Abstract
An 85-year-old woman presented with ataxia and deterioration of cognitive functions. She had no history of autoimmune diseases or viral infections. Magnetic resonance imaging showed a solitary mass lesion at the cerebral falx on contrast-enhanced T1-weighted imaging. Gross total resection of the lesion involving the dura mater was performed by bifrontal craniotomy. Histological examination showed diffuse infiltration of small lymphocytes and plasma cells. There was also some proliferation of large lymphocytes with folded nuclei, high-density chromatin, and inconspicuous nucleoli. The large atypical B lymphocytes did not demonstrate diffuse dense sheet findings. Meningothelial components were not detected. Immunohistochemistry was positive for pan B-cell antigens. The analysis of the kappa/lambda ratio indicated kappa immunoglobulin light chain-restricted B-cell proliferation. The final histopathological diagnosis was mucosa-associated lymphoid tissue lymphoma. Systemic screening examinations were then performed. Histological findings of the bone marrow showed normal findings without atypical lymphocytes. A chromosomal study of the bone marrow showed 46, XX. 18F fluoro-2-deoxyglucose positron emission tomography showed high accumulations at the left pterygoid muscle and the right transverse processes of the thoracic vertebrae, and mild accumulation at the right ilium bone, which indicated disseminated lesions. One year later, thickening of the dura mater was detected. Therefore, gamma knife surgery was performed. Two years later, she was alive without neurological deterioration, and magnetic resonance imaging showed no evidence of recurrence.
Keywords: mucosa-associated lymphoid tissue, MALT, marginal zone lymphoma, central nervous system, dural
Introduction
Primary central nervous system (CNS) lymphoma is an extranodal non-Hodgkin lymphoma. Approximately 90% of them are diffuse large B-cell lymphomas (DLBCLs), which are high-grade and aggressive neoplasms.1) On the other hand, low-grade primary CNS lymphoma is much less common, and marginal zone B-cell lymphoma is the most common type, arising from postgerminal center marginal zone B cells. Marginal zone B-cell lymphoma is classified, according to the anatomical location, into three subtypes by the World Health Organization (WHO) classification:2) extranodal or mucosa-associated lymphoid tissue (MALT), nodal, and splenic types. Therefore, marginal zone B-cell lymphoma in the CNS is MALT lymphoma. These three types share similar histopathological and immunohistochemical features. CNS MALT lymphoma is either primary at the time of initial presentation or secondary during the natural progression of the disease. Primary CNS MALT lymphoma has a lower incidence, appearing in the literature as single case reports or small series.3-8) Most primary CNS MALT lymphomas are located in the dura mater and are called dural or dural-based MALT lymphomas.
Because primary CNS MALT lymphomas are rare, information gained from cumulative case reports is useful in managing patients with this unusual disease. A case of dural MALT lymphoma mimicking falx meningioma is presented, with an update on diagnostic and therapeutic information.
Case Report
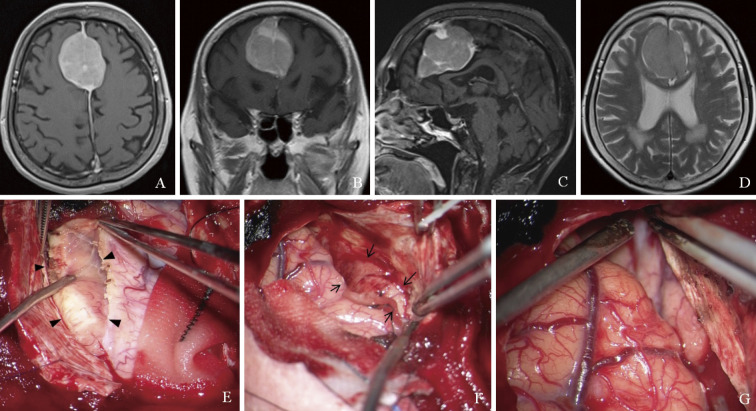
An 85-year-old woman presented with ataxia and deterioration of cognitive functions. She had no history of autoimmune diseases or viral infections such as hepatitis B and C, human T-lymphotrophic, and human immunodeficiency viruses. Magnetic resonance (MR) imaging showed a solitary mass lesion at the cerebral falx with contrast enhancement on T1-weighted imaging, and there were no apparent findings of invasion and edema around the brain on T2-weighted imaging (Fig. 1A-D). Additional examinations were not performed because the preoperative diagnosis was a falx meningioma. Gross total resection of the lesion involving the dura mater was performed by bifrontal craniotomy. The lesion was elastic soft tissue, and the margins of the brain were almost clear. First, the right side of the lesion was detached from the right frontal lobe and removed, and then the left side was also removed. Finally, the dura mater attachment of the lesion was cut and removed (Fig. 1E-G). The intraoperative microscopic findings corresponded to meningioma. However, intraoperative frozen pathology did not demonstrate typical meningioma, but proliferation of small lymphocytes.
