Abstract
Objective
We compared the characteristics and outcomes of vaccinated and nonvaccinated patients hospitalized with COVID-19.
Design
We analyzed patients hospitalized in a COVID hub during three one-month periods: (i) October 15, 2020-November 15, 2020 (prevaccination peak); (ii) October 15, 2021-November 15, 2021 (Delta wave); (iii) December 15, 2021-January 15, 2022 (Omicron wave). To define the epidemiologic context, SARS-CoV-2 infection in healthcare workers was analyzed.
Results
SARS-CoV-2 infection incidence in healthcare workers was 146 cases per 1000 persons in 2020 (prevaccination) and 67 in 2021 (postvaccination, when the Omicron variant caused most infections). There were 420 hospitalized patients in the prevaccination period, 51 during the Delta wave (52.1% vaccinated) and 165 during the Omicron wave (52.9% vaccinated). During the Delta wave, a significantly higher number of nonvaccinated (29.2%) than vaccinated patients (3.7%) were admitted to the intensive care unit (ICU) (p = 0.019). Nonvaccinated patients were younger and had a lower rate of concomitant medical conditions (53.2% vs 83.7%; p < 0.001) during the Omicron wave when 80% of patients admitted to ICU and all those who died were still infected by the Delta variant.
Conclusions
Vaccine effectiveness in fragile individuals appears to be lower because of a faster immunity decline. However, the Omicron variant seems to cause less severe COVID-19.
Keywords: SARS-CoV-2 infection, COVID-19, Vaccinated, Delta variant, Omicron variant
Abbreviations: S, Spike; ICUs, Intensive care units; IQR, Interquartile range; CI, Confidence interval
1. Introduction
Since the emergence of SARS-CoV-2 pandemic that causes COVID-19, several efforts have been made to contain and prevent the spread of infection and disease. Among the available interventions, population-based vaccination campaigns have been implemented worldwide after the development of highly effective vaccines (Baden et al., 2021; Polack et al., 2020; Sadoff et al., 2021; Voysey et al., 2021). In Italy, the vaccination campaign started on December 27, 2020, and the following vaccines were adopted for immunization: BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), ChAdOx1 (AstraZeneca), and Ad26.COV2.S (Janssen). The available vaccines showed high efficacy in protection from infection or disease in the original clinical trials, and their effectiveness has been confirmed at the population level by real-life postauthorization studies (Angel et al., 2021; Dagan et al., 2021; Haas et al., 2021; Hall et al., 2021; Lopez Bernal et al., 2021; Rovida et al., 2021b; Vasileiou et al., 2021). However, several issues are to be fully elucidated, such as the duration of the protective effectiveness and the impact of the diffusion of new viral variants on protection against severe disease. In particular, although the vaccines have been designed on the Spike (S) protein of the original wild-type strain, several viral variants arose before the vaccine implementation. Particularly, those defined as variants of concern are characterized by mutations conferring potentially higher transmissibility, immune evasiveness, or severity (https://www.ecdc.europa.eu/en/covid-19/variants-concern). At the end of 2020, the Alpha variant (B.1.1.7) was identified in the United Kingdom and became dominant in several countries, including Italy. Subsequently, the Delta variant (B.1.617.2) emerged and replaced the Alpha variant worldwide, and, more recently, the Omicron variant (B.1.1.529) was identified in South Africa and became predominant. Although vaccine effectiveness was still high against the Alpha variant (Rovida et al., 2021b), Delta and especially Omicron harbored mutations on the S protein associated with vaccine evasion (https://www.who.int/docs/default-source/coronaviruse/2022-01-07-global-technical-brief-and-priority-action-on-omicron---corr2.pdf?sfvrsn=918b09d_26). Meanwhile, on February 10, 2022, full vaccination vaccine coverage in the Italian population reached 88.61% of individuals older than 12 years and 21.47% of individuals younger than 12 years (https://www.governo.it/it/cscovid19/report-vaccini/). However, considering that vaccine effectiveness is below 100%, waning immunity, and immune escape of the new variants, the occurrence of breakthrough infections is expected to increase. However, the ratio between infections in vaccinated versus unvaccinated individuals is still unclear. The objective of this study was to compare the characteristics and outcomes of vaccinated and nonvaccinated patients hospitalized with COVID-19 in a single Italian hub, at the Fondazione IRCCS Policlinico San Matteo Hospital in Pavia, Northern Italy (Lombardy region, 10 million inhabitants), during the Delta and Omicron waves. The San Matteo Hospital is an Italian research hospital affiliated with the University of Pavia. In 2021, there were nearly 83,500 emergency visits, 30,000 admissions, 20,000 surgical procedures, 190 transplant procedures, and more than 2,500,000 outpatient clinic visits. Data were compared with those of patients hospitalized during the second pandemic wave before the implementation of the vaccination campaign. As an indicator of the epidemiological context, we analyzed the incidence of SARS-CoV-2 infection and the relevant virus genotype among healthcare workers in the same hospital.
