Highlights
-
•
Helicobacter pylori was found to be highly prevalent in asymptomatic HIV-positive and negative patients.
-
•
The success of H. pylori eradication was limited, especially in HIV-infected patients.
-
•
There are possible high rates of antibiotic resistance and drug interactions in HIV patients.
KEYWORDS: Helicobacter, Oromia, Sub-Saharan Africa, Africa, Antimicrobial resistance
Abstract
Objectives
Helicobacter pylori is a widespread pathogen and major contributor to dyspeptic disease and gastric cancer. Although the interaction between HIV and H. pylori infection is not well investigated, previous studies have suggested a decreased prevalence of H. pylori and limited efficacy of eradication therapy in HIV-positive individuals. Therefore, the objectives of this study were to describe the prevalence of H. pylori infection according to HIV status and analyze the efficacy of eradication therapy in Ethiopia.
Methods
A prospective, randomized, interventional study was performed involving HIV-positive and negative participants presenting to the Asella Referral and Teaching Hospital in Central Ethiopia between March and June 2017. A stool antigen test was used as a screening tool for H. pylori infection. Randomly selected patients received triple eradication therapy.
Results
The cumulative H. pylori prevalence was 77.3% (392/507): 78.8% (241/306) among HIV-positive individuals versus 75.1% (151/201) among HIV-negative individuals (P = 0.386). Twenty-five HIV-positive and 26 HIV-negative H. pylori-infected participants were randomized to receive standard triple therapy; three of them were lost to follow-up (one HIV-positive, two HIV-negative). The total eradication rate was 50.0%: 62.5% (15/24) among those HIV-negative versus 37.5% (9/24) among those HIV-positive [Au?1].
Conclusions
A high prevalence of H. pylori was observed among HIV-positive and negative individuals in Central Ethiopia. The efficacy of eradication therapy was low, with a trend towards lower efficacy in HIV-infected individuals.
Introduction
Helicobacter pylori is one of the most common chronic infections in the world, with a prevalence ranging from 35% (95% confidence interval 30.2–39.3) in high-income countries (Zamani et al., 2018) to79–90% in low-income countries (Asrat et al., 2004a; Hooi et al., 2017). The infection is mostly asymptomatic but can manifest as dyspeptic disease, malignant complications such as gastric and esophageal cancer (Xie et al., 2013), and non-malignant complications, for example iron-deficiency anemia (Gravina et al., 2020).
A recently published meta-analysis on H. pylori prevalence in Ethiopia showed a wide range, from 7.7% in southern Ethiopia to 91% in Addis Ababa (Melese et al., 2019). Furthermore, studies have shown a declining trend in H. pylori infection among dyspeptic patients in Ethiopia (Mathewos et al., 2013; Workineh and Andargie, 2016). The gold standard eradication therapy in areas of low clarithromycin resistance comprises the combination of a proton pump inhibitor, clarithromycin, and amoxicillin or metronidazole (Malfertheiner et al., 2017), which is also available in Ethiopia.
There is evidence of an immunological interplay between HIV and H. pylori co-infection, with an effect on the H. pylori prevalence in HIV-infected individuals, as well as HIV viral load and CD4+ cell counts. Most studies have reported decreased rates of H. pylori in HIV-infected individuals (Fialho et al., 2011; Luo et al., 2009; Sarfo et al., 2015). Furthermore, previous studies have suggested that HIV infection could be associated with a decreased efficacy of H. pylori eradication therapy (Nkuize et al., 2015). The association and interplay of these two infections have not been well studied but are of potential significance, since more than one million people live with HIV in Ethiopia (WHO, 2017). Therefore, the aim of this study was to describe the prevalence of H. pylori infection according to HIV status and to evaluate the efficacy of eradication therapy in HIV-negative versus HIV-positive individuals in a tertiary hospital in Central Ethiopia.
Methods
Study design
This prospective cohort study was conducted at the Asella Referral and Teaching Hospital (ARTH), which includes an HIV clinic as well as a voluntary counseling and HIV testing (VCT) clinic. The results presented here are part of a study investigating the effects of H. pylori on the immune response.
Study population
Consecutive HIV-positive patients aged 18–55 years presenting to the HIV clinic and consecutive individuals aged 18–55 years presenting to the VCT clinic for HIV testing and receiving a negative test result were included in a prospective, randomized interventional study between March and June 2017. After providing informed consent, the participants were screened for H. pylori infection using a stool antigen test (see below). To facilitate and expedite data examination, only limited and equal numbers (n = 140) of HIV-positive and HIV-negative participants were selected from the larger cohort; this was done through grab sampling, with screening for confounding factors. Demographic data were collected for these participants.
