Abstract
Medium-chain triglycerides contain medium-chain fatty acid esterified to the glycerol backbone. These MCFA have a shorter chain length and are quickly metabolized in the body serving as an immediate energy source. They are known to have good physiological as well as functional characteristics which help in treating various health disorders. Naturally, they are found in coconut oil, milk fat, and palm kernel oil, and they are synthetically produced by esterification and interesterification reactions. Due to their numerous health benefits, MCT is used as a functional or nutraceutical oil in various food and pharmaceutical formulations. To increase their nutraceutical benefits and food applications MCFA can be used along with polyunsaturated fatty acids in the synthesis of structured lipids. This review aims to provide information about triglycerides of MCFA, structure, metabolism, properties, synthetic routes, intensified synthesis approaches, health benefits, application, and safety of use of MCT in the diet.
Keywords: Medium-chain triglyceride, Medium-chain fatty acid, Nutraceutical, Esterification, Interesterification, Coconut oil
Introduction
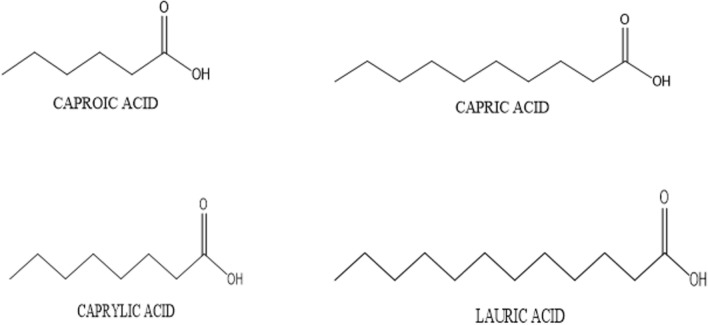
Lipids are considered one of the major food constituents required for the normal functioning and growth of the human body. Lipids are required by human beings from all age groups starting from infants till old age people. They serve as the source of essential fatty acids in the human diet and also serve as a concentrated source of energy-giving 9 kcal/g. Most lipids in the diet are in the form of triglycerides, in which three different fatty acids are esterified to the glycerol backbone, these fatty acids can be SFA, MUFA, and, PUFA. Each fatty acid has its own chemical and physical characteristics and is metabolized and absorbed by the body depending on its chain length. The fatty acid composition of triglyceride is largely decided by its source which can be a plant source or animal source. Triglycerides from plant sources are generally liquid at room temperature because they contain MUFA/PUFA. But, triglycerides from animal sources are generally solid at room temperature because they contain SFA (Dorni et al. 2018). Food processing industries make use of fat/oil for making processed food and even at home cooking is done using fat/oil. These dietary fats used at home or in industry contains LCFA. A diet containing a high amount of dietary fat or excess intake of these dietary fats is related to various diseases like cardiovascular diseases, obesity, cancer, and increased health issues which are mostly seen in the developed countries (Liu et al. 2020). Consumer across the globe needs a better alternative that will be healthier and beyond that, a ‘Nutraceutical’ MCT emerged as a ‘Nutraceutical fat’ having many health benefits (St-Onge and Jones 2002). MCT contains all three positions on the glycerol backbone occupied by medium chain-fatty acids. MCFA includes caprylic acid (C8:0), capric acid (C10:0) and lauric acid (C12:0) (Table 1). There are limited natural sources that contain medium-chain triglycerides which include coconut oil, palm kernel oil,and bovine milk (Jensen 2002). Synthetically MCT are synthesized by an esterification reaction between MCFA and glycerol (Jadhav and Annapure 2021a; Jadhav et al. 2021a). In comparison with long-chain saturated triglycerides, the MCT give less calorie i.e., 8.4 kcal/g are liquid at ambient temperature, and has a shorter chain length (Fig. 1) (Ingle et al. 1999). These unique properties of MCT make it an ideal choice for health-conscious consumer and because of such unique physicochemical properties, MCT are metabolized in different ways unlike long-chain triglycerides (Babayan 1968; Rial et al. 2020). MCTs were launched as an exceptional source of energy for various clinical nutrition like malabsorption of fat, atherosclerosis, obesity, parenteral nutrition, severe hyperchylomicronemia etc., and they were also used in infant formulations (Bach et al. 1996; Carlson et al. 2015; Zhang et al. 2016; Augustin et al. 2018; Hollis et al. 2018; Avgerinos et al. 2020; Izgelov et al. 2020; Ashton et al. 2021). US FDA has assigned GRAS status to the use of MCT in food products (Traul et al. 2000). In spite of having so many good physicochemical properties which make it superior to other fats, MCT has certain limitations in food applications. A diet containing only MCT can make human body to have deficiencies of essential fatty acids since MCT lack essential fatty acids and PUFA. This can be overcome by making designer lipid having MCFA at sn-1,3 position and EFA or PUFA at sn-2 position on the same glycerol backbone, thus providing the nutrition from both the fatty acids. Japan was the first country to use designer lipid (Jadhav and Annapure 2021b) (Fig. 1) as a healthy cooking oil which does not result in fat accumulation when taken into diet. These are available as cooking oil under the brand name Resetta in Japan since 2000. This review aims to highlight medium chain triglyceride which has many benefits over traditional fat. This review also covers various sources and synthetic routes to produce medium chain triglycerides, intensification approaches to get maximum yield in less time, health benefits of MCT, application and safety of MCT. MCT has been a research topic of great interest lately, hence this review will be of great interest to food scientists and technologists across the globe.
Table 1.
