Abstract
Autism spectrum disorder (ASD) is a neurodevelopmental disorder, whose core symptoms consist of deficits in social interaction and communication as well as restricted and repetitive behavior. Brain oxytocin (OXT) has been associated with various prosocial behaviors, and might, therefore, be involved in the pathogenesis of disorders associated with socio-emotional dysfunctions such as ASD. However, significant associations between central and peripheral OXT levels may only be present in response to physiological or stressful stimuli but were not shown under baseline conditions. In this study, we, therefore, investigated salivary and plasma OXT in response to physical exercise in adults with ASD (n = 33, mean age: 36.8 ± 10.7 years) without intellectual impairment (IQ > 70) and neurotypical controls (n = 31, mean age: 31.0 ± 11.7 years). To stimulate the OXT system, we used rapid cycling and measured cortisol (CORT) concentrations to monitor the physiological stress response. When controlling for age, neither salivary OXT (p = .469), plasma OXT (p = .297) nor CORT (p = .667) concentrations significantly differed between groups at baseline. In addition, neither OXT nor CORT concentrations significantly differed between groups after physical exercise. Social anxiety traits were negatively correlated with plasma, but not saliva OXT concentrations in neurotypicals at baseline, while empathetic traits were positively correlated with saliva, but not plasma concentrations in autistic patients at baseline. No significant correlations between salivary and plasma OXT concentrations were found at any time point. Future studies including adult participants should investigate the effect of age on CORT and OXT concentrations in response to stress.
Keywords: Autism spectrum disorder, Oxytocin, Cortisol, Stress
Highlights
-
•
Basal levels of cortisol and oxytocin did not differ in adults with ASD from controls.
-
•
After physical exercise plasma oxytocin increased in ASD with low cortisol-response.
-
•
Cortisol and oxytocin levels post-task did not significantly differ between groups.
-
•
Social phobic traits predicted lower plasma oxytocin concentrations in controls.
-
•
Empathetic traits predicted higher salivary oxytocin levels in ASD.
1. Introduction
Autism spectrum disorder (ASD) is defined by persistent impairments of social communication and interaction as well as repetitive and restricted patterns of behavior, interests or activities [1]. The existing diagnostic assessment of ASD is still very complex and time-consuming. Especially autistic individuals with at least average intelligence, also referred to as persons with high-functioning autism (HFA), can reach adulthood without being tested for ASD due to cognitive compensation mechanisms and favorable environmental factors [2]. Due to the high comorbidity rate of psychiatric conditions (e.g. depression, social phobia, and alexithymia) potentially overlapping the core symptoms of ASD, the diagnostic assessment is further complicated [2,3]. For these reasons, the development of objective and observer-independent markers would be an important break-through for autism diagnosis but could also be relevant for treatment decisions.
Until today, the exact etiology of ASD remains poorly understood [4]. The neuropeptide oxytocin (OXT), which has been frequently linked with ASD in recent years, has received considerable interest as a modulator of social behaviors on the one hand, and anxiety and stress-coping on the other [5,6]. OXT is primarily synthesized in the magnocellular neurons of the paraventricular and supraoptic nuclei of the hypothalamus, then transported to the posterior pituitary gland, and finally released into the blood stream [7]. OXT is also released via widely distributed pathways within distinct brain regions in response to social or stressful stimuli as revealed in laboratory animals, where it acts as a neuromodulator [7]. In humans, OXT is mostly measured in the periphery, i.e. in different body fluids such as plasma, saliva or urine [8].
Due to the core symptomatology of ASD, a deficiency of the OXT system has been suggested as an underlying cause of autism [9]. Analyses of peripheral OXT concentrations in autistic individuals, however, showed mixed results with lower [10,11], higher [12,13], or similar OXT values compared to neurotypicals [14,15]. Importantly, the temporal dynamics of peripheral OXT release may substantially differ from that of central release in a stimulus-dependent way [5,16]. While both central and peripheral OXT release was observed during birth, suckling, mating, physical exercise or experimentally induced stress, no correlation was found under baseline conditions [5,16,17]. Thus, previous findings observed solely at baseline need to be treated with caution as it is unclear whether they reflect the OXT (dys-)regulations of the brain. Several stimuli like sexual self-stimulation [18], psychosocial stress [18,19], and physical exercise [18,20,21] have been identified to reliably increase peripheral OXT concentrations. In adults with ASD, however, a commonly used psychosocial stress task like public speaking has failed to induce a peripheral OXT change [13]. In this study, we therefore, chose physical exercise in form of rapid cycling (ergometry) as OXT-inducing stimulus. Apart from stimulating OXT secretion, this procedure also allows to elicit stress responses measurable as cortisol (CORT) increase.
CORT is the primary glucocorticoid in humans which is released from the adrenal cortices of the hypothalamic-pituitary-adrenal (HPA) axis and responsive to physical or perceived psychological stress [[22], [23], [24]]. CORT follows a circadian rhythm with its highest levels in the early morning hours (about 30 min after waking), declining rapidly in the morning, with a slower decrease in the afternoon, and reaching its lowest level in the evening [25,26]. Measuring CORT in addition to OXT seems relevant because an increase of CORT might be essential to stimulate OXT concentrations [27,28]. To ensure the successful individual stress response a cut-off of ≥15.5% CORT baseline-to-peak increase was defined [47]. More precisely, participants reaching a CORT post-stress-to-baseline quotient of at least 1.155 were defined as CORT responders, while others below this quotient were CORT non-responders to the physical challenge.
Plasma has been the biomaterial of choice to measure OXT given the modestly invasive character and its good temporal resolution [8,18]. However, people with ASD often suffer from sensory issues [30], thus a less invasive method, e.g. saliva collection, would be advantageous. While measures of basal OXT in saliva have repeatedly been shown to be reliable [31,32], the sensitivity of saliva samples to detect dynamic changes in OXT levels in response to relevant physiological and psychological challenges needs to be further examined [18]. Therefore, we decided to collect both plasma and saliva samples to measure peripheral OXT.
In this study, we pursued five main aims: First, we tested whether the peripheral OXT and CORT concentrations of adult individuals with HFA were altered compared to those of neurotypicals under baseline conditions. Second, we examined peripheral OXT and CORT concentrations for group-related effects after physical exercise. Third, we focused on the correlation between peripheral CORT and OXT concentrations, as recently found in neurotypicals [18,19,33]. Fourth, we tested, whether the behavioral phenotype (e.g. autistic or anxious traits) was associated with peripheral OXT concentrations measured at baseline. Implementing this approach, we aimed at integrating the complex behavioral characteristics of our participants, especially of those with ASD and comorbid psychiatric conditions, into our analysis. Fifth, we were interested whether salivary and plasma OXT concentrations were correlated in this experimental set-up.
2. Subjects and methods
2.1. Study sample
Seventy-seven participants, aged 18–60 years, were recruited in the time between October 2017 and April 2019 at the Max Planck Institute of Psychiatry (MPIP). Thirteen participants were excluded from the study due to cardiovascular risks (n = 7), exceedance of cut-off values in psychometric questionnaires (this applies to alleged neurotypicals, n = 5), or intake of hormonal contraception (n = 1), leaving 64 participants for the final data analyses (Table 1). Neurotypical controls were recruited through an online study application system on the institute’s website. Individuals with autism were either recruited through the “Outpatient and Day Clinic for Disorders of Social Interaction” at the MPIP (n = 28) or the online system (n = 5). Neurotypical controls (n = 31) were defined as adults without any history of psychiatric or neurological disorders. Before inclusion in the study, the medical history of potential candidates was taken following a physical examination. They were excluded from the study in case of a suspected somatic, psychiatric or neurological disorder. Autistic patients (n = 33) received a diagnostic assessment for ASD according to the German national autism guidelines [34] (see 2.2). Only patients who met the DSM-5-criteria for ASD were admitted to participate in the study [1]. Since participants with ASD did not show any intellectual impairment and IQ values < 70 were used as an exclusion criterion, they were regarded as patients with high-functioning autism (HFA). Exclusion criteria consisted of any serious somatic illness (e.g. diabetes, diseases of the cardiac, pulmonary, renal or hepatic system, chronic inflammatory diseases etc.), a diagnosis of schizophrenia in the present or past, breast-feeding, pregnancy, and the use of hormonal contraception and/or sex hormones to control for potential hormonal effects on OXT. All study participants provided written informed consent. Ethical approval was granted by the Ethics Committee of the Ludwig-Maximilians-University (LMU) Munich (Project number: 712–15). All procedures were performed in accordance with the Declaration of Helsinki. Participants could withdraw from the study at any time and were financially compensated for their time.
