Abstract
The role of a dysregulated renin-angiotensin system (RAS) in the pathogenesis of COVID-19 is well recognized. The imbalance between angiotensin II (Ang II) and Angiotensin1-7 (Ang1,7) caused by the interaction between SARS-CoV-2 and the angiotensin converting enzyme 2 (ACE2) receptors exerts a pivotal role on the clinical picture and outcome of COVID-19.
ACE2 receptors are not the exclusive angiotensinases in nature. Other angiotensinases (PRCP, and POP) have the potential to limit the detrimental effects of the interactions between ACE2 and the Spike proteins. In the cardiovascular disease continuum, ACE2 activity tends to decrease, and POP/PRCP activity to increase, from the health status to advanced deterioration of the cardiovascular system. The failure of the counter-regulatory RAS axis during the acute phase of COVID-19 is characterized by a decrease of ACE2 expression coupled to unchanged activity of other angiotensinases, therefore failing to limit the accumulation of Ang II.
COVID-19 vaccines increase the endogenous synthesis of SARS-CoV-2 spike proteins. Once synthetized, the free-floating spike proteins circulate in the blood, interact with ACE2 receptors and resemble the pathological features of SARS-CoV-2 ("Spike effect" of COVID-19 vaccines). It has been noted that an increased catalytic activity of POP/PRCP is typical in elderly individuals with comorbidities or previous cardiovascular events, but not in younger people. Thus, the adverse reactions to COVID-19 vaccination associated with Ang II accumulation are generally more common in younger and healthy subjects. Understanding the relationships between different mechanisms of Ang II cleavage and accumulation offers the opportunity to close the pathophysiological loop between the risk of progression to severe forms of COVID-19 and the potential adverse events of vaccination.
Keywords: SARS-CoV-2, COVID-19, ACE2, Vaccines, Renin-angiotensin-aldosterone system, Therapy, Olygopeptodases, POP, PRCP
Abbreviations: ACE2, angiotensin-converting enzyme 2 receptor; Ang II, angiotensin II; Ang1,7, angiotensin1,7; AT2, angiotensin II receptor type 2; BMI, body mass index; COVID-19, Coronavirus disease 2019; POP, prolyl oligopeptidase; PRCP, prolyl carboxypeptidases; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RAS, renin-angiotensin system; VITT, vaccine-induced immune thrombotic thrombocytopenia
1. Introduction
The role of a dysregulated renin-angiotensin system (RAS) in the pathogenesis of the complications of Coronavirus Disease 2019 (COVID-19) is now well recognized [1], [2], [3]. Evidences from experimental and clinical studies have been accrued in this area of research [3]. Specifically, the imbalance between angiotensin II (Ang II) and Angiotensin1-7 (Ang1,7) caused by the interaction between Severe Acute respiratory Syndrome Coronavirus 2 (SARS-CoV-2, as mediated by the binding of the Spike protein of the virus) and the angiotensin converting enzyme 2 (ACE2) receptors exerts a pivotal role on the clinical picture and outcome of COVID-19 [1], [2], [3], [4]. Moreover, the reduced catalytic efficiency of ACE2 resulting from viral occupation and down-regulation of these receptors appears to be detrimental in patients with baseline deficiency of ACE2 receptor activity (including patients with advanced age, cardiovascular risk factors, and previous cardiovascular events) [[1], [2], [3],[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]].
Similarly, COVID-19 vaccines increase the endogenous synthesis of SARS-CoV-2 Spike proteins [16]. Once synthetized, the Spike proteins assemble in the cytoplasm, migrate towards the cell surface and protrude externally with a native-like conformation [16].
Furthermore, the free-floating Spike proteins synthetized by cells targeted by vaccine and destroyed by the immune response massively circulate in the blood and systematically interact with ACE2 receptors expressed by a variety of cells, thereby promoting ACE2 internalization and degradation [16]. These reactions may result in pathological features which resemble those of SARS-CoV-2, ultimately leading to platelet aggregation, thrombosis and inflammation ("Spike effect" of COVID-19 vaccines) [16].
However, ACE2 are not the exclusive angiotensinases in nature. Other angiotensinases involved in the processing of Ang II to Ang1,7 may exert a counterbalance influence in the detrimental interactions between Spike proteins (of both SARS-CoV-2 and vaccine-induced) and ACE2 receptors [17], [18], [19], [20]. Understanding the relationships between different mechanisms of Ang II cleavage and accumulation offer the opportunity to draw a unique pathophysiological mechanism explaining the risk of both the progression to severe forms of COVID-19 and potential adverse events following vaccination.
2. Conversion of angiotensin II to angiotensin1,7
In the last years, the knowledge of the RAS has expanded with the identification of different enzymes and peptides downstream of Ang II [21]. Ang1,7 forming enzymes do not only prevent and counteract Ang II overactivity but also foster Ang1,7 formation eliciting potentially beneficial actions of the latter peptide. Specifically, the Ang II-Ang1,7 axis of the RAS encompasses three enzymes (carboxypeptidases) that form Ang1,7 directly from (by cleavage) Ang II: ACE2, prolyl carboxypeptidases (PRCP), and prolyl oligopeptidase (POP) [21].
POP is the main enzyme responsible for Ang II conversion to Ang1,7 in the circulation and in the lungs, whereas Ang1,7 formation in the kidney is mainly ACE2-dependent [21]. POP cuts at the C-side of an internal proline and cleaves Ang I to form Ang1,7, and Ang II to form Ang1,7 [20], [21], [22], [23].
Similarly, PRCP (serine carboxyprotease) cleaves the C-terminal amino acid of Ang II [24]. PRCP is ubiquitously expressed [25,26] and it plays versatile roles in cell proliferation, autophagy, oxidative stress, inflammation, vascular homeostasis, and various diseases (including hypertension, obesity, diabetes, and thrombosis) [27], [28], [29]. PRCP has protective effects on hypertension and thrombosis by stimulating the synthesis and release of nitric oxide and prostaglandin [28,30,31].
3. Changes in angiotensinases
Older age, male sex, and the presence of comorbidities (including hypertension, chronic obstructive pulmonary disease, diabetes mellitus, and history of cardiovascular events) are significant risk factors for increased disease severity in COVID-19 [5], [6], [7], [8], [9], [10], [11], [12]. Remarkably, all these conditions are associated with RAS dysregulation and ACE2 deficiency [13], [14], [15].
