Abstract
It has been well documented that police officers are frequently engaged in a variety of high stress situations during their normal daily tasks, such as civilian encounters where force is needed or domestic violence situations, that cause significant increases in a variety of physiological and psychological stress markers. Chronic exposure to stressors increases risk for cardiovascular disease (CVD) progression. The purpose of this study was to compare male and female salivary and blood markers of stress in response to an active shooter training drill (ASD) to determine if acute stress differentially impacts men and women to better understand if interventions should be targeted. Thirty-one participants (males = 15 [mean age: 23], females = 16 [mean age: 21]) participated in an ASD involving professional actors playing the role of one active gunman, as well as four victims. The ASD lasted approximately 50 seconds. Blood samples were collected 15 min prior as well as after the ASD and analyzed for epinephrine (EPI), norepinephrine (NE), and hydrogen peroxide (H2O2) levels. Saliva samples were collected 30 and 5 min prior to the ASD and 5 and 30 min after the ASD, and were analyzed for cortisol, α-amylase, uric acid, and secretory immunoglobulin-A (SIgA). Our analysis revealed that acute (~50 sec) psychological stress in the form of an ASD resulted in significant increases in blood and salivary stress and oxidative stress markers in both men and women. However, four of the seven markers were lower in female participants (cortisol, uric acid, H2O2, and α-amylase presented significant main effects for sex). In addition, SIgA was significantly lower in women compared to men 30 min prior to, and five min post ASD. These findings suggest females may be at a lower risk to stress induced oxidative stress and CVD.
Keywords: Police, Cortisol, Scenario training, Sympathoadrenal axis, Cardiometabolic health
Highlights
-
•
Acute psychological stress results in significant increases in blood and saliva stress and oxidative stress markers.
-
•
Stress markers were attenuated in female participants.
-
•
Blood oxidative stress was lower in female participants.
1. Introduction
Individuals working in law enforcement are regularly exposed to stressors that engage the “fight or flight” response, resulting in a challenge to allostatic homeostasis as maintained by the hypothalamic pituitary adrenal (HPA) and sympathoadrenal (SA) axes [27,32]. Hans Selye (1907–1983) first identified three phases of the physiological response to stress which are identified as the following: 1) alarm, 2) resistance and 3) exhaustion [35]. Acute exposure to these stressors (i.e., alarm phase) results in increasing levels of stress markers, such as cortisol, epinephrine, and norepinephrine. Acute stress can serve a beneficial purpose, facilitating physiological homeostasis in response to stress exposure and the development of stress resistance (i.e., phase 2). However, chronic activation of these axes can result phase 3 which is associated with adverse effects to cardiometabolic health, increased risk illness and premature death. In fact, chronic stress has been linked to numerous diseases including cardiovascular disease (CVD), insulin resistant diabetes, and cancer [5,32].
In terms of CVD prevalence in law enforcement officers (LEOs), it has been shown that LEOs have higher CVD-related morbidity and mortality rates than the general public [4,8,10,13,29]. Indeed, LEOs have been shown to have poor cardiometabolic health profiles compared to other occupations [13]. For example, LEOs tend to have higher rates of obesity, metabolic syndrome, and total cholesterol levels in comparison to other occupations in the U.S [13]. Law enforcement is identified as a high stress occupation, and past work has suggested the high stress nature is a primary factor contributing to increased risk of developing CVD [10,41]. Stress induced activation of the HPA and SA axes contributes to oxidative stress and inflammation [17] and may be a primary factor in contributing to ~ 40% of atherosclerosis in individuals without traditional risk factors for CVD, such as obesity and hypertension [2]. Stress induced oxidative stress is associated with impaired vascular function, as well as increased likelihood of LDL oxidation and atherosclerotic development [18]. It is important to note that LEOs have been shown to have elevated rates of both traditional and non-traditional risk factors for CVD compared to other occupations in the U.S [13].
Several studies have reported relationships between blood and salivary biomarkers of stress in response to acute psychological stress exposure [19,38]. Salivary α-amylase is a commonly used marker of stress related to sympathetic activity or activation of the SA axis [28], while salivary cortisol (S-CORT) concentrations are used as a reflection of activation of the HPA axis [12]. Chronic exposure to stress is known to be immunosuppressive, thus, secretory immunoglobulin A (SIgA) concentrations in saliva are commonly used as a marker of stress exposure since immunoglobulin A (IgA) has a role in immune function [19]. Moreover, acute exposure to stress has been previously shown to impact salivary SIgA concentrations [25,30]. In addition, acute stress can result in oxidative stress, which is a main factor facilitating CVD progression [17,18]. Therefore, this study aims to investigate the impact of acute psychological stress on markers of stress and oxidative stress in male and female participants.
