Abstract
Pituitary adenylate cyclase-activating peptide (PACAP) is a neuropeptide that plays a key role in the neurobiology of the stress response, and prior studies suggest that its function is dysregulated in post-traumatic stress disorder (PTSD). Transcutaneous cervical vagus nerve stimulation (tcVNS) acts through PACAP and other neurobiological systems to modulate stress responses and/or symptoms of PTSD. In this pilot study, we examined the effects of tcVNS on PACAP in a three day chronic stress laboratory paradigm involving serial traumatic and mental stress exposures in healthy individuals with a history of exposure to psychological trauma (n = 18) and patients with PTSD (n = 12).
Methods
A total of 30 subjects with a history of exposure to psychological trauma experience were recruited (12 with PTSD diagnosis) for a three-day randomized double-blinded study of tcVNS or sham stimulation. Subjects underwent a protocol that included both personalized trauma recall and non-personalized mental stressors (public speaking, mental arithmetic) paired to tcVNS or sham stimulation over three days. Blood was collected at baseline and multiple time points after exposure to stressors. Linear mixed-effects models were used to assess changes in PACAP over time (in response to stressors) and its relation to active tcVNS or sham stimulation.
Results
PACAP blood levels increased over the course of three days for both active tcVNS and sham groups. This increase was statistically-significant in the sham group at the end of the second (Cohen’s drm = 0.35, p = 0.04), and third days (drm = 0.41, p = 0.04), but not in the active tcVNS group (drm = 0.21, drm = 0.18, and p > 0.20).
Conclusion
These pilot findings suggest tcVNS may attenuate this neurobiological stress-response. Larger studies are needed to investigate gender and interaction effects.
Highlights
-
•
We examined the effects of tcVNS on PACAP in a three day chronic stress paradigm involving traumatic and mental stress.
-
•
PACAP levels increased over the course of three days for both groups, the elevation of PACAP was larger in the sham group.
-
•
These findings suggest that tcVNS may be a potential intervention for stress-related psychiatric disorders.
1. Introduction
Post-Traumatic Stress Disorder (PTSD) is a neuropsychiatric condition triggered by experiencing or witnessing a terrifying event, with a lifetime prevalence of 6.4% in the United States [92]. Treatment modalities for PTSD primarily involve trauma-focused psychotherapies including cognitive behavior therapy (CBT) and medication [4,22,36,37,57,106]. A range of medications, which were generally not developed for PTSD specifically, have also shown efficacy in some cases [27,118]. Despite the tremendous burden of PTSD, the treatments are still not widespread, have high rates of non-completion, and/or limitations in efficacy [64,65,77]; U.S. Department of Veterans [_Affairs_2019 [111,118]]. Moreover, regardless of which treatment method is chosen, about 40% of the patients have a recurrence of symptoms within one year, and the risk of five-year recurrence is about 20% [7]. Therefore, new approaches are needed for the treatment of PTSD.
The etiology of PTSD is complex and likely involves interconnected molecular pathways of the sympathetic nervous system, the hypothalamic pituitary adrenal (HPA) axis, inflammatory response systems, and other neuropeptidal and neurohormonal systems [10,87]. Pituitary adenylate cyclase activating polypeptide (PACAP) is a highly conserved neuropeptide that connects these systems and regulates and integrates adaptive responses to stress [47]. A growing body of literature has pointed to dysregulation of PACAP along with its selective PAC1 receptor in PTSD [28,30,47,59,60,62,78,79,100]. Within the brain, PACAP participates in neural circuits relevant to PTSD and other stress disorders [55,100]. In the hypothalamus and hippocampus, PACAP acts as an important neuromodulator [34,75]. Anatomical and physiological studies established the importance of PACAP neurotransmission in the amygdala, which underscored the role of this peptide transmitter in fear responses and potentially PTSD [93,109]. Studies suggest that PACAP is anxiogenic and may play a role in symptoms of PTSD [55,100]. PACAP also has neuroprotective and anti-inflammatory properties [29,98] and is well-positioned for modulation by vagus nerve activity [88,99].
Electrical stimulation of the vagus nerve, also referred to as vagus nerve stimulation (VNS), has been demonstrated to be efficacious for the treatment of epilepsy [44,54], major depression [1,43,104], heart failure [96], and migraine [76]. VNS also results in improvements in cognition, memory, and adverse conditions such as tinnitus when paired with a stimulus or task [26,31,32,56,95]. Preclinical work suggested that VNS may be an effective adjunct to exposure therapy for the treatment of PTSD [82,89]. Considering the potential side effects and complications of implant surgeries, new medical devices were developed providing noninvasive transcutaneous stimulation [11,45]. Transcutaneous VNS devices target the auricular (taVNS) or cervical (tcVNS) portions of the vagus nerve. There has been a growing literature in recent years suggesting that these devices modulate central and peripheral physiology, as investigated by brain imaging [2,38,39,116,117], inflammatory serum cytokines [8,17,71], and peripheral physiological measures [3,42,49,51] in preclinical models or in humans with or without adverse chronic conditions including PTSD and paired with traumatic and mental stress tasks [5,15,24,25,41,49,51,53,69,101,112,117].
Of particular interest to the vagus nerve, PACAP innervation of the lateral central amygdala is thought to arise from PACAP containing neurons in the vagus nerve brainstem complex [93]. A growing literature on the anatomical location and physiology of PACAP suggested a close association with systems that are also regulated by VNS [66,86,88]. Therefore, PACAP is well-positioned as a mediator in pathways of the cholinergic anti-inflammatory axis and its potential modulation by VNS. PACAP may potentially serve as a dynamic and objective biochemical biomarker that could measure PTSD severity, hence the longitudinal changes in PACAP may indicate therapy response to potential treatments targeted at PTSD. The relationship between PACAP levels, stressful stimuli, and VNS (either direct or noninvasive stimulation) has not been examined in humans. We previously investigated the physiological [42,[48], [49], [50],52], inflammatory [8], and neural [115] effects of tcVNS as a part of a larger study that included three days of chronic stress applied to traumatized subjects with or without PTSD, who were randomized to either tcVNS or sham stimulation (control). In the current pilot investigation, we evaluated the effect of tcVNS treatment by comparing PACAP concentration across timepoints spanning the three days of exposure to personalized traumatic scripts and mental stress (public speaking, mental arithmetic) tasks.
2. Methods
2.1. Overview
The study was reviewed and approved by the Emory Institutional Review Board (#IRB00091171), Georgia Institute of Technology (#H17126), SPAWAR Systems Pacific, and the Department of Navy Human Research Protection Program. Data collection took place in Emory University School of Medicine between May 2017 and October 2019. This study used blood draw data from the project, Closed Loop Vagal Stimulation in Patients with Posttraumatic Stress Disorder funded by N66001-16-2-4054 (phases 1 and 2), which aimed to investigate the effects of tcVNS paired with acute stress in the context of traumatic recall and mental stress for PTSD and non-PTSD traumatized controls (ClinicalTrials.Gov Identifier # NCT02992899). This project was a parallel study, in which tcVNS or sham stimulation were randomly conducted in both patients with PTSD and healthy subjects. The study included dynamic monitoring of physiological signals, high resolution position emission tomography (HR-PET) of the brain [48,49,51], and blood biochemical collection. PACAP investigation was considered exploratory due to the relevance of PACAP in PTSD, hence it was not a part of the primary or secondary outcomes. A limited number of blood draws (baseline and end of each day) were analyzed for this study, as a pilot, phase 0 investigation.
2.2. Eligibility criteria
-
a.
Non-PTSD traumatized subjects: Subjects aged 18-70 with a history of exposure to psychological trauma, but did not meet criteria for PTSD as determined by the Structured Clinical Interview for DSM-5 (SCID) [35] and Clinician Administered PTSD Scale (CAPS) interview [6].
-
b.
Patients with PTSD: Subjects aged 18-70 met criteria for PTSD according to CAPS.
2.3. Exclusion criteria
Exclusion criteria were: 1) positive pregnancy test; 2) meningitis; 3) traumatic brain injury; 4) neurological disorder or organic mental disorder; 5) history of loss of consciousness greater than 1 min; 6) alcohol abuse or substance abuse or dependence based on the SCID within the past 12 months; 7) current or lifetime history of schizophrenia, schizoaffective disorder, or bulimia, based on the SCID; 8) a history of serious medical or neurological illness, such as cardiovascular, gastrointestinal, hepatic, renal, neurologic or other systemic illness; 9) evidence of a major medical or neurological illness on physical examination or as a result of laboratory studies (Complete blood count (CBC), blood urea nitrogen (BUN), creatinine, blood sugar, electrolytes, liver and thyroid function tests, urinalysis, and electrocardiogram (ECG)); 10) active implantable device (i.e. pacemaker); 11) carotid atherosclerosis; and 12) cervical vagotomy. Women were counseled about the risks of pregnancy during the course of the study and pregnancy tests were conducted before the study started for each female subject.
2.4. Baseline assessments
Each subject informed, provided written consent, after which they underwent a psychological and health assessment. Sociodemographic factors (age, sex, race/ethnicity, marital status, education level) and clinical information (current medications, medical history, SCID) were collected. Table 1 summarizes demographic characteristics and psychological assessment scores grouped by PTSD status and treatment. Additional psychological assessment was performed using structured interviews and standard questionnaires, including PTSD Checklist-Civilian Version (PCL-C) [103], PTSD Symptom Scale (PTSD-SS) [107,108], Clinician-Administered PTSD Scale (CAPS, only patients diagnosed with PTSD qualifies for CAPS) [6], early trauma inventory (ETI) [13], adulthood trauma inventory (ATI) [114], Hamilton Anxiety Scale (HAM-A) [74], Hamilton Depression Scale (HAM-D), Beck Depression Inventory (BDI) [63], Social Support (ESSI) [46], and Physical Activity (Baecke Questionnaire’s Work, Sports, Leisure indices, respectively) [94].
Table 1.
Demographic characteristics and psychological assessment scores by PTSD diagnosis and treatment groups.