Fig. 1.
Magnetic resonance imaging shows a solitary mass lesion at the cerebral falx with contrast enhancement on T1-weighted imaging (A: axial, B: coronal, and C: sagittal views) and no apparent findings of invasion and edema around the brain on T2-weighted imaging (D). Intraoperative photographs show that the lesion is elastic soft tissue. First, the right side of the lesion is removed (E, arrowheads), and next the left side is also removed (F, arrows). Finally, the dura mater attachment of the mass is cut and removed (G).
Histological examination showed diffuse infiltration of small lymphocytes and plasma cells (Fig. 2A). There was also some proliferation of large lymphocytes with folded nuclei, high-density chromatin, and inconspicuous nucleoli (Fig. 2B). Meningothelial components were not detected. These small and large lymphocytes were positive for CD20, CD79a, and multiple myeloma 1, suggesting B-cell lymphoma (Fig. 2C and D). However, the large atypical B lymphocytes did not demonstrate diffuse dense sheet findings. There were several cells intermixed, such as CD3-positive T-cells (Fig. 2E), CD68-positive cells, and S-100 positive cells. The atypical lymphocytes were stained for mind bomb 1 (Fig. 2F). The Ki-67 labeling index was 44%. Other immunohistochemical markers were negative, such as CD10, B-cell lymphoma 2, epithelial membrane antigen, and progesterone receptor. In situ hybridization did not demonstrate the presence of Epstein-Barr virus ribonucleic acid. The plasma cells showed dominant expression of lambda immunoglobulin light chain (IgL) and lack of kappa light chain expression on immunohistochemistry (Fig. 2G and H). Flow cytometry showed a kappa/lambda ratio of 0.5. Polymerase chain reaction analysis of the immunoglobulin heavy chain (IgH) genes showed a polyclonal pattern.
Fig. 2.
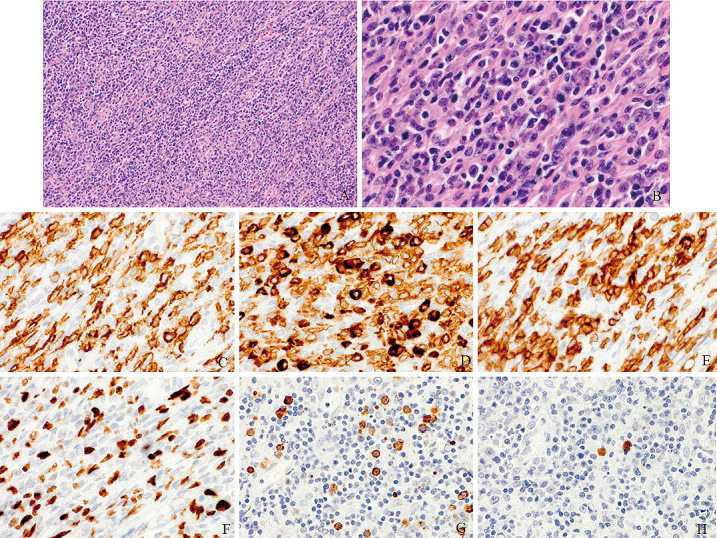
Surgical specimen stained with hematoxylin-eosin shows diffuse infiltration of small lymphocytes and plasma cells. There is some proliferation of large lymphocytes with folded nuclei, high-density chromatin, and inconspicuous nucleoli (A: ×100 and B: ×400). Immunohistochemical examinations show that these cells exhibit positive staining for CD20 (C) and CD79a (D), and there are CD3-positive T-cells (E). The atypical lymphocytes are stained for mind bomb 1 (F). The plasma cells are positive for the lambda immunoglobulin light chain (G) but lack kappa light chain expression (H).