Conversely, studies indicate that increased plasma PRCP and POP levels are associated with aging, cardiovascular risk factors (including obesity) and dysfunction, atherosclerosis, renal disease, inflammation and diabetes [32,33]. In further support of an important role of these angiotensinases, significant correlations with several metabolic and cardiovascular parameters have been determined [34,35]. The development of a sensitive immunoassay by Xu and co-workers has demonstrated that plasma protein concentrations of PRCP are increased in obese patients and are even more elevated in patients with both obesity and diabetes [34]. Strong correlations with metabolic (including body mass index and blood glucose) parameters have been reported [34]. Similarly, Kehoe and co-workers showed that serum PRCP activity was increased with a rising body mass index (BMI) and they demonstrated a correlation with BMI, body weight, waist, hip circumference, and amount of total, visceral and subcutaneous abdominal adipose tissue [35]. Tabrizian and co-workers found PRCP protein to be highly elevated in human plasma with diabetes and that anti-diabetic agents reversed it [32]. PRCP activity and body weight decreases significantly after diet but even more pronounced after bariatric surgery which is also associated with several dietary guidelines and restrictions [35,36]. PRCP expression is affected by impaired tissues within the cardiovascular system and associated with cardiovascular abnormalities and dysfunction. In a recent study, intraplaque PRCP was upregulated in unstable plaques compared to stable plaques, and PRCP transcript levels correlated positively with the reverse cholesterol transporters particularly in carotid plaque samples [37]. Finally, endothelial dysfunction may also lie at the basis of the raised PRCP protein concentrations in plasma, since PRCP has been shown to be located on the membrane of endothelial cells and to regulate endothelial cell growth [27,34].
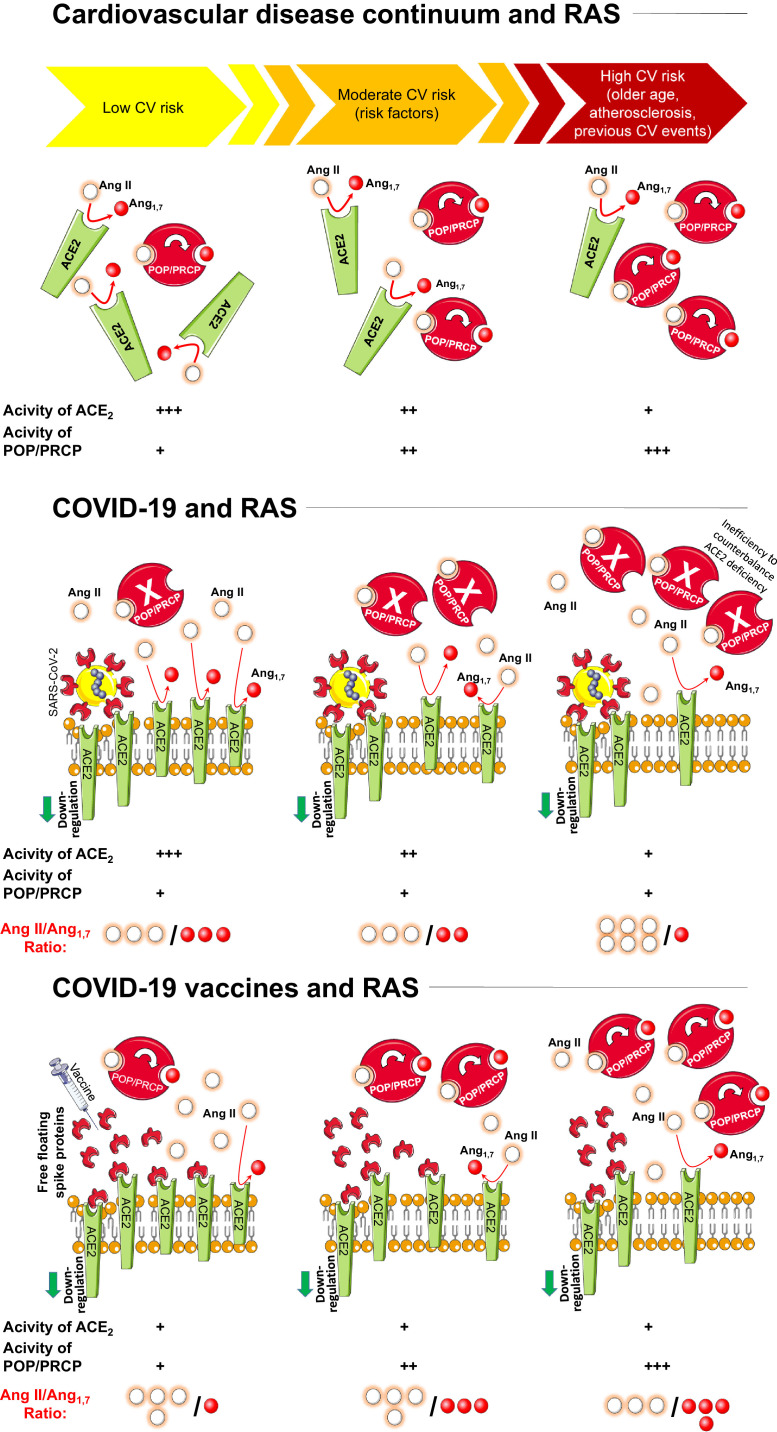
In the sequence of cardiovascular events, which begins from a cluster of cardiovascular risk factors consisting of diabetes mellitus, dyslipidemia, hypertension, smoking and visceral obesity (cardiovascular disease continuum) specific changes of angiotensinanes levels and activities may be postulated. Specifically, in the cardiovascular disease continuum [38] ACE2 activities decrease; on the other hand, POP and PRCP levels increase from the health status to advanced deterioration of the cardiovascular system (Fig. 1 , upper panel).
Fig. 1.
Mechanisms implicated in the development of Angiotensin II storm during the SARS-CoV-2 infection and after vaccination. Changes in Angiotensinases levels and activities are also depicted. See text for details.
Legend: A II=angiotensin II; A1,7=angiotensin1,7; ACE2=angiotensin coverting enzyme 2; POP= prolyl oligopeptidase; PRCP= prolyl carboxypeptidases; RAS=renin-angiotensin system; SARS-CoV-2= severe acute respiratory syndrome coronavirus-2.