Table 1. Demographic Characteristics and Psychological Assessment Scores.
| Variables | Patients with PTSD |
Non-PTSD Traumatized Control Subjects |
Total (n = 30) | |||||
|---|---|---|---|---|---|---|---|---|
| tcVNS (n = 5) | Sham (n = 5) | Total (n = 10) | tcVNS (n = 13) | Sham (n = 7) | Total (n = 20) | |||
| Age Years (Mean ± SD) | 28.6 ± 7.2 | 32.4 ± 7.2 |
30.5 ± 7.4 |
30.1 ± 8.1 | 30.4 ± 8.3 | 30.2 ± 8.2 | 30.3 ± 7.9 | |
| BMI (Mean ± SD) | 25.0 ± 7.0 | 30.6 ± 4.1 |
27.8 ± 6.4 |
26.7 ± 5.1 | 26.6 ± 4.7 | 26.6 ± 5.0 | 27.0 ± 5.5 | |
| Sex (N (%)) | ||||||||
| Male | 0 (0.0) | 3 (60.0) | 3 (30.0) | 8 (61.5) | 4 (57.1) | 12 (60.0) | 15 (50) | |
| Female | 5 (100) | 2 (40.0) | 7 (70.0) | 5 (38.5) | 3 (42.9) | 8 (40.0) | 15 (50) | |
| Race (N (%)) | ||||||||
| White/Caucasian | 2 (40.0) | 2 (40.0) | 4 (40.0) | 6 (46.2) | 3 (42.9) | 9 (45.0) | 13 (43.3) | |
| African American | 2 (40.0) | 3 (60.0) | 4 (40.0) | 2 (15.4) | 4 (57.1) | 4 (20) | 8 (26.7) | |
| Education Level (N (%)) | ||||||||
| High school - graduate | 0 (0.0) | 0 (0.0) | 9 (90.0) | 1 (7.7) | 1 (14.3) | 2 (10.0) | 2 (6.7) | |
| College -not complete | 2 (40.0) | 3 (60.0) | 0 (0.0) | 1 (7.7) | 1 (14.3) | 2 (10.0) | 7 (23.3) | |
| Associate’s degree | 0 (0.0) | 1 (20.0) | 0 (0.0) | 2 (15.4) | 0 (0.0) | 2 (10.0) | 3 (10.0) | |
| Bachelor’s degree | 2 (40.0) | 0 (0.0) | 0 (0.0) | 7 (53.8) | 2 (28.6) | 9 (45.0) | 11 (36.7) | |
| Master’s degree | 0 (0.0) | 1 (20.0) | 0 (0.0) | 2 (15.4) | 3 (42.9) | 5 (25.0) | 6 (20) | |
| Marital status (N (%)) | ||||||||
| Never Married |
4 (80.0) | 2 (40.0) | 6 (60.0) | 9 (69.2) | 5 (71.4) | 14 (70.0) | 20 (66.7) | |
| Married/Civil Partnership | 0 (0.0) | 1 (20.0) | 1 (10.0) | 3 (23.1) | 1 (14.3) | 4 (20.0) | 5 (16.7) | |
| Divorced/Separated | 1 (20.0) | 1 (20.0) | 2 (20.0) | 1 (7.7) | 1 (14.3) | 2 (10.0) | 4 (13.3) | |
| Widowed | 0 (0.0) | 1 (20.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.3) | |
| PTSD Checklist (PCL-C) | 44.8 (9.1) | 51.6 (12.7) | 48.6 (11.8) | 27.6 (9.2) | 32.4 (9.7) | 29.3 (9.7) | 35.3 (13.7) | |
| PTSD Symptom Scale (PTSD-SS) | 26.0 (7.3) | 26.0 (8.1) | 26.0 (7.6) | 18.3 (4.2) | 17.7 (2.2) | 18.1 (3.7) | 20.6 (6.4) | |
| Clinician-Administered PTSD Scale (CAPS) | 40.8 (13.4) | 39.3 (11.5) | 40.1 (12.6) | N/A | N/A | N/A | 40.1 (12.6) | |
| Early Trauma Inventory (ETI) | 25.0 (7.1) | 9.8 (2.9) | 16.3 (9.1) | 11.7 (8.3) | 9.2 (1.0) | 10.7 (6.5) | 12.7 (8.1) | |
| Adulthood Trauma Inventory (ATI) | 4.5 (1.1) | 5.4 (2.6) | 5 (2.1) | 4.1 (1.9) | 4.1 (2.5) | 4.1 (2.1) | 4.4 (2.2) | |
| Hamilton Anxiety Scale (HAM-A) | 16.5 (9.9) | 10.3 (5.9) | 13.4 (8.8) | 4.7 (8.2) | 6.4 (4.8) | 5.3 (7.2) | 7.6 (8.5) | |
| Hamilton Depression Scale (HAM-D) | 13.5 (9.9) | 10.5 (7.8) | 12.0 (9.1) | 5.7 (8.4) | 5.1 (2.6) | 5.5 (6.9) | 7.4 (8.2) | |
| Beck Depression Inventory (BDI) | 8.3 (3.3) | 17.8 (13.0) | 13.6 (11.0) | 12.0 (9.5) | 15.0 (10.3) | 13.1 (9.9) | 13.2 (10.3) | |
| ESSI (Social Support) | 27.5 (3.9) | 20.0 (7.3) | 23.3 (7.1) | 23.5 (8.0) | 23.3 (4.7) | 23.5 (7.0) | 23.4 (7.1) | |
| Baecke (Sports Index) | 0.7 (0.9) | 0.3 (0.3) | 0.4 (0.6) | 0.8 (0.8) | 1.3 (1.0) | 1.0 (0.9) | 0.8 (0.8) | |
| Baecke (Work Index) | 2.8 (0.7) | 3.1 (0.6) | 2.9 (0.7) | 2.7 (0.7) | 2.5 (0.6) | 2.6 (0.7) | 2.7 (0.7) | |
| Baecke (Leisure Index) | 3.7 (1.1) | 3.3 (0.8) | 3.4 (1.0) | 3.8 (0.9) | 4.2 (0.9) | 4.0 (0.9) | 3.8 (1) | |
The subjects who did not self-identify as either White or African American identified as multiracial, Asian, or chose not to self-identify race, and missing education data were coded as “other” in analysis. Psychometric scores were reported per the literature recommendation of each test.
2.5. Blinding and stimulation administration
The subjects were randomized into tcVNS and sham groups with a double-blind approach. The research staff and patients were blinded to the stimulus type. The “tcVNS” group was administered stimulation using the electroCore© GammaCore-S non-invasive VNS devices. The tcVNS stimulus intensity was adjusted by the researcher to the maximum tolerable level without causing pain with a burst frequency of 5 kHz, and envelope frequency of 25 Hz (five sine waves at 5 kHz for 1 ms, repeating every 40 ms, amplitude 0–30V peak, adjustable roll switch 0-5 a.u.). The sham devices were identical to the active tcVNS devices in appearance and operation, with different waveform characteristics (0.2Hz biphasic square-like waves, amplitude 0–14V peak, adjustable roll switch 0-5 a.u.), resulting in a tingling sensation. Supplementary Fig. 1 shows both active and sham stimulation waveforms.
For ensuring sham stimulation will not be as powerful as active, we used a low frequency sham waveform to minimize (possible) stimulation with sham device. From an electrical engineering standpoint, high frequency voltage signals (such as the active stimulus, 25Hz with 5 kHz bursts, waveform seen in Supplementary Fig. 1) pass through the skin with minimal power dissipation due to the low skin-electrode impedance (in the range of ohms) at kHz frequencies; in contrast, lower frequency signals (such as the sham stimulus, 0.2Hz, waveform seen in Supplementary Fig. 1) are mainly attenuated at the skin-electrode interface due to the high impedance (in the range of megaohms) [102]. Accordingly, the active device operating at higher frequencies may deliver substantial energy to facilitate nerve stimulation, while the voltage levels appearing under the skin would be expected to be orders of magnitude lower for the sham device and thus vagus nerve stimulation is unlikely with sham. Nevertheless, since the sham device does deliver relatively high voltage and current levels directly to the skin, it activates skin nociceptors, causing a similar feeling to a pinch. This sensation is necessary for blinding of the participants.
The duration of delivery was 2 min for each device and every subject received a dedicated device throughout the protocol, which was determined upon randomization before recruitment by a research coordinator who did not take part in data collection or analysis. Collar electrodes were used for ease of delivery, on left carotid pulsation which was identified by the research staff. For this sample (n = 36), 20 subjects received tcVNS (7 PTSD, 13 non-PTSD) and 16 subjects received sham (5 PTSD, 11 Non-PTSD). Across all administrations over the course of three days, active tcVNS group subjects received a mean ± SD stimulation amplitude of 2.94 ± 1.13 a.u. (corresponding to 17.67 ± 6.78V), and sham subjects received 4.58 ± 1.02 a.u. (corresponding to 12.81 ± 2.86V). No subjects reported any lack of sensation.
