Abstract
Cats are susceptible to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and risk transmitting viruses to naive cats or humans. Here, based on our novel adenovirus-vectored COVID-19 vaccine, the immunogenicity of Sad23L-nCoV-S vaccine was evaluated in cats by prime-boost vaccinations. Five cats were primed with a dose of 108 plaque-forming units (PFUs) Sad23L-nCoV-S vaccine and then boosted with an equal dose of same vaccine at a 4-week interval. Cat serum neutralizing antibody (NAb) titers (the sample dilution at which 50% inhibitory concentration [IC50]) were measured as IC50 15,849 to wild-type strain, IC50 6,591 to Alpha, IC50 2,315 to Beta, IC50 2,744 to Gamma, IC50 1,848 to Delta, and IC50 318 to Omicron variants of pseudotyped SARS-CoV-2 viruses at week 6 post-prime vaccination. All NAb levels to these five variants were ≥IC50 49 from vaccinated cats at week 10, while 48.8% to Delta and 100% to Omicron variants were <IC50 10 from human vaccinees at week 2 or 4 after receiving two injections of the inactivated SARS-CoV-2 vaccines. Robust T cell response of interferon (IFN)-γ to S peptides were detected in vaccinated cats. It was concluded that Sad23L-nCoV-S vaccine could be a promising vaccine candidate against SARS-CoV-2 infection in cats by prime or plus boost vaccinations.
Keywords: SARS-CoV-2, major variants, adenovirus vector, vaccine, cats
Graphical abstract

Cats can be symptomatically infected with SARS-CoV-2. In this study, the protective immunity of a novel adenovirus-vectored COVID-19 vaccine against wild type and five major variants of pseudotyped SARS-CoV-2 was evaluated in cats, suggesting the vaccine could be a promising candidate for cats by prime or plus boost vaccinations.
Introduction
Coronavirus disease 2019 (COVID-19) is caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); it has become a global pandemic, severely injuring human health and heavily hampering the world economy.1,2 SARS-CoV-2 is thought to be transmitted from bats to humans through intermediate animal hosts.3 There were several reports demonstrating that domestic pets (especially cats and dogs) were infected due to SARS-CoV-2-infected humans from more than 10 countries, suggesting that the SARS-CoV-2 transmission could occur from humans to animals.4,5 It is now found that cats are susceptible to SARS-CoV-2 infection with mild-to-moderate clinical symptoms and risk transmitting viruses to naive cats or humans.6,7
According to the World Health Organization (WHO) draft landscape of COVID-19 candidate vaccines, there are more than 100 COVID-19 vaccines in the development focusing on humans, including virus vector-based vaccines, mRNA and DNA vaccines, subunit vaccines, and inactivated whole-virus vaccines.8 However, few vaccines are applied for preventing SARS-CoV-2 infection in animals. Recently, Russian researchers developed the first COVID-19 vaccine for animal use, designated as Carnivac-Cov. This vaccine was based on the inactivated vaccine development platform, which could induce SARS-CoV-2 immunity in dogs, cats, foxes, and mink.9 In addition, a new subunit vaccine, named NARUVAX-C19, was reported for cats in Nur-Sultan, which was tested protective against SARS-CoV-2 and Delta variant infections in cats.10 An alphavirus replicon-based vaccine expressing the SARS-CoV-2 S protein was able to induce high level of serum NAb and prevent infection of the upper respiratory tract in cats.11 In order to fight the threat of virus through animal-to-animal or animal-to-human transmission, it is necessary to develop COVID-19 vaccines for animals.
Four adenovirus-based COVID-19 vaccines were approved for emerging use in humans, including Ad5-S from CanSino Biological and Beijing Institute of Biotechnology, Ad26-S and Ad5-S from Gamaleya Research Institute, ChAdOx1 nCoV-19 from AstraZeneca and University of Oxford, and Ad26.COV2.S from Janssen Pharmaceutical. Clinical trials have demonstrated their good safety profile and broad and strong immune responses.12, 13, 14, 15, 16 Previously, we developed simian adenovirus serotype 23 vector (Sad23L) and human adenovirus serotype 49 vector (Ad49L)-based COVID-19 vaccines (Sad23L-nCoV-S and Ad49L-nCoV-S) and examined their immunogenicity in mice and rhesus monkeys.17,18 Here, we evaluated the protective immunity of Sad23L-nCoV-S vaccine against wild-type strain and Alpha, Beta, Gamma, Delta, and Omicron variants of pseudotyped SARS-CoV-2 in cats.