The way acute stress differentially impacts male and female health has been extensively studied. In fact, stress reactivity has been linked to differences between sex morbidity rates for multiple disorders [20] such as type 2 diabetes [15,36], metabolic syndrome, depression [3], and coronary heart disease [33]. One study found female LEOs exhibited higher levels of perceived stress and job-related stress than male LEOs [42]. Because stress can be a contributing factor to CVD, it is within reason that female LEOs may be at an elevated risk for stress-related CVD [42]. Acute exposure to stress causes greater concentrations of cortisol in female rats compared to male rats [1,14]. This is likely attributed to greater activation of the HPA and autonomic responses in men compared to women [20,22]. This may be due to greater sensitivity of the adrenal cortex in women compared to men [31]. This may be one mechanism contributing to a greater prevalence of stress-related psychiatric disorders in women versus men [1]. However, the majority of data examining sex related differences in stress markers stems from animal trials. Thus, more data are needed to clarify the potential differences between men and women in relation to stress and oxidative stress responses to acute stressors. Collectively, these findings may have a meaningful impact on CVD risk for men and women working in law enforcement. Therefore, the purpose of this study was to examine differences between men and women in relation to markers of stress and oxidative stress when exposed to an acute stressor in the form of an active shooter training drill (ASD).
2. Materials and methods
2.1. Participants and experimental design
This study utilized a repeated measures two-arm design where both males and females participated in an active shooter training drill (ASD). Measurements were collected pre and post ASD. Participants (n = 31 males = 15 [mean age: 23], females = 16 [mean age: 21]) were recruited via word of mouth from the university campus. To participate in the experimental protocol, participants were required to be apparently healthy, non-cigarette smokers, and free from donating blood as well as any major life stressor in the last 30 days (e.g., death in the family, divorce, birth of a child). A post hoc power analysis using G∗Power 3.1.9.7 [9] for a repeated measures analysis of variance (RMANOVA) revealed that a sample size of 31 resulted in a reported power of 0.93 to detect a large effect (f = .5) with α at .05. All participants provided written informed consent, and all experimental procedures were approved by the University’s Institutional Review Board. These data were analyzed as a subset from a previous study where we analyzed the combined stress responses in both males and females in response to an ASD [26]. Sex differences were examined separately in this manuscript to allow for a thorough examination outside of the combined stress response manuscript.
2.2. Experimental procedures
Upon arriving at the study location, participants rested in a quiet room separate from the ASD facility for 30 minutes. They were then transported to the ASD facility by a member of the research team. Prior to starting the experiment, the member of the research team explained the experiment. First, the participant would be the first “police officer” to arrive to a “shots fired” call. Second, limited information would be provided via simulated dispatch traffic. Participants were only told that a Caucasian male was reported to be firing a gun and there were multiple casualties reported. Participants were told to shoot the attacker if he was still a threat. Third, the researcher would simulate arrival time by telling the participant when he/she “arrived” and could enter the ASD. The researcher then ensured each participant was comfortable with the Glock 17T training pistol prior to starting the ASD. The Glock 17T fired blanks (i.e., no projectile). After these instructions and acclimation period, the researcher started the dispatch recording. The active shooter began firing a blank gun from within the building approximately 20s into the dispatch recording. Additional shots were fired, a fire alarm was activated, and professional actors playing the roles of victims began to scream. Participants were then told they had “arrived” and could enter the building to stop the active shooter.
The ASD was intended to be as realistic as possible to a real active shooting. As such, professional actors played the role of the injured and dying victims. Victims wore a variety of moulage (realistic fake wounds) and realistic fake blood to increase authenticity. There were four victims in total. The first victim was laying on the floor about 10 feet past the door. The first victim presented traumatic injuries (e.g., eviscerated bowels, gunshot wound to her upper thigh, and a pool of blood on the floor) and was screaming for help. The second victim ran out of the scenario room and past the participant. The second victim had two gunshot wounds (one to her upper arm and leg) and screamed for help. The third and fourth victims were in the scenario room with the shooter. The third victim was on the floor with a traumatic head injury, laying in a pool of her blood. The fourth victim was shot multiple times before he collapsed to the floor as the participant stood in the door’s threshold. The shooter would then fall to the ground if the participant fired his/her training weapon or turn to the participant to elicit a response. The researcher then entered the scenario room, stopped the scenario, retrieved the training weapon from the participant, and returned the participant back to the resting room. Use of force simulators have been previously used to examine physiological responses to stress (see Ref. [16]). We chose to create a realistic ASD to increase the realism and better understand stress response in this medium.
