Abstract
Recent studies have suggested that subjective age—a subjective evaluation of one's own age—is a promising construct in gerontology that may contribute our understanding of risk for immune dysfunction. Nevertheless, studies documenting the association between subjective age and inflammatory biomarkers remain limited and provide mixed findings. In the present study, we revisited the relation between subjective age and systemic inflammation by utilizing a range of well-established inflammatory biomarkers (C-reactive protein, interleukin-6, fibrinogen, E-selectin, and intercellular adhesion molecule 1) through the collection of fasting blood samples before breakfast. In a large-scale dataset of midlife adults (N = 1800), we found some evidence that an older subjective age is associated with elevated inflammation when indexed by C-reactive protein and fibrinogen, as well as a composite inflammation score. However, these relations were not significant when health variables were controlled for, suggesting that the association between subjective age and systemic inflammation is fully accounted for by better health profiles among those with a younger subjective age. Additionally, the subjective age-inflammation association was influenced by slight variations in the analytic method, highlighting the importance of sensitivity analyses in this area.
Keywords: Subjective age, C-reactive protein, Interleukin-6, Fibrinogen, E-Selectin, Intercellular adhesion molecule 1
Highlights
-
•
Subjective age predicted a composite score comprising five inflammatory biomarkers.
-
•
Subjective age is linked with elevated C-reactive protein and fibrinogen.
-
•
Subjective age-systemic inflammation link is fully accounted by health profiles.
-
•
Subjective age predicted the composite score and fibrinogen after correcting for multiple comparisons.
-
•
Variation in analyses can influence subjective age-inflammation associations.
1. Introduction
Elevated levels of systemic inflammatory biomarkers, such as interleukin-6, C-reactive protein, and fibrinogen, have been shown to be associated with multiple health-related risks, such as cardiovascular disease [1,2] and dementia [3,4], in midlife and older adults. Given the importance of inflammatory biomarkers in the development of these diseases, a considerable number of studies have sought to identify modifiable psychosocial factors that are associated with systemic inflammatory biomarkers [[5], [6], [7]]. One promising psychological factor that may contribute to the elevation of inflammatory biomarkers is subjective age—how old or young individuals experience themselves to be, relative to their chronological age [8]. To date, only two studies have investigated the association between subjective age and C-reactive protein, and their findings were mixed [8,9]. Thus, the current study aims to revisit and examine the association between subjective age and systemic inflammation indexed with five well-established inflammatory biomarkers, including C-reactive protein, interleukin-6, fibrinogen, E-selectin, and intercellular adhesion molecule 1 (ICAM-1), using a large-scale dataset.
Age-related changes in inflammatory markers is one of the most robust phenomena in gerontology and geriatrics [10]. More importantly, research has consistently demonstrated the predictive value of inflammatory biomarkers on multiple age-related health outcomes, including cardiovascular disease [1,2], diabetes [11,12], and cognitive decline [13,14]. Given this, researchers have sought to examine modifiable psychological factors that may be associated with elevated levels of inflammatory markers, such as stress [6,15], affective state [16], perceived social obligation [17], and purpose in life [5].
A potential psychological factor that has emerged in the literature is subjective aging, which has been shown to be malleable and sensitive to external factors [[18], [19], [20]]. Although chronological age has been associated with elevated inflammatory markers, aging can also be construed as a subjective experience. Notably, a growing body of research has highlighted the construct of subjective age and its implications for important health outcomes, including cognitive functioning [21,22], depressive symptoms [23], and risk of mortality [24]. An older subjective age—feeling older relative to one's chronological age—was associated with worsened health outcomes. Importantly, feeling younger than one's age could suggest a more favorable health profile, thereby accounting for lower systemic inflammation [22].
Given that elevated levels of inflammation are often implicated in poor health outcomes, it stands to reason that subjective age could be associated with levels of inflammatory biomarkers. In favor of this view, Stephan et al. [8] analyzed data from the 2008 Health and Retirement Study and found a relationship between older subjective age and increased levels of inflammatory biomarkers such as C-reactive protein [22]. Notably, it was found that a more favorable health profile among those with younger subjective age—lower risk of obesity, frequent physical activity, and lower disease burden—partially accounted for the relation between subjective age and C-reactive protein. However, recent study by Thyagarajan et al. [9] analyzed data from the 2016 Health and Retirement Study and failed to find significant association between C-reactive protein and subjective age, casting doubt on the relationship between subjective age and inflammatory biomarkers. It is noteworthy that both studies are largely comparable in terms of participants’ characteristics and blood samples were similarly assayed using a standard enzyme-linked immunosorbent assay (ELISA).
In view of these mixed findings, the present study seeks to replicate the association between subjective age and systemic inflammation. More importantly, we attempt to expand existing findings in C-reactive protein by including more well-established biomarkers related to systemic inflammation including interleukin-6, fibrinogen, E-selectin, and ICAM-1 [25,26]. Taken together, we hypothesized positive relationships between an individual's subjective age and inflammation levels indicated by the biomarkers.