Table 1.
Characteristics of study participants.
| Variables | Neurotypicals |
ASD |
Statistics1 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Females | Males | p | Total | Females | Males | p | P | |
| Number of participants | n = 31 | n = 20 | n = 11 | n = 33 | n = 14 | n = 19 | .077 | ||
| Mean age (SD) in years | 31.0 (11.7) | 32.7 (13.7) | 28.1 (6.4) | .219 | 36.8 (10.7) | 39.9 (12.0) | 34.6 (9.3) | .164 | .043∗ |
| Mean BMI (SD) in kg/m2 | 23.1 (4.9) | 21.7 (4.7) | 25.6 (4.4) | .031∗ | 23.9 (4.1) | 23.1 (3.8) | 24.5 (4.3) | .330 | .492 |
| Mean urine osmolality (mosm/kg) | 767.3 (216.9) | 728.1 (243.0) | 845.9 (128.9) | .164 | 773.5 (179.4) | 708.1 (178.2) | 824.4 (167.7) | .068 | .903 |
| Mean AQ | 13.7 (5.3) | 13.7 (5.8) | 13.6 (4.4) | .993 | 35.4 (9.8) | 39.5 (7.2) | 32.3 (10.5) | .036∗ | .001∗∗ |
| Mean BDI-II | 4.5 (5.2) | 5.0 (5.8) | 3.6 (3.8) | .456 | 13.2 (11.6) | 20.6 (12.5) | 7.7 (7.3) | .006∗∗ | .001∗∗ |
| Mean EQ | 46.5 (11.3) | 47.4 (11.8) | 44.9 (10.7) | .546 | 19.1 (12.6) | 16.2 (5.8) | 21.3 (15.6) | .222 | .001∗∗ |
| Mean LSAS | 23.4 (15.4) | 22.9 (16.0) | 24.2 (15.1) | .821 | 73.1 (25.0) | 82.7 (23.4) | 66.0 (24.2) | .059 | .001∗∗ |
| Mean TAS-20 | 41.2 (9.3) | 40.7 (10.8) | 42.2 (6.0) | .617 | 58.8 (9.5) | 60.9 (9.2) | 57.2 (9.7) | .255 | .001∗∗ |
| Mean ADOS-2 | – | – | – | – | 7.4 (4.0)2 | 6.1 (3.9)3 | 8.4 (3.9)4 | .135 | – |
Note. Neurotypicals: n = 31; Autism Spectrum Disorder (ASD): n = 33. SD: Standard Deviation. BMI: Body Mass Index. AQ: Autism-Spectrum Quotient; BDI-II: Beck Depression Inventory – II; EQ: Empathy Quotient; LSAS: Liebowitz Social Anxiety Scale; TAS-20: Toronto Alexithymia Scale with 20 Items. ADOS-2: Autism Diagnostic Observation Schedule - Second Edition.
Diagnostic group statistics. 2n = 29.3n = 12.4n = 17.
Questionnaire results are based on 1000 bootstrap samples.
∗ denotes significance with p < .05. ∗∗ denotes significance with p < .01.
2.2. Diagnostic procedures
Autistic patients received a diagnostic assessment for ASD according to the German national autism guidelines [34], in which ASD related symptoms were interrogated and observed in a comprehensive diagnostic interview conducted by a psychologist or psychiatrist experienced in diagnosing ASD. In the interview, the focus was set on diagnostic criteria for ASD according to DSM-5 covering the lifespan (starting from early development to adulthood). If possible and with the patient’s consent, third parties (e.g. parents, siblings), who were familiar with the early development of the patient, were also interviewed for ASD related symptoms of the patient. As part of the diagnostic process, autistic participants were tested using the “Autism Diagnostic Observation Schedule-2” (ADOS-2) – Module 4 [35]. Approximately 61% of autistic individuals had psychiatric comorbidities known to be common for adults with HFA (Supplementary Table 1) [2,3]. 51.5% of patients took psychiatric medication on a regular basis (Supplementary Table 1). A reduction of medication and stop of intake prior to the study participation would have caused a disruption of familiar procedures in the patients’ everyday lives, causing psychological stress and potentially confounding with the experiment’s measures. Therefore, patients were asked not to take the medication in the morning prior to the experiment but afterwards. Participants of both groups filled out the same set of questionnaires to assess social functioning. The focus was set on the autistic phenotype by evaluating autistic traits with the “Autism-Spectrum Quotient (AQ)” [36] and empathetic traits with the “Empathy Quotient (EQ)” [37]. Furthermore, symptoms of psychiatric comorbidities commonly found in adults with ASD such as social phobia and depression were assessed by the “Liebowitz Social Anxiety Scale (LSAS)” [38] and “Beck Depression Inventory-II (BDI-II)” [39], respectively. Since alexithymia also represents a common co-condition in autistic individuals [40,41], alexithymic traits were measured by the “Toronto Alexithymia Scale” with 20 Items („TAS-20“) [42]. As expected, neurotypical and autistic participants significantly differed in all psychometric measures (Table 1).
2.3. Physical exercise as stress paradigm
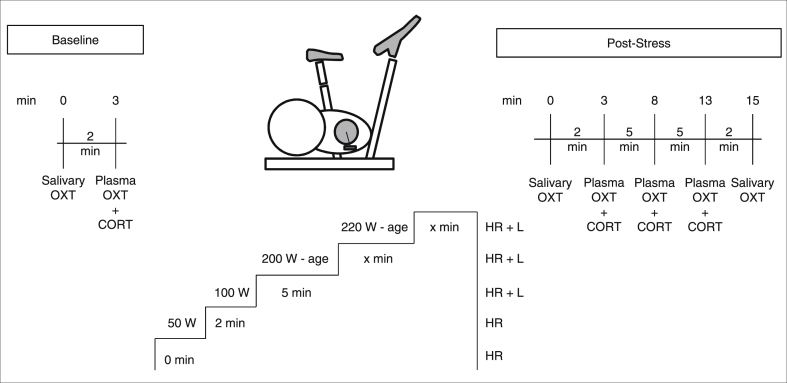
Participants arrived at the outpatient unit of the MPIP at 8:30 a.m. in a fasting state (>12 h). After a physical examination, the experiment started (Fig. 1).
Fig. 1.
Experimental set-up. Heart rate (HR) was measured at the start (0 min) and 2 min after the start of the cycling. HR and lactate levels (L) were measured 7 min after the start of the cycling and prior to the stop of the cycling depending on the individual performance.
First, salivary OXT was collected using a cylindrical chewing swab (see 2.4, Sarstedt, S-Salivette, Cat.nr.: 51.1534.500), on which participants were instructed to chew for approximately 2 min for sufficient saliva absorption. Then (+3 min), plasma was taken for plasma OXT and CORT analyses. After the baseline sample collection, participants exercised on a bicycle ergometer (Kettler Ergometer TXl, Germany) according to a strict protocol [20]. In summary, participants exercised for ≥ 7 min, and then stopped either a) when lactate levels reached ≥ 4 mmol/l which is considered to be the anaerobic threshold [43], or b) if lactate continuously remained < 4 mmol/l, then it was stopped when participants either reached physical exhaustion or after overall 25 min of exercise [20]. After the stress task, salivary OXT samples were taken at +1 and at +15 min post-stress. Plasma samples were taken at +3, 8, and 13 min post-stress for plasma OXT and CORT analyses. The time points were chosen to monitor the OXT concentrations over time considering the half-life of OXT, which is supposed to be 4–10 min in human beings [44]. All blood and saliva samples were collected within a strict time frame (9.00 a.m.– 10.30 a.m.) according to protocol. In addition to the heart rate, lactate in capillary blood was measured repeatedly (Lactate Pro2, Japan [45]) in order to monitor for individual exertion during the experiment (Fig. 1).