Results
Cat vaccination and tolerance
Ten healthy outbred British shorthair cats aged 1–3 years were randomly divided into two groups of sham (n = 5) and vaccination (n = 5). The pre-existing NAb (AdNAb) titers to Ad5 and Sad23L vectors were detected <1:20 prior to inoculation of vaccine (week 0) (Table S1). In addition, cats were detected negative for antibodies to feline enteric coronavirus and SARS-CoV-2 before immunization. The vaccination or sham cats were inoculated intramuscularly (i.m.) by prime immunization with a dose of 108 plaque-forming units (PFUs) Sad23L-nCoV-S vaccine or Sad23L-GFP control, respectively, and then boosted by the second inoculation with same vaccine or control at a 4-week interval. Blood samples were collected from cats at a 2-week interval after the first inoculation of vaccine (Figure 1A). Cat body temperature and weight (mean ± SD) were continuously monitored up to 10 weeks post-vaccination, and no significant difference was observed between vaccination and sham groups (36.58°C ± 0.43°C versus 36.55°C ± 0.39°C; 3.59 ± 0.38 kg versus 3.96 ± 0.46 kg) (Figures 1B and 1C), indicating the tolerance to Sad23L-nCoV-S vaccine.
Figure 1.
Clinical examination of Sad23L-nCoV-S-vaccinated cats
(A) Prime-boost vaccination regimen. Ten cats were randomly divided into vaccine group (n = 5) and sham group (n = 5), who were primed by injection of a dose of 108 PFUs Sad23L-nCoV-S vaccine or Sad23L-GFP vector sham control (blue), respectively, and then boosted by injection of same vaccine or vector control at a 4-week interval (red). Blood samples were collected at every 2 weeks after the first injection. (B and C) Temperature (B) and body weight (C) examinations were monitored during the course up to 10 weeks post-first inoculation.
Strong immunogenicity of Sad23L-nCoV-S vaccine in cats
Cat sera were measured for S1 binding antibody (S1-BAb) and S2 binding antibody (S2-BAb) by ELISA and NAb by pseudovirus neutralization test (pVNT), respectively. At week 6 post-prime-boost inoculations of vaccines, S1-BAb and S2-BAb immunoglobulin G (IgG) titers reached to 1:31,623 or 1:4,571 at the highest, respectively, and remained above 1:15,849 or 1:3,162 at the higher level until week 10 (Figure 2A). Both IgM titers showed 1:977 or 1:457 at the highest titers at week 2 post-prime immunization and then gradually decreased to 1:407 or 1:288 at week 10 post-prime-boost vaccinations (Figure 2B). The pseudoviruses harboring the spike (S) protein of wild-type SARS-CoV-2 strain were generated and used to test the pVNT NAb potency of vaccinated cats in comparison with vaccinated rhesus macaques, human vaccinees, and COVID-19 patients. NAb was measured as the IC50 titer (the sample dilution at which 50% inhibitory concentration) by pVNT (Figure S1). The data showed that cat NAb titers to wild-type strain rose to IC50 219 at week 2, IC50 7,762 at week 4, continually reached to IC50 15,849 at the highest at week 6 post-prime-boost vaccinations, and then maintained at the higher level >IC50 2,818 up to week 10, which was 7-fold higher than the highest titers at week 6 from immunized rhesus macaques and 58.8- or 4.8-fold higher than human NAb levels from vaccinees in 2 or 4 weeks after receiving two injections of the inactivated SARS-CoV-2 vaccines or convalescent patients of COVID-19 (p < 0.01; Figure 2C). The results suggested that Sad23L-nCoV-S vaccine elicited strong NAb response in cats.
Figure 2.
The immunity of Sad23L-nCoV-S vaccine to cats
(A and B) Detection of binding antibodies (BAbs) to SARS-CoV-2 S1 and S2 (S1-BAb or S2-BAb) IgG or IgM by ELISA. (C) Titration of NAb (IC50) in blood samples from vaccinated cats (red) to neutralize wild-type strain of pseudovirus by pVNT in comparison with vaccinated macaques (Mac.) (gray), human vaccinees (Vac.) (purple), and COVID-19 patients (Conv.) (green) is shown. Serum samples were taken from vaccinated rhesus macaques in 6 or 40 weeks post-prime-boost inoculations of Sad23L-nCoV-S and Ad49L-nCoV-S vaccines (n = 5), human vaccinees in 2 or 4 weeks after receiving two doses of inactivated SARS-CoV-2 vaccine inoculations (n = 41), and convalescent patients of COVID-19 (n = 21). (D) Examination of T cell response in PBMCs from vaccinated cats and sham control cats by ELISpot is shown. IFN-γ secreting T cells were stimulated with SARS-CoV-2 S peptides, and the reacted T cells were presented as spot-forming cells (SFCs)/million cells. Data are shown as mean ± SEM (standard errors of means). p values are analyzed by unpaired two-tailed t test and Mann-Whitney test. Statistically significant differences are indicated with asterisks (∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001). ns, not significant (p > 0.05).
Vaccine-induced cellular immunity might play an important role in protection against SARS-CoV-2 infection. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood samples of sham and vaccinated cats, of which the specific T cell response to SARS-CoV-2 S antigen was examined by ELISpot. The interferon (IFN)-γ-secreting T cell response (88.2–209.2 spot forming cells [SFCs]/million cells) was found significantly higher in Sad23L-nCoV-S vaccine immunized cats than that in Sad23L-GFP-inoculated sham cats post-prime vaccination (p < 0.05; Figure 2D).
