Highlights
-
•
The risk of diphtheria remains high in Kon Tum given the low seroprevalence.
-
•
One third of community in Kon Tum have no protective antibodies to diphtheria.
-
•
The antibodies from previous childhood vaccination gradually wane over time.
-
•
A booster dose (5-7 years) is recommended for adolescents and adults in Vietnam.
Keywords: Diphtheria, toxoid, antibodies, seroprevalence, Kon Tum, Vietnam
Abstract
Background
Despite diphtheria immunization are to apply an effective primary immunization in childhood and to maintain immunity throughout life. Cases of diphtheria have been reported in Viet Nam in recent years. The aim of this study was to evaluate the seroprevalence of IgG antibodies to diphtheria toxoid among healthy person population in Kon Tum, Viet Nam.
Methods
Blood samples were obtained from 2225 healthy persons aged 2-98 years collected in 2019 and 2020. Samples were tested for diphtheria toxoid antibodies by commercial Anti-Diphtheria Toxoid IgG Enzyme-Linked Immunosorbent Assay (ELISA).
Results
An antibody level of <0.01 IU/mL (susceptibility) was found in 802 (36.0%) of the 2225 subjects, 136 (6.1%) had antibody levels of 0.01–0.099 IU/mL (basic protection), and 1287 (57.8%) had antibody levels ≥0.1 IU/mL (full protection). The full protection level increased significantly in persons aged above 60 years with antibody levels of 70.6%. No significant difference in seroprotection prevalence was found according to gender, ethnicity, residence, education and occupation. The results also demonstrated that people with vaccination against diphtheria during past 10 years were found to have a high immunity (83.8%) compared to 54.8% (OR: 4.7; 95%CI: 3.8-6.5) and 60.7% (OR: 3.8; 95%CI: 2.6-5.7) in persons with no and unknown vaccination (p <0.0001).
Conclusions
The level of anti-diphtheria toxoid antibodies among children and adults in Kon Tum was low. The high risk of diphtheria outbreaks may occur among individuals lacking basic immunity against diphtheria.
1. Introduction
Diphtheria is a serious infection disease caused by toxigenic bacteria of Corynebacterium diphtheriae affecting to children and adults in the world. Mass vaccination programs have been highly successful in reducing drastically in morbidity and mortality caused by diphtheria. World Health Organization (WHO) reported a total of 4490 cases of diphtheria worldwide in 2013, mainly in the developing countries (Centers for Disease Control and Prevention, 2012). Therefore, diphtheria is still a great public health concerns in many developing countries. During past years, the diphtheria outbreaks have been reported in Thailand (Wanlapakorn et al., 2012), Lao PDR (Nanthavong et al., 2015) and Vietnam (Kitamura et al., 2020; Murakami et al., 2008). So, it is concern about outbreaks of these diseases especially in developing countries. With mass childhood vaccination programs, children are not a high risk group threatened by diphtheria and recent documented cases are more common in the adult population (Aue et al., 2003).
In Vietnam, WHO stated that a total of 53 cases of diphtheria reported in Vietnam only in 2019, compared with 13 cases in 2018, 21 cases in 2017 and 13 cases in 2016 (World Health Organization, 2020). Kon Tum is one of the provinces of the Central Highlands where the diphtheria outbreaks occurred in 2020 with 50 confirmed cases, 1 death. All cases documenting in both children and adults, highest rate of incidence was group aged 6-15 years old (48.0%), followed by 16-25 years old (24.0%); 26-45 years old (14.0%) and 0-5 years old (12.0%) (unpublished paper).
Vaccination against diphtheria has contributed to a dramatic decrease in morbidity and mortality due to this disease. For diphtheria vaccines, guidelines of the current Expanded Program on Immunization (EPI) in Vietnam, a primary series of 3 doses with a booster dose at 18 to 24 age months is recommended and then every 10 years, a Tetanus diphtheria (Td) vaccine booster is recommended for adult to maintain life-long protection. It is generally agreed that when more than 25% percent of a diphtheria susceptible population, there is a high risk for epidemic diphtheria occurring in that community (Plans-Rubió et al., 2012). In order to provide a data on herd immunity and to prevent the diphtheria outbreaks in future, it is necessary to conduct an investigation on the immunity levels of the general population and to identify and vaccinate the unprotected groups (Damasco et al., 2005). The aim of this study was to evaluate the immunity to diphtheria in population in Kon Tum province of the Central Highlands, Vietnam.
2. Materials and methods
2.1. Study design and sampling
In this cross-sectional study during in the period from December 2019 to November 2020. A multi-stage cluster sampling was applied to randomly select 30 clusters. In the first stage, the number of clusters were identified by wards/communes of districts according to the population size and using sampling proportional to the probability by size (PPS). In the second step, the simple random sampling to select seventy-two households in each selected cluster chosen was performed. Finally, one person within each household was randomly selected to participate in the study from all eligible persons in the household using a random number generator.