2.4. Quantification of plasma CORT, salivary and plasma OXT
2.4.1. Sample preparation
Saliva was taken into collection tubes (Sarstedt, S-Salivette, Cat.nr.: 51.1534.500) and transported at room temperature. Then saliva was centrifuged at 4 °C for 15 min with 2500×g. After centrifugation the samples were aliquoted into 2D-barcode tubes (Brooks, fluidX, Cat.nr.: 68-0703-12) and stored at −80 °C. Plasma was taken into blood collection tubes (Sarstedt, S-Monovette K3E 2.7 ml or 7.5 ml, Cat.nr.: 01.1605.001). Immediately after blood collection the tubes were transported in a cooling box. Then plasma was centrifuged at 4 °C for 15 min with 2500×g. The supernatant was filled into a 4 ml tube (Sarstedt, 92 × 15.3 mm, PP, Cat.nr.: 62.611) and centrifuged again. After centrifugation the samples were aliquoted into 2D-barcode tubes (Brooks, fluidX, Cat.nr.: 68-0703-12) and stored at −80 °C.
2.4.2. OXT analysis
All salivary and plasma OXT concentrations were quantified by an external laboratory (RIAgnosis, Sinzing, Germany) using radioimmunoassay (RIA) as previously described [18]. The analysis was performed on encoded samples without providing any additional information (including times of sample collection, matching pairs of saliva and plasma etc.). According to the provider, saliva samples (300 μl) were evaporated (Concentrator, Eppendorf, Germany) prior to analysis. Plasma samples (0.5 ml) were kept at −20 °C until extraction using LiChroprep® Si60 (Merck) heat-activated at 690 °C for 3 h. 20 mg of LiChroprep® Si60 in 1 ml distilled water were added to the sample, mixed for 30 min, washed twice with distilled water and 0.01 mol/l HCl, eluded with 60% acetone and evaporated as described above. To both, evaporated saliva samples and plasma extracts, 50 μl of assay buffer was added followed by 50 μl antibody raised in rabbits against OXT. Finally, after 60-min pre-incubation, 10 μl of 125I-labeled OXT was added. The detection limit of the RIA was in the 0.1–0.5 pg/sample range depending on the age of the tracer. Intra- and inter-assay variabilities were < 10% and cross-reactivities with related peptides < 0.7%. All samples were assayed in the same batch. Serial dilutions of samples containing high levels of endogenous OXT run strictly parallel to the standard curve indicating immuno-identity.
2.4.3. CORT analysis
Plasma CORT was determined by using an Enzyme-linked Immunosorbent Assay (ELISA) kit (RE52061, TECAN, IBL Hamburg, Germany). The Standard Range was 20–800 ng/ml. The analytical sensitivity (limit of detection) is 2.46 ng/ml, the 2 SD functional sensitivity is 4.03 ng/ml and the mean concentration is < 20% CV; cross-reactivity of other substances tested < 0.01%; intra-assay < 3.48; inter-assay < 3.42.
2.5. Statistics
Data processing and statistical analyses were performed in IBM SPSS 25.0 (IBM Corp., Armonk, NY, USA), Matlab (R2010a, The MathWorks, Inc., Natick, MA, USA), and Perseus (http://www.biochem.mpg.de/5111810/perseus). Missing values of OXT, CORT and psychometric measures (<7%) were imputed with Perseus (http://www.biochem.mpg.de/5111810/perseus) using normal distribution with width 0.3 and down shift 0 (Supplementary Table 2). Descriptive data were compared by Chi2-tests (sex) or t-tests. To analyze dimensional measures of OXT, all OXT measures were transformed by natural logarithmic transformation to achieve a normal distribution. After transformation, OXT measures were normally distributed. For easier interpretation of results, most tables and figures show the original, non-transformed data. CORT measures were normally distributed (Kolmogorov-Smirnov tests, all p-values > .05). Univariate analyses were performed to test for effects of group and sex as between-subject factors and age as covariate on baseline CORT and OXT concentrations. Repeated measures analyses of variance (rmANOVA) were conducted to test for effects of time as within-subject factor and group (Neurotypicals vs. ASD patients) as between-subject factor on CORT and OXT reactivity. In addition, the influence of sex and age on time and group effects of CORT and OXT reactivity was investigated. Sex (males vs. females) was included as between-subject factor in rmANOVAs first, while age was entered as covariate second, to test for sex effects and age effects on CORT and OXT stress response, respectively. Greenhouse Geisser corrections were implemented when necessary. In case Greenhouse Geisser corrections exceeded .75, Huynh-Feldt corrections were used [46]. Differences in CORT and OXT reactivity (p < .05) were followed by post-hoc Bonferroni-corrected pair-wise comparisons. Participants qualifying as stress responders for CORT were defined by a cut-off of ≥ 15.5% baseline-to-peak increase [47]. The CORT peak was reached at the third time point after the physical exercise (CORT +13 min). Thus, a quotient of the CORT concentrations +13 min/baseline ≥ 1.155 was defined as CORT response (= 1) with participants being CORT responders to the physical challenge. Otherwise, participants were identified as CORT non-responders (= 0). The OXT and CORT responses were operationalized as a change score (ΔOXT and ΔCORT, respectively) by subtracting the baseline value from the concentrations at different time points of the sample collection (Table 2) [33].
Table 2.
Δ Values for OXT and CORT concentrations.
| Δ Values | Minute | Calculation | Material |
|---|---|---|---|
| ΔOXT-1 | +1 | post-stress – baseline | Saliva |
| ΔOXT-3 | +3 | post-stress – baseline | Plasma |
| ΔOXT-8 | +8 | post-stress – baseline | Plasma |
| ΔOXT-13 | +13 | post-stress – baseline | Plasma |
| ΔOXT-15 | +15 | post-stress – baseline | Saliva |
| ΔCORT-3 | +3 | post-stress – baseline | Plasma |
| ΔCORT-8 | +8 | post-stress – baseline | Plasma |
| ΔCORT-13 | +13 | post-stress – baseline | Plasma |
Correlations between ΔOXT and ΔCORT values were calculated using partial correlation analyses with age and sex as control variables (no logarithmic transformations were possible due to negative values). Linear regression analyses were performed to reveal associations between the behavioral phenotype and peripheral OXT levels. Since behavioral measures, applied in this study, were rather trait than state related only basal OXT concentrations were used. First, backwards selection was applied to identify the most parsimonious model. This approach allowed us to observe the relative impact of psychometric measures on OXT concentrations, even if they were not included in the final models. Scores from AQ, BDI-II, EQ, LSAS, and TAS-20 were included in the model as independent variables. To control for potential age and sex effects, both variables were additionally entered as independent variables, while basal salivary and plasma OXT concentrations were included as dependent variables, respectively. Second, to provide more robust statistics [46], variables of the most parsimonious model were included with the enter method and 1000 resamples bootstrapping. The reason for this second approach was that tests of normality revealed that relevant measures (AQ, BDI-II, EQ, LSAS, TAS-20) were not normally distributed, and data transformation failed to improve skewness.
3. Results
3.1. Baseline concentrations of CORT, salivary and plasma OXT concentrations
An overview of baseline concentrations of CORT, salivary and plasma OXT concentrations can be found in Supplementary Tables 5a, 6a and 7a.
