Abstract
The correlational nature of previous studies on household chaos does not allow claims about causal effects of household chaos. The present study used an experimental design to assess the causal effect of household chaos on stress, negative emotions, and caregiving. Ninety-six female students (18–25 years) participated in our study. They took care of an infant simulator in a normal living room (neutral condition), and a chaotic living room (chaos condition), while caregiver sensitivity was observed, operationalized as perceiving, correctly interpreting, and responding accurately and promptly to the infant's signals. Participants reported on their current emotional state, and saliva was collected four times for analysis of salivary alpha-amylase (sAA). Results showed that there were no significant time or condition effects on negative emotional state. Yet, sAA levels were higher in the chaos condition compared to the neutral condition. We found no evidence for negative emotional state or sAA mediating the relation between household chaos and caregiver sensitivity. Because household chaos affected physiological stress in a parenting situation, it should not be ignored when using interventions aimed at reducing stress in parents. More research is needed on the effect of reduced (as opposed to increased) levels of household chaos on physiological stress levels in families with young children.
Keywords: Household chaos, Stress, Salivary alpha-amylase, Emotional state, Sensitive caregiving, Experiment
Highlights
-
•
Household chaos is causally related to physiological stress in a caregiving context.
-
•
Household chaos does not affect self-reported negative emotions.
-
•
No evidence for stress mediating the relation between household chaos and caregiving.
1. Introduction
Raising a child and the responsibilities and demands that come with it can be stressful at times. Parenting stress has been associated with a decrease in parenting quality [1], thereby affecting child functioning [2,3]. Certain environmental factors might add to parents’ sense of stress in the home, such as a chaotic home environment. Household chaos consists of poor routines and regularities, and high levels of noise, clutter, and crowding in the home [4]. Chaotic home environments have been associated with poorer parenting practices (e.g. Refs. [5,6], and higher levels of parental self-reported stress and negative emotions [7,8]. However, the answer to the question of directionality of these associations has not been established, and knowledge based on objectively assessed physiological stress is limited. Therefore, the aim of the present study is to assess the causal effect of household chaos on emotional state and physiological stress during caregiving. In addition, we investigated whether emotional state and stress mediate the association between chaos and caregiving. This study attempts to broaden the existing literature about household chaos, subjective emotional state, physiological stress, and caregiving by using an experiment in which household chaos is manipulated.
Household chaos is a proximal environmental factor that should be taken into account when studying risk factors for parenting problems, as the majority of the parent-child interactions take place in the home [9]. The inconsistency, instability, and sensory overstimulation that are characteristic of household chaos may pose a risk for maladaptive parenting and child development [10]. Indeed, household chaos has been associated with more negative parenting outcomes, such as parental negativity and anger [5,11], and less sensitive and more intrusive parenting [6,12]. Most studies on the associations of household chaos with parenting and child development are correlational [10], but there is also experimental research conforming a causal effect. A study on the same experiment as the current study found that caregiver sensitivity was influenced by household chaos; sensitivity was lower when young adults took care of an infant simulator in a chaotic living room compared to a non-chaotic living room [43]. This supports the suggestion that household chaos affects parenting, although less is known about the underlying mechanisms of this effect. It is important to understand these mechanisms to henceforth develop effective prevention and intervention programs.
Several mechanisms that could explain the effect of household chaos on parenting have been proposed (see [43]), including negative emotional state, i.e., experiencing feelings of anxiety, fear or anger. Since parents may perceive chaotic home environments as uncertain and disorganized, due to an excess of interminable stimuli such as noise and clutter, household chaos might cause negative emotions in parents, which, in turn, could affect parenting. Indeed, household chaos has been related to negative emotions and conflicts [13]. Moreover, higher levels of parental negative emotions are associated with a higher potential of child abuse, and there is evidence that negative emotions serve as a mediator between a history of childhood maltreatment and later child abuse potential in parents [14].
In addition to affecting subjective emotional distress in adults reflected by their emotional state, household chaos might also influence more objectively assessed physiological stress. Physiological biomarkers such as salivary alpha-amylase (sAA) serve as a quick and noninvasive indicator of physical stress levels. Increased sAA corresponds with autonomous nervous system (ANS) activity, as the sympathetic and parasympathetic components of the ANS play a role in the physiological response to stressors [15,16]. sAA has been proven to be a reliable and valid indicator of physiological stress [15]. Furthermore, sAA is correlated with other measures of the ANS, such as heart rate [17]. This strengthens the claim that sAA is a biomarker for ANS reactivity. In response to a stressor (e.g., Trier Social Stress Test; [18], sAA typically increases and peaks compared to resting state levels, followed by a gradual decrease. sAA is sensitive to environmental changes [18], such as infant stimuli (i.e., crying sounds; [19], which makes sAA a suitable candidate to measure stress response in response to environmental stimuli, such as household chaos.
Although household chaos has been proven to be related to physiological stress levels in children [10,20,21], research assessing the effect of household chaos on parental stress reactivity is scarce. However, associations between noise exposure, which is an element of household chaos, and physiological stress responses in adults have been reported [[22], [42]]. It is thus likely that the full range of household chaos is also related to increased physiological stress levels.
Associations between physiological stress levels and caregiving have been established for several ANS biomarkers [1,17,23], and salivary cortisol, reflecting activity of the hypothalamus-pituitary-adrenal axis. For example, parental sAA has been related to intended harsh parenting, i.e., participants who had higher salivary alpha-amylase levels in response to infant crying sounds indicated more harsh parenting responses [24]. In addition, meta-analytic evidence indicates higher physiological stress levels in maltreating compared to non-maltreating parents [25].
1.1. Current study
The present study uses an experimental design to assess associations between household chaos, negative emotions, physiological stress, and caregiving. Our experimental design allowed to create a caregiving context in which caregiving demands, including child behavior, are standardized and levels of household chaos are manipulated. We expected higher levels of negative emotions and ANS activity during the chaos condition compared to the neutral condition. Second, we assessed whether negative emotions and sAA would mediate the association between household chaos and sensitive caregiving. We hypothesized that negative emotions and physiological stress mediate the relation between household chaos and sensitive caregiving that was previously found in the same experiment as this study [43].
2. Methods
2.1. Participants
Participants were 96 female students, 18–25 years of age (M age = 20.31, SD = 1.93) from vocational schools (22%), and colleges (78%). Of these participants, 71% were Dutch, 11% were of other Western ethnicities, and 18% were of non-Western ethnicities. Students from colleges were older on average compared to students from vocational schools (t(94) = −3.15, p = .002, d = 0.83), whereas no significant differences were found in birth country, living situation, socio-economic status (SES), or current levels of household chaos. Six participants (6%) only completed the first visit, hence had missing data on all assessments of the second visit. Exclusion criteria were having children, a pregnancy at the time of recruitment or in the past, having psychopathology or a physical disability such as being in a wheelchair or having a cochlear implant, and taking courses focusing on childcare or caregiving as part of their educational program. We also assessed whether the participants had any experience in caring for children under the age of 2, including experience through relatives and babysitting. Of the total sample, 58% had caregiving experience with a higher percentage for college students (68%) compared to vocational school students (38%; p = .018). For more detailed sample information, see [43].
