Abstract
Recently, it has become clear that the tumour microenvironment (TME) is important in cancer immunotherapy. While immune checkpoint inhibitors are effective for some patients, the heterogeneous nature and status of the TME (‘cold’ tumours) play a critical role in suppressing antitumour immunity in non-responding patients. Converting ‘cold’ to ‘hot’ tumours through modulation of the TME may enable expansion of the therapeutic efficacy of immunotherapy to a broader patient population. This paper describes advances in intratumoural immunotherapy, specifically activation of nucleic acid sensing pattern recognition receptors to modulate the TME.
Keywords: Intratumoral immunotherapy, Modulation of tumour microenvironment, Nucleic acid recognising pattern recognition receptors, RIG-I, STING, Toll-like receptors
Intratumoural immunotherapy with novel immune stimulatory agents, which activate nucleic acid-sensing pattern recognition receptors, modulate tumour microenvironment to exert local and anenestic tumour responses. This approach allows converting ‘cold’ to ‘hot’ tumours and potentiate immune responses of checkpoint inhibitors.
Highlights
-
•
Intratumoural immunotherapy to modulate the tumour microenvironment.
-
•
Use of novel immunostimulatory agents which activate nucleic acid sensing pattern recognition receptors.
-
•
Harnessing innate and adaptive immunity induced by receptor-mediated immune cascade.
-
•
Intratumoural therapy leads to local and anenestic tumour responses.
Introduction
Over the last decade, immune checkpoint inhibitors (CPIs) have transformed cancer treatment. Antibodies against the key immunological checkpoints cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed death receptor-1 (PD-1)/programmed death receptor ligand-1 (PD-L1) have resulted in unprecedented durable antitumour responses with survival benefits across many cancer types [[1], [2], [3]]. However, only a minority (10–30%) of patients benefit from CPI treatment [4,5] and options for CPI relapsed or refractory patients are limited [6]. Could CPI efficacy be improved by rational combination with other agents that may potentiate T-cell activation? Intratumoural (IT) immunotherapy, where immune-activating agents are injected directly into the tumour to modulate the tumour microenvironment (TME) for antitumour T-cell priming, is a promising possibility.
The discovery of pattern recognition receptors (PRRs) and their role in activating innate and adaptive immune responses led to the development of novel immune-activating agents. These agents mimic pathogen-associated molecular patterns (PAMPs) to activate targeted receptor-mediated immune cascades [[7], [8], [9]]. This paper summarizes recent clinical data and ongoing trials of such agents.
Converting ‘cold’ to ‘hot’ tumours is beneficial for immunotherapy
Patients with a T-cell inflamed or ‘hot’ TME are likely to respond to CPI therapy, while those with a non-T-cell-inflamed or ‘cold’ TME require additional interventions to make the tumours susceptible. The TME contains cancerous cells as well as various immune cells, such as tumour-associated macrophages, T cells, B cells, dendritic cells and myeloid-derived suppressor cells. A complex interplay between antitumour immunity and immunosuppression regulates the balance between tumour growth and eradication. Growing evidence suggests that the TME can be manipulated by direct IT injection of immunostimulatory agents that increase T-cell infiltration. This should convert ‘cold’ tumours to ‘hot’ tumours that are responsive to treatment with CPIs. For example, IT immunotherapy with talimogene laherparepvec (TVEC) has been approved for treatment of unresectable melanoma [10,11], and trials are underway combining TVEC with pembrolizumab (anti-PD-1) in advanced melanoma [12].
Nucleic acid sensing PRRs
IT immunotherapy aims to create a suitable TME for optimal T-cell priming within the injected tumour, leading to systemic anenestic tumour response [13]. This could involve either the direct use of cytokines or the induction of immune responses via engaging PRRs expressed in immune cells present in the TME. Both T-cell- and B-cell-mediated antitumour responses can be generated.
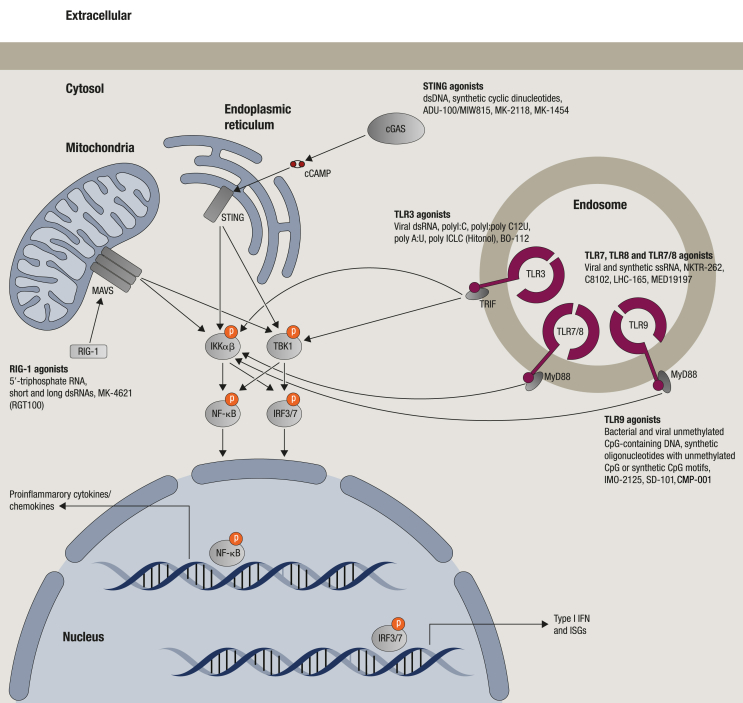
The role of individual components of bacteria and viruses in activating specific PRRs resulting in innate and adaptive immune responses has become clear [7]. These PRRs are evolutionarily conserved and expressed primarily by immune cells, but also by other cells. Specific receptors for various bacterial or viral components (e.g. lipopolysaccharide, lipopeptide, flagellin and nucleic acids) have been identified [7,8,14]. Nucleic acid sensing receptors can detect both pathogen- and host-derived nucleic acids and are broadly classified according to their location in endosomes or cytoplasm (Figure 1). For example, Toll-like receptors (TLRs) can be found in endosomal membranes, while RIG-I-like receptors (RLRs) and cyclic GMP-AMP synthase–stimulator of IFN genes (c-GAS-STING) are located in the cytoplasm [7] (Figure 1).
Figure 1.
The pattern recognition receptors (PRRs) that sense nucleic acids and their derivatives and brief signalling pathways. The natural ligands and clinical candidates for each receptor are shown. The clinical status of PRR activators and the combination agents for immuno-oncology are shown in Table 1 cGAMP, cyclic guanosine monophosphate–adenosine monophosphate; cGAS, cyclic GMP-AMP synthase; IFN, interferon; IKKα/β, inhibitor of nuclear factor κ-B kinase subunit α or β; IRF3/7, interferon regulatory factor 3 or 7; ISGs, interferon stimulatory genes; MyD88, myeloid differentiation factor 88; MAVS, mitochondrial antiviral-signalling protein; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; RIG-I, retinoic acid inducible gene I; STING, stimulator of interferon genes; TLR3/7/8/9, Toll-like receptor 3, 7, 8 or 9; TBK1, TANK binding kinase 1.
Of the 13 TLRs identified in mammals, TLR3, TLR7, TLR8, TLR9 and TLR13 detect distinct molecular patterns of RNA and DNA [7,8]. TLR3 is the receptor for viral and synthetic double-stranded RNAs [15]. TLR7 and TLR8 recognize single-stranded RNAs [16,17] but also small molecules like imidazoquinolines and certain nucleosides [18,19], while TLR9 is activated by unmethylated CpG motifs typically present in bacterial and viral DNA [20,21]. Although a human analogue has not been identified, TLR13 senses bacterial and viral 23S rRNA in mice [22,23].
TLRs are transmembrane receptors containing extracellular leucine-rich repeats and a cytoplasmic Toll/IL-1R (TIR) domain [24,25]. Activation results in TLR dimerization through the TIR domain and recruitment of adapter proteins. For TLR3, these are TIR domain-containing adapter-inducing IFN-β protein [26,27] and TNF receptor-associated factor 6 (TRAF6) [28]. Of note, the widely used ‘TLR3 agonist’ polyinosinic-polycytidylic acid (poly I:C) also activates the cytosolic RNA helicases retinoic acid inducible protein I (RIG-I) and melanoma differentiation-associate gene 5 (MDA5) [29]. In general, TLR3 agonists induce the cytokines interferon (IFN)-β, tumour necrosis factor (TNF)-α, interleukin (IL)-6 and CXCL10.
In contrast to TLR3, TLR7, TLR8 and TLR9 utilize the myeloid differentiation factor 88 as an adapter molecule [30]. Subsequent assembly of a large helical oligomer called the ‘myddosome’ [31] leads to engagement of IL-1-associated kinase, TLR-specific additional adapter proteins and TRAF6. Depending on the nature of the PAMP encountered and TLR activated, the transcription factors AP-1, ELK1, NF-κB or interferon regulatory factors are activated [30]. In general, TLR7 and TLR9 induce genes encoding Th1-type cytokines, such as IL-6, IL-12, TNFα, chemokines and type I IFNs [7,9]. However, the exact cytokine profile depends on the compound. Activation of TLR8 leads to mostly IL12, TNF, IL-6 and minimal IFN.