4. Angiotensin II accumulation in the acute phase of SARS-CoV-2 infection
The failure of the counter-regulatory RAS axis, characterized by the decrease of ACE2 expression and generation of the protective Ang1,7, appears to be strictly implicated in the development of severe forms of COVID-19 [1,2,4,39,40]. More specifically, ACE2 internalization, downregulation and malfunction predominantly due to viral occupation, dysregulates the protective RAS axis with increased generation and activity of Ang II and reduced formation of Ang1,7 [1,2,4].
This has been corroborated by the findings of recent investigations supporting the evidence of the development of an “Ang II storm” [41] or “Ang II intoxication” [42] during the SARs-CoV-2 infection [[2], [3], [4],[43], [44], [45]].
For example, Wu and co-workers investigated plasma Ang II levels in patients infected by SARS-CoV-2 and critically ill patients not infected by SARS-CoV-2 were used as controls [46]. They found increased Ang II levels in 90.2% of COVID-19 patients, and in 100% of those who were critically ill [46]. There was a direct association between plasma Ang II levels and COVID-19 severity [46]. Similarly, a clinical investigation aimed to predict disease severity in SARS-CoV-2 infected patients in Shenzen, demonstrated that Ang II levels in the plasma samples were significantly increased and linearly associated with viral load and lung damage in critically ill patients [47]. Moreover, the degree of ACE2 deficiency (more pronounced in specific conditions including older age, cardiovascular disease and risk factors) are associated with more severe forms of COVID-19 [1,2,8,11,12,48,49]. Accrued evidences in the field showed that specific conditions associated with ACE2 deficiency and RAS dysregulation [13], [14], [15] (including older age [48,[50], [51], [52]], male sex [53], the presence of diabetes [54,55], lung disease [56], and history of cardiovascular events [57], [58], [59], [60], [61]) are well established risk factors for increased disease severity in COVID-19 (2-1) [5], [6], [7], [8], [9], [10], [11], [12] (Fig. 1, middle panel).
POP and PRCP, are involved in blood pressure (BP) regulation and inflammatory pathways, which are both disturbed in COVID-19 [21,62,63]. Bracke and co-workers specifically studied whether the specific plasma activities of these peptidases are dysregulated in COVID-19 patients at the time of hospital admission or during their hospital stay [64]. They demonstrated that PRCP activity remains stable during intensive care unit stay and does not differ from the median PRCP activity in healthy controls. Furthermore, they supported the hypothesis [65] that the elevated POP levels observed in plasma of patients COVID-19 originates from cell damage associated with acute lung injury or even multiple organ failure.
The important take-home message of these studies is that the activity of these two carboxypeptidases remains substantially unchanged during the acute phase of SARS-CoV-2 infection, therefore failing to limit the accumulation of Ang II which results from ACE2 deficiency (Fig. 1, middle panel).
5. Angiotensin II accumulation after COVID-19 vaccination (the "Spike effect")
COVID-19 vaccination was the most efficient strategy to come out of the current phase of the pandemic. However, some concerns regarding the safety of SARS-CoV-2 vaccines have been recently raised, mostly based on thromboembolic events [66], [67], [68], [69], myocarditis and myopericarditis [70], [71], [72], [73], [74], [75], and raised BP [76,77] following vaccination.
It has been recently suggested that Spike proteins produced upon vaccination have the native-like mimicry of SARS-CoV-2 Spike protein's receptor binding functionality and prefusion structure [16,78]. Free-floating Spike proteins released by the destroyed cells previously targeted by vaccines may interact with ACE2 of other cells, thereby promoting ACE2 internalization and degradation [16,79]. This mechanism may enhance the imbalance between Ang II overactivity and Ang1-7 deficiency through the loss of ACE2 receptor activity, which may contribute to trigger inflammation, thrombosis, an increase in BP, and other adverse reactions ("Spike effect" of COVID-19 vaccines) [80,81]. Moreover, the detrimental effects of other angiotensinases (POP and PRCP) deficiency on BP, thrombosis and inflammation are well supported. In an experimental model of acute hypertension, BP response was altered in the genetic absence of POP (as attributed to the diminished Ang II degradation and Ang1,7 formation) [21,82]. Similarly, global PRCP deficiency is associated with a moderate rise in BP and alteration in the heart and kidney [29]. The PRCP gene variant promotes disease progression in hypertensive patients [83]. PRCP depletion also induces vascular dysfunction with hypertension and faster arterial thrombosis [84]. Very low PRCP activity was found in resting and thrombin-activated blood platelets [35]. Additionally, activation or overexpression of the Ang II receptor type 2 (AT2) receptor was found to increase PRCP expression [85].
Of note, loss of ACE2 activities due to the interaction between these receptors and free-floating Spike proteins is observed across all the strata of the cardiovascular disease continuum [16] (Fig. 1, lower panel). On the other hand, an increased catalytic activity of POP and PRCP is not typical in the young, but more pronounced in elderly individuals with comorbidities or previous cardiovascular events (Fig. 1, upper panel). Thus, the adverse reactions to COVID-19 vaccination associated with Ang II accumulation are reasonably expected to be more common in younger and healthy subjects (Fig. 1, lower panel).
Such a hypothesis ("Spike effect" of COVID-19 vaccines) is well documented by clinical studies and epidemiological data. In a study published in October 2021 in JAMA Internal Medicine, researchers identified 15 cases of post-vaccination myocarditis [70]. All cases occurred in men with a median age of 25 years [70].
Among 530 cases of post-vaccination myocarditis reported to Vaccine Adverse Events Reporting System as of June 2021, approximately 65% of patients were aged 12–24 years [86].
Schultz and co-workers reported findings in five patients who presented with venous thrombosis and thrombocytopenia 7–10 days after receiving the first dose of the ChAdOx1 nCoV-19 adenoviral vector vaccine against COVID-19 [87]. The patients were health care workers who were 32–54 years of age [87]. Other reports also suggested that individuals with vaccine-induced immune thrombotic thrombocytopenia (VITT) were younger (< 55 or 60 years) [88,89]. Furthermore, in the December 2021 report from the Advisory Committee on Immunization Practices, rates were similar between males and females in most age brackets, with the exception of females ages 30–49 years, in whom rates were higher [90].