Participants were recruited by an invitation to participate on their school's digital platform, on which a link to a website with information about the study was posted, or by a researcher visiting their classrooms. All participants received an information letter with more detailed information about the study, and written consent was obtained from all participants. A reimbursement of €40 was given after the last visit. The study was conducted in accordance with the guidelines proposed by the World Medical Association Declaration of Helsinki and was approved by the Ethics Review Board of the Institute of Education and Child Studies, Leiden University (ECPW 2015-090). The study was preregistered in Open Science Framework (DOI: 10.17605/OSF.IO/VA8WM).
2.2. Procedure
2.2.1. General procedure
Participants visited our lab at Leiden University twice to do the experiment in two different conditions (two months apart, counterbalanced order). The 2-h lab visits were divided into a baseline, experiment, and recovery part. The baseline consisted of a 30-min period in which participants filled out questionnaires, watched a 10-min relaxation movie, reported on their current emotional state, and donated saliva in a room with only a table and chairs in it. Thereafter, participants were led to another room which was set up as a living room, in which the experiment took place. Participants were asked to take care of an infant simulator, just as they would do if it was a real infant. The Real-Care Baby 3 (Realityworks, Eau Claire, WI, USA) was used as a real-life baby doll. We used a Caucasian female infant simulator, which resembles a real infant in size, weight (approximately 3 kg) and appearance. Just like an infant, the simulator has a lifelike neck that needs to be supported. The simulator can be programmed to cry (it uses realistic cry sounds), and makes other noises such as breathing and coughing. The use of the infant simulator in scientific research to assess sensitive caregiving in a standardized setting has been used and validated before [26,27].
Participants took care of the infant simulator three consecutive times for 12 min, which was videotaped. The experiment consisted of three phases: taking care of the infant simulator (phase 1), taking care of the infant simulator and simultaneously filling out a questionnaire (phase 2), and taking care of the infant simulator and simultaneously playing a game on a tablet, in which the participant was asked to reach the highest level of all participants, resulting in a prize (phase 3). The infant simulator was programmed not to respond to the care provided. During each phase, the infant simulator cried for five consecutive minutes with at least 2 min of non-crying before and after this period. The crying programme was fixed across conditions and participants, which resulted in an equal exposure duration of crying between participants. After each phase, participants informed us on their current emotional state by filling out questionnaires, and they donated saliva. After the experiment, participants were led back into the first room in which a 20-min recovery period took place.
2.2.2. Conditions
The room in which the experiment took place was set up as a living room with a table and chairs, a couch, a comfortable chair, cabinets, a floor luminaire, a playpen, an infant carrier, toys, diapers, bottles, magazines, and some living room accessories such as plants and candles (see [43]). Changes were made to the room depending on the condition of the visit. In the neutral condition, the full room was used, items in the room were orderly tidied up in sight or put away in the cabinets, neutral colors were used, and soft music was playing (average sound level of 43.4 dB).
In the chaos condition, a chaotic living environment was realized by creating clutter, crowding, and sensory overstimulation in the room. Clutter was created by leaving toys, clothes, magazines, papers, and tableware scattered around the room (with sufficient space left for the participant to walk and sit). Crowding was created by making the room smaller, using a see-through curtain, which resulted in a more crammed room. Sensory overstimulation was created by the untidied room, papers and to-do lists on the wall, using colorful props and putting on the television. On the television, commercials and music videos played that resulted in predominant, continuously changing and loud visual and auditory stimuli (average sound level of 58.1 dB). The validity of the chaos manipulation has been confirmed [43]. Participants were instructed to make no changes to the room in either condition. After the second lab visit, participants were debriefed about the infant simulator, including the fact that the infant simulator was programmed not to be responsive to the participant's actions, and the two experimental conditions.
2.3. Measures
2.3.1. Negative emotional state
Current negative emotional state was assessed using two questionnaires. First, participants reported on their emotional state on several occasions during the lab visit by filling out the 20-item Positive and Negative Affect Schedule (PANAS [28]; The subscale negative affect was used in this study (10 items, e.g., “At the present moment, I feel upset”). Participants rated the items on a 5-point Likert scale, ranging from 1 = very slightly or not at all, to 5 = extremely. The completed questionnaires of the baseline and of the three experimental phases were used for the analyses described in this paper. Mean scores for negative affect were calculated for each time point. The PANAS has high reliability, as well as excellent convergent and discriminant validity [28]. Internal consistency of negative affect in this study ranged between α = 0.71 - 0.86.
Second, the six-item short form of the State-Trait Anxiety Inventory (STAI [29,30]; was used to obtain participants’ state of anxiety on multiple time points during the lab visit. The completed questionnaires of the baseline and of the three experimental phases were used. Participants rated their current state of anxiety (e.g., “I feel worried”) on a 4-point Likert scale, ranging from 1 = not at all, to 4 = very much. Scores were reverse coded if needed, and averaged. This scale has demonstrated acceptable reliability and validity, and has been proven to be sensitive to fluctuations in anxiety levels over time [29]. In the current study, internal consistency ranged between α = 0.66 - 0.78. Both the PANAS negative affect scale and the STAI were log-transformed with base 10. Because of the high correlations between the two scales (r = 0.52 - 0.77, p < .001), they were combined into one negative emotional state score by computing unweighted mean scores.
2.3.2. Salivary alpha-amylase
Salivary alpha-amylase (sAA) was used as an indicator of autonomic nervous system activity during the lab visit. The baseline sample and the three saliva samples collected during the experimental phases were used for analyses. Participants collected saliva in their mouths for 3 min, and spit the saved-up saliva via a funnel in the tube at 60, 120, and 180 s. The tubes were sealed and stored frozen at Leiden University in a −20 °C freezer, and were placed in a −80 °C freezer within a month. sAA was analyzed at the Leiden University Medical Center, using a maltoheptaoside-nitrophenol reagent, according to the IFCC reference method, on a Roche Diagnostics Cobas c502 clinical chemistry analyzer (Roche Diagnostics, Mannheim Germany). Salivary samples were thawed on the morning of analysis and centrifugated. Supernatant was diluted 50 times with 0.9% saline to obtain values within the analytical range of the assay. All samples were analyzed twice. The two saliva scores of each sample were averaged and log-transformed with base 10.
2.3.3. Sensitive caregiving
Sensitive caregiving towards the infant simulator was assessed during the three phases of the experiment, using the Ainsworth Sensitivity Scale [31], which was slightly adjusted to caregiving situations involving the infant simulator [27]. Sensitive caregiving refers to perceiving, correctly interpreting, and responding accurately and promptly to the infant's signals. A sensitivity score, ranging from 1 = extremely insensitive, to 9 = extremely sensitive, was obtained for each of the three phases. Five coders were extensively trained and regularly supervised to prevent coder drift. Between-coder reliability was satisfying: the intraclass correlation coefficient (ICC) was 0.79 (range = 0.74 - 0.83).