The following confounding factors were considered exclusion criteria for both HIV-negative and positive patients: immunosuppressive therapy, previous malignancy, known chronic infection (e.g. hepatitis B virus (HBV), hepatitis C virus (HCV)), known tuberculosis co-infection, elevated C-reactive protein (CRP), parasitic infection, anti-helminth/antibiotic treatment in the past 6 months, upper gastrointestinal symptoms (self-reported), acute infection (e.g. pneumonia) in the past 6 months, anemia (hemoglobin <10 g/dl), and pregnancy.
Analysis of stool samples
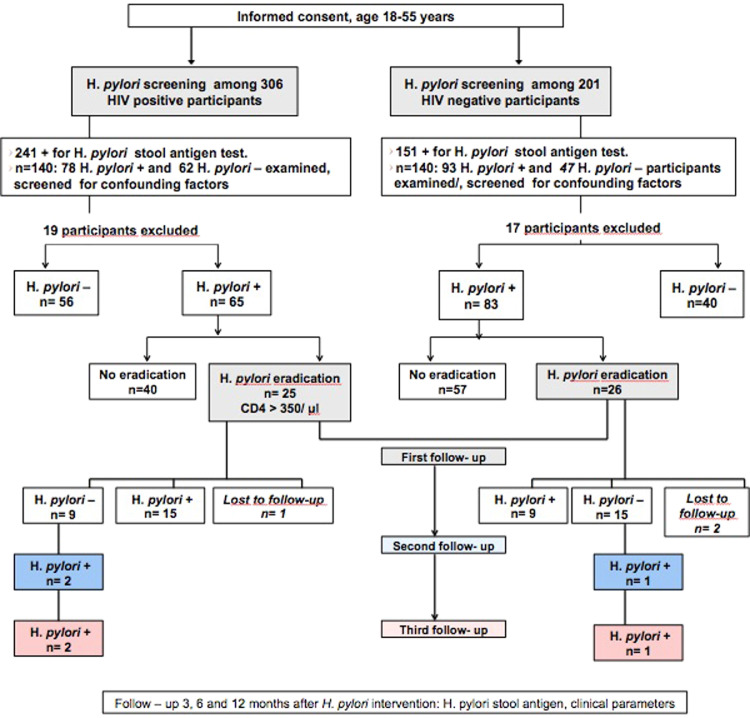
Approximately 5 g of stool were collected from all participants at baseline (study inclusion) and during follow-up at 3, 6, and 12 months after the intervention for participants who received eradication therapy (Figure 1). All stool samples were screened for H. pylori antigen using a Helicobacter Antigen Quick test (GA Generic Assays GmbH, Blankenfelde, Germany). The test was performed according to the manufacturer's instructions.
Figure 1.
Study design flow chart.
VCT: voluntary counseling and HIV testing clinic; n: number.
The stool samples were examined microscopically for parasitic infections using direct and formol ether concentrated wet mount techniques (Cheesbrough, 2005). Two to three grams of each stool specimen were preserved at −80°C and exported to Germany for further analysis.
Screening for the presence of hepatitis B surface antigen, HCV antibodies, and elevated CRP
Approximately 5 ml of venous blood were collected at baseline in a serum separator tube from the 280 participants included. Serological tests for hepatitis B surface antigen (HBsAg) and HCV antibodies (InTec Products Inc., Fujian, China), as well as semi-quantitative measurement of CRP values (NADAL CRP test; nal von minden GmbH, Moers, Germany) were performed using this serum sample. The lower limit of detection (LLD) for the CRP assay was 5 mg/l. All values above the LLD were considered elevated. Patient with co-infections or elevated CRP were excluded from further analysis.
CD4+ T cell count
A CD4+ T cell count was done for 70 out of the 140 selected HIV-positive patients. The cell count was performed according to the manufacturer's instructions, using a FACSCalibur flow cytometer (Becton Dickinson Biosciences, Franklin Lakes, NJ, USA) available at the ARTH.
H. pylori eradication therapy
In order to assess the efficacy of H. pylori eradication therapy, 25 of the 70 HIV-positive patients with an available CD4+ T cell count and >350 CD4+ cells/µl, as well as 26 HIV-negative participants out of the grab sample described above, were randomly selected and then treated using a standard triple therapy with pantoprazole 40 mg (1A Pharma GmbH, Oberhaching, Germany), metronidazole 500 mg (Lagap Pharma, Vezia, Switzerland), and clarithromycin 500 mg (Bilim Pharmaceuticals, Istanbul, Turkey). These were administered twice daily for 14 days. No sample size calculation was performed for the eradication part of this study. A metronidazole-based regimen was used as recommended in the Ethiopian national guidelines for H. pylori eradication treatment and to avoid allergic reactions in participants with an undetected penicillin allergy. The occurrence of side effects of eradication therapy was monitored during follow-up visits and quantified as a categorical variable (yes/no).
