Abstract
Therapeutic vaccines are currently at the forefront of medical innovation. Various endeavors have been made to develop more consolidated approaches to producing nucleic acid-based vaccines, both DNA and mRNA vaccines. These innovations have continued to propel therapeutic platforms forward, especially for mRNA vaccines, after the successes that drove emergency FDA approval of two mRNA vaccines against SARS-CoV-2. These vaccines use modified mRNAs and lipid nanoparticles to improve stability, antigen translation, and delivery by evading innate immune activation. Simple alterations of mRNA structure- such as non-replicating, modified, or self-amplifying mRNAs- can provide flexibility for future vaccine development. For protein vaccines, the use of long synthetic peptides of tumor antigens instead of short peptides has further enhanced antigen delivery success and peptide stability. Efforts to identify and target neoantigens instead of antigens shared between tumor cells and normal cells have also improved protein-based vaccines. Other approaches use inactivated patient-derived tumor cells to elicit immune responses, or purified tumor antigens are given to patient-derived dendritic cells that are activated in vitro prior to reinjection. This review will discuss recent developments in therapeutic cancer vaccines such as, mode of action and engineering new types of anticancer vaccines, in order to summarize the latest preclinical and clinical data for further discussion of ongoing clinical endeavors in the field.
Keywords: Cancer vaccines, SARS-CoV-2, mRNA vaccines, Neoantigens, Synthetic long peptides, Neoadjuvant, Checkpoint inhibitors
Abbreviations: NSCL, non-small cell lung cancer; SCC, squamous cell cancer; TNBC, triple negative breast cancer; mCRC, metastatic colorectal cancer; mPC, metastatic pancreatic cancer; TNBC, triple negative breast cancer, SCLC, small cell lung cancer; TAA, tumor-associated antigen; LNP, lipid nanoparticle; SNL, synthetic long peptide; MHC I and MHC II, Major Histocompatibility Complex I and II; DC, Dendritic Cells; SAM, virus-derived self-amplifying mRNAs; i.d., intradermal; i.m., intramuscular; i.n., intranodal; i.v., intravenous; s.c., subcutaneous; FDA, Federal and Drug Administration; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TME, tumor micro environment; Tregs, regulatory T cells; ROS, reactive oxygen species; SAM, self-amplifying mRNAs; IVT, synthesized through in vitro transcription; TAAs, abnormally expressed proteins; APC, antigen-presenting cells; ORF, open reading frame; UTR, untranslated region; TLR, Toll-like receptors; TCR, T-cell receptor; HPV, human papillomavirus; VLP, targets virus-like particles; VSV, vesicular stomatitis virus; HNPCC, hereditary non-polyposis colorectal cancer; MSH-2, mouse model for the lynch syndrome; PD-1/PD-L1, programmed death protein-1/ligand1; NAP, Naproxen; CTLA-4, cytotoxic T-lymphocyte antigen 4; VEGFR, vascular endothelial growth factor receptor; MDSCs, myeloid-derived suppressor cells; DC-IL12-OVA, DC-based vaccine expressing IL-12, pulsed with OVA-peptide; CEA, carcinoembryogenic antigen
Background
Cancer is a significant health problem, with nearly 10 million deaths every year [1]. Besides protecting the organism from pathogens, the immune system's role is also useful for surveying the body to maintain cellular homeostasis. However, tumor cells can escape immune surveillance either by a selection of non-immunogenic tumor cell variants (immunoselection) or by actively suppressing immune response (immunesubversion)[2]. Advancements in immunotherapy have brought forth new potential therapies and prophylactic treatments that could lead to anticancer vaccines. Tumors display on their surface specific proteins generated when certain mutations occur in tumor DNA, and these proteins are called neoantigens. The body can generate an immune response against cancer cells through the help of neoantigens. Therefore, artificially triggering an immune response against tumor neoantigens constitutes the foundation for vaccines against tumors. Neoantigens are newly formed antigens that the immune system has not previously recognized. Neoantigens can arise from somatic mutation, alternative splicing, or viral proteins.
Latest vaccine-engineering strategies include administration of antigens as inactivated tumor cell extracts, purified mutated tumor proteins, or DNA and mRNA for endogenous production of tumor antigens, combined with various adjuvants and systems of delivery [3]. Two major two challenges to the development of cancer vaccines are the identification of neoantigens and the generation of new molecular epitopes recognized as foreign by the immune system to elicit a robust immune response against tumor cells. Results from ongoing clinical trials corroborate the information on therapeutic anticancer vaccine safety, with some studies indicating a potential efficacy.
This review focuses on recent advancements in therapeutic cancer vaccines, going from latest vaccine technologies, identification of neo-antigens methods, to ongoing investigations in animals and humans.
Cancer vaccines: from biological mechanisms to engineering
Historically, vaccines are used to prevent diseases caused by infectious pathogens. Present-day vaccines are expanding to cancer as well. Early cancer therapeutic vaccines failed to amplify de novo T cell responses primarily because they targeted abnormally expressed tumor-associated antigens proteins (TAAs) or self-proteins on tumor cells [4]. Therapeutic cancer neoantigen strategies are highly advantageous since they home on an antigen while preventing central and peripheral tolerance and potential ‘off-target’ tissue damage observed in previous TAA-targeting strategies [5].
One critical step to developing a cancer vaccine is identifying and selecting appropriate neoantigens or neoepitope targets expressed exclusively by cancer cells. The immune system readily mounts a CD4+ and CD8+ T cell response to foreign proteins but tolerates self-proteins retained by cancer [6]. Generally, antigens with a heavy mutational burden make neoantigen identification more accessible and more likely to result in a tumor cell-specific immune response.
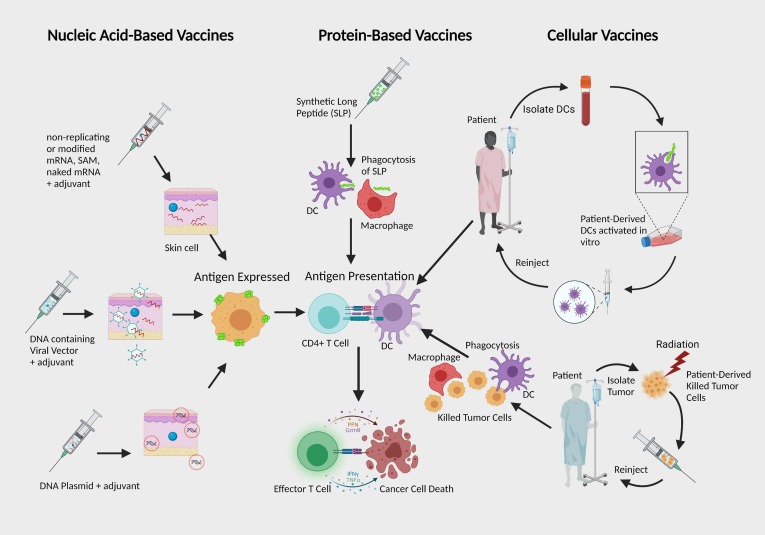
Another challenge lies with the tumor microenvironment (TME), which has many adverse qualities such as generation of hypoxia, nutrient depletion, low pH, an increase of reactive oxygen species (ROS), and a high number of regulatory T cells (Tregs). Also, solid tumors have other barriers such as tumor fibroblasts (fibrotic extracellular matrix), myeloid suppressor cells, and Tregs that further reduce the number of tumor-infiltrating T cells [7], [8], [9]. Other limitations include low tumor mutational burden, antigen escape, and antigen-presenting cells (APCs) ability. Advancements in immunotherapy have brought forth new potential therapies and prophylactic treatments that could lead to anticancer vaccines. Tumors display on their surface specific proteins generated when certain mutations occur in tumor DNA, and these proteins are called neoantigens. Vaccines with effective delivery methods, adjuvants, and appropriate antigens can potentially overcome these immunosuppressive obstacles. The most common types of therapeutic cancer vaccines that have been designed are nucleic acid vaccine (RNA or DNA), long synthetic peptide (SLP) vaccine, and cellular vaccine (tumor cell or on dendritic cell (DC)-based) (Fig. 1 ) [10], [11].
Fig. 1.
The Main Types of Cancer Therapeutic Vaccines: Cancer vaccines primarily deliver antigens either nucleic acids, proteins, peptides, or patient-derived cells. Within nucleic acid-based vaccines, RNA has various approaches that differ with RNA structure manipulation and delivery compared to DNA, which is restricted by exclusively relying on plasmids to deliver antigen-encoding genetic materials. Both mRNA and DNA are taken up by cells and eventually translated into protein antigens APCs present to activate T cells. Cellular vaccines depend on patient-derived cells to deliver isolated tumor cells that are killed, or the patient’s DCs are activated in vitro with purified tumor antigens before reinjection. All therapeutic cancer vaccine types aim for antigen presentation followed by T cell activation and tumor rejection. Abbreviations: DC, Dendritic Cells; SAM, virus-derived self-amplifying mRNAs; SLP, synthetic long peptide.
Nucleic Acid-based vaccines
mRNA encodes target antigens expressed after administered mRNA is efficiently taken up and translated by local cells. This factor is beneficial for making mRNA available as an off-the-shelf cancer vaccine and for personalized neoantigen vaccination [12]. Another significant advantage is that mRNA encoded proteins can undergo post-translation modifications such as glycosylation, acetylation, methylation, or phosphorylation to become mature folded proteins, an essential condition to be appropriately antigenic. Previously, non-formulated or “naked” mRNA injection was shown to deliver vaccine components to the lymph nodes of mice. It also was observed that RNA directly injected into lymph node tissue effectively targets APCs and promotes high IL-12 secretion and expression of CD86 on DCs. This, in turn, promoted active proliferation and infiltration of CD8+ T and CD4+ T cells in lymph node tissue compared to controls [13]. Other methods include injecting liposome-encapsulated mRNA to increase adjuvanticity, or injecting self-adjuvanted RNA to promote cellular and humoral responses and Th1 and Th2 cell activation [14]. Self-adjuvanted mRNA molecules are mRNAs whose encoded protein expression has been enhanced by 4–5 orders of magnitude by modification of nucleotide sequences with naturally occurring nucleotides (A/G/C/U) that do not affect primary aminoacid sequence. In addition, they are complexed with protamine, thus activate immune system by the involvement of toll-like receptor (TLR) 7. Self-adjuvanted RNA vaccines induce strong, balanced immune responses comprising humoral and cellular responses, effector and memory responses, and also activate important subpopulations of immune cells such as Th1 and Th2 cells [14].
Recently, a new promising platform called the KISIMA vaccine can select tumor antigen, makes cell-penetrating peptides to improve antigen delivery and epitopes presentation and finally, it uses TLR2/4 agonist as self-adjuvant. KISIMA was used in a study to produce a vaccine against achaete-scute family bHLH transcription factor 2 (Ascl2), an antigen found in early colon cancer. The vaccine could reduce colon tumor formation by stimulation of an antitumor immune response. Combining this vaccine with anti-PD-1, significantly reduced development of colon adenomas and macroadenomas, vs. negative controls, and may be used in patients at high-risk of incurring it [15].
The United States Food and Drug Administration (US FDA) has recently approved lipid nanoparticles (LNP)-loaded mRNA for COVID-19 [12], [16]. Currently, three types of LNP-loaded RNA vaccines are being studied in clinical trials for solid tumors: modified or unmodified non-replicating mRNA, and virus-derived self-amplifying mRNAs (SAM). Both non-replicating mRNA and SAM have been synthesized through in vitro transcription (IVT).
IVT uses a bacteriophage RNA polymerase and DNA template for target antigen sequences to synthesize RNA cell-free. This method has proven easy for large-scale production of eukaryotic-like mRNAs, which contain an open reading frame (ORF) for a target antigen, flanked by 5′ and 3′ untranslated regions (UTR) bearing a 5′ 7-methylguanosine cap and 3′ poly adenine poly(A) tail. The poly(A) tail is transcribed from DNA templates or is added post-IVT by enzymes. Non-replicating mRNAs contain optimized 3′ and 5′ UTR and the ORF target antigen sequence, but not additional sequences for RNA replication (i.e., the viral replication machinery). Therefore, these mRNA sequences cannot self-replicate [17].
SAM contains two ORFs, one encoding targeted antigen sequence, while the other machinery for viral replication is to support intracellular RNA amplification. After internalization, SAM enters the cytosol to be replicated or transcribed in mRNA and translated into proteins by ribosomes. Following translation and post-translational modification, the protein is folded and becomes functional [18].