An Italian prospective survey showed that among 113 health care workers who received COVID-19 vaccine, 6 subjects (5.3%) showed a rise in systolic or diastolic BP at home ≥ 10 mmHg during the first five days after the first dose of the vaccine when compared with the five days before the vaccine (the BP rise required an intensification of BP-lowering treatment in 4 subjects) [81]. Age of patients with a significant raise in BP following COVID-19 vaccination ranged from 35 to 52 years [81].
Tran and co-workers [91] performed a cross-sectional survey including 1028 subjects (899 had one ChAdOx1nCoV-19 dose and the rest received 2 doses). Abnormal BP after vaccination was recorded in 52 subjects [91]. Importantly, age of participants was a significant factor affecting raise in BP as the increase of age was associated with the decrease of self-reported adverse events [91].
6. Conclusion
A wealth of evidence clearly suggest that the RAS plays an important role in the pathophysiology of COVID-19 [1,2,4]. It is now well known that ACE2, a zinc-metalloproteinase, and its catalytic product Ang1,7 provides significant cardiovascular protection during the acute phase of the infection [1,2,4]. More specifically, the biological importance of conversion of Ang II to Ang1,7 in COVID-19 is two-fold: by lowering Ang II its potentially detrimental actions may be prevented; in addition, Ang1,7 is being formed and this peptide has tissue-protective actions that are generally opposite to the unwanted chronic effects of excessive Ang II [21]. However, pre-existing ACE2 deficiency (as documented for elderly patients, diabetes mellitus, lung disease, hypertension, and chronic disease) contributes to an unfavorable outcome in SARS-CoV-2 infection [1,2,48].
Interestingly, other angiotensinases (POP and PRCP) participate in the angiotensin cleavage pathway, sharing substrate specificity with ACE2, the entrance receptor for SARS-CoV-2 [63].
The relative activity of each of the Ang1,7 forming enzymes and their changes in the cardiovascular continuum disease, need to be taken into consideration in the pathogenesis of COVID-19 and adverse reactions following COVID-19 vaccination. The changes in the angiotensin cleavage pathway by ACE2 are not obligatorily accompanied by similar changes in other angiotensinases levels and activities.
In the acute phase of SARS-CoV-2 infection, ACE2 expression and activity are reduced among subjects at high cardiovascular risk; moreover, POP and PRCP exhibit ineffective counterbalance properties to avoid Ang II accumulation. These mechanisms explain the increased risk of severe COVID-19 among patients with specific phenotypes of ACE2 deficiency [3,8,11,12,48].
After vaccination, the free-floating Spike proteins released by the cells targeted by vaccines may interact with ACE2 of other cells, thereby promoting ACE2 internalization and degradation [16,79], Ang II accumulation, and adverse reactions ("Spike effect" of COVID-19 vaccines) [16]. However, current knowledge supports the hypothesis of the protective effect of an enhanced expression of other angiotensinases on COVID-19 vaccines injury. The relative over-expression of POP and PRCP among subjects with cardiovascular risk factors or previous cardiovascular events may limit the detrimental effect of ACE2 deficiency on Ang II accumulation. Conversely, the relative deficiency of POP and PRCP among young and healthy subjects does not counterbalance ACE2 internalization, downregulation and malfunction due to free-floating Spike proteins interactions, resulting in an increased risk of Ang II accumulation, and adverse reactions ("Spike effect" of COVID-19 vaccines).
Gaining a greater understanding of angiotensinases’ multi-faceted biological functions could open up novel therapeutic avenues. Indeed, these findings may have important implications for therapeutic purposes when targeting Ang1,7 formation from Ang II in the circulation and specific organs. For each of these Angiotensinases, specific pharmacological inhibitors are available, allowing more functional studies and investigations on their potential as therapeutic targets [64].
As recently highlighted [4], compounds with insurmountable inhibition of ACE2, blocking or attenuating the binding of the viral Spike protein to the pocket of the ACE2 receptor, have the potential to prevent viral internalization. However, pharmacological inhibition of ACE2 should be modulated without blocking the crucial protective properties of this enzyme [4]. Moreover, protein engineering approaches to identify binders to viral entry proteins may offer an alternative therapeutic strategy to ameliorate the potential detrimental effects of the interaction between ACE2 and Spike proteins [16]. The use of vaccines encoding mutated Spike proteins with conformational change might partly lose adherence to ACE2 receptors [92,93] and reduce Ang II accumulation.
Future studies are needed to fully evaluate these mechanisms and the role of inhibitors of Ang II accumulation preventive therapy. Moreover, the exact role of other processes involved in the production of Ang1,7 needs to be more clearly evaluated. Indeed, Ang1,7 can be formed directly from Ang I. Combined ACE/Neprilysin inhibition decreases Ang1,7 formation from Ang I infusion suggesting a critical role of Neprilysin on the formation of Ang1,7 directly from Ang I [94].
Declaration of Competing Interest
None of the authors of this study has financial or other reasons that could lead to a conflict of interest.