3.1.1. Plasma CORT
CORT baseline concentrations significantly differed between sexes (F(1,59) = 8.37, p = .005), with men (mean CORT = 145.74 ng/ml, SD = 39.99) presenting significantly higher CORT concentrations than women (mean CORT = 117.43 ng/ml, SD = 34.22). There was neither a significant main effect of group on CORT baseline concentrations (F(1,59) = 0.19, p = .667), nor of the interaction of group x sex (F(1,59) = 0.46, p = .502), nor of age (F(1,59) = 0.36, p = .554).
3.1.2. Salivary OXT
There was neither a significant main effect of group on salivary OXT baseline concentrations (F(1,59) = 0.53, p = .469), nor of sex (F(1,59) = 0.92, p = .341), nor of the interaction of group x sex (F(1,59) = 0.08, p = .783), nor of age (F(1,59) = 0.12, p = .731).
3.1.3. Plasma OXT
There was neither a significant main effect of group on plasma OXT baseline concentrations (F(1,59) = 1.11, p = .297), nor of sex (F(1,59) = 2.88, p = .095), nor of the interaction of group x sex (F(1,59) = 0.20, p = .656), nor of age (F(1,59) = 0.04, p = .851).
3.2. Validity of the stress paradigm
Heart rates and lactate levels significantly increased during the stress paradigm in each group, respectively (p < .001; Supplementary Table 3). Physiological parameters before and during the physical exercise did not significantly differ between groups (Supplementary Table 4).
3.3. CORT, salivary and plasma OXT concentrations in response to the stress paradigm
3.3.1. Plasma CORT
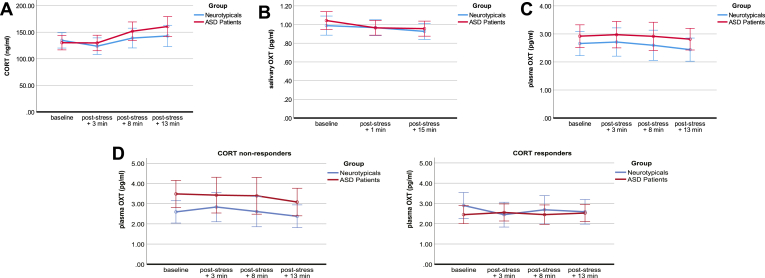
Time had a significant effect on CORT concentrations (F(1.68, 100.89) = 11.74, p < .001) with increasing CORT levels over time (Supplementary Tables 5a, b). When applying Bonferroni correction, significant effects were observed between baseline and CORT+13 min (p = .025), CORT+3 min and CORT+8 min (p < .001), and CORT+3 min and CORT+13 min (p < .001) values. When entering age as covariate, neither time nor any other main or interaction effects on CORT concentrations were significant (Fig. 2a; Supplementary Table 5c).
Fig. 2a-d.
Hormonal changes in response to stress.
Figure 2a. Cortisol (CORT) concentrations are illustrated over time for the neurotypical (blue) and autistic group (red). When adding age as covariate, the time effect for the total sample did not reach significance any more.
Figure 2b. Salivary oxytocin (OXT) concentrations are illustrated over time for the neurotypical (blue) and autistic group (red).
Figure 2c. Plasma oxytocin (OXT) concentrations are illustrated over time for the neurotypical (blue) and autistic group (red).
Figures 2d. Plasma oxytocin (OXT) concentrations are illustrated over time for the subsample of CORT non-responders (quotient of the CORT concentrations +13 min/baseline < 1.155) and CORT responders (quotient of the CORT concentrations +13 min/baseline ≥ 1.155). When adding age as covariate, the group effect of neurotypicals and patients with autism spectrum disorder (ASD) did not reach significance any more.
For Figures 2a-d ddata are shown as means ± 2 Standard Error of Means. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The physical stressor induced the defined CORT response (CORT quotient ≥ 1.155, see 2.5) in 11 out of 31 neurotypicals (8 females and 3 males) and 18 out of 33 autistic patients (9 females and 9 males).
3.3.2. Salivary OXT
Time had a significant effect on salivary OXT concentrations (F(2.00, 120.00) = 3.79, p = .025; Supplementary Tables 6a, b). When adjusting for multiple testing, the difference between baseline salivary OXT and OXT+15 min did not remain significant (p = .017; adjusted p = .051). When entering age as covariate, neither time nor any other main or interaction effects on salivary OXT concentrations were significant (Fig. 2b; Supplementary Table 6c). No significant effects were found when examining salivary OXT concentrations according to CORT responders and non-responders (Supplementary Tables 6b, c).
3.3.3. Plasma OXT
Neither main nor interaction effects had a significant impact on plasma OXT concentrations (Supplementary Tables 7a, b). When entering age as covariate, main and interaction effects remained non-significant (Fig. 2c; Supplementary Table 7c). A significant difference between ASD and controls was found in the CORT non-responder subsample (F(1, 31) = 5.14, p = .030), with elevated levels in the ASD group (Fig. 2d; Supplementary Tables 7a, b). When including age as covariate, the effect of group on plasma OXT concentrations did not remain significant (Supplementary Table 7c).
3.3.4. Correlation between ΔOXT and ΔCORT concentrations
By controlling for age and sex, no significant correlations were found between ΔOXT and ΔCORT concentrations in neurotypicals (Supplementary Table 8a). In autistic individuals, ΔOXT-3 and ΔCORT-8 (r = −0.58, p = .039) as well as ΔOXT-3 and ΔCORT-13 (r = −0.58, p = .038) were correlated in absence of a CORT response, while ΔOXT-15 and ΔCORT-13 (r = −0.52, p = .039) were correlated in the subsample with CORT response (Supplementary Table 8b). However, these results did not remain significant when correcting for multiple testing.
3.4. Behavioral measures as predictors of basal OXT concentrations
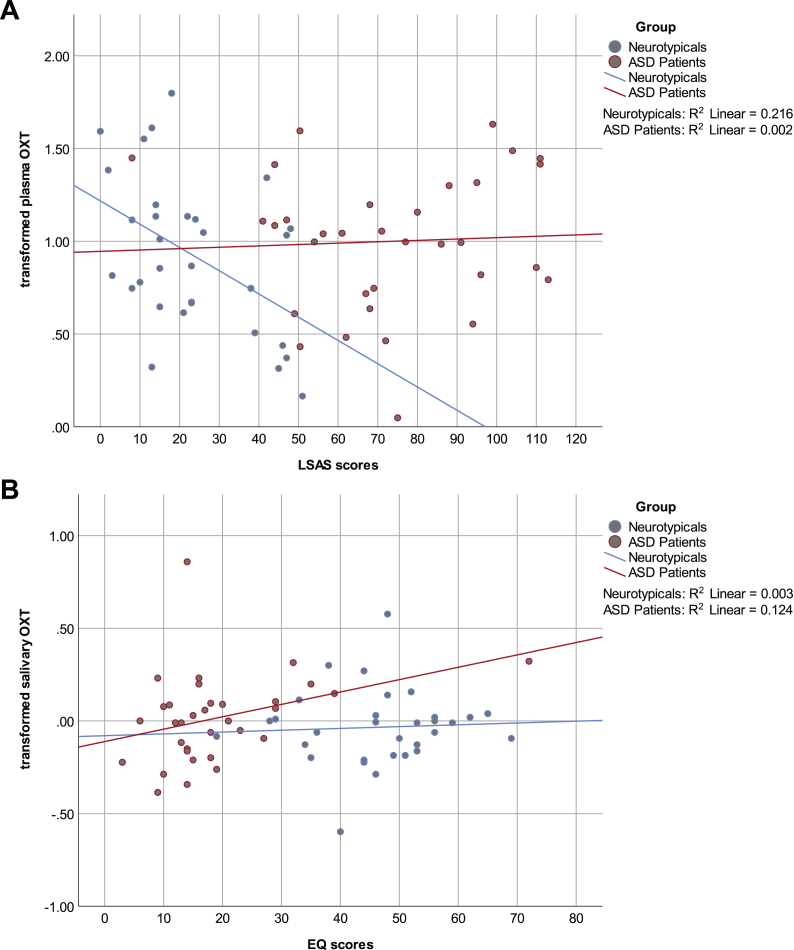
In neurotypicals, LSAS scores were identified as significant predictors (LSAS [-0.021; −0.004]: β = −0.013, p = .010) and explained 21.6% of variance (F(1,29) = 8.01, p = .008) of basal plasma OXT concentrations (Fig. 3a).