NAb and T cell responses were detected at high levels in vaccinated cats after boosting immunization at the 4th week with a second dose of homologous vaccine (Figure 2). The anti-Sad23L vector NAb (AdNAb) was highly raised by prime vaccination (Figure S2), but it did not reduce boost vaccination efficacy evidently.
Neutralizing efficacy of NAb to pseudotyped variants of SARS-CoV-2 in cats
Five major variants of SARS-CoV-2 have been classified into the Alpha, Beta, Gamma, Delta, and Omicron variants by the WHO based on increased transmissibility and pathogenicity as variants of concern (VOCs).19 These five pseudovirus variants were generated for examining neutralization efficacy of serum NAb from vaccinated cats. The measurement accuracy of antibodies was demonstrated by the strong correlation between pVNT (except for Omicron) and S1-BAb or S2-BAb titers (p < 0.001; R = 0.858–0.956) (Figure S3).
The highest IC50 titers of pVNT NAb against the variants were found at week 6, of which the NAb titers were IC50 6,607 to Alpha (Figure 3A), IC50 2,291 to Beta (Figure 3B), IC50 2,754 to Gamma (Figure 3C), IC50 1,862 to Delta (Figure 3D), and IC50 316 to Omicron variants (Figure 3E), respectively. Comparing with neutralization potency to wild-type strain over weeks 2–10, cat NAb levels were reduced by 2.7-fold to Alpha, 8.8-fold to Beta, 5.8-fold to Gamma, 10-fold to Delta, or 39.5-fold to Omicron variants (Figures 4A and S4), respectively, but all those cat NAb titers at week 10 were at least ≥IC50 49 to five variants, including Omicron (Table S2), suggesting that Sad23L-nCoV-S-vaccinated cats produced potent NAb to the major variants of pseudoviruses.
Figure 3.
Neutralization potency of vaccinated cats, macaques, human vaccinees, and convalescent COVID-19 patients to the pseudotyped SARS-CoV-2 variants
Titration of NAb (IC50) in blood samples from sham or Sad23L-nCoV-S-vaccinated cats (weeks 0–10), Mac., Vac., and Conv. to neutralize Alpha (A), Beta (B), Gamma (C), Delta (D), and Omicron (E) of pseudoviruses by pVNT. The mean titer is marked on each group, and p values are analyzed by unpaired two-tailed t test and Mann-Whitney test. Statistically significant differences are indicated with asterisks (∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001).
Figure 4.
Resistance of major variants of SARS-CoV-2 to NAb neutralization in vaccinated cats, macaques, human vaccinees, and COVID-19 patients
(A) Cat NAb titers (IC50) of serum samples from vaccinated cats at weeks 2, 4, 6, 8, and 10 post-first inoculation of vaccine. (B) Serum NAb titers (IC50) at week 6 or 40 of immunized rhesus macaques (n = 10) are shown. (C) NAb titers (IC50) of human vaccinees in 2 or 4 weeks after receiving two doses of inactivated SARS-CoV-2 vaccines (n = 41) are shown. (D) NAb titers (IC50) of convalescent sera from COVID-19 patients (n = 21) are shown. Fold change, the ratio of NAb titers by wild type than variant; Wt, wild type.
In the control groups, the NAb titers to five variants from vaccinated rhesus macaques, human vaccinees who received two injections of the inactivated SARS-CoV-2 vaccines, or COVID-19 convalescent patients were 4.5- to 65-fold lower than those from vaccinated cats (p < 0.05; Figure 3). By comparing with neutralization efficacy to wild-type strain, serum NAb levels to Alpha, Beta, Gamma, Delta, and Omicron variants were decreased for 1.5-, 5.2-, 2.9-, 11.1-, or 24.2-fold from vaccinated rhesus macaques (Figure 4B); 1.6-, 3.8-, 3.6-, 4.7-, or 18.2-fold from COVID-19 human vaccinees (Figure 4C); and 2.1-, 6.5-, 4.9-, 14.9-, or 20.2-fold from convalescent patients of COVID-19 (Figure 4D), respectively. Among vaccinated rhesus macaques at week 6 or 40 post-vaccination, 90% NAb titers to Beta and Delta and 50% to Omicron variants were >IC50 10 (Table S3). Among human vaccinees’ NAb titers, 43.9% to Beta, 36.6% to Gamma, 48.8% to Delta, and 100% to Omicron variants were <IC50 10 (Table S4), suggesting waning of neutralization potency to these five variants. Comparing with human vaccinees, more than 76% COVID-19 convalescent patients had NAb titers >IC50 10 to Beta, Gamma, Delta, and Omicron variants (Table S5).
