Abstract
Background
We aimed to assess the risk of developing new-onset seizures or seizure decompensations in people with epilepsy (PWE) associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines.
Methods
A retrospective observational study in a tertiary hospital was conducted. Clinical records of all patients attended because of seizures or epilepsy at outpatient clinics, emergency department, or admitted to our hospital from January to December 2021 were reviewed, including patients older than 16 years who received some dose of coronavirus disease 2019 (COVID-19) vaccines.
Results
A total of 418 vaccinated PWE were analyzed: 6.2% presented an increase in seizure frequency and 1% reported different seizure types during the next month after vaccination. However, 61.5% had another possible cause for this decompensation. Having monthly seizures (1–3/month) was the only associated risk factor (OR 4.9, p < 0.001) while being seizure free > 1 year had a protective role (OR 0.36, p = 0.019). Patients with epileptic encephalopathies or a history of COVID-19 infection were not at increased risk of seizure decompensation. Besides this, 15 patients presented new-onset seizures within the first month post-vaccination, mean time from vaccination 15 ± 8 days, 67% after the second dose. Again, 53.3% had another possible trigger for seizures. Eight debuted with status epilepticus or cluster of seizures.
Conclusions
A small proportion of PWE (6.2%) had an increase in seizure frequency after COVID-19 vaccination and 15 patients had new-onset seizures during the first month after vaccination, though another reason for seizure exacerbation was identified in 61.5% and 53.3%, respectively. Severe acute respiratory syndrome COVID-19 vaccines appear to have little impact on the generation or decompensation of seizures.
Keywords: COVID-19, SARS-CoV-2 vaccine, Trigger, Epilepsy, Seizures
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has caused a global sociosanitary crisis, leading to a radical change in our way of life. The recently developed vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have made a decisive contribution, changing the course of the pandemic. The regulatory agencies conferred a temporary emergency use authorization in December 2020 to the first COVID-19 vaccines, rising the biggest vaccination campaign ever [1]. In Spain, the vaccination against SARS-CoV-2 started on December 27, 2020, being one of the countries with the highest percentage of vaccinated population in the world [2]. Like all other medications, vaccines have been associated with neurological adverse events, such as acute symptomatic seizures and status epilepticus [3]. Indeed, vaccine inoculation constitutes the second most frequent cause of seizures in children [4]. However, post-vaccination seizures or status also depend on other factors, such as having a genetic epileptic syndrome, age at vaccination, having a confirmed coinfection, or the type of vaccine [3], [5].
Several observational studies have reported that during the COVID-19 pandemic, people with epilepsy (PWE) worsened their seizure control [6], [7], [8], [9], [10], [11]. However, in most cases, this decompensation occurred because of circumstances derived from the pandemic and not due to COVID-19 infection [12]. Although previous studies have associated COVID-19 with different types of neurological involvement [13], acute symptomatic seizures resulting from COVID-19 are uncommon [9], [14], [15], [16]. Despite this, vaccination against SARS-CoV-2 may associate a relevant risk of acute symptomatic seizures or an increase in seizure frequency in PWE. So far, a few studies evaluating the adverse effects of the SARS-CoV-2 vaccines in PWE have approached this last question [17], [18], [19], [20], outlining that worsening of seizure control was infrequent. However, several biases cast doubts on these reports, and further studies to corroborate the results seem necessary [21]. Otherwise, the risk of developing a first seizure or status epilepticus associated with these vaccines remains unknown.
This study aimed to assess the risk of developing new-onset seizures, as well as the risk of seizure control worsening in PWE associated with SARS-CoV-2 vaccines. We reviewed all patients who were attended at our hospital because of seizures or epilepsy during 2021, looking for those patients with new-onset seizures temporally related to COVID-19 vaccination, and those PWE who presented an increase in seizure frequency temporally related to these vaccines.
2. Methods
2.1. Study design
An observational, retrospective study was conducted in a tertiary center (Albacete University Hospital Complex) in the province of Albacete, Castilla-La Mancha, Spain. The Neurology Service of our hospital provides specialized care for a reference population of 300,000 inhabitants. This study was conducted in accordance with the Declaration of Helsinki and local governmental regulations and was approved by the local ethics committee.