2.3.4. Covariates
We controlled for participants' age and their education level, i.e., vocational school or college, because those were significantly related to caregiver sensitivity. Since caregiving experience was only related to educational level and not to caregiver sensitivity, we did not control for this. In addition, we controlled for participant's baseline negative emotional state and baseline sAA levels. Participants reported if they had drunk anything in the past half hour and if they had ever experienced the gum diseases gingivitis or periodontitis. Both of these variables can affect the quality of saliva samples [32], hence they were used as covariates in all analysis which included sAA.
2.4. Statistical analyses
The course of negative emotional state, sAA, and the role of household chaos were assessed over time by performing multilevel analyses. All analyses were performed in R version 4.0.2 [33]. Multilevel imputations were performed using the mice function from the mice package. Its results were equivalent to those obtained from three alternative methods: the MI function in the Amelia package, the panImpute and jomoImpute functions from the mitml package. Due to differences in implementation, the required number of iterations varied per method. A fixed starting seed was set for reproducibility. Pooling of results on 100 imputation sets was performed using the summary functions from mitml and miceadds, and using the summary and modelRandEffStats from the merTools package.
A series of multilevel models were estimated, incrementally comparing nested models. As preliminary analysis, non-linear models were tested with time as factor. We started with an unconditional means model (Model 1). Subsequently we added: the main effects of time, and covariates (Model 2), random slopes (Model 3), and the correlations between random effects (Model 4). For testing the effect of household chaos on emotional state and physiological stress during caregiving over time (controlled for baseline scores), linear models were tested, and we added the fixed effect of condition (Model 5), and the interaction effect between time and condition (Model 6). The anova functions from mitml and merTools were used (which yielded equivalent results). Model comparisons and effect estimates were evaluated at 5% alpha level, using the lmerTest function in merTools. Furthermore, we assessed the association between negative emotions and sAA, respectively, and the change in sensitive caregiving between the neutral and the chaos condition in order to gain information on the possible mediating roles of these variables.
For sensitivity purposes, we assessed whether the order of the conditions of the two sessions (neutral-chaos, or chaos-neutral) affected the study results. To this end, we added a model with the fixed effect of order, and the interaction term between condition and order. Neither model fit improved significantly, nor were the conditional effects of order significant. We concluded that the order of the lab visits did not affect study results and results were presented for the model without the order of conditions.
3. Results
3.1. Descriptive statistics
Descriptive statistics and correlations between the study variables are presented in Table 1, and Appendix A. For negative emotional state, correlations within the neutral condition ranged from r = 0.40 to 0.86, ps < .001, and in the chaos condition from r = 0.53 to 0.87, ps < .001. Correlations between conditions ranged from r = 0.35 to 0.78, ps < .001. For sAA, correlations within conditions ranged from r = 0.79 to 0.89, ps < .001 for the neutral condition, and r = 0.64 to 0.88, ps < .001 for the chaos condition. Correlations between conditions ranged from r = 0.58 to 0.73, ps < .001. Negative emotional state levels did not correlate significantly with sAA levels (r = −0.14 – 0.14, ps ≥ .138). For the separate time points, levels of negative emotional state, sAA levels and caregiving sensitivity did not differ between the neutral and the chaos condition.
Table 1.
Descriptive statistics and correlations of study variables between time and conditions.
| Time | Neutral condition |
Chaos condition |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M(SD) | Baseline | Phase 1 | Phase 2 | Phase 3 | Baseline | Phase 1 | Phase 2 | |||
| Negative emotional state | Neutral condition | Baseline | 0.12 (0.08) | |||||||
| Phase 1 | 0.19 (0.12) | .57*** | - | |||||||
| Phase 2 | 0.18 (0.12) | .49*** | .86*** | |||||||
| Phase 3 | 0.19 (0.13) | .40*** | .79*** | .86*** | ||||||
| Chaos condition | Baseline | 0.14 (0.09) | .53*** | .45*** | .40*** | .44*** | ||||
| Phase 1 | 0.21 (0.11) | .39*** | .58*** | .61*** | .65*** | .68*** | ||||
| Phase 2 | 0.19 (0.11) | .35** | .68*** | .68*** | .72*** | .63*** | .79*** | - | ||
| Phase 3 | 0.19 (0.12) | .40*** | .70*** | .78*** | .76*** | .53*** | .69*** | .87*** | ||
| Salivary alpha-amylase | Neutral condition | Baseline | 5.00 (0.34) | |||||||
| Phase 1 | 5.08 (0.31) | .83*** | ||||||||
| Phase 2 | 5.04 (0.31) | .79*** | .84*** | |||||||
| Phase 3 | 5.02 (0.31) | .82*** | .84*** | .89*** | ||||||
| Chaos condition | Baseline | 4.97 (0.37) | .72*** | .68*** | .70*** | .68*** | ||||
| Phase 1 | 5.11 (0.35) | .61*** | .63*** | .68*** | .65*** | .81*** | ||||
| Phase 2 | 5.03 (0.32) | .58*** | .60*** | .69*** | .64*** | .78*** | .89*** | |||
| Phase 3 | 5.04 (0.31) | .67*** | .66*** | .73*** | .75*** | .80*** | .88*** | .87*** | ||
| Sensitive caregiving | Neutral condition | Phase 1 | 6.03 (1.55) | |||||||
| Phase 2 | 4.74 (1.92) | .54*** | ||||||||
| Phase 3 | 4.09 (1.92) | .43*** | .83*** | |||||||
| Chaos condition | Phase 1 | 5.75 (1.54) | .43*** | .18 | .22* | |||||
| Phase 2 | 4.37 (1.96) | .25* | .50*** | .53*** | .46*** | |||||
| Phase 3 | 4.03 (1.89) | .25* | .50*** | .57*** | .41*** | .81*** | ||||
Note. Salivary alpha-amylase, and negative emotional state are log-transformed. Sensitive caregiving was not assessed during baseline. *p < .05, **p < .01, ***p < .001, N range = 87–95.