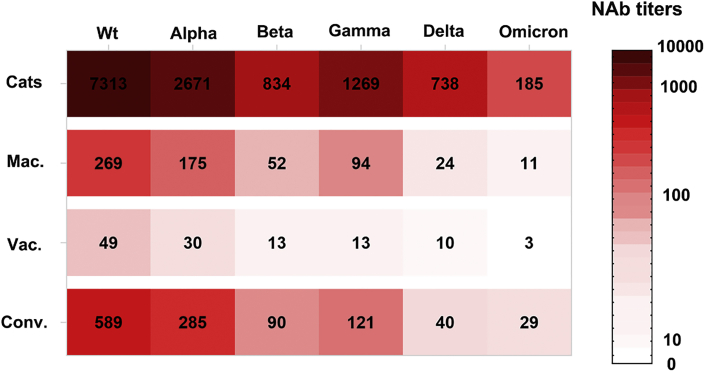
As summarized in Figure 5, the levels of NAb to wild type and variants of pseudotyped SARS-CoV-2 were shown in vaccinated cats, macaques, and humans and recovered COVID-19 patients. Comparing the resistance of variants to NAb neutralization, Omicron variant exhibited the most, Delta was the secondary, Beta and Gamma were the intermediate, and Alpha was the least. Comparing the neutralization potency between animal species and humans, the vaccinated cats were the highest and human vaccinees were the lowest. The results demonstrated that Sad23L-nCoV-S vaccine could elicit the effective NAb to neutralize pseudotyped SARS-CoV-2 in cats in vitro.
Figure 5.
The heatmap of NAb levels to wild type and major variants from Sad23L-nCoV-S-vaccinated cats and macaques, inactivated-SARS-CoV-2-vaccine-immunized human vaccinees, and COVID-19 patients
The color density in dark red to white represents the level of NAb from the high to low. The numbers indicate the mean IC50 titers of NAb from vaccinated cats, Mac., Vac., or Conv. capable of neutralizing the wild type or variant of pseudotyped SARS-CoV-2. The range of NAb titers is presented as the scale bar, in which the titer <IC50 10 is considered for no neutralization potency.
Discussion
Previous investigations found that cats were susceptible to SARS-CoV-2 and able to transmit virus to closely contacting mates.4,6 To date, all described infection cases in cats were related to the cat owners with COVID-19 or contacting with COVID-19 patients.20, 21, 22, 23 The infected domestic cats likely tend to be euthanized in many countries since no guarantee for blocking virus transmission by these cats exists. Vaccines may protect infected animal species and prevent virus transmission.9 So far, a live-inactivated SARS-CoV-2 vaccine (Carnivac-Cov) and an alphavirus-replicon-based vaccine are reported for cats.9,11 Therefore, more cat vaccines for SARS-CoV-2 are urgently demanded. In this study, the immunogenicity of previously developed Sad23L-nCoV-S vaccine has been evaluated in cats by immunizations with prime-boost regimen.
Sad23L-nCoV-S vaccine was constructed on a novel adenoviral vector (Sad23L) originated from serotype 23 simian adenovirus.24 Sad23L was already used in the development of Zika and COVID-19 vaccines and was not cross-reactive with human adenovirus serotype 5 (Ad5) vector.17,18,24 These vaccines were examined by intramuscularly inoculating mice, common marmosets, and rhesus monkeys, inducing the sufficient protection against Zika virus challenging25 or highly protective immunity to SARS-CoV-2 infection17,18 and presenting potential vaccine candidates for humans. In this study, cats were vaccinated with two injections of Sad23L-nCoV-S vaccines, acquiring the strongly specific NAb and IFN-γ secretion T cell response to SARS-CoV-2. The boosting injection with homologous Sad23L-nCoV-S vaccine enhanced immune response, which was not obviously suppressed by the pre-existing NAb (AdNAb) elicited with prime immunization. NAb levels from vaccinated cats, human vaccinees, and convalescent patients of COVID-19 were largely varied (Tables S2, S4, and S5). A recent study compared differential kinetics of immune responses in human vaccinees induced by the mRNA or adenovirus vectorial Ad26.COV2.S vaccines over an 8-month follow-up period, showing that the BNT162b2 and mRNA-1273 vaccines rapidly raised NAb response and then declined sharply by the end of 6 months, while Ad26.COV2.S vaccine initially induced lower NAb response but stayed relatively stable over 8 months.16 The reason the NAb response to Sad23L-nCoV-S vaccine in cats is much higher may be explained by its effective infectivity and long persistence of adenovirus-based vaccine in host cells and consistently expressing S proteins to induce the higher immune responses.16,17
The mRNA and novel adenovirus vectorial vaccines offered substantial protection against death in humans caused by Delta variant of SARS-CoV-2 infection,26 while the vaccine efficacy correlated with the level of NAb.27 In our study, we measured the neutralizing potency of Sad23L-nCoV-S-vaccinated cat NAb to five major variants of pseudotyped SARS-CoV-2 in comparison with the serum NAb from Sad23L-nCoV-S-vaccinated rhesus macaques, inactivated-vaccine-inoculated human vaccinees, and convalescent patients of COVID-19 (Figure 3). Our findings showed that cats were more sensitive to this vaccine immunization. At week 10 post-prime inoculation, Nab levels of five vaccinated cats to Omicron variant were between IC50 49 and 171 (mean:131) (Figure 3; Table S2), while NAb levels of 41 human vaccinees to Omicron variant were dropped to <IC50 10 or undetectable (Figure 3; Table S4). According to the predicative models that approximately 20% of the mean NAb titer of convalescent COVID-19 patients provides 50% protection against detectable SARS-CoV-2 infection,28 the cat NAb titers at week 10 were IC50 321 to Delta and IC50 131 to Omicron variants, respectively, higher than 20% of the mean NAb titer (IC50 589.2) to wild-type strain from convalescent patients of COVID-19 (Figure 3; Tables S2 and S5). Although the relatively small size samples of five vaccination and five sham cats were measured in this study, the significant differences in protective immunity were observed between two groups (Figures 2 and 3). These data suggested that Sad23L-nCoV-S vaccine could produce the sufficient neutralization potency to major variants of SARS-CoV-2 in cats, but the vaccine efficacy needed to be demonstrated further by challenge study with live SARS-CoV-2.
