Abstract
The recent successes of chimeric antigen receptor T cells in the treatment of hematological malignancies have clearly led to an explosion in the field of adoptive cell therapy for cancer. Current efforts are focused on the translation of this exciting technology to the treatment of solid tumors and the development of allogeneic ‘off-the-shelf’ therapies. γδ T cells are currently gaining considerable attention in this field as their unique biology and established role in cancer immunosurveillance place them in a unique position to potentially overcome these challenges in adoptive cell therapy. Here, we review the relevant aspects of the function of γδ T cells in cancer immunity, and summarize clinical observations and clinical trial results that highlight their emerging role as a platform for the development of safe and effective cancer immunotherapies.
Keywords: γδ T cells, Adoptive cell therapy, Immunotherapy, Cancer
γδ T cells provide tissue- and blood-resident cancer surveillance, combining adaptive T cell biology with the innate recognition of malignant events in a non-clonally selected and MHC-unrestricted fashion. This unique combination renders γδ T cells an attractive target for clinical development of fully allogeneic adoptive cell therapies, genetic manipulation and targeted immunotherapies.
Highlights
-
•
γδ T cells are a unique subset of T cells combining innate and adaptive features.
-
•
Tissue-resident γδ T cells have important functions in tissue and cancer immunosurveillance.
-
•
γδ T cells are being exploited increasingly for cancer immunotherapy.
Introduction
There is striking evidence that in addition to our adaptive immune system, the innate immune system deals with malignant cells long before visible tumor development. Special interest in this matter is given to the unconventional group of γδ T cells that share all cytotoxic features with αβ T cells but also possess innate-like features, including the expression of various natural killer cell receptors (NCRs) [1]. Evolutionary highly conserved, γδ T cells are unique in that they recognize a variety of antigens [2] in a major histocompatibility complex (MHC)-unrestricted fashion, mature in the thymus and retain a preactivated state, meaning they do not require clonal expansion or differentiation into an effector T cell phenotype upon activation [3]. Showing strong enrichment in epithelial tissues, these cells have adopted efficient ways to monitor other cells for abnormal changes in their physiology in tissues and blood –– a function that has been summarized as the ‘lymphoid stress-surveillance response’ [4], [5].
Unique contributions by γδ T cells to broader immunological processes, such as pathogen recognition and clearance [6], attraction and maturation of antigen-presenting cells [7] and direct stimulation of αβ T cells via direct antigen presentation [8], are well established. In addition, γδ T cells contribute to tissue homeostasis and wound healing [9]. Most striking is the phenotype of mice that lack the entirety or specific subtypes of γδ T cells. T cell receptor (TCR) δ chain knockout mice show a significant increase in the occurrence of papillomas which develop into carcinomas in a model of chemically induced skin cancer [10]. This increase in malignant events is not shared by mice that lack αβ T cells [11]. Similar protective effects by γδ T cells have been validated in models of colorectal cancer [12], malignant melanoma [13], B cell lymphoma [14] and prostate cancer [15]. These important contributions to tissue homeostasis and cancer immunosurveillance [16] have fuelled scientific interest to further explore the biology of γδ T cells and their potential for clinical translation [17], [18].
Self-surveillance, natural killer receptor NKG2D and other NCRs
The activating cell surface receptor NKG2D and its ligands play an important role in cytotoxic immune responses of natural killer (NK) cells, NK-T cells and γδ T cells against tumors [19]. Ligands for NKG2D include MHC class I polypeptide-related sequence A and B (MICA/B) and several UL16-binding proteins (ULBPs) that are poorly expressed in normal tissues but are strongly upregulated in stressed or transformed cells [20]. This stress signal can be induced via the DNA repair response after ultraviolet exposure [21], oncogenes such as Ras [22], osmotic shock and/or oxidative stress via epidermal growth factor receptor signalling [23]. Moreover, MICA can also be upregulated via pharmacological manipulation of the mevalonate pathway [24]. In mice, γδ T cells protect the skin from tumors by responding to increased expression of the MICA homologue Rae1 [25]. Remarkably, this protective contribution not only involves direct cytotoxicity, but also the production of interleukin (IL)-13 [26] and modulation of B cells promoting immunoglobulin E class switching and the accumulation of autoreactive antibodies [27]. Mice lacking NKG2D show more susceptibility to spontaneous development of prostate cancer [28], and T cells and NK cells rapidly clear malignant cells injected into mice when they express NKG2D ligands [29]. In human carcinomas of the lung, breast, kidney, ovary, prostate and colon, NKG2D ligands are widely expressed and prompt responses from tumor-infiltrating autologous Vδ1+ T cells [30]. In lung cancer, single nucleotide polymorphisms of MICA influence not only disease progression but also susceptibility to platinum chemotherapy [31]. Lung cancer cells that express MICA are recognized and killed by NK cells [32], and in patients with head and neck cancer, the use of cetuximab causes NKG2D+ NK cells to recognize MICA, which induces a tumor antigen-specific adaptive response through dendritic cell maturation and consequent activation of cytotoxic T lymphocytes [33].
The activating NCRs NKp30, NKp44 and NKp46 are also expressed on human Vδ1+ T cells after activation and costimulation with cytokines enhancing the production of interferon gamma (IFNγ) [34]. Furthermore, the engagement of NKp30 and NKp44 on Vδ1+ T cells promotes the recognition and killing of leukemia cells and correlates with increased granzyme expression [35]. Not limited to cytotoxicity alone, activation of NKp30 on Vδ1+ T cells induces production of the CC chemokine ligands CCL3, CCL4 and CCL5, linking target recognition with the attraction of antigen-presenting cells such as monocytes and conventional αβ T cells [36]. NCRs have also been shown to bind to self-proteins expressed on malignant or stressed cells; for example, binding of B-associated transcript 3 [37] or B7-H6 [38] on target cells by NKp30 renders these cells prone to killing by NK cells. Similarly, the multiplicity of NCRs expressed [34], especially on intra-epithelial γδ T cells [39], is expected to enable these cells to respond to markers of dysregulation and stress immediately where they reside [4], [25].
The impact of other NK cell-associated inhibitory receptors on γδ T cells (e.g. CD94 heterodimers with NKG2A), which have been shown to be strongly inhibitory for NK cells and conventional cytotoxic αβ T cells in tumors [40], remains unclear as reports investigating the expression on γδ T cells and modulation of function through NKG2A are currently lacking.
γδ T cells in humans: same but different
Human T cells expressing a γδ TCR show functional similarities with mice in that they are highly capable and primed killer cells that almost exclusively produce IFNγ upon activation [41], [42], [43]. However, there are fundamental differences between γδ T cells in humans and mice. For example, the signature subset of mouse dendritic epidermal T cells is completely absent in humans, most likely due to a premature stop codon in the Vγ5 selecting protein Skint-1 [44]. Other γδ T cell-specific tissue-selecting proteins do show conservation between mice and humans, namely butyrophilin-like (Btnl) 1/6 in mice and BTNL3/8 in humans, selecting mouse Vγ7 T cells into the intestinal epithelium or human Vγ4 T cells into the colonic epithelium, respectively [45]. Fascinatingly, these interactions of γδ TCRs and BTNLs happen through germline-encoded regions of the TCR, allowing for additional binding of clone-specific antigens through the complementarity-determining regions 1–3 [46].