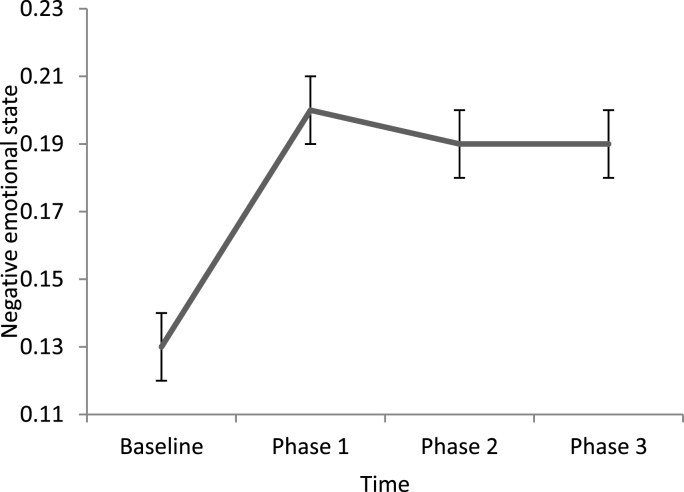
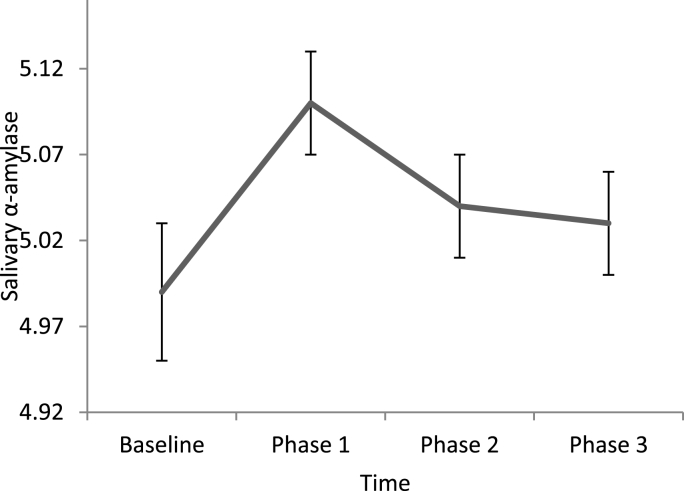
To test the course from baseline to the experiment for negative emotional state and sAA respectively, we explored the non-linear course of negative emotional state from baseline to the experimental phase. Since we were interested in a time effect, models 3 and 4 were compared with respect to model fit. For negative emotional state, Model 3 was accepted as the final model, and included a random intercept, random slopes, covariates and the main effect of time as a factor. The significant effect between baseline and phase 1 (b = 0.08, 95% CI = 0.06–0.09) indicated that negative emotional state levels increased from baseline to phase 1. For sAA, Model 4 was accepted as the final model, and included a random intercept, random slopes, covariates, correlations between effects, and the main effect of time as a factor. Again, the change between baseline and phase 1 was significant (b = 0.11, 95% CI = 0.08–0.15), indicating that sAA levels increased from baseline to phase 1. The course of negative emotional state and sAA during caregiving is displayed in Fig. 1, Fig. 2.
Fig. 1.
Levels of negative emotional state at baseline and during caregiving (M, SE).
Fig. 2.
Levels of salivary alpha-amylase at baseline and during caregiving (M, SE).
3.2. The effect of household chaos on negative emotional state
To test whether household chaos had an effect on negative emotional state, stepwise multilevel models were performed for negative emotional state, controlled for baseline levels, age, and education (Table 2). The unconditional means model showed dependency (ICC = 0.64). Since the within-person variance of negative emotional state levels was smaller than the between-person variance, it justified the use of multilevel modelling. Since adding correlations between random effects (Model 4), and adding the main effect of condition (Model 5), and the interaction between time and condition (Model 6) did not improve model fit significantly, these models were rejected. Model 3 was accepted as the final model, and included a random intercept, random slopes, covariates, and the main effect of time. The main effect of time was not significant over de course of the experiment (b = −0.04, 95% CI = 0.05 to −0.02, β = 0.001), neither were the effect of condition and the interaction effect between time and condition. This indicates that negative emotional state did not change significantly over the course of the experiment, nor did it differ between conditions, and the patterns over time were not different between conditions.
Table 2.
Linear stepwise multilevel models of negative emotional state with the moderating role of household chaos.
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|---|---|---|---|---|---|---|
| Fixed effects | b (se), 95% CI | |||||
| Intercept | 0.13 (0.01) 0.10–0.15* |
0.05 (0.10) −0.14–0.23 |
0.05 (0.10) −0.13–0.23 |
0.06 (0.09) −0.12–0.24 |
0.05 (0.10) −0.14–0.23 |
0.04 (0.10) −0.15–0.22 |
| Time | 0.00 (0.00) 0.00–0.01 |
0.00 (0.00) 0.00–0.01 |
0.00 (0.00) −0.01–0.01 |
0.00 (0.0) −0.01–0.01 |
0.01 (0.00) 0.00–0.01 |
|
| Condition | 0.00 (0.01) −0.01–0.01 |
0.02 (0.01) 0.00–0.05 |
||||
| Time*condition | −0.01 (0.01) −0.02–0.00 |
|||||
| Covariates | ||||||
| Baseline score | 0.48 (0.06) 0.36–0.61* |
0.48 (0.06) 0.35–0.60* |
0.48 (0.06) 0.35–0.60* |
0.48 (0.06) 0.36–0.61* |
0.47 (0.06) 0.34–0.59* |
0.47 (0.06) 0.34–0.59* |
| Age | 0.00 (0.01) 0.00–0.02 |
0.01 (0.01) 0.00–0.01 |
0.10 (0.01) 0.00–0.02 |
0.01 (0.01) 0.00–0.02 |
0.01 (0.01) 0.00 01–0.02 |
|
| Education | −0.06 (0.02) −0.11 to −0.02* |
−0.06 (0.02) −0.11 – -0.02* |
−0.06 (0.02) −0.10 to −0.01* |
−0.06 (0.02) −0.11 to −0.02* |
−0.06 (0.02) −0.11 to −0.02* |
|
| Variance components | Coef. (se) | |||||
| Variance residuals intercept | 0.09 (0.00) | 0.08 (0.00) | 0.08 (0.00) | 0.07 (1.71)) | 0.08 (0.00) | 0.08 (0.00) |
| Variance residuals within-person | 0.05 (0.00) | 0.05 (0.00) | 0.05 (0.00) | 0.05 (9.24) | 0.05 (0.00) | 0.05 (0.00) |
| Correlation intercept slope ρ_01 | 1.00 (2.48) | |||||
| Variance residuals slope | 0.00 (0.00) | 0.00 (5.51) | 0.00 (0.00) | 0.00 (0.00) | ||
| F(df), p | ||||||
| Comparison to previous model | 3.11 (3)* | 2.86 (1) | 0.70 (1) 1 | 2.72 (1) |
Note. Dependent variables are listed under model description. Condition coded 0 = neutral, 1 = chaos. *p < 0.05 ** p < 0.01 *** p < .001.
1 Model 5 is compared to Model 3, since Model 4 did not improve significantly compared to Model 3. Empty fields = not applicable. Model 3 and 5 are not nested and thus cannot be statistically compared.
3.3. The effect of household chaos on salivary alpha-amylase
Next, we tested whether household chaos affected sAA levels. The unconditional means model (Model 1) with random intercepts, sAA across time as outcome variable, controlled for baseline scores, age, education, drink in the past ½ hour, and gum diseases, showed dependency (ICC = 0.45). This justified the use of multilevel modelling since the within-person variance of sAA levels was smaller than the between-person variance. Model 4, which added correlations between random effects, did not improve model fit significantly, and was rejected, as was Model 6, in which the interaction between time and condition was added (b = 0.03, 95% CI = −0.04 – 0.10). This resulted in Model 5 being accepted as the final model, which included a random intercept, random slopes, covariates and the main effects of time and condition.