The emergence of Omicron has brought new challenges to fight against SARS-CoV-2. We found that only 25 of 41 vaccinated individuals maintained poorly neutralizing activity against Omicron (NAb titers <IC50 10) and the rest were undetectable (Tables S4), indicating that the human vaccinees who received two doses of inactivated vaccines almost lost their neutralizing potency against Omicron variant. The convalescent sera of COVID-19 patients (n = 21) displayed the mean NAb titer of IC50 29.3 to Omicron variant, which was decreased by 20.2-fold compared with the wild-type strain (IC50 589.2). Referring NAb titers from cats infected symptomatically with SARS-CoV-2 (wild type)29 or vaccinated with an alphavirus replicon-based vaccine,11 although cat NAb titers against Omicron variant were reduced by 39.5-fold compared with wild-type strain in our study, but the NAb titer still remained sufficiently high at IC50 131 (mean) in 10 weeks post-prime vaccination. However, due to biosafety restriction, the evaluation of vaccine efficacy in vaccinated cats was not conducted by challenging with live SARS-CoV-2.
In conclusion, Sad23L-nCoV-S vaccine could be a promising candidate against Omicron-variant-involved SARS-CoV-2 infection in cats by prime or plus boost vaccinations. A large scale clinical trial for Sad23L-nCoV-S vaccine in cats has been planned.
Materials and methods
Cats and ethics statement
Ten healthy outbred cats (Felinae, British shorthair) aged 1–3 years were randomly allocated to this study and maintained in the Ramical Pet Technology Company, Yunfu, China (Table S1). Cats tested negative for antibodies to feline enteric coronavirus and SARS-CoV-2. All animal care and experimental procedures (NFYYLASOP-037) were in accordance with national and institutional policies for animal health and well being. Animals were individually housed in spacious cages and were provided with commercial food pellets supplemented with appropriate treats. Blood samples were obtained using sterilized needle and syringe from the venous vessels of animal legs.
The care and use of cats in this study were evaluated and approved by the Animal Care and Use Committee at the Guangdong Medical Laboratory Animal Center, China (permit number: C202104-12).
Serum samples of human vaccinees, COVID-19 patients, and vaccinated rhesus macaques
Forty-one human vaccinees’ serum samples were collected from individuals in 2 or 4 weeks after receiving two injections of the inactivated SARS-CoV-2 vaccines at the university hospital. A total of 21 convalescent serum samples of COVID-19 patients were kindly provided by Shenzhen Center for Disease Control and Prevention (CDC), China.30 Macaque serum samples were collected at the 6th or 40th week from five rhesus macaques that were primed with a dose of 5 × 109 PFUs Sad23L-nCoV-S vaccine and boosted with an equal dose of Ad49L-nCoV-S vaccine at a 4-week interval as described previously.17 All serum samples were inactivated for 40 min by heating at 56°C in the water bath. All individual samples were aliquoted to several vials and stored at −80°C. This study was approved by the Medical Ethics Committee of Southern Medical University and Shenzhen CDC and followed the ethical guidelines of the 1975 Declaration of Helsinki.