2.2. Sample size calculation
The sample size is calculated using single proportion sample size formula
Where n is the sample size under simple random sample assumption; Zα/2 is the statistic corresponding to level of confidence, assumed to be 1.96 (when α = 0.05); e is precision (3%), p is the expected seroprevalence of antibodies against diphtheria among the target populations (50%), d: design effect of 2. The minimum sample size was 2135. Subjects were divided into age groups followed as 0–5 years, 6-10 years, 11–20 years, 21–30 years, 31–40 years, 41–50 years, 51– 60 years and >60 years old. Serum samples were frozen and stored at minus 20°C during 72 hours prior to transportation to the laboratory of Tay Nguyen Institute of Hygiene and Epidemiology and stored at minus 80°C until antibody testing was performed.
2.3. Enzyme-linked assay
IgG antibody levels against diphtheria were determined using commercial anti-diphtheria toxoid enzyme-linked immunosorbent assay (ELISA) (Euroimmun, Germany). Anti-diphtheria toxoid antibody levels below 0.01 IU/ml were considered susceptibility, levels of 0.01–0.099 IU/ml were considered to basic protection and levels above 0.1 IU/ml were considered to full protection against diphtheria (World Health Organization, 2009).
IgG anti-diphtheria toxoid antibody level was expressed as the geometric mean concentrations (GMC) with 95% confidence interval (95% CI). For the calculation of GMC, the undetectable values were excluded.
2.4. Ethical approval
This study was approved by the Ethical Committee of Tay Nguyen Institute of Hygiene and Epidemiology, Vietnam (Approval number: 546/CN-VTN, dated 4th October 2019). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All participants and their caregivers had provided written informed consent and assent, as appropriate, prior to study enrollment.
2.5. Statistical Analysis
The data are expressed as mean ± standard deviation (SD). Prevalence rates, geometric mean concentrations (GMCs) and 95% Confidence intervals (CIs) were calculated. The comparisons of participant characteristics and seroprevalence were performed using Chi-square, while differences in GMCs of diphtheria toxoid antibodies between groups were tested for statistical significance by Student's t-test. Mean levels of IgG antibodies between the age groups were examined by two-way ANOVA. A test probability of 5% and two-sided interval was considered statistically significant. Backward selection was used to identify risk factors independently associated with antibody levels below 0.01 IU/ml. Determinants (vaccination status, demographic and socio-economic characteristics) of seroprotection against diphtheria were analysed with multiple logistic regression. Statistics were performed using SPSS version 20 (IBM, USA).
3. Results
In total, 2225 sera (733 males and 1492 females) were included in the study. The age of the study population ranged from 2 to 98 years, with mean ages of 30.9 (± 19.1) (Table 1).
Table 1.
Studied population according to age and sex
| Age group (year) | Male | Female | Total | Mean age (years) | SD of mean age (years) |
|---|---|---|---|---|---|
| 0-5 | 37 | 45 | 82 | 3.5 | 1.2 |
| 6-10 | 125 | 108 | 233 | 8.3 | 1.4 |
| 11-20 | 208 | 249 | 457 | 14.4 | 2.8 |
| 21-30 | 96 | 353 | 449 | 25.6 | 2.6 |
| 31-40 | 96 | 282 | 378 | 35.2 | 2.8 |
| 41-50 | 54 | 185 | 239 | 45.2 | 2.9 |
| 51-60 | 54 | 136 | 190 | 55.2 | 2.9 |
| >60 | 63 | 134 | 197 | 70.1 | 7.2 |
| Total | 733 | 1492 | 2225 | 30.9 | 19.1 |
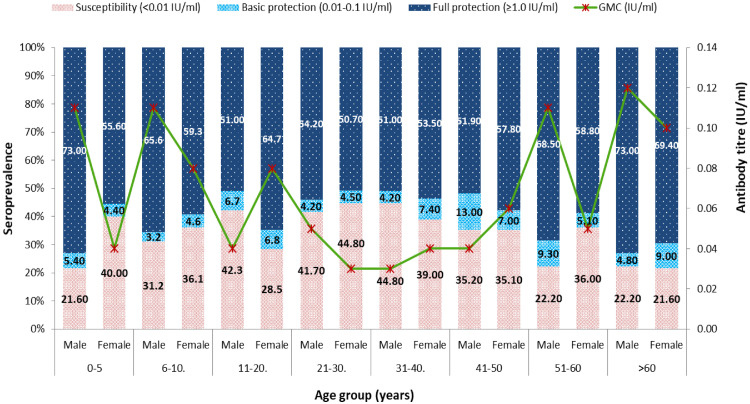
In the studied population, 57.8%, 6.1%, and 36.0% of persons had full, basic, and no diphtheria protection, respectively. No statistically difference was found between male and female in diphtheria antibodies level of full, basic and no diphtheria protection and geometric mean concentration (GMC) (Table 2). The percentage of diphtheria protected individuals (≥0.01 IU/mL) in various age groups is summarized in Table 3 and Figure 1. Overall, 63.9% (95%CI 62.7–66.6) of the study population had antibody levels of ≥0.01 IU/mL against diphtheria toxoid. Proportions of protected males (64.2%, 95%CI: 60.8–67.7) were similar to those of females (63.8%, 95%CI: 62.4–67.2) (p >0.005). The highest full protection rate was observed in the groups aged >60 years (70.6%, 95%CI: 63.6–76.8) and the lowest protection percentage was observed in the groups aged 21–30 years (51.4%, 95%CI: 46.6–56.0). The figure 1 showed that 63.4% (95%CI: 51.3–73.0) of full protection for the group aged 0–5 years decreased to 51.4% (95%CI: 46.6–56.0) for the group aged 21–30 years (p <0.05). The proportion of individuals protected against diphtheria increased gradually for individuals aged 30+ years. Thus, 52.9% (95%CI: 48.5–58.8) of individuals aged 31–40 years were regarded as full protected, compared with 56.5% (95%CI: 49.9–62.8), 61.1% (95%CI: 54.3–68.5) and 70.6% (95%CI: 63.6-76.8) for the groups aged 41–50 years; 51-60 years; and 60+ years, respectively (Table 3). The increases in the proportion of the protected individuals between the groups aged 40–50 and 51–60 years, and 60+ years, were statistically significant (p <0.05). Individuals with antibody levels of <0.01 IU/mL (unprotected) against diphtheria toxin comprised 31.7%, 33.5%, 34.8%, 44.1%, 40.5%, 35.1%, 32.1% and 21.8% of the groups aged 0–5, 6–10, 11–20, 21-30, 31-40, 41-50, 51-60, and 60+ years, respectively (Table 3).