Modified mRNAs containing pseudo nucleotides such as, 1-methylpseudouridine, 5-methylcytidine, or N4-aceylcytidine to replace uridine and cytidine, improve not only translational efficiency and stability of the mRNA but also increase immune response potency [19]. Karikó et al. found that modified nucleoside mRNAs decreased innate immunity by reducing the activation of RNA sensors such as Toll-like receptors (TLR) and RNA-dependent protein kinase (PKR). Interestingly, it was previously observed that reduced innate immune activation prevents abolition of mRNA translation into proteins. It should be noted that the phage polymerase contained in IVT can yield short RNA contaminants, which promote innate immunity by activating intracellular PRRs. However, purification by high-performance liquid chromatography (HPLC), permits the recovery of mRNA that decreases inflammatory cytokines activating innate immunity [20], [21], [22]. In general, mRNA is an innovative and powerful cancer vaccine platform currently tested for cancer vaccination after recent advances in the field.
DNA vaccines contain closed circular DNA plasmids of bacterial origin encoding desired antigens, which are transcribed into mRNAs and translated to proteins to induce antigen-specific immune responses. Like RNA vaccines, DNA vaccines promote humoral and cellular responses specific to target antigens. In addition, bacterial DNA stimulates TLRs or membrane-bound receptors that are critical in aiding DCs, B cells, and natural killer cells in recognizing pathogen-associated molecular patterns [23]. This leads to a pro-inflammatory response cascade with cytokine production. Gardasil (Merck) is one well-known vaccine quadrivalent, HPV L1 virus-like particle (VLP) recombinant vaccine, which targets oncogenic subtypes of human papillomavirus (HPV) through the production of HPV-neutralizing antibodies. The first Gardasil vaccine protected against HPV types 6, 11 and 18. Currently, Gardasil-9 vaccine contains HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58. The VLPs of Gardasil-9 are generated through the self-assembly of 360 copies of the L1 major capsid protein of the virus. Gardasil-9 VLP vaccine has been approved for use in the US against nine HPV strains and may give protection of 80% of cervical cancers [24], [25]. Although the current subunit HPV vaccination provides effective prophylaxis, they do not eradicate existing infections. Therefore, other HPV vaccination strategies have begun evaluation. DNA vaccination targeting HPV proteins E6 and E7, both associated with HPV 16 and 18, results in poor immunogenicity, possibly because the virus evades host recognition [26]. To overcome this obstacle, DNA vaccination is combined with other immunotherapies. Recently, Peng et al. tested a DNA vaccine that targets E6 and E7 HPV proteins combined with PD-1 blockade in mice. Results indicated that combined therapy led to significantly prolonged survival time [26]. One type of DNA vaccine currently undergoing preclinical investigation is chimeric DNA vaccines, which encode xenogeneic antigens, homologous with the self-orthologue, but originated from a different species [27]. The xenogeneic antigens are recognized as foreign, and may help override immune tolerance for TAAs that are recognized as self-antigens. Quaglino et al. designed a xenogeneic vaccine that consisted of a plasmid encoding chimeric rat/human ErbB2 proteins, which was administered to mice with ErbB2+ mammary tumors [28]. ErbB2 is an epidermal growth factor often highly displayed on some colorectal, pancreatic, endometrial, gastric, and breast cancers [29]. Results indicated that the chimeric/xenogeneic vaccine induced more robust antitumor responses than autologous controls [30]. Corroborating this data, in humans a phase I trial using gp100 plasmid DNA led several patients to increased levels of gp100-CD8+ T cells [31]. DNA is more stable than RNA, and its safety makes DNA vaccination an attractive strategy. However, its delivery is more complex than RNA due to its higher dimension and the need for nuclear localization. Improving transfection methods to in vivo delivery is necessary to enhance vaccine efficacy [32]. Ongoing clinical trials investigating nucleic-acid vaccines are summarized in Table 1 .
Table 1.
Ongoing clinical trials investigating nucleic acid vaccines in cancer.
| Clinical Trial Identifier Code | Investigation Plan | Vaccines, Drug/s | Clinical Setting Lines of therapy | Primary Endpoint | Stage of Development | Clinical Trials Status |
|---|---|---|---|---|---|---|
| NCT03970746 | 64 participants, Non-Randomized, Sequential Assignment, Open label | PDC*lung01, Keytruda Injectable Product, Alimta | Wash out of 4 weeks since last cycle of chemotherapy | DLT | 1/2 | Recruiting |
| NCT02439450 | 121 participants, Non-Randomized, Parallel Assignment, Open label | Viagenpumatucel-L, Nivolumab, Pembrolizumab, Pemetrexed | Second or later | TEAEs, ORR, PFS | 1/2 | Active, not recruiting |
| NCT02960230 | 49 participants, Non-Randomized, Parallel Assignment, Open label | K27M peptide, Nivolumab | Second line | K27M peptide, Nivolumab | 1/2 | Recruiting |
| NCT01773395 | 123 participants, Randomized, Parallel Assignment, Triple (Participant, Care Provider, Investigator) | GVAX, Busulfan, Fludarabine, Tacrolimus, Methotrexate | First line | 18/month PFS | 2 | Active, not recruiting |
Peptide-based vaccines
Antigens must be on the surface and be presented by the Major Histocompatibility Complex I or II (MHC I or II) molecules in order for T cells to recognize them [33]. Peptide-based vaccines are specific, safe, and rely heavily on the strong, adaptive immune response to initiate cancer-killing effects [34].
The peptide-MHC and T cell receptor interactions are highly immunogenic and result in less ‘off-target’ toxicity and central tolerance when neoantigens are targeted. The Synthetic Long Peptide (SLP) vaccines are subunit vaccines made from peptides that mimic epitopes of antigen that trigger direct or potent immune responses. SLPs containing class-I and class-II MHC restricted neoepitopes can induce neoantigen-reactive CD8+ T-cell responses and CD4+ T-cell responses which drive direct antitumor effects. Immunogenicity typically increases with peptides that include multiple epitopes and recognition motifs. This strategy avoids central tolerance and bolsters CD4 and CD8 T cell responses. Short peptides (∼9 aminoacid residues) can be exogenously loaded onto MHC-I molecules of non-professional APCs, leading to a poor T cell response [35]. Shorter peptides are easily digested by enzymes, and thereby are quickly eliminated in the human blood serum. Longer peptides (25–35 aminoacid residues) are more readily endocytosed by professional APCs, which have the proper machinery to allow complete T cell activation and antigen presentation by MHC-II molecules [36]. Short peptides also restrict HLA-type, making broad coverage of HLA-types a greater challenge, thereby making SLP more attractive overall [37].
PTHrP plays key roles in a wide variety of solid tumors, including osteosarcoma, breast cancer, lung cancer, chondrosarcoma, anaplastic thyroid cancer, medulloblastoma, adrenocortical tumor, squamous cancer and prostate cancer cell lines. Recently, it has been shown that the combination of anti-PTHrP with zoledronic acid, which is a third-generation bisphosphonate, is promising to control bone-metastasis in immunosuppressed mice depleted of NK cells inoculated with SBC-5 human small cell lung cancer (SCLC) cells, than the agent alone, therefore suggesting that the dual-target therapy is more useful [38]. The overexpression in cancer cells of Thymidylate Synthase (TS) – a crucial enzyme for DNA repair and replication- inspired the development of a 27-mer peptide vaccine named TS poly-epitope peptide (TSPP) vaccine. TSPP contains three epitopes of HLA-A2.1-binding motifs of TS. The vaccine has shown to be safe and has antitumor activity in both preclinical studies and a clinical (phase 1) investigation [39]. To corroborate this data, another phase Ib study of 29 metastatic colorectal cancer showed that the poly-epitope vaccination to TSPP and GOLFIG chemo-immunotherapy was safe and possibly effective [40].
Since malignancy-associated hypercalcemia is characterized by excessive production of parathyroid hormone-related protein (PTHrP), a peptide vaccine was developed targeting PTHrP. The first study showed that the vaccine was effective in mice bearing transplanted human PTHrP-producing tumors, as it prolonged their survival [41]. Interestingly there is a combination of cancer peptides called TAS0313. This cancer vaccine cocktail is made of overall 12 cytotoxic T lymphocyte (CTL) epitope peptides derived from the following eight cancer-associated antigens overexpressed in various types of solid cancers: EGFR, KUA, LCK, MRP3, PTHRP, SART2, SART3, and WHSC2. A Phase I/II study showed that the TAS0313 vaccine could produce an immune response with favorable safety and tolerability in 10 Glioblastoma (GBM) patients [42]. Generally, improving adaptive immune response, effector functions, and clinical efficacy of SLP vaccines, is an ongoing process at a fast pace.
Recently, a novel proteogenomic approach has been conducted to identify tumor antigens in colorectal cancer-derived cell lines, with biopsy samples having matched tumors and normal adjacent tissues. In this study, mass spectrometry analyses identified 30,000 unique MHC-I-associated peptides. The authors identified 19 tumor-specific antigens in both microsatellites stable and unstable tumors and intriguingly, 2/3 of them were from non-coding regions. Many of such peptides were from genes involved in colorectal cancer progression. Future vaccine research could use such findings to develop T-cell-based vaccines, in which T cells are primed against these antigens to target and destroy these tumors [43].
Another recent study investigated multi-epitope-based vaccines for colorectal cancer treatment and prevention. The authors designed various vaccines targeting crucial oncogenes in this cancer, namely DC25B, COX2, RCAS1, and FASCIN1 proteins. Their peptide vaccines targeted human class II MHC for each protein and T-cells specific for both peptides and corresponding recombinant protein. Only when they immunized for both CDC25B and COX2 peptides, they were able to observe a significant tumor growth inhibition in MC38 syngeneic mice vs. control. Therefore, they suggested that immunization with CDC25B and COX2 epitopes could be a good method to suppress tumor development in both treatment and prophylactic models that they were able to evaluate [44].
Recently, a study developed a recombinant anti-mKRAS scFV-fused, mutant Hydra actinoporin-like-toxin 1 (mHALT-1) immunotoxin that can recognize and eradicate cells bearing K-Ras antigen from mutated codon-12. They showed high cytotoxic efficacy on SW-480 (bearing the KRASG12V mutation) colorectal cancer cells, whereas they spared NHDF control cells [45].
A phase II clinical study specifically showed that the combination of chemotherapy with second-line telomerase peptide vaccine (GV1001) in 56 metastatic colorectal cancer patients, was tolerable and modestly effective [46].
Viral vaccines can be designed to deliver RNA, which encode peptides antigens that are later displayed by tumor cells and other cells. Also, oncolytic viruses (OVs) are used to selectively infect, replicate in, and lyse malignant cells. In addition to killing infected malignant cells, OVs may promote the destruction of the tumor’s blood cells [47]. OVs can act as an effective adjuvant and delivery platform for personalized anticancer vaccines, by using peptide antigens [48]. One OV therapy that uses an IFN-β-expressing vesicular stomatitis virus (VSV) to treat hepatocellular carcinoma (HCC), recently entered a phase I clinical trial [49]. Viral vector vaccines have shown to be a versatile strategy for promoting antitumor activity and will continue to be further developed.
Vaccines against frameshift mutations have been tested for Lynch tumors and hereditary non-polyposis colorectal cancer (HNPCC). Gebert et al. chose 10 short peptides based on frameshift modifications and immunized C57BL/6 mice, four times biweekly, to confirm the expression of the peptides through an ELISpot immunogenicity assay. In a mouse model for Lynch syndrome (MSH-2 conditional knock-out mice), vaccination improved survival and reduced tumor burden that would otherwise spontaneously develop tumors. These vaccines had to be combined with aspirin and Naproxen (NAP), two nonsteroidal anti-inflammatory drugs used for chemoprevention of HNPCC, to elicit great outcomes in the model [50]. In glioma models, current cancer vaccines needed anti-programmed death protein-1/ligand1 (PD-1/PD-L1), or anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4), to boost immune response further [51]. Ongoing clinical trials investigating peptide or viral vaccines are summarized in Table 2, Table 3 , respectively.
Table 2.