General information of medium chain fatty acids
| Medium chain fatty acid | Number of carbons | Molecular formula | Structural formula | Systematic name | Molecular weight (g/mol) |
|---|---|---|---|---|---|
| Caproic acid | C6:0 | C6H12O2 | CH3(CH2)4COOH | Hexanoic acid | 116.15 |
| Caprylic acid | C8:0 | C8H16O2 | CH3(CH2)6COOH | Octanoic acid | 144.21 |
| Capric acid | C10:0 | C10H20O2 | CH3(CH2)8COOH | Decanoic acid | 172.26 |
| Lauric acid | C12:0 | C12H24O2 | CH3(CH2)10COOH | Dodecanoic acid | 200.31 |
Fig. 1.
Chemical structure of medium chain fatty acids
Sources of medium chain triglycerides
Naturally MCT are found in coconut oil, palm kernel oil, and also in milk fat. Coconut oil contains a larger fraction of lauric acid than other MCFA. Specifically, in bovine milk caproic, caprylic and capric constitute about 4–12% of total fatty acids whereas lauric acid constitutes about 3–5%. These MCT from natural sources are hydrolysed to obtain MCFA which are then again esterified to obtain MCT. In case of coconut oil, copra is obtained by sun drying, this copra is pressed using a solvent to obtain the oil. The obtained oil is referred to as virgin coconut oil, this oil is then subjected to a refining process, which can be used for food and nutraceutical application. This oil is known as Refined, bleached and deodorized (RBD) oil. The oil obtained from coconut contains about 46–54% lauric acid, 5–10% caprylic acid and 5–8% capric acid. Similarly, oil obtained from palm kernel contains 45–50% lauric acid, 3–5% caprylic acid, 3–4% capric acid and 0.1–0.5% caproic acid. The amount of MCFA present in milk fat is very low i.e., lauric acid, capric acid, caprylic acid, caproic acid constitute about 2.5–4%, 1.5–3.5%, 0.5–1.6%, 0.5–3.0% respectively (Ransom-Painter et al. 1997; Ibrahim et al. 2003).
Synthetic routes
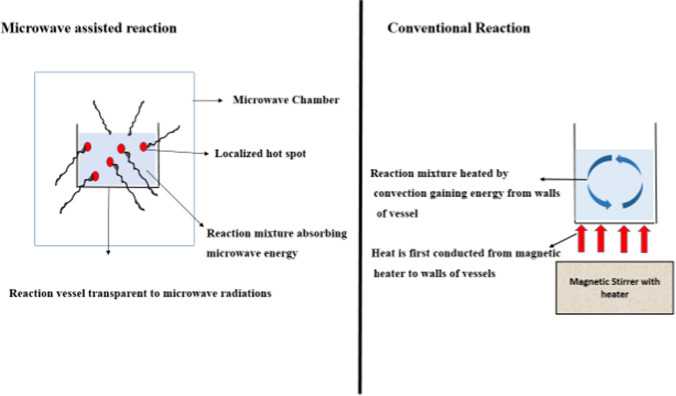
Since the natural sources of MCT are limited and demand is increasing, hence MCT is obtained through a synthetic route by the esterification reaction between alcohol (glycerol) and fatty acids (MCFAs) using an enzyme or chemical catalyst. The enzymatic esterification process for the synthesis of MCT uses lipases from various sources like animals, plants, and microorganisms (Mehta et al. 2021), these lipases are specific to the fatty acid, i.e., they can be stereospecific or regiospecific. Lipases that are specific to fatty acid will tend to have an affinity for that particular fatty acid regardless of their position on the glycerol backbone. For example, lipase from Penicillium rocquefortii shows a higher affinity towards SCFA, lipase from Geotrichum candidum shows more affinity towards a fatty acid that contains unsaturation at the cis-9 position (Jensen 1983; Mase et al. 1995). Stereospecific lipases are specific to positions on glycerol backbone. They are specific to sn-1 and sn-3 position on the glycerol backbone. Their specificity varies at sn-1 and sn-3 positions, for example, lipase from Candida antarctica B is specific to sn-3 position whereas lipase from Pseudomonas fluorescens is specific to the sn-1 position (Jensen et al. 1982; Lavayre et al. 1982; Uzawa et al. 1990; Rogalska et al. 1993). Regiospecific lipase has an affinity towards position sn-1,3 and sn-2. Lipase from Aspergillus niger has affinity towards the sn-1,3 position and cannot act on sn-2 position because of steric hindrance and lipase from Candida parapsilosis shows more affinity towards sn-2 position than sn-1,3 position (Riaublanc et al. 1993). There are non-specific lipases that do not have an affinity for specificity for a particular position on the glycerol backbone, they interact with fatty acid regardless of their position and produces triglyceride with a random distribution of fatty acid to the glycerol backbone. For example, lipase from Candida cylindraceae is not specific to any position, and also lipase from Staphylococcus aureus is also non-specific (Macrae 1983). These specific and non-specific enzymes are used for attachment of fatty acid on glycerol backbone and even hydrolysis of fatty acid from the glycerol backbone. As it is done when oil is extracted from natural sources like coconut oil, the triglycerides are first hydrolyzed and then re-esterified to get MCT. Enzymatic esterification process has many advantages over chemical esterification process, as chemical esterification reactions are carried out at higher temperature using acid catalyst, there is formation of many unwanted by-products, and quality of triglyceride is also low as compared with one formed from enzymatic processes. In chemical esterification additional processes like washing, bleaching, deodorization, and purification is required (Sivakanthan and Madhujith 2020). The chemical catalyst used are very reactive and they should be handled carefully for large-scale production of triglycerides. The acid catalyst may lead to an explosion when it comes in contact with water. Since enzymes are very specific to the position, the enzymatic synthesis does not form any undesirable by-products. The enzymatic esterification reaction is generally carried out at a moderate temperature below 50 °C, thus posing less harm and enzymes are not toxic like a chemical catalyst. The chemical or enzymatic esterification reaction (Fig. 2) forms water as a by-product, this water should be continuously removed from the reaction mixture, so that reaction proceeds in the forward direction. Esterification reaction for synthesis of the triglycerides is highly reversible in presence of water. Table 2 shows comparison between chemical and enzymatic esterification process. There are many studies reported in the literature for synthesis of MCT using either chemical or enzymatic