Table 2.
Diphtheria immunity in population
| Sample |
Susceptibility <0.01 IU/ml |
Basic protection 0.01-0.1 IU/ml |
Full protection >0.1 IU/ml |
GMC (IU/ml) | 95%CI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | N | % | 95%CI | N | % | 95%CI | N (%) | % | 95%CI | |||
| Overall | 2225 | 802 | 36.0 | 34.0-38.1 | 136 | 6.1 | 5.1-7.2 | 1287 | 57.8 | 55.8-59.9 | 0.05 | 0.04-0.06 |
| Male | 733 | 263 | 35.9 | 32.6-39.7 | 43 | 5.9 | 4.3-7.8 | 427 | 58.3 | 54.3-61.6 | 0.05 | 0.05-0.07 |
| Female | 1492 | 539 | 36.1 | 33.5-38.5 | 93 | 6.2 | 5.0-7.6 | 860 | 57.6 | 55.2-60.3 | 0.05 | 0.04-0.06 |
Table 3.
Age-specific prevalence of diphtheria immunity
| Age groups (years) | Susceptibility (<0.01 IU/ml) |
Basic protection (0.01-0.1 IU/ml) |
Full protection (>0.1 IU/ml) |
P-Value+ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | N | % | 95%CI | N | % | 95%CI | N | % | 95%CI | ||
| 0-5 | 82 | 26 | 31.7 | 21.9-42.9 | 4 | 4.9 | 1.3-12.0 | 52 | 63.4 | 52.0-73.8 | <0,001* |
| 6-10 | 233 | 78 | 33.5 | 27.4-39.9 | 9 | 3.8 | 1.8-7.2 | 146 | 62.7 | 56.1-68.9 | |
| 11-20 | 457 | 159 | 34.8 | 30.4-39.4 | 31 | 6.8 | 4.6-9.5 | 267 | 58.4 | 53.7-62.9 | |
| 21-30 | 449 | 198 | 44.1 | 39.5-48.8 | 20 | 4.5 | 2.7-6.8 | 231 | 51.4 | 46.7-56.2 | |
| 31-40 | 378 | 153 | 40.5 | 35.5-45.6 | 25 | 6.6 | 4.3-9.6 | 200 | 52.9 | 47.7-58.0 | |
| 41-50 | 239 | 84 | 35.1 | 29.1-41.5 | 20 | 8.4 | 5.2-12.6 | 135 | 56.5 | 49.9-62.8 | |
| 51-60 | 190 | 61 | 32.1 | 25.5-39.2 | 12 | 6.3 | 3.3-10.7 | 117 | 61.6 | 54.3-68.5 | |
| >60 | 197 | 43 | 21.8 | 16.3-28.2 | 15 | 7.6 | 4.3-12.2 | 139 | 70.6 | 63.6-76.8 | |
+ using Pearson Chi-Square
* denote statistical significance
Fig. 1.
Distribution of diphtheria immunity according to age group and gender, as measured by antibodies against diphtheria toxin
The overall geometric mean antibody concentration (GMC) of diphtheria toxin antibody for the entire population of 2225 individuals was 0.05 IU/mL (95%CI: 0.04-0.06). GMCs for the groups aged 0–5, 6–10, 11-20, 21–30, 31–40, 41–50, 51–60 and 60+ years were 0.07 (95%CI: 0.03-0.13), 0.10 (95%CI: 0.06-0.15), 0.06 (95%CI: 0.04-0.08), 0.04 (95%CI: 0.03-0.05), 0.04 (95%CI: 0.03-0.05), 0.05 (95%CI: 0.04-0.08), 0.06 (95%CI: 0.04-0.09) and 0.11 (95%CI: 0.07-0.16) IU/mL, respectively (Table 4). There was statistically significant difference in GMC between age groups of 21-30 and 31-40 compared to 60+ years (p <0.05).
Table 4.