The final histopathological diagnosis was MALT lymphoma. Systemic screening examinations were then performed by hematologists. Serum soluble interleukin-2 receptor was 568 (normal range: 157-474) U/mL, and β2-microglobulin was 2.3 (normal range: 1-1.9) mg/L. Histological findings of the bone marrow showed normal hematopoietic differentiation and aggregated small lymphocytes consisting of mainly CD3-positive T lymphocytes, without atypical B lymphocytes. A chromosomal study of the bone marrow showed 46, XX. Ophthalmologic evaluations showed no other areas of disease. 18F fluoro-2-deoxyglucose positron emission tomography showed high accumulations at the left pterygoid muscle and right transverse processes of the thoracic vertebrae, and mild accumulation at the right ilium bone, indicating disseminated lesions (Fig. 3A-C). According to the Groupe d'Etude des Lymphomes Folliculaires,9) additional postsurgical therapy was not performed until the appearance of local recurrence or growing, symptomatic, disseminated lesions.
Fig. 3.
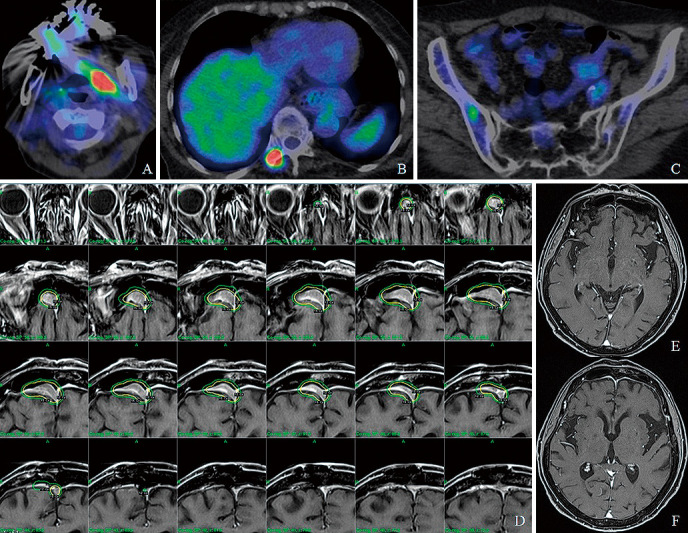
18F fluoro-2-deoxyglucose positron emission tomography shows high accumulations at the left pterygoid muscle (A) and the right transverse processes of the thoracic vertebrae (B), and mild accumulation at the right ilium bone (C). The plan of gamma knife surgery shows a maximum dose of 30 Gy and a marginal dose of 15 Gy (yellow lines) for thickening of the dura mater with contrast enhancement (D). Magnetic resonance images two years later show no evidence of recurrence (E and F).
About one year later, MR imaging showed thickening of the dura mater with contrast enhancement, which suggested local recurrence. Therefore, gamma knife surgery was performed with a maximum dose of 30 Gy and a marginal dose of 15 Gy (Fig. 3D). Two years later, she was alive without neurological deterioration, and MR imaging showed no recurrence (Fig. 3E and F). The last 18F fluoro-2-deoxyglucose positron emission tomography showed no growing findings of the high accumulated lesions.
Discussion
MALT lymphoma commonly arises from mucosal organs including the stomach, lungs, bladders, salivary glands, conjunctiva, and lacrimal glands. Interestingly, MALT lymphoma can also develop from tissue sites without mucosa, including the liver, breasts, thyroid, orbit, skin, and CNS.5,10) The pathogenesis of MALT lymphoma outside the CNS is believed to result from chronic inflammation or autoimmune diseases. However, primary CNS MALT lymphoma has not been definitely associated with any chronic inflammation or autoimmune diseases. It has been hypothesized that meningothelial cells of the arachnoid membrane are analogous to epithelial cells at other sites from where MALT lymphomas arise.4) Primary CNS MALT lymphoma typically presents as a solitary dural mass. According to the latest literature review, dural MALT lymphomas have been reported in 38 articles describing 126 cases.3) The mean age of dural MALT lymphoma presentation was 51.3 (26-78) years, with female predominance (3:1). Clinical presentations depend on the anatomic region of involvement, such as headache, ataxia, nausea, vomiting, fatigue, seizure, and focal sensory and motor deficits.3-8) The radiological pattern seems to be hypointensity on T1-weighted MR images with homogeneous contrast enhancement, mimicking a meningioma.