A striking functional difference in mice is that γδ T cells develop into two functional lineages in the thymus that produce high levels of either INFγ or IL-17 upon activation [47]; the latter is abundant in the dermis, together with its INFγ-producing counterpart. IL-17 producing γδ T cells have been shown to have undesirable effects on tumor growth and promotion in mouse models of breast cancer [48] and ovarian cancer [49]. Although there has been a report of human γδ T cells producing IL-17 in colorectal cancer [50], humans lack the mouse counterpart of the dedicated IL-17+ γδ T cells at steady state, which is identified by the lack of CD27 expression in mice.
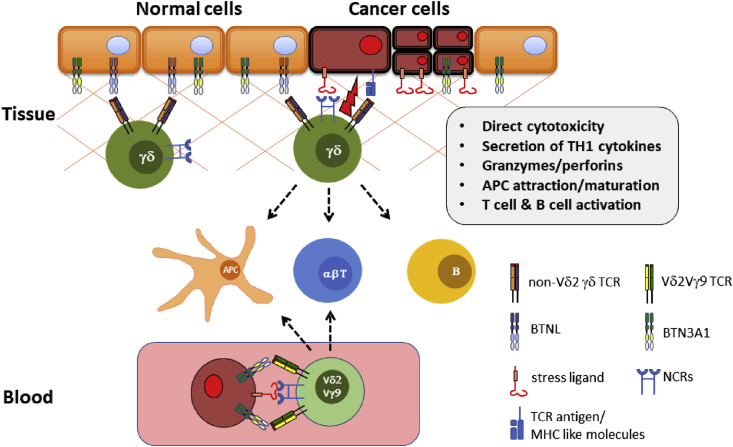
The main difference between γδ T cells in humans and mice is the fact that humans, among other primates, have an additional subset of γδ T cells which express a Vγ9 chain paired to a Vδ2 chain to form the TCR [51]. Rodents completely lack this type of invariant T cell, whereas in humans, this cell type dominates the composition of γδ T cell subtypes in the blood, representing up to 5% of all T cells (Figure 1).
Figure 1.
γδ T cells, predominantly Vδ1+ T cells, are rich in tissues such as colon and skin, where they interact with tissue-selecting proteins of the BTNL family. High expression of NCRs (e.g. NKG2D, NKp30, DNAM-1) allows these cells to respond to stress ligands [MICA/B, ULBPs] in an MHC-unrestricted and non-clonal fashion, and at the same time to recognize TCR-specific ligands. Activation of γδ T cells involves direct cytolysis of target cells as well as the production and release of cytolytic granules, chemokines and TH1 cytokines. Bridging the innate and adaptive systems, γδ T cells have been shown to attract and activate antigen-presenting cells (APCs), αβ T cells and B cells, thereby orchestrating adaptive immune responses. In blood, mostly Vδ2Vγ9 T cells survey for metabolically hyperactive cells, sensing intermediates of the mevalonate pathway through interactions with BTN3A1.
T cells expressing the Vδ2Vγ9 TCR recognize the bacterial metabolite (E)-4-hydroxy-3-methyl-but-2-enyl and show cross-reactivity with the mevalonate pathway metabolites isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate [52]. The mevalonate pathway is the exclusive metabolic source for prenyl residues for the post-translational prenylation of proteins, which is crucial for the function of multiple members of the RAS superfamily [53]. In human malignancies with a mutated p53 oncogene, representing ∼50% of human cancers, it has been reported that the mevalonate pathway is significantly upregulated and maintains a malignant phenotype of tumor cells [54]. Vδ2+ T cells recognize increased levels of IPP, resulting in cytotoxic responses against malignant cells but not normal tissues [55]. Targeting the mevalonate pathway using aminobisphosphonates (N-bis) (e.g. zoledronic acid, which is commonly used in the treatment of osteoporosis) results in accumulation of IPP in cancer cells, thereby further increasing the immunogenicity of cancer cells towards Vδ2+ T cells [5]. Moreover, it has been demonstrated that γδ T cells respond to various mevalonate pathway intermediates; this process is influenced by stress-related cytokines [56], [57]. Interestingly, the recognition of phosphoantigens by Vδ2Vγ9 T cells involves the modulation of BTN 3A1, 2 and 3 [58], [59], further supporting the idea of γδ T cell regulation and activation via the family of butyrophilin and butyrophilin-like molecules [60], [61].
The above functional aspects of Vδ2+ T cell biology and the fact that these cells can easily be grown and expanded ex vivo using N-bis [62] and synthetic phosphoantigens (pAgs), such as bromohydrin pyrophosphate (BrHPP) [63], have motivated investigators to exploit Vδ2+ T cells for cancer immunotherapy.
Clinical experiences with Vδ2+ T cell immunotherapy in cancer
Two strategies of Vδ2+ T cell cancer immunotherapy have been developed and applied. The first is to stimulate and expand Vδ2+ T cells in vivo by systemic administration of pAgs or N-bis. This approach has been tested in eight pilot/phase 1 clinical trials in hematological malignancies and solid tumors over the last years (Table 1). The use of BrHPP or N-bis (pamidronate or zoledronate), mainly in combination with IL-2, was found to be safe and resulted in Vδ2+ T cell expansions in vivo and/or maturation towards an IFNγ-producing effector phenotype in most patients. Eight out of a total of 121 patients (7%) showed objective responses, but no complete responses were observed.
Table 1.
Pilot/Phase 1 trials evaluating safety and clinical activity of in vivo activation of Vγ9Vδ2 T cells
| Year | Disease | Treatment | n | OR | CR | Reference |
|---|---|---|---|---|---|---|
| 2003 | MM NHL |
Pamidronate + IL-2 | 19 | 3/19 | 0/19 | [94] |
| 2003 | Prostate cancer Breast cancer |
Zoledronate | 9 | 0/9 | 0/9 | [95] |
| 2007 | Prostate cancer | Zoledronate vs zoledronate + IL-2 | 18 | 3/18 | 0/18 | [96] |
| 2010 | Breast cancer | Zoledronate + IL-2 | 10 | 0/10 | 0/10 | [97] |
| 2010 | RCC Colon cancer Esophagus cancer Gastric cancer Ovarian cancer Breast cancer |
BrHPP + IL-2 | 28 | 0/28 | 0/28 | [98] |
| 2011 | RCC | Zoledronate + IL-2 | 12 | 0/12 | 0/12 | [99] |
| 2012 | RCC MM AML |
Zoledronate + IL-2 | 21 | 2/21 | 0/21 | [100] |
| 2016 | Neuroblastoma | Zoledronate + IL-2 | 4 | 0/4 | 0/4 | [101] |
MM, multiple myeloma; NHL, non-Hodgkin lymphoma; RCC, renal cell cancer; AML, acute myeloid leukemia.
The second approach that has been clinically applied is the adoptive transfer of autologous Vδ2+ T cells after ex vivo expansion using synthetic pAgs or N-bis. Infusion of ex vivo expanded autologous Vδ2+ T cells alone or in combination with BrHPP or zoledronate and IL-2 was well tolerated across nine different clinical trials (Table 2), and resulted in six objective responses (8%; n=86) and two complete responses (2%; n=86) in total.
Table 2.