2.2. Study population and data collection
The study period was from January 2021 to December 2021, since the COVID-19 vaccination campaign in our country began on December 27, 2020. All patients who successively visited the Neurology outpatient clinic because of seizures or epilepsy during the study period were reviewed. In addition, all patients discharged from the emergency department or after hospitalization with a diagnosis of seizures or epilepsy were systematically reviewed. Data were collected through a review of the hospital and primary care medical records. The medical records review was carried out by senior neurologists and neurology or neurophysiology residents. Diagnosis of epilepsy was made according to the current criteria [22]. Patients under 16 years of age and those who had not received any dose of the SARS-CoV-2 vaccine were excluded. Also, patients with resolved epilepsy [22] and with episodes highly suggestive of non-epileptic paroxysmal events that could not be clearly differentiated from their seizures were excluded. Two groups were conformed: a group of patients with epilepsy diagnosed prior to the vaccination, in whom a change in seizure control along the first month after vaccination was assessed, and a group of patients without previous epilepsy who had new-onset seizures during the first month after COVID-19 vaccination. Previous studies have reported that the temporal relationship between vaccination and seizures is close, but the time of increased risk of seizures after vaccination is not clearly defined [23]. We selected the first month after vaccination to certify that all seizures related to vaccination were included in the study. In addition, seizure frequency is usually recorded in number of seizures per month and a greater precision would be impossible due to the retrospective nature of the study.
2.3. Variables collected
In the group of PWE, the main variable studied was the proportion of patients who presented a significant increase in seizure frequency during the first month after SARS-CoV-2 vaccination. We considered a significant increase in seizure frequency those increases of more than 50% compared to the pre-vaccination period. Seizure frequency was calculated as the average number of seizures per month. Pre-vaccination seizure frequency was obtained by reviewing medical records and according to patients' calendar of seizures during the previous year. In case of changes of baseline seizure frequency during the months prior to vaccination, we considered only those months for seizure count. Post-vaccination seizure frequency was based on the best estimation of the number of seizures during the month after vaccination recalled by the patients. Pre-vaccination seizure frequency was also classified into no seizures for more than 1 year, annual (1 to 11 seizures per year), monthly (1 to 3 seizures per month), weekly (1 to 6 seizures per week), and daily (at least 1 seizure per day). Other possible factors that could have influenced the seizure control decompensation were recorded. Likewise, the proportion of PWE who developed new types of seizures different from their usual ones was analyzed. The type of epilepsy (according to the current ILAE classifications of seizures and epilepsies [24], etiology of epilepsy, and the type and number of antiseizure medication (ASM) were revised. The proportion of patients with active epilepsy (defined as the presence of some seizures in the last 5 years [25]), drug-refractory epilepsy (defined as the persistence of seizures despite the trial of 2 or more ASM properly indicated and adequately dosed), and those who underwent epilepsy surgery were also recorded. In the group of patients with new-onset seizures, the time from vaccination to the seizures was measured. Also, other possible causes for the seizure onset were investigated and noted. For all patients, the type of vaccine received, the number of doses administered, and the date of vaccinations were recorded. Also, those who contracted COVID-19 and the date of infection were noted. Other variables such as sex, age, intellectual disability, the presence of comorbidities such as hypertension, diabetes mellitus, dyslipidemia, immunosuppression, cardiological, neoplastic, or pneumological disease, and neurological disease other than epilepsy were documented in all cases.
2.4. Statistical analysis
The statistical analysis was performed using the SPSS software, version 26 (SPSS, Chicago, IL). A descriptive analysis of the variables collected was performed: the qualitative variables were expressed as a percentage and the quantitative variables using mean ± standard deviation (SD) or median-interquartile range (IQR) whether they had a normal distribution. An analysis of the variables associated with the increase in seizure frequency was performed: the ratios were compared using the chi-squared test or Fisher exact test, quantitative variables were compared using Student’s t-test or the U-Mann Whitney test when appropriate. Finally, a multivariate analysis for classification of increasing seizures or not was performed, using binary logistic regression including the variables significantly or nearly significantly associated with seizure frequency increase in univariate analyses (basal seizure frequency, number of ASM, intellectual disability, active epilepsy, and drug-refractory epilepsy). To assess the fit of the logistic regression model, we used the Hosmer-Lemeshow goodness-of-fit test (data did not show multicollinearity). The odds ratios (OR) and confidence intervals (CI) were calculated. In all cases, p values less than 0.05 were considered statistically significant.