The researcher explained the study to each participant following the ASD. No participant reported the ASD as traumatic; however, had any participant expressed or appeared to have experienced potential trauma they would have been directed to the university’s mental health services listed on the IRB approved informed consent form.
2.3. Saliva and blood collection and analysis
Blood samples were collected 15 minutes before and 15 minutes after the ASD and were collected into sealed sodium heparin vacutainers. Sample tubes were immediately cooled on dry ice and centrifuged at 1600 g for 15 minutes. Plasma samples were stored in multiple aliquots at −80 °C until analysis.
Saliva samples of ~500 μL were collected via passive drool technique (Salimetrics, PA, USA) a total of four times: 30 and 5 minutes prior to the ASD, as well as 5 and 30 minutes after completion of the ASD. Saliva samples were immediately stored on dry ice and subsequently stored at −80 °C until analysis.
Saliva samples were thawed and centrifuged at 1500 g (4 °C) for 15 minutes and subsequently analyzed in duplicate for S-CORT, α-amylase, SIgA and uric acid (UA) using commercially available kits (Salimetrics, PA, USA). Thawed plasma samples were analyzed for epinephrine (EPI) and norepinephrine (NE) using an enzyme linked immunosorbent assay (ELISA) which included an extraction procedure (ALPCO, NH, USA). Plasma concentrations of hydrogen peroxide (H2O2) were determined using the Amplex red assay by methods described by the manufacturer (Molecular Probes, Invitrogen Detection Technologies, Eugene, OR, USA). Absorbance was read using a colorimetric plate reader (BioTek, Winooski, VT, USA).
2.4. Statistical analysis
Statistical procedures were conducted with SAS 9.4 (Cary, NC). Differences between men and women pre and post ASD were analyzed with 2 4 (gender time) repeated measures analysis of variance (RMANOVA) for salivary markers S-CORT, α-amylase, SIgA, and UA. Changes in blood EPI, NE, and H2O2 were analyzed with 2 2 RMANOVAs. Fisher’s Least Significant Difference post hoc test was used to further compare means following a significant interaction or main effect. Effect sizes were calculated and reported as partial eta squared (ηp2).
3. Results
All data are reported as mean ± standard deviation. Descriptive characteristics for study participants are as follows: men (n = 15, age = 23.1 ± 4.7 yrs) and women (n = 16, age = 21 ± 0.9 yrs). All 31 participants provided saliva samples; however, 25 participants provided blood samples. Six participants requested to voluntarily skip the blood draw due to needle phobia.
3.1. Salivary markers
With respect to mean S-CORT levels, no gender time interaction was noted (p = 0.132). However, there was a significant main effect for gender (F = 32.2, p < 0.001, ηp2 = 0.27) and time (F = 8.0, p < 0.001, ηp2 = 0.21). S-CORT concentrations were significantly lower at 5- and 30-min post ASD compared to pre ASD (p < 0.01), and overall, concentrations were significantly higher in men compared to women (p < 0.01). S-CORT data are shown in Fig. 1. The average intra- and inter-assay %CV for this assay was 7.2 and 6.1% respectively.
Fig. 1.
Data are shown as mean ± SD. Changes in salivary cortisol across time for men and women. ASD = active shooter training drill. (−30) = 30 min prior to ASD, (−5) = 5 min prior to ASD, (+5) = five min post ASD, (+30) = 30 min post ASD. ∗Denotes significantly (p < 0.05) lower values post ASD compared to pre ASD, and “Ω” indicates a main effect for gender with significantly (p < 0.001) lower S-CORT concentrations in women compared to men.
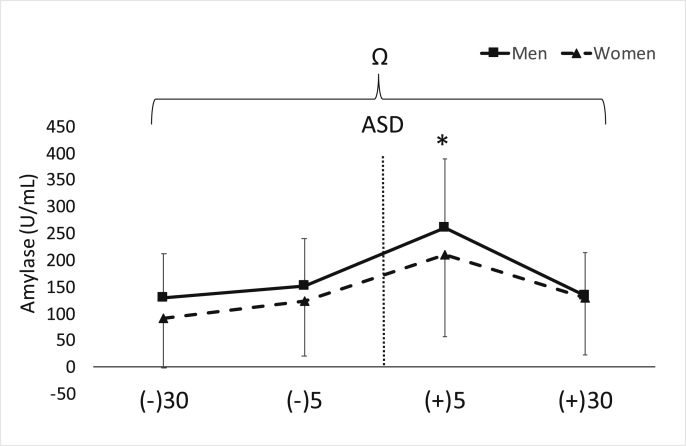
No gender time interaction was found for α-amylase (p = 0.58). However, there was a main effect for gender (F = 6.23, p = 0.01, ηp2 = 0.06) and time (F = 21.6, p < 0.001, ηp2 = 0.43). Concentrations of α-amylase were significantly higher at 5-min post ASD compared to all other timepoints (p < 0.01), and overall, men demonstrated significantly higher levels (p = 0.01). Data for α-amylase are shown in Fig. 2. The average intra- and inter-assay %CV for this assay was ~4.5 and 5.0% respectively.