2. Materials and methods
2.1. Study design
We analyzed the characteristics of patients admitted for COVID-19 at the San Matteo Hospital in Pavia, Northern Italy, during three one-month periods: (i) October 15, 2020-November 15, 2020 (prevaccination), corresponding to the peak of the second epidemic wave in Italy, which was sustained by the ancestral virus strain (with the D614G mutation); (ii) October 15, 2021-November 15, 2021, when >70% of the population was fully vaccinated, and the Delta variant accounted for almost all cases of infections (https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-rapporti-periodici-10-dicembre-2021.pdf), this period was defined as “Delta wave” in this study; (iii) December 15, 2021-January 15, 2022, when the Omicron variant accounted for most of the circulating strains (https://www.epicentro.iss.it/coronavirus/pdf/sars-cov-2-monitoraggio-varianti-indagini-rapide-3-gennaio-2022.pdf), this period was defined as “Omicron wave” in this study. Patients hospitalized for other diagnoses who were found positive for SARS-CoV-2 during recurring screening performed in hospital wards with an asymptomatic infection were excluded from the analysis. Patients’ data are collected within the routine Regional Healthcare system surveillance of SARS-CoV-2 infection; therefore, informed consent was not required. The study was approved by the Medical Direction of Fondazione IRCCS Policlinico, San Matteo.
2.2. Data collection
Patients’ data were retrieved and anonymized from electronic medical records and the Regional vaccination registry. The following information was collected: age, sex, vaccination status, concomitant chronic medical conditions (hypertension, cardiovascular disease, pulmonary disease, nephropathy, diabetes mellitus, obesity, neoplastic disease, immune depression), admission to intensive care units (ICUs), and death. For healthcare workers with SARS-CoV-2 infection, previous SARS-CoV-2 infection and vaccination status were collected.
2.3. Surveillance of SARS-CoV-2 infection in healthcare workers
Data on the occurrence of SARS-CoV-2 infection were available for 3832 healthcare workers of Fondazione IRCCS Policlinico, San Matteo, in 2020 and 4066 healthcare workers in 2021 (Rovida et al., 2021a; Rovida et al., 2021b; Lilleri et al., 2022). Naso-pharyngeal swabs were collected and tested for SARS-CoV-2 RNA positivity in subjects with symptoms suggestive of SARS-CoV-2 infection or in case of contact with infected subjects, as previously reported (Giardina et al., 2021). Moreover, in compliance with the local healthcare workers' surveillance protocol, personnel working in clinical wards dedicated to fragile patients undergo routine screening for SARS-CoV-2 infection every 14 days, whereas monitoring was scheduled every 30 days for healthcare workers in the other wards. The health condition of all workers was regularly monitored, and data on symptoms were collected during an interview by a physician and inserted into a specific database.
2.4. Virus genotyping and sequencing
SARS-CoV-2 variants were determined in samples from vaccinated healthcare workers with breakthrough infections and as part of a national surveillance program by the Istituto Superiore di Sanità. Multiplex real-time reverse transcription–PCR tests specific for mutations characteristic of Delta (478K and 452R) and Omicron (501Y and 484A) were performed.
In addition, whole-genome sequencing was performed in selected samples using next-generation sequencing as previously reported (Rovida et al., 2021a). Viral variants were also determined in patients admitted to ICU during the Omicron wave period.