Ongoing clinical trials investigating peptide vaccines in cancer.
| Clinical Trial Identifier Code | Investigation Plan | Vaccines, Drug/s | Clinical Setting Lines of therapy | Primary Endpoint | Stage of Development | Clinical Trials Status |
|---|---|---|---|---|---|---|
| NCT01789099 | 18 participants, Single Group Assignment, Open Label |
UV1 synthetic peptide vaccine and GM-CSF | Second or later lines | Safety and tolerability, Immunological response | 1/2 | Active, not recruiting |
| NCT01784913 | 22 participants, Single Group Assignment, Open Label |
UV1/hTERT2012P | First line | Safety and tolerability | 1/2 | Active, not recruiting |
| NCT03012100 | 280 participants, Randomized, Parallel Assignment, Triple masking, Double Blind |
Cyclophosphamide, Multi-epitope Folate Receptor Alpha Peptide Vaccine, Sargramostim |
Second or later lines | DFS | 2 | Recruiting |
| NCT01720836 | 30 participants, Non-Randomized, Parallel Assignment, Open Label |
Vaccine + PolyICLC | Second or later lines | Immunologic response | 1/2 | Recruiting |
| NCT00194714 | 26 participants, Single Group Assignment, Open Label |
HER-2/neu Peptide Vaccine | Second or later lines | Immunologic response, AE |
1/2 | Active, not recruiting |
| NCT03606967 | 70 participants, Randomized, Parallel Assignment, Open Label |
Carboplatin, Durvalumab, Gemcitabine Hydrochloride, Nab-paclitaxel, Personalized Synthetic Long Peptide Vaccine, Poly ICLC, Tremelimumab |
First line | Clinical response | 2 | Recruiting |
| NCT04998474 | 15 participants, Single Group Assignment, Open Label |
FRAME-001 personalized vaccine | Fourth or later lines | FRAME-001-specific immune responses | 2 | Not yet recruiting |
| NCT02795988 | 36 participants, Randomized, Parallel Assignment, Partially blinded (outcomes assessor) |
IMU-131, Cisplatin and either Fluorouracil (5-FU) or Capecitabine or Oxaliplatin and capecitabine. | First line | Safety and tolerability, Recommended Phase 2 dose and clinical efficacy of IMU-131 |
1/2 | Active, not recruiting |
| NCT04747002 | 100 participants, Randomized, Parallel Assignment, Opel label |
DSP-7888 | Second line | RFS | 2 | Recruiting |
| NCT03761914 | 90 participants, Non-Randomized, Parallel Assignment, Opel label |
galinpepimut-S, Pembrolizumab |
Second or later lines | TRAEs, ORR, CR | 1/2 | Active, not recruiting |
| NCT02396134 | 133 participants, Randomized, Parallel Assignment, Triple masking |
CMVpp65-A*0201 peptide vaccine | First line | CMV reactivation and CD8+ T cells binding | 2 | Active, not recruiting |
| NCT02636582 | 13 participants, Randomized, Parallel Assignment, Single masking (Participant) |
Nelipepimut-S Plus GM-CSF Vaccine, Sargramostim |
First line | Evaluate CTL | 2 | Active, not recruiting |
| NCT05127824 | 42 participants, Randomized, Factorial Assignment, Open label |
Autologous alpha-DC1/TBVA vaccine, Cabozantinib |
First line | Immune response, Safety |
2 | Not yet recruiting |
| NCT04197687 | 480 participants, Randomized, Parallel Assignment, Double blinded (participant, care provider) |
Multi-epitope HER2 Peptide Vaccine TPIV100, Pertuzumab, Sargramostim, Trastuzumab, Trastuzumab Emtansine |
Second or later lines | iDFS | 2 | Recruiting |
| NCT05243862 | 28 participants, Single Group Assignment, Open label |
PolyPEPI1018, Atezolizumab |
Third or later lines | AEs, Safety | 2 | Not yet recruiting |
| NCT02126579 | 62 participants, Randomized, Parallel Assignment, Open label |
Peptide Vaccine (LPV7) + Tetanus peptide, PolyICLC |
Second or later lines | Safety and toxicity, T cell response | 1/2 | Active, not recruiting |
| NCT00703105 | 36 participants, Single Group Assignment, Open label |
DC vaccination | Second or later lines | Immune response | 2 | Recruiting |
| NCT02818426 | 54 participants, Single Group Assignment, Open label |
UCPVax | Third or later lines | DLT | 1/2 | Recruiting |
| NCT03047928 | 50 participants, Single Group Assignment, Open label |
Nivolumab, PD-L1/IDO peptide vaccine |
Second or later lines | AEs | 1/2 | Recruiting |
| NCT02960230 | 49 participants, Non-randomized, Parallel Assignment, Open label |
K27M peptide, Nivolumab | Second or later lines | AEs, OS |
1/2 | Recruiting |
| NCT04206254 | 80 participants, Randomized, Parallel Assignment, Open label |
gp96 | Second or later lines | 2-years RFS | 2/3 | Not yet recruiting |
| NCT04364230 | 44 participants, Single Group Assignment, Open label |
6MHP, NeoAg-mBRAF, PolyICLC, CDX-1140 |
First line | Safety of CDX-1140 + melanoma peptide vaccine, Immunogenicity |
1/2 | Recruiting |
| NCT04280848 | 28 participants, Single Group Assignment, Open label |
UCPVax | Second or later lines | Immunogenicity | 1/2 | Active, not recruiting |
| NCT03946358 | 47 participants, Single Group Assignment, Open label |
Atezolizumab, UCPVax | Second or later lines | ORR | 2 | Recruiting |
| NCT03560752 | 36 participants, Single Group Assignment, Open label |
Multi-peptide CMV-Modified Vaccinia Ankara Vaccine | Second or later lines | AEs | 2 | Recruiting |
| NCT04051307 | 48 participants, Single Group Assignment, Open label |
PD-L1 peptide: PD-L1 Long(19–27), Arginase1 peptide: ArgLong2(169–206) | First line | Immune response | 1/2 | Recruiting |
| NCT03617328 | 30 participants, Randomized, Parallel Assignment, Open label |
6MHP, Montanide ISA-51, polyICLC, CDX-1127 | First or second line | Safety and immunogenicity | 1/2 | Recruiting |
| NCT01885702 | 25 participants, Non-Randomized, Parallel Assignment, Open label |
DC vaccination | First or second line | Safety and feasibility | 1/2 | Active, not recruiting |
| NCT02802943 | 56 participants, Non-Randomized, Parallel Assignment, Open label |
Peptide Vaccine, Imiquimod | First or second line | Induction of peptide-specific T cell responses | 2 | Recruiting |
| NCT03715985 | 12 participants, Sequential Assignment, Open label |
EVAX-01-CAF09b | Second or later line | Safety and tolerability | 1/2 | Active, not recruiting |
| NCT05096481 | 120 participants, Single Group Assignment, Open label |
PEP-CMV, Temozolomide, Tetanus Diphtheria Vaccine |
Second line | PFS, OS | 2 | Not yet recruiting |
| NCT04580771 | 35 participants, Single Group Assignment, Open label |
Cisplatin, Liposomal HPV-16 E6/E7 Multipeptide Vaccine PDS0101, Radiation Therapy | First line | Toxicity | 2 | Recruiting |
| NCT02455557 | 66 participants, Single Group Assignment, Open label |
Montanide ISA 51 VG, Sargramostim, SVN53-67/M57-KLH Peptide Vaccine, Temozolomide | Second line | PFS |
2 | Active, not recruiting |
| NCT03821272 | 20 participants, Randomized, Parallel Assignment, Open label |
PepCan | Second line | AEs | 1/2 | Recruiting |
| NCT02358187 | 25 participants, Randomized, Single Group Assignment, Open label |
HLA-A2 Restricted Glioma Antigen-Peptides with Poly-ICLC | Third line | Tumor shrinkage or stable disease | 2 | Recruiting |
| NCT04114825 | 180 participants, Randomized, Parallel Assignment, Quadruple masking |
RV001V | Second or later line | Time to PSA progression | 2 | Active, not recruiting |
| NCT05232851 | 24 participants, Randomized, Parallel Assignment, Open label |
Liposomal HPV-16 E6/E7 Multipeptide Vaccine PDS0101, Pembrolizumab | First line | ctHPVDNA response | 1/2 | Recruiting |
| NCT01697527 | 6 participants, Single Group Assignment, Open label |
Aldesleukin, fludarabine phosphate, cyclophosphamide, NY-ESO-1 reactive TCR retroviral vector transduced autologous PBL, DC therapy, fludeoxyglucose F 18, PET | Second or later line | Clinical response | 2 | Active, not recruiting |
| NCT03384914 | 110 participants, Randomized Parallel Assignment, Open label |
DC1 Vaccine, WOKVAC Vaccine | Second or later line | Immunogenicity | 2 | Recruiting |
| NCT05163080 | 265 participants, Randomized Parallel Assignment, Double-Blind |
SurVaxM | Second or later line | OS | 2 | Recruiting |
| NCT01814813 | 90 participants, Randomized, Parallel Assignment, Open label |
HSPPC-96, bevacizumab | First line | OS | 2 | Active, not recruiting |
| NCT04912765 | 60 participants, Single Group Assignment, Open label |
Neoantigen DC Vaccine, Nivolumab | Second or later line | 24-months Relapse Free Survival | 2 | Recruiting |
| NCT03633110 | 24 participants, Non-Randomized, Single Group Assignment, Open label |
GEN-009 Adjuvanted Vaccine, Nivolumab, Pembrolizumab | First line | AEs, T-cell responses | 1/2 | Active, not recruiting |
| NCT02506933 | 102 participants, Randomized, Parallel Assignment, Double blinded (Participant and Investigator) |
Multi-peptide CMV-Modified Vaccinia Ankara Vaccine | First line | CMV events, Severe AEs | 2 | Active, not recruiting |
| NCT02134925 | 110 participants, Randomized, Parallel Assignment, Double blinded (Participant and Investigator) |
MUC1 Peptide-Poly-ICLC Vaccine | First or second line | Change in Anti-MUC1 Immunoglobulin G (IgG) Levels | 2 | Active, not recruiting |
| NCT04060277 | 128 participants, Randomized, Parallel Assignment, Double blinded (Participant and Care Provider) |
Letermovir, Multi-peptide CMV-Modified Vaccinia Ankara Vaccine | First line | Clinically significant cytomegalovirus | 2 | Recruiting |
| NCT03284866 | 536 participants, Randomized, Parallel Assignment, Double blinded (Participant and Investigator) |
Recombinant Human Papillomavirus Nonavalent Vaccine | Second or later line | Lesions occurrences | 3 | Recruiting |
| NCT02543749 | 30 participants, Single Group Assignment, Open Label | DC vaccine | First line | DC toxicity Parameters using CTC | 1/2 | Recruiting |
| NCT02334735 | 36 participants, Randomized, Parallel Assignment, Open Label | DC Vaccine, Montanide Vaccine, Poly-ICLC | First line | Humoral immune response | 2 | Active, not recruiting |
| NCT04445064 | 11 participants, Randomized, Parallel Assignment, Open Label | IO102 | First line | Number of participants with a T-cell peptide-specific response to the vaccine | 2 | Recruiting |
Table 3.