Fig. 2.
Data are shown as mean ± SD. Changes in salivary α-amylase across time. ASD = active shooter training drill. (−30) = 30 min prior to ASD, (−5) = 5 min prior to ASD, (+5) = five min post ASD, (+30) = 30 min post ASD. ∗Denotes significant (p < 0.05) increase for both men and women compared to all other time points and “Ω” indicates a significant (p = 0.01) main effect for gender with higher concentrations in men compared to women.
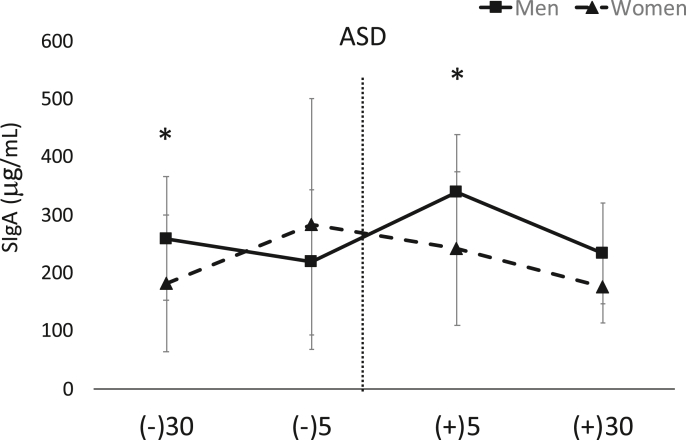
In terms of mean SIgA levels, a significant gender time interaction was found (F = 3.7, p = 0.01, ηp2 = 0.11). Post hoc analysis demonstrated significantly higher concentrations in men compared to women 30-min pre ASD (p = 0.04) as well as 5-min post ASD (p = 0.01). Data showing changes across time between genders are shown in Fig. 3. The average intra- and inter-assay % CV for this assay was ~5.9 and 6.1% respectively.
Fig. 3.
Data are shown as mean ± SD. Changes in salivary secreted immunoglobulin A (SIgA) across time. ASD = active shooter training drill. (−30) = 30 min prior to ASD, (−5) = 5 min prior to ASD, (+5) = five min post ASD, (+30) = 30 min post ASD. ∗Denotes significant (p < 0.05) gender × time interaction for −30 and + 5 timepoints whereby men showed significantly higher concentrations.
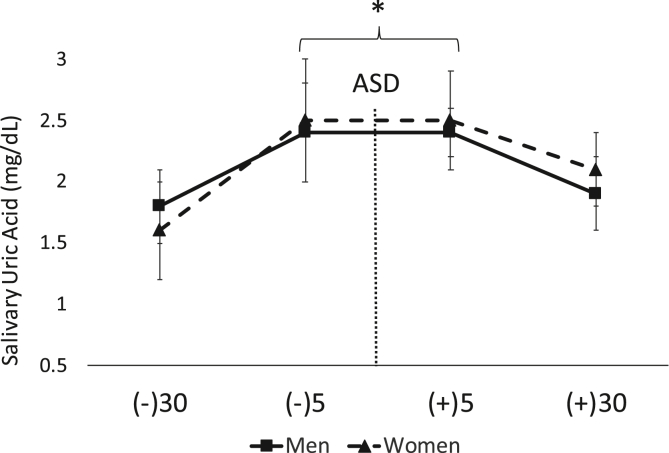
No gender time interaction was found for UA concentrations (p = 0.41). There was no main effect for gender (p = 0.83). However, there was a main effect for time (F = 6.69, p = 0.001, ηp2 = 0.17). A significant increase in salivary UA was noted from 30-min pre to 5-min pre ASD (p < 0.01) and 5-min post ASD (p < 0.01). Further, there was a significant decrease in UA concentrations from 5-min post ASD to 30-min post ASD (p = 0.01). These results demonstrate an increase due to anticipation of the ASD since concentrations were significantly elevated at 5-min pre ASD. Salivary UA concentrations and shown in Fig. 4. The average intra- and inter-assay % CV was ~10 and 11% respectively.