2. Methods
2.1. Participants
The present study involved data from the National Survey of Midlife Development in the United States (MIDUS) projects, namely the MIDUS 2: Biomarker Project (N = 1054) and MIDUS Refresher Biomarker studies (N = 746). The MIDUS 2: Biomarker Project [27] was conducted from 2004 to 2009 and is a subset of the original MIDUS 1 baseline cohort comprising a national probability sample of 7108 non-institutionalized English-speaking American adults, aged 24 to 74, recruited through random digit sampling. The MIDUS Refresher: Biomarker Project [28] was conducted from 2012 to 2016 with a younger distinct cohort and is a subset of a national probability sample of 3577 adults, aged 25 to 74, designed to replenish the original MIDUS 1 baseline cohort. In the MIDUS 2: Biomarker Project and MIDUS Refresher Biomarker studies, participants were invited to visit one of the three clinical research centers (University of Wisconsin-Madison; University of California, Los Angeles; and Georgetown University) for an overnight hospital stay for a comprehensive health assessment that included levels of interleukin-6, C-reactive protein, fibrinogen, E-selectin, and ICAM-1 through the collection of a fasting blood sample before breakfast.
Given that both studies utilized the same data collection methodology, the datasets were combined to strengthen the power of the analyses. Participants’ characteristics are summarized in Table 1. Data collection for both studies were approved by the Health Sciences IRBs at the University of Wisconsin-Madison. All participants provided written consent prior to participation.
Table 1.
Descriptive statistics for demographics, health status, health behaviors, subjective age, and inflammation levels.
| N | M | (SD) | Range | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 1800 | 53.75 | (12.69) | 25–84 | |
| Sex (% male) | 1800 | 47.28% | |||
| Race (% white) | 1795 | 87.91% | |||
| Race (% black) | 1795 | 4.62% | |||
| Race (% other) | 1795 | 7.47% | |||
| Educationa | 1796 | 8.02 | (2.43) | 1–12 | |
| Health | |||||
| Depressive symptoms | 1796 | 8.30 | (7.61) | 0–49 | |
| Vigorous physical activity | 1788 | 3.30 | (1.86) | 1–6 | |
| Moderate physical activity | 1779 | 2.58 | (1.65) | 1–6 | |
| Smoking (% current/former smokers) | 1800 | 46.72% | |||
| BMI | 1740 | 28.26 | (6.15) | 14.23–66.26 | |
| Disease burden | 1788 | 2.46 | (2.58) | 0–29 | |
| Subjective age discrepancyb | 1763 | 0.15 | (0.36) | −12.51–0.95 | |
| Inflammation biomarkers | |||||
| Interleukin-6 (pg/mL) | 1784 | 2.76 | (2.61) | 0.12–23.00 | |
| C-reactive protein (ug/mL) | 1779 | 2.73 | (4.71) | 0.03–79.30 | |
| Fibrinogen (mg/dL) | 1779 | 340.38 | (79.38) | 45.00–759.00 | |
| E-selectin (ng/mL) | 1784 | 41.01 | (20.34) | 0.09–175.00 | |
| ICAM-1 (ng/mL) | 1784 | 279.17 | (140.53) | 30.00–3334.92 | |
Education attainment was rated on a scale of 1 (No school) to 12 (PhD, EdD, MD, LLB, LLD, JD, or other professional degree).
Higher values represent younger subjective age. Three participants who were outliers on subjective age were excluded from the analyses.
2.2. Measures
2.2.1. Subjective age
Consistent with previous studies [8,29], subjective age was operationalized by a question: “Many people feel older or younger than they actually are. What age do you feel most of the time?“. Proportional discrepancy scores were calculated by subtracting each participant's reported subjective age from their chronological age and dividing the obtained difference by their chronological age [8,29,30]. Proportional discrepancy scores more than three standard deviations from the mean were excluded from our analysis as outliers (n = 3). Higher proportional discrepancy scores reflect lower subjective age.
2.2.2. Inflammation biomarkers
Systemic inflammation level was indexed by all five available inflammatory biomarkers in the original MIDUS Biomarker Project: interleukin-6, C-reactive protein, fibrinogen, E-selectin, and ICAM-1, consistent with the previous studies examining inflammatory markers using the same dataset [[31], [32], [33]]. All biomarker measurements were log-transformed and winsorized at 3 SD to normalize their distributions. Interleukin-6 was measured using the Quantikine® High-sensitivity ELISA kit #HS600B with inter-assay and intra-assay CVs of 12.31% and 3.25% in MIDUS 2 and 15.66% and 3.73% in MIDUS Refresher. C-reactive protein was measured using the BNII nephelometer from Dade Behring with inter-assay and intra-assay CVs of 2.1–5.7% and 2.3–4.4% in MIDUS 2 and 1.08–4.3% and 2.3–4.4% in MIDUS Refresher. However, if participants’ measures were below the assay range measured with the BNII nephelometer, the Meso Scale Diagnostics #K151STG high-sensitivity kit was used instead. Fibrinogen was measured using the BNII nephelometer by Dade Behring (MIDUS 2) and Siemens (MIDUS Refresher) with inter-assay and intra-assay CVs of 2.6% and 2.7% in MIDUS 2 and 4.13–6.64% and 2.7% in MIDUS Refresher. Soluble E-selectin was measured by a high sensitivity ELISA assay (Parameter Human sE-Selectin Immunoassay) in MIDUS 2 with inter-assay and intra-assay CVs of 5.7–8.8% and 4.7–5.0%, and by sandwich ELISA using Quantikine® kit #SSLE00 in MIDUS Refresher with inter-assay and intra-assay CVs of 7.1–11.15% and 5.2–6.6%. ICAM-1 was measured by an ELISA assay in MIDUS 2 with an inter-assay CV of 5.0%, and by a Quantikine® kit #SCD540 in MIDUS Refresher with inter-assay and intra-assay CVs of 7.49–8.16% and 3.7–5.2% respectively. Based on Hostinar et al. [31], we also computed a composite inflammatory biomarker score from interleukin-6, C-reactive protein, fibrinogen, E-selectin, and ICAM-1. The score was constructed by standardizing each of the five individual biomarkers and then averaging the standardized scores.