routes. Boulos (2013) reported synthesis of MCT using metal catalyst. The author reported maximum yield of triglycerides of caproic acid as 85% with use of tungsten oxide as catalyst, whereas triglyceride of caprylic acid gave maximum yield of 93% with tungsten oxide and triglyceride of capric acid gave highest yield of 93%, 92%, 91%, 90% with tungsten oxide, tungsten chloride, calcium oxide, zinc chloride respectively. Triglycerides of lauric acid gave highest yield (%) of 78% with calcium oxide as catalyst. Author reported different yield with use of different catalyst at 160–180 °C in 22–24 h. Thus, in esterification reaction the yield of final triglyceride will depend on the type of catalyst used and activity of catalyst will be dependent on the substrate of reaction. The chemical esterification reaction gives yield more than 90% at higher temperature with reaction time of more than 20 h. In order to reduce this reaction time and increase the yield there is recent advancement in the synthesis of MCT with application of microwave and or ultrasound irradiation technology (Table 3). In recent study MCT (tricaprylin) was synthesized using amberlyst − 15 which is an acid resin catalyst with application of microwave. The authors reported conversion (%) of more than 95% in just 16 min (Jadhav and Annapure 2021a). Microwave was used as an intensified approach for synthesis of triglyceride. In microwave the microwave energy is directly coupled with the reaction mixture and there is formation of hotspots which results in enhanced rate of heat and mass transfer (Fig. 3) (Surat et al. 2012). Another approach for intensified synthesis is with use of sonication process. In ultrasonication there is formation, growth and collapse of cavities which results in generation of shock wave which travels through the reaction mixture and creates turbulence which increases rate of mass transfer thus achieving intensified yield of product in short reaction time (Gharat and Rathod 2020). There are many recently published studies on ultrasound assisted synthesis of MCT. Mohod et al. (2018) reported intensified synthesis of MCT using chemical catalyst in presence of ultrasound. The authors reported yield (%) of 77.8% in 5 h of ultrasound irradiation at 90 °C. In order to highlight the intensification approach by ultrasound and microwave Mohod et al. (2017) reported synthesis of medium chain triglyceride using microwave, ultrasound and conventional approaches. The authors reported that microwave assisted synthesis gave 97.8% yield in 20 min, ultrasound gave 97.3% yield in 120 min whereas conventional process gave only 40% yield in 180 min. Similar studies using chemical catalyst and intensification approach were reported by Deshmane et al. (Deshmane et al. 2008; Jadhav and Annapure 2021c). Langone et al. (2002) reported synthesis of MCT with use of immobilized lipase. The authors reported that higher selectivity of lipase was observed at 5–9% concentration and at different temperature i.e., 70 °C for capric acid, 80 °C for lauric acid and 90 °C for myristic acid. Further the authors reported 50%, 70% and 70% as yield (%) of triglycerides of capric acid, lauric and myristic acid respectively. The difference in the yield (%) is due to affinity of lipases towards the particular substrate. The enzymatic synthesis is time consuming; the enzymatic process requires around 48 h for completion because in enzyme catalysed reaction the rate of mass transfer is very slow, however this can be overcomed with use of newer synthetic technologies like ultrasound, supercritical carbondioxide. One such recent study by More et al. (2018) reported that with the use of supercritical carbondioxide at 100 bar pressure for 60 min at 50 °C has been shown to intensify yield (%) of triglyceride of caprylic acid by enzymatic esterification using immobilized enzymes. The authors reported intensified yield of 97.3% in 6 h of reaction time. The supercritical carbondioxide increases the diffusivity of the enzyme, which result in increase in rate of mass transfer, thus the reaction is pulled in forward direction and supercritical CO2 is a green alternative to organic solvents usually used in enzymatic synthesis. Similarly, ultrasound is also used to intensify the yield of MCT in less reaction time. More et al. (2017b) reported intensified synthesis of triglyceride of caprylic acid by enzymatic esterification using Novozyme 435 and Lipozyme IM RM with 70% ultrasound duty cycle at 50 °C. Author reported maximum yield (%) of 94.8% in 420 min. MCT can also be synthesized by interesterification reaction between two triglycerides. In interesterification the fatty acids may change position on same glyceride molecule or there may be interchange of fatty acid between two triglyceride molecules (Sivakanthan and Madhujith 2020). These reactions are also catalysed by enzyme or chemical catalyst, mostly alkali metals are used for chemical interesterification reaction (Pires et al. 2008). But here also enzymatic process is preferred for synthesis if end application is meant for food or nutraceutical products. Lu et al. (2017) reported synthesis of MCT by interesterification reaction between MCT and soyabean oil using Lipozyme 435. The authors reported yield (%) of 74.9% at 90 °C in 300 min of reaction time. The interesterification reaction may produce triglyceride molecule enriched in MCFA with one LCFA. Interesterification of coconut oil produced a triglyceride containing MCFA, this chemical interesterification reaction resulted in reshuffling or exchange of fatty acid with on the glyceride of coconut oil, thus giving a new triglyceride molecule with different fatty acid composition (Nugrahini and Soerawidjaja 2015).