2.5. Statistical analysis
The incidence of SARS-CoV-2 infection in healthcare workers was expressed as number of cases per 1000 persons. The annual incidence rate was calculated for the entire 2020 and 2021. Since July 2020, when routine, standardized surveillance of the personnel of the hospital was implemented, data on monthly incidence rates were also calculated. Age was reported as median and range or interquartile range (IQR) and was compared with the Mann-Whitney U-test when two groups were compared or the Kruskal-Wallis test and Dunn's post-test with correction for multiple comparisons when more than two groups were compared.
Categorical variables were expressed as percentage and compared by the Fisher's exact test or the chi-square test when more than two groups were compared.
2.6. Role of the funding source
This work was partially supported by the European Union's Horizon 2020 Research and Innovation Program (ATAC, No. 101003650). The funding source had no role in the study design, conduct, and report.
3. Results
3.1. SARS-CoV-2 infections in healthcare workers as an indicator of the epidemiological context
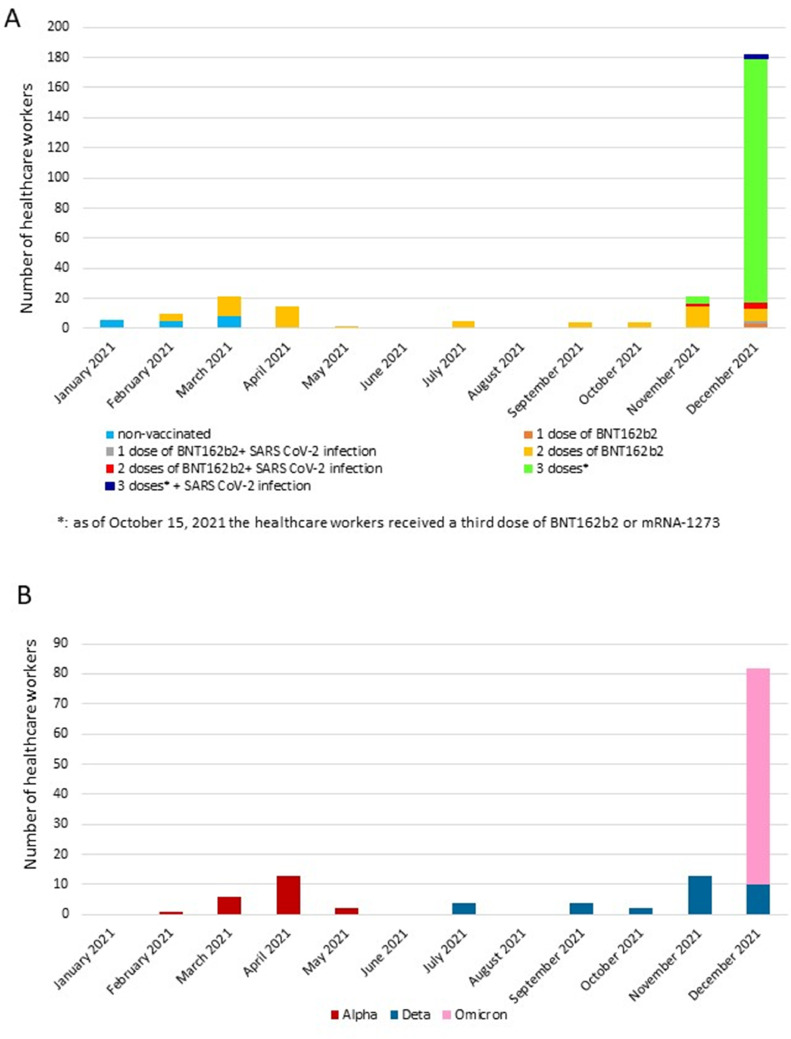
Data relevant to the surveillance of SARS-CoV-2 infection in healthcare workers of San Matteo Hospital are partially reported in previous work (Rovida et al., 2021a; Rovida et al., 2021b; Lilleri et al., 2022). For 2020, data relevant to the occurrence of SARS-CoV-2 infection are available for 3832 healthcare workers. A serological screening conducted during April 29, 2020-June 30, 2020, showed that 334 subjects (87 per 1000 persons; 95% confidence interval [CI]: 79-97) were infected during the first pandemic wave. Subsequently, during July 2020-December 2020, monitoring of SARS-CoV-2 infection by testing of nasal swabs showed that an additional 237 subjects (10 SARS-CoV-2-seropositive after the first wave and 227 seronegative) were infected during the second wave, for a total annual incidence of 561 SARS-CoV-2 infected subjects (146 per 1000 persons; 95% CI: 136-158). Among the 237 healthcare workers infected during the second wave, three subjects (0.8 per 1000 persons, 95% CI: 0.2-2.3) were infected in September, 85 (22 per 1000 persons; 95% CI: 18-27) in October, 148 (39 per 1000 persons; 955 CI: 33-45) in November, and one (0.3 per 1000 persons; 95% CI: 0.0-1.5) in December 2020. The peak of infections occurred during October 15th, 2020-November 15, 2020, when 167 subjects (44 per 1000 persons; 95% CI: 38-51) were infected. In 2021, after the implementation of the vaccination