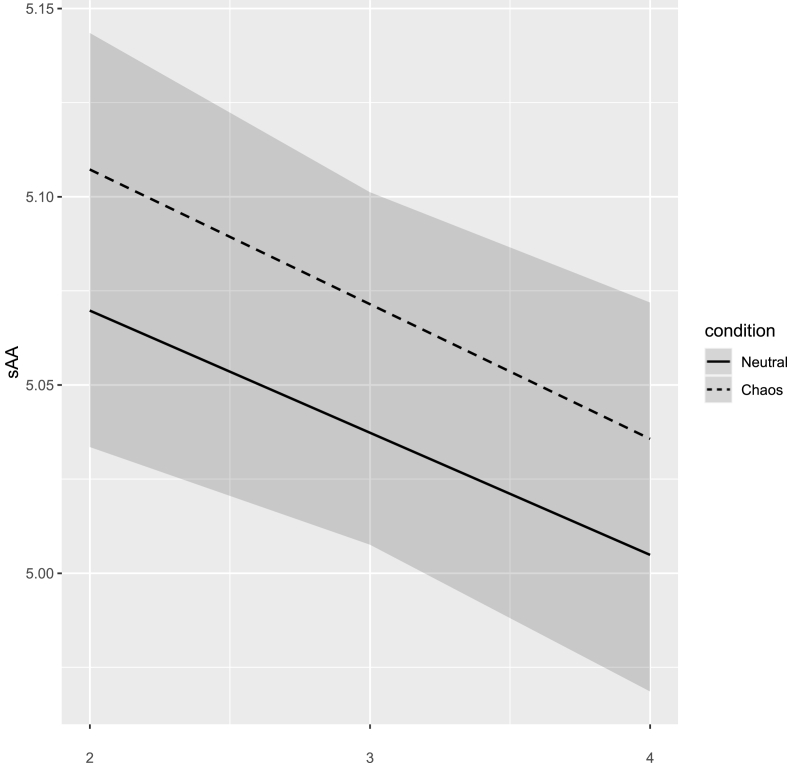
Results of the final model are presented in Table 3. The significant main effect of time (b = 0.01, 95% CI = −0.04 to −0.02, β = −0.11) indicates that sAA levels decreased over the course of the experiment. Condition showed a significant main effect as well (b = 0.03, 95% CI = 0.001–0.05, β = 0.06), indicating that sAA levels differed between the two conditions. As is shown in Fig. 3, baseline corrected sAA levels were higher during the chaos condition compared to the neutral condition.
Table 3.
Linear stepwise multilevel models of salivary alpha-amylase with the moderating role of household chaos.
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |
|---|---|---|---|---|---|---|
| Fixed effects | b (se), 95% CI | |||||
| Intercept | 1.98 (0.18) 1.63–2.33* |
2.22 (0.25) 1.73–2.71* |
2.22 (0.25) 1.73–2.71* |
2.23 (0.25) 1.74–2.72* |
2.16 (0.25) 1.66–2.65* |
2.16 (0.25) 1.66–2.65* |
| Time | −0.04 (0.00) -.05 to −.02* |
−0.04 (0.0) −0.05 to −0.02* |
0.01 (0.01) −0.02 to −0.02* |
0.01 (0.01) −0.05 – -0.02* |
−0.03 (0.01) −0.06 to −0.01* |
|
| Condition |
0.03 (0.01) 0.001–0.05*1 |
0.03 (0.03) −0.04–0.10 |
||||
| Time*condition | 0.00 (0.02) −0.03–0.03 |
|||||
| Covariates | ||||||
| Baseline score | 0.62 (0.04) 0.55–0.68* |
0.60 (0.04) 0.53–0.67* |
0.60 (0.04) 0.53–0.67* |
0.60 (0.04) 0.53–0.67 |
0.61 (0.04) 0.54–0.68* |
−0.10 (0.06) −0.21–0.02 |
| Age | 0.00 (0.00) −0.01–0.02 |
0.00 (0.01) −0.01–0.02 |
0.00 (0.01) −0.01–0.02 |
0.00 (0.01) −0.01–0.02 |
0.00 (0.02) −0.01–0.02 |
|
| Education | −0.06 (0.04) −0.13–0.01 |
−0.06 (0.04) −0.13–0.11 |
−0.06 (0.04) −0.13–0.01 |
−0.06 (0.04) −0.13–0.01 |
−0.06 (0.04) −0.13–0.01 |
|
| Gingivitis/periodontitis | −0.06 (0.03) −0.12–0.01 |
−0.06 (0.03) −0.04–0.04 |
−0.06 (0.03) −0.12–0.01 |
−0.06 (0.03) −0.12–0.01 |
−0.06 (0.03) −0.12–0.01 |
|
| Drink past ½ hour | 0.00 (0.02) −0.04–0.04 |
0.00 (0.02) −0.04–0.04 |
0.00 (0.02) −0.04–0.04 |
0.00 (0.02) −0.04–0.04 |
0.00 (0.02) −0.04–0.04 |
|
| Variance components | Coef. (se) | |||||
| Variance residuals intercept | 0.13 (0.00) | 0.13 (0.00) | 0.13 (0.00) | 0.14 (0.00) | 0.13 (0.00) | 0.31 (0.00) |
| Variance residuals within-person | 0.15 (0.00) | 0.15 (0.00) | 0.15 (0.00) | 0.15 (0.00) | 0.15 (0.00) | 0.15 (0.00) |
| Correlation intercept slope ρ_01 | 0.00 (0.00) | −0.66 (0.75) | ||||
| Variance residuals slope | 0.00 (0.00) | 0.00 (0.00) | 0.00 (0.00) | |||
| F(df), p | ||||||
| Comparison to previous model | N. A. | 5.10 (5)*** | 0.17 (1) | 4.17 (1)*2 | 0.03(1) |
Note. Dependent variables are listed under model description. Condition coded 0 = neutral, 1 = chaos. *p < 0.05 ** p < 0.01 *** p < .001.
1 To show that this CI did not include zero, the value is rounded off an extra decimal point.
2 Model 5 is compared to Model 3, since Model 4 did not improve significantly compared to Model 3. Empty fields = not applicable. Model 3 and 5 are not nested and thus cannot be statistically compared.
Fig. 3.
Levels of salivary alpha-amylase at baseline and during the experiment in the neutral and chaos conditions (M, 95% CI).
3.4. Relations with sensitive caregiving
Next we tested whether negative emotional state and sAA were related to sensitive caregiving. Within the separate phases in the neutral condition, there were no significant correlations. Within the three phases in the chaos condition, only during phase 1 were negative emotions related to lower levels of sensitivity (r = −0.23, p < .050). For sAA, there were no significant correlations with caregiver sensitivity within phases per condition (see Appendix A). Furthermore, the difference scores between sensitive caregiving in the neutral and chaos conditions (for each of the phases) were not related to the difference scores of negative emotional state (rs = −0.03 – 0.11, ps = .306–779), or sAA (rs = 0.03–0.18, ps = .091–0.673).
