previous study among healthy men reported HRV indices of elevated PNS activation (increased HF-HRV) after inOT [4]. In contrast, we found that oxytocin lowers RMSSD, indicating reduced parasympathetic activity, although no effects on HF-HRV were found. In addition, reduced LF-HRV may point to reduced activation of the sympathetic nervous system, although there is controversy regarding the interpretation of the LF component, as it includes both sympathetic and parasympathetic (baroreflex-related) components of the ANS [6]. Our contradicting findings, reduced parasympathetic and sympathetic activity after inOT in women, could be interpreted in multiple ways. First, because previous studies indicate that low HRV is related to attentional state (e.g. [3]), our findings may indicate that oxytocin increases alertness or arousal in women during rest. Consistent with this explanation, a recent study showed that oxytocin decreased LF-HRV and HF-HRV during a mild state of stress as induced by an arithmetic task among individuals with chronic neck or shoulder pain [16], possibly indicating that oxytocin enhanced attentional control and the salience of external stimuli. Second, only among those with positive childhood rearing experiences, the lowering effects of oxytocin on the LF-HRV reflect the cardiac pattern that may be typical for the tend-and-befriend response in women during stress [5]. Future studies are needed to examine the effects of inOT on HRV components reflecting different aspects of the ANS in women exposed to stress, ideally taking into account childhood rearing experiences. Lastly, an alternative explanation for the mixed findings of inOT on HRV may be that childhood rearing experiences were not included in previous studies.
This study has a few limitations. A between-subject design was used and pre-existing group differences may therefore have influenced the results, although randomization reduced this risk. Furthermore, we used a retrospective self-report questionnaire to assess childhood rearing experiences, whereas interviews might yield more valid data. Lastly, our sample consisted of female undergraduate students with only mild negative childhood rearing experiences. Results cannot be generalized to male samples or clinical samples with more severe childhood maltreatment. Future studies should include both sexes in order to test possible sex differences in the cardiac effects of oxytocin. Lastly, it should be noted that studies increasingly point to inconsistent findings with regard to influences of childhood rearing experiences on intranasal oxytocin effects, with studies pointing to either less [17] or more pronounced effects of oxytocin after childhood adversity [18]. More research is therefore needed examining oxytocinergic dysregulations after childhood adversity in both males and females, as effects may be sex-specific and it is still unknown how such changes emerge.
Taken together, the present results indicate that inOT affects HRV by lowering RMSSD and LF-HRV among women with positive childhood rearing experiences. Contrary, among those with negative childhood rearing experiences (higher maternal love withdrawal and higher childhood trauma) RMSSD and LF-HRV were unaffected by oxytocin, possibly because changes in oxytocin receptor expression after childhood adversity result in a dysregulated oxytocin system and altered sensitivity to inOT. Our study is the first to show that childhood rearing experiences shape oxytocin effects at cardiac patterns even in the absence of external demands. In addition, our results may suggest that oxytocin, among other neuroendocrine factors, plays a role in previously found sex differences in HRV.