References
- 1.Verdecchia P., Cavallini C., Spanevello A., Angeli F. COVID-19: ACE2 centric infective disease? Hypertension. 2020;76:294–299. doi: 10.1161/HYPERTENSIONAHA.120.15353. (Dallas, Tex : 1979) [DOI] [PubMed] [Google Scholar]
- 2.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angeli F., Zappa M., Reboldi G., Trapasso M., Cavallini C., Spanevello A., Verdecchia P. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection: one year later. Eur J Intern Med. 2021;93:28–34. doi: 10.1016/j.ejim.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angeli F., Reboldi G., Verdecchia P. SARS-CoV-2 infection and ACE2 inhibition. J Hypertens. 2021;39:1555–1558. doi: 10.1097/HJH.0000000000002859. [DOI] [PubMed] [Google Scholar]
- 5.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin J.M., Bai P., He W., Wu F., Liu X.F., Han D.M., Liu S., Yang J.K. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopel J., Perisetti A., Roghani A., Aziz M., Gajendran M., Goyal H. Racial and gender-based differences in COVID-19. Front Public Health. 2020;8:418. doi: 10.3389/fpubh.2020.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angeli F., Marazzato J., Verdecchia P., Balestrino A., Bruschi C., Ceriana P., Chiovato L., Dalla Vecchia L.A., De Ponti R., Fanfulla F., La Rovere M.T., Perego F., Scalvini S., Spanevello A., Traversi E., Visca D., Vitacca M., Bachetti T. Joint effect of heart failure and coronary artery disease on the risk of death during hospitalization for COVID-19. Eur J Intern Med. 2021;89:81–86. doi: 10.1016/j.ejim.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Y.D., Ding M., Dong X., Zhang J.J., Kursat Azkur A., Azkur D., Gan H., Sun Y.L., Fu W., Li W., Liang H.L., Cao Y.Y., Yan Q., Cao C., Gao H.Y., Brüggen M.C., van de Veen W., Sokolowska M., Akdis M., Akdis C.A. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76:428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 10.Guo L., Shi Z., Zhang Y., Wang C., Do Vale Moreira N.C., Zuo H., Hussain A. Comorbid diabetes and the risk of disease severity or death among 8807 COVID-19 patients in China: a meta-analysis. Diabetes Res Clin Pract. 2020;166 doi: 10.1016/j.diabres.2020.108346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Angeli F., Masnaghetti S., Visca D., Rossoni A., Taddeo S., Biagini F., Verdecchia P. Severity of COVID-19: the importance of being hypertensive. Monaldi Arch Chest Dis. 2020;90 doi: 10.4081/monaldi.2020.1372. [DOI] [PubMed] [Google Scholar]
- 12.Angeli F., Spanevello A., De Ponti R., Visca D., Marazzato J., Palmiotto G., Feci D., Reboldi G., Fabbri L.M., Verdecchia P. Electrocardiographic features of patients with COVID-19 pneumonia. Eur J Intern Med. 2020;78:101–106. doi: 10.1016/j.ejim.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abassi Z., Assady S., Khoury E.E., Heyman S.N. Letter to the editor: angiotensin-converting enzyme 2: an ally or a trojan horse? Implications to SARS-CoV-2-related cardiovascular complications. Am J Physiol Heart Circ Physiol. 2020;318:H1080–H1083. doi: 10.1152/ajpheart.00215.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K., Gheblawi M., Oudit G.Y. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020;142:426–428. doi: 10.1161/CIRCULATIONAHA.120.047049. [DOI] [PubMed] [Google Scholar]
- 16.Angeli F., Spanevello A., Reboldi G., Visca D., Verdecchia P. SARS-CoV-2 vaccines: lights and shadows. Eur J Intern Med. 2021;88:1–8. doi: 10.1016/j.ejim.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gass J., Khosla C. Prolyl endopeptidases. Cell Mol Life Sci. 2007;64:345–355. doi: 10.1007/s00018-006-6317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grobe N., Weir N.M., Leiva O., Ong F.S., Bernstein K.E., Schmaier A.H., Morris M., Elased K.M. Identification of prolyl carboxypeptidase as an alternative enzyme for processing of renal angiotensin II using mass spectrometry. Am J Physiol Cell Physiol. 2013;304:C945–C953. doi: 10.1152/ajpcell.00346.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welches W.R., Santos R.A., Chappell M.C., Brosnihan K.B., Greene L.J., Ferrario C.M. Evidence that prolyl endopeptidase participates in the processing of brain angiotensin. J Hypertens. 1991;9:631–638. doi: 10.1097/00004872-199107000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Velez J.C., Ierardi J.L., Bland A.M., Morinelli T.A., Arthur J.M., Raymond J.R., et al. Enzymatic processing of angiotensin peptides by human glomerular endothelial cells. Am J Physiol Ren. Physiol. 2012;302:F1583–F1594. doi: 10.1152/ajprenal.00087.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serfozo P., Wysocki J., Gulua G., Schulze A., Ye M., Liu P., Jin J., Bader M., Myohanen T., Garcia-Horsman J.A., Batlle D. Ang II (Angiotensin II) conversion to angiotensin-(1-7) in the circulation Is POP (Prolyloligopeptidase)-dependent and ACE2 (Angiotensin-converting enzyme 2)-independent. Hypertension. 2020;75:173–182. doi: 10.1161/HYPERTENSIONAHA.119.14071. (Dallas, Tex : 1979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greene L.J., Spadaro A.C., Martins A.R., Perussi De Jesus W.D, Camargo A.C. Brain endo-oligopeptidase B: a post-proline cleaving enzyme that inactivates angiotensin I and II. Hypertension. 1982;4:178–184. doi: 10.1161/01.hyp.4.2.178. (Dallas, Tex : 1979) [DOI] [PubMed] [Google Scholar]
- 23.Welches W.R., Brosnihan K.B., Ferrario C.M. A comparison of the properties and enzymatic activities of three angiotensin processing enzymes: angiotensin converting enzyme, prolyl endopeptidase and neutral endopeptidase 24.11. Life Sci. 1993;52:1461–1480. doi: 10.1016/0024-3205(93)90108-f. [DOI] [PubMed] [Google Scholar]
- 24.Odya C.E., Marinkovic D.V., Hammon K.J., Stewart T.A., Erdos E.G. Purification and properties of prolylcarboxypeptidase (angiotensinase C) from human kidney. J Biol Chem. 1978;253:5927–5931. [PubMed] [Google Scholar]