2.3. Data analysis
The current study aimed to examine the association between subjective age and systemic inflammation levels indexed by five inflammatory biomarkers: interleukin-6, C-reactive protein, fibrinogen, E-selectin, and ICAM-1. For each inflammatory biomarker, ordinary least squares regression was performed with the proportional discrepancy score of subjective age as the predictor. Similar analyses were also conducted for inflammation composite score as a criterion. The analytic method in our main analyses closely followed the seminal study by Stephan et al. [8] to minimize potential data analytic flexibility. Similar to Stephan et al. [8], two separate models were estimated for each criterion to ensure the robustness of the associations between subjective age and inflammation levels. In the first model, we controlled for demographic variables, such as age, gender, education attainment, and race, that may be associated with inflammation levels [34,35].
In the second model, we controlled for health status and health-related behaviors, including BMI, smoking status, depressive symptoms, disease burden (the total number of chronic diseases experienced in the past 12 months), and frequency of vigorous and moderate physical activity, due to their potential mediating role on the association between subjective age and inflammation levels [8]. For example, studies have shown that acute phase protein, such as C-reactive protein and fibrinogen, as well as plasma levels of the upstream regulators of C-reactive protein, such as interleukin-6, are sensitive inflammatory biomarkers for cigarette smoke-induced inflammation and found to be significantly elevated in smokers [[36], [37], [38]]. A number of studies have also found that regular and chronic physical activity helps modulate inflammatory processes and associated with reduction of inflammatory biomarkers such as C-reactive protein, interleukin-6, fibrinogen, E-selectin, and intercellular adhesion molecule-1 [[39], [40], [41]]. Similarly, higher BMI and higher depressive symptoms have been shown to be associated with higher levels of multiple inflammatory biomarkers, such as interleukin-6 and C-reactive protein [39,[42], [43], [44]]. Subsequently, we also tested whether the associations between subjective age and inflammation composite score were moderated by age, gender, race, and education.
Lastly, sensitivity analyses were conducted to ensure the robustness of the results to slight variations in the analytic method and adjustment for multiple comparisons. Analyses were conducted in R version 3.6.3 [45], and interactions were investigated using the R package interactions version 1.1.3 [46]. Additionally, Bayes Factors were computed using JASP version 0.13.1 [47].
3. Results
We conducted ordinary least squares regression to examine the predictability of proportional discrepancy score of subjective age on each inflammatory biomarker. As shown in Table 2, after controlling for demographics (Model 1), we found that proportional discrepancy score of subjective age significantly predicted lower levels of interleukin-6 (b = −0.09, SE = 0.04, 95% CI = [-0.17, −0.004], β = −0.05, p = .040, BF10 = 0.97), C-reactive protein (b = −0.16, SE = 0.07, 95% CI = [-0.29, −0.03], β = −0.06, p = .019, BF10 = 2.14), and fibrinogen (b = −0.03, SE = 0.01, 95% CI = [-0.06, −0.008], β = −0.06, p = .010, BF10 = 3.47), but not E-selectin (b = −0.03, SE = 0.03, 95% CI = [-0.09, 0.02], β = −0.03, p = .243, BF10 = 0.30) and ICAM-1 (b = −0.02, SE = 0.02, 95% CI = [-0.06, 0.02], β = −0.02, p = .348, BF10 = 0.21). The results suggest that younger subjective age is associated with lower levels of C-reactive protein and fibrinogen. However, after we controlled for health status and health behaviors (Model 2), the proportional discrepancy score of subjective age did not predict any inflammatory biomarkers; interleukin-6 (b = 0.03, SE = 0.04, 95% CI = [-0.05, 0.11], β = 0.02, p = .459, BF10 = 0.17), C-reactive protein (b = 0.03, SE = 0.06, 95% CI = [-0.10, 0.16], β = 0.01, p = .664, BF10 = 0.15), fibrinogen (b = −0.01, SE = 0.01, 95% CI = [-0.04, 0.02], β = −0.02, p = .499, BF10 = 0.20), E-selectin (b = 0.01, SE = 0.03, 95% CI = [-0.05, 0.07], β = 0.01, p = .638, BF10 = 0.20) and ICAM-1 (b = 0.01, SE = 0.02, 95% CI = [-0.04, 0.04], β = 0.01, p = .792, BF10 = 0.19).
Table 2.
Standard coefficient estimates of the subjective age discrepancy scores on Interleukin-6, fibrinogen, C-reactive protein, E-selectin, and ICAM-1.