Evaluation of the rapid H. pylori stool antigen test used routinely in the hospital laboratory
The prevalence of H. pylori reported in 2017 by the ARTH, as shown using data collected retrospectively from the hospital laboratory, was conspicuously low (15.7%) according to tests performed using with the Wondfo One Step H. pylori stool test kit (Guangzhou Wondfo Biotech Co., Ltd, [Au?3], China) when compared to other prevalence rates reported from Ethiopia. A sub-study was therefore performed to evaluate the performance of the Wondfo stool test kit used in the hospital laboratory. A total of 137 stool samples (convenience sample) were tested simultaneously using the Wondfo kit and the Serazym H. pylori second-generation ELISA (Seramun Diagnostica GmbH, Heidesee, Germany). The ELISA test was performed at the Heinrich Heine University in Düsseldorf, Germany.
Since monoclonal enzyme immunoassays such as the Serazym ELISA provide more reliable results when compared to monoclonal immunochromatography assays such as the H. pylori antigen stool test (accuracy 90–100% vs under 90%), the sensitivity and specificity of the Wondfo test kit were calculated using the results of the ELISA test as reference (Wang et al., 2015).
Statistical analysis
The study data were analyzed using IBM SPSS Statistics for Mac OS version 25.0 (IBM Corp., Armonk, NY, USA). Simple frequencies and a descriptive analysis were performed. The association between variables as well as specificity and sensitivity were analyzed by Chi-square test. Continuous variables were compared by Wilcoxon signed-rank test. Multivariate logistic regression was used to establish possible risk factors for H. pylori infection. Odds ratios (OR) and 95% confidence intervals (CI) for the OR were calculated to assess the strengths of association and statistical significance. Cohen's kappa coefficient of agreement was used to compare test performance. Differences were considered statistically significant at a two-tailed P-value of <0.05.
Results
Socio-demographic parameters
Socio-demographic parameters including sex, age, area of residence, educational status, family size, accessibility of latrine facility, and source of water were collected from 140 HIV-positive and 140 HIV-negative participants selected as a grab sample from the larger cohort of HIV-positive and HIV-negative patients. The sex distribution in this sample was 180 female and 100 male, and the mean participant age was 36 years (range 18–55 years). The majority of participants (83.6%) lived in an urban area, had a latrine in their compound (99.6%), and had tap water as the main water source (99.2%). All of the 280 selected participants affirmed that they washed their hands before eating and after using the latrine. More than 30% of the participants had a family size of more than four and 52.3% had attended secondary school or a higher form of education.
Prevalence of H. pylori and risk factors
Among the 306 HIV-positive and 201 HIV-negative participants being screened for H. pylori, the cumulative H. pylori prevalence was 77.3% (n = 392): 78.8% (241/306) among HIV-positive participants versus 75.1% (151/201) among HIV-negative participants (P = 0.386). There was no significant difference in H. pylori prevalence according to sex or age in this study (Table 1). Applying multivariate logistic regression for the analysis of risk factors, no significant association was detected between socio-demographic parameters and H. pylori status (Table 2).
Table 1.
Prevalence of Helicobacter pylori according to sex, age, and HIV status.
|
H. pylori status |
P-value | ||
|---|---|---|---|
| Positiven (%) | Negativen (%) | ||
| Sex | 0.657 | ||
| Male | 140 (78.7) | 38 (21.3) | |
| Female | 252 (76.6) | 77 (23.4) | |
| Age (years) | 0.086 | ||
| ≤30 | 130 (81.8) | 29 (18.2) | |
| 31–40 | 134 (70.5) | 56 (29.5) | |
| >40 | 128 (81.0) | 30 (19.0) | |
| HIV status | 0.386 | ||
| HIV-positive | 241 (78.8) | 65 (21.2) | |
| HIV-negative | 151 (75.1) | 50 (24.9) | |
Table 2.