Funding
Madelon Riem was supported for this study by the Tilburg University Alumni Fund, the Department of Medical and Clinical Psychology, and the Center of Research on Psychological and Somatic disorders, Tilburg University, The Netherlands. The sponsors did not have involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Dclaration of competing interest
The authors report no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpnec.2020.100007.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Norman G.J., Cacioppo J.T., Morris J.S., Malarkey W.B., Berntson G.G., DeVries A.C. Oxytocin increases autonomic cardiac control: moderation by loneliness. Biol. Psychol. 2011;86(3):174–180. doi: 10.1016/j.biopsycho.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Kubzansky L.D., Mendes W.B., Appleton A.A., Block J., Adler G.K. A heartfelt response: oxytocin effects on response to social stress in men and women. Biol. Psychol. 2012;90(1):1–9. doi: 10.1016/j.biopsycho.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luft C.D.B., Takase E., Darby D. Heart rate variability and cognitive function: effects of physical effort. Biol. Psychol. 2009;82(2):186–191. doi: 10.1016/j.biopsycho.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Kemp A.H., Quintana D.S., Kuhnert R.-L., Griffiths K., Hickie I.B., Guastella A.J. Oxytocin increases heart rate variability in humans at rest: implications for social approach-related motivation and capacity for social engagement. PloS One. 2012;7(8) doi: 10.1371/journal.pone.0044014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adjei T., Xue J., Mandic D.P. The Female Heart: Sex Differences in the Dynamics of ECG in Response to Stress. Frontiers in Physiology. 2018;9(1616) doi: 10.3389/fphys.2018.01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koenig J., Thayer J.F. Sex differences in healthy human heart rate variability: a meta-analysis. Neurosci. Biobehav. Rev. 2016;64:288–310. doi: 10.1016/j.neubiorev.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Bakermans-Kranenburg M.J., Van IJzendoorn M.H. Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl. Psychiatry. 2013;3:e258. doi: 10.1038/tp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bos P.A. The endocrinology of human caregiving and its intergenerational transmission. Dev. Psychopathol. 2017;29(3):971–999. doi: 10.1017/S0954579416000973. [DOI] [PubMed] [Google Scholar]
- 9.Riem M.M.E., Van IJzendoorn M.H., Tops M., Boksem M.A., Rombouts S.A., Bakermans-Kranenburg M.J. Oxytocin effects on complex brain networks are moderated by experiences of maternal love withdrawal. Eur. Neuropsychopharmacol. 2013;23(10):1288–1295. doi: 10.1016/j.euroneuro.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Riem M.M.E., Kunst L., Bekker M.H.J., Fallon M., Kupper N. Intranasal oxytocin enhances stress-protective effects of social support in women with negative childhood experiences during a virtual Trier Social Stress Test. Psychoneuroendocrinology. 2020;111:104482. doi: 10.1016/j.psyneuen.2019.104482. [DOI] [PubMed] [Google Scholar]
- 11.Salonia A., Nappi R.E., Pontillo M., Daverio R., Smeraldi A., Briganti A.…Montorsi F. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm. Behav. 2005;47(2):164–169. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Beyers W., Goossens L. Psychological separation and adjustment to university: moderating effects of gender, age, and perceived parenting style. J. Adolesc. Res. 2003;18(4):363–382. [Google Scholar]
- 13.Hoffman M.L., Saltzstein H.D. Parent discipline and childs moral development. J. Pers. Soc. Psychol. 1967;5(1):45. doi: 10.1037/h0024189. -&. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein D.P., Fink L., Handelsman L., Foote J., Lovejoy M., Wenzel K., et al. Initial reliability and validity of a new retrospective measure of child-abuse andneglect. Am. J. Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein D.P., Stein J.A., Newcomb M.D., Walker E., Pogge D., Ahluvalia T. Development and validation of a brief screening version of the ChildhoodTrauma Questionnaire. Child Abuse Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 16.Tracy L.M., Gibson S.J., Labuschagne I., Georgiou-Karistianis N., Giummarra M.J. Intranasal oxytocin reduces heart rate variability during a mental arithmetic task: a randomised, double-blind, placebo-controlled cross-over study. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 2018;81:408–415. doi: 10.1016/j.pnpbp.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Bakermans-Kranenburg M.J., van IJzendoorn, Riem M.M.E., Tops M., Alink L.R. Oxytocin decreases handgrip force in reaction to infant crying in females without harsh parenting experiences. Soc. Cognit. Affect Neurosci. 2012;7(8):951–957. doi: 10.1093/scan/nsr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwaiger M., Heinrichs M., Kumsta R. Oxytocin administration and emotion recognition abilities in adults with a history of childhood adversity. Psychoneuroendocrinology. 2019;99:66–71. doi: 10.1016/j.psyneuen.2018.08.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.