4. Discussion
The aim of this study was to assess relations between household chaos, negative emotions and sAA during caregiving, using an experimental design in order to get insight into the directionality of the assessed relations. Young adult females (non-mothers) took care of an infant simulator, and household chaos was experimentally manipulated. Negative emotional state and sAA both showed an increase from baseline to the experiment. In addition, we assessed the effect of household chaos on emotional state and physiological stress. We found no effect of chaos on negative emotions, but sAA levels were higher in the chaos condition compared to the neutral condition, although effects were small in magnitude. Furthermore, although we found effects of household chaos on sAA, we found no evidence for sAA mediating the relation between household chaos and caregiving. In addition, we found that a stronger negative emotional state was correlated with less sensitive caregiving during the first phase of the chaos condition.
Both negative emotional state and sAA showed a significant increase from baseline to the start of the experiment. In other words, participants indicated that they felt stronger negative emotions and experienced more physiological stress from baseline levels to the experiment. The steep increase from baseline to the start of the experiment found for sAA as well as the further course of sAA reactivity resembles a typical stress response to a social stressor [16,18]. Because of the experimental nature of our study, it can be stated that taking care of an infant simulator that cries a significant portion of time, and being videotaped doing so, is a social stressor that results in a physiological stress response over both conditions. This is in line with earlier studies finding an ANS response to crying sounds [34,35]. We also found a slight decline in sAA levels after the initial increase. It is likely that this reflects the initial extra stress resulting from insecurity about what is going to happen in the living room lab.
When focusing on negative emotional state, analyses assessing the role of household chaos during the experiment yielded no significant effects. Thus, although participants did report a peak in negative emotions during the experiment in both conditions, negative emotional state seems to be unaffected by household chaos. Yet, we found we found that a stronger negative emotional state was correlated with less sensitive caregiving during the first phase of the chaos condition. Because this result was not obtained using multilevel analyses but using a correlation analysis and we only found it in one of the three phases, we must interpret this with caution. Thus, we can carefully conclude that there might be an indication that negative emotions are related to less sensitive caregiving in a chaotic home environment. This is in line with the association between negative emotions and parenting problems, including maltreatment, found in earlier studies [36,37].
Focusing on sAA, we found a time effect in which sAA levels decreased over the course of the experiment, and we found an effect of household chaos on sAA: levels were higher in the chaos condition than in the neutral condition. The experimental nature of our study implies that household chaos can be seen as an environmental stressor that affects ANS reactivity, on top of the physiological response to the crying infant. Household chaos can thus be seen as a causal factor that leads to changes in the ANS response. The unpredictability, instability and sensory overload caused by household chaos may be perceived as a threatening environment by caregivers, which seems to trigger an ANS response in order to be prepared for challenge or threat [38]. This implies that household chaos is a component of family life that can cause stress in parents. Household chaos might thus be an intervention target for reducing stress in parents. The reported effect sizes are, however, small in magnitude. This implies that decreasing household chaos is only one of the elements in the proximal environment of families that has the potential to reduce stress, and should be one of the elements amongst other targets when intervening. Future research should assess whether reducing household chaos leads to less stress in parents during parent-child interactions and should test for which parents this has the largest effect.
Moreover, negative emotional state and sAA were uncorrelated in this study. This suggests that negative emotional state and ANS reactivity are two distinct constructs that cannot be used interchangeably to assess stress. This has been argued before. For example, ANS reactivity in response to infant crying differed between parents with and without a history of childhood maltreatment, but their perception of the crying sounds did not differ [34]. This underlines the importance of distinguishing physiological arousal by physiological markers, such as sAA, from the perception of emotional state when assessing stress. This means that self-report questionnaires assessing emotional state cannot replace the assessment of physiological stress response.
We did not find evidence to support the hypothesis that sAA levels are related to sensitive caregiving, therefore we can conclude that sAA levels did not mediate the association between household chaos and caregiving. In contrast to our study, previous research has shown that physiological stress in parents is related to caregiving. For example, mother's heart rate and respiratory sinus arrhythmia has been associated with sensitive caregiving to their child [39]. In addition, a meta-analysis showed that higher ANS levels are associated with a risk for child maltreatment [25]. It has to be noted that in our study, participants took care of an infant simulator and not their own child, which may explain differences in findings. In addition, in our lab experiment, household chaos was created by the experimenters. When parents shape their own environments, including the levels of household chaos, it could be that household chaos does affect parenting via physiological stress.
Some limitations should be taken into account with regard to this study. First, in order to control for past caregiver experiences with their own children, we only included female adults who did not have children, which might raise questions regarding the generalizability of these results to parents. However, it has been argued that taking care of an infant simulator by mothers is significantly associated with parenting their own child in mothers [26]. In this light, it can be expected that parents show similar parenting when taking care of their own crying child. It has yet to be studied whether physiological stress responses are similar when taking care of one's own child compared to the infant simulator.
Second, it is possible that the videotaping elicited such a stress response in the participants already that it overshadowed the effects of chaos on stress levels. Being videotaped has been used with the goal of eliciting stress in participants, e.g., when performing the Trier Social Stress Test [40]. Nonetheless, there is evidence that infant behavior such as crying, elicits an ANS stress response [24,35], as well as a negative emotional state [41]. In addition, if being videotaped was the predominant stressor, one would expect an effect of the order of the visits, with a stronger stress response during the first lab visit. Yet, we did not find such an effect. Although it remains difficult to disentangle the effects of multiple stressors, it seems that in our study household chaos elicits at least some ANS response on top of the crying infant simulator, and being videotaped.
5. Conclusions
In sum, this study provides insight in the relations between household chaos, negative emotions, physiological stress, and caregiving. Our experimental design allowed us to standardize caregiving demands and manipulate levels of household chaos. In addition, we controlled for past parenting experiences by only including women who were not mothers. This enabled us to make claims about the causality of the effects. The course of negative emotional state and sAA showed an increase from baseline to the experiment. In addition, although effects were small, sAA during the caregiving situation was affected by chaos. Also, we found no significant effect of household chaos on negative emotional state. Future research should extend these results to parents, and assess ANS reactivity and negative emotional state in the home setting. Results imply that household chaos serves as a stressor in caregiving situations. Therefore, it is important to assess whether reduced levels of household chaos, by interventions targeting household chaos in the home, reduce physiological stress in parents. Household chaos should not be overlooked when applying or designing interventions aimed at reducing stress in parents, as well as in children. It could be worthwhile to implement it as a module in existing interventions intended de reduce stress and improve parenting in families in the home environment.
Declarations of competing interest
None.
Acknowledgements
FB, SA, MP, and LA were supported by an NWO VIDI grant awarded to LA (016.145.360). The authors have no competing interests to declare. We thank Marinus van IJzendoorn for his input at the early stages of this study.
Appendix A.