Fig. 2.
General esterification reaction for synthesis of triglycerides
Table 2.
Points of comparison between chemical and enzymatic esterification reaction
| Characteristics | Chemical | Enzymatic |
|---|---|---|
| Type of catalyst | Chemical catalyst like sulphuric acid, hydrochloric acid, sodium methoxide, para-toluene sulphonic acid etc | Lipases from animal, plant and microorganisms |
| Temperature | Reaction is usually carried out at higher temperature (150–200 °C) | Reaction is carried out at moderate temperature below 50 °C |
| Bi-product formation | Many undesirable by-products are formed and hence product needs purification | Insignificant or no by-products are formed |
| Eco-friendly | No, since it produces many by-products which are toxic in nature | Yes, it is cleaner and eco-friendly |
| Challenges | It produces toxic products and final product is dark in color hence processes like bleaching, deodorization, purification is necessary | Little change in temperature, pH of reaction medium affects the activity of enzyme |
| Rate of reaction | Rate of reaction is faster; hence less time is required for completion of reaction | Rate of reaction is very slow; hence more time is required for completion of reaction |
| Economical aspect | Chemical catalyst are inexpensive, hence the process is economical as compared with enzymatic process | Enzymes are very expensive, which increases the cost of production |
Table 3.
Synthetic routes for synthesis of medium chain triglycerides
| Reaction | Substrate | Catalyst/enzyme | Reaction temp (°C) and time | Intensification approach | Yield (%) | References |
|---|---|---|---|---|---|---|
| Esterification | Caproic acid, caprylic acid, capric acid, lauric acid and glycerol | Tungsten oxide, calcium oxide, tungsten chloride | 160–180 °C in 22–24 h | Conventional reaction | 90–93% | Boulos (2013) |
| Caprylic acid and glycerol | Amberlyst-15 | 80 °C in 16 min | Microwave assisted synthesis | 99.7% | Jadhav and Annapure (2021a) | |
| Lauric acid and glycerol | Sulphuric acid | 90 °C in 300 min | Ultrasound assisted synthesis | 77.8% | Mohod and Gogate (2018) | |
| Lauric acid and glycerol | Sulphuric acid |
90 °C in 1. 20 min 2. 120 min 3. 180 min |
1.Microwave assisted 2.Ultrasound assisted 3.Conventional synthesis |
1. 99.7% 2. 97.5% 3. 40% |
Mohod and Gogate (2017) | |
| Caprylic acid (60.35%), Capric acid (38.11%), lauric acid (1.15%) and glycerol | Sulphuric acid | 90 °C in 300 min | Ultrasound assisted synthesis | 98.5% | Deshmane et al. (2008) | |
| Caprylic acid and glycerol | Sulphuric acid, hydrochloric acid, PTSA, Sodium methoxide | 170 °C in 9 h | Ultrasound assisted synthesis (More et al. 2017a) | 96.6% | More et al. (2017a) | |
| Capric acid, lauric acid, myristic acid and glycerol | Immobilized lipase (Lipozyme IM 20) | 60–90 °C in 26 h | Conventional synthesis |
50%-Tricaprin 70%- Trilaurin 70%-Trimyristin |
Langone and Sant’Anna (2002); Langone et al. (2002) | |
| Caprylic acid and glycerol | Novozyme 435, Lipozyme IM RM | 50 °C in 6 h | Supercritical CO2 Technology | 97.3% | More et al. (2018) | |
| Caprylic acid and glycerol | Novozyme 435 and Lipozyme RM IM | 50 °C in 7 h | Ultrasound assisted synthesis | 94.8% | More et al. (2017b) | |
| Interesterification | Medium chain triglycerides and soyabean oil | Lipozyme 435 | 90 °C in 300 min | Conventional synthesis | 74.9% | Lu et al. (2017) |
| Coconut oil | Potassium methoxide | 40–60 °C in 12 h | Conventional synthesis | 24% | Nugrahini and Soerawidjaja (2015) |
Fig. 3.
Figure representing comparison between microwave and conventional reaction
Metabolism of medium chain triglyceride
MCT contain MCFA attached to glycerol molecule. The metabolism, digestion and absorption of MCT is different than that of LCT. Human endogenous enzyme lipase brings about hydrolysis of MCT. After hydrolysis the MCFA are released from glycerol backbone and because of its hydrophilic nature and shorten carbon chain these MCFA are directly transported via hepatic portal vein to liver. These fatty acids undergo β-oxidation process. This quick metabolism of MCFA results in formation of ketones body. These ketone body act as an immediate energy source to body. The hydrolysed medium chain fatty acid does not go to lymphatic system for re-synthesis of triglyceride molecule which has ability to be stored in the form of adipose tissue as fat, leading to obesity (Babayan 1968).