Risk factors for H. pylori infection.
| Factors | Sig. | OR | 95% CI |
|---|---|---|---|
| Age | 0.142 | ||
| ≤30 | 0.508 | 0.760 | 0.336-1.715 |
| 31-40 | 0.172 | 1.530 | 0.831-2.819 |
| >40 | * | ||
| Area of residence | 0.078 | ||
| Urban | 0.088 | 0.418 | 0.153-1.139 |
| Rural | * | ||
| Marital Status | 0.134 | ||
| Single | 0.403 | 1.634 | 0.517-5.169 |
| Married | 0.034 | 2.639 | 1.078-6.641 |
| Divorced | 0.189 | 2.250 | 0.671-7.543 |
| Widowed | * | ||
| Gender | 0.793 | 0.922 | 0.502-1.693 |
| Family size ≤4 |
0.714 |
0.894 |
0.492-1.626 |
| Educational status | 0.462 | ||
| Illiterate | 0.329 | 1.547 | 0.644-3.714 |
| Elementary School | 0.758 | 0.904 | 0.474-1.723 |
| Secondary school and above | * |
Sig: Significance; OR: odds ratio; CI: confidence interval.
Redundant parameter.
Co-infections
Four out of 280 (1.4%) participants were infected with intestinal parasites: three of them with Entamoeba histolytica/dispar and one with Taenia spp.
HBsAg was reactive in 6.8% (19/280) of participants. None of the participants had detectable HCV antibodies. Elevated CRP was detected in 6.1% (17/280) of participants (range 6–192 mg/l). There was no significant correlation between HBsAg status (OR 1.89, 95% CI 0.66–5.43, P = 0.235) or CRP level (OR 0.67, 95% CI 0.25–1.82, P = 0.441) and H. pylori status.
CD4+ T cell count
A CD4+ T cell count was performed for 70 HIV-positive patients. Although H. pylori-positive patients had numerically higher numbers of CD4+ T cells (median 683 cells/µl, interquartile range 386–1442 cells/µl) when compared to H. pylori-negative patients (median 589 cells/µl, interquartile range 217–1011 cells/µl), the difference was not statistically significant (P = 0.11).
Efficacy of standard triple eradication therapy
As mentioned previously, 25 HIV-positive and 26 HIV-negative H. pylori-infected participants were selected to receive standard triple eradication therapy. One HIV-positive and two HIV-negative participants were lost to follow-up (Figure 1). The cumulative eradication rate was 50.0% (24/48; 95% CI 35.2–64.8%). The per-protocol analysis showed that 62.5% (15/24; 95% CI 40.1–81.2%) of the HIV-negative participants versus 37.5% (9/24; 95% CI 18.8–59.4%) of the HIV-positive participants had successful eradication at 3 months after the intervention (OR 2.77, 95% CI 0.86–8.93, P = 0.148).
Six months after the intervention, three participants (two HIV-positive, one HIV-negative) who tested negative for H. pylori after 3 months tested positive again for H. pylori. Recurrence could not be differentiated from re-infection. No new eradication therapy was initiated, as the participants continued to be asymptomatic. Twelve months after the intervention the H. pylori status of the participants remained unchanged.
Comparison of the rapid H. pylori test used in clinical routine with the Serazym second-generation ELISA
A total of 137 conveniently chosen stool samples were tested concurrently with the Wondfo test used routinely in the hospital and the Serazym second-generation ELISA considered as a reference standard for H. pylori antigen testing. In comparison to the Serazym assay, the Wondfo test had a sensitivity of 85.1% (95% CI 76.3–91.6%) and a low specificity of 53.5% (95% CI 37.7–68.8%), and the degree of agreement between the two tests was low (Cohen's kappa = 0.401, 95% CI0.235–0.568).
Discussion
In recent years, several meta-analyses on H. pylori prevalence worldwide and in Africa have been published, but differences in H. pylori prevalence and success rates of H. pylori eradication therapy according to HIV status remain disputed. Hooi et al. reported that Africa has the highest H. pylori prevalence in the world (71%) (Hooi et al., 2017), matching the results in the present study. However, different results were documented by Zamani et al., who described the highest H. pylori prevalence in Latin America and the Caribbean (56.3%) (Zamani et al., 2018).
It is generally understood that the prevalence of H. pylori infection is usually higher in symptomatic patients with dyspeptic disease when compared to asymptomatic patients. However, the prevalence of H. pylori in asymptomatic individuals is highly variable, with reported rates ranging from 2.4% to 79% (Dilnessa and Amentie, 2017; Melese et al., 2019). Data on the prevalence of asymptomatic H. pylori infection are influenced by the varying sensitivities of the different laboratory methods. Although PCR, culture, and histopathology from biopsies of the gastric mucosa are considered to be the most sensitive methods, they are usually not available for asymptomatic participants, since no gastroscopy is performed. However, antigen testing from stool samples has also been shown to provide a reliable detection of H. pylori infection, if properly evaluated test kits are utilized, such as those used for this study (Stool Antigen Tests for Helicobacter pylori infection 2015).