The diagnosis of CNS MALT lymphoma is confirmed mainly by histopathological and immunohistochemical features. Pathological findings of extranodal MALT lymphoma consist of perivascular proliferation of small atypical lymphoid B cells with plasma cell differentiation accompanied by reactive T-cells under non-neoplastic conditions.3-8) Recently, detailed histological characteristics of CNS MALT have been proposed:11) (1) small B-lymphoid cells that show atypical cell features with indented, cleaved, slightly irregular nuclei despite their small size compared with DLBCL of the CNS; (2) small atypical B-lymphoid cells with a low Ki-67 labeling index and minimal invasion from the perivascular space to brain parenchyma accompanied by surrounding reactive T lymphocytes compared with DLBCL or T-cell lymphoma of the CNS; (3) perivascular expansive monotonous proliferation of small atypical B-lymphoid cells with plasma cell differentiation different from vasculitis and demyelinating diseases; and (4) no vascular changes such as glomeruloid changes, obliterative fibrointimal proliferation, and intramural lymphocytic infiltration different from vasculitis. On immunohistochemistry, CNS MALT lymphomas express pan B-cell antigens such as CD19, CD20, and CD79a, and some complement receptors, CD21 and CD35, are positive. However, they are usually negative, including CD3, CD5, CD10, CD23, or cyclin D1.3-8) The Ki-67 labeling index ranged between 2% and 25%.3) The Ki-67 labeling index was much higher than 25% in the present case because both atypical B-lymphoid cells and reactive T lymphocytes would have been counted as Ki-67 positive cells.
Dural MALT lymphoma is an entity within the group of B-cell chronic lymphoproliferative disorders characterized by the proliferation of mature-appearing B cells.12) The neoplastic B lymphocytes constitute a clone in which all cells are related by possessing the original transforming mutation, possessing an identical IgH gene, and showing restricted IgL expressions, such as kappa or lambda.13) The analysis of protein markers by flow cytometry is a sensitive method to assess the clonality of B-cell chronic lymphoproliferative disorders. Analysis of the kappa/lambda ratio of cell surface immunoglobulin gene products has been one of the most frequently used. Deviation from normal ranges of the kappa/lambda ratio indicates IgL restriction resulting from monoclonal B-cell proliferation. The recent development of investigations of IgH gene arrangements has been successfully applied to study the clonality and cell lineage of various lymphoid neoplasms.14,15) Reactive lymphoproliferative disorders have polyclonal rearranged Ig or T-cell receptor genes, whereas neoplastic lymphoproliferative disorders show monoclonal rearrangements.13-15) These molecular studies provide highly supportive findings for accurate diagnosis. In the present case, the kappa/lambda ratio indicated kappa light chain-restricted B-cell proliferation, although the polyclonal pattern of IgH may be false negative.
A variety of treatments for primary dural MALT lymphoma have been reported in previously published examples.3-8) However, standardized treatment regimens of primary dural MALT lymphoma are not still defined, because there have been no trials that evaluated different treatment options. The majority of cases underwent surgical resection (77.8%) or biopsy (16.7%), followed by chemotherapy or radiotherapy.3) The two-year progression-free and overall survival rates of primary dural MALT lymphoma were reported to be 59% and 80%, respectively.7) Dural MALT lymphoma is very radiosensitive and requires a relatively lower dose of radiation to achieve disease control.16-19) A recent review showed that localized treatment with radiotherapy ± surgery was the most common therapeutic approach for primary dural MALT lymphoma.17) Therefore, it has been suggested that patients with disease isolated to the dura may not require systemic chemotherapy.16-19) In cases of secondary or relapsed dural MALT lymphoma, chemotherapy may be indicated because focal radiotherapy and surgery were insufficient. Dural MALT lymphomas tend to develop in elderly females;3) however, chemotherapy is highly risky for them.