Pilot/phase 1 trials evaluating safety and clinical activity of adoptively transferred autologous ex vivo expanded Vγ9Vδ2 T cells
| Year | Disease | Treatment | n | OR | CR | Reference |
|---|---|---|---|---|---|---|
| 2007 | RCC | Vγ9Vδ2 T cells + zoledronate + IL-2 | 7 | 3/7 | 0/7 | [102] |
| 2008 | RCC | Vγ9Vδ2 T cells + BrHPP + IL-2 | 10 | 0/10 | 0/10 | [103] |
| 2009 | MM | Vγ9Vδ2 T cells + zoledronate + IL-2 | 6 | 0/6 | 0/6 | [104] |
| 2010 | NSCLC | Vγ9Vδ2 T cells + zoledronate + IL-2 | 10 | 0/10 | 0/10 | [105] |
| 2011 | RCC | Vγ9Vδ2 T cells + zoledronate + IL-2 | 11 | 1/11 | 1/11 | [106] |
| 2011 | Melanoma Colon cancer Breast cancer Cervical cancer Ovarian cancer Gastrointestinal cancer |
Vγ9Vδ2 T cells + zoledronate | 18 | 3/12 | 1/12 | [107] |
| 2011 | NSCLC | Vγ9Vδ2 T cells + zoledronate + IL-2 | 15 | 0/12 | 0/12 | [108] |
| 2013 | Colon cancer | Vγ9Vδ2 T cells | 6 | 0/6 | 0/6 | [109] |
| 2014 | NSCLC | Vγ9Vδ2 T cells | 15 | 0/12 | 0/12 | [110] |
| 2014 | Gastric cancer | Vγ9Vδ2 T cells + zoledronate | 7 | [64] |
BrHPP, bromohydrin pyrophosphate; CR, complete response; IL, interleukin; MM, multiple myeloma; NSCLC, non-small cell lung cancer; OR, objective response; RCC, renal cell cancer.
Intraperitoneal injections of ex vivo expanded autologous Vδ2+ T cells in combination with zoledronate for the treatment of malignant ascites have been reported for seven patients with gastric cancer, resulting in a significant reduction in the number of tumor cells in the ascites and a significant reduction in the volume of ascites in two patients [64].
Allogeneic Vδ2+ T cells have also been used as part of a more heterogeneous cell population in a small pilot study [65]. Four patients with advanced refractory hematological malignancies received CD4+/CD8+-depleted infusions of haploidentical leukapheresis products highly enriched for Vδ2+ T cells after lymphodepleting chemotherapy with cyclophosphamide and fludarabine. A marked in vivo expansion of donor Vδ2+ T cells was observed in all patients without any signs of graft versus host disease (GvHD). Although refractory to all prior therapies, three of four patients achieved complete remissions, which lasted for 8 months in a patient with plasma cell leukemia.
Most recently, Alnaggar et al. [66] published a case report of a patient with stage IV cholangiocarcinoma showing recurrent mediastinal lymph node metastasis after liver transplantation. The patient received eight consecutive infusions of allogeneic Vδ2+ T cells that were expanded from peripheral blood mononuclear cells (PBMCs) of a healthy donor. No adverse effects were observed after cell infusion, and the authors reported a complete response with no detectable peritoneal lymph node metastasis at the end of treatment.
In summary, these clinical results clearly demonstrate that Vδ2+ T cell-based immunotherapy is safe and well tolerated, but the signs of clinical efficacy are highly variable. This might be explained by the very heterogeneous group of diseases treated and the variation in protocols used for ex vivo or in vivo expansion of Vδ2+ T cells, or in the variability of treatment regimens applied in these studies. In vivo activation of Vδ2+ T cells by pAgs or N-bis clearly resulted in activation of circulating Vδ2+ T cells, but no study could provide evidence that this approach also resulted in activation of the small number of tissue-resident Vδ2+ T cells or resulted in recruitment of Vδ2+ T cells from the circulation to the tumor site. In addition, Vδ2+ T cells are dysfunctional in some cancer patients, are susceptible to activation-induced anergy, and repeated stimulation of Vδ2+ T cells may induce terminal differentiation and exhaustion [67], [68], [69]. This might explain why the adoptive transfer of ex vivo expanded Vδ2+ T cells seems to be the more effective approach resulting in complete responses in some patients. Strikingly, four of five patients treated with allogeneic Vδ2+ T cells showed complete responses, compared with only two complete responses observed in 98 patients treated with autologous Vδ2+ T cells. Although the number of patients treated with allogeneic cells is too small to draw definitive conclusions, these results might indicate that allogenic Vδ2+ T cells expanded from healthy donors have a functionally superior phenotype compared with autologous patient-derived Vδ2+ T cells. The fact that allogeneic Vδ2+ T cells do not induce GvHD, together with the possibility to generate large numbers and batches of cells from a single healthy donor, will certainly advance the use of healthy donor-derived Vδ2+ T cells in future clinical studies.
Role of Vδ1+ T cells in cancer
The fact that mice are protected from malignant events by tissue-resident Vδ1+ TCR chain-expressing T cells and lack the Vδ2+ T cell subtype entirely has sparked great interest in studying the tumor-protective role of Vδ1+ T cells in human cancer. Human tissues contain large numbers of Vδ1+ T cells, especially the intestine, colon and dermis [3], [46], but preclinical research on Vδ1+ T cells was held back in the past by a lack of imaging reagents to discriminate γδ T cells from αβ T cells and, more importantly, to differentiate Vδ1+ T cells from Vδ2+ T cells. Although commercial antibodies to stain γδ T cells in tissues are available [45], [70], we still rely on tissue digestion or PBMC isolation and flow cytometry to identify γδ T cell clonotypes.
Several studies have shown that γδ T cells are an important component of tumor-infiltrating lymphocytes (TILs) in patients with different types of cancer, and a recent analysis of ∼18 000 transcriptomes from 39 human tumors identified tumor-infiltrating γδ T cells as the most significant favorable cancer-wide prognostic factor. The same study also showed NKG2D to be positively associated with better outcome [71]. Although this study could not discriminate between Vδ1+ and Vδ2+ T cells, other studies showed that Vδ1+ T cells represent the predominant tumor-infiltrating γδ T cell subtype [72], [73].
More direct clinical evidence to support the tumor-protective features of Vδ1+ T cells comes from a larger study in patients with acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) who received T cell-depleted bone marrow grafts from partially human leukocyte antigen (HLA)-mismatched donors [74]. Disease-free survival at 30 months post transplant was significantly better in those patients in whom the percentage of γδ T cells exceeded 10% of the total lymphocyte count in the blood. No significant difference in the incidence of acute or chronic GvHD was observed, suggesting an enhanced graft versus leukemia effect in the absence of GvHD. In an extended 42-month follow-up study, these data were confirmed [75], and a further 8-year follow-up study with additional patients (n=153) showed significantly better 5-year leukemia-free survival and overall survival for patients who recovered with an increased proportion of γδ T cells [76]. The expanded γδ T cell subtype in >90% of long-term survivors in this study was predominantly Vδ1+, suggesting that these cells were involved in long-term clearance of leukemia.
Increases in Vδ1+ T cells have also been correlated with cytomegalovirus (CMV) reactivation in patients with leukemia following allogeneic hematopoietic stem cell transplantation (HSCT) [77], [78]. When isolated, these Vδ1+ T cells not only kill CMV-infected cells but also leukemic cells and other tumor cells in vitro via HLA- and NKG2D-independent mechanisms [78], [79]. Moreover, Vδ1+ T cells that are specifically expanded in patients with CMV reactivation are more cytotoxic against primary ALL and AML cells compared with Vδ1+ T cells from patients without CMV reactivation [80]. This may explain, at least in part, the favourable effect of CMV reactivation after HSCT on the risk of relapse [81], further supported by a 2–6-year follow-up study in patients after kidney transplantation, where expanding numbers of Vδ1+ T cells associated with CMV reactivation strongly correlated with a significantly reduced occurrence rate of malignancies [82].