Health benefits of medium chain triglycerides
MCT were first launched as a source of energy in 1950. Since MCT are metabolized in different way they provide immediate energy and can be used to control obesity. MCT has high satiety value, which prevents over consumption of food (Kinsella et al. 2017). Zhang et al. (2015) reported that diet rich in MCT can lead to increase in fat oxidation and increase in energy expenditure in healthy adults fed with 2% MCT in diet for 3 months. MCT serve as an immediate energy source for sports person, athletes and also for humans having inability in metabolizing sugar due to old age. Consumption of MCT is diet is related to increase psychological health by boosting memory. A recent study on rats showed that diet rich in MCT can reduces anxiety and leads to improve social behaviour in rats (Hollis et al. 2018; Ashton et al. 2021). These effects are closely related to metabolism of MCT which generates ketone body namely β-hydroxybutyrate. Increase in formation of this ketone body results in such positive effects (Reger et al. 2004). MCT is very effective in managing epilepsy. The ketone bodies generated after metabolism of MCT are transported to brain cell shows good results in reduction of seizures as compared to ketone bodies generated by glucose metabolism. MCT is also found to be effective against SARS Coronavirus-2. MCT is able to change the metabolism of lipids in virus. Virus replicates quickly and for this replication energy is needed which is extracted from long chain fatty acids. Long chain fatty acids are also needed for attachment of virus envelope with the host. MCT decreases formation of long chain fatty acid thereby making it unavailable to virus, leading to death of virus. MCFA released from glycerol backbone after lipase hydrolysis are believed to have antimicrobial properties. MCFA destroys the bacterial colony in intestinal tract by decreasing the pH. Recent research reported that 3% MCT supplement results in decreasing the total coliform bacteria present in rectum and colon. MCT can also be used as an effective antibiotics against the bacterial growth (Yen et al. 2015). But to use MCT as antibiotics needs further study as this is just a single study reported in literature for MCFA as potential antimicrobial agent.
Application
MCT is available in the market in the form of liquid oil (Table 4) which is largely used for therapeutic purpose and as a potential agent against obesity. MCT is also used as a salad dressing oil, it is used in yogurt, shakes, smoothies etc. MCT is also used as a cooking oil, there are certain brands available in market like Viola oil which is a blend containing about 65–70% MCT along with small amount of plant sterols, Delta oil which is a blend of MCT and canola oil with plant sterols. The physical blend of MCT along with other oil and plant sterol will give additional benefits of fatty acids from both the oil and the plant sterols will regulate the level of cholesterol in human body. MCT are also converted in to powdered form by spray drying for their better application in food products. Interesterified designer lipids produced contains medium chain fatty acid at sn-1, 3 position and long chain fatty acid at sn-2 position (Jadhav et al. 2021c). Such modification of triglyceride molecule produces a new triglyceride enriched in MCFA which can be used in confectionaries, bakery, as a better substitute to plastic fats (Heydinger and Nakhasi 1996). The di and monoglycerides of MCFA are also a good emulsifiers and can be used in making flavour emulsion having higher stability and these flavour emulsion can be used in bakery and beverage industries (Jadhav et al. 2021b). Since, MCT provides immediate energy when taken in diet, MCT can be added to food like cookies, biscuits, jellies, puff pastries for the patients suffering from critical illness and cannot metabolize carbohydrates easily. MCFA can be incorporated in designer lipid along with EFA and can be used for patients having cardiovascular problem, obesity, cancer, inflammation etc. MCT are hydrophilic as compared with LCT; hence they can be effective solvent to dissolve food colour, flavour etc. Thera are studies which have shown that the MCT show anti-bacterial activity and are even active against viruses. Malaysian palm oil board have recently notified that they will be making palm oil MCT based anti-viral drug against covid-19. Even Philippines which are largest producer of coconut have initiated their clinical trials to see effectiveness of virgin coconut oil against covid-19. Thus, MCT is an excellent lipid which can be used in formulation of various food products; thus, consumer will get processed food with added benefits of MCT in it.
Table 4.
Commercially available MCT products in market
| MCT oil brand | Application | Producer |
|---|---|---|
| MCT oil | Act as a fuel to brain and body | Nature way, USA |
| Powdered MCT Oil | Ketogenic diet | Quest Nutrition USA |
| MCT Oil (mixture of C8 and C10) | Food applications like bakery and confectionary | AAK Kamani, India |
| Joymix MCT Oil | For weight management | Malaysia |
| Keto products | Source of energy | 360 Nutrition, USA |
| Keto organic MCT Oil | Used for weight loss | Ancient Nutrition, USA |
| MCT powder | Help to control cardiovascular diseases | Ogranika, Canada |
| Liquid MCT Oil | Supplement to be used in food formulation | Supplement manufacturer, UK |
| Spring valley oil | Management of weight and for athletes | Spring valley, USA |
| Soft gel (MCT Oil) | Improved fat metabolism | Carlson Lab, USA |
| Melrose MCT powder and MCT oil | Energy for brain and body | Melrose, Australia |
| MCT Oil | Source of energy | Bioglan, Australia |
| Max-C8 | Proper metabolism, digestion, energy, weight management | Zenwise health, Germany |
| Pure tricaprylin oil | Weight management | Weight world, UK |
| Diet MCT Oil | Nutraceutical fat and source of energy | Diet works, USA |
Safety of medium chain triglyceride
MCT has been the interested area of research for food scientist. But there is lack of knowledge about the dosage and ill effect of MCT on human body. Safety and toxicity of MCT still remains an unexplored area. There is one study reported in literature which demonstrates that the designer lipids with 30% MCFA can help in reducing weight without any adverse effect on the body of mice. There are some reports available in literature demonstrating the effect of MCFA on increasing high density lipoprotein but the research lack in providing evidences of effect of these fatty acid on the level of low-density lipoprotein and on level of total cholesterol. Food Scientist should carry out extensive research in this area to find out how MCFA effects total cholesterol level in body. MCT cannot be used as a single substitute for traditional cooking oil. MCT need to blended in certain amount with other edible oil and then it can be used for cooking and frying food because it generates higher amount of smoke when heated due to presence of MCFA and they easily form foams. This can be overcomed by designing of designer lipid having combination of MCFA and PUFA attached to single glycerol backbone.