The cytosolic RIG-I senses viral RNA [32,33] with a 5′-triphosphate (5′-ppp) moiety combined with short blunt-ended double-stranded RNA (dsRNA) stretches; critical patterns that discriminate non-self from self RNA [34,35]. Activation of RIG-I and MDA5 leads to type I IFN gene expression [36]. The cGAS–STING pathway detects the presence of cytosolic DNA leading to expression of inflammatory genes, including type 1 IFN [[37], [38], [39], [40], [41], [42]].
This increased understanding of nucleic acid sensing PRRs has allowed the development of novel immunostimulatory agents that can trigger receptor-mediated immune cascades. Table 1 lists the status of all nucleic acid sensing PRR agonists currently in clinical trials following IT immunotherapy. However, this paper will only discuss compounds for which clinical data are available in more detail.
Table 1.
Clinical trials of intratumoural immunotherapy of compounds that activate nucleic acid sensing pattern recognition receptors (PRRs) alone or in combination with checkpoint inhibitors.
| PRR activator | Combination agent | Patient population | Clinical stage | Clinical trial number/status |
|---|---|---|---|---|
| TLR9 agonistf | ||||
| IMO-2125 | Ipilimumab | Anti-PD-1 refractory melanoma | Phase 3 | NCT03445533a |
| Ipilimumab or pembrolizumab | Metastatic melanoma | Phase 1/2 | NCT02644967b | |
| Ipilimumab + nivolumab | Solid tumours | Phase 2 | NCT03865082c | |
| Monotherapy | Melanoma, advanced solid tumours | Phase 1b | NCT03052205b | |
| SD-101 | Pembrolizumab | Metastatic melanoma, recurrent/metastatic SCCHN | Phase 1b/2 | NCT02521870b |
| Pembrolizumab + radiation | Hormone-naive OMPC | Phase 2 | NCT03007732a | |
| BMS986178 (anti-OX40 antibody) | Advanced solid malignancies | Phase 1 | NCT03831295a | |
| BMS986178 + radiation | Low-grade B-cell NHL | Phase 1 | NCT03410901a | |
| Epacadostat + radiation | Advanced solid tumours and lymphoma | Phase 1/2 | NCT03322384a | |
| Ipilimumab + radiation | MALT lymphoma, NMZL, MZL, SLL, grade 1/2 FL and SMZL | Phase 1/2 | NCT02254772d | |
| Pembrolizumab | Breast cancer | Phase 2 | NCT01042379a | |
| CMP-001 | Pembrolizumab | Melanoma | Phase 1 | NCT02680184a |
| Pembrolizumab | Advanced melanoma | Phase 1b | NCT03084640a | |
| Nivolumab | Melanoma with lymph node disease | Phase 2 | NCT03618641a | |
| Radiosurgery + nivolumab + ipilimumab | Metastatic CRC | Phase 1 | NCT03507699a | |
| Atezolizumab | NSCLC | Phase 1 | NCT03438318a | |
| AST-008f | Pembrolizumab | Advanced/metastatic melanoma, MCC, SCCHN, cSCC and solid tumours | Phase 1b/2 | NCT03684785a |
| MGN1703f | Ipilimumab | Melanoma, advanced solid tumours | Phase 1 | NCT02668770a |
| TLR3 agonist | ||||
| Poly-ICLC (Hiltonol) | Monotherapy | Prostate cancer | Phase 1 | NCT03262103a |
| Tremelimumab/durvalumab (MEDI4736) | SCCHN, MCC, CTCL, breast cancer, melanoma, renal cancer, bladder cancer, prostate cancer, testicular cancer and other solid tumours | Phase 1/2 | NCT02643303a | |
| Monotherapy (in situ vaccination) | Melanoma, head and neck cancer, sarcoma and non-melanoma skin cancers | Phase 2 | NCT02423863a | |
| Pembrolizumab | MRP colon cancer | Phase 1/2 | NCT02834052a | |
| BO-112g | Pembrolizumab, nivolumab | Aggressive solid tumours | Phase 1 | NCT02828098b |
| TLR7/8 agonists | ||||
| NKTR-262g (TLR7/8) | Peg-CD-122 agonist (NKTR-214) + nivolumab | Melanoma, MCC, TNBC, RCC, CRC, ovarian cancer, urothelial carcinoma and sarcoma | Phase 1/2 | NCT03435640a |
| CV8102g,h (TLR7/8/RIG-I) | Anti-PD-1 antibody | Advanced melanoma, SCC, SCCHN or ACC | Phase 1 | NCT03291002a |
| LHC-165 (TLR7) | Spartalizumab | Solid tumours | Phase 1/1b | NCT03301896a |
| MEDI9197 (TLR7/8) | Durvalumab | CTCL and solid tumours | Phase 1 | NCT02556463e |
| RIG-I agonist | ||||
| RGT100 (MK-4621) | Monotherapy | Advanced solid tumours | Phase 1/2 | NCT03065023e |
| Pembrolizumab | Advanced solid tumours | Phase 1/1b | NCT03739138a | |
| STING agonist | ||||
| ADU-S100/MIW815 | Spartalizumab | Advanced/metastatic solid tumours or lymphomas | Phase 1 | NCT03172936a |
| Pembrolizmab | Metastatic/recurrent SCCHN | Phase 2 | NCT03937141a | |
| Monotherapy and with ipilimumab | Advanced/metastatic solid tumours and lymphomas | Phase 1 | NCT02675439a | |
| MK-2118 | Monotherapy and with pembrolizumab | Advanced/metastatic solid tumours | Phase 1 | NCT03249792a |
| MK-1454 | Monotherapy and with pembrolizumab | Advanced/metastatic solid tumours or lymphomas | Phase 1 | NCT03010176a |
| SB 11285i | Nivolumab | Melanoma, SCCHN and solid tumours | Phase 1 | NCT04096638c |
| GSK3745417i | Monotherapy and with pembrolizumab | Solid tumours | Phase 1 | NCT03843359a |
ACC, adenoid cystic carcinoma; CRC, colorectal cancer; CTCL, cutaneous T-cell lymphoma; FL, follicular lymphoma; MALT lymphoma, extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue; MCC, Merkel cell carcinoma; MRP, mismatch repair proficient; MZL, marginal zone lymphoma; NHL, non-Hodgkin lymphoma; NMZL, nodal marginal zone B-cell lymphoma; NSCLC, non-small cell lung cancer; OMPC, oligometastatic prostate cancer; RCC, renal cell carcinoma; SCCHN, squamous cell carcinoma of head and neck; SLL, small lymphocytic lymphoma; SMZL, splenic marginal zone lymphoma; TNBC, triple negative breast cancer.
Recruiting.
Active, not recruiting.
Not yet recruiting.
Completed.
Terminated.
Additional TLR9 agonists in clinical trials include DV281 by inhalation, and IT AST-008 and MGN1703 are in early trials with no data reported yet.
Multiple modes of action.
Single-stranded RNA-based compound.
By intravenous administration.
TLR9 agonists
TLR9 expression is limited to plasmacytoid dendritic cells (pDCs) and B cells in humans, but is more widespread in rodent immune cells [43]. As discussed above, bacterial DNA or synthetic oligodeoxynucleotides (ODN) containing unmethylated CpG motifs are known agonists of TLR9. Although TLR9 was not discovered until 2000 [21], the first evidence of immune activation in humans by a CpG ODN was observed in the mid-1990s [44,45].
The cytokine profile resulting from TLR9 activation is dependent on the nucleotide sequence, chemical modifications of the DNA backbone, CpG flanking sequences and secondary structures of the agonist [9,[46], [47], [48], [49], [50]]. Various categories of TLR9 agonists have been synthesized and studied [9,47]. Compounds may preferentially activate pDCs, leading to the secretion of type I IFNs [51,52] or B cells, thus producing proinflammatory cytokines, IL-6, TNF and minimal type I IFNs [20,53]. Certain agonists activate both pDC and B cells, inducing secretion of IFN-α and other inflammatory cytokines [54,55].
In preclinical models, systemic treatment with TLR9 agonists (either alone or in combination with chemotherapeutic agents), targeted therapies and monoclonal antibodies showed very encouraging antitumour activity [9,47,48,[56], [57], [58], [59], [60], [61], [62], [63], [64]]. However, in clinical trials, systemic treatment with TLR9 agonists alone or in combination yielded disappointing results [[65], [66], [67], [68], [69], [70], [71], [72], [73]], although local treatment with TLR9 agonists may exert better antitumour activity [74,75]. For example, TLR9 agonists administered locally in melanoma patients after excision of the primary tumour showed improvements in recurrence-free survival for over 10 years in two phase II trials [76]. Thus, the focus shifted to IT application of TLR9 agonists. This mode of administration resulted in strong antitumour activity in the injected tumour in preclinical models [74,[77], [78], [79]], and – more importantly – systemic immune responses and anenestic antitumour activity. Responses were more potent when TLR9 agonists were combined with CPIs [75,[80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91]].
Marabelle et al. showed that IT immunotherapy with CpG ODN and anti-CTLA4 and/or anti-OX-40 agonists not only reduced tumour burden at the injected site but also distant tumours in mouse models of lymphoma and breast [75].