- 25.Jeong J.K., Diano S. Prolyl carboxypeptidase mRNA expression in the mouse brain. Brain Res. 2014;1542:85–92. doi: 10.1016/j.brainres.2013.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan N.D., Qiu Y., Xing X.B., Ghosh S., Chen M.H., Mao R. Associations between angiotensin-converting enzyme inhibitors and angiotensin II receptor blocker use, gastrointestinal symptoms, and mortality among patients with COVID-19. Gastroenterology. 2020;159:1170–1172. doi: 10.1053/j.gastro.2020.05.034. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams G.N., Stavrou E.X., Fang C., Merkulova A., Alaiti M.A., Nakajima K., Morooka T., Merkulov S., Larusch G.A., Simon D.I., Jain M.K., Schmaier A.H. Prolylcarboxypeptidase promotes angiogenesis and vascular repair. Blood. 2013;122:1522–1531. doi: 10.1182/blood-2012-10-460360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chajkowski S.M., Mallela J., Watson D.E., Wang J., McCurdy C.R., Rimoldi J.M., Shariat-Madar Z. Highly selective hydrolysis of kinins by recombinant prolylcarboxypeptidase. Biochem Biophys Res Commun. 2011;405:338–343. doi: 10.1016/j.bbrc.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maier C., Schadock I., Haber P.K., Wysocki J., Ye M., Kanwar Y., Flask C.A., Yu X., Hoit B.D., Adams G.N., Schmaier A.H., Bader M., Batlle D. Prolylcarboxypeptidase deficiency is associated with increased blood pressure, glomerular lesions, and cardiac dysfunction independent of altered circulating and cardiac angiotensin II. J Mol Med. 2017;95:473–486. doi: 10.1007/s00109-017-1513-9. (Berl) [DOI] [PubMed] [Google Scholar]
- 30.Mallela J., Yang J., Shariat-Madar Z. Prolylcarboxypeptidase: a cardioprotective enzyme. Int J Biochem Cell Biol. 2009;41:477–481. doi: 10.1016/j.biocel.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Sharma J.N. Hypertension and the bradykinin system. Curr Hypertens Rep. 2009;11:178–181. doi: 10.1007/s11906-009-0032-7. [DOI] [PubMed] [Google Scholar]
- 32.Tabrizian T., Hataway F., Murray D., Shariat-Madar Z. Prolylcarboxypeptidase gene expression in the heart and kidney: effects of obesity and diabetes. Cardiovasc Hematol Agents Med Chem. 2015;13:113–123. doi: 10.2174/1871525713666150911112916. [DOI] [PubMed] [Google Scholar]
- 33.Agirregoitia N., Gil J., Ruiz F., Irazusta J., Casis L. Effect of aging on rat tissue peptidase activities. J Gerontol A Biol Sci Med Sci. 2003;58:B792–B797. doi: 10.1093/gerona/58.9.b792. [DOI] [PubMed] [Google Scholar]
- 34.Xu S., Lind L., Zhao L., Lindahl B., Venge P. Plasma prolylcarboxypeptidase (angiotensinase C) is increased in obesity and diabetes mellitus and related to cardiovascular dysfunction. Clin Chem. 2012;58:1110–1115. doi: 10.1373/clinchem.2011.179291. [DOI] [PubMed] [Google Scholar]
- 35.Kehoe K., Noels H., Theelen W., De Hert E., Xu S., Verrijken A., Arnould T., Fransen E., Hermans N., Lambeir A.M., Venge P., Van Gaal L., De Meester I. Prolyl carboxypeptidase activity in the circulation and its correlation with body weight and adipose tissue in lean and obese subjects. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shannon C., Gervasoni A., Williams T. The bariatric surgery patient–nutrition considerations. Aust Fam Physician. 2013;42:547–552. [PubMed] [Google Scholar]
- 37.Rinne P., Lyytikainen L.P., Raitoharju E., Kadiri J.J., Kholova I., Kahonen M., et al. Pro-opiomelanocortin and its processing enzymes associate with plaque stability in human atherosclerosis - tampere vascular study. Sci Rep. 2018;8:15078. doi: 10.1038/s41598-018-33523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chrysant S.G., Chrysant G.S., Chrysant C., Shiraz M. The treatment of cardiovascular disease continuum: focus on prevention and RAS blockade. Curr Clin Pharmacol. 2010;5:89–95. doi: 10.2174/157488410791110742. [DOI] [PubMed] [Google Scholar]
- 39.Verdecchia P., Reboldi G., Cavallini C., Mazzotta G., Angeli F. ACE-inhibitors, angiotensin receptor blockers and severe acute respiratory syndrome caused by coronavirus. G Ital Cardiol (Rome) 2020;21:321–327. doi: 10.1714/3343.33127. [DOI] [PubMed] [Google Scholar]
- 40.Verdecchia P., Angeli F., Reboldi G. Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers and coronavirus. J Hypertens. 2020;38:1190–1191. doi: 10.1097/HJH.0000000000002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramos S.G., Rattis B., Ottaviani G., Celes M.R.N., Dias E.P. ACE2 down-regulation may act as a transient molecular disease causing RAAS dysregulation and tissue damage in the microcirculatory environment among COVID-19 patients. Am J Pathol. 2021;191:1154–1164. doi: 10.1016/j.ajpath.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sfera A., Osorio C., Jafri N., Diaz E.L., Campo Maldonado J.E. Intoxication with endogenous angiotensin II: a COVID-19 hypothesis. Front Immunol. 2020;11:1472. doi: 10.3389/fimmu.2020.01472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angeli F., Verdecchia P., Reboldi G. Pharmacotherapy for hypertensive urgency and emergency in COVID-19 patients. Expert Opin Pharmacother. 2022;23:235–242. doi: 10.1080/14656566.2021.1990264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angeli F., Verdecchia P., Reboldi G. RAAS inhibitors and risk of COVID-19. N Engl J Med. 2020;383:1990–1991. doi: 10.1056/NEJMc2030446. [DOI] [PubMed] [Google Scholar]
- 45.Angeli F., Verdecchia P., Balestrino A., Bruschi C., Ceriana P., Chiovato L., et al. Renin angiotensin system blockers and risk of mortality in hypertensive patients hospitalized for COVID-19: an Italian registry. J Cardiovasc Dev Dis. 2022;9:15. doi: 10.3390/jcdd9010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Z., Hu R., Zhang C., Ren W., Yu A., Zhou X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit Care. 2020;24:290. doi: 10.1186/s13054-020-03015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angeli F., Reboldi G., Verdecchia P. Ageing, ACE2 deficiency and bad outcome in COVID-19. Clin Chem Lab Med. 2021;(0658) doi: 10.1515/cclm-2021-0658. [DOI] [PubMed] [Google Scholar]