Sad23L-nCoV-S vaccine
The replication-defective adenovirus-vectored Sad23L-nCoV-S vaccine encoding the full-length S protein gene of SARS-CoV-2 (Wuhan-1 isolate; GenBank: MN908947.3) was constructed and produced as described previously.17,18 Briefly, based on the genome backbone of simian adenovirus serotype 23 (GenBank: AY530877.1), the Sad23L vector was constructed by deleting the E1 and E3 regions, and the open reading frame 6 (ORF6) within E4 region was replaced by Ad5 element in order to improve the virus propagation efficiency. The S protein gene of SARS-CoV-2 was inserted into the deleted E1 site of Sad23L vector, which was designated as Sad23L-nCoV-S. The recombinant adenovirus strain was rescued and amplified by HEK293 cells. Sad23L-nCoV-S vaccine was purified by cesium chloride gradient centrifugation and aliquoted and stored at −80°C. The manipulations of adenovirus packaging, amplification, and purification were carried out in the biosafety level 2 (BSL-2) laboratory.
Adenovirus neutralizing antibody assay
Cat serum samples were collected and tested on HEK-293A cells for NAbs (AdNAb) to Ad5-GFP and Sad23L-GFP viruses by green fluorescent activity assay as previously described.17 AdNAb titers were defined as the maximum serum dilution that neutralized 50% of green fluorescent activity in comparison with Ad5-GFP- or Sad23L-GFP-infected cells under a fluorescence microscope.17,31
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) was used to detect the binding antibodies to SARS-CoV-2. The microtiter plates (Corning, USA) were coated at 4°C overnight with 1 μg/mL of SARS-CoV-2 S1 (Sino Biological, 40591-V08H) or S2 proteins (Sino Biological, 40590-V08H1). The plates were blocked with 3% BSA PBS buffer (pH 7.4) at 37°C for 2 h. Serum samples were 3-fold serially diluted with 1% BSA PBS buffer and added to the plates for incubating for 1 h. After washing the plates five times, the properly diluted goat anti-cat IgM-horseradish peroxidase (HRP) (Abcam, UK) or rabbit anti-cat IgG-HRP (Bioss, China) was added to the plates for 1 h at 37°C. After washing five times, 100 μL of 3,3',5,5'-tetramethylbenzidine (TMB) substrate was added to the plates for 10 min color development in dark at room temperature and then were terminated by adding 50 μL of 2% sulfuric acid. Finally, the microtiter plates were read at 450 nm by a BioTek Epoch microplate reader. Endpoint titer was defined as the highest reciprocal serum dilution that yielded an absorbance >0.2 and a ratio of signal than cutoff (S/CO) >1.17,32
Pseudovirus neutralization test
Pseudovirus harboring SARS-CoV-2 S protein of wild-type (Wuhan-1) strain, Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), or Omicron (B.1.1.529) variants were generated for measuring the NAb to SARS-CoV-2 by pVNT.17,32 Briefly, 5 μg HIV backbone plasmid psPAX2 (Addgene), 4 μg envelope plasmid pcDNA3.1-SARS-CoV-2 S (wild type or variants), and 2 μg luciferase reporter plasmid pLenti-CMV Puro-luc (Addgene) were co-transfected into 5 × 106 293T cells in a 10 cm2 plate by Lipofectamine 3000 transfection reagent (Invitrogen, USA) following the manufacturer’s instruction. After 48 h, the supernatants containing pseudovirus were collected, filtered through 0.45 μm filter, aliquoted, and stored at −80°C. For measuring the NAb activity, 2-fold serial dilutions of heat-inactivated serum sample were prepared and mixed with 50 μL of pseudovirus. The mixture was incubated at 37°C for 1 h and then added to 3 × 104 HEK293T-hACE2 cells (Sino Biological) in 96-well microplate. After 48 h incubation, the cells were lysed by Bright-Glo luciferase assay (Promega) according to the manufacturer’s instructions. The NAb titer was defined as the sample dilution at which a IC50 was observed in relative luciferase activity (RLU) to the average of pseudovirus-infected cell control wells. Inhibition rate (%) = (1 − sample RLU/virus control RLU) × 100.17,32
Isolation of peripheral blood mononuclear cells
PBMCs were freshly isolated within 6 h from whole blood of cats using a Vacutainer and Cat lymphocyte-separating kit (Solarbio Company, Beijing, China). An equal volume of PBS was mixed with cat blood in a centrifuge tube. Lymphocyte-separating medium was added to the tube, and the diluted blood was gently added over the separating medium and centrifuged by 1,800 rpm for 20 min at room temperature. The buffer coat was aspirated, diluted in 10 mL of PBS, and centrifuged again. The cell pellet was resuspended in RPMI 1640 (Gibco) with 10% fetal bovine serum (FBS), 1% L-glutamine, and penicillin/streptomycin.