campaign, we analyzed the occurrence of SARS-CoV-2 infections among 4066 healthcare workers at San Matteo Hospital, along with the genotype of the infecting virus (Figure 1 ). These data were considered as proxy of the variants circulation in the general population of the territory of Pavia. A significantly lower incidence of SARS-CoV-2 infection (p <0.001) was observed in 2021 compared to 2020: 271 of 4066 healthcare workers (67 per 1000 persons; 95% CI: 59-75) were positive for SARS-CoV-2 RNA with nasal swab testing during the entire 2021. The number of infected subjects per month increased from January to March 2021, when a peak of 21 cases was observed (5 per 1000 persons; 95% CI: 3-8), and subsequently decreased, maintaining a low, steady state between May and October 2021. The incidence of infection started increasing again in November 2021, when 21 cases were detected (5 per 1000 persons; 95% CI: 3-8), reaching the maximum level in December 2021, with 182 cases detected (45 per 1000 persons; 95% CI: 39-52; Figure 1A). Regarding the vaccination status, we already reported the differential incidence of infections in vaccinated and nonvaccinated subjects during January-May 2021(Rovida et al., 2021a), showing a vaccine effectiveness of 83% in protecting from infection with the Alpha variant. Subsequently, all the study population was vaccinated; therefore, we could not compare the incidence of infection in vaccinated versus nonvaccinated subjects thereafter. Since October 15 2021, healthcare workers have received a third dose of BNT162b2 or mRNA-1273 vaccine. At the end of the year, most healthcare workers had received three vaccine doses, and most infections in December 2021 occurred in three-dose vaccinated subjects (Figure 1A). No vaccinated healthcare worker developed pneumonia or required hospitalization for COVID-19. Among the 271 SARS-CoV-2-infected subjects, identification of the infecting variant was available for 127 subjects (Figure 1B). Between February and May 2021, the Alpha variant was detected in all the subjects whose viral RNA content was sufficient for genotyping analysis. Between July and November 2021, only the Delta variant was detected, whereas, in December 2021, the Omicron variant appeared and accounted for 89% of the genotyped strains, whereas the remaining 11% of cases harbored the Delta variant.
Figure 1.
SARS-CoV-2 infections in healthcare workers of Fondazione IRCCS Policlinico San Matteo, Pavia, in 2021. (A) Monthly number of infections and vaccination status. (B) SARS-CoV-2 variants detected.