| Interleukin-6 | C-reactive protein | Fibrinogen | E-selectin | ICAM-1 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |||||||||||
| (N = 1739) | (N = 1648) | (N = 1734) | (N = 1644) | (N = 1734) | (N = 1644) | (N = 1739) | (N = 1648) | (N = 1739) | (N = 1648) | |||||||||||
| Predictor | ||||||||||||||||||||
| Subjective age discrepancy a | −0.05 | * | 0.02 | −0.06 | * | 0.01 | −0.06 | ** | −0.02 | −0.03 | 0.01 | −0.02 | 0.01 | |||||||
| Covariates | ||||||||||||||||||||
| Age | 0.33 | *** | 0.28 | *** | 0.08 | ** | 0.04 | 0.21 | *** | 0.20 | *** | −0.05 | * | −0.08 | ** | −0.01 | −0.04 | |||
| Age squared | 0.02 | 0.04 | −0.02 | 0.01 | −0.05 | * | −0.02 | −0.03 | −0.02 | −0.01 | −0.01 | |||||||||
| Sex | 0.01 | 0.01 | 0.13 | *** | 0.16 | *** | 0.13 | *** | 0.16 | *** | −0.13 | *** | −0.13 | *** | 0.00 | −0.01 | ||||
| Race (black) | 0.08 | *** | 0.03 | 0.04 | −0.02 | 0.09 | *** | 0.03 | 0.04 | −0.01 | −0.19 | *** | −0.21 | *** | ||||||
| Race (other) | 0.04 | 0.03 | 0.01 | 0.00 | 0.04 | 0.03 | −0.01 | −0.02 | −0.06 | * | −0.07 | ** | ||||||||
| Education | −0.09 | *** | 0.00 | −0.13 | *** | −0.05 | * | −0.08 | ** | −0.04 | −0.10 | *** | −0.05 | −0.17 | *** | −0.11 | *** | |||
| BMI | 0.32 | *** | 0.40 | *** | 0.25 | *** | 0.22 | *** | 0.10 | *** | ||||||||||
| Smoking | 0.05 | * | 0.03 | −0.00 | 0.02 | 0.06 | ** | |||||||||||||
| Depressive symptoms | 0.05 | * | 0.02 | 0.05 | 0.00 | 0.06 | * | |||||||||||||
| Disease burden | 0.06 | * | 0.00 | −0.05 | * | 0.03 | 0.05 | |||||||||||||
| Vigorous physical activity | 0.08 | ** | 0.04 | 0.03 | 0.06 | 0.04 | ||||||||||||||
| Moderate physical activity | 0.05 | 0.07 | * | 0.03 | 0.05 | 0.04 | ||||||||||||||
Values reflected are standardized regression coefficients.
a Higher values represent younger subjective age.
*p < .05, **p < .01, ***p < .001.
We also examined the predictability of the proportional discrepancy score of subjective age on the composite score of inflammation (Table 3). After controlling for demographic covariates, we found that the proportional discrepancy score of subjective age significantly predicted the composite score of inflammation level (b = −0.23, SE = 0.08, 95% CI = [-0.39, −0.06], β = −0.06, p = .008, BF10 = 4.50). However, we did not find a significant association between the proportional discrepancy score of subjective age and the composite score of inflammation after controlling for health covariates (b = 0.04, SE = 0.08, 95% CI = [-0.12, 0.19], β = 0.01, p = .649, BF10 = 0.14).
Table 3.
Standard coefficient estimates of the subjective age discrepancy scores on the composite biomarker.
| Composite |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1 (N = 1740) | Model 2 (N = 1649) | ||||||||
| β | 95% CILB | 95% CIUB | p | β | 95% CILB | 95% CIUB | p | ||
| Predictor | |||||||||
| Subjective age discrepancy a | −0.06 | −0.11 | −0.02 | .008 | 0.01 | −0.03 | 0.06 | .649 | |
| Covariates | |||||||||
| Age | 0.17 | 0.12 | 0.22 | <.001 | 0.12 | 0.07 | 0.17 | <.001 | |
| Age squared | −0.03 | −0.08 | 0.02 | .206 | 0.00 | −0.05 | 0.04 | .896 | |
| Sex | 0.04 | 0.00 | 0.09 | .068 | 0.06 | 0.02 | 0.10 | .006 | |
| Race (black) | 0.02 | −0.03 | 0.06 | .430 | −0.05 | −0.10 | −0.01 | .014 | |
| Race (other) | 0.01 | −0.04 | 0.05 | .820 | −0.01 | −0.05 | 0.03 | .651 | |
| Education | −0.18 | −0.22 | −0.13 | <.001 | −0.08 | −0.12 | −0.03 | .001 | |
| BMI | 0.40 | 0.36 | 0.44 | <.001 | |||||
| Smoking | 0.05 | 0.01 | 0.09 | .023 | |||||
| Depressive symptoms | 0.06 | 0.01 | 0.10 | .020 | |||||
| Disease burden | 0.03 | −0.02 | 0.08 | .196 | |||||
| Vigorous physical activity | 0.07 | 0.02 | 0.13 | .011 | |||||
| Moderate physical activity | 0.08 | 0.02 | 0.13 | .008 | |||||
β = standardized regression coefficient.
95% CILB = 95% confidence interval, lower bound.
95% CIUB = 95% confidence interval, upper bound.
a Higher values represent younger subjective age.