3. Results
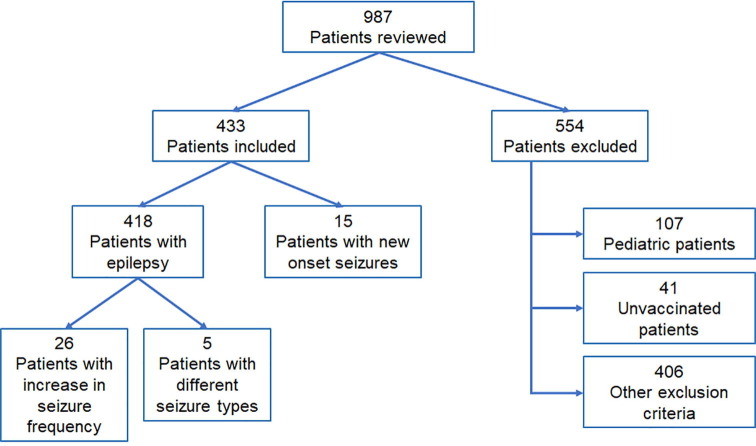
Between January 2021 and December 2021, a total of 987 patients were attended at the Neurology outpatient clinic, or discharged from the emergency department or from hospitalization with the diagnosis of epilepsy or seizure. From these, 433 patients were included in the study, 418 with epilepsy diagnosed before vaccination and 15 with new onset of seizures. The flow chart of patient selection can be seen in Fig. 1 . Only 41 patients (4.6%) older than 16 years were excluded because they were not vaccinated.
Fig. 1.
Flow chart of patient selection. Classification of patients according to inclusion/exclusion criteria.
3.1. Increase in seizure frequency in PWE
A total of 418 vaccinated PWE were attended at least a month after SARS-CoV-2 vaccination. The mean age of the group was 48.2 years, SD 19 (range 16–93), 51.9% were men. Etiology of epilepsy according to the ILAE classification [22] was: 40.2% structural, 16% genetic, 2.9% infectious, 0.2% metabolic, 1.2% immune, and 39.5% unknown. A more detailed epilepsy etiology classification and the type of epilepsy are described in Table 1 . Almost half of the patients (49.3%) were seizure free for more than one year, 20.8% had yearly seizures, and 18.9% had monthly seizures (Table 1). The mean number of ASM used was 1.7 (range 0–5) and the most frequently employed were levetiracetam (38.3% of PWE), lamotrigine (20.1%), and valproic acid (16.7%). The characteristics of their epilepsy can be seen in Table 1 and other medical comorbidities are described in Table 2 . Also, COVID-19 related information is shown in Table 2. Only 26 patients (6.2%) reported an increase of >50% in their seizure frequency along the month after vaccination (Table 1). Of these cases, two consulted without visit at the neurology outpatient clinics, four consulted at the emergency department, and three were admitted to hospital due to seizures decompensation. On the other hand, in 16 of the 26 cases (61.5%), another possible cause for decompensation other than vaccination was detected (Table 4). The basal seizure frequency was the only variable statistically associated with a post-vaccination increase in seizure frequency (p < 0.001): being seizure free for more than one year prior to vaccination had a protective role (OR 0.36, 95% CI 0.15–0.87, p = 0.019), but having monthly seizures (1–3 per month) was a significant risk factor for an increase in seizure frequency (OR 4.9, 95% CI 2.2–11.1, p < 0.001). No other clinical variable was related to an increase in seizure frequency (Table 1) as neither having suffered COVID-19, the type of vaccine, or the number of doses received (Table 2). In the multivariate analysis, only having monthly seizures was statistically associated with post-vaccination seizure increase (OR 1.8, 95% CI 1.1–3.9, p = 0.007) (Table 1).
Table 1.
Demographic characteristics of PWE.