Fig. 3a and b.
Behavioral phenotype in relation to basal oxytocin (OXT) concentrations.
Figure 3a. Social anxious traits on plasma OXT concentrations. Regression of Liebowitz Social Anxiety Scale (LSAS) scores upon transformed plasma OXT concentrations at baseline for the neurotypical (blue) and autistic group (red).
Figure 3b. Empathetic traits on salivary OXT concentrations. Regression of empathy quotient (EQ) scores upon transformed salivary OXT concentrations at baseline for the neurotypical (blue) and autistic group (red). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
In autistic individuals, EQ scores were identified as significant predictors (EQ [0.002; 0.012]: β = 0.007, p = .008) and explained 12.4% of variance (F(1,31) = 4.38, p = .045) of basal salivary OXT concentrations (Fig. 3b).
3.5. Methodological aspects - correlations between salivary and plasma OXT
Only values from plasma OXT +8 min and salivary OXT +15 min were significantly correlated (r = 0.306, p = .014). However, this result did not remain significant when correcting for multiple testing. Thus, there was no correlation between plasma and saliva OXT concentrations at any time point (Supplementary Table 9).
4. Discussion
The present study examined the OXT response to physical exercise in neurotypical compared to autistic adults. Five main study aims were hereby addressed: First, we compared basal peripheral CORT and OXT concentrations, but found no group related differences. Second, we examined CORT and OXT responses after physical exercise. CORT concentrations significantly increased over time in the total sample, while no time related effects were observed in salivary and plasma OXT concentrations. When subdividing the sample into CORT responders and non-responders, autistic patients presented significantly higher post-stress levels of plasma OXT than neurotypicals, when lacking a stress-induced increase in CORT concentrations (CORT quotient < 1.155). However, both factors, “time” on CORT and “group” on plasma OXT concentrations, did not have a significant effect any more when “age” was included as covariate, highlighting the relevance of “age” in our analysis. Third, when correcting for multiple testing, no correlations between CORT and OXT reactivity were observed irrespective of CORT subsamples. Fourth, we found a higher degree of social anxiety predicting lower OXT levels in neurotypicals, and a higher degree of empathy predicting higher OXT levels in autistic patients. Fifth, we did not observe any correlation between salivary and plasma OXT concentrations irrespective of time of sample collection.
In contrast to studies investigating CORT responses in neurotypical and autistic children [[48], [49], [50]], we did not observe any group related differences of CORT concentrations in our sample with adult participants. In accordance with our findings, other studies including neurotypical and autistic adolescents or adults did not find group related differences of CORT concentrations either [13,51]. Similar to our results, Jansen et al. [13] observed a significant time effect of CORT concentrations due to the implemented stress task. In our study, the mentioned time effect did not remain significant when we controlled for age. Age has been discussed as modulating factor of stress responses in autistic children [[48], [49], [50]] and neurotypical adolescents [52,53] with higher CORT concentrations in older participants. No age effects in relation to provoked stress were found in neurotypical adults [54,55], where the hormonal status remains largely stable except for later in life [56]. In general, the literature of age effects on CORT concentrations in adolescents and adults with ASD is very limited. In the study by Jansen et al. [13] the diagnostic groups did not significantly differ in age and no age-related effect on CORT levels was reported. Since the diagnostic groups in our study significantly differed in age with autistic patients being significantly older, the factor “age” might act as an additional group related factor.
The majority of previous studies have focused on children and adolescents with equivocal results [11,12,14], when investigating peripheral OXT concentrations in ASD under baseline conditions. While some studies found lower peripheral OXT concentrations [11,57,58], others observed higher peripheral OXT concentrations [12] or similar OXT values in autistic children and adolescents compared to neurotypical peers at baseline [14,15,22,29]. So far, only two studies have examined peripheral OXT levels in autistic adults with contrasting results. While Andari et al. [10] found significantly lower basal OXT concentrations in 13 adults with ASD compared to neurotypicals, Jansen et al. [13] observed increased levels of peripheral OXT in 10 autistic individuals at baseline. In this regard, our findings add to previous literature of peripheral OXT concentrations in adults with ASD by reporting no group-related differences under baseline conditions in a much bigger sample of 33 autistic adults. Furthermore, no significant associations were revealed between OXT levels and the degree of core autism symptoms in a study with autistic adults [59]. These results are in line with observations in children and adolescents with ASD [14,15,22,29], and further support the notion that differing OXT baseline levels do not represent a defining feature of autism.
Successful OXT stimulation has previously been reported in response to running [18,21] and rapid cycling [20]. Other studies used social tasks (e.g. Trier Social Stress Task [TSST]) to stimulate the OXT system [18,19,33]. However, these public speaking tasks were mostly tested in neurotypicals [60], and those studies which included autistic patients either failed to induce a stress [61,62] or OXT response [13]. Thus, an objective stimulus in form of physical exercise was applied in this study, related on former study designs, to increase the chances of a successful OXT and CORT stimulation in autistic adults. Against our expectations, after physical exercise, we found significantly higher plasma OXT concentrations in autistic individuals than in neurotypicals. This supports the assumption that a stimulating task is required in order to reveal group related differences of the OXT responsiveness. However, this group effect was only observed in the subsample of ASD and neurotypicals who did not show the defined CORT response to exercise. As concluded from adrenalectomized rats, glucocorticoids seem to inhibit the stress-induced secretion of OXT into blood [28], but seem essential for OXT release within the hypothalamus. This could indicate that the intensity of the applied stimulus needs to be moderate and should not induce a physiological stress response. This would be in line with previous findings of an OXT increase during a “moderate running” task in healthy participants [18]. However, the results did not hold up when including age as factor in our statistical model. So far, the existing research of age effects on peripheral OXT concentrations in human beings is limited and inconsistent. While some found age-related changes on peripheral OXT concentrations [11], others did not [10,15]. Nevertheless, the results are surprising for two reasons: first, we expected lower OXT concentrations in patients with ASD compared to neurotypicals, bearing in mind the hypothesis of an OXT deficiency in ASD [9]. Secondly, we assumed that we would reveal group-related differences in the subsample of CORT responders than among CORT non-responders because individuals with autism have been shown to exhibit heightened stress responses to experimental procedures [63].
According to the stress buffer theory, which suggests that OXT would be released as a buffer for the induced CORT increase [64,65], our preliminary and non-significant results indicate that OXT might not be sufficiently released to buffer the CORT increase or might even be suppressed in patients with ASD. This would be in line with previous findings in autistic children where, contrary to neurotypicals, OXT failed to serve as a stress buffer after a physiological challenge with hydrocortisone [22].
In addition to biological factors (e.g. sex, age and peripheral CORT concentrations), we looked for potential associations between behavioral traits and peripheral OXT concentrations. In line with former results [66,67], a higher degree of social anxiety was associated with lower basal plasma OXT concentrations in neurotypicals. In autistic participants, a higher degree of empathy was associated with higher basal salivary OXT concentrations which is in line with previous literature [14,68].