- 49.Angeli F., Reboldi G., Spanevello A., De Ponti R., Visca D., Marazzato J., Zappa M., Trapasso M., Masnaghetti S., Fabbri L.M., Verdecchia P. Electrocardiographic features of patients with COVID-19: one year of unexpected manifestations. Eur J Intern Med. 2022;95:7–12. doi: 10.1016/j.ejim.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon H.E., Kim E.N., Kim M.Y., Lim J.H., Jang I.A., Ban T.H., Shin S.J., Park C.W., Chang Y.S., Choi B.S. Age-associated changes in the vascular renin-angiotensin system in mice. Oxid Med Cell Longev. 2016;2016 doi: 10.1155/2016/6731093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie X., Chen J., Wang X., Zhang F., Liu Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78:2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J., Jiang Q., Xia X., Liu K., Yu Z., Tao W., Gong W., Han J.J. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell. 2020;19 doi: 10.1111/acel.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tukiainen T., Villani A.C., Yen A., Rivas M.A., Marshall J.L., Satija R., Aguirre M., Gauthier L., Fleharty M., Kirby A., Cummings B.B., Castel S.E., Karczewski K.J., Aguet F., Byrnes A., Consortium G.T., Laboratory D.A. Coordinating center -analysis working G, statistical methods groups-analysis working G, enhancing Gg, fund NIHC, Nih/Nci, Nih/Nhgri, Nih/Nimh, Nih/Nida, biospecimen collection source site N, biospecimen collection source site R, biospecimen core resource V, brain bank repository-university of miami brain endowment B, leidos biomedical-project M, study E, genome browser data I, visualization EBI, genome browser data I, visualization-Ucsc genomics institute UoCSC, lappalainen T, regev A, ardlie KG, hacohen N and MacArthur DG. landscape of X chromosome inactivation across human tissues. Nature. 2017;550:244–248. doi: 10.1038/nature24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamagata R., Nemoto W., Nakagawasai O., Takahashi K., Tan-No K. Downregulation of spinal angiotensin converting enzyme 2 is involved in neuropathic pain associated with type 2 diabetes mellitus in mice. Biochem Pharmacol. 2020;174 doi: 10.1016/j.bcp.2020.113825. [DOI] [PubMed] [Google Scholar]
- 55.Pal R., Bhansali A. COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020;162 doi: 10.1016/j.diabres.2020.108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prieto-Fernández E., Egia-Mendikute L., Vila-Vecilla L., Lee S.Y., Bosch A., Barreira-Manrique A., et al. Hypoxia reduces cell attachment of SARS-CoV-2 spike protein by modulating the expression of ACE2 and heparan sulfate. BioRxiv. 2021;2021:426021. doi: 10.1080/22221751.2021.1932607. 01.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-dos-Santos A.J., da Costa J., Zhang L., Pei Y., Scholey J., Ferrario C.M., Manoukian A.S., Chappell M.C., Backx P.H., Yagil Y., Penninger J.M. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 59.Danilczyk U., Penninger J.M. Angiotensin-converting enzyme II in the heart and the kidney. Circ Res. 2006;98:463–471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- 60.Kassiri Z., Zhong J., Guo D., Basu R., Wang X., Liu P.P., Scholey J.W., Penninger J.M., Oudit G.Y. Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail. 2009;2:446–455. doi: 10.1161/CIRCHEARTFAILURE.108.840124. [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto K., Ohishi M., Katsuya T., Ito N., Ikushima M., Kaibe M., Tatara Y., Shiota A., Sugano S., Takeda S., Rakugi H., Ogihara T. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension. 2006;47:718–726. doi: 10.1161/01.HYP.0000205833.89478.5b. (Dallas, Tex : 1979) [DOI] [PubMed] [Google Scholar]
- 62.Waumans Y., Baerts L., Kehoe K., Lambeir A.M., De Meester I. The dipeptidyl peptidase family, prolyl oligopeptidase, and prolyl carboxypeptidase in the immune system and inflammatory disease, including atherosclerosis. Front Immunol. 2015;6:387. doi: 10.3389/fimmu.2015.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Hert E., Bracke A., Lambeir A.M., Van der Veken P., De Meester I. The C-terminal cleavage of angiotensin II and III is mediated by prolyl carboxypeptidase in human umbilical vein and aortic endothelial cells. Biochem Pharmacol. 2021;192 doi: 10.1016/j.bcp.2021.114738. [DOI] [PubMed] [Google Scholar]
- 64.Bracke A., De Hert E., De Bruyn M., Claesen K., Vliegen G., Vujkovic A., van Petersen L., De Winter F.H.R., Hotterbeekx A., Brosius I., Theunissen C., Van Ierssel S., van Frankenhuijsen M., Vlieghe E., Vercauteren K., Van der Veken P., Hendriks D., Kumar-Singh S., De Meester I. Proline-specific peptidase activities (DPP4, PRCP, FAP and PREP) in plasma of hospitalized COVID-19 patients. Clin Chim Acta. 2022;531:4–11. doi: 10.1016/j.cca.2022.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Triposkiadis F., Starling R.C., Xanthopoulos A., Butler J., Boudoulas H. The counter regulatory axis of the lung renin-angiotensin system in severe COVID-19: pathophysiology and clinical implications. Heart Lung Circ. 2021;30:786–794. doi: 10.1016/j.hlc.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wise J. COVID-19: European countries suspend use of oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021;372:n699. doi: 10.1136/bmj.n699. [DOI] [PubMed] [Google Scholar]
- 67.Joint CDC and FDA Statement on Johnson & Johnson COVID-19 Vaccine. https://www.fda.gov/news-events/press-announcements/joint-cdc-and-fda-statement-johnson-johnson-covid-19-vaccine (Last access on date April 14, 2021 ).