The TLR9 agonist tilsotolimod (IMO-2125) was evaluated in preclinical models of colon, lung and pancreatic cancer, and melanoma [[83], [84], [85], [86], [87], [88], [89]]. IT immunotherapy with IMO-2125 led to dose-dependent growth inhibition of the injected lesion as well as anenestic tumours. Antitumour activity was associated with increased CD3+ tumour infiltrating lymphocytes, CD8+ T cells and CTL responses against tumour antigens [82,83]. Several immune checkpoint genes showed increased expression (IDO-1, PD-L1, TIM3, LAG3 and CTLA4) in both treated and distant tumours [82,85]. IMO-2125 in combination with anti-CTLA4 [[82], [83], [84],89], anti-PD-1 [86], IDO-1 inhibitor [87] or both anti-PD1 and IDO-1 inhibitor [88] showed more potent antitumour activity than any agent alone.
Similarly, IT injection of another TLR9 agonist, SD-101, in the anti-PD-1 non-responder colon cancer model CT-26 resulted in complete durable responses in all injected tumours and a majority of distant tumours [81]. IT immunotherapy of a TLR9 agonist in combination with anti-OX40 antibody or anti-CTLA4 has also shown very potent antitumour activity [75,91].
Several TLR9 agonists are currently in clinical trials, either alone or in combination with CPIs (see Table 1). These are the ODNs SD-101, CMP-001 and IMO-2125, although each contains different features. SD-101 contains phosphorothioate backbone modifications, CpG motifs and palindromic sequences that allow formation of duplex and/or hairpin structures [92]. CMP-001 (previously known as ‘CYT003/QbG10’) is an ODN containing CpG motifs and regions of poly-dG nucleotides, formulated within a virus-like particle [93]. IMO-2125 contains synthetic immunostimulatory motifs and two 5′ ends for increased metabolic stability, and forms double-stranded structures [9,47,48].
There are limited clinical data using IT-delivered TLR9 agonists alone. In a clinical trial of IMO-2125 monotherapy, patients with histologically or cytologically confirmed metastatic refractory solid tumours were injected with doses of 8, 16, 23 or 32 mg into a single lesion. Treatment was well tolerated, and no dose-limiting toxicities or treatment-related adverse events were observed. Flow cytometry of tumour biopsies from two of the three analysed patients showed HLA-DR (major histocompatibility complex class II) upregulation at 24 h compared with pre-treatment. Robust activation and upregulation of the type I IFN pathway was demonstrated by increased expression of IFN and IFN-related genes. There were no clinical responses, although 13 of the 29 (45%) evaluable patients had stable disease according to RECIST v1.1 [[94], [95], [96]].
However, several studies using TLR9 agonists in combination with CPIs are in progress, including a phase 1/2 trial of IMO-2125 in combination with ipilimumab or pembrolizumab in patients with PD-1 refractory metastatic melanoma. Over 12 weeks, six doses of 4, 8, 16 or 32 mg IMO-2125 were injected intratumourally into single lesions while patients were treated with the standard dose of ipilimumab or pembrolizumab from the second week onwards. Patients showed a strong type 1 IFN gene signature, macrophage influx and robust dendritic cell maturation 24 h post injection of IMO-2125 alone, before standard CPI treatment was initiated. The combination therapy induced strong CD8+ T-cell proliferation and activation that was preferential to the tumour and not present in peripheral blood mononuclear cells. In responding patients, the main T-cell clones expanding on therapy were shared between local and distant lesions, indicating that priming/reactivation was to a shared antigen [97,98].
Based on these clinical findings, the 8-mg dose in combination with the standard dosing regimen for ipilimumab was selected for a phase 2 trial. The interim data from this trial show a 32.4% overall response rate (ORR) for the first 34 (of 52 to date) patients, including 9% (N = 3) who achieved a complete response (CR), 24% (N = 8) with a partial response (PR) and 76.5% (N = 26) who achieved disease control [97,98].
A phase 3 trial of IMO-2125 in combination with ipilimumab versus ipilimumab alone in PD-1 refractory metastatic melanoma with ORR and overall survival as primary endpoints is ongoing (Table 1). There is also a phase 2 trial combining IMO-2125 with both nivolumab and ipilimumab for the treatment of recurrent/metastatic squamous cell carcinoma of the head and neck and microsatellite stable colorectal cancer (Table 1).
SD-101 is being studied in multiple trials in combination with pembrolizumab. In advanced melanoma patients naïve to anti-PD-1/L1 therapy, 47 patients received ≤2 mg of SD-101 in one to four lesions and 40 patients received 8 mg in a single lesion along with standard pembrolizumab dosing. The results showed 70% and 48% ORR in patients who had received ≤2 mg in one to four lesions and a single 8-mg dose of SD-101, respectively. The combination was well tolerated, with adverse events related to SD-101 being transient, mild-to-moderate flu-like symptoms [99]. In 23 PD-1/PD-L1-resistant melanoma patients who received 2 mg of SD-101 intratumourally per lesion in one to four lesions (weekly x 4 doses followed by q3w x 7) with pembrolizumab, the best overall response was 88% with PD, 8% PR/CR and 4% stable disease [92,99]. The results in the PD-1/PD-L1-resistant patients are somewhat disappointing considering a recent report on response rates in this patient population [6].
Interim data of IT-administered SD-101 in combination with pembrolizumab in patients with squamous cell carcinoma of the head and neck from an ongoing phase 1b/2 study showed a 40% ORR in evaluable patients (N = 10) (four PR, one stable disease, and five PD). Biomarker analyses showed induction of broad immune activity, including increases in CD8+ T cells and a Th1 response in the TME [100]. Additionally, IT SD-101 in combination with pembrolizumab is being studied in a phase 2 trial for breast cancer (Table 1).
Interim results of CMP-001 in patients with advanced melanoma resistant to anti-PD-1 checkpoint inhibition have been reported [101]. CMP-001 was administered at doses of 1, 3, 5, 7.5 or 10 mg in two dosing schedules (weekly for 7 weeks, followed by q3w; or weekly for 2 weeks, followed by q3w). The combination of CMP-001 and pembrolizumab was generally well tolerated. Results in 69 patients showed 22.0% ORR (15/69, 95% confidence interval 13–33%), including two CR and 13 PR. Serum levels of IP-10, a chemokine induced by IFNs, were increased, as were CD8+ T cells and PD-L1 expression in injected and non-injected post-treatment biopsies [101].
TLR3 agonists
TLR3 is expressed in the endosomes of human myeloid dendritic cells, monocytes and natural killer cells [102,103]. TLR3 recognizes dsRNA and synthetic nucleic acid polymers such as poly I:C [15]. Several analogues of poly I:C, including poly I:poly C12U [104] and poly A:U [15], as well as formulations of poly I:C with poly-L-lysine-carboxymethylcellulose (poly-ICLC) [105], polyarginine (BO-112) [106] or kanamycin-calcium ions [107], have been used as TLR3 agonists. Synthetic TLR3 agonists of defined length have also been reported [108]. These TLR3 agonists have mainly been studied as anti-infective and anticancer agents, and as vaccine adjuvants [[109], [110], [111], [112], [113], [114], [115], [116]], but none have evaluated IT TLR3 agonists in preclinical cancer models.
Poly-ICLC (also known as ‘Hiltonol’) and BO-112 have advanced into the clinic for IT immunotherapy (Table 1). As with poly I:C, BO-112 is an agonist of MDA5 and RIG-I in addition to TLR3. IT immunotherapy with BO-112 alone or in combination with anti PD-1 in 28 anti-PD-1 refractory cancer patients showed disease control rate in seven of 12 (58%) at 9–10 weeks and an objective response in two of 12 (17%; one melanoma, one renal cancer) [117].
In a case study, a patient with facial embryonal rhabdomyosarcoma was treated with IT administration of poly-ICLC followed by intramuscular poly-ICLC as maintenance therapy. Antitumour efficacy was observed and was correlated with induction of immune markers [118].
TLR7/8 agonists
In primates and humans, TLR7 is expressed in pDCs and B cells, and TLR8 is expressed in human myeloid dendritic cells and monocytes. In rodents, TLR8 is non-functional and TLR7 is expressed more widely in several types of immune cells. Known agonists of TLR7 and TLR8 are short synthetic single-stranded RNAs that mimic viral RNA segments [16,17], imidazoquinolines [Imiquimod, Aldara, 052; 852; S-34240; 3M-052 (injectable)/MEDI9197; 854A], and nucleoside analogues such as loxoribine, 7-thia-8-oxo-guanosine and 7-deazaguanosine [18,19]. Extensive structure-activity relationship studies have supported the design of potent agonists of TLR7, TLR8 or TLR7/8 [9,47,[119], [120], [121], [122], [123], [124]].
Compounds that activate both TLR7 and TLR8 have shown potent antitumour activity in preclinical cancer models. For example, IT immunotherapy of MEDI9197 induces innate and adaptive immunity in the injected tumour and tumour-draining lymph nodes [125]. Importantly, in cancer models that respond poorly to agents targeting either PD-L1 or CTLA-4, combination of these agents with MEDI9197 significantly improved antitumour activity [125]. Cellular depletion studies revealed that CD8+ T cells were required for therapeutic activity. NKTR-262, a TLR7/8 agonist resiquimod (R848) conjugate, in combination with NKTR-214 (CD122-biased cytokine agonist) has shown potent antitumour activity and associated changes in immune biomarkers [126].