Taken together, these data warrant clinical testing of Vδ1+ T cells as a novel effector cell type for cancer immunotherapy, and the period following HSCT in patients with leukemia seems to be a promising therapeutic window for adoptive transfer of Vδ1+ T cells to prevent relapse. However, the lack of clinical-grade protocols to selectively expand Vδ1+ T cells in vivo or ex vivo has prevented the conduct of clinical trials to harness the therapeutic potential of Vδ1+ T cells to date.
Vδ1+ T cells and their development for cancer immunotherapy
The use of Vδ1+ T cells for preclinical research and clinical development is currently limited to isolation of very small cell numbers from human PBMCs or isolation from human tissues using enzymatic digestion or alternative methods. Vδ1+ T cells expanded from blood using a combination of IL-7 and phytohemaglutinin controlled tumor growth in an NSG mouse model for colon cancer much better than Vδ2+ T cells [83], but this protocol is not applicable for clinical-grade expansion of Vδ1+ T cells. A system using genetically modified antigen-presenting cells linked to anti-γδ TCR antibodies generated a mixed population of expanded γδ T cells comprising Vδ2+ T cells and non-Vδ2+ T cells. Whilst all γδ T cells in this system exerted cytotoxicity against GD2-expressing neuroblastoma cells, Vδ2+ T cells relied on antibody-dependent cellular cytotoxicity via the expression of CD16, whilst non-Vδ2+, including Vδ1+ T cells, did not [84]. A more easily translatable system for the expansion of Vδ1+ T cells specifically, using a combination of common γ chain cytokines and the CD3 engaging antibody OKT3, was developed recently [35]. These Vδ1+ T cells show favourable expression of NCRs, exhibit cytotoxicity against hematological tumor lines in vitro and show the capacity to infiltrate the tumor core, bone marrow, liver and spleen thus controlling tumor growth over several weeks in an NSG mouse model of subcutaneously induced chronic lymphoid leukemia [85]. These blood-derived and expanded Vδ1+ T cells show cytotoxicity against primary, patient-derived AML cells that are resistant to chemotherapy. Whilst the mechanism of recognition and killing most likely did not depend on the TCR, it was dependent on the expression of NKp30 and B7-H6 on target cells. Adoptive transfer of Vδ1+ T cells into human AML xenograft mice improved survival significantly, decreasing tumor load in the blood and target organs [86].
Although human clinical studies testing the safety and efficacy of enriched and purified preparations of autologous or allogeneic Vδ1+ T cells have yet to be conducted, patients have been treated with high numbers of Vδ1+ T cells as part of a more heterogenous cell population. Adoptive transfer of autologous TILs has shown impressive clinical results in patients with metastatic melanoma. The efficacy of this personalized immunotherapy based on preconditioning chemotherapy followed by infusion of TILs and IL-2 has been confirmed in several independent studies. Objective response rates of 40–50%, including complete tumor regressions in 10–20% of treated patients, have been reported consistently [87], [88]. In metastatic melanoma, Vδ1+ T cells can represent the major TIL subset, accounting for ∼50% of the total CD3+ population [72]. Indeed, detectable amounts of Vδ1+ T cells in clinical-grade TIL preparations were found in 20 of 27 patients analysed in a recent study of adoptive TIL transfer, and infusion products from 10 patients contained, on average >1 x 109 of these cells [89]. Notably, one patient achieving a complete response was infused with 7.8% Vδ1+ T cells, approximately 6.5 x 109 cells in total. Since all cell products also contained CD8+ T cells, no conclusions can be drawn on the contribution of Vδ1+ T cells to the clinical antitumor activity observed, but when tested, these cells showed high cytotoxicity against melanoma cells in vitro. In summary, infusion of Vδ1+ T cells together with high numbers of αβ T cells in a clinical trial was safe and well tolerated, and the authors concluded that Vδ1+ T cells should be further scrutinized as a potentially useful tool for the treatment of patients with metastatic melanoma.
Conclusion and outlook
Harnessing the unique biology of γδ T cells for cellular or targeted immunotherapy holds considerable promise for the treatment of different types of cancer. First, γδ T cells do not recognize and kill tumor cells dependent on the expression of a single antigen. In contrast, they recognize most cancer types through a broad pattern of different NCRs expressed on their cell surface in a non-clonally expanded fashion, minimizing tumor immune escape mediated by single antigen loss. Second, γδ T cells distribute and reside in abundance within tissues. The natural tissue tropism of γδ T cells, especially Vδ1+ T cells, could give these cells an advantage over conventional αβ T cells to migrate into tissues and infiltrate solid tumors more efficiently and execute their functions in more hypoxic environments. Third, the ability to recognize target cells in an MHC-independent manner and the low risk for alloreactivity will allow the development of allogeneic cell products without the need for further genetic engineering. Finally, γδ T cells have been shown to interact with antigen-presenting cells and other members of the adaptive immune system, enabling the orchestration of secondary immune responses post activation.
Whilst these combined features of γδ T cells make them an attractive source for unmodified cell-based adoptive immunotherapy approaches, γδ T cells may also be harnessed for genetic manipulation. Either as a vehicle for chimeric antigen receptors (CARs) or αβ T cell-derived TCRs [90], γδ T cells could combine tissue resident biology and innate target recognition with antigen-specific activation and selection. Furthermore, a better understanding of γδ Τ cell interaction with BTN/BTNL molecules, as well as their regulation and activation in normal tissues and tumors, may allow for therapeutic manipulation in situ using targeted or checkpoint therapies.
Not surprisingly, several companies are now developing next-generation γδ T cell immunotherapies (Table 3). Most of these approaches focus on Vδ2+ T cells as a platform for autologous or allogeneic cell therapies engineered to express CARs. Alternative methods of expanding and/or activating Vδ2+ T cells in vivo that do not depend on the administration of N-bis or pAgs are also in development. A better understanding of Vδ2+ TCR biology and the role of BTN3A in Vδ2+ T cell activation has led to the development of activating antibodies that potentially eliminate the need for TCR overstimulation [91]. Other approaches include the redirection of Vδ2+ T cells towards specific tumor antigens using bispecific Vδ2+ T cell-engaging molecules [92], or genetically engineered chemotherapy resistance of Vδ2+ T cells that can be administered during the therapeutic window when chemotherapy increases the immunogenicity of tumors by upregulating NKG2D ligands [93].
Table 3.
Companies developing γδT-cell-based immunotherapies
| Company | Modality | T-cell type | Source | Autologous/allogeneic | Engineering | Comments |
|---|---|---|---|---|---|---|
| Adicet Bio | Cell therapy | Vδ1 | Blood | Allogeneic | CAR | - |
| Beijing Doing Biomedical | Cell therapy | Vδ2 | Blood | Autologous | Unmodified/CAR | - |
| Cytomed Therapeutics | Cell therapy | Vδ2 | Blood | Allogeneic | CAR | |
| Gadeta | Cell therapy | αβ | Blood | Autologous | Vδ2 TCR | - |
| GammaCell Biotechnologies | Cell therapy | Vδ2 | Blood | Autologous/allogeneic | Unmodified | - |
| GammaDelta Therapeutics | Cell therapy | Vδ1 | Skin/blood | Allogeneic | Unmodified/CAR | - |
| Hebei Senlang Biotechnology | Cell therapy | Vδ2 | Blood | Autologous | CAR/αβ TCR | - |
| Immatics | Cell therapy | Vδ2 | Blood | Allogeneic | αβ TCR | - |
| Incysus Therapeutics |
Cell therapy | Vδ2 | Blood | Autologous | Engineered | Engineered for chemotherapy resistance |
| PhosphoGam | Cell therapy | Vδ2 | Blood | Allogeneic | Unmodified | - |
| TC BioPharm | Cell therapy | Vδ1/Vδ2 | Blood | Autologous/allogeneic | Unmodified/CAR | - |
| Imcheck Therapeutics | Antibodies | Vδ2 | - | - | - | Activation of Vδ2 T cells (BTN3A) |
| Lava Therapeutics | T-cell engager | Vδ2 | - | - | - | Redirection of Vδ2 T cells against tumors |
| Nybo | Antibodies | Pan γδ | - | - | - | Depletion of inhibitory γδ T cells |
CAR, chimeric antigen receptor; TCR, T cell receptor.