Ongoing clinical trials investigating viral vaccines in cancer.
| Clinical Trial Identifier Code | Investigation Plan | Viral vaccine/ Drug | Clinical Setting Line | Primary Endpoint | Stage of Development | Clinical Trials Status |
|---|---|---|---|---|---|---|
| NCT04745377 | 300 participants, Observational, Case-Control |
SARS-COV-2 | First line | Rate of Covid19 Infection post vaccination | Case-Control | Recruiting |
| NCT04410874 | 45 participants, Sequential Assignment, Open Label |
Imvamune | First line | MTD | 2 | Recruiting |
| NCT03315975 | 40 participants, Interventional, Single group Assignment, Open Label |
Inactivated influenza vaccine | First line | Neutralizing antibody response | 4 | Active, not recruiting |
| NCT04521764 | 33 participants, Interventional, Single group Assignment, Open Label |
modified measles virus (MV-s-NAP) | Second line or later line | MTD | 1 | Recruiting |
| NCT03848039 | 1220 participants, Interventional, Randomized Assignment, Open Label |
Gardasil-9 | First line | Evaluation of DRR | 3 | Not yet recruiting |
| NCT01376505 | 100 participants, Non-Randomized, Parallel Assignment, Open Label |
HER-2 vaccine | First line | Safety and duration of immune response | 1 | Recruiting |
| NCT04410900 | 41 participants, Non-Randomized, Parallel Assignment, Open Label |
Wistar Rabies Virus | First line | Positive vaccine response | 1 | Recruiting |
| NCT03113487 | 28 participants, Interventional, Single group Assignment, Open Label |
Vaccinia Virus expressing p53, Pembrolizumab | Second line or later line | PFS | 2 | Recruiting |
| NCT02432963 | 19 participants, Interventional, Single group Assignment, Open Label |
Vaccinia Virus expressing p53, Pembrolizumab | First line | Tolerability | 1 | Active, not recruiting |
| NCT02285816 | 56 participants, Non-Randomized, Parallel Assignment, Open Label |
MG1MA3 AdMA3 |
Second line or later line | MFD | 2 | Active, not recruiting |
| NCT03439085 | 77 participants, Interventional, Single Group Assignment, Open Label |
IL-12 DNA plasmids, MEDI0457, Durvalumab | First line | ORR | 2 | Active, not recruiting |
| NCT04836793 | 300 participants, observational, Cohort |
Additional biological samples | First line | IgG levels after Covid19 vaccination | Recruiting | |
| NCT02700230 | 30 participants, Interventional, Single Group Assignment, Open Label |
Measles Virus Encoding Thyroidal Sodium Iodide Symporter | First line | Dose Response | 1 | Recruiting |
| NCT02865135 | 11 participants, Interventional, Single Group Assignment, Open Label |
DPX-E7 vaccine | First line | SAE | 2 | Active, not recruiting |
| NCT04355806 | 160 participants, Observational, Prospective Assignment, |
PD-1/PD-L1 inhibitors, Inactivated trivalent influenza vaccine | First line | IgG levels | Not yet recruiting | |
| NCT04667702 | 330 participants, Observational, Prospective Assignment, |
HPV | First line | Vaccine Hesitancy | Recruiting | |
| NCT04774887 | 1200 participants, Interventional, Single group Assignment, Open Label |
HPV | First line | Risk to HPV | Not Applicable | Not yet recruiting |
| NCT00092534 | 12,167 participants, Interventional, Randomized, Single Group Assignment, Double Masking |
Gardasil, HPV | First line | Incidence of Endpoint of HPV | 3 | Active, not recruiting |
| NCT02977156 | 22 participants, Interventional, Single group Assignment, Open Label |
Pexa-Vec, Ipilimumab | First line | DLTs, ORR | 1 | Active, not recruiting |
| NCT03618953 | 75 participants, Non-Randomized, Parallel Assignment, Open Label |
Ad-E6E7 MG1-E6E7 Atezolizumab |
First line | Safety | 1 | Active, not recruiting |
| NCT03560752 | 36 participants, Interventional, Single Group Assignment, Open Label |
CMV-Modified Vaccinia Ankara Vaccine | First line | Safety | 2 | Recruiting |
| NCT02653118 | 4453 participants, Observational, Cohort, Open Label |
V503, GARDASIL | First line | Incidence of HPV | Active, not recruiting | |
| NCT04847050 | 220 participants, Non-Randomized, Parallel Assignment, Open Label |
mRNA-1273 | First line | Safety | 2 | Recruiting |
| NCT04854980 | 55 participants, Observational, Prospective Assignment, |
Blood | First line | Immune response to vaccine | Recruiting | |
| NCT04580771 | 35 participants, Interventional, Single Group Assignment, Open label |
Cisplatin Liposomal HPV-16 E6/E7 Multi-peptide Vaccine PDS0101 |
First line | Rate of grade | 2 | Recruiting |
| NCT03547999 | 78 participants, Parallel Assignment, Interventional, Randomized, Open Label | mFOLFOX6, MVA-BN-CV301, FPV-CV301, Nivolumab | First line | OS | 2 | Active, not recruiting |
| NCT02415387 | 180 participants, Crossover Assignment, Interventional, Randomized, Quadruple | typhoid vaccine | First line | Change in IL6 levels | Not Applicable | Recruiting |
| NCT04935528 | 430 participants, Single group Assignment, Interventional, Randomized, Open Label | ELISPOT, Serology | First line | seroprevalence of SARS-CoV-2 | Not Applicable | Recruiting |
| NCT05237947 | 5000 participants, Parallel Assignment, Interventional, Randomized, Double | DTP, Questionnaire, HPV | First line | Incidence of persistent HPV infection | 4 | Enrolling by invitation |
| NCT02649439 | 97 participants, Parallel Assignment, Interventional, Randomized, Open Label | PROSTVAC –V, PROSTVAC-F | First line | Tumor growth rate | 2 |
Active, not recruiting |
| NCT01867333 | 57 participants, Parallel Assignment, Interventional, Randomized, Open Label | PROSTVAC-F/TRICOM PROSTVAC-V/TRICOM, Enzalutamide (Xtandi) |
First line | Increase in time to progression | 2 | Active, not recruiting |
| NCT05078866 | 45 participants, Single group Assignment, Interventional, Randomized, Open Label | GAd-209-FSP, MVA-209-FSP | First line | Adverse events | 2 | Not yet recruiting |
| NCT04041310 | 84 participants, Sequential Assignment, Interventional, Non-Randomized | GAd-209-FSP, MVA-209-FSP | First line | Toxicity | 2 | Recruiting |
| NCT04977024 | 240 participants, Parallel Assignment, Interventional, Triple masking | COVID-19 Vaccine | First line | biological activity | 2 | Recruiting |
| NCT02002182 | 15 participants, Non-randomized Parallel Assignment, Interventional, Open Label | ADXS11-001 (ADXS-HPV) | First line | HPV-Specific T Cell Response Rate | 2 | Active, not recruiting |
| NCT04442048 | 195 participants, Randomized Parallel Assignment, Interventional, Open Label | IMM-101 | First line | rate of “flu-like illness” | 3 | Active, not recruiting |
| NCT03603808 | 80 participants, Single group Assignment, Interventional, Randomized, Open Label | HPV DNA Plasmids (VGX-3100) | First line | ORR | 2 | Recruiting |
| NCT05173324 | 8000 participants, Randomized, Parallel Assignment, Quadruple |
HPV vaccine HAV vaccine | First line | HPV prevalent infections | 3 | Not yet recruiting |
| NCT03350698 | 100 participants, Randomized, Single group Assignment, Open label |
Gardasil-9 | First line | Prevention | 4 | Recruiting |
| NCT04635423 | 1050 participants, Randomized, Parallel Assignment, Triple |
V503 | First line | Combined incidence of HPV 6/11/16/18-related anogenital persistent infection | 3 | Active, not recruiting |
| NCT04274153 | 130 participants, Single group Assignment, Interventional, Open label |
Gardasil9 | First line | Immunogenicity of HPV vaccine | 4 | Recruiting |
| NCT02834637 | 930 participants, Parallel Assignment, Interventional, Randomized, Open Label | bivalent HPV vaccine, nonavalent HPV vaccine | First line | Proportion with HPV 16/18-specific seropositivity | 3 | Active, not recruiting |
| NCT03284866 | 536 participants, Parallel Assignment, Interventional, Randomized, Double | Gardasil 9 | First line | Occurrence of cervical cancer | 3 | Recruiting |
| NCT02649855 | 74 participants, Parallel Assignment, Interventional, Randomized, Open Label | PROSTVAC-V, PROSTVAC-F, Docetaxel | First line | Response/efficacy | 2 | Active, not recruiting |
| NCT03315871 | 34 participants, Parallel Assignment, Interventional, Non-Randomized, Open Label | PROSTVAC-V, PROSTVAC-F, MSB0011359C | First line | Response of combination immunotherapy | 2 | Recruiting |
| NCT02396134 | 133 participants, Parallel Assignment, Interventional, Non-Randomized, Triple masking | CMVpp65-A*0201 peptide vaccine | First line | non-relapse mortality | 2 | Active, not recruiting |
| NCT05266898 | 150 participants, Single group Assignment, Interventional, Open Label | Human papillomavirus 9-valent vaccine | First line | change in serological response to Gardasil-9 | 4 | Not yet recruiting |
| NCT01824537 | 1000 participants, Randomized, Factorial Assignment, Interventional, Quadruple | Gardasil 9, Hepatitis A vaccine | First line | Reduction in HPV type concordance | 4 | Recruiting |
| NCT04534205 | 285 participants, Randomized, Parallel Assignment, Interventional, Open Label | BNT113, Pembrolizumab | First line | TEAE and ORR | 2 | Recruiting |
| NCT04060277 | 128 participants, Randomized, Parallel Assignment, Interventional, Open Label | Letermovir, Multi-peptide CMV-Modified Vaccinia Ankara Vaccine | First line | Non-relapse mortality | 2 | Recruiting |
| NCT03702231 | 116 participants, Non-Randomized, Parallel Assignment, Interventional, Open Label | Zoster Vaccine Recombinant, Adjuvanted | First line | Safety and Tolerability | 2 | Active, not recruiting |
| NCT04484532 | 200 participants, Single group Assignment, Interventional, Open Label | Trivalent Influenza Vaccine | Second or later line | Antibody response | 4 | Recruiting |
| NCT03180034 | 25,000 participants, Randomized, Parallel Assignment, Interventional, Double | DTP adsorbed, HPV bivalent, HPV Nonavalent | Second or later line | Incidence of persistent human papillomavirus (HPV)-16 or 18 cervical infections | 4 | Active, not recruiting |
| NCT00834093 | 18 participants, Single group Assignment, Interventional, Open Label | Epstein-Barr Virus Specific Immunotherapy | First line | ORR | 2 | Active, not recruiting |
| NCT03728881 | 1240 participants, Non-Randomized, Parallel Assignment, Interventional, Open Label | Quadrivalent HPV virus, bivalent HPV vaccine | First line | Antibody levels of HPV16 | 3 | Active, not recruiting |
| NCT02506933 | 102 participants, Randomized, Parallel Assignment, Double masking | Multi-peptide CMV-Modified Vaccinia Ankara Vaccine | First line | CMV events encompassing any CMV reactivation | 2 | Active, not recruiting |
| NCT04046445 | 96 participants, Non-Randomized, Parallel Assignment, Interventional, Open Label | ATP128, BI 754091, VSV-GP128 | First line | safety and tolerability and SAEs | 2 | Recruiting |
| NCT02481414 | 125 participants, Randomized, Parallel Assignment, Interventional, Open Label | PepCan, Candin | Second or later line | Efficacy | 2 | Active, not recruiting |
| NCT03391921 | 170 participants, Randomized, Parallel Assignment, Interventional, Open Label | Vaccine | Second or later line | Rate of seroconversion in HPV antibodies against HPV | 4 | Active, not recruiting |
| NCT05262010 | 13,500 participants, Randomized, Parallel Assignment, Interventional, Open Label | 11-valent recombinant human papilloma virus vaccine | First line | Person-years incidence of CIN2 + associated with HPV6/11/16/18 | 3 | Not yet recruiting |
| NCT04436133 | 480 participants, Randomized, Parallel Assignment, Interventional, Double | 11-valent recombinant human papilloma virus vaccine, Gardasil 9 | First line | Anti-HPV neutralizing antibodies GMT | 2 | Active, not recruiting |
| NCT03943875 | 512 participants, Randomized, Parallel Assignment, Interventional, Open Label | 9-valent HPV vaccine | First line | Efficacy | 4 | Recruiting |
| NCT04482933 | 30 participants, single group Assignment, Interventional, Open Label | Biological G207 | Second line or later line | Efficacy | 2 | Not yet recruiting |
| NCT04199689 | 6000 participants, Randomized, Parallel Assignment, Interventional, Triple masking | 9vHPV Vaccine | Second line or later line | Incidence of HPV | 3 | Active, not recruiting |
| NCT03903562 | 1990 participants, Randomized, Single group Assignment, Interventional, Open Label | V503 | First line | Serum antibody titers for HPV | 3 | Active, not recruiting |
| NCT04953130 | 10,400 participants, Randomized, Parallel Assignment, Interventional, Open Label | Gardasil HPV vaccine | First line | Impact of HPV vaccination | 4 | Not yet recruiting |
| NCT04951323 | anti-COVID19 mRNA-based vaccine | anti-COVID19 mRNA-based vaccine (BNT162b2) | First line | Quantification of anti-SARS-CoV-2 receptor binding domain specific IgG | 3 | Recruiting |
| NCT02750202 | 75 participants, Randomized, Parallel Assignment, Interventional, single masking | Quadrivalent HPV vaccine, Hepatitis B vaccine | First line | Change in the genital wart lesion | 3 | Recruiting |
| NCT05291845 | 75 participants, Randomized, Factorial Assignment, Interventional, open label | Candida antigen vaccine, Bivalent HPV vaccine | Second line or later line | complete response | 2 | Not yet recruiting |
| NCT05027776 | 1348 participants, Randomized, Parallel Assignment, Interventional, open label | HPV vaccine | First line | Primary immunogenicity | 3 | Recruiting |
| NCT04708041 | 700 participants, Randomized, Parallel Assignment, Interventional, open label | 9vHPV vaccine | First line | GMT of HPV | 3 | Active, not recruiting |
| NCT04474821 | 300 participants, Randomized, Single group Assignment, Interventional, open label | Human Papillomavirus Infection | Second line or later line | Acceptance and completion rates of free HPV vaccination | 4 | Recruiting |
| NCT04895020 | 1200 participants, Single group Assignment, Interventional, open label | 9-valent HPV vaccine | First line | primary immunogenicity | 3 | Recruiting |
| NCT04422366 | 8000 participants, Parallel group Assignment, Interventional, open label | 9-valent Human Papillomavirus, GARDASIL | First line | person-year incidence of HPV | 3 | Recruiting |
| NCT03998254 | 6000 participants, Parallel group Assignment, Interventional, double label | V503, Gardasil | Second line or later line | Combined Incidence of HPV related 12-month Persistent Infection | 3 | Active, not recruiting |
| NCT05285826 | 8100 participants, Parallel group Assignment, Interventional, double label | 9vHPV vaccine | First line | Combined Incidence of HPV 58-related External Genital and Intra-anal 12-month Persistent Infection | 3 | Recruiting |
| NCT05279248 | 300 participants, Parallel Assignment, Interventional, Open label | HPV + MMR,HPV | First line | GMT of anti-HPV 16 and 18 at 7 months | 4 | Active, not recruiting |
| NCT04870333 | 5000 participants, Parallel Assignment, Randomized, Interventional, Open label |
Niclosamide, Ciclesonide, Sotrovimab | First line | Prevention | 3 | Recruiting |
| NCT05119855 | 400 participants, Parallel Assignment, Randomized, Interventional, Open label |
9vHPV Vaccine, mRNA-1273 Vaccine | First line | GMT of HPV | 3 | Recruiting |
Cellular vaccines
In DC-vaccine development, DCs are loaded with tumor antigens in the forms of mRNAs, proteins, peptides, or tumor lysates [52]. Normally, antigen delivery to the DCs occurs ex-vivo, where they are activated and reinjected. However, this process may impair dendritic cell trafficking to secondary lymphoid organs. An alternative strategy is to infect patient DCs with viral vectors encoding desired antigens or fuse their DCs with tumor cells [53]. Further investigation is required to standardize an effective DC-based vaccine engineering.