MEDI9197 (3M-052), NKTR-262, LHC-165 (a benzonapthyridine-based compound adsorbed on aluminum hydroxide) and CV8102 (a single-stranded RNA complexed with a cationic peptide) are in early clinical studies (Table 1). In a dose escalation trial (0.005–0.055 mg; q4w) of MEDI9197 IT immunotherapy in patients with subcutaneous/cutaneous tumours, the maximum tolerated dose was 0.037 mg [127]. Immunohistochemistry in the 0.037-mg cohort showed an increase in CD8 (T cells), CD40 (myeloid and B cells), CD56 (natural killer cells) or PD-L1 (tumour and immune cells) 3 weeks after treatment initiation. RNAseq analysis of paired tumour biopsies also showed an increase in innate and adaptive immune-activation signatures [127]. LHC-165 is currently in a phase 1/1b trial in combination with PDR001 (spartalizumab), an anti PD-1 agent, while CV8102 is in a phase 1 trial in patients with advanced solid tumours either alone or in combination with systemic anti-PD antibody [[128], [129], [130]].
RIG-I agonists
RIG-I belongs to the RLR family of receptors and is broadly expressed in most cells, including haematopoietic and cancer cells. Other members of the RLR family are MDA5 and LGP2. RIG-I recognizes viral and synthetic blunt-ended dsRNA structures with a 5′-triphosphate group [131].
The RIG-I agonist RGT100, a synthetic oligoribonucleotide, induces cytokine expression including IFN-α and IFN-β. In preclinical models, IT immunotherapy with RGT100 led to antitumour activity in treated and untreated contralateral tumours, and induced a strong and durable type I IFN response. Depletion of natural killer cells blocked antitumour activity of RGT100 [132]. In a phase 1/2 study, 0.2, 0.4, 0.6 and 0.8 mg of MK-4621 (RGT100) were administered IT twice per week over a 4-week period in patients with advanced or recurrent tumours. There were no dose-limiting toxicities and the safety profile was favourable at all dose levels. Treatment with MK-4621 increased circulating chemokine levels in serum and expression of genes involved in IFN signalling in tumours. Interim data showed that four of 15 patients (26.7%) experienced best overall response of stable disease [133]. It is also under clinical evaluation in combination with pembrolizumab (Table 1), although no data have been announced.
cGAS-STING agonists
STING is expressed in the endoplasmic reticulum membranes of various epithelial and endothelial cells as well as in haematopoietic cells, T cells, dendritic cells and macrophages [134]. There are significant differences between mouse (mSTING) and human STING (hSTING). Thus, agonists of mSTING may not show any activity for hSTING, and compounds need to be optimized for hSTING activation [42,135,136]. STING is activated by endogenously generated or exogenously provided synthetic cyclic dinucleotides (CDNs), leading to the production of type 1 IFNs and other inflammatory cytokines [39]. Synthetic CDN analogues [41,137] and small molecules that act as agonists of STING are available [138].
In preclinical models, IT immunotherapy with the STING agonists ADU-S100 and MK-1454 induced regression of established tumours in mice, and generated systemic immune responses capable of rejecting distant metastases and providing long-lived immunologic memory [41,136,139]. Agonists of STING have also shown antitumour activity [[140], [141], [142]] in combination with radiation [139], vaccines [143] and CPIs [41,42,144].
Clinical trials of the STING agonists ADU-S100 (also referred to as ‘MIW815’), MK-2118 and MK-1454 via IT administration (Table 1), and GSK3745417 and SB 11285 via systemic administration are ongoing. A trial of ADU-S100 at doses of 50–3200 μg on days 1, 8 and 15 of a 28-day cycle enrolled 41 patients with more than 20 different types of cancer. No dose-limiting toxicities were reported [145], and ADU-S100 IT immunotherapy increased key systemic cytokines, including IL-6, MCP-1 and IFN-β, indicating activation of the STING pathway. Two of the 40 patients (5%) had a PR: one patient with Merkel cell carcinoma and one patient with parotid gland cancer with prior anti-PD-1 therapy [145].
Interim data from a dose escalation trial of MK-1454 in patients with advanced solid tumour or lymphoma data were presented recently. Doses were 10–3000 μg for monotherapy and 90–2000 μg in combination with intravenous injection of 200 mg pembrolizumab every 3 weeks [146]. There were no responses in the monotherapy arm, but 24% of patients (N = 6/25) in the combination arm had PRs with median reductions of 83% in the size of both injected and non-injected tumours. Twenty percent and 48% of patients in the monotherapy and combination arms achieved disease control, respectively, while treatment-related adverse events occurred in 82.6% (N = 19/23) and 82.1% (N = 23/28) of patients, respectively [146].
MK-2118 is being evaluated in a study as IT injection as monotherapy, and in combination with pembrolizumab or by subcutaneous injection in combination with pembrolizumab for the treatment of adults with advanced/metastatic solid tumours or lymphomas (Table 1).
Conclusion and future prospects
Despite many notable successes in the treatment of cancer, many patients treated with CPIs have either primary resistance or recurrence. Low tumour mutational burden or tumours ignored by the immune system may be the cause, particularly in patients who are refractory to CPI therapy [147]. IT immunotherapy using immune-activating agents that modulate the TME (acting as in situ vaccination) and generate or expand pre-existing antitumour T cells could overcome this resistance and potentiate the response to CPIs.
Compounds designed to activate nucleic acid sensing PRRs and thus receptor-mediated immune responses are a novel class of immune-activating agents. Based on the available data, the type of immune cascade induced depends on the nature of the compound, targeted PRR, immune cells in which the PRR is expressed, and the presence of these cells in the TME. Most compounds induce a milieu of cytokines, including type I IFNs and cell surface markers. IFNs play a critical role in modulating the TME; however, the function of IFNs may be impacted by the levels of other cytokines (e.g. TNF, IL-6, IL-10). While IT immunotherapy with these agents alone modulates the TME and causes some responses in the injected tumour lesion, monotherapy has not resulted in systemic responses. However, IT immunotherapy with nucleic acid sensing PRRs in combination with anti-PD1 or anti- CTLA-4 has resulted in both local and systemic immune responses as well as significant clinical responses. Translational data show the similarities of the IT-treated TME in the injected and distant tumour sites, clearly demonstrating systemic immune responses. Draining lymph nodes are likely to play a very important role; however, no data are available to date for lymph node biopsies from any of these studies.
Although IT immunotherapy was initially administered to peripheral or surface lesions alone, image-guided IT immunotherapy now allows injection of deep visceral lesions [148] for broader applicability.
Gaining an understanding of the dose and dosing regimen requirements of IT immunotherapy is important; the clinical data indicate that lower doses may be more effective than higher doses [92,[94], [95], [96], [97], [98],101]. This could be due to negative feedback between regulatory factors controlling inflammation. If so, it might be helpful to target such feedback regulatory factors to achieve maximal benefit of the agent. As discussed above, in the case of TLR9 agonists, inhibition of IDO-1 has been shown to potentiate efficacy [87,88]. Remaining questions also include frequency and duration of treatment, injection volume, site of injection, etc.
In conclusion, the discovery of PRRs has allowed the development of a novel class of immune-activating agents for IT immunotherapy of cancer. The first phase 3 trial of a TLR9 agonist, IMO-2125, in combination with ipilimumab, is in progress in patients with anti-PD1 refractory melanoma (Table 1). While the available data are encouraging, ongoing trials will further guide development to realize the full potential of these approaches. Combinations of IT innate immune modulator therapies with CPIs have the potential to revolutionize cancer treatments.
Acknowledgements
The authors are very appreciative of the contributions made by their former colleagues and collaborators whose names appear in the references cited herein. The authors thank Dr Petra Disterer for editorial assistance and Ms Beth Mellor for the figure.
Funding
None declared.
Declaration of interests
The authors have declared no conflicts of interest.