3.2. Characteristics of vaccinated and nonvaccinated patients hospitalized for COVID-19
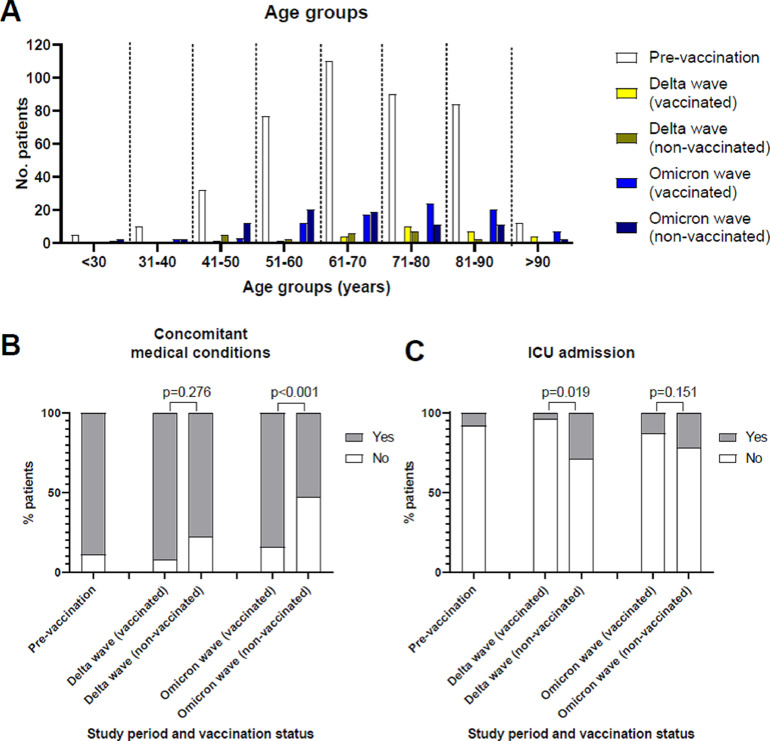
In the prevaccination period, 420 patients were hospitalized, whereas 51 patients were hospitalized during the Delta wave and 165 during the Omicron wave. Patients’ characteristics are listed in Table 1 . Among the 51 patients of the Delta wave, 27 (52.9%) were vaccinated, and among the 165 patients of the Omicron wave, 86 (52.1%) were vaccinated. Nonvaccinated patients of the Delta wave (median age 66 years; IQR 55-74 years) were younger than vaccinated patients (median age 78 years; IQR 71-86 years; p = 0.003). Similarly, nonvaccinated patients of the Omicron wave (62, IQR 54-73 years) were younger than vaccinated patients (median age 74 years; IQR 63-84 years, p < 0.001). In addition, patients of the prevaccination period (median age 68 years; IQR 59-79 years) were younger than vaccinated patients of both the Delta (p = 0.004) and Omicron waves (p = 0.024). In the Delta and Omicron waves, there were more nonvaccinated patients than vaccinated patients in the age group of 61-70 years and the younger age groups, whereas more vaccinated than nonvaccinated patients were observed in the older age groups (Figure 2 ). Concomitant chronic medical conditions (Figure 2B) were present in 375 (89.3%) patients in the prevaccination period. Among patients of the Delta wave, concomitant medical conditions were present in 18 (75%) nonvaccinated patients and 24 (88.9%) vaccinated patients (p = 0.276). Among patients of the Omicron wave, a significantly lower number of nonvaccinated (n = 42; 53.2%) than vaccinated patients (n = 72; 83.7%) had concomitant medical conditions (p <0.001).
Table 1.
Characteristics of the study population
| Characteristics | Period of hospitalization of COVID-19 patients |
||
|---|---|---|---|
| Prevaccinationa (n = 420) | Delta waveb (n = 51) | Omicron wavec (n = 165) | |
| Age, median (IQR), years | 68 (59-79) | 71 (62-82) | 69 (58-80) |
| Sex, M/F n. (%) | 294/126 (70/30) | 30/21 (59/41) | 98/67 (68/32) |
| Patients without chronic medical conditions, n. (%) | 45 (10.7) | 9 (17.6) | 51 (30.9) |
| Patients with chronic medical conditions, n. (%) | 375 (89.3) | 42 (82.4) | 114 (69.1) |
| Hypertension | 270 (72.0) | 30 (71.4) | 83 (74.5) |
| Cardiovascular disease | 120 (32.0) | 15 (35.7) | 39 (34.2) |
| Pulmonary disease | 84 (22.5) | 9 (21.4) | 14 (12.2) |
| Nephropathy | 60 (16.0) | 14 (33.3) | 16(14.0) |
| Diabetes | 101 (26.9) | 10(23.8) | 28 (24.5) |
| Obesity | 71 (18.9) | 8 (19.0) | 13 (11.4) |
| Neoplastic disease | 67 (17.8) | 11 (26.1) | 9 (7.8) |
| Immune depression | 24 (6.4) | 2 (4.7) | 11 (9.6) |
| Patients with one chronic medical conditions, n. (%) | 53 (14.1) | 4 (9.5) | 25 (21.9) |
| Patients with two chronic medical conditions, n. (%) | 63 (16.8) | 6 (14.3) | 38 (33.3) |
| Patients with ≥3 chronic medical conditions, n. (%) | 259 (69.1) | 32 (76.2) | 51 (44.7) |
| Vaccinated, n. (%) | 0 (0) | 27 (52.9) | 86 (52.1) |
October 15, 2020-November 15, 2020
October 15, 201-November 15, 2021
December 15, 2021-January 15, 2022
F = female; M = male; IQR = interquartile range.