Moreover, advances in the isolation and expansion of Vδ1+ T cells from blood and the very first protocol to isolate and grow tissue-resident Vδ1+ T cells in large numbers for clinical application (authors' unpublished data) have paved the way to add Vδ1+ T cells to the growing armamentarium of cancer immunotherapy.
It will be exciting to see these different approaches being tested in clinical trials over the coming years to prove that γδ T cells provide a safe and effective platform for allogeneic ‘off-the-shelf’ cell therapies for cancer.
Funding
None declared.
Disclosure
ON is a scientific co-founder and an employee of GammaDelta Therapeutics. MK is an employee of GammaDelta Therapeutics.
References
- 1.Bonneville M., O'Brien R.L., Born W.K. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–478. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 2.Willcox B.E., Willcox C.R. gammadelta TCR ligands: the quest to solve a 500-million-year-old mystery. Nat Immunol. 2019;20:121–128. doi: 10.1038/s41590-018-0304-y. [DOI] [PubMed] [Google Scholar]
- 3.Vantourout P., Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayday A.C. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Thurnher M., Nussbaumer O., Gruenbacher G. Novel aspects of mevalonate pathway inhibitors as antitumor agents. Clin Cancer Res. 2012;18:3524–3531. doi: 10.1158/1078-0432.CCR-12-0489. [DOI] [PubMed] [Google Scholar]
- 6.Wang T., Gao Y., Scully E., Davis C.T., Anderson J.F., Welte T., et al. Gamma delta T cells facilitate adaptive immunity against West Nile virus infection in mice. J Immunol. 2006;177:1825–1832. doi: 10.4049/jimmunol.177.3.1825. [DOI] [PubMed] [Google Scholar]
- 7.Ismaili J., Olislagers V., Poupot R., Fournie J.J., Goldman M. Human gamma delta T cells induce dendritic cell maturation. Clin Immunol. 2002;103:296–302. doi: 10.1006/clim.2002.5218. [DOI] [PubMed] [Google Scholar]
- 8.Meuter S., Eberl M., Moser B. Prolonged antigen survival and cytosolic export in cross-presenting human gammadelta T cells. Proc Natl Acad Sci U S A. 2010;107:8730–8735. doi: 10.1073/pnas.1002769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen M.M., Witherden D.A., Havran W.L. gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol. 2017;17:733–745. doi: 10.1038/nri.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girardi M., Oppenheim D.E., Steele C.R., Lewis J.M., Glusac E., Filler R., et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 11.Girardi M., Glusac E., Filler R.B., Roberts S.J., Propperova I., Lewis J., et al. The distinct contributions of murine T cell receptor (TCR)gammadelta+ and TCRalphabeta+ T cells to different stages of chemically induced skin cancer. J Exp Med. 2003;198:747–755. doi: 10.1084/jem.20021282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tie G., Yan J., Khair L., Messina J.A., Deng A., Kang J., et al. Hypercholesterolemia Increases Colorectal Cancer Incidence by Reducing Production of NKT and gammadelta T Cells from Hematopoietic Stem Cells. Cancer Res. 2017;77:2351–2362. doi: 10.1158/0008-5472.CAN-16-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanca T., Costa M.F., Goncalves-Sousa N., Rei M., Grosso A.R., Penido C., et al. Protective role of the inflammatory CCR2/CCL2 chemokine pathway through recruitment of type 1 cytotoxic gammadelta T lymphocytes to tumor beds. J Immunol. 2013;190:6673–6680. doi: 10.4049/jimmunol.1300434. [DOI] [PubMed] [Google Scholar]
- 14.Street S.E., Hayakawa Y., Zhan Y., Lew A.M., MacGregor D., Jamieson A.M., et al. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J Exp Med. 2004;199:879–884. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z., Eltoum I.E., Guo B., Beck B.H., Cloud G.A., Lopez R.D. Protective immunosurveillance and therapeutic antitumor activity of gammadelta T cells demonstrated in a mouse model of prostate cancer. J Immunol. 2008;180:6044–6053. doi: 10.4049/jimmunol.180.9.6044. [DOI] [PubMed] [Google Scholar]
- 16.Silva-Santos B., Serre K., Norell H. Gammadelta T cells in cancer. Nat Rev Immunol. 2015;15:683–691. doi: 10.1038/nri3904. [DOI] [PubMed] [Google Scholar]
- 17.Van Acker H.H., Campillo-Davo D., Roex G., Versteven M., Smits E.L., Van Tendeloo V.F. The role of the common gamma-chain family cytokines in gammadelta T cell-based anti-cancer immunotherapy. Cytokine Growth Factor Rev. 2018;41:54–64. doi: 10.1016/j.cytogfr.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Deniger D.C., Moyes J.S., Cooper L.J. Clinical applications of gamma delta T cells with multivalent immunity. Front Immunol. 2014;5:636. doi: 10.3389/fimmu.2014.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nausch N., Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27:5944–5958. doi: 10.1038/onc.2008.272. [DOI] [PubMed] [Google Scholar]
- 20.Shafi S., Vantourout P., Wallace G., Antoun A., Vaughan R., Stanford M., et al. An NKG2D-mediated human lymphoid stress surveillance response with high interindividual variation. Sci Transl Med. 2011;3:113ra124. doi: 10.1126/scitranslmed.3002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasser S., Orsulic S., Brown E.J., Raulet D.H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature. 2005;436:1186–1190. doi: 10.1038/nature03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X.V., Ho S.S., Tan J.J., Kamran N., Gasser S. Ras activation induces expression of Raet1 family NK receptor ligands. J Immunol. 2012;189:1826–1834. doi: 10.4049/jimmunol.1200965. [DOI] [PubMed] [Google Scholar]
- 23.Vantourout P., Willcox C., Turner A., Swanson C.M., Haque Y., Sobolev O., et al. Immunological visibility: posttranscriptional regulation of human NKG2D ligands by the EGF receptor pathway. Sci Transl Med. 2014;6:231ra249. doi: 10.1126/scitranslmed.3007579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pich C., Teiti I., Rochaix P., Mariame B., Couderc B., Favre G., et al. Statins Reduce Melanoma Development and Metastasis through MICA Overexpression. Front Immunol. 2013;4:62. doi: 10.3389/fimmu.2013.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strid J., Roberts S.J., Filler R.B., Lewis J.M., Kwong B.Y., Schpero W., et al. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol. 2008;9:146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 26.Dalessandri T., Crawford G., Hayes M., Castro Seoane R., Strid J. IL-13 from intraepithelial lymphocytes regulates tissue homeostasis and protects against carcinogenesis in the skin. Nat Commun. 2016;7:12080. doi: 10.1038/ncomms12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crawford G., Hayes M.D., Seoane R.C., Ward S., Dalessandri T., Lai C., et al. Epithelial damage and tissue gammadelta T cells promote a unique tumor-protective IgE response. Nat Immunol. 2018;19:859–870. doi: 10.1038/s41590-018-0161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerra N., Tan Y.X., Joncker N.T., Choy A., Gallardo F., Xiong N., et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diefenbach A., Jensen E.R., Jamieson A.M., Raulet D.H. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groh V., Rhinehart R., Secrist H., Bauer S., Grabstein K.H., Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci U S A. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J., Tian S., Yin Z., Wu S., Liu L., Qian Y., et al. MicroRNA-binding site SNPs in deregulated genes are associated with clinical outcome of non-small cell lung cancer. Lung cancer. 2014;85:442–448. doi: 10.1016/j.lungcan.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Busche A., Goldmann T., Naumann U., Steinle A., Brandau S. Natural killer cell-mediated rejection of experimental human lung cancer by genetic overexpression of major histocompatibility complex class I chain-related gene A. Hum Gene Ther. 2006;17:135–146. doi: 10.1089/hum.2006.17.135. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava R.M., Lee S.C., Andrade Filho P.A., Lord C.A., Jie H.B., Davidson H.C., et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19:1858–1872. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hudspeth K., Silva-Santos B., Mavilio D. Natural cytotoxicity receptors: broader expression patterns and functions in innate and adaptive immune cells. Front Immunol. 2013;4:69. doi: 10.3389/fimmu.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Correia D.V., Fogli M., Hudspeth K., da Silva M.G., Mavilio D., Silva-Santos B. Differentiation of human peripheral blood Vdelta1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood. 2011;118:992–1001. doi: 10.1182/blood-2011-02-339135. [DOI] [PubMed] [Google Scholar]
- 36.Hudspeth K., Fogli M., Correia D.V., Mikulak J., Roberto A., Della Bella S., et al. Engagement of NKp30 on Vdelta1 T cells induces the production of CCL3, CCL4, and CCL5 and suppresses HIV-1 replication. Blood. 2012;119:4013–4016. doi: 10.1182/blood-2011-11-390153. [DOI] [PubMed] [Google Scholar]
- 37.Pogge von Strandmann E., Simhadri V.R., von Tresckow B., Sasse S., Reiners K.S., Hansen H.P., et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27:965–974. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Brandt C.S., Baratin M., Yi E.C., Kennedy J., Gao Z., Fox B., et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satoh-Takayama N., Vosshenrich C.A., Lesjean-Pottier S., Sawa S., Lochner M., Rattis F., et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Gooden M.J., van Hall T. Infiltrating CTLs are bothered by HLA-E on tumors. Oncoimmunology. 2012;1:92–93. doi: 10.4161/onci.1.1.17961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wrobel P., Shojaei H., Schittek B., Gieseler F., Wollenberg B., Kalthoff H., et al. Lysis of a broad range of epithelial tumour cells by human gamma delta T cells: involvement of NKG2D ligands and T-cell receptor- versus NKG2D-dependent recognition. Scand J Immunol. 2007;66:320–328. doi: 10.1111/j.1365-3083.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- 42.Todaro M., D'Asaro M., Caccamo N., Iovino F., Francipane M.G., Meraviglia S., et al. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287–7296. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- 43.Gertner-Dardenne J., Castellano R., Mamessier E., Garbit S., Kochbati E., Etienne A., et al. Human Vgamma9Vdelta2 T cells specifically recognize and kill acute myeloid leukemic blasts. J Immunol. 2012;188:4701–4708. doi: 10.4049/jimmunol.1103710. [DOI] [PubMed] [Google Scholar]
- 44.Mohamed R.H., Sutoh Y., Itoh Y., Otsuka N., Miyatake Y., Ogasawara K., et al. The SKINT1-like gene is inactivated in hominoids but not in all primate species: implications for the origin of dendritic epidermal T cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Marco Barros R., Roberts N.A., Dart R.J., Vantourout P., Jandke A., Nussbaumer O., et al. Epithelia Use Butyrophilin-like Molecules to Shape Organ-Specific gammadelta T Cell Compartments. Cell. 2016;167:203–218. doi: 10.1016/j.cell.2016.08.030. e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melandri D., Zlatareva I., Chaleil R.A.G., Dart R.J., Chancellor A., Nussbaumer O., et al. The gammadeltaTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat Immunol. 2018;19:1352–1365. doi: 10.1038/s41590-018-0253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chien Y.H., Zeng X., Prinz I. The natural and the inducible: interleukin (IL)-17-producing gammadelta T cells. Trends Immunol. 2013;34:151–154. doi: 10.1016/j.it.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coffelt S.B., Kersten K., Doornebal C.W., Weiden J., Vrijland K., Hau C.S., et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rei M., Goncalves-Sousa N., Lanca T., Thompson R.G., Mensurado S., Balkwill F.R., et al. Murine CD27(-) Vgamma6(+) gammadelta T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc Natl Acad Sci U S A. 2014;111:E3562–3570. doi: 10.1073/pnas.1403424111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu P., Wu D., Ni C., Ye J., Chen W., Hu G., et al. gammadeltaT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity. 2014;40:785–800. doi: 10.1016/j.immuni.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karunakaran M.M., Herrmann T. The Vgamma9Vdelta2 T Cell Antigen Receptor and Butyrophilin-3 A1: Models of Interaction, the Possibility of Co-Evolution, and the Case of Dendritic Epidermal T Cells. Front Immunol. 2014;5:648. doi: 10.3389/fimmu.2014.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reichenberg A., Hintz M., Kletschek Y., Kuhl T., Haug C., Engel R., et al. Replacing the pyrophosphate group of HMB-PP by a diphosphonate function abrogates Its potential to activate human gammadelta T cells but does not lead to competitive antagonism. Bioorg Med Chem Lett. 2003;13:1257–1260. doi: 10.1016/s0960-894x(03)00138-0. [DOI] [PubMed] [Google Scholar]
- 53.Thurnher M., Gruenbacher G., Nussbaumer O. Regulation of mevalonate metabolism in cancer and immune cells. Biochim Biophys Acta. 2013;1831:1009–1015. doi: 10.1016/j.bbalip.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 54.Freed-Pastor W.A., Mizuno H., Zhao X., Langerod A., Moon S.H., Rodriguez-Barrueco R., et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell. 2012;148:244–258. doi: 10.1016/j.cell.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gober H.J., Kistowska M., Angman L., Jeno P., Mori L., De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nussbaumer O., Gruenbacher G., Gander H., Komuczki J., Rahm A., Thurnher M. Essential requirements of zoledronate-induced cytokine and gammadelta T cell proliferative responses. J Immunol. 2013;191:1346–1355. doi: 10.4049/jimmunol.1300603. [DOI] [PubMed] [Google Scholar]