As dural MALT lymphomas are radiosensitive, stereotactic radiosurgery is one of the safe and effective salvage treatments for recurrent intracranial lesions, particularly in elderly patients. In the current case, the recurrent dural lesion remained disappeared at the time of two years after gamma knife surgery. Currently, there are few reports on gamma knife surgery for dural MALT lymphomas. Karschnia et al. reported 20 cases of primary dural lymphomas, and one patient with a marginal zone lymphoma achieved complete remission with subtotal resection followed by gamma knife surgery.20) They concluded that the outcomes of primary dural lymphomas are excellent with multimodality treatment including surgery, radiotherapy, and chemotherapy, and the extent of surgical resection represented a positive prognostic marker for the overall survival. Therefore, local control is essential for achieving a good prognosis. Dural MALT lymphomas are low-grade radiosensitive extra-axial lesions that rarely invade brain parenchyma. Gamma knife surgery is effective to achieve local control of dural MALT lymphomas with minimal brain damage. Even if intracranial tumor recurrence repeatedly occurred, repeat gamma knife surgery could be an acceptable salvage treatment option to control local tumor recurrence.
In the present case, MR imaging findings indicated a falx meningioma, and pathological findings were mainly the proliferation of small size B lymphocytes and plasma cells. Therefore, the first differential diagnoses were either a category of meningioma or B-cell lymphoproliferative disorders. First, lymphoplasmacyte-rich meningioma was excluded because of the lack of a meningothelial component and negative for both the epithelial membrane antigen and progesterone receptor, which commonly stain meningioma. Differential diagnoses of B-cell lymphoproliferative disorders included DLBCL, lymphoplasmacytic lymphoma, follicular lymphoma, and MALT lymphoma. DLBCL was excluded because there were no findings of sheeted proliferation of atypical large B lymphocytes. The existence of T lymphocytes around the proliferation of B lymphocytes is one of the aspects of low-grade B-cell lymphomas. On immunohistochemistry, follicular lymphoma is usually positive for CD10 and B-cell lymphoma 2.21) MALT lymphoma and lymphoplasmacytic lymphoma have similar morphological and immunohistochemical profiles, but lymphoplasmacytic lymphoma typically involves the bone marrow and is associated with Waldenstrom's macroglobulinemia.22) In the present case, bone marrow findings were normal, and there was no clinical history of Waldenstrom's macroglobulinemia. Given these findings, the diagnosis was consistent with dural MALT lymphoma. Although this patient was diagnosed as having stage IV primary dural MALT lymphoma, chemotherapy was not performed because of her high age and no clinical symptoms.
Informed Consent
Informed consent for publication was obtained from the patient.
Conflicts of Interest Disclosure
The authors have no conflicts of interest directly relevant to the content of this article.
Acknowledgments
The authors would like to thank Professor Naoya Nakamura (Tokai University School of Medicine, Kanagawa, Japan) who gave valuable pathological information.
References
- 1). Batchelor TT: Primary central nervous system lymphoma: A curable disease. Hematol Oncol 37: 15-18, 2019 [DOI] [PubMed] [Google Scholar]
- 2). Swerdlow SH, Campo E, Pileri SA, et al. : The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127: 2375-2390, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Bayraktar S, Stefanovic A, Montague N, Davis J, Murray T, Lossos IS: Central nervous system manifestations of marginal zone B-cell lymphoma. Ann Hematol 89: 1003-1009, 2010 [DOI] [PubMed] [Google Scholar]
- 4). Kamoshima Y, Sawamura Y, Sugiyama T, Yamaguchi S, Houkin K, Kubota K: Primary central nervous system mucosa-associated lymphoid tissue lymphoma--case report. Neurol Med Chir (Tokyo) 51: 527-530, 2011 [DOI] [PubMed] [Google Scholar]