Figure 2.
Characteristics of vaccinated and nonvaccinated patients with SARS-CoV-2 infection in the three study periods. (A) Number of patients in the different age groups. (B) Rate of patients with concomitant chronic medical conditions. (C) Rate of patients requiring admission to the intensive care unit (ICU). Prevaccination: October 15, 2020-November 15, 2020; Delta: October 15, 2021-November 15, 2021; Omicron: December 15, 2021-January 15, 2022.
3.3. Outcome of COVID-19 in vaccinated and nonvaccinated hospitalized patients
In the prevaccination period, 32 (7.6%) patients were admitted to ICU wards (Figure 2C). Among patients of the Delta wave, a significantly higher number of nonvaccinated (n = 7; 29.2%) than vaccinated patients (n = 1; 3.7%) were admitted to the ICU (p = 0.019). Among patients of the Omicron wave, 17 (21.5%) nonvaccinated and 11 (12.8%) vaccinated patients were admitted to the ICU (p = 0.151). During the prevaccination period, 90 (21.4%) patients died because of COVID-19 (Table 2 ). A similar rate of death was observed among patients of the Delta wave (n = 10, 19.6%). No difference was observed between vaccinated and nonvaccinated patients. Nonvaccinated patients who died had a median age of 74 (IQR 65-83) years and were younger than vaccinated patients (median age 86 years, IQR 71-92 years; p = 0.071). Among patients of the Omicron wave, 41 (24.8%) died because of COVID-19. In all the periods analyzed, most patients who died had concomitant chronic medical conditions, except for nonvaccinated patients who died during the Omicron wave, when about half of them were not affected by other medical conditions (Table 2).
Table 2.
Outcome of COVID-19 in vaccinated and nonvaccinated patients
| Period of hospitalization of COVID-19 patients | n. (%) deceased patients | Median age, years (range) | n. (%) deceased patients with chronic medical conditions |
|---|---|---|---|
| Prevaccinationa (n = 420) | 90 (21.4) | 80 (39-95) | 87 (96.7) |
| Delta waveb (n = 51) | 10 (19.6) | 79 (65-92) | 8 (80.0) |
| -vaccinated (n = 27) | 5 (18.5) | 86 (71-92) | 4 (80.0) |
| -nonvaccinated(n = 24) | 5 (20.8) | 74 (65-83) | 4 (80.0) |
| Omicron wavec (n = 165) | 41 (24.8) | 81 (47-95) | 30 (73.2) |
| -vaccinated (n = 86) | 26 (30.2) | 78 (58-95) | 23 (88.5) |
| -nonvaccinated (n = 79) | 15 (19.0) | 77 (8-92) | 7 (46.7) |
October 15, 2020-November 15, 2020
October 15, 2021-November 15, 2021
December 15, 2021-January 15, 2022
3.4. SARS-CoV-2 variant in patients admitted to ICU during the Omicron wave
The SARS-CoV-2 variant in 20 of 28 patients admitted to ICU during the Omicron period was successfully determined. The Omicron variant was detected in four (20%) cases, whereas the Delta variant was detected in the remaining 16 (80%) cases. The virus genotype was available for 9 of 41 patients who died during the Omicron wave (i.e., patients who were admitted to ICU), and in all nine cases, the Delta variant was detected. Notwithstanding the predominance of the Omicron variant among the general population, most patients required ICU admission, and all patients who died were infected with the Delta variant.