2.6. Study design
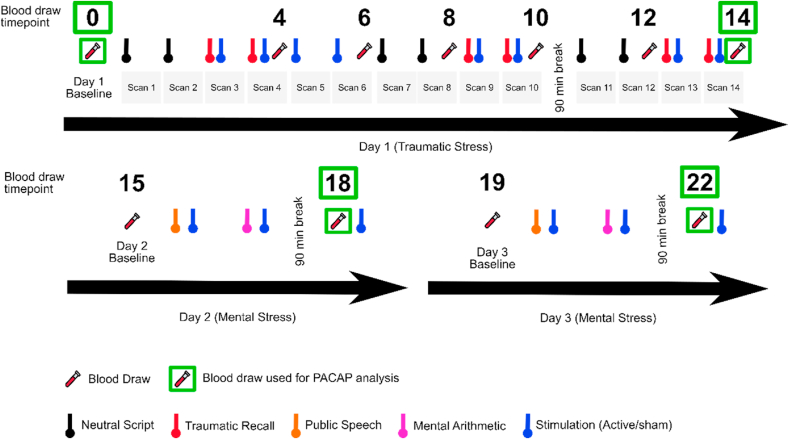
Protocol consisted of three consecutive days with continuous physiologic monitoring, intermittent blood draws, and one day of HR-PET imaging (Fig. 1). Day 1 focused on personalized traumatic stress and HR-PET imaging, days two and three focused on mentally stressful, non-personalized tasks without HR-PET imaging. Physiologic monitoring and blood draws were carried out on each day. Prior to the protocol, subjects were asked to prepare scripts of their personal traumatic experiences in written form. The scripts were transcribed, edited to last 60 s each, and recorded by a research associate in normal voice, in the first person, present tense. On the first day, after the initial set up with physiologic monitors and intravenous catheter (IV), subjects rested for 30 min and baseline measurements were collected. Laying down in HR-PET scanner, subjects underwent 14 HR-PET scans with contents detailed in Fig. 1 in order. Six of these scans included pleasant scenery recordings (termed as “neutral script”, ~60 s, scan # 1, 2, 7, 8, 11, 12), other six included listening to the personalized traumatic stress recordings (~60 s, scan # 3, 4, 9, 10, 13, 14), each traumatic stress recording was immediately followed by stimulation (active or sham, 120 s). Lastly, the remaining two scans included only stimulation (active or sham, scan # 5,6) without any playback or acute stress. The subjects were instructed to image each event as vividly as possible. Blood was drawn (total 194.5 cc) at baseline (referred as timepoint 0 in this manuscript), and during timepoints 4, 6, 8, 10, 12, 14 as expressed in Fig. 1 (immediately after the aforementioned event ended, such as the end of recordings or stimulation). The second and third days were identical to each other. After initial set up and resting, baseline (timepoints 15 and 19 for days 2 and 3, respectively) blood draws were taken both mornings. Afterwards, subjects underwent a public speech task and mental arithmetic tasks, as described previously [49]. Stimulations were applied immediately after each task. After two mental stressors and two stimulation administrations, the subjects were given a 90-min break. After the break, a second blood draw was taken (timepoints 18 and 22 for days 2 and 3, respectively). A total 57 cc of blood was drawn per day on these days. It should be noted that a third stimulation was applied after the blood draw of 90-min break to monitor the physiologic changes to stimulation without acute stress, however this administration was after the blood draws (after timepoints 18 and 22). Since the PACAP investigation was exploratory, PACAP analysis used baseline (day 1, timepoint 0), end of day 1 (timepoint 14), end of day 2 (timepoint 18), and end of day 3 (timepoint 22) due to budget constraints.
Fig. 1.
Timeline depiction of the study assessing the potency and kinetics of tcVNS treatment in the context of traumatic and mental stress. Day 1 happened inside a HR-PET scanner where subjects underwent 14 scans, each lasting approximately 8 min. Six scans included the neutral scenery recordings, six scans included traumatic stress recordings followed by stimulation, and two scans included stimulation without acute stress. On days 2 and 3, subjects underwent public speech and mental arithmetic tasks, each followed by stimulation. Each day included 90-min breaks. Blood was drawn at the baseline and the numbered timepoints (0-22) over the course of three days. PACAP data was analyzed for timepoints baseline (timepoint #0), end of day 1 (timepoint #14), end of day 2 (timepoint #18), and end of day 3 (timepoint #22).
2.7. Biomarker measurement
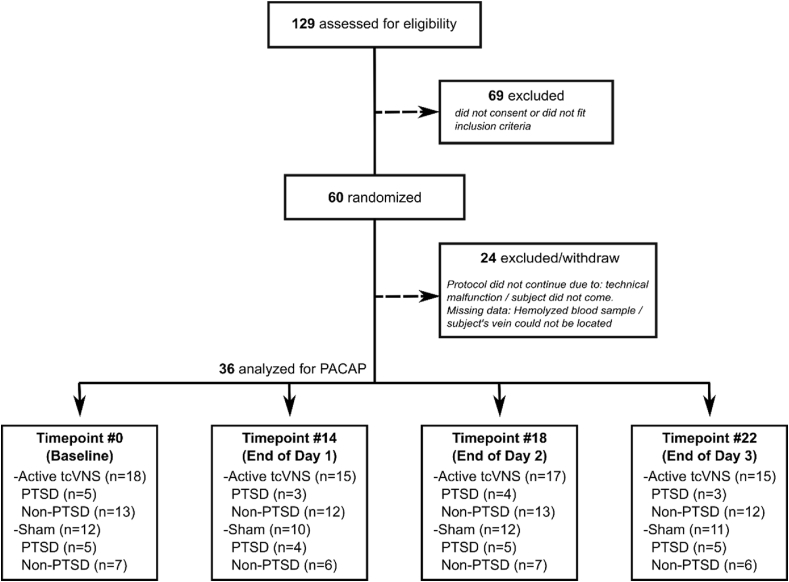
Human PACAP was assayed with a competitive ELISA using kits purchased from LS Bio (Seattle, Washington) for blood draws at timepoints 0, 14, 18, 22. Fig. 2 depicts the analyzed PACAP timepoints with number of subjects and PTSD status for each timepoint. All experimental operations were in accordance with standard protocols. Intra-assay coefficient of variation (CV) was 9.89%, sensitivity was 2.55 pg/ml, and the detection range was 6.17–500 pg/ml. The polyclonal antibody used in this assay does not differentiate between the PACAP-27 and PACAP-38 isoforms. However, since the PACAP-38 is the most abundant isoform in mammals [23], it is likely that most of the detected peptide is PACAP-38.
Fig. 2.
Consolidated Standards of Reporting Trials (CONSORT) diagram of the study.
2.8. Statistical methods
Since the distribution of PACAP concentration was skewed, log-transformation was applied to PACAP concentrations to achieve normality and better interpretation in all the subsequent analyses. These (log) PACAP data were confirmed to be normal using Shapiro-Wilk test. Initially, we computed partial correlations between (log) PACAP concentration (baseline, maximum, quartiles (lower (Q1, 25th percentile), medium (Q2, 50th percentile), upper (Q3, 75th percentile)) for each subject) and psychometric scales, controlling for age, sex, BMI, race, and education and reported correlation coefficients (rho, ρ) and p-values. To assess the trajectory of PACAP concentration over time, we computed the delta (from baseline) at each timepoint. We used a linear mixed model to regress the delta PACAP values on timepoints (0, 14, 18, 22), PTSD status (yes/no), device type (active tcVNS/sham), as well as other covariates (BMI) with subject-specific random intercepts to account for within-subject correlations. When a significant main effect or interaction was present, pairwise post-hoc comparisons with Bonferroni-Holm corrections were used to determine location of differences. For this analysis, an a priori planned comparison approach yielded the number of tests as five: successive time points (e.g., 14 vs 18) and all numbers different from 0. Effect sizes (Cohen’s d) were reported for results, regardless of the significance of p-value, based on group means, standard deviation, and group sample size for device and PTSD status main effects [68]. For post-hoc analyses that include timepoints, repeated measures Cohen’s d (drm) was calculated that includes a correction factor based on the correlation of PACAP concentrations between two timepoints (for example, correlation of delta PACAP between timepoints 0 and 18 and between timepoints 0 and 22 were included as a correction factor) [68]. Missing biomarker data were assumed to be missing completely at random for reasons such as hemolyzed sample or missing baseline data, and all analyses were based on available data. P-values less than or equal to 0.05 were considered statistically significant. Statistical analyses were done using R (v 3.6.0) and MATLAB R2020a.
3. Results
A total of 36 subjects who completed or at least partly completed the trauma tasks were involved in these analyses. Fig. 2 depicts the CONSORT diagram of the study, Table 2 breaks down the PACAP concentrations per PTSD status and treatment groups, and Supplementary Fig. 2 shows normalized PACAP concentration separated by device and PTSD status. Subject groups were similar in age, body mass index, race, education level and marital status (Table 1). Although subjects were randomly assigned to the tcVNS treatment or sham treatment groups, only female PTSD patients received tcVNS treatment. The gender proportion in the other groups was similar. The average age of this population was 30.3 (SD = 7.9) years, and the average BMI was 27.0 (SD = 5.5) kg/m2. Among all the 30 subjects, 15 (50.0%) were female, 13 (43.3%) White/Caucasian, 17 (56.7%) had a Bachelor’s or higher degree, and 20 (66.7%) had never married.
Table 2.
PACAP concentrations by PTSD diagnosis and treatment groups.
Table 2. Mean (SD) Concentrations of log Pituitary adenylate cyclase-activating peptide (log-PACAP) Over Time in PTSD and Non-PTSD Participants with Active tcVNS or Sham.
| Group | Baseline | End of Day 1 | End of Day 2 | End of Day 3 |
|---|---|---|---|---|
| PTSD Active | 4.34 (0.4) | 4.41 (0.42) | 4.46 (0.43) | 4.46 (0.39) |
| PTSD Sham | 4.43 (0.47) | 4.41 (0.44) | 4.53 (0.51) | 4.52 (0.34) |
| Non-PTSD Active | 4.23 (0.43) | 4.33 (0.45) | 4.33 (0.32) | 4.36 (0.41) |
| Non-PTSD Sham | 4.43 (0.41) | 4.55 (0.41) | 4.62 (0.31) | 4.66 (0.33) |
First, the association between PACAP baseline concentration and scores of psychological and functional scales were examined. Table 3 lists partial correlation coefficients and p-values between each psychological scale and related PACAP concentrations. Baseline PACAP concentrations were significantly positively correlated with total PTSD Symptom Score (PTSD-SS) (ρ = 0.45, p = 0.04) and significantly negatively correlated with Baecke Sports Index (ρ = −0.46, p = 0.05). The maximum PACAP concentrations (from timepoints 0 to 22 for each subject) were significantly positively correlated with Hamilton Anxiety Scale (HAM-A) (ρ = 0.43, p = 0.05) and Hamilton Depression Scale (HAM-D) (ρ = 0.45, p = 0.04). Upper quartile PACAP concentrations (from timepoints 0 to 22 for each subject) were significantly negatively correlated with Baecke Leisure Index (ρ = −0.53, p = 0.03). None of the other psychological and functional scales were statistically significant with the examined PACAP concentrations.
Table 3.