The geometric mean concentration (GMC) level according to age groups
| Age groups (years) | Total | GMC (IU/ml) | 95%CI | P value+ |
|---|---|---|---|---|
| 0-5 | 82 | 0.07 | 0.03-0.13 | 0.474 |
| 6-10 | 233 | 0.10 | 0.06-0.15 | 0.597 |
| 11-20 | 457 | 0.06 | 0.04-0.08 | 0.199 |
| 21-30 | 449 | 0.04 | 0.03-0.05 | 0.011* |
| 31-40 | 378 | 0.04 | 0.03-0.05 | 0.002* |
| 41-50 | 239 | 0.05 | 0.04-0.08 | 0.558 |
| 51-60 | 190 | 0.06 | 0.04-0.09 | 0.350 |
| >60 | 197 | 0.11 | 0.07-0.16 | Reference |
+using Mann–Whitney test
*denote statistical significance
In this study, a statistically significant difference was found between diphtheria immunity age group, ethnicity, residence, education, occupation, diphtheria vaccination during last 10 years. In contrast, no significant difference was found according to gender, ethnicity, residence, education and occupation (Tables 5 and 6). The highest susceptibility rate was aged 21-30 years (44.1%) with OR =2.7 (95%CI: 1.5-5.0), followed by 31-40 years (40.5%) with OR=2.5 (95%CI: 1.5-4.9) compared to above 60 years; while the lowest rate was aged above 60 years (21.8%) (p< 0.001). The susceptibility to diphtheria was identified in 30.9% of urban and 38.6% of rural person (p>0.05). Susceptibility proportion were detected in 34.0% of the people who were illiteracy, while this rate was only 16.9% in people who had graduated from college/university (p>0.05). The highest susceptibility rate was observed among farmers (39.2%) and self-employed/unemployment (35.9%) compared to other occupations. Of 2550 participants, 553 of the 660 (83.8%) people informed that they had been vaccinated against diphtheria during past 10 years were found to be immune compared to 54.8% (OR: 4.7; 95%CI: 3.8-6.5) and 60.7% (OR: 3.8; 95%CI: 2.8-5.7) in persons with no and unknown vaccination (p <0.0001) (Tables 5 and 6).
Table 5.
Univariate logistic regression analysis of variables for diphtheria immunity
| Characteristics | Immunitya (n = 1423) | Susceptibility (n = 802) | OR (95%CI) | p-Valuec |
|---|---|---|---|---|
| Gender | ||||
| Male (Ref) | 470 (64.1%) | 263 (35.9%) | - | - |
| Female | 953 (63.9%) | 539 (36.1%) | 1.0 (0.8-1.2) | 0.91 |
| Age groups | ||||
| 0-5 | 56 (68.3%) | 26 (31.7%) | 1.6 (0.9-2.9) | 0.08 |
| 6-10 | 155 (66.5%) | 78 (33.5%) | 1.8 (1,2-2,8) | <0.01 |
| 11-20 | 298 (65.2%) | 159 (34.8%) | 1.9 (1.3-2.8) | <0.01 |
| 21-30 | 251 (55.9%) | 198 (44.1%) | 2.8 (1.2-4.1) | <0.01 |
| 31-40 | 225 (59.5%) | 153 (40.5%) | 2.4 (1.6-3.6) | <0.001 |
| 41-50 | 155 (64.9%) | 84 (35.1%) | 1.9(1.2-2.9) | <0.001 |
| 51-60 | 129 (67.9%) | 61 (32.1%) | 1.7 (1.1-2.7) | 0.02 |
| >61 (Ref) | 154 (78.2%) | 43 (21.8%) | - | - |
| Ethnicity | ||||
| Kinh (Ref) | 459 (70.2%) | 195 (29.8%) | - | - |
| Ba Na | 329 (72.3%) | 126 (27.7%) | 0,9 (0.7-1.2) | 0.44 |
| So Dang | 322 (59.0%) | 224 (41.0%) | 1.6 (1.3-2.1) | <0.001 |
| Others | 313 (54.9%) | 257 (45.1%) | 1.9 (1.5-2.3) | <0.001 |
| Residence | ||||
| Rural (Ref) | 912 (61.4%) | 573 (38.6%) | - | - |
| Urban | 511 (69.1%) | 229 (30.9%) | 0.7 (0.6-0.9) | <0.001 |
| Education | ||||
| Illiteracy (Ref) | 132 (66.0%) | 68 (34.0%) | - | - |
| No school | 59 (66.3%) | 30 (33.7%) | 1.0 (0.6-1.7) | 0.96 |
| Primary school | 525 (60.8%) | 339 (39.2%) | 1,2 (0.9-1.7) | 0.17 |
| Secondary school | 517 (66.9%) | 256 (33.1%) | 0.9 (0.7-1.3) | 0.81 |
| High school | 126 (56.8%) | 96 (43.2%) | 1.5 (1.0-2.2) | 0.52 |
| College/University | 64 (83.1%) | 13 (16.9%) | 0.4 (0.2-0.7) | 0.06 |
| Occupation | ||||
| Farmer/worker (Ref) | 784(60.8%) | 506 (39.2%) | - | - |
| Pupil/Student | 460 (67.8%) | 218 (32.2%) | 0.7 (0.6-0.9) | <0.05 |
| Employer | 88 (76.5%) | 27 (23.5%) | 0.5 (0.3-0.7) | <0.05 |
| Self-employed/unemployment | 91 (64.1%) | 51 (35.9%) | 0.8 (0.6-1.2) | 0.44 |
| History of diphtheria vaccination during last 10 years | ||||
| Yes (Ref) | 553 (83.8%) | 107 (16.2%) | - | - |
| No | 737 (54.8%) | 609 (45.2%) | 4.3 (3.4-5.4) | <0.0001 |
| Unknown | 133 (60.7%) | 86 (39.3%) | 3.3 (2.4-4.7) | <0.0001 |
aImmunity: IgG antibody titre against diphtheria toxin ≥ 0.01 IU/mL.
bSusceptibility: IgG titre against diphtheria toxin < 0.01 IU/mL.
cChi-Square test
Table 6.