References
- 1.Szostak B., Machaj F., Rosik J., Pawlik A. CTLA4 antagonists in phase I and phase II clinical trials, current status and future perspectives for cancer therapy. Expert Opin Investig Drugs. 2019;28:149–159. doi: 10.1080/13543784.2019.1559297. [DOI] [PubMed] [Google Scholar]
- 2.Baraibar I., Melero I., Ponz-Sarvise M., Castanon E. Safety and tolerability of immune checkpoint inhibitors (PD-1 and PD-L1) in cancer. Drug Saf. 2019;42:281–294. doi: 10.1007/s40264-018-0774-8. [DOI] [PubMed] [Google Scholar]
- 3.Wolchok J. Putting the immunologic brakes on cancer. Cell. 2018;175:1452–1454. doi: 10.1016/j.cell.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Postow M.A., Callahan M.K., Wolchok J.D. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weichenthal M., Ugurel S., Leiter U.M., Satzger I., Kähler K.C., Welzel J., et al. Salvage therapy after failure from anti-PD-1 single agent treatment: a study by the German ADOReg melanoma registry. J Clin Oncol. 2019;37(suppl) Abstract no 9505. [Google Scholar]
- 7.Vanpouille-Box C., Giffnabb J.A., Galluzzi L. Pharmacological modulation of nucleic acid sensors – therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2019 doi: 10.1038/s41573-019-0043-2. [DOI] [PubMed] [Google Scholar]
- 8.O'Neill L.A., Golenbock D., Bowie A.G. The history of Toll-like receptors – redefining innate immunity. Nat Rev Immunol. 2013;13:453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 9.Agrawal S., Kandimalla E.R. In: Advances in nucleic acids therapeutics. Agrawal S., Gait M.J., editors. RSC Publishers; Cambridge: 2019. Synthetic agonists of Toll-like receptors and therapeutic applications; pp. 306–338. [Google Scholar]
- 10.Andtbacka R.H., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 11.Fukuhara H., Ino Y., Todo T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016;107:1373–1379. doi: 10.1111/cas.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun L., Funchain P., Song J.M., Rayman P., Tannenbaum C., Ko J., et al. Talimogene laherparepvec combined with anti-PD-1 based immunotherapy for unresectable stage III–IV melanoma: a case series. J Immunother Cancer. 2018;6:36. doi: 10.1186/s40425-018-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marabelle A., Andtbacka R., Harrington K., Melero I., Leidner R., de Baere T., et al. Starting the fight in the tumor: expert recommendations for the development of human intratumoral immunotherapy (HIT-IT) Ann Oncol. 2018;29:2163–2174. doi: 10.1093/annonc/mdy423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janeway C.A., Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 15.Alexopoulou L., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 16.Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S., et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 17.Diebold S.S., Kaisho T., Hemmi H., Akira S., Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1539. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 18.Hemmi H., Kaisho T., Takeuchi O., Sato S., Sanjo H., Hoshino K., et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 19.Lee J., Chuang T.H., Redecke V., She L., Pitha P.M., Carson D.A., et al. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proc Natl Acad Sci USA. 2003;100:6646–6651. doi: 10.1073/pnas.0631696100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieg A.M., Yi A.K., Matson S., Waldschmidt T.J., Bishop G.A., Teasdale R., et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 21.Hemmi H., Takeuchi O., Kawai T., Sato S., Sanjo H., Hoshino K., et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 22.Oldenburg M., Krüger A., Ferstl R., Kaufmann A., Nees G., Sigmund A., et al. TLR13 recognizes bacterial 23S rRNA devoid of erythromycin resistance-forming modification. Science. 2012;337:1111–1115. doi: 10.1126/science.1220363. [DOI] [PubMed] [Google Scholar]
- 23.Song W., Wang J., Han Z., Zhang Y., Zhang H., Wang W., et al. Structural basis for specific recognition of single-stranded RNA by Toll-like receptor 13. Nat Struct Mol Biol. 2015;22:782–787. doi: 10.1038/nsmb.3080. [DOI] [PubMed] [Google Scholar]
- 24.Lasker M.V., Nair S.K. Intracellular TLR signaling: a structural perspective on human disease. J Immunol. 2006;177:11–16. doi: 10.4049/jimmunol.177.1.11. [DOI] [PubMed] [Google Scholar]
- 25.Botos I., Segal D.M., Davies D.R. The structural biology of Toll-like receptors. Structure. 2011;19:447–459. doi: 10.1016/j.str.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowie A., O’Neill L.A. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J Leukoc Biol. 2000;67:508–514. doi: 10.1002/jlb.67.4.508. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto M., Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv Drug Del Rev. 2008;60:805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Shim J.H., Xiao C., Paschal A.E., Bailey S.T., Rao P., Hayden M.S., et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palchetti S., Starace D., De Cesaris P., Filippini A., Ziparo E., Riccioli A. Transfected poly(I:C) activates different dsRNA receptors, leading to apoptosis or immunoadjuvant response in androgen-independent prostate cancer cells. J Biol Chem. 2015;290:5470–5483. doi: 10.1074/jbc.M114.601625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenny E.F., O’Neill L.A. Signalling adaptors used by Toll-like receptors: an update. Cytokine. 2008;43:342–349. doi: 10.1016/j.cyto.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Kagan J.C., Magupalli V.G., Wu H. SMOCs: supramolecular organizing centres that control innate immunity. Nat Rev Immunol. 2014;14:821–826. doi: 10.1038/nri3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 33.Yoneyama M., Kikuchi M., Matsumoto K., Imaizumi T., Miyagishi M., Taira K., et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 34.Hornung V., Ellegast J., Kim S., Brzózka K., Jung A., Kato H., et al. 5'-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 35.Baum A., Sachidanandam R., García-Sastre A. Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proc Natl Acad Sci USA. 2010;107:16303–16308. doi: 10.1073/pnas.1005077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loo Y.-M., Gale M., Jr. Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishikawa H., Barber G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouyang S., Song X., Wang Y., Ru H., Shaw N., Jiang Y., et al. Structural analysis of the STING adaptor protein reveals a hydrophobic dimer interface and mode of cyclic di-GMP binding. Immunity. 2012;36:1073–1086. doi: 10.1016/j.immuni.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J., Sun L., Chen X., Du F., Shi H., Chen C., et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–830. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corrales L., Glickman L.H., McWhirter S.M., Kanne D.B., Sivick K.E., Katibah G.E., et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell Rep. 2015;11:1018–1030. doi: 10.1016/j.celrep.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conlon J., Burdette D.L., Sharma S., Bhat N., Thompson M., Jiang Z., et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J Immunol. 2013;190:5216–5225. doi: 10.4049/jimmunol.1300097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klinman D.M. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249–258. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 44.Agrawal S., Kandimalla E.R. Role of Toll-like receptors in antisense and siRNA. Nat Biotechnol. 2004;22:1533–1537. doi: 10.1038/nbt1042. [DOI] [PubMed] [Google Scholar]
- 45.Agrawal S., Martin R.R. Was induction of HIV-1 through TLR9? J Immunol. 2003;171:1621. doi: 10.4049/jimmunol.171.4.1621. [DOI] [PubMed] [Google Scholar]
- 46.Kandimalla E.R., Agrawal S. In: Toll receptors – an immunologic perspective. Rich T., editor. Kluwer Academic/Plenum Publishers; New York, NY: 2005. Agonists of Toll-like receptor 9. Modulation of host immune responses with synthetic oligodeoxynucleotides; pp. 181–212. [Google Scholar]
- 47.Kandimalla E.R., Agrawal S. Modulation of endosomal Toll-like receptor-mediated immune responses by synthetic oligonucleotides. Adv Polym Sci. 2012;249:61–94. [Google Scholar]
- 48.Kandimalla E.R., Bhagat L., Li Y., Yu D., Wang D., Cong Y.P., et al. Immunomodulatory oligonucleotides containing a cytosine-phosphate-2'-deoxy-7-deazaguanosine motif as potent Toll-like receptor 9 agonists. Proc Natl Acad Sci USA. 2005;102:6925–6930. doi: 10.1073/pnas.0501729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Struthers M., Bett A.J., Wisniewski T., Dubey S.A., Precopio M., Jiang W., et al. Synthesis and immunological activities of novel agonists of Toll-like receptor 9. Cell Immunol. 2010;263:105–113. doi: 10.1016/j.cellimm.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Yu D., Putta M.R., Bhagat L., Dai M., Wang D., Trombino A.F., Sullivan T., Kandimalla E.R., Agrawal S. Impact of secondary structure of Toll-like receptor 9 agonists on interferon alpha induction. Antimicrob Agents Chemother. 2008;52:4320–4325. doi: 10.1128/AAC.00701-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verthelyi D., Ishii K.J., Gursel M., Takeshita F., Klinman D.M. Human peripheral blood cells differentially recognize and respond to two distinct CPG motifs. J Immunol. 2001;166:2372–2377. doi: 10.4049/jimmunol.166.4.2372. [DOI] [PubMed] [Google Scholar]