Partial correlation results for psychological scales and log-normalized baseline, maximum, or upper quartile PACAP concentrations of all timepoints from each subject, ∗p ≤ 0.05.
Table 3. Partial correlations (rho (ρ), p-value) controlling for age, sex, BMI, race, education. ρ: partial correlation coefficient, ∗p ≤ 0.05.
| Psychological Assessment Total Score | PACAP data | ρ | p-value |
|---|---|---|---|
| PTSD Symptom Scale (PTSD-SS) | Baseline | 0.45 | 0.04∗ |
| PTSD Checklist (PCL-C) | Baseline | 0.21 | 0.35 |
| Clinician-Administered PTSD Scale (CAPS) | Baseline | 0.72 | 0.49 |
| Early Trauma Inventory (ETI) | Baseline | 0.22 | 0.49 |
| Adulthood Trauma Inventory (ATI) | Baseline | 0.24 | 0.29 |
| Hamilton Anxiety Scale (HAM-A) | Maximum | 0.43 | 0.05∗ |
| Hamilton Depression Scale (HAM-D) | Maximum | 0.45 | 0.04∗ |
| Beck Depression Inventory (BDI) | Baseline | 0.28 | 0.21 |
| ESSI (Social Support Inventory) | Baseline | 0.28 | 0.21 |
| Baecke Questionnaire (Sports) | Baseline | −0.46 | 0.05∗ |
| Baecke Questionnaire (Work) | Baseline | −0.15 | 0.54 |
| Baecke Questionnaire (Leisure) | Upper Quartile (Q3) | −0.53 | 0.03∗ |
When examining the PACAP values within the overall sample, the main effect of device type (p = 0.26, d = 0.46) and PTSD status (p = 0.13, d = 0.12) along with interactions of device type by time (p = 0.53), device type by time by PTSD status (p = 0.59), PTSD status by time (p = 0.48), and PTSD status by device type (p = 0.63) were not significant. However, the main effect of time was statistically significant (p = 0.008). Post-hoc comparisons revealed elevated PACAP at timepoints 18 (p = 0.03, drm = 0.27) and 22 (p = 0.03, drm = 0.28), but not at timepoint 14 (p = 0.27, drm = 0.18). No other significant differences were observed at any timepoint for the overall sample (p > 0.05).
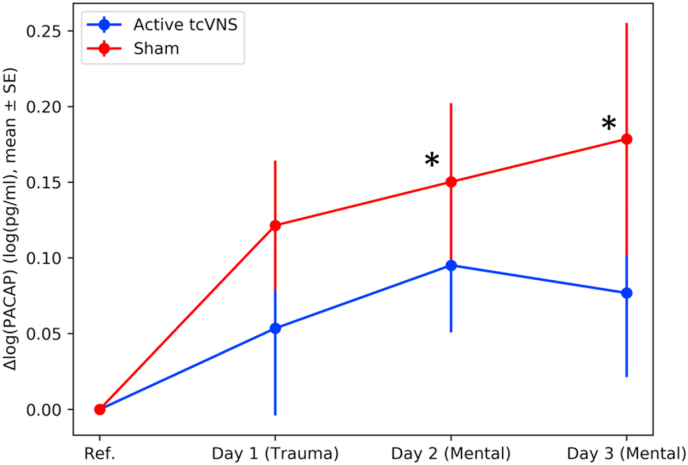
Although the device type by time interaction was not significant, a subsequent analysis was completed using an isolated dataset with just sham or active across time to better determine the contributions of treatment group to the overall time effect (Fig. 3). In our stratified analysis, a significant main effect of time was observed in the sham group (p = 0.04). Post hoc tests revealed significant differences at timepoints 18 (p = 0.04, drm = 0.35) and 22 (p = 0.04, drm = 0.41) compared to timepoint 0. No other significant differences were observed in sham including timepoint 14 (p = 0.13, drm = 0.27).
Fig. 3.
Change in PACAP concentration over three days for active tcVNS and sham groups. ∗ indicate p ≤ 0.05 after post-hoc corrections, error bars are standard error of the mean. Sham group had a marked increase in PACAP, consistently increasing over the course of three days. This elevation was less in active tcVNS group.
In contrast, no significant main effects or interactions were observed for active tcVNS (p = 0.21). The tcVNS active group effect sizes were drm = 0.12, drm = 0.21, and drm = 0.18, for days 1, 2, and 3, respectively.
4. Discussion
In this randomized, double blind, pilot study of trauma recall and other psychological stressors and longitudinal assessment of plasma PACAP levels, we found that PACAP increased over the course of the stress protocol—an effect attenuated by tcVNS (but not sham stimulation) in traumatized individuals. Increased PACAP concentrations were correlated with elevated PTSD symptoms at baseline, replicating earlier findings [100]. We also found that elevated PACAP was associated with increased symptoms of anxiety and depression and impairments in social and physical function. As PACAP is known to regulate stress response [30,100], longitudinal evaluation of PACAP may be helpful in tcVNS treatment monitoring.
This investigation touches on two main points. First, to our knowledge, this is the first report of PACAP in humans undergoing a trauma recall and mental stress paradigm over multiple days. Trauma recall and stressful tasks were associated with a steady increase in PACAP blood levels, regardless of the treatment status. Second, notably, the sham group’s PACAP increase was higher, compared to active tcVNS group, which suggests that tcVNS may reduce PACAP elevation in response to stress. These results, along with correlations between PACAP and psychological scales (PTSD-SS, HAM-A, HAM-D, Baecke Sports Index, Baecke Leisure Index) suggest that PACAP may play an integral role in stress and PTSD, supporting relevant literature [30,100].
PTSD is associated with poor health behaviors, notably physical inactivity [119], also recognized in our study with negative correlations of baseline PACAP with Baecke Sports Index. We did not find significant correlations with other assessments of PTSD symptoms (CAPS, PCL-C, ETI, ATI), we believe that this could be due to small sample size. For example, only patients with PTSD can have a total CAPS score, which significantly decreased the sample size for this correlation. Regardless of the p-values, all psychological surveys indicating increased severity with higher scores were positively correlated with baseline PACAP. Similarly, physical activity scales (Baecke Questionnaire) were negatively correlated.
The source of PACAP in circulating blood or plasma is not known [66,67,105]. PACAP has a close association with systems that are also modulated by VNS. PACAP is involved in a number of processes including limbic, autonomic, neuroendocrine functions, and regulation of circadian pacemaker [93]. Another review study noted that PACAP is an important regulator of hippocampal circuits [58]. Our HR-PET results from the same study showed that tcVNS attenuated the increased neural activity in the fear memory circuitry (brain scans obtained within seven-eight minutes after traumatic stress stimuli). Greater activation was observed during sham stimulation compared to active tcVNS within the limbic and other brain areas involved in stress, including bilateral prefrontal and orbitofrontal cortex, premotor cortex, temporal lobe, parahippocampal gyrus, insula, and left anterior cingulate [115]. In addition, we investigated physiological measures that reflect autonomic nervous system activity for the same study, and our findings suggest that active tcVNS attenuates autonomic reactivity to stress, as observed in data obtained during stimulation or within minutes after stimulation [42,48,49,52]. The attenuation in limbic and autonomic activity could potentially have led to less induction of PACAP in the tcVNS group, though it would largely be a speculation given the unclear source of PACAP in circulating blood. If we consider the measurement timescales, our PACAP results have a delay as we processed the blood samples at baseline (day 1 morning) and at the end of each day (days 1, 2, 3). As tcVNS attenuated both limbic and autonomic activity based on our previous investigations within seconds (physiological activity) and minutes (HR-PET scan resolution), one might think that the attenuation in these activities decreased PACAP as PACAP due to dampening stress responses. Nevertheless, understanding the source of PACAP and the details of a sequential relationship is beyond the scope of this paper.
The use of traumatic stress scripts or imagery is a standard practice in trauma and PTSD research [9,12,14,16,70,72,85,97]. As a part of the brain imaging investigation of this protocol, we previously reported the effects of traumatic stress scripts on brain’s fear circuitry, regardless of the device status (active and sham collapsed together). Our results indicated that listening to the personalized trauma scripts elicited similar brain activation patterns to what was previously reported in healthy traumatized individuals and patients with PTSD.
We paid substantial attention for the selection of the sham device. Our study does not use an inert sham device (i.e., no power delivery as sham). Use of inert devices as controls could lead to participants’ perception that they were not getting active interventions, increasing the risk of positive results that are only due to a placebo effect, a constant risk in device research. Furthermore, the use of “active” sham stimulation controls has been used in other studies to date, including for transcutaneous auricular VNS, so our study is consistent with the literature and standard practice in use of sham devices [71,116].
In our study, stimulations occurred after the traumatic or mental stressors, not during or before. The discussion surrounding timing (i.e., when to stimulate when paired with stress) is unclear given the clinical application and current state of knowledge surrounding tcVNS. Realistically, patients undergoing traumatic flashbacks can only use tcVNS during or following a traumatic memory, as they cannot predict the onset. While previous studies indicate lasting effects of stressful protocols, and thus presenting theoretical basis for stimulation after the traumatic memory cessation, it is unclear whether the effects are similar when compared to stimulating during traumatic stress. We applied stimulation after the trauma recall because we wanted to assess whether the groups had similar traumatic reactivity when the stimuli was first initiated, as detailed in our physiological outcomes work [49]. Unlike physiological signals, PACAP measurement does not have a second-based time resolution, and is likely capturing PACAP levels due to cumulative effect of these stressors. Previous studies in animals (direct VNS) applied stimulation both during — which also may be applicable in humans —and before traumatic stress paradigms [33,89]. No current study has examined the efficacy and/or changes in any sort of outcome when stimulation is applied either during or following traumatic stress in humans, and therefore it is unclear how the timing affects the findings in the current study. Given that the effects of traumatic stress, as autonomic parameters, persists beyond the initial presentation of the adverse stimulus, it is possible that stimulation during or after a traumatic script elicits similar changes in arousal. This is consistent with findings across various studies reporting autonomic dampening with implanted or noninvasive VNS, despite variations within stimulation timing and protocols [15,19,20,69,83,90,91].