Although the exact mechanisms of OXT entering saliva remain poorly understood, studies applying mass spectrometry confirmed the identity of OXT in saliva [69]. Furthermore, salivary OXT has been shown to respond to stimuli known to induce OXT release [18]. Thus, saliva collection seems to be an attractive alternative to blood sampling. However, contrary to results by other research groups [31,70], we did not observe any correlations between salivary and plasma OXT concentrations at any time point in our study. A possible explanation could be that enzyme immunoassays (EIA) were used to measure OXT in unextracted [70] and extracted plasma [31], while we measured OXT in extracted plasma with RIA. An extracting or non-extracting process prior to analysis as well as the kind of assay (EIA or RIA) used to assess OXT can lead to great discrepancies of measured OXT concentrations [8,71]. Comparing the complexity of OXT measurements and diversity of results with the parable of the blind men and the elephant, MacLean et al. [71] concluded that multiple valid OXT measures exist which might not covary. In this regard, our results might support their statement.
4.1. Strengths and limitations
A strength of our study lies in the assessment of both peripheral CORT and OXT concentrations at baseline and in response to physical exercise. Furthermore, participants were neurotypical and autistic adults who have hardly been set in the focus of this research context before, contrary to children and adolescents on whom most existing literature is based.
Despite these strengths, there are also some limitations in our study which need to be considered. First, neurotypicals and autistic patients were not ideally matched regarding age and sex distribution which is a common challenge in clinical trials with naturalistic designs [72]. Nevertheless, sex was considered as between-subject factor and age as covariate in the univariate and rmANOVAs. There was neither a sex nor an age-related effect on OXT concentrations. However, when entering age as covariate into the rmANOVA for CORT and plasma OXT in the last step, the described effects of time and group, respectively, turned non-significant. Thus, our findings need to be interpreted with caution and future studies are warranted to investigate the impact of age on CORT and OXT concentrations in adult participants. Second, the high rate of psychiatric comorbidities adequately represents the autistic phenotype in adulthood and stands for a very naturalistic study design [2,3]. Given the limited sample size of 33 autistic patients, we did not control for potential psychotropic effects on CORT and OXT concentrations. A binary categorical division (psychopharmacological intake: yes/no) appeared as no appropriate solution for this potential confounding factor because the prescribed substances varied among patients (Supplementary Table 1) targeting different neurotransmitter systems. Including each drug as covariate alternatively, did not appear useful either, as various different drugs were taken with different dosages (Supplementary Table 1). Therefore, we only reported this information without further including it into our analyses. However, existing research on the prescribed medication in this study suggested that it does not affect peripheral OXT concentrations [15,73]. In this regard, the effect of psychiatric medication might not have had such an important impact on the peripheral OXT concentrations overall. Third, the sample collection and stress paradigm were carried out in the morning. CORT concentrations are known to follow a circadian rhythm with higher levels in the morning following a decrease over the day [74]. Although material for CORT analyses was taken from the same samples as for OXT plasma analyses (Fig. 1), we could not control for the biological circadian rhythm of CORT which might have additionally influenced the CORT concentrations measured in relation to the paradigm. In this context, it might also be debatable that the defined CORT threshold of 15.5% baseline-to-peak increase applied in this study derives from samples collected in the afternoon [47]. However, it has been shown that CORT stress responses are comparable between morning and evening hours [75,76]. Furthermore, the defined CORT threshold of 15.5% baseline-to-peak increase is based upon a systematic evaluation of previous research including 504 healthy adults and thus, providing reliability in its application [47].
The present study has helped to extend our understanding of the OXT and stress system in autistic adults and neurotypicals. In line with previous studies, we found no evidence for group-related differences of peripheral CORT and OXT concentrations at baseline. After physical exercise, however, plasma OXT concentrations were significantly higher in autistic participants when the physical stressor had not induced an increase in CORT levels, while no such group-related differences were found under a CORT response. However, these observations did not hold up when including age as factor in the statistical model. Thus, these findings need to be interpreted with caution. Preliminary and non-significant results of the correlation analyses between ΔOXT and ΔCORT levels in autistic individuals indicate that OXT might not be sufficiently released to buffer the CORT increase or might even be suppressed in patients with ASD. Furthermore, levels of social anxiety were predictive of basal plasma OXT concentrations in neurotypicals, while empathetic traits were predictive of salivary OXT levels in autistic individuals.
Declaration of competing interest
None.
Acknowledgments
We would like to thank our clinical colleagues from the Outpatient and Day Clinic for Disorders of Social Interaction at the Max Planck Institute of Psychiatry (MPIP) for the referral of potential study participants. We would also like to thank our colleagues from the BioPrep core unit and the Study Center at the MPIP, namely Alina Tontsch, Marketa Reimann, Angelika Sangl, Larysa Teplytska, Norma Grandi, Karin Hofer, Melanie Huber, Elisabeth Kappelmann and Gertrud Ernst-Jansen, as well as Prof. Dr. Ludwig Schaaf for his medical advice. Furthermore, we want to thank Raoul Haaf, Leonie Weindel and Erica Westenberg for their support in data collection.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.cpnec.2021.100027.
Funding
This work was supported by the Max Planck Society via a grant for an Independent Max Planck Research Group awarded to Leonhard Schilbach. Laura Albantakis was funded via the Else-Kröner-Fresenius-Stiftung (EKFS) as part of a joint residency/PhD program in translational psychiatry at the LMU Munich and the Max Planck Institute of Psychiatry.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.American Psychiatric Association . fifth ed. America Psychiatric Publishing; Arlington, VA: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 2.Lai M.C., Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. The Lancet Psychiatry. 2015;2:1013–1027. doi: 10.1016/S2215-0366(15)00277-1. [DOI] [PubMed] [Google Scholar]
- 3.Albantakis L., Parpart H., Thaler H., Krankenhagen M., Böhm J., Zillekens I.C., Schilbach L. Depression bei Erwachsenen mit Autismus-Spektrum-Störung. Nervenheilkunde. 2018;37:587–593. [Google Scholar]
- 4.Bhandari R., Paliwal J.K., Kuhad A. Neuropsychopathology of autism spectrum disorder: complex interplay of genetic, epigenetic, and environmental factors. Adv. Neurobiol. 2020;24:97–141. doi: 10.1007/978-3-030-30402-7_4. [DOI] [PubMed] [Google Scholar]
- 5.Neumann I.D., Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety. depression, and social behaviors. 2012 doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Neumann I.D., Slattery D.A. Oxytocin in general anxiety and social fear: a translational approach. Biol. Psychiatr. 2016;79:213–221. doi: 10.1016/J.BIOPSYCH.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Jurek B., Neumann I.D. The oxytocin receptor: from intracellular signaling to behavior. Physiol. Rev. 2018;98:1805–1908. doi: 10.1152/physrev.00031.2017. [DOI] [PubMed] [Google Scholar]
- 8.McCullough M.E., Churchland P.S., Mendez A.J. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev. 2013;37:1485–1492. doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Modahl C., Fein D., Waterhouse L., Newton N. Does oxytocin deficiency mediate social deficits in autism? J. Autism Dev. Disord. 1992;22:449–451. doi: 10.1007/BF01048246. [DOI] [PubMed] [Google Scholar]