| Increase >50% seizure frequency during the first month after vaccination | No increase in seizure frequency during the first month after vaccination | p | p | ||
|---|---|---|---|---|---|
| univariate | multivariate | ||||
| N (%) | 26 (6.2%) | 392 (93.8%) | |||
| Sex | Man | 15 (57.7%) | 202 (51.5%) | 0.543 | |
| Woman | 11 (42.3%) | 190 (48.5%) | |||
| Age | Mean (SD) | 49.73 (12.3) | 48.12 (10.2) | 0.675 | |
| Epilepsy type | Generalized | 3 (11.5%) | 62 (15.8%) | 0.810 | |
| IGE with GTCS only | 1 (33.3%) | 10 (16.4%) | |||
| JME | 0 | 18 (29.5%) | |||
| Childhood absence | 1 (33.3%) | 9 (14.8%) | |||
| Juvenile absence | 0 | 4 (6.6%) | |||
| Epileptic encephalopathy | 1 (33.3%) | 11 (18.0%) | |||
| Other genetic generalized epilepsies# | 0 | 4 (6.6%) | |||
| Others | 0 | 5 (8.2%) | |||
| Focal | 20 (76.9%) | 299 (76.3%) | 0.773 | ||
| Frontal | 4 (20.0%) | 71 (23.7%) | |||
| Temporal | 9 (45.0%) | 100 (33.4%) | |||
| Posterior quadrant | 2 (10.0%) | 35 (11.7%) | |||
| Unknown | 5 (25.0%) | 93 (31.1%) | |||
| Unknown | 3 (11.5%) | 31 (7.9%) | 0.711 | ||
| Etiology | Unknown | 9 (34.6%) | 148 (37.8%) | 0.815 | |
| Genetic | 1 (3.8%) | 23 (5.9%) | |||
| MCD | 4 (15.4%) | 21 (5.4%) | |||
| Perinatal | 1 (3.8%) | 20 (5.1%) | |||
| Vascular | 4 (15.4%) | 49 (12.5%) | |||
| Infectious | 0 | 12 (3.1%) | |||
| TBI | 1 (3.8%) | 15 (3.8%) | |||
| Autoimmune | 0 | 5 (1.3%) | |||
| Tumoral | 2 (7.7%) | 34 (8.7%) | |||
| Others | 2 (7.7%) | 24 (6.1%) | |||
| IGE | 2 (7.7%) | 41 (10.5%) | |||
| Pre-vaccination seizure frequency | No seizure >1 year | 7 (26.9%) | 199 (50.8%) | 0.018 | 0.859 |
| Annual | 6 (23.1%) | 81 (20.7%) | 0.771 | ||
| Monthly | 13 (50.0%) | 66 (16.8%) | 0.001 | 0.007 | |
| Weekly | 0 | 28 (7.1%) | 0.158 | ||
| Daily | 0 | 18 (4.6%) | 0.262 | ||
| ASM | 0 | 1 (3.8%) | 19 (4.9%) | 0.093 | 0.185 |
| 1 | 10 (38.5%) | 180 (46.2%) | |||
| 2 | 11 (42.3%) | 104 (26.7%) | |||
| >2 | 4 (15.3%) | 87 (22.4%) | |||
| Intellectual disability | 2 (7.7%) | 77 (19.6%) | 0.194 | 0.179 | |
| Active epilepsy | 24 (92.3%) | 307 (78.3%) | 0.131 | 0.452 | |
| Drug-refractory epilepsy | 13 (50.0%) | 132 (33.7%) | 0.090 | 0.116 | |
| Epilepsy surgery | 1 (3.8%) | 24 (6.1%) | 0.999 | ||
ASM: anti-seizure medication; GTCS: generalized tonic-clonic seizure; IGE: idiopathic generalized epilepsy; JME: juvenile myoclonic epilepsy; MCD: malformation of cortical development; TBI: traumatic brain injury.
Including generalized epilepsies with genetic confirmation different than IGE and epileptic encephalopathies.
Table 2.
Comorbidities, COVID-19 infection, and vaccination status of PWE during the first month after vaccination.
| Increase >50% seizure frequency during the first month after vaccination | No increase in seizure frequency during the first month after vaccination | p | ||
|---|---|---|---|---|
| (n = 26) | (n = 392) | |||
| Comorbidities | Hypertension | 8 (30.8%) | 88 (22.4%) | 0.329 |
| Diabetes mellitus | 3 (11.5%) | 26 (6.6%) | 0.411 | |
| Dyslipidemia | 6 (23.1%) | 78 (19.9%) | 0.695 | |
| Neoplastic disease | 4 (15.4%) | 47 (12.0%) | 0.542 | |
| Pneumological disease | 5 (19.2%) | 52 (13.3%) | 0.391 | |
| Neurological disease (different from epilepsy) | 9 (34.6%) | 141 (36.0%) | 0.889 | |
| Cardiological disease | 4 (15.4%) | 46 (11.7%) | 0.539 | |
| Immunosuppression | 2 (7.7%) | 17 (4.3%) | 0.334 | |
| History of positive test for SARS-CoV-2 | 3 (11.5%) | 75 (19.2%) | 0.441 | |
| Vaccine type | Pfizer | 16 (61.5%) | 255 (65.1%) | 0.973 |
| AZ | 1 (3.8%) | 10 (2.5%) | ||
| Moderna | 3 (11.5%) | 45 (11.5%) | ||
| Jansen | 0 | 3 (0.8%) | ||
| Heterologous | 6 (23.1%) | 79 (20.1%) | ||
| Total number of doses received | 1 dose | 1 (3.8%) | 23 (5.9%) | 0.945 |
| 2 doses | 14 (53.8%) | 219 (55.9%) | ||
| (including Jansen’s 2nd dose- booster) | ||||
| 3 doses | 11 (42.4%) | 150 (38.2%) | ||
AZ: AstraZeneca. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Table 4.