- 5). La Rocca G, Auricchio AM, Mazzucchi E, et al. : Intracranial dural based marginal zone MALT-type B-cell lymphoma: a case - Based update and literature review. Br J Neurosurg 26: 1-7, 2021 [DOI] [PubMed] [Google Scholar]
- 6). Razaq W, Goel A, Amin A, Grossbard ML: Primary central nervous system mucosa-associated lymphoid tissue lymphoma: case report and literature review. Clin Lymphoma Myeloma 9: E5-E9, 2009 [DOI] [PubMed] [Google Scholar]
- 7). Sunderland AJ, Steiner RE, Al Zahrani M, et al. : An international multicenter retrospective analysis of patients with extranodal marginal zone lymphoma and histologically confirmed central nervous system and dural involvement. Cancer Med 9: 663-670, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Tu PH, Giannini C, Judkins AR, et al. : Clinicopathologic and genetic profile of intracranial marginal zone lymphoma: a primary low-grade CNS lymphoma that mimics meningioma. J Clin Oncol 23: 5718-5727, 2005 [DOI] [PubMed] [Google Scholar]
- 9). Brice P, Bastion Y, Lepage E, et al. : Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d'Etude des Lymphomes Folliculaires. Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol 15: 1110-1117, 1997 [DOI] [PubMed] [Google Scholar]
- 10). Khalil MO, Morton LM, Devesa SS, et al. : Incidence of marginal zone lymphoma in the United States, 2001-2009 with a focus on primary anatomic site. Br J Haematol 165: 67-77, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Sugita Y, Hashimoto G, Fukuda K, et al. : primary nondural central nervous system marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue type mimicking CNS inflammatory diseases. J Neuropathol Exp Neurol 80: 789-799, 2021 [DOI] [PubMed] [Google Scholar]
- 12). Sanchez ML, Almeida J, Gonzalez D, et al. : Incidence and clinicobiologic characteristics of leukemic B-cell chronic lymphoproliferative disorders with more than one B-cell clone. Blood 102: 2994-3002, 2003 [DOI] [PubMed] [Google Scholar]
- 13). Delville JP, Heimann P, El Housni H, et al. : Biclonal low grade B-cell lymphoma confirmed by both flow cytometry and karyotypic analysis, in spite of a normal kappa/lambda Ig light chain ratio. Am J Hematol 82: 473-480, 2007 [DOI] [PubMed] [Google Scholar]
- 14). Braunschweig R, Baur AS, Delacrétaz F, Bricod C, Benhattar J: Contribution of IgH-PCR to the evaluation of B-cell lymphoma involvement in paraffin-embedded bone marrow biopsy specimens. Am J Clin Pathol 119: 634-642, 2003 [DOI] [PubMed] [Google Scholar]
- 15). Thériault C, Galoin S, Valmary S, et al. : PCR analysis of immunoglobulin heavy chain (IgH) and TcR-gamma chain gene rearrangements in the diagnosis of lymphoproliferative disorders: results of a study of 525 cases. Mod Pathol 13: 1269-1279, 2000 [DOI] [PubMed] [Google Scholar]
- 16). Ayanambakkam A, Ibrahimi S, Bilal K, Cherry MA: Extranodal marginal zone lymphoma of the central nervous system. Clin Lymphoma Myeloma Leuk 18: 34-37. e8, 2018 [DOI] [PubMed] [Google Scholar]
- 17). Chihara D, Fowler NH, Oki Y, et al. : Impact of histologic subtypes and treatment modality among patients with primary central nervous system lymphoma: a SEER database analysis. Oncotarget 9: 28897-28902, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Iwamoto FM, Abrey LE: Primary dural lymphomas: a review. Neurosurg Focus 21: E5, 2006 [DOI] [PubMed] [Google Scholar]
- 19). Puri DR, Tereffe W, Yahalom J: Low-dose and limited-volume radiotherapy alone for primary dural marginal zone lymphoma: treatment approach and review of published data. Int J Radiat Oncol Biol Phys 71: 1425-1435, 2008 [DOI] [PubMed] [Google Scholar]
- 20). Karschnia P, Batchelor TT, Jordan JT, et al. : Primary dural lymphomas: Clinical presentation, management, and outcome. Cancer 126: 2811-2820, 2020 [DOI] [PubMed] [Google Scholar]
- 21). Freedman A, Jacobsen E: Follicular lymphoma: 2020 update on diagnosis and management. Am J Hematol 95: 316-327, 2020 [DOI] [PubMed] [Google Scholar]
- 22). Owen RG, Treon SP, Al-Katib A, et al. : Clinicopathological definition of Waldenstrom's macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol 30: 110-115, 2003 [DOI] [PubMed] [Google Scholar]