We believe that specific biomarkers such as PACAP or other objective measures (blood biomarkers, brain imaging, physiological biomarkers) have the potential to be more reliable than self-reported scales in clinical investigations since they include objective biological indices While the PACAP investigation did not have self-reported PTSD symptom outcomes matching the PACAP timepoints, we recently published behavioral outcomes that suggest that patients with PTSD experience lower perceived anger with active tcVNS, compared to sham [8]. Perceived anger was measured by visual analog scores after each traumatic stress paired with stimulation on day 1, as a part of our main investigation. Recent neuroscience studies using implanted VNS in rat models trained with fear conditioning reported enhancement of fear extinction based on freezing time [89,91]. Along similar lines, fear conditioning studies on human subjects using transcutaneous auricular VNS reported acceleration in fear extinction [20], and enhanced processing of safety cues based on US expectancy ratings [18]. Objective measures will gain value in complementing. PTSD diagnosis based on clinician administered PTSD instruments. These instruments such as CAPS, PCL-C, or ATI have well-studied repeatability [6,103,113,114], however they could only be administered between long time scales (i.e., months), hence they are in the current practice most useful long term longitudinal clinical studies. Dynamic biomarkers such as PACAP that could quantify treatment effects in shorter time scales are needed for psychiatry research.
The limitations of this pilot study could be listed as follows. Our sample included 12 patients with PTSD, and all three male patients were randomly assigned to the sham group. Due to this small sample size and the fact that none of the PTSD male subjects received the tcVNS treatment, we cannot evaluate the effect of tcVNS treatment on PACAP concentrations among PTSD males. Moreover, as gender may be an effect modifier of PACAP concentrations and PTSD, this interaction could not be evaluated within the tcVNS treatment group. Prior work has suggested that blood levels of PACAP are associated with PTSD diagnosis among females and stress-regulation pathways may vary between men and women [100]. Although levels of PACAP were lower at all times points taken after traumatic stress and mental stress in the tcVNS group compared to the sham group, the interaction term for device by time was not significant. This is also likely related to the small sample size, and therefore our results should be considered preliminary and in need of replication. PACAP is involved in circadian rhythm regulation [21,40,61]. Although our blood draws were scheduled in the A.M., our study lasted for three days, and the precise blood draw time for each subject varied somewhat, as would be anticipated with a multi-day human investigation. In partial correlations between PACAP data (baseline, maximum, quartiles) and psychometric scales, we did not control for multiple testing, which may result in a Type I error. These results should be interrupted very cautiously since correction for multiple comparisons would yield all of these findings statistically non-significant. Again, this is considered an exploratory analysis, and results need to be established in a larger study.
Due to the clinical nature of this study, we did not evaluate whether vagal stimulation occurred, rather, we relied on previous literature that extensively studied to what extent vagal stimulation was achievable noninvasively. There are multiple studies that reported the ability to reach the vagal afferents using tcVNS using the same device [39,80]. We replicated the stimulation application reported in Ref. [39] throughout the protocol, by locating the carotid artery as reference. In addition, a study using the studied device was reported to reliably create vagal somatosensory evoked potentials, which —are also activated with VNS implant, associated with vagal afferent activation [84]. While these studies favor our methods, we also recognize a recent pig model study that suggested noninvasive vagal nerve stimulation (Aδ- and B-fiber activation) is largely not achievable with clinically tolerable current levels defined by the researchers [81], though not necessarily proving that noninvasive stimulation does not activate human vagal fibers with any certainty.
In this study, we showed that acute traumatic and mental stressors are associated with increased PACAP concentrations in the peripheral blood in traumatized individuals both with and without PTSD. PACAP appears to be a modifiable biochemical biomarker, and its temporal changes may predict tcVNS treatment effect to acute stress or neuropsychiatric disorders showing significant PACAP dysregulation. Moreover, longitudinal monitoring of PACAP may potentially be used to follow personalized, adaptive dosing strategies for larger trials, or to identify respondent and non-respondent patients to potential treatments. Future studies should investigate sex differences in PACAP concentrations with acute and longitudinal tcVNS treatment using larger sample sizes.
CRediT authorship contribution statement
Nil Z. Gurel: Conceptualization, Methodology, Data curation, Formal analysis, Visualization, Writing - original draft, Writing - review & editing. Yunshen Jiao: Conceptualization, Methodology, Data curation, Formal analysis, Visualization, Writing - original draft, Writing - review & editing. Matthew T. Wittbrodt: Methodology, Formal analysis, Validation, Writing - review & editing. Yi-An Ko: Methodology, Validation, Writing - review & editing. Allison Hankus: Resources, Data curation, Writing - review & editing. Emily G. Driggers: Resources, Data curation, Writing - review & editing. Stacy L. Ladd: Resources, Data curation, Writing - review & editing. Lucy Shallenberger: Project administration, Resources, Writing - review & editing. Nancy Murrah: Project administration, Resources, Writing - review & editing. Minxuan Huang: Methodology, Formal analysis, Writing - review & editing. Ammer Haffar: Resources, Writing - review & editing. Mhmtjamil Alkhalaf: Resources, Writing - review & editing. Oleksiy Levantsevych: Resources, Writing - review & editing. Jonathon A. Nye: Project administration, Resources, Writing - review & editing. Viola Vaccarino: Funding acquisition, Resources, Supervision, Writing - review & editing. Amit J. Shah: Funding acquisition, Resources, Supervision, Writing - review & editing. Omer T. Inan: Funding acquisition, Resources, Supervision, Writing - review & editing. J. Douglas Bremner: Funding acquisition, Resources, Supervision, Writing - review & editing. Bradley D. Pearce: Funding acquisition, Investigation, Supervision, Resources, Writing - review & editing.
Acknowledgments
This work was supported by the Defense Advanced Research Projects Agency, Arlington, VA, under Cooperative Agreement N66001-16-2-4054. Dr. Shah is sponsored by the National Institutes of Health, Award K23 HL127251. We would like to acknowledge and thank Margie Jones, CNMT, and Steven Rhodes, RN, for their assistance with clinical research.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cpnec.2020.100012.
Contributor Information
Nil Z. Gurel, Email: nil@gatech.edu.
Bradley D. Pearce, Email: bpearce@emory.edu.
Disclosures
JDB is currently conducting research on noninvasive vagus nerve stimulation with applications to posttraumatic stress disorder with research support from an investigator-initiated research contract with ElectroCore LLC and a Distinguished Investigator Award from the Brain and Behavior Research Foundation (BBRF)/National Alliance for Research on Schizophrenia and Affective Disorders (NARSAD). There are no other relevant royalty, advisory board, consulting, patents, or stock ownership to disclose.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Aaronson S.T., Sears P., Ruvuna F., Bunker M., Conway C.R. A 5-year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am. J. Psychiatr. 2017;174:640–648. doi: 10.1176/appi.ajp.2017.16010034. [DOI] [PubMed] [Google Scholar]
- 2.Badran B.W., Dowdle L.T., Mithoefer O.J., LaBate N.T., Coatsworth J., Brown J.C., DeVries W.H., Austelle C.W., McTeague L.M., George M.S. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: a concurrent taVNS/fMRI study and review. Brain Stimul. 2018;11:492–500. doi: 10.1016/j.brs.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badran B.W., Mithoefer O.J., Summer C.E., LaBate N.T., Glusman C.E., Badran A.W., DeVries W.H., Summers P.M., Austelle C.W., McTeague L.M., Borckardt J.J., George M.S. Short trains of transcutaneous auricular vagus nerve stimulation (taVNS) have parameter-specific effects on heart rate. Brain Stimul. 2018;11:699–708. doi: 10.1016/j.brs.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballenger J.C., Davidson J.R.T., Lecrubier Y., Nutt D.J., Foa E.B., Kessler R.C., McFarlane A.C. Consensus statement on posttraumatic stress disorder from the international consensus group on depression and anxiety. J. Clin. Psychiatr. 2000;61:60–66. [PubMed] [Google Scholar]