- 68.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schultz N.H., Sorvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simone A., Herald J., Chen A., Gulati N., Shen A.Y., Lewin B., Lee M.S. Acute myocarditis following COVID-19 mRNA vaccination in adults aged 18 years or older. JAMA Intern Med. 2021;181:1668–1670. doi: 10.1001/jamainternmed.2021.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cushion S., Arboleda V., Hasanain Y., Demory Beckler M., Hardigan P., Kesselman M.M. Comorbidities and symptomatology of SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2)-related myocarditis and SARS-CoV-2 vaccine-related myocarditis: a review. Cureus. 2022;14:e24084. doi: 10.7759/cureus.24084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nassar M., Nso N., Gonzalez C., Lakhdar S., Alshamam M., Elshafey M., Abdalazeem Y., Nyein A., Punzalan B., Durrance R.J., Alfishawy M., Bakshi S., Rizzo V. COVID-19 vaccine-induced myocarditis: case report with literature review. Diabetes Metab Syndr. 2021;15 doi: 10.1016/j.dsx.2021.102205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sulemankhil I., Abdelrahman M., Negi S.I. Temporal association between the COVID-19 Ad26.COV2.S vaccine and acute myocarditis: a case report and literature review. Cardiovasc Revasc Med. 2022;38:117–123. doi: 10.1016/j.carrev.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu C.T., Chin S.C., Chu P.H. Acute fulminant myocarditis After ChAdOx1 nCoV-19 vaccine: a case report and literature review. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.856991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lane S., Yeomans A., Shakir S. Reports of myocarditis and pericarditis following mRNA COVID-19 vaccination: a systematic review of spontaneously reported data from the UK, Europe and the USA and of the scientific literature. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2021-059223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Angeli F., Reboldi G., Trapasso M., Santilli G., Zappa M., Verdecchia P. Blood pressure increase following COVID-19 vaccination: a systematic overview and meta-analysis. J Cardiovasc Dev Dis. 2022;9:150. doi: 10.3390/jcdd9050150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Angeli F., Reboldi G., Trapasso M., Verdecchia P. Hypertension after COVID-19 vaccination. G Ital Cardiol. 2022;23:10–14. doi: 10.1714/3715.37055. (Rome) [DOI] [PubMed] [Google Scholar]
- 78.Watanabe Y., Mendonca L., Allen E.R., Howe A., Lee M., Allen J.D., Chawla H., Pulido D., Donnellan F., Davies H., Ulaszewska M., Belij-Rammerstorfer S., Morris S., Krebs A.S., Dejnirattisai W., Mongkolsapaya J., Supasa P., Screaton G.R., Green C.M., Lambe T., Zhang P., Gilbert S.C. and Crispin M. Native-like SARS-CoV-2 spike glycoprotein expressed by ChAdOx1 nCoV-19/AZD1222 vaccine. BioRxiv. 2021.
- 79.Deshotels M.R., Xia H., Sriramula S., Lazartigues E., Filipeanu C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014;64:1368–1375. doi: 10.1161/HYPERTENSIONAHA.114.03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., Liu M., Zhao X., Xie Y., Yang Y., Zhang S., Fan Z., Dong J., Yuan Z., Ding Z., Zhang Y., Hu L. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13:120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zappa M., Verdecchia P., Spanevello A., Visca D., Angeli F. Blood pressure increase after Pfizer/BioNTech SARS-CoV-2 vaccine. Eur J Intern Med. 2021;90:111–113. doi: 10.1016/j.ejim.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wysocki J., Ye M., Rodriguez E., Gonzalez-Pacheco F.R., Barrios C., Evora K., Schuster M., Loibner H., Brosnihan K.B., Ferrario C.M., Penninger J.M., Batlle D. Targeting the degradation of angiotensin II with recombinant angiotensin-converting enzyme 2: prevention of angiotensin II-dependent hypertension. Hypertension. 2010;55:90–98. doi: 10.1161/HYPERTENSIONAHA.109.138420. (Dallas, Tex : 1979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang L., Feng Y., Zhang Y., Zhou H., Jiang S., Niu T., Wei L.J., Xu X., Xu X., Wang X. Prolylcarboxypeptidase gene, chronic hypertension, and risk of preeclampsia. Am J Obstet Gynecol. 2006;195:162–171. doi: 10.1016/j.ajog.2006.01.079. [DOI] [PubMed] [Google Scholar]
- 84.Adams G.N., LaRusch G.A., Stavrou E., Zhou Y., Nieman M.T., Jacobs G.H., Cui Y., Lu Y., Jain M.K., Mahdi F., Shariat-Madar Z., Okada Y., D'Alecy L.G., Schmaier A.H. Murine prolylcarboxypeptidase depletion induces vascular dysfunction with hypertension and faster arterial thrombosis. Blood. 2011;117:3929–3937. doi: 10.1182/blood-2010-11-318527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu L., Carretero O.A., Liao T.D., Harding P., Li H., Sumners C., XP Yang. Role of prolylcarboxypeptidase in angiotensin II type 2 receptor-mediated bradykinin release in mouse coronary artery endothelial cells. Hypertension. 2010;56:384–390. doi: 10.1161/HYPERTENSIONAHA.110.155051. (Dallas, Tex : 1979) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wallace M., Oliver S. COVID-19 mRNA vaccines in adolescents and young adults: benefit-risk discussion. Proceedings of the United States Advisory Committee on Immunization Practices (US ACIP) COVID-19 vaccines work group conference; Atlanta, GA; 2021. Corporate Authors(s) Author(s): US ACIP MeetingMay 12, 2021 Published June 23, 2021. [Google Scholar]
- 87.Schultz N.H., Sorvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., Wiedmann M., Aamodt A.H., Skattor T.H., Tjonnfjord G.E., Holme P.A. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bourguignon A., Arnold D.M., Warkentin T.E., Smith J.W., Pannu T., Shrum J.M., Al Maqrashi Z.A.A., Shroff A., Lessard M.C., Blais N., Kelton J.G., Nazy I. Adjunct immune globulin for vaccine-induced immune thrombotic thrombocytopenia. N Engl J Med. 2021;385:720–728. doi: 10.1056/NEJMoa2107051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pavord S., Scully M., Hunt B.J., Lester W., Bagot C., Craven B., Rampotas A., Ambler G., Makris M. Clinical Features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385:1680–1689. doi: 10.1056/NEJMoa2109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf (Accessed on May 31, 2022).
- 91.Tran V.N., Nguyen H.A., Le T.T.A., Truong T.T., Nguyen P.T., Nguyen T.T.H. Factors influencing adverse events following immunization with AZD1222 in Vietnamese adults during first half of 2021. Vaccine. 2021;39:6485–6491. doi: 10.1016/j.vaccine.2021.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mercurio I., Tragni V., Busto F., De Grassi A., Pierri C.L. Protein structure analysis of the interactions between SARS-CoV-2 spike protein and the human ACE2 receptor: from conformational changes to novel neutralizing antibodies. Cell Mol Life Sci. 2021;78:1501–1522. doi: 10.1007/s00018-020-03580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yamamoto K., Chappell M.C., Brosnihan K.B., Ferrario C.M. In vivo metabolism of angiotensin I by neutral endopeptidase (EC 3.4.24.11) in spontaneously hypertensive rats. Hypertension. 1992;19:692–696. doi: 10.1161/01.hyp.19.6.692. (Dallas, Tex : 1979) [DOI] [PubMed] [Google Scholar]