Fig. 4.
Data are shown as mean ± SD. Changes in uric acid (UA) across time. ASD = active shooter training drill. (−30) = 30 min prior to ASD, (−5) = 5 min prior to ASD, (+5) = five min post ASD, (+30) = 30 min post ASD. ∗Denotes significantly (p < 0.05) higher UA values −5 and +5 compared to −30 and + 30.
3.2. Blood EPI, NE, & H2O2
With respect to mean EPI concentrations, no gender time interaction was found (p = 0.93). Further, no main effect for gender was noted (p = 0.19). However, there was a main effect for time (F = 7.7, p = 0.01, ηp2 = 0.25). Post hoc analysis demonstrated a significant increase from pre to post ASD (p = 0.01). Mean EPI concentrations are shown in Fig. 5A.
Fig. 5.
A and 5B. Data are shown as mean ± SD. Changes in blood epinephrine (5A) and norepinephrine (5B) levels for men and women before and after the active shooter training drill (ASD). (−15) = 15 min before the ASD, (+15) = 15 min after the ASD. ∗Denotes a significant (p < 0.05) increase from 15 min pre to 15 min post ASD for epinephrine (5A) but not norepinephrine (5B).
In terms of mean NE concentrations, no gender time interaction was found (p = 0.20). No main effect for gender was noted (p = 0.09), and the main effect for time approached significance (p = 0.06). Mean NE concentrations for men and women are shown in Fig. 5B. The average %CV for EPI and NE was 20%.
Finally, in terms of mean H2O2 concentrations, there was no gender time interaction (p = 0.10) or main effect for time (p = 0.60). However, there was a main effect for gender (F = 27.7, p < 0.001, ηp2 = 0.54). Post hoc analysis indicated women demonstrate significantly lower concentrations of H2O2 (p < 0.01). Plasma H2O2 concentrations are shown in Fig. 6. The average intra-and inter-assay %CV for H2O2 was ~4.9 and 5.7% respectively.
Fig. 6.
Data are shown as mean ± SD. Changes in plasma hydrogen peroxide (H2O2) levels for men and women before and after the active shooter training drill (ASD). (−15) = 15 min before the ASD, (+15) = 15 min after the ASD. “Ω” Denotes a significant (p < 0.001) difference between men and women with women demonstrating lower concentrations of H2O2.
4. Discussion
The main findings of this study suggest higher levels of physiological stress in men compared to women when exposed to acute psychological stress in the form of a ~50 second long ASD. These findings are supported by lower S-CORT, α-amylase, UA, and H2O2 concentrations in women compared to men. Salivary concentrations of SIgA were also lower post ASD in women compared to men. Both men and women demonstrated significant increases in EPI in response to the ASD. Finally, it is important to note that the anticipation of ASD resulted in a significant increase in oxidative stress in both men and women as measured by UA. Overall sex differences were found in four of the seven stress markers, suggesting generally lower stress levels in women compared to men. In addition, the significant gender time interaction in relation to mean SIgA levels indicate a lower response to stress in women compared to men. The differences in these biomarkers in women compared to men should be considered in future work seeking to study physiological impacts of psychological stress. The implications in relation to risk for developing CVD are also significant given the role of stress and oxidative stress in CVD progression.
Individuals working in high stress occupations (firefighters, law enforcement & military personnel, etc.) are regularly exposed to psychological and physiological stressors. Chronic exposure to such stressors increases risk for CVD progression due to the increase in stress hormones and oxidative stress [18]. In fact, law enforcement officers have been shown to demonstrate aspects of CVD including hypertension, diabetes, and hypercholesterolemia [11,41]. It is important to note that much of the risk of CVD progression is attributed to stress induced oxidative stress and inflammation [18]. Indeed, psychological and physiological stress results in significant increases in concentrations of biomarkers of stress which can lead to oxidative stress [17]. The current findings are meaningful in that they demonstrate that acute exposure to brief (~50 sec) psychological stress in the form of an ASD results in significant increases in blood and salivary stress and oxidative stress markers, and those markers are attenuated in women. Therefore, women working in high stress tactical occupations may have attenuated risk of developing stress-induced cardiovascular dysfunction. It should be noted that past work has shown higher levels of reported stress in female LEOs and it has been speculated that female LEOs may be at greater risk for developing CVD due to greater potential for perceived stress [42]. It is possible that women in the general population react differently to acute stress than female LEOs. These conflicting results warrant additional work in the areas of stress and CVD in male and female LEOs.