Possible causes of seizures during the first month after vaccination.
| Patients with increase >50% seizure frequency | Patients with different seizure type | Patients with new-onset seizures | |
|---|---|---|---|
| n = 26 | n = 5 | n = 15 | |
| No evident cause | 10 (38.5%) | 4 (80.0%) | 7 (46.7%) |
| ASM (missed dose, change doses, malabsorption disease) | 10 (38.5%) | 1 (20.0%) | 0 |
| Acute cerebrovascular | 0 | 2 (13.3%) | |
| Metabolic disturbances | 1 (3.8%) | 0 | 2 (13.3%) |
| Neurotoxic substances | 0 | 0 | 1 (6.7%) |
| Infectious | 1 (3.8%) | 0 | 1 (6.7%) |
| Tumoral | 2 (7.7%) | 0 | 2 (13.3%) |
| Stress and insomnia | 2 (7.7%) | 0 | 0 |
ASM: antiseizure medication.
3.2. Change in seizures type in PWE
Of the 418 vaccinated PWE included, 5 cases (1%) had a change in their usual type of seizures during the month after vaccination. The mean age was 40.4 years (range 17–72), 60% men. All five had focal epilepsy, the etiology was structural in 4 cases (80%), and one unknown (20%). Four cases presented their first focal to bilateral tonic-clonic seizure and one case had a first focal impaired awareness seizure. In one case, a concomitant change of ASM could have been the cause of the new seizure type (Table 4). Three patients had monthly seizures, one had weekly seizures and one was seizure free for more than one year. The five patients had active epilepsy, but only one was drug-refractory, and none of them underwent epilepsy surgery. Comorbidities were similar to those reported in the rest of the patients. According to the COVID-19 history, all cases received the Pfizer vaccine and none of them had a history of COVID-19 infection. None of the studied variables showed a significant association with having a change in the type of seizures.
3.3. Post-vaccination new-onset seizures in people without previous epilepsy
During the study period, 15 patients were discharged from the emergency department or hospitalization or attended the Neurology outpatient clinic because of a first seizure within the first month after COVID-19 vaccination. The mean age was 65.8 years, SD 21.7 (range 20–90), 8 (53%) were women (Table 3 ). Comorbidities are described in Table 3. Noteworthy, having a neurological disease other than epilepsy was the comorbidity most frequently associated with new-onset seizures, present in more than half of the patients (60%), followed by hypertension and dyslipidemia (53.3% both of them). In more than half of the cases (54%) a different possible precipitant factor for the seizure other than vaccination was found (Table 4 ). Of the 15 patients, five debuted with a status epilepticus and three with a cluster of seizures. Within the patients with status, one case was attributed to a brain tumor progression and other to urinary sepsis. Of the cases of cluster of seizures, one was attributed to an acute subdural hemorrhage. The most frequent seizure type was a bilateral tonic-clonic seizure, whether a focal or generalized origin could not always be known (Table 3). The mean time from vaccination to seizure was 15 ± 8 days, ranging from 1 to 28 days. The different types and doses of vaccines received before the seizure are described in Table 3. In 33% of cases, seizures occurred after the first dose of the SARS-CoV-2 vaccine and in the remaining 67% after the second dose. In many of the reviewed patients, the third dose of the vaccine was already administered at the moment of this review, but no case of new-onset of seizures after this dose was found.
Table 3.