Multiple logistic regression analysis of variables for diphtheria immunitya
| Characteristics | OR (95%CI) | 95%CI | p-Valueb |
|---|---|---|---|
| Age groups | |||
| 0-5 | 1.5 | 0.7-2.6 | 0.09 |
| 6-10 | 1.6 | 0.6-2.5 | <0.001 |
| 11-20 | 1.8 | 0.8-3.3 | <0.001 |
| 21-30 | 2.7 | 1.5-5.0 | <0.001 |
| 31-40 | 2.5 | 1.5-4.9 | <0.001 |
| 41-50 | 1.8 | 1.6-4.0 | <0.001 |
| 51-60 | 1.6 | 1.2-3.1 | <0.01 |
| >61 (Ref) | - | - | - |
| Ethnicity | |||
| Kinh (Ref) | - | - | - |
| Ba Na | 0.9 | 0.6-1.3 | 0.62 |
| So Dang | 1.3 | 0.9-1.8 | 0.13 |
| Others | 1.3 | 0.9-1.7 | 0.09 |
| Residence | |||
| Rural (Ref) | - | - | - |
| Urban | 0.8 | 0.6-1.3 | 0.06 |
| Occupation | |||
| Farmer/worker (Ref) | - | - | - |
| Pupil/Student | 0.7 | 0.3-1.1 | 0.3 |
| Employer | 0.6 | 0.4-1.2 | 0.5 |
| Self-employed/unemployment | 0.9 | 0.5-1.4 | 0.4 |
| History of diphtheria vaccination during last 10 years | |||
| Yes | - | - | - |
| No | 4.7 | 3.8-6.5 | <0.0001 |
| Unknown | 3.8 | 2.6-5.7 | <0.0001 |
Susceptibility: IgG antibody titre against diphtheria toxin < 0.01 IU/mL
Logistic regression analysis, main effects model. All variables are listed which attained a statistical significance of p < 0.05
4. Discussion
Diphtheria is well-controlled in countries where have implemented diphtheria vaccination. However, Vietnam has been affected by diphtheria outbreaks with 87 cases being reported only in between 2016 and 2019 (World Health Organization, 2020). The number of diphtheria cases and asymptomatic carriers are still remaining as being high in Vietnam, although the Expanded Program on Immunization (EPI) in Vietnam started in 1981(Nguyen et al., 2015).
Since 2009, the national vaccine policy for Vietnam has recommended a DPT-VGB-Hib (Diphtheria-Tetanus Toxoids–whole cell Pertussis, Hepatitis B and Hemophilus influenzae) vaccine for infants aged 2, 4 and 4 months, a DTP for children aged 18 months to 24 months. However, the immunity declines over time. World Health Organization (WHO) recommends people living in low endemic and non-endemic areas need a booster every 10 years to maintain life-long protection (Scheifele and Ochnio, 2009). A study reported that adults who has not yet received any diphtheria dose or had an unknown vaccination history recommend at least 3 doses of dT or Td to provide a protection (Ang et al., 2015). In Vietnam, the current EPI has been running a Td booster dose for individuals who have been vaccinated during childhood and then every 10 years. This study further provides evidence for the importance of adhering to the current recommendations.
Overall, in this study showed that proportion of susceptible persons was 35.3%, it was accordant with seronegative prevalence found in Lao PDR (Nanthavong et al., 2015), this rate was lower compared to 74.0% in Nha Trang, Vietnam (Kitamura et al., 2022) and 57,0% in Malaysia (Yusoff et al., 2021). However, it was much higher compared to only 8.0% in Singapore (Ang et al., 2015) and 17.0% in Thailand (Wanlapakorn et al., 2012). This is a great concern as a high percentage of population with an unprotective level of anti-diphtheria antibodies creates a high risk for potential diphtheria outbreaks. In fact, diphtheria outbreaks occurred in Kon Tum province in the period of 2019 and 2020 (unpublished paper). This raises questions about vaccination policy, including both timely immunization and a number of boosters doses required to protect children and adults. A study by An et al. (2016) in Vietnam concentrated that proportions of children under five who had timely immunization completion were low and recommended that the EPI should include ‘timely immunization completion’ as a quality indicator (An et al., 2016). WHO estimated that a threshold for sufficient herd immunity requires at least immunity rate of 90% in children and 75% in adults (Begg, 1994). Several studies proved that after primary vaccination against diphtheria, in the absence of the natural booster, the anti-diphtheria toxoid antibody concentration decreased continuously after 15 years (Kjeldsen et al., 1985; Kjeldsen et al., 1988). It was general agreed that 25 years after primary vaccination, over 20% of vaccinated people would be unprotected to disease due to without the protective immunity. It is noted that from 25% susceptible subjects in a population is sufficient for the spread of infection.