Another type of cellular vaccine is created using irradiated allogeneic whole tumor cells or autologous patient-derived tumor cells to induce antitumor immune responses [54]. To enhance immune response against whole tumor cells, new generations of tumor cell vaccines have been genetically modified to either produce co-stimulatory molecules, chemokines, cytokines, or reduce inhibitory molecule production [55]. For example, the FANG vaccine contains autologous-tumor cells modified with a plasmid that encodes a bi-functional short hairpin RNAi that targets furin convertase resulting in downregulation of TGF-β, an immunosuppressive transforming growth factor [56]. After tumor cells or lysate is delivered, DCs initiate T cell activation by cross-priming CD8+ T cells [55]. This strategy allows multiple tumor antigens to be targeted simultaneously without neoantigen identification prior to administration. However, neoantigen mutational burden and quality are still considered to be associated with good prognosis and are good predictors of treatment success. One study showed that neoantigen vaccine effectiveness was limited by a low mutational burden [57]. Even checkpoint inhibitor antibodies targeting PD-1 and CTLA-4 have improved clinical response when tumors have a higher mutation frequency [58], [59]. Salewski et al. showed mice administered with autologous-cell line-derived tumor lysates from 328 and A7450 T1 M1 cell lines, with high-quality neoantigens, are more effective at promoting a prophylactic effect on gastrointestinal tumor formation [60]. Therefore, neoantigen identification may still add some value to tumor cell vaccine design. Regardless, improvements will need to be made to further enhance antitumor response associated with cellular vaccines.
From sequence mutations to the identification of neoantigens for vaccines
Neoantigens identification depends on several fundamental factors besides somatic mutations: translation, post-translational modifications and affinity between mutated peptide and patients’ MHC molecules, and affinity between mutant peptide-MHC complex with T-cell receptor (TCR) [61]. Prediction of neoantigens needs to combine both genomic mutations and MHC information on patients, and different software has shown this conjunction to be useful, as summarized in Table 4 [62], [63], [64], [65].
Table 4.
Neoantigen prediction softwares.
| Software (references) | Principle | Year |
|---|---|---|
| NeoPredPipe [140] | Connects commonly used bioinformatics software using custom python scripts giving neoantigen burden, immune stimulation potential, tumor heterogeneity and HLA haplotype of patients. | 2019 |
| Strelka2 [141] | Estimates error or deletion parameters of each sample improved tumor liquid analysis | 2018 |
| MuPeXI [142] | Identifies tumor-specific peptides through the extraction and induction of mutant peptides, it can predict immunogenicity and evaluate the potential of novel peptides | 2017 |
| CloudNeo pipeline [143] | The docker container executes the tasks. After giving as an input mutant VCF file and bam FILE representing HLA typing, the software predicts HLA affinity all mutant peptides. | 2017 |
| pVAC-Seq [144] | Integrates tumor mutation and expression data to identify personalized mutagens through personalized sequencing. | 2016 |
| NetMHCpan [145] | The sequences are compared using artificial intelligence neural network and predict affinity of molecular peptide-MHC-I type | 2016 |
| VariantEffect Predictor Tool [146] | It uses automated annotations to manual review time and prioritize variants | 2016 |
| Somaticseq [147] | It uses a randomized enhancement algorithm, which has more than 70 individual genome sequence features based on candidate sites to accurately detect somatic mutations | 2015 |
| OptiType [148] | It uses an HLA type algorithm with a linear programming that gives sequencing databases comprising RNA, exome and whole genome sequencings. | 2014 |
| ATHLATES [149] | It assembles allele recognition, pair interface applied to short sequences and HLA genotyping at allele level achieved via exon sequencing | 2013 |
| VarScan2 [150] | It detects somatic and copy number mutations within tumor-normal exome data using a heuristic statistical algorithm. | 2012 |
| HLAminer [151] | Through a shotgun sequencing Illumina database platform, predicts HLA type through an orientation of the assembly of the shotgun sequence data to then compare it with databases of allele sequences used as references. | 2012 |
| Strelka [152] | It uses a Bayesian model that matches normal-tumor sample sequencing data to analyze and predict with high accuracy and sensitivity somatic cellular variations | 2012 |
| SMMPMBEC [153] | Through a Beyesian matrix based on optimal neural network they can predict peptide molecules with MHC-I | 2009 |
| UCSC browser [154] | The fusion of various databases can give fast and accurate access to any gene sequence. | 2002 |
Discovery of personalized neoantigens from patients
Next-generation sequencing has advanced cancer therapy to allow patients to receive personalized therapies such as cancer vaccine, to generate a robust immune response against a patient’s cancer cells based on their unique molecular profile.
Existing immunotherapies reactivating the immune system work for only 30% of patients [66]. Hence, other ways to boost antitumor immune response using a targeted vaccine for specific patient genetics and MHCs, are urgently needed [67], [68], [69], [70]. Additionally, tumors expressing more neoantigens are associated with a stronger immune response and better survival [71], [72], [73]. Therefore, harnessing the natural ability of the immune system to detect and kill cancer cells [74].
Creating neoantigen-based vaccines
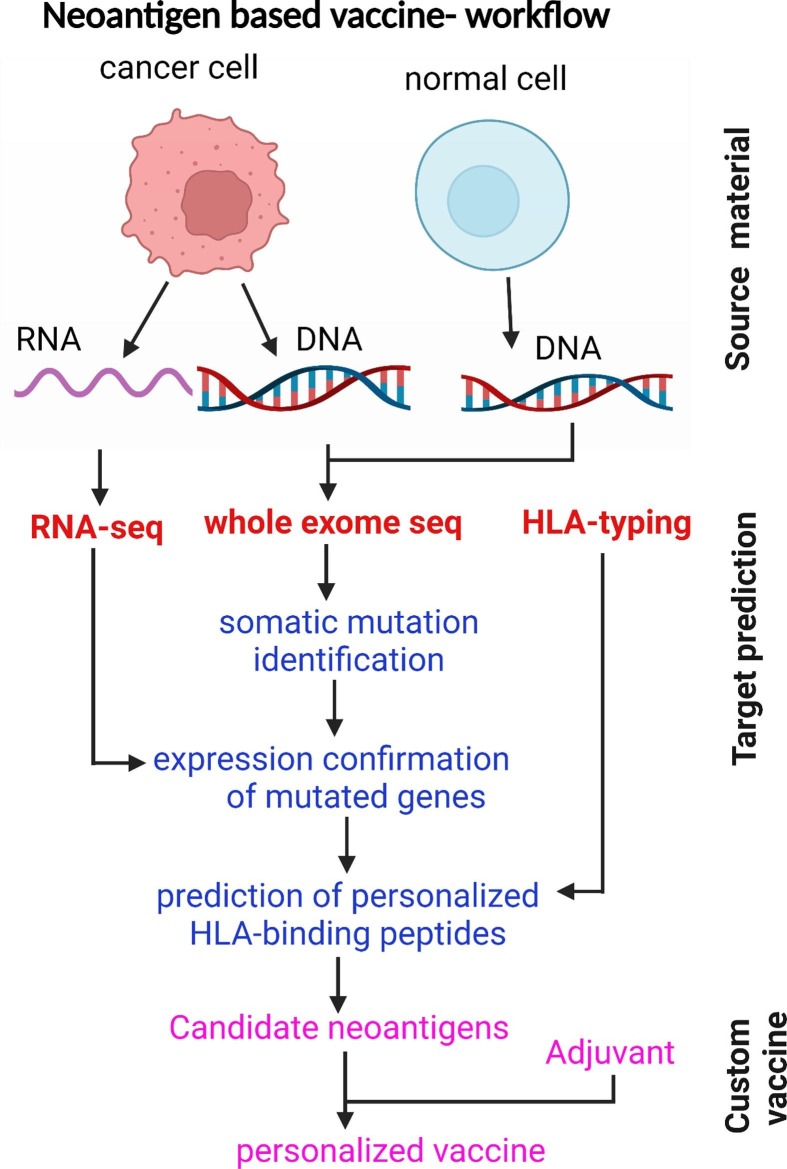
Due to the application of NGS, discovering tumor neoantigens has become a valuable tool for developing a personalized neoantigen vaccine. To manufacture a neoantigen vaccine, it is essential to compare patients’ tumor cells to their normal cells using whole-exome sequencing to identify mutations uniquely present in tumor cells [74]. Neoantigens are made from mutated proteins from DNA mutations. However, not all DNA mutations are missense or nonsense to produce mutated proteins. Techniques such as RNA-seq can be used to discriminate those mutations that lead to the formation of neoantigens to target vaccines. Secondly, only some peptides from processed mutated proteins can bind to HLA class I molecules. Neural network-based algorithms were used to predict which mutant proteins are most likely to undergo this transformation, and be presented on the surface of tumor cells or APCs as neoantigens [71], [75], [76]. These neoantigens are most likely to be detected by T cells to produce a strong tumor-specific immune response. The number of neoantigens included in a vaccine varies by patient, but, so far, up to 20 different neoantigens have been included in a single personalized vaccine [45] (Fig. 2 ).
Fig. 2.
Identification of neoantigens: Tumor-specific mutations are identified using whole-exome sequencing (WES), confirmed by RNA sequencing, then ranked by predicted affinity binding to HLA types; finally, neoantigens are synthesized based on mutated alleles followed by ex vivo T-cell reactivity analysis to confirm the immunogenicity.
Neoantigen vaccines stimulate tumor-specific T cells
Clinical trials of personalized cancer vaccines in solid tumors have shown that neoantigen vaccines can generate tumor-specific T cells that only recognize the tumor without serious side effects [66], [77], [78], [79], [80]. Recent glioblastoma clinical trials have revealed that peripheral stimulation by vaccine-generated T-cells with neoantigen specificity could be tracked inside the tumor [78]. To overcome immune resistance, many current clinical trials have combined personalized vaccines with immunotherapies. For example, Roche has started a clinical trial combining a personalized neoantigen vaccine with PD-L1 therapy to treat melanoma and non-small cell lung cancer [81]. Neoantigens predicting algorithms like HLA-thena, have improved based on mass spectrometry data and can better predict HLA-binding preferences for various types of patients [82].