4. Discussion
Results of this study show that in our study population, a resurgence of SARS-CoV-2 infection in vaccinated individuals occurred with the Omicron but not with the Delta variant. However, a lower number of patients was hospitalized during both the Delta and Omicron waves, when >70% of the population was vaccinated, than in the prevaccination era. Vaccinated patients hospitalized for COVID-19 were older than nonvaccinated patients hospitalized during the Delta and Omicron waves and in the prevaccination era. In addition, vaccinated patients hospitalized during the Omicron wave were more frequently affected by concomitant chronic medical conditions than nonvaccinated patients. Finally, vaccinated patients had a significantly lower rate of admission to ICU than nonvaccinated patients during the Delta wave. Some real-life studies showed only a modest reduction in the effectiveness of BNT162b2 and ChAdOx1 against Delta compared to the Alpha variant (Lopez Bernal et al., 2021; Sheikh et al., 2021), especially in protection from severe infections requiring hospitalization (Sheikh et al., 2021), whereas other studies documented a major reduction in vaccine effectiveness (Keehner et al., 2021; Rosenberg et al., 2022). Waning of immunity with time after vaccination is another factor contributing to reduced protection (Collier et al., 2021; Goldberg et al., 2021; Khoury et al., 2021; Pouwels et al., 2021; Thomas et al., 2021; Wall et al., 2021), which is difficult to differentiate from reduced effectiveness against the Delta or Omicron variants. Notwithstanding the decline of the antibody response observed six months after vaccination and the partial immune evasiveness of the Delta variant, we did not observe a resurgence of SARS-CoV-2 infections and hospitalizations during the Delta wave, as it was observed elsewhere (Keehner et al., 2021; Rosenberg et al., 2022). The peak monthly incidence of SARS-CoV-2 infection in vaccinated healthcare workers was similar during periods dominated by the Alpha or Delta variants. The high vaccination coverage at the population level, coupled with nonpharmacological measures, such as the persistence of indoor masking requirements, may have contributed to the reduction of virus circulation in the general population during the Delta wave, avoiding a significant resurgence of infections both in vaccinated and nonvaccinated individuals. A modeling study showed that increasing the rate of vaccination could have prevented substantial hospitalizations and deaths, even in the Delta-driven wave (Vilches et al., 2022). In addition, in our study, infections with the Delta variant appeared more severe in nonvaccinated patients because a higher proportion of nonvaccinated than vaccinated patients required intensive care, as also reported in other studies (Taylor et al., 2021; Tenforde et al., 2021). The vaccine evasion of the Omicron variant was even higher, as reflected by the increased breakthrough infections in vaccinated healthcare workers and the number of hospitalized patients. However, our data support the lower pathogenicity of this variant, as was suggested in early studies from South Africa (Maslo et al., 2022; Wolter et al., 2022). An indirect data is the fact that, although the monthly incidence of infection in vaccinated healthcare workers increased by almost 10 times from the Delta to the Omicron wave, the number of hospitalized patients only increased by about three times. Moreover, during the Omicron wave, most of the ICU-admitted patients were infected with the Delta variant, and the Delta variant was detected in all the patients with fatal outcomes, although Omicron accounted for more than 80% of the strains circulating in the general population. The viral variants detected in the hospitalized population may reflect those circulating in the general population about one to two weeks before hospitalization. Nevertheless, the prevalence of Omicron among patients admitted to the ICU appears to be lower than expected. Finally, hospitalized vaccinated patients during the Delta and Omicron waves were older than nonvaccinated patients admitted to hospital in both the pre- and postvaccination eras. In addition, during the Omicron wave, a higher proportion of vaccinated patients had concomitant chronic medical conditions than nonvaccinated patients. Also, among patients who died during the Omicron wave, a higher proportion of vaccinated patients had concomitant chronic medical conditions than nonvaccinated patients. These observations confirm lower vaccine effectiveness and faster waning of immunity in older individuals and those with underlying clinical conditions (Andrews et al., 2022) and more sustained protection in younger subjects. Limitations of this study reside in its observational retrospective nature, the lack of a noninfected control group to estimate vaccine effectiveness, the relatively low number of patients examined, and the partial availability of virus genotype. In addition, different behaviors and containment measures in the population occurring during the three study periods have influenced virus circulation. However, the stringency of containment measures was lower in the vaccination era, whereas a partial lockdown was implemented during the second wave of the prevaccination era. This may have potentially increased the number of hospitalizations concerning what may have occurred if, instead, the same measures applied during the second wave were maintained in the vaccination era. In conclusion, vaccinated patients hospitalized for COVID-19 during the Delta and Omicron waves are older and more fragile; the risk of developing more severe COVID-19 is lower in vaccinated individuals with Delta variant breakthrough infections than in nonvaccinated subjects, and the Omicron variant seems to cause less severe COVID-19. Vaccine effectiveness in fragile individuals appears to be lower because of a faster immunity decline and may take advantage of periodical vaccine boosters, which could be adjourned on the newly identified viral variants.