- 5.Ben-Menachem E., Revesz D., Simon B.J., Silberstein S. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur. J. Neurol. 2015;22:1260–1268. doi: 10.1111/ene.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. The development of a clinician-administered PTSD scale. J. Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 7.Bourla A., Mouchabac S., El Hage W., Ferreri F.J.E. 2018. E-PTSD: an Overview on How New Technologies Can Improve Prediction and Assessment of Posttraumatic Stress Disorder (PTSD) 9, 1424448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bremner J.D., Gurel N.Z., Jiao Y., Wittbrodt M.T., Levantsevych O.M., Huang M., Jung H., Shandhi M.H., Beckwith J., Herring I., Rapaport M.H., Murrah N., Driggers E., Ko Y.-A., Alkhalaf M.L., Soudan M., Song J., Ku B.S., Shallenberger L., Hankus A.N., Nye J.A., Park J., Vaccarino V., Shah A.J., Inan O.T., Pearce B.D. Transcutaneous vagal nerve stimulation blocks stress-induced activation of interleukin-6 and interferon-γ in posttraumatic stress disorder: a double-blind, randomized, sham-controlled trial. Brain, Behav. Immunity - Health. 2020:100138. doi: 10.1016/j.bbih.2020.100138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bremner J.D., Narayan M., Staib L.H., Southwick S.M., McGlashan T., Charney D.S. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am. J. Psychiatr. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bremner J.D., Pearce B. Wiley-Blackwell; Hoboken, New Jersey: 2016. Neurotransmitter, Neurohormonal, and Neuropeptidal Function in Stress and PTSD, Posttraumatic Stress Disorder: from Neurobiology to Treatment; pp. 181–232. [Google Scholar]
- 11.Bremner J.D., Rapaport M.H. Vagus nerve stimulation: back to the future. Am. J. Psychiatr. 2017;124:609–610. doi: 10.1176/appi.ajp.2017.17040422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bremner J.D., Staib L.H., Kaloupek D., Southwick S.M., Soufer R., Charney D.S. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol. Psychiatr. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bremner J.D., Vermetten E., Mazure C.M. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the Early Trauma Inventory. Depress. Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 14.Bremner J.D., Vythilingam M., Vermetten E., Southwick S.M., McGlashan T., Nazeer A., Khan S., Vaccarino L.V., Soufer R., Garg P.K., Ng C.K., Staib L.H., Duncan J.S., Charney D.S. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am. J. Psychiatr. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- 15.Bretherton B., Atkinson L., Murray A., Clancy J., Deuchars S., Deuchars J. Effects of transcutaneous vagus nerve stimulation in individuals aged 55 years or above: potential benefits of daily stimulation. Aging (Albany NY) 2019;11:4836–4857. doi: 10.18632/aging.102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Britton J.C., Phan K.L., Taylor S.F., Fig L.M., Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol. Psychiatr. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 17.Brock C., Brock B., Aziz Q., Moller H.J., Pfeiffer Jensen M., Drewes A.M., Farmer A.D. Transcutaneous cervical vagal nerve stimulation modulates cardiac vagal tone and tumor necrosis factor-alpha. Neuro Gastroenterol. Motil. 2017;29 doi: 10.1111/nmo.12999. [DOI] [PubMed] [Google Scholar]
- 18.Burger A.M., Van Diest I., van der Does W., Hysaj M., Thayer J.F., Brosschot J.F., Verkuil B. Transcutaneous vagus nerve stimulation and extinction of prepared fear: a conceptual non-replication. Sci. Rep. 2018;8:11471. doi: 10.1038/s41598-018-29561-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burger A.M., Verkuil B., Fenlon H., Thijs L., Cools L., Miller H.C., Vervliet B., Van Diest I. Mixed evidence for the potential of non-invasive transcutaneous vagal nerve stimulation to improve the extinction and retention of fear. Behav. Res. Ther. 2017;97:64–74. doi: 10.1016/j.brat.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Burger A.M., Verkuil B., Van Diest I., Van der Does W., Thayer J.F., Brosschot J.F. The effects of transcutaneous vagus nerve stimulation on conditioned fear extinction in humans. Neurobiol. Learn. Mem. 2016;132:49–56. doi: 10.1016/j.nlm.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Cagampang F.R.A., Piggins H.D., Sheward W.J., Harmar A.J., Coen C.W.J.B.r. Circadian changes in PACAP type 1 (PAC1) receptor mRNA in the rat suprachiasmatic and supraoptic nuclei. 1998;813:218–222. doi: 10.1016/s0006-8993(98)01044-0. [DOI] [PubMed] [Google Scholar]
- 22.Cahill S.P., Foa E.B., Hembree E.A., Marshall R.D., Nacash N. Dissemination of exposure therapy in the treatment of posttraumatic stress disorder. J. Trauma Stress. 2006;19:597–610. doi: 10.1002/jts.20173. [DOI] [PubMed] [Google Scholar]
- 23.Cardoso J.C.R., Garcia M.G., Power D.M. Tracing the origins of the pituitary adenylate-cyclase activating polypeptide (PACAP) Front. Neurosci. 2020;14 doi: 10.3389/fnins.2020.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S.P., Ay I., de Morais A.L., Qin T., Zheng Y., Sadeghian H., Oka F., Simon B., Eikermann-Haerter K., Ayata C. Vagus nerve stimulation inhibits cortical spreading depression. Pain. 2016;157:797–805. doi: 10.1097/j.pain.0000000000000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clancy J.A., Mary D.A., Witte K.K., Greenwood J.P., Deuchars S.A., Deuchars J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 2014;7:871–877. doi: 10.1016/j.brs.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 26.Clark K.B., Naritoku D.K., Smith D.C., Browning R.A., Jensen R.A. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat. Neurosci. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 27.Davis L., Hamner M., Bremner J.D. Wiley-Blackwell; Hoboken, NJ: 2016. Pharmacotherapy for PTSD: Effects on PTSD Symptoms and the Brain. Posttraumatic Stress Disorder: from Neurobiology to Treatment; pp. 389–412. [Google Scholar]
- 28.Davis M., Walker D.L., Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex: possible relevance to PTSD a. Ann. N. Y. Acad. Sci. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- 29.Delgado M., Abad C., Martinez C., Juarranz M.G., Leceta J., Ganea D., Gomariz R.P. PACAP Immunity Inflammation. 2003;992:141–157. doi: 10.1111/j.1749-6632.2003.tb03145.x. [DOI] [PubMed] [Google Scholar]
- 30.Dias B.G., Ressler K.J. PACAP and the PAC1 receptor in post-traumatic stress disorder. Neuropsychopharmacology. 2013;38:245–246. doi: 10.1038/npp.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engineer C.T., Engineer N.D., Riley J.R., Seale J.D., Kilgard M.P. Pairing speech sounds with vagus nerve stimulation drives stimulus-specific cortical plasticity. Brain Stimul. 2015;8:637–644. doi: 10.1016/j.brs.2015.01.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engineer N.D., Riley J.R., Seale J.D., Vrana W.A., Shetake J.A. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engineer N.D., Riley J.R., Seale J.D., Vrana W.A., Shetake J.A., Sudanagunta S.P., Borland M.S., Kilgard M.P. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ergang P., Vodička M., Soták M., Klusoňová P., Behuliak M., Řeháková L., Zach P., Pácha J.J.P. vol. 53. 2015. pp. 49–59. (Differential Impact of Stress on Hypothalamic–Pituitary–Adrenal axis: Gene Expression Changes in Lewis and Fisher Rats). [DOI] [PubMed] [Google Scholar]
- 35.First M.B., Gibbon M. In: Comprehensive Handbook of Psychological Assessment. Segal M.J.H.D.L., editor. John Wiley & Sons Inc.; Hoboken, NJ, US: 2004. The structured clinical interview for DSM-IV Axis I disorders (SCID-I) and the structured clinical interview for DSM-IV Axis II disorders (SCID-II) pp. 134–143. [Google Scholar]
- 36.Foa E., Hembree E., Rothbaum B.O. Oxford University Press; 2007. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences Therapist Guide. [Google Scholar]
- 37.Foa E.B. Prolonged exposure therapy: past, present, and future. Depress. Anxiety. 2011;28:1043–1047. doi: 10.1002/da.20907. [DOI] [PubMed] [Google Scholar]
- 38.Frangos E., Ellrich J., Komisaruk B.R. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 2015;8:624–636. doi: 10.1016/j.brs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frangos E., Komisaruk B.R. Access to vagal projections via cutaneous electrical stimulation of the neck: fMRI evidence in healthy humans. Brain Stimul. 2017;10:19–27. doi: 10.1016/j.brs.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Fukuhara C., Suzuki N., Matsumoto Y., Nakayama Y., Aoki K., Tsujimoto G., Inouye S.-I.T., Masuo Y.J.N.l. Day-night variation of pituitary adenylate cyclase-activating polypeptide (PACAP) level in the rat suprachiasmatic nucleus. 1997;229:49–52. doi: 10.1016/s0304-3940(97)00415-1. [DOI] [PubMed] [Google Scholar]
- 41.Garcia R.G., Lin R.L., Lee J., Kim J., Barbieri R., Sclocco R., Wasan A.D., Edwards R.R., Rosen B.R., Hadjikhani N., Napadow V. Modulation of brainstem activity and connectivity by respiratory-gated auricular vagal afferent nerve stimulation in migraine patients. Pain. 2017;158:1461–1472. doi: 10.1097/j.pain.0000000000000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gazi A.H., Gurel N.Z., Richardson K.L.S., Wittbrodt M.T., Shah A.J., Vaccarino V., Bremner J.D., Inan O.T. Digital cardiovascular biomarker responses to transcutaneous cervical vagus nerve stimulation: state-space modeling, prediction, and simulation. JMIR Mhealth Uhealth. 2020;8(9) doi: 10.2196/20488. PMID: 32960179. PMCID: 7539162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.George M.S., Rush A.J., Marangell L.B., Sackeim H.A., Brannan S.K., Davis S.M., Howland R., Kling M.A., Moreno F., Rittberg B., Dunner D. A one-year comparison of vagus nerve stimulation with treatment as usual for treatment-resistant depression. Biol. Psychiatr. 2005;58:364–373. doi: 10.1016/j.biopsych.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 44.George R., Salinsky M., Kuzniecky R., Rosenfeld W., Bergen D., Tarver W.B., Wernicke J.F. Vagus nerve stimulation for treatment of partial seizures: 3. Long-term follow-up on first 67 patients exiting a controlled study. First Int. Vagus Nerve Stimul. Study Group. Epilepsia. 1994;35:637–643. doi: 10.1111/j.1528-1157.1994.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 45.Giordano F., Zicca A., Barba C., Guerrini R., Genitori L.J.E. vol. 58. 2017. pp. 85–90. (Vagus Nerve Stimulation: Surgical Technique of Implantation and Revision and Related Morbidity). [DOI] [PubMed] [Google Scholar]