Enzyme-linked immunospot
Cat IFN-γ ELISpotPLUS kits (MabTech) were used to determine SARS-CoV-2 S antigen-specific T lymphocyte response by enzyme-linked immunospot (ELISpot). The epitopes within S protein were predicted (http://www.iedb.org/), and 79 peptides (14 amino acids per peptide except for a peptide carrying 18 amino acids) were synthesized by Guangzhou IGE Biotechnology (Table S6). Each peptide was dissolved in water or DMSO to obtain a 5 mg/mL stock solution, and all peptides were mixed equally, aliquoted, and stored at −80°C. Peptide aliquots were thawed once and used immediately. PBMCs (1 × 105 cells/well) were stimulated with S peptide mixture (5 μg/mL) in duplicate and incubated with 5% CO2 for 48 h at 37°C. Spots were developed according to the kit’s instruction and then counted with a CTL Immunospot Reader (Cellular Technology). The results were expressed as SFCs per million cells.17,25
Statistical analyses
Antibody titers between groups were compared with unpaired two-tailed t test and Mann-Whitney test. Antibody titers between variants and wild-type strains were compared with the Wilcoxon signed-rank test. Antibody titers between S1-BAb (ELISA) and NAb (pVNT) or S2-BAb (ELISA) and NAb (pVNT) were analyzed using Pearson’s correlation coefficients.33 Statistically significant differences are indicated with asterisks (∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001). All graphs are generated with GraphPad Prism 8 software.
Data availability statement
All data are available from the main article or supplemental information.
Acknowledgments
The authors thank Guangdong Medical Laboratory Animal Center for ethical evaluation and approval of use of cats in this study and Dr. Ruiai Chen and Dr. Guangcai Ren (Agricultural University of South China) for recommending the laboratory cats and maintenance. This work was supported by the grants from the National Natural Science Foundation of China (no. 32070929, 81871655, and 31770185), the Postdoctoral Science Foundation of China (2021M691474), Guangdong Basic and Applied Basic Research Foundation (2021A1515110991), and Guangzhou Bai Rui Kang (BRK) Biological Science and Technology Limited Company (Guangzhou, China).
Author contributions
C.Li, T.L., S.L., and P. Zhang designed the research. P. Zhang, S.L., L.Z., P. Zou, C. Liang, and C.W. carried out the experiments. J.L., Y.L., and G.W. provided COVID-19 patients or human vaccinees blood samples. P. Zhang, L.Z., T.L., and C. Li analyzed data and wrote the paper.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtm.2022.06.011.
Contributor Information
Ling Zhang, Email: zhangling1982@163.com.
Tingting Li, Email: apple-ting-007@163.com.
Chengyao Li, Email: chengyaoli@hotmail.com.
Supplemental information
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halfmann P.J., Hatta M., Chiba S., Maemura T., Fan S., Takeda M., Kinoshita N., Hattori S.I., Sakai-Tagawa Y., Iwatsuki-Horimoto K., et al. Transmission of SARS-CoV-2 in domestic cats. N. Engl. J. Med. 2020;383:592–594. doi: 10.1056/nejmc2013400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Q., Zhang H., Gao J., Huang K., Yang Y., Hui X., He X., Li C., Gong W., Zhang Y., et al. A serological survey of SARS-CoV-2 in cat in Wuhan. Emerg. Microb. Infect. 2020;9:2013–2019. doi: 10.1080/22221751.2020.1817796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B., Liu R., He X., Shuai L., Sun Z., et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. 2020;368:1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzales J.L., de Jong M.C.M., Gerhards N.M., Van der Poel W.H.M. The SARS-CoV-2 reproduction number R0 in cats. Viruses. 2021;13:2480. doi: 10.3390/v13122480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization Draft Landscape of COVID-19 Candidate Vaccines on 5 November 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines World Health Organization.
- 9.Chavda V.P., Feehan J., Apostolopoulos V. A veterinary vaccine for SARS-CoV-2: the first COVID-19 vaccine for animals. Vaccines. 2021;9:631. doi: 10.3390/vaccines9060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabynov K., Orynbassar M., Yelchibayeva L., Turebekov N., Yerubayev T., Matikhan N., Yespolov T., Petrovsky N., Tabynov N. A Spike protein-based subunit SARS-CoV-2 vaccine for pets: safety, immunogenicity, and protective efficacy in juvenile cats. Front. Vet. Sci. 2022;9:815978. doi: 10.3389/fvets.2022.815978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langereis M.A., Albulescu I.C., Stammen-Vogelzangs J., Lambregts M., Stachura K., Miller S., Bosco-Lauth A.M., Hartwig A.E., Porter S.M., Allen M., et al. An alphavirus replicon-based vaccine expressing a stabilized Spike antigen induces protective immunity and prevents transmission of SARS-CoV-2 between cats. NPJ Vaccines. 2021;6:122. doi: 10.1038/s41541-021-00390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu F.C., Li Y.H., Guan X.H., Hou L.H., Wang W.J., Li J.X., Wu S.P., Wang B.S., Wang Z., Wang L., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. 