Conclusion
MCT are lipids with multiple health benefits owing to its fatty acid composition which are metabolized in such a manner that it produces ketone bodies which serves as a quick energy source and there is no reformation of triglyceride which accumulate as a fat. These MCFA are found in coconut oil, milkfat and palm kernel oil only. Due to limited natural sources and increasing demand, these are synthetically made by esterification and interesterification reaction using enzyme or chemical catalyst. Use of MCT in to foods like confectionary, cookies, biscuits, plastic fats etc. reduces the calorific value of food thereby managing diseases like obesity. The application of MCT in cooking and frying can be increased by synthesis of designer lipid having MCFA and PUFA or essential fatty acid esterified on same glycerol backbone. So, that the nutrition of PUFA and MCFA can be enjoyed in a single triglyceride molecule and also the problem of foam formation and low smoke point in frying will be overcomed. Still, there need to extensive research in the area to know the exact effect of MCFA on the total cholesterol level in body and what ill effect does MCFA cause to body when taken in high amount because of their saturation. Thus, by overcoming these lacunas, food processing industries will be able to serve the consumer with processed food containing functional or nutraceutical MCT.
Acknowledgements
Author is thankful to Department of Science and technology, Government of India for providing DST- Inspire fellowship for doing doctoral research.
Abbreviations
- MCT
Medium-chain triglycerides
- MCFA
Medium-chain fatty acids
- MUFA
Monounsaturated fatty acids
- PUFA
Polyunsaturated fatty acids
- EFA
Essential fatty acids
- SFA
Saturated fatty acids
- LCPFA
Long-chain polyunsaturated fatty acids
- LCT
Long-chain triglycerides
- LCFA
Long-chain fatty acids
- USFDA
United States Food & Drug Administration
- GRAS
Generally Recognized as Safe
Author contributions
H.B.J.: Conceived, did the critical review of literature and wrote manuscript. U.S.A.: Supervision, Proof reading.
Funding
Author is thankful to Department of Science and technology, Government of India for providing DST- Inspire fellowship for doing doctoral research.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors confirm that they have no conflicts of interest with respect to the work described in this manuscript.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Harsh B. Jadhav, Email: harshjadhav.ict@gmail.com
Uday S. Annapure, Email: us.annapure@ictmumbai.edu.in
References
- Ashton JS, Roberts JW, Wakefield CJ, et al. The effects of medium chain triglyceride (MCT) supplementation using a C8:C10 ratio of 30:70 on cognitive performance in healthy young adults. Physiol Behav. 2021;229:113252. doi: 10.1016/j.physbeh.2020.113252. [DOI] [PubMed] [Google Scholar]
- Augustin K, Khabbush A, Williams S, et al. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018;17:84–93. doi: 10.1016/S1474-4422(17)30408-8. [DOI] [PubMed] [Google Scholar]
- Avgerinos KI, Egan JM, Mattson MP, Kapogiannis D. Medium Chain Triglycerides induce mild ketosis and may improve cognition in Alzheimer’s disease. A systematic review and meta-analysis of human studies. Ageing Res Rev. 2020;58:101001. doi: 10.1016/j.arr.2019.101001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babayan VK. Medium-chain triglycerides-their composition, preparation, and application. J Am Oil Chem Soc. 1968;45:23–25. doi: 10.1007/BF02679040. [DOI] [PubMed] [Google Scholar]
- Bach AC, Ingenbleek Y, Frey A. The usefulness of dietary medium-chain triglycerides in body weight control: fact or fancy? J Lipid Res. 1996;37:708–726. doi: 10.1016/s0022-2275(20)37570-2. [DOI] [PubMed] [Google Scholar]
- Boulos Z. Method for preperation of triglycerides of medium chain fatty acids. Int Pat Treaty. 2013;12:1–20. [Google Scholar]
- Carlson SJ, Nandivada P, Chang MI, et al. The addition of medium-chain triglycerides to a purified fish oil-based diet alters inflammatory profiles in mice. Metabolism. 2015;64:274–282. doi: 10.1016/j.metabol.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane VG, Gogate PR, Pandit AB. Process intensification of synthesis process for medium chain glycerides using cavitation. Chem Eng J. 2008;145:351–354. doi: 10.1016/j.cej.2008.08.012. [DOI] [Google Scholar]
- Dorni C, Sharma P, Saikia G, Longvah T. Fatty acid profile of edible oils and fats consumed in India. Food Chem. 2018;238:9–15. doi: 10.1016/j.foodchem.2017.05.072. [DOI] [PubMed] [Google Scholar]
- Gharat NN, Rathod VK. Ultrasound-assisted organic synthesis. Amsterdam: Elsevier Inc; 2020. [Google Scholar]