The seroprotection rates in some age groups in our study were lower than protective threshold, especially among those aged 21–30 years and the 31–40 years. The 67.4% frequency of protected individuals aged 0–11 years decreased to 56.6% and 61.3% among age groups of 21–30 years and 31-40 years; respectively. These results demonstrate that the majority of elderly groups are unprotected to diphtheria and suggest that a recrudescence of the disease would involve almost entirely persons. In 2016, our pilot study examined in persons aged 6-25 years in a small district of Kon Tum Le showed that 47.5% subjects were unprotective to diphtheria (Be et al., 2017). 10.5% of children in India were nonimmune to diphtheria. This low population-level immunity possibly reflects incomplete coverage of diphtheria vaccination, especially booster doses, and a decline in acquired immunity by primary and booster vaccination (Murhekar et al., 2021). The other studies reported a higher incidence of diphtheria in adults than young people, suggesting that the immunological memory declines with age (Golaz et al., 2000; Gower et al., 2020; Kjeldsen et al., 1988 and Yusoff et al., 2021). Aue et al. showed that 66.4% of blood donors in Germany were not immune against diphtheria (Aue et al., 2003). This study also showed the increased frequency of immune individuals among elderly adults (>51-year-olds). It is probably associated with the fact that natural immunity of C. diphtheriae occurs throughout life (Galazka, 2000). Other studies indicated that elderly people (>60 years) were less likely to appear susceptible than the middle-aged (Ang et al., 2015, Wanlapakorn et al., 2012). The obtained results in the agreement with previous studies (Mohammed et al., 2018; Skogen et al., 2000). In Kon Tum, the Expanded Program on Immunization (EPI) only started in 1985. Therefore, people over 60 years of age have been unvaccinated against diphtheria. Moreover, diphtheria outbreaks reported occasionally in Kon Tum during the period 1990-2020. It is probably associated with that the elderly persons have developed their natural immunity to diphtheria during past epidemic. However, our finding is contrast to studies conducted in European countries where showed the level of antibodies to diphtheria toxin decreases significantly in persons above 40 years old (Aue et al., 2003; Di Giovine et al., 2013; Edmunds et al., 2000), reaching more than 67% of seronegative individuals (Di Giovine et al., 2013; Edmunds et al., 2000; Pachon et al., 2002; Zasada et al., 2013).
The risk of a diphtheria outbreaks could occur when there is a combination of low vaccination coverage in childhood and an immunity gap in the adult population (Dittmann et al., 2000; Galazka, 2000). In this study showing only 67.4% children aged 0–10 years had protective immunity. A low proportion of children without protective immunity was also identified in Laos (Nanthavong et al., 2015) and Czech (Di Giovine et al., 2013). These data provide the evidence that basic immunization without booster doses may result in insufficient protection among children. In addition, asymptomatic carrier continues to put on a threat to susceptible children and adults (Ang et al., 2015). It is clearly important that booster doses should be implemented in providing protective antibody level against diphtheria.
Our study showed no statistical significant difference among men and women in protective immunity against diphtheria, this result is accordance with other study (Galazka, 2000), and in contrast to others (de Melker et al., 1999; Kjeldsen et al., 1988). Some countries observed males had the higher protective immunity than females. This could be explained that males in those countries had been vaccinated against diphtheria in military services (Aue et al., 2003; de Melker et al., 1999; Mossong et al., 2006).
In Vietnam, Ministry of Health recommends booster doses every 10 years in adults. Kon Tum is one of the mountainous and remote provinces of the Central Highlands of Vietnam. Difficult access to the villages, districts can explain difficulties related to monitor and vaccinate target populations (both children and adults) for diphtheria booster vaccination.
In our study, a significant difference in immunity to diphtheria were observed among different age group, ethnicity, education, occupation, diphtheria vaccination during last 10 years (p <0.05). These results were in accordant with the study conducted in Germany (Völzkea et al., 2006). In addition, the immunity proportion was significantly lower in people living in rural when compared to those living in urban areas, it was in contrast to other study in Turkey (Cavus et al., 2007). It is possible to be a difficulty access to vaccination services in rural areas. This study also showed that no significant difference was observed according to gender, ethnicity, residence, education and occupation. There are limitations in our study. Firstly, we excluded known immunocompromised persons from participating in the study, this may result in underestimation of the susceptible proportion. We used ELISA for quantitating anti-diphtheria toxoid antibody level instead of in vitro toxin neutralization Vero cell assay, which is the standard method. Vero cell assay was not performed due to cost and laboratory capacities constraint. Considering the large sample size and time constraint, the ELISA method was employed. Secondly, we assessed the validity of parental recall by childhood vaccination card-based data were the reference, but these data are sometimes incomplete or inaccurate. Lastly, the history of diphtheria vaccination of older children and adults was not validated due to vaccination records are not available, therefore, the recall bias might be large.
In conclusion, this study revealed an unsatisfactory level of protective immunity to diphtheria among children and adults in Kon Tum population, exclusion of individuals aged >50 years creates the high risk of diphtheria outbreaks. Active immunization remains the most important way of prevention of potential diphtheria outbreaks. Administration of a booster dose of vaccine against diphtheria for adults every 10 years should be recommended.