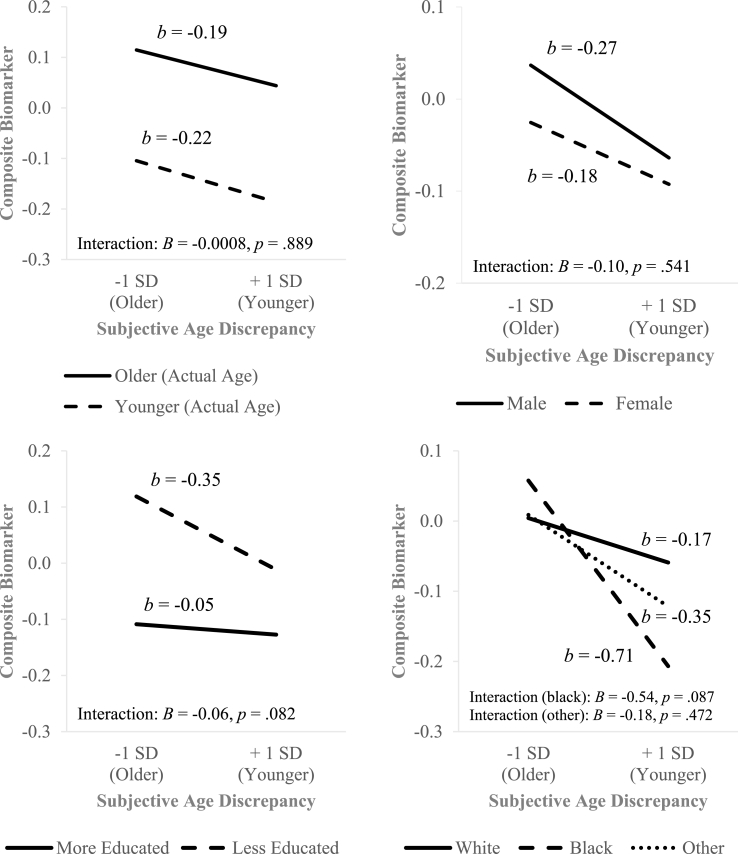
Furthermore, we conducted moderation analyses to examine the interaction between subjective age and demographic variables on inflammation levels in Model 1. We found that the relationship between the composite biomarker and subjective age discrepancy was not moderated by age, sex, education or race (see Fig. 1). We also did not find any evidence of the moderating effect of age, sex, education, or race on the association between subjective age and each individual inflammatory biomarker (interleukin-6, C-reactive protein, fibrinogen, E-selectin, and ICAM-1), with an exception for the interaction between subjective age and education in interleukin-6 (p = .005).
Fig. 1.
Association between subjective age discrepancy and composite inflammation biomarker across age, sex, education or race.
We also conducted sensitivity analyses to examine whether the significant associations between the proportional discrepancy score of subjective age and the inflammatory biomarkers in the Model 1 remained robust when we varied our method of analysis, such as using winsorization instead of excluding the outliers in the subjective age discrepancy score, using missing data imputation, or analyzing the MIDUS 2 and MIDUS Refresher datasets separately. When we winsorized rather than excluded the three outliers who had proportional discrepancy scores more than three standard deviations from the mean, we found that subjective age was still a significant predictor of lower levels of interleukin-6, fibrinogen, and the composite inflammatory biomarker score (ps < .05), except for C-reactive protein levels (b = −0.13, SE = 0.07, 95% CI = [-0.26, 0.00], β = −0.05, p = .051). In contrast, when multiple imputation (Rubin, 1987) was performed using a Markov chain Monte Carlo algorithm with a fully conditional specification to create five imputed datasets (each with N = 1797), subjective age significantly predicted levels of C-reactive protein, fibrinogen, as well as the composite score (ps < .05), but not interleukin-6 (b = −0.08, SE = 0.04, 95% CI = [-0.16, 0.01], β = −0.04, p = .070). Multiple imputation and pooling of results were carried out in R using mice version 3.10.0 [48] and miceadds version 3.9–14 [49].
When comparing subsamples, we found that subjective age was significantly associated with fibrinogen (b = −0.05, SE = 0.02, 95% CI = [-0.09, −0.003], β = −0.07, p = .038), but not with interleukin-6, C-reactive protein, or the composite score (ps > .05) in the MIDUS 2 dataset. In comparison, in the MIDUS Refresher dataset, we found significant associations between subjective age and C-reactive protein (b = −0.23, SE = 0.10, 95% CI = [-0.43, −0.04], β = −0.09, p = .016) as well as the composite score (b = −0.33, SE = 0.12, 95% CI = [-0.57, −0.09], β = −0.10, p = .008), while the associations with interleukin-6 and fibrinogen were non-significant (ps > .05). These findings from the sensitivity analyses as a whole suggest that slight variations of the analysis method may influence the association between subjective age and inflammatory biomarkers.
Lastly, we computed adjusted p-values for the six outcomes in Model 1 using the Hommel procedure based on recommendations for multiple outcomes [50]. Based on the adjusted p-values, the proportional discrepancy score of subjective age significantly predicted only fibrinogen (p = .045) and the composite score (p = .040). Interleukin-6 (p = .117), C-reactive protein (p = .076), E-selectin (p = .380), and ICAM-1 (p = .380) were not significantly predicted by subjective age after adjustments for multiple comparisons.1
4. Discussion and conclusions
In the present study, we examined the relationship between subjective age and systemic inflammation using a large sample of midlife adults. By employing five inflammatory biomarkers—interleukin-6, C-reactive protein, fibrinogen, E-selectin, and ICAM-1—we found some evidence supporting the association between subjective age and systemic inflammation. Specifically, higher subjective age was significantly associated with elevated levels of C-reactive protein and fibrinogen, as well as the composite inflammation score after controlling for demographics and socioeconomic status (SES). These findings are consistent with prior literature demonstrating the association between subjective age and C-reactive protein [8].
However, while Stephan et al. [8] found that better health profiles partially accounted for the association between subjective age and levels of C-reactive protein, the links between subjective age and levels of inflammatory biomarkers in the current study were not significant once we controlled for health status and health-related behaviors. These findings suggest that the association between younger subjective age and lower levels of systemic inflammation were fully accounted for by better health profiles among those with a younger subjective age, such as lower depressive symptoms [23] and lower BMI [51]. Therefore, individuals' health profiles are likely to be an important pathway that links subjective age and systemic inflammation. While younger subjective age may increase one's tendency to engage in healthier lifestyles, such as more physical exercises [52], it is also plausible that subjective age may simply reflect biomarker profiles that are indicative of one's health status.