- Heydinger JA, Nakhasi DK. Medium chain triacylglycerols. J Food Lipids. 1996;3:251–257. doi: 10.1111/j.1745-4522.1996.tb00072.x. [DOI] [Google Scholar]
- Hollis F, Mitchell ES, Canto C, et al. Medium chain triglyceride diet reduces anxiety-like behaviors and enhances social competitiveness in rats. Neuropharmacology. 2018;138:245–256. doi: 10.1016/j.neuropharm.2018.06.017. [DOI] [PubMed] [Google Scholar]
- Ibrahim NA, Kuntom A, Tang TS, Siew WL. Current status of malaysian crude palm kernel oil characteristics. Oil Palm Bull. 2003;47:15–27. [Google Scholar]
- Ingle DL, Driedger A, Traul KA, Nakhasi DK. Dietary energy value of medium-chain triglycerides. J Food Sci. 1999;64:960–963. doi: 10.1111/j.1365-2621.1999.tb12259.x. [DOI] [Google Scholar]
- Izgelov D, Shmoeli E, Domb AJ, Hoffman A. The effect of medium chain and long chain triglycerides incorporated in self-nano emulsifying drug delivery systems on oral absorption of cannabinoids in rats. Int J Pharm. 2020;580:119201. doi: 10.1016/j.ijpharm.2020.119201. [DOI] [PubMed] [Google Scholar]
- Jadhav H, Annapure U. Greener route for intensified synthesis of Tricaprylin using Amberlyst-15. J Chem Sci. 2021;133:1. doi: 10.1007/s12039-020-01869-z. [DOI] [Google Scholar]
- Jadhav HB, Annapure U. Designer lipids -synthesis and application – a review. Trends Food Sci Technol. 2021;116:884–902. doi: 10.1016/j.tifs.2021.08.020. [DOI] [Google Scholar]
- Jadhav HB, Annapure U. Process intensification for synthesis of triglycerides of capric acid using green approaches. J Indian Chem Soc. 2021;98:100030. doi: 10.1016/j.jics.2021.100030. [DOI] [Google Scholar]
- Jadhav H, Gogate P, Annapure U. Intensification of synthesis of triglyceride of Decanoic acid in the presence of amberlyst 15 as catalyst based on the use of ultrasound and microwave irradiations. Chem Eng Process Process Intensif. 2021;165:108424. doi: 10.1016/j.cep.2021.108424. [DOI] [Google Scholar]
- Jadhav H, Waghmare J, Annapure U. Effect of mono and diglyceride of medium chain fatty acid on the stability of flavour emulsion. Food Res. 2021;5:214–220. doi: 10.26656/fr.2017.5(2).589. [DOI] [Google Scholar]
- Jadhav HB, Gogate PR, Waghmare JT, Annapure US. Intensified synthesis of palm olein designer lipids using sonication. Ultrason Sonochem. 2021 doi: 10.1016/j.ultsonch.2021.105478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RG. Detection and determination of lipase (acylglycerol hydrolase) activity from various sources. Lipids. 1983;18:650–657. doi: 10.1007/BF02534677. [DOI] [PubMed] [Google Scholar]
- Jensen RG. The composition of bovine milk lipids. J Dairy Sci. 2002;85:295–350. doi: 10.3168/jds.S0022-0302(02)74079-4. [DOI] [PubMed] [Google Scholar]
- Jensen RG, Dejong FA, Clark RM, et al. Stereospecificity of premature human infant lingual lipase. Lipids. 1982;17:570–572. doi: 10.1007/BF02535386. [DOI] [PubMed] [Google Scholar]
- Kinsella R, Maher T, Clegg ME. Coconut oil has less satiating properties than medium chain triglyceride oil. Physiol Behav. 2017;179:422–426. doi: 10.1016/j.physbeh.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Langone MAP, Sant’Anna GL. Process development for production of medium chain triglycerides using immobilized lipase in a solvent-free system. Appl Biochem Biotechnol Part A Enzym Eng Biotechnol. 2002;98–100:997–1008. doi: 10.1385/ABAB. [DOI] [PubMed] [Google Scholar]
- Langone MAP, De Abreu ME, Rezende MJC, Sant’Anna GL. Enzymatic synthesis of medium chain monoglycerides in a solvent-free system. Appl Biochem Biotechnol Part A Enzym Eng Biotechnol. 2002;98–100:987–996. doi: 10.1385/ABAB:98-100:1-9:987. [DOI] [PubMed] [Google Scholar]
- Lavayre J, Verrier J, Baratti J. Stereospecific hydrolysis by soluble and immobilized lipases. Biotechnol Bioeng. 1982;24:2175–2188. doi: 10.1002/bit.260241006. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lu X, Li X, et al. High-fat diet triggers obesity-related early infiltration of macrophages into adipose tissue and transient reduction of blood monocyte count. Mol Immunol. 2020;117:139–146. doi: 10.1016/j.molimm.2019.11.002. [DOI] [PubMed] [Google Scholar]
- Lu J, Jin Q, Wang X, Wang X. Preparation of medium and long chain triacylglycerols by lipase-catalyzed interesterification in a solvent-free system. Process Biochem. 2017;54:89–95. doi: 10.1016/j.procbio.2016.12.015. [DOI] [Google Scholar]
- Macrae AR. Lipase-catalyzed interesterification of oils and fats. J Am Oil Chem Soc. 1983;60:291–294. doi: 10.1007/BF02543502. [DOI] [Google Scholar]
- Mase T, Matsumiya Y, Matsuura A. Purification and characterization of penicillium roqueforti IAM 7268 lipase. Biosci Biotechnol Biochem. 1995;59:329–330. doi: 10.1271/bbb.59.329. [DOI] [PubMed] [Google Scholar]
- Mehta A, Guleria S, Sharma R, Gupta R. The lipases and their applications with emphasis on food industry. Amsterdam: Elsevier Inc; 2021. [Google Scholar]