- 10.Andari E., Duhamel J.-R., Zalla T., Herbrecht E., Leboyer M., Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Modahl C., Green L., Fein D., Morris M., Waterhouse L., Feinstein C., Levin H. Plasma oxytocin levels in autistic children. Biol. Psychiatr. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson J.D., Ellerbeck K.A., Kelly K.A., Fleming K.K., Jamison T.R., Coffey C.W., Smith C.M., Reese R.M., Sands S.A. Evidence for alterations in stimulatory G proteins and oxytocin levels in children with autism. Psychoneuroendocrinology. 2014;40:159–169. doi: 10.1016/j.psyneuen.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansen L.M.C., Gispen-De Wied C.C., Wiegant V.M., Westenberg H.G.M., Lahuis B.E., Van Engeland H. Autonomic and neuroendocrine responses to a psychosocial stressor in adults with autistic spectrum disorder. J. Autism Dev. Disord. 2006;36:891–899. doi: 10.1007/s10803-006-0124-z. [DOI] [PubMed] [Google Scholar]
- 14.Parker K.J., Garner J.P., Libove R.A., Hyde S.A., Hornbeak K.B., Carson D.S., Liao C.-P., Phillips J.M., Hallmayer J.F., Hardan A.Y. Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc. Natl. Acad. Sci. U. S. A. 2014;111:12258–12263. doi: 10.1073/pnas.1402236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taurines R., Schwenck C., Lyttwin B., Schecklmann M., Jans T., Reefschläger L., Geissler J., Gerlach M., Romanos M. Oxytocin plasma concentrations in children and adolescents with autism spectrum disorder: correlation with autistic symptomatology. Atten. Defic. Hyperact. Disord. 2014;6:231–239. doi: 10.1007/s12402-014-0145-y. [DOI] [PubMed] [Google Scholar]
- 16.Landgraf R., Neumann I.D. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004;25:150–176. doi: 10.1016/J.YFRNE.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Valstad M., Alvares G.A., Egknud M., Matziorinis A.M., Andreassen O.A., Westlye L.T., Quintana D.S. The correlation between central and peripheral oxytocin concentrations: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2017 doi: 10.1016/j.neubiorev.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 18.De Jong T.R., Menon R., Bludau A., Grund T., Biermeier V., Klampfl S.M., Jurek B., Bosch O.J., Hellhammer J., Neumann I.D. Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: the Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology. 2015;62:381–388. doi: 10.1016/j.psyneuen.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Bernhard A., van der Merwe C., Ackermann K., Martinelli A., Neumann I.D., Freitag C.M. Adolescent oxytocin response to stress and its behavioral and endocrine correlates. Horm. Behav. 2018;105:157–165. doi: 10.1016/j.yhbeh.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Gebert D., Auer M.K., Stieg M.R., Freitag M.T., Lahne M., Fuss J., Schilbach K., Schopohl J., Stalla G.K., Kopczak A. De-masking oxytocin-deficiency in craniopharyngioma and assessing its link with affective function. Psychoneuroendocrinology. 2018;88:61–69. doi: 10.1016/j.psyneuen.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Hew-Butler T., Noakes T.D., Soldin S.J., Verbalis J.G. Acute changes in endocrine and fluid balance markers during high-intensity, steady-state, and prolonged endurance running: unexpected increases in oxytocin and brain natriuretic peptide during exercise. Eur. J. Endocrinol. 2008;159:729–737. doi: 10.1530/EJE-08-0064. [DOI] [PubMed] [Google Scholar]
- 22.Corbett B.A., Bales K.L., Swain D., Sanders K., Weinstein T.A.R., Muglia L.J. Comparing oxytocin and cortisol regulation in a double-blind, placebo-controlled, hydrocortisone challenge pilot study in children with autism and typical development. J. Neurodev. Disord. 2016;8 doi: 10.1186/s11689-016-9165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hennessy J.W., Levine S. In: Progress in Psychobiology and Physiological Psychology. eighth ed. Sprague J.M., Epstein A.N., editors. Academic Press; New York: 1979. Stress, arousal, and the pituitary-adrenal system: a psychoendocrine hypothesis; pp. 133–178. [Google Scholar]
- 24.Herman J.P., Cullinan W.E. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 25.Corbett B.A., Mendoza S., Wegelin J.A., Carmean V., Levine S., Corbett B. Variable cortisol circadian rhythms in children with autism and anticipatory stress. J. Psychiatry Neurosci. 2008 [PMC free article] [PubMed] [Google Scholar]
- 26.Smyth J.M., Ockenfels M.C., Gorin A.A., Catley D., Porter L.S., Kirschbaum C., Hellhammer D.H., Stone A.A. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22:89–105. doi: 10.1016/S0306-4530(96)00039-X. [DOI] [PubMed] [Google Scholar]
- 27.Brown C.A., Cardoso C., Ellenbogen M.A. A meta-analytic review of the correlation between peripheral oxytocin and cortisol concentrations. Front. Neuroendocrinol. 2016 doi: 10.1016/j.yfrne.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Torner L., Plotsky P.M., Neumann I.D., de Jong T.R. Forced swimming-induced oxytocin release into blood and brain: effects of adrenalectomy and corticosterone treatment. Psychoneuroendocrinology. 2017;77:165–174. doi: 10.1016/j.psyneuen.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Miller M., Bales K.L., Taylor S.L., Yoon J., Hostetler C.M., Carter C.S., Solomon M. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: sex differences and associations with symptoms. Autism Res. 2013;6:91–102. doi: 10.1002/aur.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crane L., Goddard L., Pring L. Sensory processing in adults with autism spectrum disorders. Autism. 2009;13:215–228. doi: 10.1177/1362361309103794. [DOI] [PubMed] [Google Scholar]
- 31.Grewen K.M., Davenport R.E., Light K.C. An investigation of plasma and salivary oxytocin responses in breast- and formula-feeding mothers of infants. Psychophysiology. 2010;47:625–632. doi: 10.1111/j.1469-8986.2009.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White-Traut R., Watanabe K., Pournajafi-Nazarloo H., Schwertz D., Bell A., Carter C.S. Detection of salivary oxytocin levels in lactating women. Dev. Psychobiol. 2009;51:367–373. doi: 10.1002/dev.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engert V., Koester A.M., Riepenhausen A., Singer T. Boosting recovery rather than buffering reactivity: higher stress-induced oxytocin secretion is associated with increased cortisol reactivity and faster vagal recovery after acute psychosocial stress. Psychoneuroendocrinology. 2016;74:111–120. doi: 10.1016/j.psyneuen.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 34.AWMF . vol. 1. der DGKJP und der DGPPN Teil; Di: 2015. Autismus-Spektrum-Störungen im Kindes-, Jugend-und Erwachsenenalter Teil 1: Diagnostik S3-Leitlinie AWMF-Registernummer: 028 -018. Interdiszip. S3-leitlin; p. 252. [Google Scholar]
- 35.Hus V., Lord C. The autism diagnostic observation schedule, module 4: revised algorithm and standardized severity scores. J. Autism Dev. Disord. 2014;44:1996–2012. doi: 10.1007/s10803-014-2080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. The autism-spectrum quotient (AQ): evidence from asperger syndrome/high-functioning autism, malesand females, scientists and mathematicians. J. Autism Dev. Disord. 2001;31:5–17. doi: 10.1023/A:1005653411471. [DOI] [PubMed] [Google Scholar]
- 37.Baron-Cohen S., Wheelwright S. The empathy quotient: an investigation of adults with asperger syndrome or high functioning autism, and normal sex differences. J. Autism Dev. Disord. 2004;34:163–175. doi: 10.1023/B:JADD.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- 38.Fresco D.M., Coles M.E., Heimberg R.G., Liebowitz M.R., Hami S., Stein M.B., Goetz D. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychol. Med. 2001;31:1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- 39.Beck A., Steer R., Brown G. The Psychological Corporation; San Antonio, TX: 1996. Beck Depression Inventory-II. [Google Scholar]