Despite the positive findings related to the associations between subjective age and systemic inflammation, we also observed that slight variations of the analysis method may influence the association between subjective age and levels of inflammatory biomarkers. For instance, we found that the association between subjective age and interleukin-6 was not significant once missing data was imputed using multiple imputation. The findings from our sensitivity analyses may suggest that different analytic methods could possibly contribute to the mixed findings in the previous studies [8,9], especially since both studies area largely comparable in terms of participants’ demographics and data collection methodology, thereby highlighting the importance of sensitivity analyses in examining the association between subjective age and systemic inflammation.
The mixed findings also could be contributed by the possibility that the effect size in the relations between subjective age and inflammatory biomarkers are small. In the current study, we observed that the standardized beta coefficients ranged between −0.05 and −0.06 from a relatively large sample. This could explain why the results were inconsistent within our subsamples. Furthermore, it is noteworthy that the links between subjective age and elevated C-reactive protein as well as interleukin-6 were no longer significant after the adjustment for multiple comparisons.
Although the current study employed a large sample size and indexed systemic inflammation with five well-established inflammatory biomarkers, the current study was not without limitations. For instance, given the cross-sectional design of the current study, reverse causation is plausible, in that individuals’ levels of inflammation could also affect how young they feel. Further, the participants were predominantly American midlife adults, and thus our findings may not be generalizable to other cultures, racial/ethnic groups, and age cohorts. Taken together, it is imperative for future studies to replicate and ascertain the direction of the association between subjective age and systemic inflammation with more diverse samples and rigorous sensitivity analyses.
Funding
The data of this research was supported by grants from the NIH National Institute on Aging (P01-AG020166) to conduct the MIDUS II and MIDUS Refresher baseline surveys. The biomarker projects were further supported by the NIH National Center for Advancing Translational Sciences (NCATS) Clinical and Translational Science Award (CTSA) program (UL1TR001409, UL1TR001881, & 1UL1RR025011) as well as the NIH National Institute on Aging (5P01AG020166).
Declaration of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
In the Supplementary Materials, we also reported exploratory analyses on serum cytokine concentrations that were newly added in the dataset using a V-plex Custom Human Cytokine Kit (catalog #K151A0H-2) manufactured by Meso Scale Diagnostics (MSD), including interleukin-6, interleukin-8, interleukin-8, and tumor necrosis factor alpha (via Immunoelectrochemiluminescent). We thank our reviewer for the suggestion.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpnec.2021.100072.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Anderson L. Candidate-based proteomics in the search for biomarkers of cardiovascular disease: candidate-based proteomics in cardiovascular disease. J. Physiol. 2005;563:23–60. doi: 10.1113/jphysiol.2004.080473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cesari M., Penninx B.W.J.H., Newman A.B., Kritchevsky S.B., Nicklas B.J., Sutton-Tyrrell K., Rubin S.M., Ding J., Simonsick E.M., Harris T.B., Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC Study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 3.Ader R., Godbout J.P., Johnson R.W. In: Aging, Neuroinflammation and Behavior. Ader R., editor. Elsevier/Academic Press; Boston: 2009. pp. 322–345. (Psychoneuroimmunology). [Google Scholar]
- 4.Godbout J.P., Johnson R.W. Age and neuroinflammation: a lifetime of psychoneuroimmune consequences. Neurol. Clin. 2006;24:521–538. doi: 10.1016/j.ncl.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Friedman E.M., Hayney M., Love G.D., Singer B.H., Ryff C.D. Plasma interleukin-6 and soluble IL-6 receptors are associated with psychological well-being in aging women. Health Psychol. 2007;26:305–313. doi: 10.1037/0278-6133.26.3.305. [DOI] [PubMed] [Google Scholar]
- 6.Hänsel A., Hong S., Cámara R.J.A., von Känel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci. Biobehav. Rev. 2010;35:115–121. doi: 10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Kitayama S., Park J., Boylan J.M., Miyamoto Y., Levine C.S., Markus H.R., Karasawa M., Coe C.L., Kawakami N., Love G.D., Ryff C.D. Expression of anger and ill health in two cultures: an examination of inflammation and cardiovascular risk. Psychol. Sci. 2015;26:211–220. doi: 10.1177/0956797614561268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephan Y., Sutin A.R., Terracciano A. Younger subjective age is associated with lower C-reactive protein among older adults. Brain Behav. Immun. 2015;43:33–36. doi: 10.1016/j.bbi.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Thyagarajan B., Shippee N., Parsons H., Vivek S., Crimmins E., Faul J., Shippee T. How does subjective age get “under the skin”? The association between biomarkers and feeling older or younger than one's age: the Health and Retirement Study. Innovation in Aging. 