- 52.Krug A., Rothenfusser S., Hornung V., Jahrsdörfer B., Blackwell S., Ballas Z.K., et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 53.Klinman D.M., Yi A.K., Beaucage S.L., Conover J., Krieg A.M. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hartmann G., Battiany J., Poeck H., Wagner M., Kerkmann M., Lubenow N., et al. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-alpha induction in plasmacytoid dendritic cells. Eur J Immunol. 2003;33:1633–1641. doi: 10.1002/eji.200323813. [DOI] [PubMed] [Google Scholar]
- 55.Marshall J.D., Fearon K., Abbate C., Subramanian S., Yee P., Gregorio J., et al. Identification of a novel CpG DNA class and motif that optimally stimulate B cell and plasmacytoid dendritic cell functions. J Leukoc Biol. 2003;73:781–792. doi: 10.1189/jlb.1202630. [DOI] [PubMed] [Google Scholar]
- 56.Wang H., Rayburn E.R., Wang W., Kandimalla E.R., Agrawal S., Zhang R. Chemotherapy and chemosensitization of non-small cell lung cancer with a novel immunomodulatory oligonucleotide targeting Toll-like receptor 9. Mol Cancer Ther. 2006;5:1585–1592. doi: 10.1158/1535-7163.MCT-06-0094. [DOI] [PubMed] [Google Scholar]
- 57.Wang D., Li Y., Yu D., Song S.S., Kandimalla E.R., Agrawal S. Immunopharmacological and antitumor effects of second-generation immunomodulatory oligonucleotides containing synthetic CpR motifs. Int J Oncol. 2004;24:901–908. [PubMed] [Google Scholar]
- 58.Melisi D., Frizziero M., Tamburrino A., Zanotto M., Carbone C., Piro G., et al. Toll-like receptor 9 agonists for cancer therapy. Biomedicines. 2014;2:211–228. doi: 10.3390/biomedicines2030211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H., Rayburn E.R., Wang W., Kandimalla E.R., Agrawal S., Zhang R. Immunomodulatory oligonucleotides as novel therapy for breast cancer: pharmacokinetics, in vitro and in vivo anticancer activity, and potentiation of antibody therapy. Mol Cancer Ther. 2006;5:2106–2114. doi: 10.1158/1535-7163.MCT-06-0158. [DOI] [PubMed] [Google Scholar]
- 60.Damiano V., Caputo R., Bianco R., D'Armiento F.P., Leonardi A., De Placido S., et al. Novel Toll-like receptor 9 agonist induces epidermal growth factor receptor (EGFR) inhibition and synergistic antitumor activity with EGFR inhibitors. Clin Cancer Res. 2006;12:577–583. doi: 10.1158/1078-0432.CCR-05-1943. [DOI] [PubMed] [Google Scholar]
- 61.Damiano V., Caputo R., Garofalo S., Bianco R., Rosa R., Merola G., et al. TLR9 agonist acts by different mechanisms synergizing with bevacizumab in sensitive and cetuximab-resistant colon cancer xenografts. Proc Natl Acad Sci USA. 2007;104:12468–12473. doi: 10.1073/pnas.0705226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Damiano V., Garofalo S., Rosa R., Bianco R., Caputo R., Gelardi T., et al. A novel Toll-like receptor 9 agonist cooperates with trastuzumab in trastuzumab-resistant breast tumors through multiple mechanisms of action. Clin Cancer Res. 2009;15:6921–6930. doi: 10.1158/1078-0432.CCR-09-1599. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H., Liu L., Yu D., Kandimalla E.R., Sun H.B., Agrawal S., et al. An in situ autologous tumor vaccination with combined radiation therapy and TLR9 agonist therapy. PLoS One. 2012;7 doi: 10.1371/journal.pone.0038111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saha S., Bhanja P., Liu L., Alfieri A.A., Yu D., Kandimalla E.R., et al. TLR9 agonist protects mice from radiation-induced gastrointestinal syndrome. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hirsh V., Paz-Ares L., Boyer M., Rosell R., Middleton G., Eberhardt W.E., et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:2667–2674. doi: 10.1200/JCO.2010.32.8971. [DOI] [PubMed] [Google Scholar]
- 66.Guha M. Anticancer TLR agonists on the ropes. Nat Rev Drug Disc. 2012;11:503–505. doi: 10.1038/nrd3775. [DOI] [PubMed] [Google Scholar]
- 67.Smith D.A., Conkling P., Richards D.A., Nemunaitis J.J., Boyd T.E., Mita A.C., et al. Antitumor activity and safety of combination therapy with the Toll-like receptor 9 agonist IMO-2055, erlotinib, and bevacizumab in advanced or metastatic non-small cell lung cancer patients who have progressed following chemotherapy. Cancer Immunol Immunother. 2014;63:787–796. doi: 10.1007/s00262-014-1547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Machiels J.P., Kaminsky M.C., Keller U., Brümmendorf T.H., Goddemeier T., Forssmann U., et al. Phase Ib trial of the Toll-like receptor 9 agonist IMO-2055 in combination with 5-fluorouracil, cisplatin, and cetuximab as first-line palliative treatment in patients with recurrent/metastatic squamous cell carcinoma of the head and neck. Invest New Drugs. 2013;31:1207–1216. doi: 10.1007/s10637-013-9933-z. [DOI] [PubMed] [Google Scholar]
- 69.Ruzsa A., Sen M., Evans M., Lee L.W., Hideghety K., Rottey S., et al. Phase 2, open-label, 1:1 randomized controlled trial exploring the efficacy of EMD 1201081 in combination with cetuximab in second-line cetuximab-naïve patients with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN) Invest New Drugs. 2014;32:1278–1284. doi: 10.1007/s10637-014-0117-2. [DOI] [PubMed] [Google Scholar]
- 70.Chan E., Kwak E.L., Hwang J., Heiskala M., de La Bourdonnaye G., Mita M. Open-label phase 1b study of FOLFIRI plus cetuximab plus IMO-2055 in patients with colorectal cancer who have progressed following chemotherapy for advanced or metastatic disease. Cancer Chemother Pharmacol. 2015;75:701–709. doi: 10.1007/s00280-015-2682-2. [DOI] [PubMed] [Google Scholar]
- 71.Manegold C., van Zandwijk N., Szczesna A., Zatloukal P., Au J.S., Blasinska-Morawiec M., et al. A phase III randomized study of gemcitabine and cisplatin with or without PF-3512676 (TLR9 agonist) as first-line treatment of advanced non-small-cell lung cancer. Ann Oncol. 2012;23:72–77. doi: 10.1093/annonc/mdr030. [DOI] [PubMed] [Google Scholar]
- 72.Weber J.S., Zarour H., Redman B., Trefzer U., O'Day S., van den Eertwegh A.J., et al. Randomized phase 2/3 trial of CpG oligodeoxynucleotide PF-3512676 alone or with dacarbazine for patients with unresectable stage III and IV melanoma. Cancer. 2009;115:3944–3954. doi: 10.1002/cncr.24473. [DOI] [PubMed] [Google Scholar]
- 73.Manegold C., Gravenor D., Woytowitz D., Mezger J., Hirsh V., Albert G., et al. Randomized phase II trial of a Toll-like receptor 9 agonist oligodeoxynucleotide, PF-3512676, in combination with first-line taxane plus platinum chemotherapy for advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3979–3986. doi: 10.1200/JCO.2007.12.5807. [DOI] [PubMed] [Google Scholar]
- 74.Lou Y., Liu C., Lizée G., Peng W., Xu C., Ye Y., et al. Antitumor activity mediated by CpG: the route of administration is critical. J Immunother. 2011;34:279–288. doi: 10.1097/CJI.0b013e31820d2a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marabelle A., Kohrt H., Sagiv-Barfi I., Ajami B., Axtell R.C., Zhou G., et al. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest. 2013;123:2447–2463. doi: 10.1172/JCI64859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koster B.D., van den Hout M.F.C.M., Sluijter B.J.R., Molenkamp B.G., Vuylsteke R.J.C.L.M., Baars A., et al. Local adjuvant treatment with low-dose CpG-B offers durable protection against disease recurrence in clinical stage I–II melanoma: data from two randomized phase II trials. Clin Cancer Res. 2017;23:5679–5686. doi: 10.1158/1078-0432.CCR-17-0944. [DOI] [PubMed] [Google Scholar]
- 77.Li J., Song W., Czerwinski D.K., Varghese B., Uematsu S., Akira S., et al. Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. J Immunol. 2007;179:2493–2500. doi: 10.4049/jimmunol.179.4.2493. [DOI] [PubMed] [Google Scholar]
- 78.Grauer O.M., Molling J.W., Bennink E., Toonen L.W., Sutmuller R.P., Nierkens S., et al. TLR ligands in the local treatment of established intracerebral murine gliomas. J Immunol. 2008;181:6720–6729. doi: 10.4049/jimmunol.181.10.6720. [DOI] [PubMed] [Google Scholar]
- 79.Shirota Y., Shirota H., Klinman D.M. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188:1592–1599. doi: 10.4049/jimmunol.1101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marabelle A., Kohrt H., Caux C., Levy R. Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res. 2014;20:1747–1756. doi: 10.1158/1078-0432.CCR-13-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang S., Campos J., Gallotta M., Gong M., Crain C., Naik E., et al. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc Natl Acad Sci USA. 2016;113:E7240–E7249. doi: 10.1073/pnas.1608555113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sato-Kaneko F., Yao S., Ahmadi A., Zhang S.S., Hosoya T., Kaneda M.M., et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight. 2017;2:93397. doi: 10.1172/jci.insight.93397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang D., Jiang W., Zhu F., Mao X., Agrawal S. Modulation of the tumor microenvironment by intratumoral administration of IMO-2125, a novel TLR9 agonist, for cancer immunotherapy. Int J Oncol. 2018;53:1193–1203. doi: 10.3892/ijo.2018.4456. [DOI] [PubMed] [Google Scholar]