Comorbidities, COVID-19 infection, and vaccination status of patients with new-onset seizures.
| New-onset seizures during the first month after vaccination | |||
|---|---|---|---|
| n = 15 (%) | |||
| Sex | Male | 7 (47.0%) | |
| Female | 8 (53.0%) | ||
| Mean age (SD) | 65.8 (21.7) | ||
| Seizure type | Focal aware | 1 (6.7%) | |
| Focal impaired awareness | 4 (26.7%) | ||
| Focal to bilateral tonic-clonic | 5 (33.3%) | ||
| Generalized tonic-clonic with unknown onset | 5 (33.3%) | ||
| Status epilepticus | 5 (33.0%) | ||
| Cluster of seizures | 3 (20.0%) | ||
| Comorbidities | Hypertension | 8 (53.3%) | |
| Diabetes mellitus | 7 (46.6%) | ||
| Dyslipidemia | 8 (53.3%) | ||
| Neoplastic disease | 4 (26.6%) | ||
| Pneumological disease | 2 (13.3%) | ||
| Neurological disease (different from epilepsy) | 9 (60.0%) | ||
| Cardiological disease | 5 (33.3%) | ||
| Immunosuppression | 0 | ||
| History of a positive test for SARS-CoV-2 | 4 (26.6%) | ||
| Vaccine type and dose before seizure | Pfizer | 1st dose | 5 (33.3%) |
| 2nd dose | 7 (47.0%) | ||
| Moderna | 1st dose | 0 | |
| 2nd dose | 3 (20.0%) | ||
| AZ | 1st dose | 0 | |
| 2nd dose | 0 | ||
| Jansen | 1st dose | 0 | |
| 2nd dose- booster | 0 | ||
AZ: AstraZeneca. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
4. Discussion
Since the first SARS-CoV-2 vaccines were approved, numerous concerns have arisen about their possible side effects, including a potential proconvulsant effect. Studies evaluating the safety and efficacy of COVID-19 vaccines in people with neurological diseases are urgently required [21]. In the present study, we systematically analyzed a large sample of PWE attended after vaccination against COVID-19, studying its relationship with seizure control. Only 26/418 (6.2%) of PWE had a significant increase in their seizure frequency shortly after vaccination. Indeed, other possible reasons for seizure exacerbation could be identified in 16 of these cases. The group of patients at risk of seizure exacerbation after COVID-19 vaccination were those who had monthly seizures, with no other risk factors related. Interestingly, in our population, a 4-fold higher proportion of PWE (27%) reported an increase in their seizure frequency in the first months of the COVID-19 pandemic [9]. In most cases, this was related to different factors attributable to the pandemic and home confinement (increased stress/anxiety, sleep deprivation, decreased physical activity) rather than attributable to the infection itself [9]. In comparison, SARS-CoV-2 vaccines had little impact on seizure control in PWE. Similarly, in our study, the number of patients who presented changes in their normal seizure types was very low, with no significant risk factors associated.
Furthermore, we reviewed all cases of new-onset seizures seen in our tertiary hospital potentially attributable to the COVID-19 vaccine. Fifteen patients were attended in our hospital because of a first seizure within the first month after vaccination throughout the year 2021. Again, more than half of them had another plausible trigger for seizure occurrence. Although the data from the present study do not allow us to calculate the incidence of first seizures in our hospital, considering our reference population, this number of first seizures does not seem to be higher than the incidence of first seizures described in other European regions (102/100,000 persons/year) [26]. Noteworthy, these seizures occurred as status epilepticus in 5 cases. The incidence of status according to literature reviews is around 10.0–15.3 cases/100,000 persons/year [27]. According to our registries, in our center, approximately 51 cases of status are diagnosed per year (non-published own data), so the number of status reported in the present study is not above the expected range. However, in some cases, the causal relationship between the vaccine and these seizures/status cannot be excluded. Although infrequent, this potential adverse effect of SARS-CoV-2 vaccines should be taken into account.