2020;395:1845–1854. doi: 10.1016/s0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/nejmoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., et al. Oxford COVID Vaccine Trial Group Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logunov D.Y., Dolzhikova I.V., Shcheblyakov D.V., Tukhvatulin A.I., Zubkova O.V., Dzharullaeva A.S., Kovyrshina A.V., Lubenets N.L., Grousova D.M., Erokhova A.S., et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/s0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Collier A.R.Y., Yu J., McMahan K., Liu J., Chandrashekar A., Maron J.S., Atyeo C., Martinez D.R., Ansel J.L., Aguayo R., et al. Differential kinetics of immune responses elicited by covid-19 vaccines. N. Engl. J. Med. 2021;385:2010–2012. doi: 10.1056/nejmc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo S., Zhang P., Liu B., Yang C., Liang C., Wang Q., Zhang L., Tang X., Li J., Hou S., et al. Prime-boost vaccination of mice and rhesus macaques with two novel adenovirus vectored COVID-19 vaccine candidates. Emerg. Microb. Infect. 2021;10:1002–1015. doi: 10.1080/22221751.2021.1931466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo S., Zhang P., Zou P., Wang C., Liu B., Wu C., Li T., Zhang L., Zhang Y., Li C. A self-biomineralized novel adenovirus vectored COVID-19 vaccine for boosting immunization of mice. Virol. Sin. 2021;36:1113–1123. doi: 10.1007/s12250-021-00434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization 2021. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants
- 20.Segalés J., Puig M., Rodon J., Avila-Nieto C., Carrillo J., Cantero G., Terrón M.T., Cruz S., Parera M., Noguera-Julián M., et al. Detection of SARS-CoV-2 in a cat owned by a COVID-19-affected patient in Spain. Proc. Natl. Acad. Sci. U S A. 2020;117:24790–24793. doi: 10.1073/pnas.2010817117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garigliany M., Van Laere A.S., Clercx C., Giet D., Escriou N., Huon C., van der Werf S., Eloit M., Desmecht D. SARS-CoV-2 natural transmission from human to cat, Belgium, March 2020. Emerg. Infect. Dis. 2020;26:3069–3071. doi: 10.3201/eid2612.202223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sit T.H.C., Brackman C.J., Ip S.M., Tam K.W.S., Law P.Y.T., To E.M.W., Yu V.Y.T., Sims L.D., Tsang D.N.C., Chu D.K.W., et al. Infection of dogs with SARS-CoV-2. Nature. 2020;586:776–778. doi: 10.1038/s41586-020-2334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenz O.C., Marques A.D., Kelly B.J., Rodino K.G., Cole S.D., Perera R.A.P.M., Weiss S.R., Bushman F.D., Lennon E.M. SARS-CoV-2 Delta variant (AY.3) in the feces of a domestic cat. Viruses. 2022;14:421. doi: 10.3390/v14020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo S., Zhang P., Ma X., Wang Q., Lu J., Liu B., Zhao W., Allain J.P., Li C., Li T. A rapid strategy for constructing novel simian adenovirus vectors with high viral titer and expressing highly antigenic proteins applicable for vaccine development. Virus Res. 2019;268:1–10. doi: 10.1016/j.virusres.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Luo S., Zhao W., Ma X., Zhang P., Liu B., Zhang L., Wang W., Wang Y., Fu Y., Allain J.P., et al. A high infectious simian adenovirus type 23 vector based vaccine efficiently protects common marmosets against Zika virus infection. PLoS Neglected Trop. Dis. 2020;14:e0008027. doi: 10.1371/journal.pntd.0008027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheikh A., Robertson C., Taylor B. BNT162b2 and ChAdOx1 nCoV-19 vaccine effectiveness against death from the Delta variant. N. Engl. J. Med. 2021;385:2195–2197. doi: 10.1056/nejmc2113864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert P.B., Montefiori D.C., McDermott A.B., Fong Y., Benkeser D., Deng W., Zhou H., Houchens C.R., Martins K., Jayashankar L., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 29.Cardillo L., de Martinis C., Brandi S., Levante M., Cozzolino L., Spadari L., Boccia F., Carbone C., Pompameo M., Fusco G. SARS-CoV-2 serological and biomolecular analyses among companion animals in campania region (2020-2021) Microorganisms. 2022;10:263. doi: 10.3390/microorganisms10020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang C., Liu B., Li J., Lu J., Zhang E., Deng Q., Zhang L., Chen R., Fu Y., Li C., Li T. A nanoenzyme linked immunochromatographic sensor for rapid and quantitative detection of SARS-CoV-2 nucleocapsid protein in human blood. Sens. Actuators Chem. 2021;349:130718. doi: 10.1016/j.snb.2021.130718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Q., Sun Y., Xu Y., Wang Y., Wang H., Fu Y., Allain J.P., Li C., Li T. Seroprevalence of human adenovirus type 5 neutralizing antibody in common marmosets determined by a new set of two assays. Viral Immunol. 2019;32:348–354. doi: 10.1089/vim.2019.0054. [DOI] [PubMed] [Google Scholar]
- 32.Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H., Nkolola J.P., Liu J., Li Z., Chandrashekar A., et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.I.C., Tiu C., Hu Z., Chen V.C.W., Young B.E., Sia W.R., et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the main article or supplemental information.