Funding
This work was partially supported by the European Union's Horizon 2020 Research and Innovation Program (ATAC, No. 101003650).
Ethical approval statement
Patients’ data are collected within the routine Regional Healthcare system surveillance of SARS-CoV-2 infection; therefore, informed consent was not required. The study was approved by the Medical Direction of Fondazione IRCCS Policlinico, San Matteo.
Author contributions
Conceptualization: FB.
Methodology: DL.
Formal analysis: FR, GLE, and DL.
Investigation: FR, GLE, MR, CR, EB, MD, AP, SP, GC, GF, FG, and FZ.
Resources: AMG, GG, and AO.
Data curation: FR, MR, VN, SC, and AM.
Writing – original draft preparation: DL.
Writing – review, and editing: FR and FZ.
Visualization: FR and DL.
Supervision: CM and FB.
Funding acquisition: FB.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.
Conflicts of interest
The authors have no competing interests to declare.
Acknowledgments
We thank all the technical staff of the Microbiology and Virology Unit.
References
- Andrews N, et al. Duration of protection against mild and severe disease by COVID-19 vaccines. N Engl J Med. 2022;386:340–350. doi: 10.1056/NEJMoa2115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel Y, et al. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325:2457–2465. doi: 10.1001/jama.2021.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden LR, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AY, et al. Differential kinetics of immune responses elicited by COVID-19 vaccines. N Engl J Med. 2021;385:2010–2012. doi: 10.1056/NEJMc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagan N, et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardina F, et al. No evidence of SARS-CoV-2 circulation in the framework of influenza surveillance between October 2019 and February 2020 in Lombardy, Italy. Travel Med Infect Dis. 2021;40 doi: 10.1016/j.tmaid.2021.102002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg Y, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385:e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas EJ, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall VJ, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehner J, et al. Resurgence of SARS-CoV-2 infection in a highly vaccinated health system workforce. N Engl J Med. 2021;385:1330–1332. doi: 10.1056/NEJMc2112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury DS, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Lilleri D, et al. SARS-CoV-2 infection in vaccinated health care workers. N Engl J Med. 2022;386:199–200. doi: 10.1056/NEJMc2117119. [DOI] [PubMed] [Google Scholar]
- Lopez Bernal J, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Bernal J, et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslo C, et al. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 omicron wave compared with previous waves. JAMA. 2022;327:583–584. doi: 10.1001/jama.2021.24868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack FP, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels KB, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27:2127–2135. doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg ES, et al. COVID-19 vaccine effectiveness in New York State. N Engl J Med. 2022;386:116–127. doi: 10.1056/NEJMoa2116063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovida F, et al. Incidence of SARS-CoV-2 infection in health care workers from Northern Italy based on antibody status: immune protection from secondary infection- a retrospective observational case-controlled study. Int J Infect Dis. 2021;109:199–202. doi: 10.1016/j.ijid.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovida F, et al. SARS-CoV-2 vaccine breakthrough infections with the alpha variant are asymptomatic or mildly symptomatic among health care workers. Nat Commun. 2021;12:6032. doi: 10.1038/s41467-021-26154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N Engl J Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh A, et al. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CA, et al. Severity of disease among adults hospitalized with laboratory-confirmed COVID-19 before and during the period of SARS-CoV-2 B.1.617.2 (delta) predominance - COVID-NET, 14 states, January–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1513–1519. doi: 10.15585/mmwr.mm7043e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenforde MW, et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326:2043–2054. doi: 10.1001/jama.2021.19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SJ, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasileiou E, et al. Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397:1646–1657. doi: 10.1016/S0140-6736(21)00677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilches TN, et al. COVID-19 hospitalizations and deaths averted under an accelerated vaccination program in northeastern and southern regions of the USA. Lancet Reg Health Am. 2022;6 doi: 10.1016/j.lana.2021.100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall EC, et al. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet. 2021;397:2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter N, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399:437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.