Although current research mainly focuses on HLA class-I molecule to generate T cells that kill cancer cells, research on class II HLA to induce memory T cells, had been conducted to induce a long-lasting response.
Currently, not all neoantigens in a vaccine produce T cell response, as neoantigens must bind to HLA class I molecules (on the surface of cancer cells), or to class II (on the surface of APCs), as well as to T cell receptors (TCRs) [82]. There are still several opportunities to improve the selection of neoantigen to elicit the best antitumor response.
As NGS and predictive algorithms continue to advance, personalized cancer vaccines will continue to impact the field of immunotherapy. The rationale is to act against cancer cells by promoting immunity by vaccines and removing suppression immunity by inhibitor drugs, besides conventional chemotherapies.
Cancer vaccines targeting immune checkpoint proteins
Immunotherapy has significantly revolutionized cancer therapy by using monoclonal antibodies targeted to immune checkpoint molecules that are very active, even in advanced stages of the disease [84], [85], [86], [87], [88], [89], [90]. These results led to the study of peptide vaccines capable of generating antibodies against immune checkpoint proteins in the body.
PD-L1-based vaccine made from the fusion of extracellular domain of PD-L1 (PD-L1E) to C-terminal region of translocation domain of diphtheria toxin (DTT), showed to elicit CD4+ T cell response inducing Th1 antitumor immunity in mouse tumor models. In this study, PD-L1E was extracted from sera, blocked the binding of PD-L1 to PD-1 in vitro, which revealed a specific interaction. Moreover, PD-L1E vaccination induced an increase in TILs levels and a decrease in LAG3 + PD-1 + levels and CD8+ T cells. The data suggest that the PD-L1 vaccine reverses tumor suppression and could be a promising strategy for cancer therapy [83].
Another strategy consists of DCs loaded with an immunogenic PD-L1 (PD-L1-Vax), which has been shown to induce anti-PD-L1 immune responses and tumor inhibition in cancer cells expressing PD-L1 [84].
Kaumaya et al. recently developed a novel chimeric B-cell peptide epitope capable of targeting PD-1 linked to measles fusion protein (MVF, sequence 288–302) T-cell epitope, that could elicit polyclonal antibodies in the body, enabling blockage of PD-1 signaling, and mimicking the effects of nivolumab. The authors observed that their vaccine candidate with epitope sequence 92–110 (PD-1-Vax), significantly reduced tumor growth in the syngeneic BALB/c CT26 mouse model [85].
Neoantigen vaccines potentiating the immune response
Although neoantigens vaccines have been extensively studied for personalized immunotherapy, the vast majority of neoantigens have very minimal to no immunogenicity. An important role is played by adjuvants, because they can elicit a powerful immune response. Among other approaches that can potentiate immunogenicity of neoantigens to develop powerful and durable cancer response, there are: synergistic modulation of multiple immune signaling pathways, presence of multiepitope antigens that elicit a broad spectrum of immune responses, and cancer-specific antigens capable of inducing a specific adaptive immune response.
Currently, adjuvants such as polyinosinic-polycytidylic acid-poly-L-Lysine carboxy methyl cellulose (poly-ICLC) in combination with anti-CD40 have been used in neoantigen vaccines. However, not all adjuvants can induce a robust immune response, and some soluble vaccine formulations may also limit the immunogenicity of the vaccine itself [86], [87]. To overcome these challenges, pathogen-imitating nanovaccines were created and seem to have a great potential in improving the immunogenicity of neoantigens for cancer immunotherapy. Due to their small size (5–100 nm), nanovaccines can be effectively delivered to secondary lymphoid tissues like lymph nodes and APCs, where they can be retained for a long time. Delivering neoantigens with multiple synergistic adjuvants into lymph nodes [106-109] or APCs [88], [89], [90] is an essential step for ideal immunotherapy.
The administration through encapsulation of adjuvants and neoantigens can improve the pharmacokinetic properties of drug payloads, further enhancing immunomodulation. Recently, bi-adjuvant neoantigen nanovaccines (banNVs) co-delivered a peptide neoantigen with two adjuvants, TLR7/8 agonist R848, and TLR9 agonist CpG oligos, were developed to enhance immunogenicity. The combination of banNVs together anti-PD-1-induced potent and durable cancer immunotherapy when combined with anti-PD-1 [91]. TLR7/8 agonists used for cancer treatment in clinics [91], especially imiquimod and resiquimod (R848) are US FDA-approved drugs to treat topical skin lesions. The combination of these immune adjuvants exhibited synergistic therapeutic efficacy through the TLR-Myd88 pathway [91]. However, these drugs’ poor solubility and unfavorable pharmacokinetics have desisted them from being used together with immunotherapy [88], [89], [90]. The engineering of these drugs using nanocarriers could overcome this problem. Indeed, nanoparticles can be used to effectively encode more adjuvants and neoantigens and to enhance immune response. Adjuvants in neoantigenic vaccines promoted the response of cytotoxic T cells to specific neoantigens, leading to complete tumor destruction when combined with immune checkpoint blockade.
Ongoing clinical trials for neoantigens vaccines
The in vitro transcribed mRNAs have been administered differently and formulated as naked mRNA in buffer or LNP. These studies are based on the knowledge generated by Theilemans et al. showing that it is possible to generate powerful, clinical-grade IVT mRNA DC vaccines through electroporation [92], [93], [94], [95], [96]. Various studies have been conducted to find a way to directly deliver neo-antigen mRNA to APCs [97], [98].
Shahin et al. identified somatic mutations in tumor biopsies of 13 patients with stage III/IV using whole genome/exome and RNA sequencing techniques compared to control. After ranking mutations, they predicted binding affinity to patients’ HLA-I/II molecules. mRNA vaccines were generated against HLA-I and HLA-II, and mRNA doses of 0.5–1 µg per vaccination course and injected in the inguinal lymph nodes. Patients with tumors displaying TAAs such as NY-ESO-1 and tyrosinase received an mRNA-based vaccine targeting these TAAs [99]. The results were encouraging: eight patients had no radiologically detectable tumors when neoepitope vaccination started and remained without recurrence in 12–33 -months follow-up; five patients had the metastatic disease before vaccination, and two of them experienced objective responses, a third patient exhibited complete response for combined treatment of PD-1 blocking antibody. All patients showed Tcell responses against neoepitopes with 60% response. The same strategy was tested in a subsequent clinical trial with mRNA developed based on somatic mutations and LNP adjuvant delivered intravenously in patients with triple-negative breast cancer. Various drug administration methods could impact the efficacy of mRNA vaccination, as previously observed in animal studies using nanoparticle vaccines with neoantigen peptides linked to TLR7/8 agonist [100]. The authors showed that intravenous injection (i.v.) vaccination induced a higher proportion of CF1 + PD-1 + CD8+ T cells versus subcutaneous injection. Additionally, stem cells were induced by i.v injection, whereas effector genes were induced by subcutaneous injection [100]. Various clinical trials are currently investigating safety and efficacy of neo-antigens mRNA using different delivery methods and formulations either alone or in combination with other therapies. Ongoing clinical trials for neoantigen vaccinations for cancer therapy are summarized in Table 5 .
Table 5.
Ongoing clinical trials investigating cancer neo-antigen vaccines.
| Ongoing clinical trials investigating cancer neo-antigen vaccines Study | Cancer Type | Phase | Neo Antigen | Modality | Co-treatment | Status |
|---|---|---|---|---|---|---|
| NCT03639714 | Solid tumors | I/II | GRT-C901/2 | NN | Nivolumab/ipilimumab | Recruiting |
| NCT04864379 | Solid tumors | I | iNeo-Vac-P01 | NN | Anti-PD-1 | Recruiting |
| NCT04072900 | Melanoma | I | rhGM-CSF | NN | Anti-PD-1 | Recruiting |
| NCT02287428 | Glioblastoma | I | Personalized NeoAntigen Vaccine | NN | Pembrolizumab/ Temozolomide/Radiation Tehrapy | Recruiting |
| NCT03361852 | Follicular Lymphoma | I | Neo Vax | s.c. | Rituximab | Not yet recruiting |
| NCT03219450 | Lymphocytic Leukemia | I | NeoVax | s.c. | Pembrolimuzab/Cyclophosphamide | Not yet recruiting |
| NCT02950766 | Kidney Cancer | I | NeoVax | s.c. | Ipilimumab | Recruiting |
| NCT04810910 | Resectable Pancreatic Cancer | I | iNeo-Vac-P01/ GM-CSF | NN | NA | Not yet recruiting |
| NCT04024878 | Ovarian Cancer | I | NeoVax | s.c. | Nivolumab | Recruiting |
| NCT03953235 | Solid tumors | I/II | GRT-C903/4 | NN | Nivolumab/ipilimumab | Recruiting |
| NCT03807102 | Lung Cancer | I/II | Neoantigen Tumor Vaccine | NN | NA | Recruiting |
| NCT04087252 | Solid tumors | I | Tumor neoantigen | i.m. | NA | Recruiting |
| NCT03359239 | Urothelial/Bladder Cancer, NOS | I | Multipeptide Personalized Neoantigen Vaccine (PGV001, ICLC) | NN | Atezolizumab | Recruiting |
| NCT04749641 | Diffuse Intrinsic Pontine Glioma | I | Histone H3.3-K27M Neoantigen Vaccine Therapy | s.c. | NA | Recruiting |
| NCT04799431 | mPCmCRC | I | Neoantigen Vaccine with Poly-ICLC adjuvant | s.c. | Retifanlimab | Not yet recruiting |
| NCT04912765 | Solid Tumors | II | Neoantigen Dendritic Cell Vaccine | i.d.. | Nivolumab | Recruiting |
| NCT04397926 | NSCLC | I | Individualized neoantigen peptides vaccine | s.c. | NA | Recruiting |
| NCT04487093 | NSCLC | I | neoantigen vaccine | s.c. |
|
Recruiting |
| NCT03122106 | Pancreatic cancer | I | Personalized neoantigen DNA vaccine | NN | NA | Active, not recruiting |
| NCT03956056 | Pancreatic cancer | Neoantigen Peptide Vaccine, Poly ICLC | s.c. | NA | Recruiting | |
| NCT03199040 | TNBC | Neoantigen DNA vaccine, TDS-IM system (Inchor Medical Systems) | NN | Durvalumab | Recruiting | |
| NCT03655756 | Melanoma Stage III/IV | I | IFx-Hu2.0 | s.c. | NA | Active, not recruiting |
| NCT04397003 | Extensive-stage SCLC | II | Neoantigen DNA vaccine | NN | Durvalumab | Not yet recruiting |
| NCT02129075 | Cutaneous, Mucosal and Ocular Melanoma | II | DEC-205/NY-ESO-1 Fusion Protein CDX-1401, Neoantigen-based Melanoma-Poly-ICLC Vaccine | s.c. | NA | Active, not recruiting |
| NCT04266730 | Squamous NSCLC SCC of Head and Neck |
I | PANDA-VAC | s.c. | Pembrolizumab | Not yet recruiting |
| NCT03558945 | Pancreatic Tumor | I | Personalized neoantigen vaccine | s.c. | NA | Recruiting |
| NCT03468244 | Solid tumors, lymphoma | NN | Naked mRNA | s.c. | NA | Recruiting |
| NCT03815058 | Metastatic melanoma | II | LNP | i.v. | Pembrolizumab | Recruiting |
| NCT03897881 | High-risk melanoma | II | NN | NN | Pembrolizumab | Recruiting |
| NCT03908671 | Esophageal cancer, NSCLC | NN | LNP | s.c. | NA | Not yet Recr4 times uiting |
| NCT04161755 | Pancreas cancer | I | NN | NN | Atezolizumab, chemotherapy | Recruiting |
Abbreviations:i.d., intradermal; i.m., intramuscular; i.n., intranodal; i.v., intravenous; s.c., subcutaneous; NN, not known, NSCL, non-small cell lung cancer; SCC, squamous cell cancer; TAA, tumor-associated antigen; TNBC, triple negative breast cancer; LNP, lipid nanoparticle; NA, not applicable; mCRC, metastatic colorectal cancer; mPC, metastatic pancreatic cancer; TNBC, triple negative breast cancer, SCLC, small cell lung cancer.