Correlations between sAA, negative emotional state, and caregiver sensitivity.
| Salivary alpha-amylase |
Caregiver sensitivity |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutral condition |
Chaos condition |
Neutral condition |
Chaos condition |
|||||||||||||
| B | 1 | 2 | 3 | B | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |||
| Negative emotional state | Neutral condition | B | .06 | .14 | .06 | .06 | .13 | .05 | -.01 | .06 | .04 | .13 | .21* | -.20 | -.09 | .01 |
| 1 | .03 | .06 | .06 | .07 | .05 | -.03 | -.01 | .08 | -.05 | .03 | .11 | -.24* | -.06 | -.03 | ||
| 2 | .05 | .05 | .09 | .05 | .11 | .02 | .05 | .11 | .05 | .16 | .21* | -.13 | .08 | .09 | ||
| 3 | .12 | .12 | .14 | .12 | .11 | .09 | .08 | .16 | -.06 | .08 | .15 | -.18 | .07 | .09 | ||
| Chaos condition | B | -.13 | -.08 | -.14 | -.10 | .10 | .08 | -.01 | .07 | .06 | .15 | .24** | -.30** | .02 | .00 | |
| 1 | -.10 | -.04 | -.03 | -.03 | .08 | .09 | .07 | .13 | .06 | .13 | .30** | -.23* | .07 | .10 | ||
| 2 | .00 | .02 | .02 | .02 | .09 | .06 | .04 | .12 | -.01 | .11 | .20 | -.21 | .01 | .02 | ||
| 3 | .09 | .08 | .11 | .10 | .14 | .08 | .06 | .14 | .10 | .21* | .28** | -.20 | .10 | .12 | ||
| Caregiver sensitivity | Neutral condition | 1 | .06 | .14 | .04 | .03 | .05 | .01 | -.01 | .00 | ||||||
| 2 | -.07 | -.02 | -.04 | -.06 | -.15 | -.20 | -.22* | -.20 | ||||||||
| 3 | -.01 | .04 | -.10 | .00 | -.07 | -.10 | -.06 | -.07 | ||||||||
| Chaos condition | 1 | .06 | .06 | .02 | .13 | .06 | .01 | .09 | .02 | |||||||
| 2 | -.07 | -.11 | -.04 | -.10 | -.07 | -.04 | .01 | -.05 | ||||||||
| 3 | -.07 | -.10 | -.05 | -.04 | -.07 | -.05 | -.05 | -.10 | ||||||||
Note. B = Baseline, 1 = phase 1, 2 = phase 2, 3 = phase 3. N range = 87–95. *p < .05, **p < .01.
References
- 1.Sturge‐Apple M.L., Skibo M.A., Rogosch F.A., Ignjatovic Z., Heinzelman W. The impact of allostatic load on maternal sympathovagal functioning in stressful child contexts: implications for problematic parenting. Dev. Psychopathol. 2011;23:831–844. doi: 10.1002/ana.22528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deater-Deckard K. Parenting stress and child adjustment: some old hypotheses and new questions. Clin. Psychol. Sci. Pract. 1998;5:314–332. doi: 10.1111/j.1468-2850.1998.tb00152.x. [DOI] [Google Scholar]
- 3.Rieder J.K., Goshin L.S., Sissoko D.R.G., Kleshchova O., Weierich M.R. Salivary biomarkers of parenting stress in mothers under community criminal justice supervision. Nurs. Res. 2019;68:48–56. doi: 10.1097/NNR.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans G.W., Wachs T.D. American Psychological Association; 2010. Chaos and its Influence on Children's Development. [DOI] [Google Scholar]
- 5.Coldwell J., Pike A., Dunn J. Household chaos–links with parenting and child behaviour. JCPP (J. Child Psychol. Psychiatry) 2006;47(11):1116–1122. doi: 10.1111/j.1469-7610.2006.01655.x. [DOI] [PubMed] [Google Scholar]
- 6.Barnes J., Gardiner J., Sutcliffe A., Melhuish E. The parenting of preschool children by older mothers in the United Kingdom. Eur. J. Dev. Psychol. 2014;11(4):397–419. doi: 10.1080/17405629.2013.863728. [DOI] [Google Scholar]
- 7.Park J.L., Johnston C. The relations among stress, executive functions, and harsh parenting in mothers. J. Abnorm. Child Psychol. 2020;48:619–632. doi: 10.1007/s10802-020-00622-x. [DOI] [PubMed] [Google Scholar]
- 8.Hruska V., Ambrose T., Darlington G., Ma D.W., Haines J., Buchholz A.C. Stress is associated with adiposity in parents of young children. Obesity. 2020;28(3):655–659. doi: 10.1002/oby.22710. [DOI] [PubMed] [Google Scholar]
- 9.Bronfenbrenner U. Toward an experimental ecology of human development. Am. Psychol. 1977;32(7):513. [Google Scholar]
- 10.Marsh S., Dobson R., Maddison R. The relationship between household chaos and child, parent, and family outcomes: a systematic scoping review. BMC Publ. Health. 2020;20:1–27. doi: 10.1186/s12889-020-08587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deater‐Deckard K., Mullineaux P.Y., Beekman C., Petrill S.A., Schatschneider C., Thompson L.A. Conduct problems, IQ, and poor family routines: a longitudinal multi‐informant study. JCPP (J. Child Psychol. Psychiatry) 2009;50(10):1301–1308. doi: 10.1111/j.1469-7610.2009.02108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zvara B.J., Lathren C., Mills-Koonce R. Maternal and paternal attachment style and chaos as risk factors for parenting behavior. Fam. Relat. 2020;69(2):233–246. doi: 10.1111/fare.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson J.A., O'Brien M., Blankson A.N., Calkins S.D., Keane S.P. Family stress and parental responses to children's negative emotions: tests of the spillover, crossover, and compensatory hypotheses. J. Fam. Psychol. 2009;23:671–679. doi: 10.1037/a0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith A.L., Cross D., Winkler J., Jovanovic T., Bekh B. Emotional dysregulation and negative affect mediate the relationship between maternal history of child maltreatment and maternal child abuse potential. J. Fam. Violence. 2014;29:483–494. doi: 10.1007/s10896-014-9606-5. [DOI] [Google Scholar]
- 15.Nater U.M., Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009;34(4):486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Rohleder N., Nater U.M., Wolf J.M., Ehlert U., Kirschbaum C. Psychosocial stress-induced activation of salivary alpha-amylase: an indicator of sympathetic activity. Ann NY Acad Sci. 2004;1032(1):258–263. doi: 10.1196/annals.1314.033. [DOI] [PubMed] [Google Scholar]
- 17.Reijman S., Alink L.R.A., Compier-de Block L.H.C.G., Werner C.D., Maras A., Rijnberk C., Van IJzendoorn M.H., Bakermans-Kranenburg M.J. Autonomic reactivity to infant crying in maltreating mothers. Child. Maltreat. 2014;19:101–112. doi: 10.1177/1077559514538115. [DOI] [PubMed] [Google Scholar]