In a meta-analysis on H. pylori prevalence in Ethiopia published by Melese et al. (Melese et al., 2019), the pooled prevalence of H. pylori in both asymptomatic and dyspeptic patients was 52.2%, with the highest prevalence in the Somali region (71%) and the lowest in Oromia (39.9%), where ARTH is located. In contrast, a much higher prevalence of almost 78% was found in the current study, confirming significant regional and inter-study differences. An even higher prevalence of up to 90% has been found in dyspeptic patients from Ethiopia (Asrat et al., 2004a). In comparison, in Eastern Africa, a study on dyspeptic patients from Kenya showed a prevalence of 54.8% in adults versus 73.3% in children (Kimang'a et al., 2010).
According to the comparison of the tests utilized for H. pylori antigen diagnostics, these different findings for H. pylori prevalence might be explained, at least in part, by differences in sensitivity and specificity. A low level of consistency was found between the H. pylori test used in clinical routine and the H. pylori stool ELISA test used in this study, for which the manufacturer states a sensitivity and specificity of more than 90%, irrespective of co-infections (Seramun, 2021). The only other study from Ethiopia utilizing the Wondfo test to diagnose H. pylori infection showed a prevalence of 37.6%, lower than other published rates (Kasew et al., 2017).
The prevalence of H. pylori is influenced by various factors such as age, sex, location, and ethnicity (Grad et al., 2021). Risk factors that have been described for H. pylori infection include overcrowding (Bello et al., 2018; Smith et al., 2018), low socio-economic status with low income and education levels (Bello et al., 2018; Kouitcheu Mabeku et al., 2018), family history of chronic gastritis (Kouitcheu Mabeku et al., 2018), open-air defecation (Awuku et al., 2017), unclean water sources (Awuku et al., 2017; Kouitcheu Mabeku et al., 2018; Romanelli et al., 2007), smoking, and alcohol consumption (Bello et al., 2018). In the present study, there was no influence of sex, age, or socio-economic status on H. pylori prevalence. The finding with regard to age differs from those of previous publications in which the prevalence of H. pylori increased with age, but the assumption is that these effects are due to a cohort effect, since most infections occur early in life (Melese et al., 2019). The absence of other significant risk factors can most likely be explained by the homogeneous population, since most patients lived in an urban area, had access to a toilet and clean water, and also had a higher level of education.
No significant difference in H. pylori prevalence was observed between HIV-positive and negative participants. In this regard, previous studies have reported inconsistent results. Most have reported lower H. pylori prevalence rates in HIV-positive individuals and especially in those with low CD4+ cell counts (Fialho et al., 2011; Luo et al., 2009; Nevin et al., 2014; Romanelli et al., 2007; Sarfo et al., 2015), while some have reported similar prevalence rates in HIV-positive and negative participants (Kafil et al., 2011; Moges et al., 2006), in line with the findings of the present study. All HIV patients with an available CD4+ cell count had >350 CD4 cells/µl, and thus the influence of severe immunosuppression on the prevalence of H. pylori could not be investigated in this study. Likewise, the effect of combined antiretroviral therapy (cART) on H. pylori prevalence could not be assessed in this study, since all HIV-positive participants received cART. However, an effect of cART on H. pylori prevalence has been described. Radovanović Spurnić et al. reported a higher prevalence in patients receiving cART, similar to that in HIV-negative participants, when compared to HIV-infected patients from the pre-highly active antiretroviral therapy (HAART) era (Radovanović Spurnić et al., 2017).
Another factor that could have influenced the absence of demographic risk factors and association of H. pylori prevalence with HIV status was the sampling method used in this study. The use of convenience sampling, in this case a limited selection of consecutively presenting patients, is associated with a higher degree of bias: there is a higher chance that participants with easier access to health facilities (such as individuals from urban areas and with higher income) as well as healthier patients (with higher CD4+ cell counts) may have been included in the study.
The cumulative per-protocol eradication rate in this study was 50%, which is much lower than the optimal eradication cut-off rate (≥ 90%) for a per-protocol analysis (Graham et al., 2007) and the rate reported from a different study performed in Bahir-dar, Ethiopia (90%) (Gebeyehu et al., 2019). In the aforementioned study, an amoxicillin-based triple therapy was used, and a very low rate of amoxicillin resistance was reported (6%). In the present study, a metronidazole-based triple therapy was used, as recommended in the Ethiopian national H. pylori eradication therapy guidelines and in order to avoid the risk of complications in patients with a previously undetected penicillin allergy. However, a meta-analysis on worldwide antibiotic resistance of H. pylori showed high rates of resistance to metronidazole (Kasahun et al., 2020) and two studies from Ethiopia also showed that metronidazole-based therapy was a risk factor for eradication failure, with resistance rates ranging from 76% to 94.7% (Asrat et al, 2004b; Henriksen et al., 1999). Thus, the low eradication rate documented in the current study might be associated with metronidazole resistance. However, no resistance testing was performed and other factors such as compliance with the eradication treatment were not directly observed. Nevertheless, there were no reported side effects with the eradication therapy and the participants declared compliance with the eradication treatment during the follow-up visits.