In terms of differences between men and women’s physiological response to stress, both human and animal trials have consistently demonstrated sex differences [39]. In relation to rodent studies, female rats demonstrate higher average daily concentrations of corticosterones compared to male rats [7]. Moreover, acute stress exposure has been shown to cause greater concentrations of corticosterones, cortisol, and adrenocorticotropic hormone activity in female compared to male rats [1,14]. As discussed by Ref. [1]; rodent studies consistently show greater physiological responses to stress in rodent trials; however, human trials provide equivocal results. The present findings are in line with past work showing higher cortisol concentrations in response to acute stress in men compared to women [6,23]. However, these sex differences in human trials are inconsistent [1]. It is important to note these findings may be impacted by age, as young men exhibit greater responses to stress compared to young women, while among older adults, women may demonstrate greater increases [34]. These sex differences are likely due to the role of female sex hormones in modulating the activity of the HPA axis [1]. In addition, glucocorticoid binding may be reduced in females due to fewer hypothalamic receptors [37]. Therefore, findings may also be impacted by menstrual phase [21]. For a more detailed discussion on the impact of androgenic and estrogenic hormones on the HPA axis, readers are directed to a recent review by Ref. [14]. While the present findings do not support past work showing greater HPA activation in response to acute stress in men compared to women [6,23], the present findings are similar in that generalized differences between men and women in terms of S-CORT are present during an ASD. Therefore, more human trials are needed to further examine these differences.
The present study sought to study the potential differences between men and women in terms of the biological response to acute psychological stress. However, it should be noted that the current study was limited by several factors. First, time of day for testing was not consistent for all participants and some of the measured analytes such as S-CORT can be impacted by circadian changes. In addition, menstrual cycle phase as well as oral contraceptive use can impact cortisol responses to stress [24]. These factors were not accounted for and should also be viewed as limitations. Age of participants is another factor that can impact such results; however, the age range of participants was similar in both male and females as both groups were college aged (mean ages: males = 23.1, females = 21). As this study seeks to understand stress response to a law enforcement centric ASD, it is worth noting that both groups are also demographically similar to law enforcement recruits (average age of recruits: 20–25; [40]. However, participants were not sworn law enforcement officers, they were undergraduate college students. It is possible that experienced law enforcement officers with experience in active shooter situations could exhibit lower baseline stress markers as well as potentially exhibit more extreme responses due to accumulated stress over their career. Finally, it is also important to consider that the sex of the victims may impact the emotional/stress response by the responding police officer. Future work should examine this hypothesis.
5. Conclusions
Several studies have investigated differences between men and women in relation to potential differences in stress markers and acute response to stress. Furthermore, extensive work has shown a relationship between psychological stress, oxidative stress, and risk for developing CVD. While it has been shown that women tend to be slightly protected against oxidative stress compared to men, the findings from the current study suggest acute psychological stress results in significant increases in salivary and blood stress markers as well as salivary oxidative stress in both men and women. However, most stress markers were lower in women compared to men. Given the role of stress and oxidative stress in CVD progression, these findings should be used as a template for future work to further study potential sex related differences in CVD progression especially among high stress tactical occupations.
Funding
The authors disclose receipt of partial financial support for the execution of this project: U.S. DOJ – Office of Community Oriented Policing Services (COPS Office) Award # 2019ASWXK001.
Author contributions
Conceptualization (MJM & MHM); Data Curation (MJM & MHM); Formal Analysis (MJM); Investigation (MJM & MHM); Methodology (MJM & MHM); Project Administration (MJM & MHM); Original Draft (MJM & MHM); Review & Editing (MJM & MHM).
Declaration of competing interest
None.