Currently, only a few studies addressed the relationship between PWE and SARS-CoV-2 vaccination [17], [18], [19], [20]. These studies aimed to explore the safety and tolerability of vaccination among PWE, as well as the vaccination rates and the reasons for refusing. Besides that, seizure frequency was also analyzed, and none of these studies reported a high proportion of PWE with a post-vaccination worsening of seizure control. Nevertheless, several biases limit the external validity of these results. First of all, these studies were based on questionnaires completed by the patients, associating an inevitable selection bias. The first studies [18], [19], [20] included small and poorly extrapolable samples. In addition, vaccination rates were relatively low at the time. They reported a 6.1%, 3.7%, and 2.2% of patients with epilepsy who presented a seizure worsening, with a single case of status epilepticus in the first, and a cluster of seizures in the second, results that are in line with those seen in our study. A posterior study compared the tolerability of COVID-19 vaccines in a larger cohort of 491 PWE [17]. An increase in seizure frequency was seen in 9.2% of vaccinated PWE, but almost one-third of them stopped or reduced their ASM concomitantly because of fear of interactions with the vaccine. Interestingly, a very low vaccination rate was observed among PWE, only 42% received some dose of the SARS-CoV-2 vaccine compared with 93% of controls. In contrast, vaccination rates were very high in our population, with 95.4% of the studied adult population that have received at least one dose of the vaccines against SARS-CoV-2 by the end of 2021 (Fig. 1). This proportion was even higher than the vaccination rate of the national population (83.7% of the total population with at least one dose and 79.8% with a complete vaccination schedule, as of December 30, 2021) [2].
On the other hand, patients with intellectual disability were excluded from previous studies [17], [18], [19], which may be a subgroup on risk of seizure decompensation after vaccination (e.g. due to fever sensitivity in patients with Dravet’s syndrome). Noteworthy, we did not find differences in seizure exacerbation in patients diagnosed with intellectual disability nor some epileptic encephalopathy. A recent study evaluated the tolerability of SARS-CoV-2 vaccines in people with Dravet syndrome [28]: 3/15 (20%) of individuals reported an increase in seizure frequency after the first dose of the vaccine (all three received the Oxford/AstraZeneca vaccine). Overall, SARS-CoV-2 vaccines seemed to be safe in terms of seizure decompensation in these patients.
Another variable that was not previously analyzed was the history of COVID-19 infection. It has been questioned whether these patients are more susceptible to vaccine side effects because of their already existing natural immunity against SARS-CoV-2. Our results confirm that vaccination does not associate a higher risk of seizures in this subgroup of patients.
4.1. Study limitations
The main limitations of this study derive from its retrospective nature. Therefore, the direct association between seizures and vaccination should be treated with caution. The low proportion of PWE non-vaccinated in our population made impossible the selection of a valid control group, preventing a better analysis of the correlation between vaccination and seizures occurrence. The low number of patients who suffered the main outcomes (seizure frequency increase and new-onset seizures) limits the validity of the analyses of variable association, especially multivariate tests. Also, patients under 16 years of age were not evaluated in this study, so the results cannot be extrapolated to this population. On the other hand, other uncontrolled factors may have influenced the occurrence of new-onset seizures or changes in seizure frequency. Moreover, in many cases, the seizure frequency documented at the follow-up visits was based on patients’ subjective recall of the previous period. Finally, the circumstances of the seizures could not always be properly investigated due to the retrospective design.
5. Conclusions
Vaccination rates in our population of adult PWE were very high, with 95.4% of the studied patients having received at least one dose of the COVID-19 vaccine. A small proportion of PWE had a relevant increase in their seizure frequency after COVID-19 vaccination (6.2%), and indeed, in 61.5% of these cases other possible reasons for seizures exacerbation different than vaccination could be identified. The group of patients at higher risk of seizure exacerbation after COVID-19 vaccination were those who had monthly seizures. Similarly, the number of patients who presented some different seizure types after vaccination was very low (1%). Patients with epileptic encephalopathies, cognitive impairment, or a history of past COVID-19 infection were not at increased risk of seizure decompensation. In addition, 15 patients presented new-onset seizures within the first month after COVID-19 vaccination throughout the year 2021 in our hospital. Among them, more than half (53.3%) had another plausible precipitant for seizure occurrence. Five of the cases debuted with a status epilepticus and three with a cluster of seizures. Altogether, SARS-CoV-2 vaccines appear to have little impact on the generation or decompensation of seizures.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lamb Y.N. BNT162b2 mRNA COVID-19 vaccine: first approval. Drugs. 2021;81:495–501. doi: 10.1007/s40265-021-01480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuadro de mando resumen de datos de vacunación, Ministerio de Sanidad, https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/pbiVacunacion.htm [accessed 26 March 2022].