There are very few reports on the efficacy of triple H. pylori eradication therapy in Ethiopia. In addition, drug interactions with cART might influence drug levels and thus influence eradication success. This study is novel in analyzing the efficacy of eradication according to HIV status in Ethiopia. There was a numerically lower success rate of H. pylori eradication in HIV-positive participants, but this finding was not statistically significant. However, the results are similar to a previous study from Belgium (Nkuize et al., 2015), which showed that HIV-infected individuals and individuals of African ethnicity had higher rates of antimicrobial resistance among H. pylori isolates. In this previous study, it was assumed that the patients were previously infected in Africa with a metronidazole-resistant strain without previous exposure to the antibiotic. Moreover, patients receiving cART had a smaller chance of eradication than cART-naïve patients, which suggests that possible drug–drug interactions might have played a role. It is known, that protease inhibitors can increase and nevirapine (a non-nucleoside reverse transcriptase inhibitor) can decrease the levels of clarithromycin (Ceschi et al., 2010). All HIV-positive participants in the present study were receiving cART at baseline and after the intervention, which might thus have contributed to the low H. pylori eradication rate.
Three participants in whom H. pylori was successfully eradicated, tested positive for H. pylori at the 6-month follow-up visit. Within this investigation, it was not possible to differentiate between recurrence and re-infection, since no genotypic differentiation of H. pylori strains was performed.
This study was limited by the fact that socio-demographic data were only available for a convenience sample of patients. Furthermore, data regarding antibiotic resistance or adherence to eradication treatment were not available.
In conclusion, in the investigated setting, H. pylori infection was common in both HIV-negative and HIV-positive patients, and the success rate of standard metronidazole-based triple eradication therapy was low. While the prevalence of H. pylori infection was not associated with HIV status in this study, there was a trend towards lower efficacy of the eradication therapy in HIV-infected individuals. Further studies in larger patient populations are required in order to investigate differences in H. pylori prevalence and the success of eradication therapy according to HIV status, efficacy of different therapy alternatives, and resistance patterns of the pertinent strains, especially in the vulnerable sub-group of HIV-infected patients.
Authors contributions
All authors have made substantial contributions to the publication. SG, MGM, TF, and AF drafted the article, revised it critically for important intellectual content, and approved the final version for submission. MGM, AF, HMO, AS, and TF contributed to the conception and design of the study and acquisition of data. MGM and SG performed the statistical analysis. TL revised the manuscript for important intellectual content and approved the final version for submission.
Ethical considerations
Ethical approval was obtained from the ethics review board of the College of Health Sciences of Arsi University (reference number A/U/H/S/C/87/6392), the National Research Ethics Review Committee (reference number 3.10/271/2017), and the research and ethics commission of the Medical Faculty of Heinrich Heine University (study number 5728).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
Million G. Mesfun received a doctoral fellowship from the German Academic Exchange Service and a grant from the Bayer Foundation. Material and personal costs were supported by a third-party account of the Hirsch Institute of Tropical Medicine.