- 84.Wang D., Zhu F.-G., Mao X., Agrawal S. Intratumoral administration of IMO-2125, a novel TLR9 agonist, modulates the tumor microenvironment and exerts systemic antitumor activity alone and in combination with an anti-CTLA-4 mAb. Cancer Immunol Res. 2016;4(Suppl. l) Abstract no B094. [Google Scholar]
- 85.Wang D., Mao X., Argon E.K., Zhu F.-G., Agrawal S. 2017. Intratumoral IMO-2125 treatment in combination with anti-CTLA-4 mAb induces durable anti-tumor responses associated with tumor-specific memory in preclinical studies, poster presented at the third CRI-CIMT-EATI-AACR International Cancer Immunotherapy Conference. Mainz, Germany, September 6–9. [Google Scholar]
- 86.Jiang W., Wang D., Zhu F.G., Bhagat L., DiMuzio J., Agrawal S. Modulation of checkpoint expression in tumor microenvironment by intratumoral administration of a novel TLR9 agonist: rationale for combination therapy. Cancer Immunol Res. 2016;4(Suppl. l) Abstract no B159. [Google Scholar]
- 87.Wang D., Zhu F.-G., DiMuzio J., Agrawal S. Intratumoral administration of IMO-2125, a novel TLR9 agonist, modulates tumor microenvironment and potentiates antitumor activity of anti-PD-1 mAb in a murine colon carcinoma model. Mol Cancer Ther. 2015;14(Suppl. 2) Abstract no B196. [Google Scholar]
- 88.Wang D., Jiang W., Bhagat L., DiMuzio J., Zhu F.-G., Agrawal S. Creating the tumor microenvironment for effective immunotherapy: antitumor activity of intratumoral IMO-2125, a TLR9 agonist is further enhanced by inhibition of indoleamine-pyrrole 2,3-dioxygenase (IDO) Cancer Res. 2016;76(Suppl. l) abstract no 3847. [Google Scholar]
- 89.Argon E.K., Zhu F.-G., Agrawal S., Yingling J., Wang D. Poster presentation at AACR annual meeting. 2018. Triple combination of IMO-2125, epacadostat and anti-PD-1 antibody demonstrates maximal antitumor efficacy and eradicates large established tumors in preclinical models. April 14–18, 2018, Chicago, IL. Abstract no. 4704. [Google Scholar]
- 90.Wang D., Argon E.K., Zhu F.-G., Agarwal S. Local treatment with novel TLR9 agonist IMO-2125 demonstrates antitumor activity in preclinical models of pancreatic cancer [abstract] Cancer Res. 2017;77(Suppl. l) Abstract no 5659. [Google Scholar]
- 91.Sagiv-Barfi I., Czerwinski D.K., Levy S., Alam I.S., Mayer A.T., Gambhir S.S., et al. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ribas A., Medina T., Kummar S., Amin A., Kalbasi A., Drabick J.J., et al. SD-101 in combination with pembrolizumab in advanced melanoma: results of a phase Ib, multicenter study. Cancer Discov. 2018;8:1250–1257. doi: 10.1158/2159-8290.CD-18-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Casale T.B., Cole J., Beck E., Vogelmeier C.F., Willers J., Lassen C., et al. CYT003, a TLR9 agonist, in persistent allergic asthma – a randomized placebo-controlled phase 2b study. Allergy. 2015;70:1160–1168. doi: 10.1111/all.12663. [DOI] [PubMed] [Google Scholar]
- 94.Haymaker C., Uemura M., Murthy R., James M., Wang D., Brevard J., et al. Presentation at SITC 2016 annual meeting, november 9–13. National Harbor; MD: 2016. Reactivating the anti-tumor immune response by targeting innate and adaptive immunity in a phase I/II study of intratumoral IMO-2125 in combination with systemic ipilimumab in patients with anti-PD-1 refractory metastatic melanoma. [Google Scholar]
- 95.Haymaker C.L., Uemura M., Murthy R., James M., Wang D., Brevard J., et al. Translational evidence of reactivated innate and adaptive immunity with intratumoral IMO-2125 in combination with systemic checkpoint inhibitors from a phase I/II study in patients with anti-PD-1 refractory metastatic melanoma. Cancer Res. 2017;77(Suppl. l) Abstract no 5652. [Google Scholar]
- 96.Haymaker C. Presentation at SITC 2017 annual meeting, november 8–12. National Harbor; MD: 2017. TLR9 agonist harnesses innate immunity to drive tumor-infiltrating T-cell expansion in distant lesions in a phase 1/2 study of intratumoralIMO-2125+ipilimumab in anti-PD1 refractory melanoma patients. [Google Scholar]
- 97.Diab A., Rahimian S., Haymaker C.L., Bernatchez C., Andtbacka R.H.I., James M., et al. A phase 2 study to evaluate the safety and efficacy of intratumoral (IT) injection of the TLR9 agonist IMO-2125 (IMO) in combination with ipilimumab (ipi) in PD-1 inhibitor refractory melanoma. J Clin Oncol. 2018;36(Suppl. 15):abs9515. [Google Scholar]
- 98.Diab A., Haymaker C., Hwu W.-J., Uemura M., Murthy R., James M., et al. Presented at the European society for medical oncology congress. September, 2017. A phase 1/2 trial of intratumoral (i.t.) IMO-2125 (IMO) in combination with checkpoint inhibitors (CPI) in PD-(L)1-refractory melanoma. [Google Scholar]
- 99.Milhem M.M., Long G.V., Hoimes C.J., Amin A., Lao C.D., Conry R.M., et al. Phase 1b/2, open label, multicenter, study of the combination of SD-101 and pembrolizumab in patients with advanced melanoma who are naïve to anti-PD-1 therapy. J Clin Oncol. 2019;37(Suppl. l):abs9534. [Google Scholar]
- 100.Cohen E., Bishnoi S., Laux D.E., Wong D., Amin A., Nabell L., et al. Phase Ib/II, open label, multicenter study of intratumoral SD-101 in combination with pembrolizumab in anti-PD-1 treatment naïve patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) Cancer Res. 2018;78(Suppl. l) Abstract no CT098. [Google Scholar]
- 101.Milhem M., Gonzales R., Medina T., Kirkwood J.M., Buchbinder E., Mehmi I., et al. Intratumoral Toll-like receptor 9 (TLR9) agonist, CMP-001, in combination with pembrolizumab can reverse resistance to PD-1 inhibition in a phase Ib trial in subjects with advanced melanoma. Cancer Res. 2018;78(Suppl. l) Abstract no CT144. [Google Scholar]
- 102.Matsumoto M., Funami K., Tanabe M., Oshiumi H., Shingai M., Seto Y., et al. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol. 2003;171:3154–3162. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- 103.Schmidt K.N., Leung B., Kwong M., Zarember K.A., Satyal S., Navas T.A., et al. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J Immunol. 2004;172:138–143. doi: 10.4049/jimmunol.172.1.138. [DOI] [PubMed] [Google Scholar]
- 104.Stahl-Hennig C., Eisenblätter M., Jasny E., Rzehak T., Tenner-Racz K., Trumpfheller C., et al. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boukhvalova M.S., Sotomayor T.B., Point R.C., Pletneva L.M., Prince G.A., Blanco J.C. Activation of interferon response through Toll-like receptor 3 impacts viral pathogenesis and pulmonary Toll-like receptor expression during respiratory syncytial virus and influenza infections in the cotton rat Sigmodon hispidus model. J Interferon Cytokine Res. 2010;30:229–242. doi: 10.1089/jir.2009.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Da Silva D.M., Woodham A.W., Rijkee L.K., Skeate J.G., Taylor J.R., Koopman M.E., et al. Human papillomavirus-exposed Langerhans cells are activated by stabilized Poly-I:C. Papillomavirus Res. 2015;1:12–21. doi: 10.1016/j.pvr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lau Y., Tang L., McCall A., Ooi E., Subbarao K. An adjuvant for the induction of potent, protective humoral responses to an H5N1 influenza virus vaccine with antigen-sparing effect in mice. J Virol. 2010;84:8639–8649. doi: 10.1128/JVI.00596-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lan T., Wang D., Bhagat L., Philbin V.J., Yu D., Tang J.X., et al. Design of synthetic oligoribonucleotide-based agonists of Toll-like receptor 3 and their immune response profiles in vitro and in vivo. Org Biomol Chem. 2013;11:1049–1058. doi: 10.1039/c2ob26946e. [DOI] [PubMed] [Google Scholar]
- 109.Glavan T.M., Pavelic J. The exploitation of Toll-like receptor 3 signaling in cancer therapy. Curr Pharm Des. 2014;20:6555–6564. doi: 10.2174/1381612820666140826153347. [DOI] [PubMed] [Google Scholar]
- 110.Martins K.A., Bavari S., Salazar A.M. Vaccine adjuvant uses of poly-IC and derivatives. Expert Rev Vaccines. 2015;14:447–459. doi: 10.1586/14760584.2015.966085. [DOI] [PubMed] [Google Scholar]
- 111.Lampkin B.C., Levine A.S., Levy H., Krivit W., Hammond D. Phase II trial of a complex polyriboinosinic-polyribocytidylic acid with poly-L-lysine and carboxymethyl cellulose in the treatment of children with acute leukemia and neuroblastoma: a report from the Children’s Cancer Study Group. Cancer Res. 1985;45:5904–5909. [PubMed] [Google Scholar]
- 112.Levine A.S., Sivulich M., Wiernik P.H., Levy H.B. Initial clinical trials in cancer patients of polyriboinosinic-polyribocytidylic acid stabilized with poly-L-lysine, in carboxymethylcellulose [poly(ICLC)], a highly effective interferon inducer. Cancer Res. 1979;39:1645–1650. [PubMed] [Google Scholar]
- 113.De Clercq E. Interferon: ten stories in one. A short review of some of the highlights in the history of an almost quinquagenarian. Acta Microbiol Immunol Hung. 2005;52:273–289. doi: 10.1556/AMicr.52.2005.2.6. [DOI] [PubMed] [Google Scholar]
- 114.Matsumoto M., Seya T. TLR3: interferon induction by double-stranded RNA including poly(I:C) Adv Drug Deliv Rev. 2008;60:805–812. doi: 10.1016/j.addr.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 115.Homan E.R., Zendzian R.P., Schott L.D., Levy H.B., Adamson R.H. Studies on poly I:C toxicity in experimental animals. Toxicol Appl Pharmacol. 1972;23:579–588. doi: 10.1016/0041-008x(72)90098-1. [DOI] [PubMed] [Google Scholar]