- 57.Gruenbacher G., Nussbaumer O., Gander H., Steiner B., Leonhartsberger N., Thurnher M. Stress-related and homeostatic cytokines regulate Vgamma9Vdelta2 T-cell surveillance of mevalonate metabolism. Oncoimmunology. 2014;3 doi: 10.4161/21624011.2014.953410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vantourout P., Laing A., Woodward M.J., Zlatareva I., Apolonia L., Jones A.W., et al. Heteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing gammadelta T cell biology. Proc Natl Acad Sci U S A. 2018;115:1039–1044. doi: 10.1073/pnas.1701237115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sandstrom A., Peigne C.M., Leger A., Crooks J.E., Konczak F., Gesnel M.C., et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vgamma9Vdelta2 T cells. Immunity. 2014;40:490–500. doi: 10.1016/j.immuni.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abeler-Dorner L., Swamy M., Williams G., Hayday A.C., Bas A. Butyrophilins. an emerging family of immune regulators. Trends Immunol. 2012;33:34–41. doi: 10.1016/j.it.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 61.Blazquez J.L., Benyamine A., Pasero C., Olive D. New Insights Into the Regulation of gammadelta T Cells by BTN3A and Other BTN/BTNL in Tumor Immunity. Front Immunol. 2018;9:1601. doi: 10.3389/fimmu.2018.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson K., Roelofs A.J., Jauhiainen M., Monkkonen H., Monkkonen J., Rogers M.J. Activation of gammadelta T cells by bisphosphonates. Adv Exp Med Biol. 2010;658:11–20. doi: 10.1007/978-1-4419-1050-9_2. [DOI] [PubMed] [Google Scholar]
- 63.Chargui J., Combaret V., Scaglione V., Iacono I., Peri V., Valteau-Couanet D., et al. Bromohydrin pyrophosphate-stimulated Vgamma9delta2 T cells expanded ex vivo from patients with poor-prognosis neuroblastoma lyse autologous primary tumor cells. J Immunother. 2010;33:591–598. doi: 10.1097/CJI.0b013e3181dda207. [DOI] [PubMed] [Google Scholar]
- 64.Wada I., Matsushita H., Noji S., Mori K., Yamashita H., Nomura S., et al. Intraperitoneal injection of in vitro expanded Vgamma9Vdelta2 T cells together with zoledronate for the treatment of malignant ascites due to gastric cancer. Cancer Med. 2014;3:362–375. doi: 10.1002/cam4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilhelm M., Smetak M., Schaefer-Eckart K., Kimmel B., Birkmann J., Einsele H., et al. Successful adoptive transfer and in vivo expansion of haploidentical gammadelta T cells. J Transl Med. 2014;12:45. doi: 10.1186/1479-5876-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alnaggar M., Xu Y., Li J., He J., Chen J., Li M., et al. Allogenic Vgamma9Vdelta2 T cell as new potential immunotherapy drug for solid tumor: a case study for cholangiocarcinoma. J Immunother Cancer. 2019;7:36. doi: 10.1186/s40425-019-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gnant M., Mlineritsch B., Stoeger H., Luschin-Ebengreuth G., Heck D., Menzel C., et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12:631–641. doi: 10.1016/s1470-2045(11)70122-x. [DOI] [PubMed] [Google Scholar]
- 68.Coscia M., Vitale C., Peola S., Foglietta M., Rigoni M., Griggio V., et al. Dysfunctional Vgamma9Vdelta2 T cells are negative prognosticators and markers of dysregulated mevalonate pathway activity in chronic lymphocytic leukemia cells. Blood. 2012;120:3271–3279. doi: 10.1182/blood-2012-03-417519. [DOI] [PubMed] [Google Scholar]
- 69.Morgan G.J., Davies F.E., Gregory W.M., Cocks K., Bell S.E., Szubert A.J., et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet (London, England) 2010;376:1989–1999. doi: 10.1016/s0140-6736(10)62051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jungbluth A.A., Frosina D., Fayad M., Pulitzer M.P., Dogan A., Busam K.J., et al. Immunohistochemical Detection of gamma/delta T Lymphocytes in Formalin-fixed Paraffin-embedded Tissues. Appl Immunohistochem Mol Morphol. 2018 Mar 6 doi: 10.1097/PAI.0000000000000650. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gentles A.J., Newman A.M., Liu C.L., Bratman S.V., Feng W., Kim D., et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cordova A., Toia F., La Mendola C., Orlando V., Meraviglia S., Rinaldi G., et al. Characterization of human gammadelta T lymphocytes infiltrating primary malignant melanomas. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meraviglia S., Lo Presti E., Tosolini M., La Mendola C., Orlando V., Todaro M., et al. Distinctive features of tumor-infiltrating gammadelta T lymphocytes in human colorectal cancer. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2017.1347742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamb L.S., Jr., Henslee-Downey P.J., Parrish R.S., Godder K., Thompson J., Lee C., et al. Increased frequency of TCR gamma delta + T cells in disease-free survivors following T cell-depleted, partially mismatched, related donor bone marrow transplantation for leukemia. J Hematother. 1996;5:503–509. doi: 10.1089/scd.1.1996.5.503. [DOI] [PubMed] [Google Scholar]
- 75.Lamb L.S., Jr., Gee A.P., Hazlett L.J., Musk P., Parrish R.S., O'Hanlon T.P., et al. Influence of T cell depletion method on circulating gammadelta T cell reconstitution and potential role in the graft-versus-leukemia effect. Cytotherapy. 1999;1:7–19. doi: 10.1080/0032472031000141295. [DOI] [PubMed] [Google Scholar]
- 76.Godder K.T., Henslee-Downey P.J., Mehta J., Park B.S., Chiang K.Y., Abhyankar S., et al. Long term disease-free survival in acute leukemia patients recovering with increased gammadelta T cells after partially mismatched related donor bone marrow transplantation. Bone Marrow Transplant. 2007;39:751–757. doi: 10.1038/sj.bmt.1705650. [DOI] [PubMed] [Google Scholar]
- 77.Knight A., Madrigal A.J., Grace S., Sivakumaran J., Kottaridis P., Mackinnon S., et al. The role of Vdelta2-negative gammadelta T cells during cytomegalovirus reactivation in recipients of allogeneic stem cell transplantation. Blood. 2010;116:2164–2172. doi: 10.1182/blood-2010-01-255166. [DOI] [PubMed] [Google Scholar]
- 78.Scheper W., van Dorp S., Kersting S., Pietersma F., Lindemans C., Hol S., et al. gammadeltaT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia. 2013;27:1328–1338. doi: 10.1038/leu.2012.374. [DOI] [PubMed] [Google Scholar]
- 79.Halary F., Pitard V., Dlubek D., Krzysiek R., de la Salle H., Merville P., et al. Shared reactivity of V{delta}2(neg) {gamma}{delta} T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J Exp Med. 2005;201:1567–1578. doi: 10.1084/jem.20041851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Airoldi I., Bertaina A., Prigione I., Zorzoli A., Pagliara D., Cocco C., et al. gammadelta T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-alphabeta+/CD19+ lymphocytes. Blood. 2015;125:2349–2358. doi: 10.1182/blood-2014-09-599423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Elmaagacli A.H., Steckel N.K., Koldehoff M., Hegerfeldt Y., Trenschel R., Ditschkowski M., et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118:1402–1412. doi: 10.1182/blood-2010-08-304121. [DOI] [PubMed] [Google Scholar]
- 82.Couzi L., Levaillant Y., Jamai A., Pitard V., Lassalle R., Martin K., et al. Cytomegalovirus-induced gammadelta T cells associate with reduced cancer risk after kidney transplantation. J Am Soc Nephrol. 2010;21:181–188. doi: 10.1681/ASN.2008101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu D., Wu P., Wu X., Ye J., Wang Z., Zhao S., et al. Ex vivo expanded human circulating Vdelta1 gammadeltaT cells exhibit favorable therapeutic potential for colon cancer. Oncoimmunology. 2015;4 doi: 10.4161/2162402X.2014.992749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fisher J.P., Yan M., Heuijerjans J., Carter L., Abolhassani A., Frosch J., et al. Neuroblastoma killing properties of Vdelta2 and Vdelta2-negative gammadeltaT cells following expansion by artificial antigen-presenting cells. Clin Cancer Res. 2014;20:5720–5732. doi: 10.1158/1078-0432.CCR-13-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Almeida A.R., Correia D.V., Fernandes-Platzgummer A., da Silva C.L., da Silva M.G., Anjos D.R., et al. Delta One T Cells for Immunotherapy of Chronic Lymphocytic Leukemia: Clinical-Grade Expansion/Differentiation and Preclinical Proof of Concept. Clin Cancer Res. 2016;22:5795–5804. doi: 10.1158/1078-0432.CCR-16-0597. [DOI] [PubMed] [Google Scholar]