Conclusions
A new age of cancer vaccines has started with the first LNP mRNA vaccines being FDA approved as safe and effective in preventing infections by SARS-CoV-2 causing COVID-19. Such new methods could be important to other medical fields, especially therapeutic anticancer vaccines. Several clinical trials are testing LNP mRNA anti-cancer vaccines after encouraging in vitro results. Current methods for delivery of nucleic acid-based vaccines (RNA and DNA), like electroporation and intradermal needle-free system, are also advancing rapidly. Moreover, with the advent of immune therapies, stimulation of the immune system through checkpoint inhibition provides a valid rationale for the combination of therapeutic cancer vaccines together with immune-stimulating agents. Additional methods to further stabilize vaccines in the blood system should be considered in future research. DNA vaccines with more efficient delivery methods and combined with nanoparticles’ adjuvants could become a valid and potentially superior alternative to current RNA vaccines. In fact, DNA vaccines are simpler to design. With the impetus to the vaccination field, significant improvements are also expected in cellular and peptide-based anticancer vaccines.
CRediT authorship contribution statement
Navid Sobhani: Conceptualization, Supervision, Data curation, Visualization, Writing – original draft, Reviewing and editing. Bruna Scaggiante: Conceptualization, Data curation, Formal analysis, Supervision, Writing and editing the draft. Rachel Morris: Data curation, Conceptualization, Software, Visualization, Writing – original draft. Dafei Chai: Conceptualization, Investigation, Visualziation, Writing and editing. Martina Catalano: Data curation, Investigation, Resources, Software, Visualization, Writing – review & editing. Dana Rae Tardiel-Cyril: Data curation, Formal analysis, Methodology, Project administartion, Writing – review & editing. Praveen Neeli: Conceptualization, Software, Visualization, Writing and editing. Giandomenico Roviello: Conceptualization, Formal analysis, Supervision, Writing – original draft. Giuseppina Mondani: Conceptualization, Validation, Writing – review & editing. Yong Li: Conceptualization, Formal analysis, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Zitvogel L., Tesniere A., Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nature Reviews Immunology. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 3.Duarte J.H. Individualized neoantigen vaccines. Nat Res. 2021;2020 [Google Scholar]
- 4.Zamora A.E., Crawford J.C., Thomas P.G. Hitting the target: how T cells detect and eliminate tumors. Journal of Immunology. 2018;200(2):392–399. doi: 10.4049/jimmunol.1701413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedersen S.R., Sørensen M.R., Buus S., Christensen J.P., Thomsen A.R. Comparison of Vaccine-Induced Effector CD8 T Cell Responses Directed against Self- and Non–Self-Tumor Antigens: Implications for Cancer Immunotherapy. Journal of Immunology. 2013;191:3955–3967. doi: 10.4049/jimmunol.1300555. [DOI] [PubMed] [Google Scholar]
- 6.Hollingsworth R.E., Jansen K. Turning the corner on therapeutic cancer vaccines. npj Vaccines. 2019;4:1–10. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belli C., Trapani D., Viale G., D'Amico P., Duso B.A., Della Vigna P., et al. Targeting the microenvironment in solid tumors. Cancer Treatment Reviews. 2018;65:22–32. doi: 10.1016/j.ctrv.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Vedenko A., Panara K., Goldstein G., Ramasamy R., Arora H. Tumor microenvironment and nitric oxide: Concepts and mechanisms. Advances in Experimental Medicine and Biology. 2020;1277:143–158. doi: 10.1007/978-3-030-50224-9_10. [DOI] [PubMed] [Google Scholar]
- 9.Roma-Rodrigues C., Mendes R., Baptista P., Fernandes A. Targeting tumor microenvironment for cancer therapy. International Journal of Molecular Sciences. 2019;20(4):840. doi: 10.3390/ijms20040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng M., Mo Y., Wang Y., Wu P., Zhang Y., Xiong F., et al. Neoantigen vaccine: An emerging tumor immunotherapy. Mol Cancer. 2019;18(1) doi: 10.1186/s12943-019-1055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jou J., Harrington K.J., Zocca M.B., Ehrnrooth E., Cohen E.E.W. The changing landscape of therapeutic cancer vaccines-novel platforms and neoantigen identification. Clinical Cancer Research. 2021;27:689–703. doi: 10.1158/1078-0432.CCR-20-0245. [DOI] [PubMed] [Google Scholar]
- 12.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreiter S., Selmi A., Diken M., Koslowski M., Britten C.M., Huber C., et al. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Research. 2010;70:9031–9040. doi: 10.1158/0008-5472.CAN-10-0699. [DOI] [PubMed] [Google Scholar]
- 14.Kallen K.-J., Heidenreich R., Schnee M., Petsch B., Schlake T., Thess A., et al. A novel, disruptive vaccination technology: Self-adjuvanted RNActive ® vaccines. Hum Vaccines Immunother. 2013;9(10):2263–2276. doi: 10.4161/hv.25181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belnoue E., Leystra A.A., Carboni S., Cooper H.S., Macedo R.T., Harvey K.N., et al. Novel Protein-Based Vaccine against Self-Antigen Reduces the Formation of Sporadic Colon Adenomas in Mice. Cancers (Basel) 2021;13(4):845. doi: 10.3390/cancers13040845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wadhwa A., Aljabbari A., Lokras A., Foged C., Thakur A. Opportunities and Challenges in the Delivery of mRNA-Based Vaccines. Pharmaceutics. 2020;12(2):102. doi: 10.3390/pharmaceutics12020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines — a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao L., Zhang Y., Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20:1–23. doi: 10.1186/S12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karikó K., Muramatsu H., Ludwig J., Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Research. 2011;39:e142. doi: 10.1093/NAR/GKR695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA Recognition by Toll-like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/J.IMMUNI.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Karikó K., Muramatsu H., Welsh F.A., Ludwig J., Kato H., Akira S., et al. Incorporation of Pseudouridine Into mRNA Yields Superior Nonimmunogenic Vector With Increased Translational Capacity and Biological Stability. Molecular Therapy. 2008;16:1833. doi: 10.1038/MT.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang B., Jeang J., Yang A., Wu T.C., Hung C.F. DNA vaccine for cancer immunotherapy. Hum Vaccines Immunother. 2014;10:3153–3164. doi: 10.4161/21645515.2014.980686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singhal P., Marfatia Y.S. Human papillomavirus vaccine. Indian J Sex Transm Dis AIDS. 2009;30:51. doi: 10.4103/2589-0557.62761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monsonégo J. Prévention du cancer du col utérin : enjeux et perspectives de la vaccination antipapillomavirus. Gynécologie Obs Fertil. 2006;34:189–201. doi: 10.1016/J.GYOBFE.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 26.Peng S., Ferrall L., Gaillard S., Wang C., Chi W.-Y., Huang C.-H., et al. Development of DNA Vaccine Targeting E6 and E7 Proteins of Human Papillomavirus 16 (HPV16) and HPV18 for Immunotherapy in Combination with Recombinant Vaccinia Boost and PD-1 Antibody. MBio. 2021;12:1–19. doi: 10.1128/MBIO.03224-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strioga M.M., Darinskas A., Pasukoniene V., Mlynska A., Ostapenko V., Schijns V. Xenogeneic therapeutic cancer vaccines as breakers of immune tolerance for clinical application: To use or not to use? Vaccine. 2014;32:4015–4024. doi: 10.1016/J.VACCINE.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Quaglino E., Riccardo F., Macagno M., Bandini S., Cojoca R., Ercole E., et al. Chimeric DNA Vaccines against ErbB2+ Carcinomas: From Mice to Humans. Cancers (Basel) 2011;3:3225. doi: 10.3390/CANCERS3033225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.English D.P., Roque D.M., Santin A.D. HER2 Expression Beyond Breast Cancer: Therapeutic Implications for Gynecologic Malignancies. Mol Diagn Ther. 2013;17:85. doi: 10.1007/S40291-013-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopes A., Vandermeulen G., Préat V. Cancer DNA vaccines: current preclinical and clinical developments and future perspectives. Journal of Experimental & Clinical Cancer Research. 2019;38 doi: 10.1186/S13046-019-1154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan J., Ku G.Y., Gallardo H.F., Orlandi F., Manukian G., Rasalan T.S., et al. Safety and immunogenicity of a humanand mouse gp100 DNA vaccine in a phase I trial of patients with melanoma. Cancer Immun a J Acad Cancer Immunol. 2009;9:5. [PMC free article] [PubMed] [Google Scholar]
- 32.Rice J., Ottensmeier C.H., Stevenson F.K. DNA vaccines: Precision tools for activating effective immunity against cancer. Nature Reviews Cancer. 2008;8:108–120. doi: 10.1038/nrc2326. [DOI] [PubMed] [Google Scholar]
- 33.Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology - 10th ed.; 2021. p. 600.
- 34.Tay R.E., Richardson E.K., Toh H.C. Revisiting the role of CD4+ T cells in cancer immunotherapy—new insights into old paradigms. Cancer Gene Therapy. 2021;28:5–17. doi: 10.1038/s41417-020-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slingluff C.L. The present and future of peptide vaccines for cancer: Single or multiple, long or short, alone or in combination? Cancer Journal. 2011;17:343–350. doi: 10.1097/PPO.0b013e318233e5b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diao L., Meibohm B. Pharmacokinetics and pharmacokinetic-pharmacodynamic correlations of therapeutic peptides. Clinical Pharmacokinetics. 2013;52:855–868. doi: 10.1007/s40262-013-0079-0. [DOI] [PubMed] [Google Scholar]
- 37.Southwood S., Sidney J., Kondo A., del Guercio M.F., Appella E., Hoffman S., et al. Several common HLA-DR types share largely overlapping peptide binding repertoires. Journal of Immunology. 1998;160:3363–3373. [PubMed] [Google Scholar]
- 38.Luparello C. Parathyroid Hormone-Related Protein (PTHrP): A Key Regulator of Life/Death Decisions by Tumor Cells with Potential Clinical Applications. Cancers (Basel) 2011;3:396. doi: 10.3390/CANCERS3010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cusi M.G., Botta C., Pastina P., Rossetti M.G., Dreassi E., Guidelli G.M., et al. Phase I trial of thymidylate synthase poly-epitope peptide (TSPP) vaccine in advanced cancer patients. Cancer Immunology, Immunotherapy. 2015;64:1159–1173. doi: 10.1007/S00262-015-1711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Correale P., Botta C., Martino E.C., Ulivieri C., Battaglia G., Carfagno T., et al. Phase Ib study of poly-epitope peptide vaccination to thymidylate synthase (TSPP) and GOLFIG chemo-immunotherapy for treatment of metastatic colorectal cancer patients. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2015.1101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato K., Yamakawa Y., Shizume K., Satoh T., Nohtomi K., Demura H., et al. Passive immunization with anti-parathyroid hormone-related protein monoclonal antibody markedly prolongs survival time of hypercalcemic nude mice bearing transplanted human PTHrP-producing tumors. Journal of Bone and Mineral Research. 1993;8:849–860. doi: 10.1002/JBMR.5650080711. [DOI] [PubMed] [Google Scholar]
- 42.Arakawa Y, Okita Y, Narita Y. Efficacy finding cohort of a cancer peptide vaccine, TAS0313, in treating recurrent glioblastoma, vol. 39; 2021. p. 2038-2038. 10.1200/JCO.2021.39.15_SUPPL.2038. [DOI]