References
- 1.Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35(3):303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black P.H., Garbutt L.D. Stress, inflammation and cardiovascular disease. J. Psychosom. Res. 2002;52(1):1–23. doi: 10.1016/s0022-3999(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 3.Brown E.S., Varghese F.P., McEwen B.S. Association of depression with medical illness: does cortisol play a role? Biol. Psychiatr. 2004;55(1):1–9. doi: 10.1016/s0006-3223(03)00473-6. [DOI] [PubMed] [Google Scholar]
- 4.Calvert G.M., Merling J.W., Burnett C.A. Ischemic heart disease mortality and occupation among 16-to 60-year-old males. J. Occup. Environ. Med. 1999;41(11):960–966. doi: 10.1097/00043764-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Cohen S., Janicki-Deverts D., Miller G.E. Psychological stress and disease. J. Am. Med. Assoc. 2007;298(14):1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 6.Collins A., Frankenhaeuser M. Stress responses in male and female engineering students. J. Hum. Stress. 1978;4(2):43–48. doi: 10.1080/0097840X.1978.9934986. [DOI] [PubMed] [Google Scholar]
- 7.Critchlow V., Liebelt R.A., Bar-Sela M., Mountcastle W., Lipscomb H.S. Sex difference in resting pituitary-adrenal function in the rat. American Journal of Physiology-Legacy Content. 1963;205(5):807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- 8.Dubrow R., Burnett C.A., Gute D.M., Brockert J.E. Ischemic heart disease and acute myocardial infarction mortality among police officers. J. Occup. Med.: Official Publication of the Industrial Medical Association. 1988;30(8):650–654. doi: 10.1097/00043764-198808000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Faul F., Erdfelder E., Lang A.G., Buchner A. G∗ Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 10.Franke W.D., Collins S.A., Hinz P.N. Cardiovascular disease morbidity in an Iowa law enforcement cohort, compared with the general Iowa population. J. Occup. Environ. Med. 1998;40(5):441–444. doi: 10.1097/00043764-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Franke W.D., Ramey S.L., Shelley M.C. Relationship between cardiovascular disease morbidity, risk factors, and stress in a law enforcement cohort. J. Occup. Environ. Med. 2002;44(12):1182–1189. doi: 10.1097/00043764-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Golden S.H., Wand G.S., Malhotra S., Kamel I., Horton K. Reliability of hypothalamic–pituitary–adrenal axis assessment methods for use in population-based studies. Eur. J. Epidemiol. 2011;26(7):511–525. doi: 10.1007/s10654-011-9585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartley T.A., Burchfiel C.M., Fekedulegn D., Andrew M.E., Violanti J.M. Health disparities in police officers: comparisons to the US general population. Int. J. Emerg. Ment. Health. 2011;13(4):211. [PMC free article] [PubMed] [Google Scholar]
- 14.Heck A.L., Handa R.J. Sex differences in the hypothalamic–pituitary–adrenal axis’ response to stress: an important role for gonadal hormones. Neuropsychopharmacology. 2019;44(1):45–58. doi: 10.1038/s41386-018-0167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heim C., Ehlert U., Hellhammer D.H. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25(1):1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 16.Hope L. Evaluating the effects of stress and fatigue on police officer response and recall: a challenge for research, training, practice and policy. Journal of applied research in memory and cognition. 2016;5(3):239–245. [Google Scholar]
- 17.Huang C.J., Webb H.E., Evans R.K., McCleod K.A., Tangsilsat S.E., Kamimori G.H., Acevedo E.O. Psychological stress during exercise: immunoendocrine and oxidative responses. Exp. Biol. Med. 2010;235(12):1498–1504. doi: 10.1258/ebm.2010.010176. [DOI] [PubMed] [Google Scholar]
- 18.Huang C.J., Webb H.E., Zourdos M.C., Acevedo E.O. Cardiovascular reactivity, stress, and physical activity. Front. Physiol. 2013;4:314. doi: 10.3389/fphys.2013.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivković N., Božović Đ., Račić M., Popović-Grubač D., Davidović B. Biomarkers of stress in saliva. Acta Fac. Med. Naissensis. 2015;32(2):91–99. [Google Scholar]
- 20.Kajantie E., Phillips D.I. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Kirschbaum C., Kudielka B.M., Gaab J., Schommer N.C., Hellhammer D.H. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Kudielka B.M., Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Kudielka B.M., Hellhammer J., Hellhammer D.H., Wolf O.T., Pirke K.M., Varadi E., Pilz J., Kirschbaum C. Sex differences in endocrine and psychological responses to psychosocial stress in healthy elderly subjects and the impact of a 2-week dehydroepiandrosterone treatment. J. Clin. Endocrinol. Metab. 1998;83(5):1756–1761. doi: 10.1210/jcem.83.5.4758. [DOI] [PubMed] [Google Scholar]