2019;3 doi: 10.1093/geroni/igz035. igz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh T., Newman A.B. Inflammatory markers in population studies of aging. Ageing Res. Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertoni A.G., Burke G.L., Owusu J.A., Carnethon M.R., Vaidya D., Barr R.G., Jenny N.S., Ouyang P., Rotter J.I. Inflammation and the incidence of type 2 diabetes: the multi-ethnic study of atherosclerosis (MESA) Diabetes Care. 2010;33:804–810. doi: 10.2337/dc09-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dehghan A., Kardys I., de Maat M.P.M., Uitterlinden A.G., Sijbrands E.J.G., Bootsma A.H., Stijnen T., Hofman A., Schram M.T., Witteman J.C.M. Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes. 2007;56:872–878. doi: 10.2337/db06-0922. [DOI] [PubMed] [Google Scholar]
- 13.Weaver J.D., Huang M.-H., Albert M., Harris T., Rowe J.W., Seeman T.E. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi: 10.1212/WNL.59.3.371. [DOI] [PubMed] [Google Scholar]
- 14.Yaffe K., Lindquist K., Kluse M., Cawthon R., Harris T., Hsueh W.-C., Simonsick E.M., Kuller L., Li R., Ayonayon H.N., Rubin S.M., Cummings S.R. Telomere length and cognitive function in community-dwelling elders: findings from the Health ABC Study. Neurobiol. Aging. 2011;32:2055–2060. doi: 10.1016/j.neurobiolaging.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marsland A.L., Walsh C., Lockwood K., John-Henderson N.A. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav. Immun. 2017;64:208–219. doi: 10.1016/j.bbi.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stellar J.E., John-Henderson N., Anderson C.L., Gordon A.M., McNeil G.D., Keltner D. Positive affect and markers of inflammation: discrete positive emotions predict lower levels of inflammatory cytokines. Emotion. 2015;15:129–133. doi: 10.1037/emo0000033. [DOI] [PubMed] [Google Scholar]
- 17.Hartanto A., Lau Y.-M.I., Yong J.C. Culture moderates the link between perceived obligation and biological health risk: evidence for culturally distinct pathways to achieving positive health outcomes. Soc. Sci. Med. 2020;244:1–9. doi: 10.1016/j.socscimed.2019.112644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eibach R.P., Mock S.E., Courtney E.A. Having a “senior moment”: induced aging phenomenology, subjective age, and susceptibility to ageist stereotypes. J. Exp. Soc. Psychol. 2010;46:643–649. doi: 10.1016/j.jesp.2010.03.002. [DOI] [Google Scholar]
- 19.Geraci L., De Forrest R., Hughes M., Saenz G., Tirso R. The effect of cognitive testing and feedback on older adults' subjective age. Aging Neuropsychol. Cognit. 2018;25:333–350. doi: 10.1080/13825585.2017.1299853. [DOI] [PubMed] [Google Scholar]
- 20.Stephan Y., Chalabaev A., Kotter-Grühn D., Jaconelli A. “Feeling younger, being stronger”: an experimental study of subjective age and physical functioning among older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2013;68:1–7. doi: 10.1093/geronb/gbs037. [DOI] [PubMed] [Google Scholar]
- 21.Stephan Y., Sutin A.R., Caudroit J., Terracciano A. Subjective age and changes in memory in older adults. GERONB. 2016;71:675–683. doi: 10.1093/geronb/gbv010. [DOI] [PubMed] [Google Scholar]
- 22.Stephan Y., Boiché J., Canada B., Terracciano A. Association of personality with physical, social, and mental activities across the lifespan: findings from US and French samples. Br. J. Psychol. 2014;105:564–580. doi: 10.1111/bjop.12056. [DOI] [PubMed] [Google Scholar]
- 23.Choi N.G., DiNitto D.M. Felt age and cognitive-affective depressive symptoms in late life. Aging Ment. Health. 2014;18:833–837. doi: 10.1080/13607863.2014.886669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stephan Y., Sutin A.R., Terracciano A. Subjective age and mortality in three longitudinal samples. Psychosom. Med. 2018;80:659–664. doi: 10.1097/PSY.0000000000000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crimmins E., Vasunilashorn S., Kim J.K., Alley D. Biomarkers related to aging in human populations. Adv. Clin. Chem. 2008;46:161–216. doi: 10.1016/s0065-2423(08)00405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pahwa R., Goyal A., Bansal P., Jialal I. In: StatPearls, editor. StatPearls Publishing; Treasure Island (FL: 2020. Chronic inflammation.http://www.ncbi.nlm.nih.gov/books/NBK493173/ accessed. [Google Scholar]
- 27.Ryff C.D., Seeman T., Weinstein M. 2019. Midlife in the United States (MIDUS 2): Biomarker Project; pp. 2004–2009. [DOI] [Google Scholar]
- 28.Weinstein M., Ryff C.D., Seeman T.E. 2019. Midlife in the United States (MIDUS Refresher): Biomarker Project; pp. 2012–2016. [DOI] [Google Scholar]
- 29.Chen Y.T., Holahan C.K., Holahan C.J., Li X. Leisure-time physical activity, subjective age, and self-rated memory in middle-aged and older adults. Int. J. Aging Hum. Dev. 2018;87:377–391. doi: 10.1177/0091415017752939. [DOI] [PubMed] [Google Scholar]
- 30.Segel-Karpas D., Palgi Y. ‘It is nothing more than a senior moment’: the moderating role of subjective age in the effect of change in memory on self-rated memory. Aging Ment. Health. 2019;23:272–276. doi: 10.1080/13607863.2017.1399350. [DOI] [PubMed] [Google Scholar]