- 40.Albantakis L., Brandi M.L., Zillekens I.C., Henco L., Weindel L., Thaler H., Schliephake L., Timmermans B., Schilbach L. Alexithymic and autistic traits: relevance for comorbid depression and social phobia in adults with and without autism spectrum disorder. Autism. 2020 Nov;24(8):2046–2056. doi: 10.1177/1362361320936024. Epub 2020 Jul 14. PMID: 32662285; PMCID: PMC7543015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinnaird E., Stewart C., Tchanturia K. Investigating alexithymia in autism: a systematic review and meta-analysis. Eur. Psychiatr. 2019;55:80–89. doi: 10.1016/j.eurpsy.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bagby R.M., Parker J.D., Taylor G.J. The twenty-item Toronto Alexithymia Scale--I. Item selection and cross-validation of the factor structure. J. Psychosom. Res. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 43.Beneke R. Anaerobic threshold, individual anaerobic threshold, and maximal lactate steady state in rowing. Med. Sci. Sports Exerc. 1995;27:863–867. doi: 10.1249/00005768-199506000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Leng G., Sabatier N. Measuring oxytocin and vasopressin: bioassays, immunoassays and random numbers. J. Neuroendocrinol. 2016;28 doi: 10.1111/jne.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pyne D.B., Boston T., Martin D.T., Logan A. Evaluation of the Lactate Pro blood lactate analyser. Eur. J. Appl. Physiol. 2000;82:112–116. doi: 10.1007/s004210050659. [DOI] [PubMed] [Google Scholar]
- 46.Field A. vol. 5. Sage Publications Inc; Thousand Oaks, CA: 2018. Discovering Statistics Using IBM SPSS Statistics. [Google Scholar]
- 47.Miller R., Plessow F., Kirschbaum C., Stalder T. Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress: evaluation of salivary cortisol pulse detection in panel designs. Psychosom. Med. 2013;75:832–840. doi: 10.1097/PSY.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 48.Corbett B.A., Schupp C.W., Lanni K.E. Comparing biobehavioral profiles across two social stress paradigms in children with and without autism spectrum disorders. Mol. Autism. 2012 doi: 10.1186/2040-2392-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corbett B.A., Schupp C.W., Simon D., Ryan N., Mendoza S. Elevated cortisol during play is associated with age and social engagement in children with autism. Mol. Autism. 2010;1:13. doi: 10.1186/2040-2392-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schupp C.W., Simon D., Corbett B.A. Cortisol responsivity differences in children with autism spectrum disorders during free and cooperative play. J. Autism Dev. Disord. 2013;43:2405–2417. doi: 10.1007/s10803-013-1790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bakker-Huvenaars M.J., Greven C.U., Herpers P., Wiegers E., Jansen A., van der Steen R., van Herwaarden A.E., Baanders A.N., Nijhof K.S., Scheepers F., Rommelse N., Glennon J.C., Buitelaar J.K. Saliva oxytocin, cortisol, and testosterone levels in adolescent boys with autism spectrum disorder, oppositional defiant disorder/conduct disorder and typically developing individuals. Eur. Neuropsychopharmacol. 2018 doi: 10.1016/j.euroneuro.2018.07.097. [DOI] [PubMed] [Google Scholar]
- 52.Gunnar M.R., Wewerka S., Frenn K., Long J.D., Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: normative changes and associations with puberty. Dev. Psychopathol. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stroud L.R., Foster E., Papandonatos G.D., Handwerger K., Granger D.A., Kivlighan K.T., Niaura R. Stress response and the adolescent transition: performance versus peer rejection stressors. Dev. Psychopathol. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dickerson S.S., Kemeny M.E. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 55.Kudielka B.M., Buske-Kirschbaum A., Hellhammer D.H., Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/S0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 56.Otte C., Hart S., Neylan T.C., Marmar C.R., Yaffe K., Mohr D.C. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30:80–91. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Al-Ayadhi L.Y. Altered oxytocin and vasopressin levels in autistic children in Central Saudi Arabia. Neurosciences. 2005;10:47–50. [PubMed] [Google Scholar]
- 58.Green L., Fein D., Modahl C., Feinstein C., Waterhouse L., Morris M. Oxytocin and autistic disorder: alterations in peptide forms. Biol. Psychiatr. 2001;50:609–613. doi: 10.1016/S0006-3223(01)01139-8. [DOI] [PubMed] [Google Scholar]
- 59.Alaerts K., Bernaerts S., Vanaudenaerde B., Daniels N., Wenderoth N. Amygdala–hippocampal connectivity is associated with endogenous levels of oxytocin and can Be altered by exogenously administered oxytocin in adults with autism. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2019;4:655–663. doi: 10.1016/j.bpsc.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 60.Allen A.P., Kennedy P.J., Dockray S., Cryan J.F., Dinan T.G., Clarke G. Elsevier Inc; 2017. The Trier Social Stress Test: Principles and Practice. Neurobiology of Stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lanni K.E., Schupp C.W., Simon D., Corbett B.A. Verbal ability, social stress, and anxiety in children with Autistic Disorder. Autism. 2012;16:123–138. doi: 10.1177/1362361311425916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levine T.P., Sheinkopf S.J., Pescosolido M., Rodino A., Elia G., Lester B. Physiologic arousal to social stress in children with Autism Spectrum Disorders: a pilot study. Res. Autism Spectr. Disord. 2012;6:177–183. doi: 10.1016/j.rasd.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lam K.S.L., Aman M.G., Arnold L.E. Neurochemical correlates of autistic disorder: a review of the literature. Res. Dev. Disabil. 2006;27:254–289. doi: 10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 64.Engelmann M., Landgraf R., Wotjak C.T. The hypothalamic-neurohypophysial system regulates the hypothalamic- pituitary-adrenal axis under stress: an old concept revisited. Front. Neuroendocrinol. 2004;25(3–4):132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 65.Neumann I.D. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog. Brain Res. 2002;139 doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- 66.Carson D.S., Berquist S.W., Trujillo T.H., Garner J.P., Hannah S.L., Hyde S.A., Sumiyoshi R.D., Jackson L.P., Moss J.K., Strehlow M.C., Cheshier S.H., Partap S., Hardan A.Y., Parker K.J. Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Mol. Psychiatr. 2015;20:1085–1090. doi: 10.1038/mp.2014.132. [DOI] [PubMed] [Google Scholar]
- 67.Weisman O., Zagoory-Sharon O., Schneiderman I., Gordon I., Feldman R. Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology. 2013;38:694–701. doi: 10.1016/j.psyneuen.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 68.Mariscal M.G., Oztan O., Rose S.M., Libove R.A., Jackson L.P., Sumiyoshi R.D., Trujillo T.H., Carson D.S., Phillips J.M., Garner J.P., Hardan A.Y., Parker K.J. Blood oxytocin concentration positively predicts contagious yawning behavior in children with autism spectrum disorder. Autism Res. 2019;12:1156–1161. doi: 10.1002/aur.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.MacLean E.L., Gesquiere L.R., Gee N., Levy K., Martin W.L., Carter C.S. Validation of salivary oxytocin and vasopressin as biomarkers in domestic dogs. J. Neurosci. Methods. 2018;293:67–76. doi: 10.1016/j.jneumeth.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 70.Feldman R., Gordon I., Schneiderman I., Weisman O., Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology. 2010;35:1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 71.MacLean E.L., Wilson S.R., Martin W.L., Davis J.M., Nazarloo H.P., Carter C.S. Challenges for measuring oxytocin: the blind men and the elephant? Psychoneuroendocrinology. 2019;107:225–231. doi: 10.1016/j.psyneuen.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fogel D.B. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review. Contemp. Clin. Trials Commun. 2018 doi: 10.1016/j.conctc.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Keating C., Dawood T., Barton D.A., Lambert G.W., Tilbrook A.J. Effects of selective serotonin reuptake inhibitor treatment on plasma oxytocin and cortisol in major depressive disorder. BMC Psychiatr. 2013;13:124. doi: 10.1186/1471-244X-13-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oster H., Challet E., Ott V., Arvat E., de Kloet E.R., Dijk D.J., Lightman S., Vgontzas A., Van Cauter E. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr. Rev. 2017 doi: 10.1210/er.2015-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kunz-Ebrecht S.R., Mohamed-Ali V., Feldman P.J., Kirschbaum C., Steptoe A. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain Behav. Immun. 2003;17:373–383. doi: 10.1016/S0889-1591(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 76.Kudielka B.M., Schommer N.C., Hellhammer D.H., Kirschbaum C. Acute HPA axis responses, heart rate, and mood changes to psychosocial stress (TSST) in humans at different times of day Brigitte. Psychoneuroendocrinology. 2004;29:983–992. doi: 10.1016/j.psyneuen.2003.08.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.