- 86.Di Lorenzo B., Simoes A.E., Caiado F., Tieppo P., Correia D.V., Carvalho T., et al. Broad Cytotoxic Targeting of Acute Myeloid Leukemia by Polyclonal Delta One T Cells. Cancer Immunol Res. 2019;7(4):552–558. doi: 10.1158/2326-6066.CIR-18-0647. [DOI] [PubMed] [Google Scholar]
- 87.Rosenberg S.A., Yang J.C., Sherry R.M., Kammula U.S., Hughes M.S., Phan G.Q., et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.Ccr-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Besser M.J., Shapira-Frommer R., Itzhaki O., Treves A.J., Zippel D.B., Levy D., et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res. 2013;19:4792–4800. doi: 10.1158/1078-0432.Ccr-13-0380. [DOI] [PubMed] [Google Scholar]
- 89.Donia M., Ellebaek E., Andersen M.H., Straten P.T., Svane I.M. Analysis of Vdelta1 T cells in clinical grade melanoma-infiltrating lymphocytes. Oncoimmunology. 2012;1:1297–1304. doi: 10.4161/onci.21659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fisher J., Anderson J. Engineering Approaches in Human Gamma Delta T Cells for Cancer Immunotherapy. Front Immunol. 2018;9:1409. doi: 10.3389/fimmu.2018.01409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Starick L., Riano F., Karunakaran M.M., Kunzmann V., Li J., Kreiss M., et al. Butyrophilin 3A (BTN3A, CD277)-specific antibody 20.1 differentially activates Vgamma9Vdelta2 TCR clonotypes and interferes with phosphoantigen activation. Eur J Immunol. 2017;47:982–992. doi: 10.1002/eji.201646818. [DOI] [PubMed] [Google Scholar]
- 92.Oberg H.H., Peipp M., Kellner C., Sebens S., Krause S., Petrick D., et al. Novel bispecific antibodies increase gammadelta T-cell cytotoxicity against pancreatic cancer cells. Cancer Res. 2014;74:1349–1360. doi: 10.1158/0008-5472.CAN-13-0675. [DOI] [PubMed] [Google Scholar]
- 93.Lamb L.S., Jr., Bowersock J., Dasgupta A., Gillespie G.Y., Su Y., Johnson A., et al. Engineered drug resistant gammadelta T cells kill glioblastoma cell lines during a chemotherapy challenge: a strategy for combining chemo- and immunotherapy. PLoS One. 2013;8 doi: 10.1371/journal.pone.0051805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilhelm M., Kunzmann V., Eckstein S., Reimer P., Weissinger F., Ruediger T., et al. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 95.Dieli F., Gebbia N., Poccia F., Caccamo N., Montesano C., Fulfaro F., et al. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102:2310–2311. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 96.Dieli F., Vermijlen D., Fulfaro F., Caccamo N., Meraviglia S., Cicero G., et al. Targeting human {gamma}delta} T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.Can-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meraviglia S., Eberl M., Vermijlen D., Todaro M., Buccheri S., Cicero G., et al. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161:290–297. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bennouna J., Levy V., Sicard H., Senellart H., Audrain M., Hiret S., et al. Phase I study of bromohydrin pyrophosphate (BrHPP, IPH 1101), a Vgamma9Vdelta2 T lymphocyte agonist in patients with solid tumors. Cancer Immunol Immunother. 2010;59:1521–1530. doi: 10.1007/s00262-010-0879-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lang J.M., Kaikobad M.R., Wallace M., Staab M.J., Horvath D.L., Wilding G., et al. Pilot trial of interleukin-2 and zoledronic acid to augment gammadelta T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1447–1460. doi: 10.1007/s00262-011-1049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kunzmann V., Smetak M., Kimmel B., Weigang-Koehler K., Goebeler M., Birkmann J., et al. Tumor-promoting versus tumor-antagonizing roles of gammadelta T cells in cancer immunotherapy: results from a prospective phase I/II trial. J Immunother. 2012;35:205–213. doi: 10.1097/CJI.0b013e318245bb1e. [DOI] [PubMed] [Google Scholar]
- 101.Pressey J.G., Adams J., Harkins L., Kelly D., You Z., Lamb L.S., Jr. In vivo expansion and activation of gammadelta T cells as immunotherapy for refractory neuroblastoma: A phase 1 study. Medicine. 2016;95 doi: 10.1097/md.0000000000004909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kobayashi H., Tanaka Y., Yagi J., Osaka Y., Nakazawa H., Uchiyama T., et al. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56:469–476. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bennouna J., Bompas E., Neidhardt E.M., Rolland F., Philip I., Galea C., et al. Phase-I study of Innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1599–1609. doi: 10.1007/s00262-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abe Y., Muto M., Nieda M., Nakagawa Y., Nicol A., Kaneko T., et al. Clinical and immunological evaluation of zoledronate-activated Vgamma9gammadelta T-cell-based immunotherapy for patients with multiple myeloma. Exp Hematol. 2009;37:956–968. doi: 10.1016/j.exphem.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 105.Nakajima J., Murakawa T., Fukami T., Goto S., Kaneko T., Yoshida Y., et al. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous gammadelta T cells. Eur J Cardiothorac Surg. 2010;37:1191–1197. doi: 10.1016/j.ejcts.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 106.Kobayashi H., Tanaka Y., Yagi J., Minato N., Tanabe K. Phase I/II study of adoptive transfer of gammadelta T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1075–1084. doi: 10.1007/s00262-011-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nicol A.J., Tokuyama H., Mattarollo S.R., Hagi T., Suzuki K., Yokokawa K., et al. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br J Cancer. 2011;105:778–786. doi: 10.1038/bjc.2011.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sakamoto M., Nakajima J., Murakawa T., Fukami T., Yoshida Y., Murayama T., et al. Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded gammadeltaTcells: a phase I clinical study. J Immunother. 2011;34:202–211. doi: 10.1097/CJI.0b013e318207ecfb. [DOI] [PubMed] [Google Scholar]
- 109.Izumi T., Kondo M., Takahashi T., Fujieda N., Kondo A., Tamura N., et al. Ex vivo characterization of gammadelta T-cell repertoire in patients after adoptive transfer of Vgamma9Vdelta2 T cells expressing the interleukin-2 receptor beta-chain and the common gamma-chain. Cytotherapy. 2013;15:481–491. doi: 10.1016/j.jcyt.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 110.Kakimi K., Matsushita H., Murakawa T., Nakajima J. gammadelta T cell therapy for the treatment of non-small cell lung cancer. Transl Lung Cancer Res. 2014;3:23–33. doi: 10.3978/j.issn.2218-6751.2013.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]