- 24.Maki P.M., Mordecai K.L., Rubin L.H., Sundermann E., Savarese A., Eatough E., Drogos L. Menstrual cycle effects on cortisol responsivity and emotional retrieval following a psychosocial stressor. Horm. Behav. 2015;74:201–208. doi: 10.1016/j.yhbeh.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matos-Gomes N., Katsurayama M., Makimoto F.H., Santana L.L.O., Paredes-Garcia E., Becker M.A.D.Á., Dos-Santos M.C. Psychological stress and its influence on salivary flow rate, total protein concentration and IgA, IgG and IgM titers. Neuroimmunomodulation. 2010;17(6):396–404. doi: 10.1159/000292064. [DOI] [PubMed] [Google Scholar]
- 26.McAllister M.J., Martaindale M.H., Rentería L.I. Active shooter training drill increases blood and salivary markers of stress. Int. J. Environ. Res. Publ. Health. 2020;17(14):5042. doi: 10.3390/ijerph17145042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McEwen B.S. Stressed or stressed out: what is the difference? J. Psychiatry Neurosci. 2005;30(5):315. [PMC free article] [PubMed] [Google Scholar]
- 28.Petrakova L., Doering B.K., Vits S., Engler H., Rief W., Schedlowski M., Grigoleit J.S. Psychosocial stress increases salivary alpha-amylase activity independently from plasma noradrenaline levels. PloS One. 2015;10(8) doi: 10.1371/journal.pone.0134561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramey S.L., Downing N.R., Franke W.D. Milwaukee police department retirees: cardiovascular disease risk and morbidity among aging law enforcement officers. AAOHN J. 2009;57(11):448–453. doi: 10.3928/08910162-20091019-02. [DOI] [PubMed] [Google Scholar]
- 30.Ring C., Carroll D., Hoving J., Ormerod J., Harrison L.K., Drayson M. Effects of competition, exercise, and mental stress on secretory immunity. J. Sports Sci. 2005;23(5):501–508. doi: 10.1080/02640410410001729955. [DOI] [PubMed] [Google Scholar]
- 31.Roelfsema F., Van den Berg G., Frölich M., Veldhuis J.D., Van Eijk A., Buurman M.M., Etman B.H. Sex-dependent alteration in cortisol response to endogenous adrenocorticotropin. J. Clin. Endocrinol. Metab. 1993;77(1):234–240. doi: 10.1210/jcem.77.1.8392084. [DOI] [PubMed] [Google Scholar]
- 32.Rohleder N. Stress and inflammation–The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology. 2019;105:164–171. doi: 10.1016/j.psyneuen.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz A.R., Gerin W., Davidson K.W., Pickering T.G., Brosschot J.F., Thayer J.F., Christenfeld N., Linden W. Toward a causal model of cardiovascular responses to stress and the development of cardiovascular disease. Psychosom. Med. 2003;65(1):22–35. doi: 10.1097/01.psy.0000046075.79922.61. [DOI] [PubMed] [Google Scholar]
- 34.Seeman T.E., Singer B., Wilkinson C.W., McEwen B. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 2001;26(3):225–240. doi: 10.1016/s0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 35.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138(3479) doi: 10.1176/jnp.10.2.230a. 32-32. [DOI] [PubMed] [Google Scholar]
- 36.Tsigos C., Chrousos G.P. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 37.Turner B.B. Sexual dimorphism of glucocorticoid binding in rat brain. Brain Res. 1985;343(1):16–23. doi: 10.1016/0006-8993(85)91153-9. [DOI] [PubMed] [Google Scholar]
- 38.Tzira D., Prezerakou A., Papadatos I., Vintila A., Bartzeliotou A., Apostolakou F., Papassotiriou I., Papaevangelou V. vol. 6. SAGE open medicine; 2018. (Salivary Biomarkers May Measure Stress Responses in Critically Ill Children). 2050312118802452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verma R., Balhara Y.P.S., Gupta C.S. Gender differences in stress response: role of developmental and biological determinants. Ind. Psychiatr. J. 2011;20(1):4. doi: 10.4103/0972-6748.98407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Violanti J.M., Hartley T.A., Gu J.K., Fekedulegn D., Andrew M.E., Burchfiel C.M. Life expectancy in police officers: a comparison with the US general population. Int. J. Emerg. Ment. Health. 2013;15(4):217. [PMC free article] [PubMed] [Google Scholar]
- 41.Williams M.A., Petratis M.M., Baechle T.R., Ryschon K.L., Campain J.J., Sketch M.H. Frequency of physical activity, exercise capacity, and atherosclerotic heart disease risk factors in male police officers. J. Occup. Med.: official publication of the Industrial Medical Association. 1987;29(7):596–600. [PubMed] [Google Scholar]
- 42.Yoo H., Franke W.D. Stress and cardiovascular disease risk in female law enforcement officers. Int. Arch. Occup. Environ. Health. 2011;84(3):279–286. doi: 10.1007/s00420-010-0548-9. [DOI] [PubMed] [Google Scholar]