- 46.Gottlieb B.H., Bergen A.E. Social support concepts and measures. J. Psychosom. Res. 2010;69:511–520. doi: 10.1016/j.jpsychores.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Gray S.L., Cline D.L. Elsevier; 2019. PACAP: Regulator of the Stress Response, Stress: Physiology, Biochemistry, and Pathology; pp. 279–291. [Google Scholar]
- 48.Gurel N.Z., Gazi A.H., Scott K.L., Wittbrodt M.T., Shah A.J., Vaccarino V., Bremner J.D., Inan O.T. Timing considerations for noninvasive vagal nerve stimulation in clinical studies. AMIA Annu Symp Proc. 2020;2019:1061–1070. [PMC free article] [PubMed] [Google Scholar]
- 49.Gurel N.Z., Huang M., Wittbrodt M.T., Jung H., Ladd S.L., Shandhi M.M.H., Ko Y.-A., Shallenberger L., Nye J.A., Pearce B., Vaccarino V., Shah A.J., Bremner J.D., Inan O.T. Quantifying acute physiological biomarkers of transcutaneous cervical vagal nerve stimulation in the context of psychological stress. Brain Stimul.: Basic, Transl. Clinic. Res. Neuromodulation. 2020;13:47–59. doi: 10.1016/j.brs.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gurel N.Z., Shandhi M.M.H., Bremner J.D., Vaccarino V., Ladd S.L., Shallenberger L., Shah A., Inan O.T. IEEE 15th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Las Vegas, NV. 2018. Toward closed-loop transcutaneous vagus nerve stimulation using peripheral cardiovascular physiological biomarkers: a proof-of-concept study; pp. 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gurel N.Z., Wittbrodt M.T., Jung H., Ladd S.L., Shah A.J., Vaccarino V., Bremner J.D., Inan O. Automatic detection of target engagement in transcutaneous cervical vagal nerve stimulation for traumatic stress triggers. IEEE J. Biomed. Health Informatics. 2020;24(7):1917–1925. doi: 10.1109/JBHI.2020.2981116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gurel N.Z., Wittbrodt M.T., Jung H., Ladd S.L., Shah A.J., Vaccarino V., Bremner J.D., Inan O.T. Automatic detection of target engagement in transcutaneous cervical vagal nerve stimulation for traumatic stress triggers. IEEE J. Biomed. Health Informatics. 2020;24:1917–1925. doi: 10.1109/JBHI.2020.2981116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamer H.M., Bauer S. Lessons learned from transcutaneous vagus nerve stimulation (tVNS) Epilepsy Res. 2019;153:83–84. doi: 10.1016/j.eplepsyres.2019.02.015. [DOI] [PubMed] [Google Scholar]
- 54.Handforth A., DeGiorgio C.M., Schachter S.C., Uthman B.M., Naritoku D.K., Tecoma E.S., Henry T.R., Collins S.D., Vaughn B.V., Gilmartin R.C., Labar D.R., Morris G.L., 3rd, Salinsky M.C., Osorio I., Ristanovic R.K., Labiner D.M., Jones J.C., Murphy J.V., Ney G.C., Wheless J.W. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/wnl.51.1.48. [DOI] [PubMed] [Google Scholar]
- 55.Hashimoto H., Shintani N., Tanida M., Hayata A., Hashimoto R., Baba A.J.C.p.d. Vol. 17. 2011. PACAP is implicated in the stress axes; pp. 985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hays S.A., Rennaker R.L., Kilgard M.P. Targeting plasticity with vagus nerve stimulation to treat neurological disease. Prog. Brain Res. 2013;207:275–299. doi: 10.1016/B978-0-444-63327-9.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hembree E.A., Foa E.B., Dorfan N.M., Street G.P., Kowalski J., Tu X. Do patients drop out prematurely from exposure therapy for PTSD? J. Trauma Stress. 2003;16:555–562. doi: 10.1023/B:JOTS.0000004078.93012.7d. [DOI] [PubMed] [Google Scholar]
- 58.Johnson G.C., Parsons R., May V., Hammack S.E. The role of pituitary adenylate cyclase-activating polypeptide (PACAP) signaling in the hippocampal dentate gyrus. Front. Cell. Neurosci. 2020;14 doi: 10.3389/fncel.2020.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jovanovic T., Norrholm S.D., Blanding N.Q., Phifer J.E., Weiss T., Davis M., Duncan E., Bradley B., Ressler K. Fear potentiation is associated with hypothalamic–pituitary–adrenal axis function in PTSD. Psychoneuroendocrinology. 2010;35:846–857. doi: 10.1016/j.psyneuen.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jovanovic T., Norrholm S.D., Davis J., Mercer K.B., Almli L., Nelson A., Cross D., Smith A., Ressler K.J., Bradley B. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Mol. Psychiatr. 2013;18:742–743. doi: 10.1038/mp.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kallo I., Kalamatianos T., Piggins H., Coen C.W. vol. 16. 2004. pp. 758–766. (Ageing and the Diurnal Expression of mRNAs for Vasoactive Intestinal Peptide and for the VPAC2 and PAC1 Receptors in the Suprachiasmatic Nucleus of Male Rats). [DOI] [PubMed] [Google Scholar]
- 62.Kamkwalala A., Norrholm S.D., Poole J.M., Brown A., Donley S., Duncan E., Bradley B., Ressler K.J., Jovanovic T. Dark-enhanced startle responses and heart rate variability in a traumatized civilian sample: putative sex-specific correlates of posttraumatic stress disorder. Psychosom. Med. 2012;74:153. doi: 10.1097/PSY.0b013e318240803a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kendall P.C., Hollon S.D., Beck A.T., Hammen C.L., Ingram R.E. Issues and recommendations regarding use of the Beck depression inventory. Cognit. Ther. Res. 1987;11:289–299. [Google Scholar]
- 64.Kessler R.C., Aguilar-Gaxiola S., Alonso J., Benjet C., Bromet E.J., Cardoso G., Degenhardt L., de Girolamo G., Dinolova R.V., Ferry F.J. vol. 8. 2017. p. 1353383. (Trauma and PTSD in the WHO World Mental Health Surveys). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kessler R.C., Sonnega A., Bromet E., Hughes M., Nelson C.B. vol. 52. 1995. pp. 1048–1060. (Posttraumatic Stress Disorder in the National Comorbidity Survey). [DOI] [PubMed] [Google Scholar]
- 66.Koves K. Springer; 2016. Distribution of PACAP in the Mammalian Nervous System, Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; pp. 179–203. [Google Scholar]
- 67.Köves K., Szabó E., Kántor O., Heinzlmann A., Szabó F., Csáki Á. Current state of understanding of the role of PACAP in the hypothalamo-hypophyseal gonadotropin functions of mammals. Front. Endocrinol. 2020;11 doi: 10.3389/fendo.2020.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lamb D.G., Porges E.C., Lewis G.F., Williamson J.B. Non-invasive vagal nerve stimulation effects on hyperarousal and autonomic state in patients with posttraumatic stress disorder and history of mild traumatic brain injury: preliminary evidence. Front. Med. 2017;4:124. doi: 10.3389/fmed.2017.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lanius R.A., Williamson P.C., Densmore M., Boksman K., Neufeld R.W., Gati J.S., Menon R.S. The nature of traumatic memories: a 4-T fMRI functional connectivity analysis. Am. J. Psychiatr. 2004;161:36–44. doi: 10.1176/appi.ajp.161.1.36. [DOI] [PubMed] [Google Scholar]
- 71.Lerman I., Hauger R., Sorkin L., Proudfoot J., Davis B., Huang A., Lam K., Simon B., Baker D.G. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: a randomized, blinded, healthy control pilot trial. Neuromodulation. 2016;19:283–290. doi: 10.1111/ner.12398. [DOI] [PubMed] [Google Scholar]
- 72.Liberzon I., Britton J.C., Luan Phan K. Neural correlates of traumatic recall in posttraumatic stress disorder. Stress. 2003;6:151–156. doi: 10.1080/1025389031000136242. [DOI] [PubMed] [Google Scholar]
- 74.Maier W., Buller R., Philipp M., Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J. Affect. Disord. 1988;14:61–68. doi: 10.1016/0165-0327(88)90072-9. [DOI] [PubMed] [Google Scholar]
- 75.Masuo Y., Matsumoto Y., Tokito F., Tsuda M., Fujino M.J.B.r. Effects of vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) on the spontaneous release of acetylcholine from the rat hippocampus by brain microdialysis. 1993;611:207–215. doi: 10.1016/0006-8993(93)90504-g. [DOI] [PubMed] [Google Scholar]
- 76.Mauskop A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia. 2005;25:82–86. doi: 10.1111/j.1468-2982.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- 77.Medicine I.o. Treatment for posttraumatic stress disorder in military and veteran populations: final assessment. Mil. Med. 2014;179:1401–1403. doi: 10.7205/MILMED-D-14-00418. [DOI] [PubMed] [Google Scholar]
- 78.Morgan C., Grillon C., Lubin H. Exaggerated acoustic startle reflex in Gulf War veterans with posttraumatic stress disorder. Am. J. Psychiatr. 1996;153:64–68. doi: 10.1176/ajp.153.1.64. [DOI] [PubMed] [Google Scholar]
- 79.Morgan C.A., Grillon C., Lubin H., Southwick S.M. Startle reflex abnormalities in women with sexual assault-related posttraumatic stress disorder. Am. J. Psychiatr. 1997;154:1076–1080. doi: 10.1176/ajp.154.8.1076. [DOI] [PubMed] [Google Scholar]
- 80.Mourdoukoutas A.P., Truong D.Q., Adair D.K., Simon B.J., Bikson M. High-resolution multi-scale computational model for non-invasive cervical vagus nerve stimulation. Neuromodulation: Technol. Neural Interface. 2018;21:261–268. doi: 10.1111/ner.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nicolai E.N., Settell M.L., Knudsen B.E., McConico A.L., Gosink B.A., Trevathan J.K., Baumgart I.W., Ross E.K., Pelot N.A., Grill W.M. Sources of off-target effects of vagus nerve stimulation using the helical clinical lead in domestic pigs. J. Neural. Eng. 2020;17(4) doi: 10.1088/1741-2552/ab9db8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noble L.J., Gonzalez I., Meruva V., Callahan K.A., Belfort B.D., Ramanathan K., Meyers E., Kilgard M.P., Rennaker R.L., McIntyre C.K.J.T.p. Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms in rats. 2017;7 doi: 10.1038/tp.2017.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noble L.J., Gonzalez I.J., Meruva V.B., Callahan K.A., Belfort B.D., Ramanathan K.R., Meyers E., Kilgard M.P., Rennaker R.L., McIntyre C.K. Effects of vagus nerve stimulation on extinction of conditioned fear and post-traumatic stress disorder symptoms in rats. Transl. Psychiatry. 2017;7:e1217. doi: 10.1038/tp.2017.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nonis R., D’Ostilio K., Schoenen J., Magis D. Evidence of activation of vagal afferents by non-invasive vagus nerve stimulation: an electrophysiological study in healthy volunteers. Cephalalgia. 2017;37:1285–1293. doi: 10.1177/0333102417717470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Osuch E.A., Benson B., Geraci M., Podell D., Herscovitch P., McCann U.D., Post R.M. Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biol. Psychiatr. 2001;50:246–253. doi: 10.1016/s0006-3223(01)01107-6. [DOI] [PubMed] [Google Scholar]