- 43.Cleyle J., Hardy M.-P., Minati R., Courcelles M., Durette C., Lanoix J., et al. Immunopeptidomic analyses of colorectal cancers with and without microsatellite instability. Molecular and Cellular Proteomics. 2022:100228. doi: 10.1016/J.MCPRO.2022.100228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corulli L.R., Cecil D.L., Gad E., Koehnlein M., Coveler A.L., Childs J.S., et al. Multi-Epitope-Based Vaccines for Colon Cancer Treatment and Prevention. Frontiers in Immunology. 2021;12:3533. doi: 10.3389/FIMMU.2021.729809/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mun Teo M.Y., Ceen Ng J.J., Fong J.Y., Hwang J.S., Song A.A.L., Hong Lim R.L., et al. Development of a single-chain fragment variable fused-mutant HALT-1 recombinant immunotoxin against G12V mutated KRAS c olorectal cancer cells. PeerJ. 2021;9 doi: 10.7717/PEERJ.11063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim S., Kim B.J., Kim I., Kim J.H., Kim H.K., Ryu H., et al. A phase II study of chemotherapy in combination with telomerase peptide vaccine (GV1001) as second-line treatment in patients with metastatic colorectal cancer. J Cancer. 2022;13:1363–1369. doi: 10.7150/JCA.70385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell S.J., Peng K.-W., Bell J.C. Oncolytic virotherapy. Nature Biotechnology. 2012;30:658. doi: 10.1038/NBT.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roy D.G., Geoffroy K., Marguerie M., Khan S.T., Martin N.T., Kmiecik J., et al. Adjuvant oncolytic virotherapy for personalized anti-cancer vaccination. Nature Communications. 2021;12:1–11. doi: 10.1038/s41467-021-22929-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pol J.G., Zhang L., Bridle B.W., Stephenson K.B., Rességuier J., Hanson S., et al. Maraba Virus as a Potent Oncolytic Vaccine Vector. Molecular Therapy. 2014;22:420–429. doi: 10.1038/MT.2013.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gebert J., Gelincik O., Oezcan-Wahlbrink M., Marshall J.D., Hernandez-Sanchez A., Urban K., et al. Recurrent frameshift neoantigen vaccine elicits protective immunity with reduced tumor burden and improved overall survival in a Lynch syndrome mouse model. Gastroenterology. 2021 doi: 10.1053/J.GASTRO.2021.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antonios J.P., Soto H., Everson R.G., Orpilla J., Moughon D., Shin N., et al. PD-1 blockade enhances the vaccination-induced immune response in glioma. JCI Insight. 2016;1:87059. doi: 10.1172/JCI.INSIGHT.87059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le D.T., Pardoll D.M., Jaffee E.M. Cellular vaccine approaches. Cancer Journal. 2010;16:304–310. doi: 10.1097/PPO.0b013e3181eb33d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamamoto L., Amodio N., Gulla A., Anderson K.C. Harnessing the Immune System Against Multiple Myeloma: Challenges and Opportunities. Frontiers in Oncology. 2021;10:3160. doi: 10.3389/fonc.2020.606368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keenan B.P., Jaffee E.M. Whole cell vaccines - Past progress and future strategies. Seminars in Oncology. 2012;39:276–286. doi: 10.1053/j.seminoncol.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiang C.L.-L., Coukos G., Kandalaft L.E. Whole Tumor Antigen Vaccines: Where Are We? Vaccines. 2015;3:344. doi: 10.3390/VACCINES3020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Senzer N., Barve M., Kuhn J., Melnyk A., Beitsch P., Lazar M., et al. Phase I Trial of “bi-shRNAifurin/GMCSF DNA/Autologous Tumor Cell” Vaccine (FANG) in Advanced Cancer. Molecular Therapy. 2012;20:679. doi: 10.1038/MT.2011.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin S.D., Brown S.D., Wick D.A., Nielsen J.S., Kroeger D.R., Twumasi-Boateng K., et al. Low Mutation Burden in Ovarian Cancer May Limit the Utility of Neoantigen-Targeted Vaccines. PLoS ONE. 2016;11 doi: 10.1371/JOURNAL.PONE.0155189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J., et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science (80-) 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A., et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. New England Journal of Medicine. 2014;371:2189–2199. doi: 10.1056/nejmoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salewski I., Gladbach Y.S., Kuntoff S., Irmscher N., Hahn O., Junghanss C., et al. In vivo vaccination with cell line-derived whole tumor lysates: neoantigen quality, not quantity matters. J Transl Med. 2020;18:402. doi: 10.1186/s12967-020-02570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science (80-) 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 62.Yadav M., Jhunjhunwala S., Phung Q.T., Lupardus P., Tanguay J., Bumbaca S., et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 63.Sahin U., Derhovanessian E., Miller M., Kloke B.P., Simon P., Löwer M., et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 64.Ott P.A., Hu Z., Keskin D.B., Shukla S.A., Sun J., Bozym D.J., et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gubin M.M., Zhang X., Schuster H., Caron E., Ward J.P., Noguchi T., et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei S.C., Duffy C.R., Allison J.P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 67.Campbell P.J., Pleasance E.D., Stephens P.J., Dicks E., Rance R., Goodhead I., et al. Subclonal phylogenetic structures in cancer revealed by ultra-deep sequencing. Proc Natl Acad Sci U S A. 2008;105:13081. doi: 10.1073/PNAS.0801523105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alexandrov L.B., Nik-Zainal S., Wedge D.C., Aparicio S.A.J.R., Behjati S., Biankin A.V., et al. Signatures of mutational processes in human cancer. Nat. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lawrence M.S., Stojanov P., Polak P., Kryukov G.V., Cibulskis K., Sivachenko A., et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nat. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vogelstein B., Papadopoulos N., Velculescu V.E., Zhou S., Diaz L.A., Kinzler K.W. Cancer genome landscapes. Science (80-) 2013;340:1546–1558. doi: 10.1126/SCIENCE.1235122/SUPPL_FILE/VOGELSTEIN.SM.COVER.PAGE.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rooney M.S., Shukla S.A., Wu C.J., Getz G., Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/J.CELL.2014.12.033/ATTACHMENT/6429F90E-4497-4300-B031-42EEEF626695/MMC15.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown S.D., Warren R.L., Gibb E.A., Martin S.D., Spinelli J.J., Nelson B.H., et al. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Research. 2014;24:743. doi: 10.1101/GR.165985.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giannakis M., Mu X.J., Shukla S.A., Qian Z.R., Cohen O., Nishihara R., et al. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep. 2016;15:857–865. doi: 10.1016/J.CELREP.2016.03.075/ATTACHMENT/A727252A-0701-48AD-9A01-7044E54B66A7/MMC2.XLSX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu Z., Ott P.A., Wu C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nature Reviews Immunology. 2017;18:168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fritsch E.F., Rajasagi M., Ott P.A., Brusic V., Hacohen N., Wu C.J. HLA-Binding Properties of Tumor Neoepitopes in Humans. Cancer Immunol Res. 2014;2:522–529. doi: 10.1158/2326-6066.CIR-13-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rajasagi M., Shukla S.A., Fritsch E.F., Keskin D.B., DeLuca D., Carmona E., et al. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood. 2014;124:453–462. doi: 10.1182/BLOOD-2014-04-567933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sonntag K., Hashimoto H., Eyrich M., Menzel M., Schubach M., Döcker D., et al. Immune monitoring and TCR sequencing of CD4 T cells in a long term responsive patient with metastasized pancreatic ductal carcinoma treated with individualized, neoepitope-derived multipeptide vaccines: a case report. J Transl Med. 2018;16 doi: 10.1186/S12967-018-1382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keskin D.B., Anandappa A.J., Sun J., Tirosh I., Mathewson N.D., Li S., et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nat. 2018;565:234–239. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hilf N., Kuttruff-Coqui S., Frenzel K., Bukur V., Stevanović S., Gouttefangeas C., et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nat. 2018;565:240–245. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 80.Johanns T.M., Miller C.A., Liu C.J., Perrin R.J., Bender D., Kobayashi D.K., et al. Detection of neoantigen-specific T cells following a personalized vaccine in a patient with glioblastoma. Oncoimmunology. 2019;8 doi: 10.1080/2162402X.2018.1561106/SUPPL_FILE/KONI_A_1561106_SM4321.XLSX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.A Study of Autogene Cevumeran (RO7198457) as a Single Agent and in Combination With Atezolizumab in Participants With Locally Advanced or Metastatic Tumors - Full Text View - ClinicalTrials.gov; n.d.
- 82.Sarkizova S., Klaeger S., Le P.M., Li L.W., Oliveira G., Keshishian H., et al. A large peptidome dataset improves HLA class I epitope prediction across most of the human population. Nature Biotechnology. 2019;38:199–209. doi: 10.1038/s41587-019-0322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin Z., Zhang Y., Cai H., Zhou F., Gao H., Deng L., et al. A PD-L1-based cancer vaccine elicits antitumor immunity in a mouse melanoma model. Molecular Therapy - Oncolytics. 2019;14:222. doi: 10.1016/J.OMTO.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen J., Liu H., Jehng T., Li Y., Chen Z., Lee K.D., et al. A Novel Anti-PD-L1 Vaccine for Cancer Immunotherapy and Immunoprevention. Cancers (Basel) 2019;11 doi: 10.3390/CANCERS11121909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaumaya P.T.P., Guo L., Overholser J., Penichet M.L., Bekaii-Saab T. Immunogenicity and antitumor efficacy of a novel human PD-1 B-cell vaccine (PD1-Vaxx) and combination immunotherapy with dual trastuzumab/pertuzumab-like HER-2 B-cell epitope vaccines (B-Vaxx) in a syngeneic mouse model. Oncoimmunology. 2020;9 doi: 10.1080/2162402X.2020.1818437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thompson E.A., Liang F., Lindgren G., Sandgren K.J., Quinn K.M., Darrah P.A., et al. Human Anti-CD40 Antibody and Poly IC:LC Adjuvant Combination Induces Potent T Cell Responses in the Lung of Nonhuman Primates. Journal of Immunology. 2015;195:1015–1024. doi: 10.4049/jimmunol.1500078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Melssen M.M., Petroni G.R., Chianese-Bullock K.A., Wages N.A., Grosh W.W., Varhegyi N., et al. A multipeptide vaccine plus toll-like receptor agonists LPS or polyICLC in combination with incomplete Freund’s adjuvant in melanoma patients. Journal for ImmunoTherapy of Cancer. 2019;7:163. doi: 10.1186/s40425-019-0625-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pockros P.J., Guyader D., Patton H., Tong M.J., Wright T., McHutchison J.G., et al. Oral resiquimod in chronic HCV infection: Safety and efficacy in 2 placebo-controlled, double-blind phase IIa studies. Journal of Hepatology. 2007;47:174–182. doi: 10.1016/j.jhep.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 89.Kasturi S.P., Skountzou I., Albrecht R.A., Koutsonanos D., Hua T., Nakaya H.I., et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–550. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lynn G.M., Laga R., Darrah P.A., Ishizuka A.S., Balaci A.J., Dulcey A.E., et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nature Biotechnology. 2015;33:1201–1210. doi: 10.1038/nbt.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ni Q., Zhang F., Liu Y., Wang Z., Yu G., Liang B., et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Science Advances. 2020;6 doi: 10.1126/sciadv.aaw6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilgenhof S., Van Nuffel A.M.T., Benteyn D., Corthals J., Aerts C., Heirman C., et al. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Annals of Oncology. 2013;24:2686–2693. doi: 10.1093/annonc/mdt245. [DOI] [PubMed] [Google Scholar]
- 93.Wilgenhof S., Corthals J., Heirman C., Van Baren N., Lucas S., Kvistborg P., et al. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patientswith pretreated advanced melanoma. Journal of Clinical Oncology. 2016;34:1330–1338. doi: 10.1200/JCO.2015.63.4121. [DOI] [PubMed] [Google Scholar]
- 94.Wilgenhof S., Corthals J., Van Nuffel A.M.T., Benteyn D., Heirman C., Bonehill A., et al. Long-term clinical outcome of melanoma patients treated with messenger RNA-electroporated dendritic cell therapy following complete resection of metastases. Cancer Immunology, Immunotherapy. 2015;64:381–388. doi: 10.1007/s00262-014-1642-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wilgenhof S., Van Nuffel A.M.T., Corthals J., Heirman C., Tuyaerts S., Benteyn D., et al. Therapeutic vaccination with an autologous mRNA electroporated dendritic cell vaccine in patients with advanced melanoma. Journal of Immunotherapy. 2011;34:448–456. doi: 10.1097/CJI.0b013e31821dcb31. [DOI] [PubMed] [Google Scholar]
- 96.Jansen Y., Kruse V., Corthals J., Schats K., van Dam P.J., Seremet T., et al. A randomized controlled phase II clinical trial on mRNA electroporated autologous monocyte-derived dendritic cells (TriMixDC-MEL) as adjuvant treatment for stage III/IV melanoma patients who are disease-free following the resection of macrometastases. Cancer Immunology, Immunotherapy. 2020;69:2589–2598. doi: 10.1007/s00262-020-02618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dörrie J., Schaft N., Schuler G., Schuler-Thurner B. Therapeutic cancer vaccination with ex vivo rna-transfected dendritic cells—an update. Pharmaceutics. 2020;12:92. doi: 10.3390/pharmaceutics12020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kowalski P.S., Rudra A., Miao L., Anderson D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Molecular Therapy. 2019;27:710–728. doi: 10.1016/j.ymthe.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C., et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 100.Baharom F., Ramirez-Valdez R.A., Tobin K.K., Yamane H., Dutertre C.-A., Khalilnezhad A., et al. Intravenous Nanoparticle Vaccination Generates Stem-Like TCF1+ Neoantigen-Specific CD8+ T Cells. Nature Immunology. 2021;22:41. doi: 10.1038/S41590-020-00810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]