- Mohod AV, Gogate PR. Intensified synthesis of medium chain triglycerides using novel approaches based on ultrasonic and microwave irradiations. Chem Eng J. 2017;317:687–698. doi: 10.1016/j.cej.2017.02.102. [DOI] [Google Scholar]
- Mohod AV, Gogate PR. Intensified synthesis of medium chain triglycerides using ultrasonic reactors at a capacity of 4L. Ultrason Sonochem. 2018;42:347–355. doi: 10.1016/j.ultsonch.2017.11.044. [DOI] [PubMed] [Google Scholar]
- More SB, Gogate PR, Waghmare JS. Intensification of acid catalyzed synthesis of tricaprylin using ultrasound pretreatment. Chem Eng Process Process Intensif. 2017;120:317–329. doi: 10.1016/j.cep.2017.07.027. [DOI] [Google Scholar]
- More SB, Waghmare JT, Gogate PR. Ultrasound pretreatment as a novel approach for intensification of lipase catalyzed esterification of tricaprylin. Ultrason Sonochem. 2017;36:253–261. doi: 10.1016/j.ultsonch.2016.11.036. [DOI] [PubMed] [Google Scholar]
- More SB, Waghmare JS, Gogate PR, Naik SN. Improved synthesis of medium chain triacylglycerol catalyzed by lipase based on use of supercritical carbon dioxide pretreatment. Chem Eng J. 2018;334:1977–1987. doi: 10.1016/j.cej.2017.11.122. [DOI] [Google Scholar]
- Nugrahini AD, Soerawidjaja TH. Directed interesterification of coconut oil to produce structured lipid. Agric Agric Sci Procedia. 2015;3:248–254. doi: 10.1016/j.aaspro.2015.01.048. [DOI] [Google Scholar]
- Pires AS, Osório NM, Nascimento AC, et al. Pattern recognition of lipase-catalyzed or chemically interesterified fat blends containing n-3 polyunsaturated fatty acids. Eur J Lipid Sci Technol. 2008;110:893–904. doi: 10.1002/ejlt.200700270. [DOI] [Google Scholar]
- Ransom-Painter KL, Williams SD, Hartel RW. Incorporation of milk fat and milk fat fractions into compound coatings made from palm kernel Oil. J Dairy Sci. 1997;80:2237–2248. doi: 10.3168/jds.S0022-0302(97)76172-1. [DOI] [Google Scholar]
- Reger MA, Henderson ST, Hale C, et al. Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25:311–314. doi: 10.1016/S0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]
- Rial SA, Jutras-Carignan A, Bergeron KF, Mounier C. A high-fat diet enriched in medium chain triglycerides triggers hepatic thermogenesis and improves metabolic health in lean and obese mice. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865:158582. doi: 10.1016/j.bbalip.2019.158582. [DOI] [PubMed] [Google Scholar]
- Riaublanc A, Ratomahenina R, Galzy P, Nicolas M. Peculiar properties of lipase from Candida parapsilosis (Ashford) langeron and talice. J Am Oil Chem Soc. 1993;70:497–500. doi: 10.1007/BF02542583. [DOI] [Google Scholar]
- Rogalska E, Cudrey C, Ferrato F, Verger R. Stereoselective hydrolysis of triglycerides by animal and microbial lipases. Chirality. 1993;5:24–30. doi: 10.1002/chir.530050106. [DOI] [PubMed] [Google Scholar]
- Sivakanthan S, Madhujith T. Current trends in applications of enzymatic interesterification of fats and oils: a review. LWT Food Sci Technol. 2020;132:109880. doi: 10.1016/j.lwt.2020.109880. [DOI] [Google Scholar]
- St-Onge MP, Jones PJH. Physiological effects of medium-chain triglycerides: potential agents in the prevention of obesity. J Nutr. 2002;132:329–332. doi: 10.1093/jn/132.3.329. [DOI] [PubMed] [Google Scholar]
- Surat MA, Jauhari S, Desak KR. A brief review: Microwave assisted organic reaction. Appl Sci Res. 2012;4:645–661. [Google Scholar]
- Traul KA, Driedger A, Ingle DL, Nakhasi D. Review of the toxicologic properties of medium-chain triglycerides. Food Chem Toxicol. 2000;38:79–98. doi: 10.1016/S0278-6915(99)00106-4. [DOI] [PubMed] [Google Scholar]
- Uzawa H, Nishida Y, Ohrui H, Meguro H. a New approach to determine the crosslinking in polyethylene vinyl. Biochem Biophys Res Commun. 1990;168:506–511. doi: 10.1016/0006-291X(90)92350-9. [DOI] [PubMed] [Google Scholar]
- Yen HC, Lai WK, Lin CS, Chiang SH. Medium-chain triglyceride as an alternative of in-feed colistin sulfate to improve growth performance and intestinal microbial environment in newly weaned pigs. Anim Sci J. 2015;86:99–104. doi: 10.1111/asj.12248. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu Q, Liu YH, et al. Medium-chain triglyceride activated brown adipose tissue and induced reduction of fat mass in C57BL/6J mice fed high-fat diet. Biomed Environ Sci. 2015;28:97–104. doi: 10.3967/bes2015.012. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang Y, Liu Y, et al. Medium-chain triglycerides promote macrophage reverse cholesterol transport and improve atherosclerosis in ApoE-deficient mice fed a high-fat diet. Nutr Res. 2016;36:964–973. doi: 10.1016/j.nutres.2016.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.