- 18.Nater U.M., Rohleder N., Gaab J., Berger S., Jud A., Kirschbaum C., Ehlert U. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int. J. Psychophysiol. 2005;55:333–342. doi: 10.1016/j.ijpsycho.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Out D., Bakermans-Kranenburg M.J., Granger D.A., Cobbaert C.M., Van IJzendoorn M.J. State and trait variance in salivary alpha-amylase: a behavioral genetic study. Biological Psyhology. 2011;1(88):147–154. doi: 10.1016/j.biopsycho.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Doom J.R., Cook S.H., Sturza J., Kaciroti N., Gearhardt A.N., Vazquez D.M., Lumeng J.C., Miller A.L. Family conflict, chaos, and negative life events predict cortisol activity in low-income children. Dev. Psychobiol. 2018;60(4):364–379. doi: 10.1002/dev.21602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown E.D., Anderson K.E., Garnett M.L., Hill E.M. Economic instability and household chaos relate to cortisol for children in poverty. J. Fam. Psychol. 2019;33(6):629–639. doi: 10.1037/fam0000545. [DOI] [PubMed] [Google Scholar]
- 22.Seelander J., Bluhm G., Theorell T., Pershagen G., Babisch W., Seiffert I., Houthuijs D., Breugelmans O., Vigna-Taglianti F., Antoniotti M.C., Velonakis E., Davou E., Dudley M.L., Jarup L. Saliva cortisol and exposure to aircraft noise in six European countries. Environ. Health Perspect. 2009;117:1713–1717. doi: 10.1289/ehp.0900933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crouch J.L., Hiraoka R., McCanne T.R., Reo G., Wagner M.F., Krauss A., Milner J.S., Skowronski J.J. Heart rate and heart rate variability in parents at risk for child physical abuse. J. Interpers Violence. 2018;33:1629–1652. doi: 10.1177/0886260515619169. [DOI] [PubMed] [Google Scholar]
- 24.Out D., Bakermans-Kranenburg M.J., Van Pelt J., Van IJzendoorn M.J. Salivary α-amylase and intended harsh caregiving in response to infant crying: evidence for physiological hyperreactivity. Child. Maltreat. 2012;17(4):295–305. doi: 10.1177/1077559512464427. [DOI] [PubMed] [Google Scholar]
- 25.Reijman S., Bakermans-Kranenburg M.J., Hiraoka R., Crouch J.L., Milner J.S., Alink L.R.A., Van IJzendoorn M.H. Baseline functioning and stress reactivity in maltreating parents and at-risk adults: review and meta-analyses of autonomic nervous system studies. Child. Maltreat. 2016;21:327–342. doi: 10.1177/1077559516659937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakermans‐Kranenburg M.J., Alink L.R., Biro S., Voorthuis A., van IJzendoorn M.H. The leiden infant simulator sensitivity assessment (LISSA): parenting an infant simulator as your own baby. Infant Child Dev. 2015;24:220–227. doi: 10.1002/icd.1905. [DOI] [Google Scholar]
- 27.Voorthuis A., Out D., van der Veen R., Bhandari R., van IJzendoorn M.H., Bakermans-Kranenburg M.J. One doll fits all: validation of the leiden infant simulator sensitivity assessment (LISSA) Am. J. Bioeth. 2013;15:603–617. doi: 10.1080/14616734.2013.820897. [DOI] [PubMed] [Google Scholar]
- 28.Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 1988;54(6):1063. doi: 10.1037//0022-3514.54.6.1063. 0022-35l4/88/$00.75. [DOI] [PubMed] [Google Scholar]
- 29.Marteau T.M., Bekker H. The development of a six‐item short‐form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI) Br. J. Clin. Psychol. 1992;31(3):301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 30.Spielberger C.D. Assessment of state and trait anxiety: conceptual and methodological issues. Southern Psychologist. 1985;2(4):6–16. [Google Scholar]
- 31.Ainsworth M.D.S., Bell S.M., Stayton D.F. The Integration of a Child into a Social World. Cambridge University Press; 1974. Infant-mother attachment and social development: socialization as a product of reciprocal responsiveness to signals; pp. 99–135. [Google Scholar]
- 32.Strahler J., Skoluda N., Kappert M.B., Nater U.M. Simultaneous measurement of salivary cortisol and alpha-amylase: application and recommendations. Neurosci. Biobehav. Rev. 2017;83:657–677. doi: 10.1016/j.neubiorev.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 33.R Core Team . 2020. R: A Language and Environment for Statistical Computing.http://www.R-project.org/ Retrieved from. [Google Scholar]
- 34.Buisman R.S.M., Pittner K., Compier-de Block L.H.C.G., van den Berg L.J.M., Bakermans-Kranenburg M.J., Alink L.R.A. The past is present: the role of maltreatment history in perceptual, behavioral and autonomic responses to infant emotional signals. Child Abuse Negl. 2018;77:23–34. doi: 10.1016/j.chiabu.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Reijman S., Alink L.R.A., Compier-de Block L.H.C.G., Werner C.D., Maras A., Rijnberk C., Van IJzendoorn M.H., Bakermans-Kranenburg M.J. Salivary α-amylase reactivity to infant crying in maltreating mothers. Child Psychiatr. Hum. Dev. 2015;46:589–599. doi: 10.1007/s10578-014-0499-6. [DOI] [PubMed] [Google Scholar]
- 36.Leerkes E.M. Predictors of maternal sensitivity to infant distress. Parenting: Science and Practice. 2010;10:219–239. doi: 10.1080/15295190903290840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stith S.M., et al. Risk factors in child maltreatment: a meta-analytic review of the literature. Aggress. Violent Behav. 2009;14:13–29. [Google Scholar]
- 38.Boyce W.T., Ellis B.J. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- 39.Joosen K.J., Mesman J., Bakermans‐Kranenburg M.J., Pieper S., Zeskind P.S., Van IJzendoorn M.H. Physiological reactivity to infant crying and observed maternal sensitivity. Infancy. 2013;18(3):414–431. doi: 10.1111/j.1532-7078.2012.00122.x. [DOI] [Google Scholar]
- 40.Kirschbaum C., Pirke K.M., Hellhammer D.H. The ‘Trier Social Stress Test’: a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 41.Soltis J. The signal functions of early infant crying. Behav. Brain Sci. 2004;27(4):443–458. doi: 10.1017/S0140525X0400010X. [DOI] [PubMed] [Google Scholar]
- 42.Hohmann C., Grabenhenrich L., De Kluizenaar Y., Tischer C., Heinrich J., Chen C., Thijs C., Nieuwenhuijsen M., Keil T. Health effects of chronic noise exposure in pregnancy and childhood: a systematic review initiated by ENRIECO. Int. J. Hyg Environ. Health. 2013;216:217–229. doi: 10.1016/j.ijheh.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Andeweg S.M., Bodrij F.F., Prevoo M.J.L., Rippe R.C.A., Alink L.R.A. Does sensory-processing sensitivity moderate the effect of household chaos on caregiver sensitivity? An experimental design. J. Fam. Psychol. 2021;35(3):356–365. doi: 10.1037/fam0000766. [DOI] [PubMed] [Google Scholar]