References
- Asrat D, Kassa E, Mengistu Y, Nilsson I, Wadström T. Antimicrobial susceptibility pattern of Helicobacter pylori strains isolated from adult dyspeptic patients in Tikur Anbassa University Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2004;42(2):79–85. PMID: 16895024. [PubMed] [Google Scholar]
- Asrat D, Nilsson I, Mengistu Y, Ashenafi S, Ayenew K, WA Al-Soud, Wadström T, Kassa E. Prevalence of Helicobacter pylori infection among adult dyspeptic patients in Ethiopia. Ann Trop Med Parasitol. 2004;98(2):181–189. doi: 10.1179/000349804225003190. [DOI] [PubMed] [Google Scholar]
- Awuku YA, Simpong DL, Alhassan IK, Tuoyire DA, Afaa T, Adu P. Prevalence of helicobacter pylori infection among children living in a rural setting in Sub-Saharan Africa. BMC Public Health. 2017;17:360. doi: 10.1186/s12889-017-4274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello AK, Umar AB, Borodo MM. Prevalence and risk factors for Helicobacter pylori infection in gastroduodenal diseases in Kano, Nigeria. Afr J Med Health Sci. 2018;17:41–46. doi: 10.5897/AJMHS.9000010. [DOI] [Google Scholar]
- Ceschi A, Curkovic I, Kirchheiner J, Kullak-Ublick GA, Jetter A. Arzneimittel interaktionen mit antiretroviralen Medikamenten [Interactions with antiretroviral drugs] Internist (Berl) 2010;51(1):94–99. doi: 10.1007/s00108-009-2528-2. [DOI] [PubMed] [Google Scholar]
- Cheesbrough M. Cambridge University Press; New York: 2005. Formol ether concentration technique. District laboratory practice in tropical countries part 1. 2. [Google Scholar]
- Dilnessa T, Amentie M. Prevalence of Helicobacter pylori and risk factors among dyspepsia and non-dyspepsia adults at Assosa general hospital, West Ethiopia: a comparative study. Ethiop J Health Dev. 2017;31(1):4–12. [Google Scholar]
- Fialho ABC, Braga-Neto MB, Guerra EJC, Fialho AMN, Fernandes KC, Sun JLM, Takeda CFV, Silva CIS, Queiroz DMM, Braga LLBC. Low prevalence of H. pylori infection in HIV-positive patients in the northeast of Brazil. BMC Gastroenterology. 2011;11(1):13. doi: 10.1186/1471-230X-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebeyehu E, Nigatu D, Engidawork E. Helicobacter pylori eradication rate of standard triple therapy and factors affecting eradication rate at Bahir Dar city administration, Northwest Ethiopia: A prospective follow up study. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0217645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol. 2021;175:54–59. doi: 10.1093/aje/kwr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275–278. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- Gravina AG, Priadko K, Ciamarra P, Granata L, Facchiano A, Miranda A, Dallio M, Federico A, Romano M. Extra-Gastric Manifestations of Helicobacter pylori Infection. J Clin Med. 2020;9(12):3887. doi: 10.3390/jcm9123887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen TH, Nysaeter G, Madebo T, Setegn D, Brorson O, Kebede T, Berstad A. Peptic ulcer disease in south Ethiopia is strongly associated with Helicobacter pylori. Trans R Soc Trop Med Hyg. 1999;93(2):171–173. doi: 10.1016/s0035-9203(99)90297-3. [DOI] [PubMed] [Google Scholar]
- Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global prevalence of helicobacter pylori infection: systematic review and meta analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. https://www.seramun.com/de/produkte/elisa-teste/gastrointestinale infektionskrankheiten html. GAL_E-114-A_2021-02-25_DE.docx, 2021 (accessed 22 december 2021) [DOI] [PubMed] [Google Scholar]
- Kafil HS, Jahromi FF, Hajikhani B, Pirayeh SN, Aghazadeh M. Screening for the presence of Helicobacter pylori in stool of HIV-positive patients. Journal of AIDS and HIV Research. 2011;3(4):3. http://www.academicjournals.org/jahr [Google Scholar]
- Kasahun GG, Demoz GT, Desta DM. Primary Resistance Pattern of Helicobacter pylori to Antibiotics in Adult Population: A Systematic Review. Infect Drug Resist. 2020;13:1567–1573. doi: 10.2147/IDR.S250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasew D, Abebe A, Munea U, Deressa T, Tegegne Y, Alemayehu M, Melku M. Magnitude of Helicobacter pylori among Dyspeptic Patients Attending at University of Gondar Hospital, Gondar, Northwest Ethiopia. Ethiop J Health Sci. 2017;27(6):571–580. doi: 10.4314/ejhs.v27i6.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimang'a AN, Revathi G, Kariuki S, Sayed S, Devani S. Helicobacter pylori: Prevalence and antibiotic susceptibility among Kenyans. S Afr Med J. 2010;100:53–57. [PubMed] [Google Scholar]
- Kouitcheu Mabeku LB, Noundjeu Ngamga ML, Leundji H. Potential risk factors and prevalence of Helicobacter pylori infection among adult patients with dyspepsia symptoms in Cameroon. BMC Infect Dis. 2018;18:278. doi: 10.1186/s12879-018-3146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo HB, Hu ZW, Guo JW. [Helicobacter pylori infection in the gastric mucosa of patients with HIV/AIDS in different clinical stages] Nan Fang Yi Ke Da Xue Xue Bao. 2009;29(7):1397–1399. PMID: 19620064. [PubMed] [Google Scholar]
- Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht V/Florence consensus report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- Mathewos B, Moges B, Dagnew M. Seroprevalence and trend of Helicobacter pylori infection in Gondar University Hospital among dyspeptic patients, Gondar, North West Ethiopia. BMC Res Notes. 2013;6:346. doi: 10.1186/1756-0500-6-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melese A, Genet C, Zeleke B, Andualem T. Helicobacter pylori infections in Ethiopia; prevalence and associated factors: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19:8. doi: 10.1186/s12876-018-0927-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moges F, Kassu A, Mengistu Getahun, Adugna Solomon, Andualem Berhanu, Nishikawa Takeshi, Ota Fusao. Seroprevalence of Helicobacter pylori in dyspeptic patients and its relationship with HIV infection, ABO blood groups and lifestyle in a university hospital, Northwest Ethiopia. World J Gastroenterol. 2006;12(12):1957–1961. doi: 10.3748/wjg.v12.i12.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin DT, Morgan CJ, Graham DY, Genta RM. Helicobacter pylori gastritis in HIV-infected patients: a review. Helicobacter. 2014;19(5):323–329. doi: 10.1111/hel.12131. [DOI] [PubMed] [Google Scholar]
- Nkuize M, De Wit S, Muls V, Delforge M, Miendje Deyi VY, Cadiere GB, Buset M. HIV-Helicobacter pylori co-infection: Antibiotic resistance, Prevalence and Risk Factors. Plos one. 2015;10(12):e0145119. doi: 10.1371/journal.pone.0145119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovanović Spurnić A, Brmbolić B, Stojšić Z, Pekmezović T, Bukumirić Z, Korać M, Salemović D, Pešić-Pavlović I, Stevanović G, Milošević I, Jevtović D. The increasing prevalence of HIV/Helicobacter pylori co-infection over time, along with the evolution of antiretroviral therapy (ART) Peer J. 2017;5:e3392. doi: 10.7717/peerj.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli F, Smith KM, Murphy BS. Does HIV infection alter the incidence or pathology of Helicobacter pylori infection? AIDS patient care and STDs. 2007;21(12):908–919. doi: 10.1089/apc.2006.0215. [DOI] [PubMed] [Google Scholar]
- Sarfo FS, Eberhardt KA, Dompreh A, Kuffour EO, Soltau M, Schachscheider M, Drexler JF, Eis-Hübinger AM, Häussinger D, Oteng-Seifah EE, Bedu-Addo G, Phillips RO, Norman B, Burchard G, Feldt T. Helicobacter pylori Infection Is Associated with Higher CD4 T Cell Counts and Lower HIV-1 Viral Loads in ART-Naïve HIV-Positive Patients in Ghana. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0143388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Jolaiya T, Fowora M, Palamides P, Ngoka F, Bamidele M, Lesi O, Onyekwere C, Ugiagbe R, Agbo I, Ndububa D, Adekanle O, Adedeji A, Adeleye I, Harrison U. Clinical and Socio- Demographic Risk Factors for Acquisition of Helicobacter pylori Infection in Nigeria. Asian Pac J Cancer Prev. 2018;19:1851–1857. doi: 10.22034/APJCP.2018.19.7.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stool Antigen Tests for Helicobacter pylori infection . Canadian Agency for Drugs and Technologies in Health (CADTH); OttawaON: 2015. A Review of Clinical and Cost-Effectiveness and Guidelines [Internet]https://www.ncbi.nlm.nih.gov/books/NBK269453/table/T4/ Jan 8. PMID: 25632493.Available from. [PubMed] [Google Scholar]
- Wang YK, Kuo FC, Liu CJ, Wu MC, Shih HY, Wang SS, Wu JY, Kuo CH, Huang YK, Wu DC. Diagnosis of Helicobacter pylori infection: Current options and developments. World J Gastroenterol. 2015;21(40):11221–11235. doi: 10.3748/wjg.v21.i40.11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workineh M, Andargie D. A 5-year trend of Helicobacter pylori seroprevalece among dyspeptic patients at Bahir Dar Felege Hiwot Referral Hospital, Northwest Ethiopia. Research and Reports in Tropical Medicine. 2016;7:17–22. doi: 10.2147/RRTM.S105361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation . WHO; Geneva: 2017. Analytical summary- HIV/AIDS.https://www.who.int/hiv/data/Country_profile_Ethiopia.pdf?ua=1 (accessed 22 december 2021) [Google Scholar]
- Xie FJ, Zhang YP, Zheng QQ, Jin HC, Wang FL, Chen M, Shao L, Zou DH, Yu XM, Mao M. Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World Journal of gastroenterology. 2013;19(36):6098–6107. doi: 10.3748/wjg.v19.i36.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani M, Ebrahimtabar F, Zamani V, Miller WH, Alizadeh-Navaei R, Shokri-Shirvani J, Derakhshan MH. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47(7):868–876. doi: 10.1111/apt.14561. [DOI] [PubMed] [Google Scholar]