- 86.Ozawa M., Aono M., Mizuta K., Moriga M., Okuma M. Central administration of PACAP stimulates gastric secretion mediated through the vagal pathway in anesthetized rats. Dig. Dis. Sci. 1997;42:2552–2559. doi: 10.1023/a:1018824931267. [DOI] [PubMed] [Google Scholar]
- 87.Pace T.W.W., Heim C.M. A short review on the psychoneuroimmunology of posttraumatic stress disorder: from risk factors to medical comorbidities. Brain Behav. Immun. 2011;25:6–13. doi: 10.1016/j.bbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 88.Palkovits M., Somogyvári-Vigh A., Arimura A. Concentrations of pituitary adenylate cyclase activating polypeptide (PACAP) in human brain nuclei. Brain Res. 1995;699:116–120. doi: 10.1016/0006-8993(95)00869-r. [DOI] [PubMed] [Google Scholar]
- 89.Pena D.F., Childs J.E., Willett S., Vital A., McIntyre C.K., Kroener S. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front. Behav. Neurosci. 2014;8:327. doi: 10.3389/fnbeh.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peña D.F., Childs J.E., Willett S., Vital A., McIntyre C.K., Kroener S. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front. Behav. Neurosci. 2014;8:327. doi: 10.3389/fnbeh.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Peña D.F., Engineer N.D., McIntyre C.K. Rapid remission of conditioned fear expression with extinction training paired with vagus nerve stimulation. Biol. Psychiatr. 2013;73:1071–1077. doi: 10.1016/j.biopsych.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pietrzak R.H., Goldstein R.B., Southwick S.M., Grant B.F. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: results from wave 2 of the national epidemiologic survey on alcohol and related conditions. J. Anxiety Disord. 2011;25:456–465. doi: 10.1016/j.janxdis.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piggins H.D., Stamp J.A., Burns J., Rusak B., Semba K. Distribution of pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in the hypothalamus and extended amygdala of the rat. J. Comp. Neurol. 1996;376:278–294. doi: 10.1002/(SICI)1096-9861(19961209)376:2<278::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 94.Pols M.A., Peeters P.H., Bueno-de-Mesquita H.B., Ocke M.C., Wentink C.A., Kemper H.C., Collette H.J. Validity and repeatability of a modified Baecke questionnaire on physical activity. Int. J. Epidemiol. 1995;24:381–388. doi: 10.1093/ije/24.2.381. [DOI] [PubMed] [Google Scholar]
- 95.Porter B.A., Khodaparast N., Fayyaz T., Cheung R.J., Ahmed S.S., Vrana W.A., Rennaker R.L., II Kilgard M.P. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cerebr. Cortex. 2011;22:2365–2374. doi: 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- 96.Premchand R.K., Sharma K., Mittal S., Monteiro R., Dixit S., Libbus I., DiCarlo L.A., Ardell J.L., Rector T.S., Amurthur B., KenKnight B.H., Anand I.S. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J. Card. Fail. 2014;20:808–816. doi: 10.1016/j.cardfail.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 97.Rauch S.L., van der Kolk B.A., Fisler R.E., Alpert N.M., Orr S.P., Savage C.R., Fischman A.J., Jenike M.A., Pitman R.K. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch. Gen. Psychiatr. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 98.Reglodi D., Tamas A., Jungling A., Vaczy A., Rivnyak A., Fulop B., Szabo E., Lubics A., Atlasz T. Protective effects of pituitary adenylate cyclase activating polypeptide against neurotoxic agents. Neurotoxicology. 2018;66:185–194. doi: 10.1016/j.neuro.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 99.Reimer M., Moller K., Sundler F., Hannibal J., Fahrenkrug J., Kanje M. Increased expression, axonal transport and release of pituitary adenylate cyclase-activating polypeptide in the cultured rat vagus nerve. Neuroscience. 1999;88:213–222. doi: 10.1016/s0306-4522(98)00240-1. [DOI] [PubMed] [Google Scholar]
- 100.Ressler K.J., Mercer K.B., Bradley B., Jovanovic T., Mahan A., Kerley K., Norrholm S.D., Kilaru V., Smith A.K., Myers A.J.J.N. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. 2011;470:492. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reuter U., McClure C., Liebler E., Pozo-Rosich P. Non-invasive neuromodulation for migraine and cluster headache: a systematic review of clinical trials. J. Neurol. Neurosurg. Psychiatry. 2019;90(7):796–804. doi: 10.1136/jnnp-2018-320113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rosell J., Colominas J., Riu P., Pallas-Areny R., Webster J.G. Skin impedance from 1 Hz to 1 MHz. IEEE (Inst. Electr. Electron. Eng.) Trans. Biomed. Eng. 1988;35:649–651. doi: 10.1109/10.4599. [DOI] [PubMed] [Google Scholar]
- 103.Ruggiero K.J., Del Ben K., Scotti J.R., Rabalais A.E. Psychometric properties of the PTSD checklist—civilian version. J. Trauma Stress. 2003;16:495–502. doi: 10.1023/A:1025714729117. [DOI] [PubMed] [Google Scholar]
- 104.Rush A.J., Sackeim H.A., Marangell L.B., George M.S., Brannan S.K., Davis S.M., Lavori P., Howland R., Kling M.A., Rittberg B., Carpenter L., Ninan P., Moreno F., Schwartz T., Conway C., Burke M., Barry J.J. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: a naturalistic study. Biol. Psychiatr. 2005;58:355–363. doi: 10.1016/j.biopsych.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 105.Sarszegi Z., Szabo D., Gaszner B., Konyi A., Reglodi D., Nemeth J., Lelesz B., Polgar B., Jungling A., Tamas A. Examination of pituitary adenylate cyclase-activating polypeptide (PACAP) as a potential biomarker in heart failure patients. J. Mol. Neurosci. 2019;68:368–376. doi: 10.1007/s12031-017-1025-7. [DOI] [PubMed] [Google Scholar]
- 106.Schnurr P.P., Friedman M.J., Engel C.C., Foa E.B., Shea M.T., Chow B.K., Resick P.A., Thurston V., Orsillo S.M., Haug R., Turner C., Bernardy N. Cognitive behavioral therapy for posttraumatic stress disorder in WomenA randomized controlled trial. J. Am. Med. Assoc. 2007;297:820–830. doi: 10.1001/jama.297.8.820. [DOI] [PubMed] [Google Scholar]
- 107.Southwick S.M., Krystal J.H., Bremner J.D., Morgan C.A., 3rd, Nicolaou A.L., Nagy L.M., Johnson D.R., Heninger G.R., Charney D.S. Noradrenergic and serotonergic function in posttraumatic stress disorder. Arch. Gen. Psychiatr. 1997;54:749–758. doi: 10.1001/archpsyc.1997.01830200083012. [DOI] [PubMed] [Google Scholar]
- 108.Southwick S.M., Krystal J.H., Morgan C.A., Johnson D., Nagy L.M., Nicolaou A., Heninger G.R., Charney D.S. Abnormal noradrenergic function in posttraumatic stress disorder. Arch. Gen. Psychiatr. 1993;50:266–274. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- 109.Starr E.R., Margiotta J.F. Pituitary Adenylate Cyclase Activating Polypeptide—PACAP. Springer; 2016. PACAP modulates distinct neuronal components to induce cell-specific plasticity at central and autonomic synapses; pp. 83–107. [Google Scholar]
- 111.Va B.J.F., Health A.T.S.o.M. vol. 10. 2013. p. 22. (Posttraumatic Stress Disorder in the National Comorbidity Survey). [Google Scholar]
- 112.Warren C.M., Tona K.D., Ouwerkerk L., van Paridon J., Poletiek F., van Steenbergen H., Bosch J.A., Nieuwenhuis S. The neuromodulatory and hormonal effects of transcutaneous vagus nerve stimulation as evidenced by salivary alpha amylase, salivary cortisol, pupil diameter, and the P3 event-related potential. Brain Stimul. 2019;12:635–642. doi: 10.1016/j.brs.2018.12.224. [DOI] [PubMed] [Google Scholar]
- 113.Weathers F.W., Bovin M.J., Lee D.J., Sloan D.M., Schnurr P.P., Kaloupek D.G., Keane T.M., Marx B.P. The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol. Assess. 2018;30:383–395. doi: 10.1037/pas0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wittbrodt M., Vaccarino V., Shah A., Mayer E., Bremner J. American Psychological Association; 2020. Psychometric Properties of the Adulthood Trauma Inventory. Health Psychology: Official Journal of the Division of Health Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wittbrodt M.T., Gurel N.Z., Nye J.A., Ladd S., Shandhi M.M.H., Huang M., Shah A.J., Pearce B.D., Alam Z.S., Rapaport M.H., Murrah N., Ko Y.-A., Haffer A.A., Shallenberger L.H., Vaccarino V., Inan O.T., Bremner J.D. Non-invasive vagal nerve stimulation decreases brain activity during trauma scripts. Brain Stimul. 2020;13:1333–1348. doi: 10.1016/j.brs.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yakunina N., Kim S.S., Nam E.C. Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation. 2017;20:290–300. doi: 10.1111/ner.12541. [DOI] [PubMed] [Google Scholar]
- 117.Yakunina N., Kim S.S., Nam E.C. BOLD fMRI effects of transcutaneous vagus nerve stimulation in patients with chronic tinnitus. PloS One. 2018;13 doi: 10.1371/journal.pone.0207281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yehuda R., Hoge C.W., McFarlane A.C., Vermetten E., Lanius R.A., Nievergelt C.M., Hobfoll S.E., Koenen K.C., Neylan T.C., Hyman S.E. Post-traumatic stress disorder. Nat. Rev. Dis. Prim. 2015;1:15057. doi: 10.1038/nrdp.2015.57. [DOI] [PubMed] [Google Scholar]
- 119.Zen A.L., Whooley M.A., Zhao S., Cohen B.E. Post-traumatic stress disorder is associated with poor health behaviors: findings from the Heart and Soul Study. Health Psychol. 2012;31:194–201. doi: 10.1037/a0025989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.