- 116.Robinson R.A., DeVita V.T., Levy H.B., Baron S., Hubbard S.P., Levine A.S. A phase I–II trial of multiple-dose polyriboinosic-polyribocytidylic acid in patients with leukemia or solid tumors. J Natl Cancer Inst. 1976;57:599–602. doi: 10.1093/jnci/57.3.599. [DOI] [PubMed] [Google Scholar]
- 117.Rodas I.M., Longo F., Rodriguez-Ruiz M., Calles A., Perez-Garcia J.L., Gomez-Rueda A., et al. Presentation at the European Society for medical oncology 2018 congress, Munich, Germany. October 19–23, 2018. Intratumoral BO-112, a double-stranded RNA (dsRNA), alone and in combination with systemic anti-PD-1 in solid tumors. [Google Scholar]
- 118.Salazar A.M., Erlich R.B., Mark A., Bhardwaj N., Herberman R.B. Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: case report, hypothesis, and clinical trial. Cancer Immunol Res. 2014;2:720–724. doi: 10.1158/2326-6066.CIR-14-0024. [DOI] [PubMed] [Google Scholar]
- 119.Lan T., Kandimalla E.R., Yu D., Bhagat L., Li Y., Wang D., et al. Stabilized immune modulatory RNA compounds as agonists of Toll-like receptors 7 and 8. Proc Natl Acad Sci USA. 2007;104:13750–13755. doi: 10.1073/pnas.0706059104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Agrawal S., Kandimalla E.R. Synthetic agonists of Toll-like receptors 7, 8 and 9. Biochem Soc Trans. 2007;35:1461–1467. doi: 10.1042/BST0351461. [DOI] [PubMed] [Google Scholar]
- 121.Lan T., Dai M., Wang D., Zhu F.G., Kandimalla E.R., Agrawal S. Toll-like receptor 7 selective synthetic oligoribonucleotide agonists: synthesis and structure-activity relationship studies. J Med Chem. 2009;52:6871–6879. doi: 10.1021/jm901145s. [DOI] [PubMed] [Google Scholar]
- 122.Kandimalla E.R., Struthers M., Bett A.J., Wisniewski T., Dubey S.A., Jiang W., et al. Synthesis and immunological activities of novel Toll-like receptor 7 and 8 agonists. Cell Immunol. 2011;270:126–134. doi: 10.1016/j.cellimm.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 123.Lan T., Bhagat L., Wang D., Dai M., Kandimalla E.R., Agrawal S. Synthetic oligoribonucleotides containing arabinonucleotides act as agonists of TLR7 and 8. Bioorg Med Chem Lett. 2009;19:2044–2047. doi: 10.1016/j.bmcl.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 124.Lan T., Putta M.R., Wang D., Dai M., Yu D., Kandimalla E.R., et al. Synthetic oligoribonucleotides-containing secondary structures act as agonists of Toll-like receptors 7 and 8. Biochem Biophys Res Commun. 2009;386:443–448. doi: 10.1016/j.bbrc.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 125.Fakhari A., Nugent S., Elvecrog J., Vasilakos J., Corcoran M., Tilahun A., et al. Thermosensitive gel-based formulation for intratumoral delivery of Toll-like receptor 7/8 dual agonist, MEDI9197. J Pharm Sci. 2017;106:2037–2045. doi: 10.1016/j.xphs.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 126.Kivimae S., Hennessy M., Pena R., Kirksey Y., Nieves W., Quatch P., et al. Comprehensive antitumor immune activation by a novel TLR7/8 targeting agent NKTR-262 combined with CD122-biased immunostimulatory cytokine NKTR-214. Cancer Res. 2018;78(Suppl. l) Abstract no 3755. [Google Scholar]
- 127.Gupta S., Grilley-Olson J., Hong D., Marabelle A., Munster P., Aggarwal R., et al. Safety and pharmacodynamic activity of MEDI9197, a TLR 7/8 agonist, administered intratumorally in subjects with solid tumors. Cancer Res. 2017;77(Suppl. l) Abstract no CT091. [Google Scholar]
- 128.Heidenreich R., Jasny E., Kowalczyk A., Lutz J., Probst J., Baumhof P., et al. A novel RNA-based adjuvant combines strong immunostimulatory capacities with a favorable safety profile. Int J Cancer. 2015;137:372–384. doi: 10.1002/ijc.29402. [DOI] [PubMed] [Google Scholar]
- 129.Ziegler A., Soldner C., Lienenklaus S., Spanier J., Trittel S., Riese P., et al. A new RNA-based adjuvant enhances virus-specific vaccine responses by locally triggering TLR- and RLH-dependent effects. J Immunol. 2017;198:1595–1605. doi: 10.4049/jimmunol.1601129. [DOI] [PubMed] [Google Scholar]
- 130.Terheyden P., Weishaupt C., Heinzerling L., Klinkhardt U., Jkrauss Mohr P., et al. Presentation at the European Society for medical oncology 2018 congress, Munich, Germany. October 19–23, 2018. Phase I dose escalation and expansion study of intratumoral CV8102, a RNA-based TLR and RIG-1 agonist, in patients with advanced solid tumors. [Google Scholar]
- 131.Schlee M., Roth A., Hornung V., Hagmann C.A., Wimmenauer V., Barcher W., et al. Recognition of 5’- triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009;31:25–34. doi: 10.1016/j.immuni.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barsoum J., Renn M., Schuberth C., Jakobs C., Schwickart A., Schlee M., et al. Selective stimulation of RIG-I with a novel synthetic RNA induces strong anti-tumor immunity in mouse tumor models. Cancer Immunol Res. 2017;5(Suppl. l) Abstract no B44. [Google Scholar]
- 133.Middleton M.R., Wermke M., Calvo E., Chartash E., Zhou H., Zhao X., et al. Presentation at the European Society for medical oncology 2018 congress, Munich, Germany. October 19–23, 2018. Phase 1/2 multicenter, open-label study of intratumoral/intralesional administration of the retinoic acid inducible gene-I (RIG-I) activator, MK-4621 in patients with advanced or recurrent tumors. [Google Scholar]
- 134.Barber G.N. STING: infection, inflammation and cancer. Nat Rev Immunol. 2015;15:760–770. doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gaidt M.M., Ebert T.S., Chauhan D., Ramshorn K., Pinci F., Zuber S., et al. The DNA inflammasome in human myeloid cells is initiated by a STING-cell death program upstream of NLRP3. Cell. 2017;171:1110–1124. doi: 10.1016/j.cell.2017.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao D., Wu J., Wu Y.T., Du F., Aroh C., Yan N., et al. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schwede F., Genieser H.G., Rentsch A. The chemistry of the noncanonical cyclic dinucleotide 2'3'-cGAMP and its analogs. Handb Exp Pharmacol. 2017;238:359–384. doi: 10.1007/164_2015_43. [DOI] [PubMed] [Google Scholar]
- 138.Ramanjulu J.M., Pesiridis G.S., Yang J., Concha N., Singhaus R., Zhang S.Y., et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018;564:439–443. doi: 10.1038/s41586-018-0705-y. [DOI] [PubMed] [Google Scholar]
- 139.Kim S., Li L., Maliga Z., Yin Q., Wu H., Mitchison T.J. Anticancer flavonoids are mouse-selective STING agonists. ACS Chem Biol. 2013;8:1396–1401. doi: 10.1021/cb400264n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li A., Yi M., Qin S., Song Y., Chu Q., Wu K. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J Hematol Oncol. 2019;12:35. doi: 10.1186/s13045-019-0721-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sallets A., Robinson S., Kardosh A., Levy R. Enhancing immunotherapy of STING agonist for lymphoma in preclinical models. Blood Adv. 2018;2:2230–2241. doi: 10.1182/bloodadvances.2018020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jing W., McAllister D., Vonderhaar E.P., Palen K., Riese M.J., Gershan J., et al. STING agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. J Immunother Cancer. 2019;7:115. doi: 10.1186/s40425-019-0573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kinkead H.L., Hopkins A., Lutz E., Wu A.A., Yarchoan M., Cruz K., et al. Combining STING-based neoantigen-targeted vaccine with checkpoint modulators enhances antitumor immunity in murine pancreatic cancer. JCI Insight. 2018;3:122857. doi: 10.1172/jci.insight.122857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Corrales L., Gauthier K.S., Desbien A.L., Reiner G., Glickman L.H., Hudson T.E., et al. Intratumoral activation of STING with a synthetic cyclic dinucleotide elicits antitumor CD8 T-cell immunity that effectively combines with checkpoint inhibitors. Cancer Res. 2018;78(Suppl. l) Abstract no 631. [Google Scholar]
- 145.Meric-Bernstam F., Werner T.L., Hodi F.S., Messersmith W., Lewis N., Talluto C., et al. Presentation at SITC 2017 annual meeting. November 7–11, 2018. Phase I dose-finding study of MIW815 (ADU-S100), an intratumoral STING agonist, in patients with advanced solid tumors or lymphomas. Washington, DC. [Google Scholar]
- 146.Harrington K.J., Brody J., Ingham M., Strauss J., Cemerski S., Wang M., et al. Presentation at the European Society for medical oncology 2018 congress, Munich, Germany. October 19–23, 2018. Preliminary results of the first in human (FIH) study of MK 1454, an agonist of stimulator of intereferon genes (STING), as monotherapy or in combination with pembrolizumab pembro in patients with advanced solid tumors or lymphomas. [Google Scholar]
- 147.Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Diab A., Haymaker C., Bernatchez C., Johnson D.H., Andtbacka R., James M., et al. Presentation at the European society for medical oncology 2018 congress, munich, Germany, october 19–23. 2018. The safety and efficacy of intratumoral injection of the TLR9 agonist tilsotolimod (IMO-2125) in combination with ipilimumab in patients with PD-1 inhibitor refractory metastatic melanoma: an analysis of efficacy in injected and uninjected lesions. [Google Scholar]