Authors' contributions
TVL conceived the idea and designed the study; TVL, VTTN, QHN, TTTN, TTND, TTTL, TNP, VLN collected the data; TVL, CCV analyzed the data and drafted the manuscript. All authors commented the paper and approved the final manuscript.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgements
This work was supported by Kon Tum Department of Science and Technology (Grant No: 319/SKHCN-2019). We would like to thank all participants for their contribution to the study.
REFERENCES
- An DTM, Lee JK, Minh HV, Trang NT, Huong NTT, Nam YS, et al. Timely immunization completion among children in Vietnam from 2000 to 2011: a multilevel analysis of individual and contextual factors. Glob Health Action. 2016;29(9):29189. doi: 10.3402/gha.v9.29189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang LW, James L, Goh KT. Prevalence of diphtheria and tetanus antibodies among adults in Singapore: a national serological study to identify most susceptible population groups. J Public Health (Oxf) 2015;38:99–105. doi: 10.1093/pubmed/fdv011. (1741-3850 (Electronic)) [DOI] [PubMed] [Google Scholar]
- Aue A, Hennig H, Krüger S, Closius B, Kirchner HMS. Immunity against diphtheria and tetanus in German blood donors. Med Microbiol Immunol. 2003;192(2):93–97. doi: 10.1007/s00430-002-0163-9. [DOI] [PubMed] [Google Scholar]
- Be LV, Phuong NLT, Duoc PT, Tuan LV. Evaluation of antibody responses to diphtheria among persons aged 6-25 years after Tetanus-diphtheria (Td) vaccine immunization in Kon Plong district, Kon Tum province, from May 2016 to March 2017. Vietnam Journal of preventive Medicine. 2017;27(8):465. [Google Scholar]
- Begg NT. World Health Organisation; Copenhagen: 1994. Manual for the Management and Control of Diphtheria in the European Region. [Google Scholar]
- Cavus AS, Oguz AV, Yuce A. The seroprevalence of diphtheria among adults in Izmir-Turkey. Vaccine. 2007;25(19):3851–3854. doi: 10.1016/j.vaccine.2007.01.104. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Diphtheria. In: Atkinson W, Wolfe S, Hamborsky J (eds). Epidemiology and Prevention of Vaccine-Preventable Diseases, 12th edn. Washington, DC: Public Health Foundation 2012(12):75-86.
- Damasco PV, Pimenta F, Filardy A, Brito S, Andrade A, Lopes G, et al. Prevalence of IgG diphtheria antitoxin in blood donors in Rio de Janeiro. Epidemiol Infect. 2005;133:911–914. doi: 10.1017/S0950268805003997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Melker HE, Berbers GA, Nagelkerke NJ, Conyn-van Spaendonck MA. Diphtheria Antibody Levels in the Netherlands: a Population-Based Study. Emerg Infect Dis. 1999;5:694–700. doi: 10.3201/eid0505.990511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giovine P, Kafatos G, Nardone A, Andrews N, Olander RM, Alfarone G, et al. Comparative seroepidemiology of diphtheriae in six European countries and Israel. Epidemiol Infect. 2013;141:132–142. doi: 10.1017/S0950268812000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmann S, Wharton M, Vitek C. Successful control of pidemic diphtheria in the states of the former Union of Soviet Socialist Republics: lessons learned. J Infect Dis. 2000;181(1):S10–S22. doi: 10.1086/315534. [DOI] [PubMed] [Google Scholar]
- Edmunds WJ, Pebody RG, Aggerback H, Baron S, Berbers G, Conyn-van Spaendonck MAE, et al. The sero-epidemiology of diphtheria in Western Europe. Epidemiol Infect. 2000;125:113–125. doi: 10.1017/s0950268899004161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galazka A. The changing epidemiology of diphtheria in the vaccine era. J Infect Dis. 2000;181(1):20–29. doi: 10.1086/315533. [DOI] [PubMed] [Google Scholar]
- Galazka A. Implications of the diphtheria epidemic in the Former Soviet Union for immunization programs. J Infect Dis. 2000;181(1):244–248. doi: 10.1086/315570. [DOI] [PubMed] [Google Scholar]
- Golaz A, Hardy I, Strebel P, Bisgard K, Vitek C, Popovic T, et al. Epidemic diphtheria in the Newly Independent States of the Former Soviet Union: implications for diphtheria control in the United States. J Infect Dis. 2000;181:237–243. doi: 10.1086/315569. [DOI] [PubMed] [Google Scholar]
- Gower CM, Scobie A, Fry NK, Litt DJ, Cameron JC, Chand MA, et al. The changing epidemiology of diphtheria in the United Kingdom, 2009 to 2017. LID - 10.2807/1560-7917.ES.2020.25.11.1900462 [doi] LID - 1900462. Euro Surveill. 2020;25(11) doi: 10.2807/1560-7917.ES.2020.25.11.1900462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N, Thao TTL, Lien TL, Luong DN, Anh TD, Thanh TH, et al. Diphtheria Outbreaks in Schools in Central Highland Districts, Vietnam, 2015–2018. EID Journal. 2020;26(3) doi: 10.3201/eid2603.191027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N, Le LT, Le TTL, Nguyen HAT, Edwards T, Madaniyazi L, et al. The seroprevalence, waning rate, and protective duration of anti-diphtheria toxoid IgG antibody in Nha Trang, Vietnam. Int J Infect Dis. 2022;116:273–280. doi: 10.1016/j.ijid.2022.01.025. Jan 19. [DOI] [PubMed] [Google Scholar]