- 31.Hostinar C.E., Lachman M.E., Mroczek D.K., Seeman T.E., Miller G.E. Additive contributions of childhood adversity and recent stressors to inflammation at midlife: findings from the MIDUS study. Dev. Psychol. 2015;51:1630–1644. doi: 10.1037/dev0000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong A.D., Williams D.R. Lifetime discrimination, global sleep quality, and inflammation burden in a multiethnic sample of middle-aged adults. Cult. Divers Ethnic Minor. Psychol. 2019;25:82–90. doi: 10.1037/cdp0000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ransome Y., Slopen N., Karlsson O., Williams D.R. Elevated inflammation in association with alcohol abuse among Blacks but not Whites: results from the MIDUS biomarker study. J. Behav. Med. 2018;41:374–384. doi: 10.1007/s10865-017-9905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartanto A., Lee S.T.H., Yong J.C. Dispositional gratitude moderates the association between socioeconomic status and interleukin-6. Sci. Rep. 2019;9:802. doi: 10.1038/s41598-018-37109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Connor M.-F., Bower J.E., Cho H.J., Creswell J.D., Dimitrov S., Hamby M.E., Hoyt M.A., Martin J.L., Robles T.F., Sloan E.K., Thomas K.S., Irwin M.R. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersson B.-Å., Sayardoust S., Löfgren S., Rutqvist L.E., Laytragoon-Lewin N. Cigarette smoking affects microRNAs and inflammatory biomarkers in healthy individuals and an association to single nucleotide polymorphisms is indicated. Biomarkers. 2019;24:180–185. doi: 10.1080/1354750X.2018.1539764. [DOI] [PubMed] [Google Scholar]
- 37.Peres L.C., Bandera E.V., Qin B., Guertin K.A., Shivappa N., Hebert J.R., Abbott S.E., Alberg A.J., Barnholtz‐Sloan J., Bondy M., Cote M.L., Funkhouser E., Moorman P.G., Peters E.S., Schwartz A.G., Terry P.D., Camacho F., Wang F., Schildkraut J.M. Dietary inflammatory index and risk of epithelial ovarian cancer in African American women. Int. J. Canc. 2017;140:535–543. doi: 10.1002/ijc.30467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tibuakuu M., Kamimura D., Kianoush S., DeFilippis A.P., Rifai M.A., Reynolds L.M., White W.B., Butler K.R., Mosley T.H., Turner S.T., Kullo I.J., Hall M.E., Blaha M.J. The association between cigarette smoking and inflammation: the Genetic Epidemiology Network of Arteriopathy (GENOA) study. PloS One. 2017;12 doi: 10.1371/journal.pone.0184914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geffken D.F., Cushman M., Burke G.L., Polak J.F., Sakkinen P.A., Tracy R.P. Association between physical activity and markers of inflammation in a healthy elderly population. Am. J. Epidemiol. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 40.Gleeson M., Bishop N.C., Stensel D.J., Lindley M.R., Mastana S.S., Nimmo M.A. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 41.Saetre T., Enoksen E., Lyberg T., Stranden E., Jørgensen J.J., Sundhagen J.O., Hisdal J. Supervised exercise training reduces plasma levels of the endothelial inflammatory markers E-selectin and ICAM-1 in patients with peripheral arterial disease. Angiology. 2011;62:301–305. doi: 10.1177/0003319710385338. [DOI] [PubMed] [Google Scholar]
- 42.Wium-Andersen M.K., Ørsted D.D., Nielsen S.F., Nordestgaard B.G. Elevated C-reactive protein levels, psychological distress, and depression in 73 131 individuals. JAMA Psychiatry. 2013;70:176–184. doi: 10.1001/2013.jamapsychiatry.102. [DOI] [PubMed] [Google Scholar]
- 43.Kohut M.L., McCann D.A., Russell D.W., Konopka D.N., Cunnick J.E., Franke W.D., Castillo M.C., Reighard A.E., Vanderah E. Aerobic exercise, but not flexibility/resistance exercise, reduces serum IL-18, CRP, and IL-6 independent of β-blockers, BMI, and psychosocial factors in older adults. Brain Behav. Immun. 2006;20:201–209. doi: 10.1016/j.bbi.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Visser M., Bouter L.M., McQuillan G.M., Wener M.H., Harris T.B. Elevated C-reactive protein levels in overweight and obese adults. J. Am. Med. Assoc. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 45.R Core Team R. R Foundation for Statistical Computing; Vienna, Austria: 2020. A Language and Environment for Statistical Computing.https://www.r-project.org/ [Google Scholar]
- 46.Long J.A. 2019. Interactions: Comprehensive, User-Friendly Toolkit for Probing Interactions.https://cran.r-project.org/package=interactions [Google Scholar]
- 47.JASP Team . 2020. JASP.https://jasp-stats.org/ [Computer software] [Google Scholar]
- 48.van Buuren S., Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J. Stat. Software. 2011;45:1–67. doi: 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- 49.Robitzsch A., Grund S. 2020. Miceadds: Some Additional Multiple Imputation Functions, Especially for “Mice.https://CRAN.R-project.org/package=miceadds accessed. [Google Scholar]
- 50.Vickerstaff V., Omar R.Z., Ambler G. Methods to adjust for multiple comparisons in the analysis and sample size calculation of randomised controlled trials with multiple primary outcomes. BMC Med. Res. Methodol. 2019;19:129. doi: 10.1186/s12874-019-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stephan Y., Sutin A.R., Terracciano A. Subjective age and adiposity: evidence from five samples. Int. J. Obes. 2019;43:938–941. doi: 10.1038/s41366-018-0179-x. [DOI] [PubMed] [Google Scholar]
- 52.Wienert J., Kuhlmann T., Fink S., Hambrecht R., Lippke S. Testing principle working mechanisms of the health action process approach for subjective physical age groups. Res. Sports Med. 2016;24:67–83. doi: 10.1080/15438627.2015.1126277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.