- Kjeldsen K, Simonsen O, Heron I. Immunity against diphtheria 25-30 years after primary vaccination in childhood. Lancet. 1985;i:1755. doi: 10.1016/s0140-6736(85)91675-7. [DOI] [PubMed] [Google Scholar]
- Kjeldsen K, Simonsen O, Heron I. Immunity against diphtheria and tetanus in the age group 30-70 years. Scand J Infect Dis. 1988;20:177–185. doi: 10.3109/00365548809032435. [DOI] [PubMed] [Google Scholar]
- Mohammed A, Redwan EM, Almehdar HA. Status of Diphtheria Immunity Among Saudi Population. Journal of Pure and Applied Microbiology. 2018;11(1) [Google Scholar]
- Mossong J, Putz L, Shkedy Z, Scheider F. Seroepidemiology of diphtheria and pertussis in Luxembourg in 2000. Epidemiol Infect. 2006;134:573–578. doi: 10.1017/S0950268805005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murhekar MV, Kamaraj P, Kumar MS, Siraj Ahmed Khan SA, Allam RR, Barde PV, et al. Immunity against diphtheria among children aged 5–17 years in India, 2017–18: a cross-sectional, population-based serosurvey. The Lancet. 2021;21(6):868–875. doi: 10.1016/S1473-3099(20)30595-8. [DOI] [PubMed] [Google Scholar]
- Murakami DR, Phuong DR, Thang HV, Chau NV, Giao PN, Tho ND. Combating Endemic Diphtheria in a Tropical Metropolis: A Matched Case-Control Study in Ho Chi Minh City, Vietnam. International Journal of Infectious Diseases. 2008;12 [Google Scholar]
- Nanthavong N, Black AP, Nouanthong P, Souvannaso C, Vilivong K, Muller CP, et al. Diphtheria in Lao PDR: Insufficient Coverage or Ineffective Vaccine? PLoS One. 2015;10 doi: 10.1371/journal.pone.0121749. (1932-6203 (Electronic))e0121749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TD, Dang A, Van Damme P, Nguyen CV, Duong HT, Goossens H, et al. Coverage of the expanded program on immunization in Vietnam: Results from 2 cluster surveys and routine reports. Hum Vaccin Immunother. 2015;11:1526–1533. doi: 10.1080/21645515.2015.1032487. (2164-554X (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachon I., Amela C., De Ory F. Age-specific seroprevalence of poliomyelitis, diphtheria and tetanus antibodies in Spain. Epidemiol Infect. 2002;129:535–541. doi: 10.1017/s0950268802007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheifele DW, Ochnio JJ. Module 2: Diphtheria (update 2009). In: The immunological basis for immunization series. Geneva: World Health Organization 2009.
- Plans-Rubió P. Evaluation of the establishment of herd immunity in the population by means of serological surveys and vaccination coverage. Hum Vaccin Immunother. 2012;8(2):184–188. doi: 10.4161/hv.18444. [DOI] [PubMed] [Google Scholar]
- Skogen V, Jenum PA, Danilova E, Koroleva VN, Halvorsen DS, Sjursen H. Immunity to diphtheria among children in Northern Norway and NorthWestern Russia. Vaccine. 2000;19:197–203. doi: 10.1016/s0264-410x(00)00176-6. [DOI] [PubMed] [Google Scholar]
- Völzkea H, Klokerab KM, Kramer L, Guertler L, Dörend M., Baumeister SE, et al. Susceptibility to diphtheria in adults: prevalence and relationship to gender and social variables. Clinical Microbiology and Infec. 2006;22(10):961–967. doi: 10.1111/j.1469-0691.2006.01477.x. [DOI] [PubMed] [Google Scholar]
- Wanlapakorn N, Pornsak Y, Tharmaphornpilas P, Theamboonlers A. YP. Diphtheria outbreak in Thailand, 2012; seroprevalence of diphtheria antibodies among Thai adults and its implications for immunization programs. Southeast Asian J Trop Med Public Health. 2012;45:1132–1141. (0125-1562 (Print)) [PubMed] [Google Scholar]
- World Health Organization. The Immunological Basis for Immunization Series: Module 2: Diphtheria. Alvaible at http://whqlibdoc.who.int/publications/2009/9789241597869_eng.pdf (2009) 2009. [accessed 11 February 2022].
- World Health Organization . WHO; Geneva: 2020. Diphtheria reported cases by WHO region.https://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencediphtheria.html Available at. [accessed 11 February 2022] [Google Scholar]
- Yusoff AF, Mohd Sharani Z, Kee C, Md Iderus NH, Md Zamri ASS, Nagalingam T, et al. Seroprevalence of diphtheria toxoid IgG antibodies in the Malaysian population. BMC Infect Dis. 2021:581. doi: 10.1186/s12879-021-06285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasada AA, Rastawicki W, Rokosz N, Jagielski M. Seroprevalence of diphtheria toxoid IgG antibodies in children, dolescents and adults in Poland. BMC Infect Dis. 2013;13:551. doi: 10.1186/1471-2334-13-551. [DOI] [PMC free article] [PubMed] [Google Scholar]