- 3.Deng L, Danchin M, Lewis G, Wen CHS, Doyle R, Barnett M, et al. Status epilepticus following vaccination in children aged ≤24 months: A five-year retrospective observational study. Epilepsy Behav. 2022; 128: 108579. https://doi.org/10.1016/j.yebeh.2022.108579. [DOI] [PubMed]
- 4.Li X., Lin Y., Yao G., Wang Y. The influence of vaccine on febrile seizure. Curr Neuropharmacol. 2018;16:59–65. doi: 10.2174/1570159X15666170726115639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Top K.A., Righolt C.H., Hawken S., Donelle J., Pabla G., Brna P., et al. Adverse events following immunization among children with epilepsy: A self-controlled case series. Pediatr Infect Dis J. 2020;39(5):454–459. doi: 10.1097/INF.0000000000002553. [DOI] [PubMed] [Google Scholar]
- 6.Aledo-Serrano Á., Mingorance A., Jiménez-Huete A., Toledano R., García-Morales I., Anciones C., et al. Genetic epilepsies and COVID-19 pandemic: Lessons from the caregiver perspective. Epilepsia. 2020;61:1312–1314. doi: 10.1111/epi.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conde Blanco E., Manzanares I., Centeno M., Khawaja M., Betrán O., Donaire A., et al. Epilepsy and lockdown: A survey of patients normally attending a Spanish centre. Acta Neurol Scand. 2021;143(2):206–209. doi: 10.1111/ane.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonseca E., Quintana M., Lallana S., Luis Restrepo J., Abraira L., Santamarina E., et al. Epilepsy in time of COVID-19: A survey-based study. Acta Neurol Scand. 2020;142(6):545–554. doi: 10.1111/ane.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Larsen A., Gonzalez-Villar E., Díaz-Maroto I., Layos-Romero A., Martínez-Martín Á., Alcahut-Rodriguez C., et al. Influence of the COVID-19 outbreak in people with epilepsy: Analysis of a Spanish population (EPICOVID registry) Epilepsy Behav. 2020;112:107396. doi: 10.1016/j.yebeh.2020.107396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alkhotani A., Siddiqui M.I., Almuntashri F., Baothman R. The effect of COVID-19 pandemic on seizure control and self-reported stress on patient with epilepsy. Epilepsy Behav. 2020;112:107323. doi: 10.1016/j.yebeh.2020.107323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosengard J.L., Donato J., Ferastraoaru V., Zhao D., Molinero I., Boro A., et al. Seizure control, stress, and access to care during the COVID-19 pandemic in New York City: The patient perspective. Epilepsia. 2021;62(1):41–50. doi: 10.1111/epi.16779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez-Larsen A., Conde-Blanco E., Viloria-Alebesque A., Sánchez-Vizcaíno Buendía C., Espinosa Oltra T., Alvarez-Noval A., et al. COVID-19 prevalence and mortality in people with epilepsy: A nation-wide multicenter study. Epilepsy Behav. 2021;125:108379. doi: 10.1016/j.yebeh.2021.108379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero-Sánchez C.M., Díaz-Maroto I., Fernández-Díaz E., Sánchez-Larsen Á., Layos-Romero A., García-García J., et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry: The ALBACOVID registry. Neurology. 2020;95:e1060–e1070. doi: 10.1212/WNL.0000000000009937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu L., Xiong W., Liu D., Liu J., Yang D., Li N., et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: A retrospective multicenter study. Epilepsia. 2020;61(6):e49–e53. doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keshavarzi A., Janbabaei G., Kheyrati L., Ghavamabad L.H., Asadi-Pooya A.A. Seizure is a rare presenting manifestation of COVID-19. Seizure. 2021;86:16–18. doi: 10.1016/j.seizure.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asadi-Pooya A.A., Simani L., Shahisavandi M., Barzegar Z. COVID-19, de novo seizures, and epilepsy: a systematic review. Neurol Sci. 2021;42:415–431. doi: 10.1007/s10072-020-04932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu L., Zhang Q., Xiao J., Zhang Y., Peng W., Han X., et al. COVID-19 vaccine take-up rate and safety in adults with epilepsy: Data from a multicenter study in China. Epilepsia. 2022;63:244–251. doi: 10.1111/epi.17138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massoud F., Ahmad S.F., Hassan A.M., Alexander K.J., Al-Hashel J., Arabi M. Safety and tolerability of the novel 2019 coronavirus disease (COVID-19) vaccines among people with epilepsy (PwE): A cross-sectional study. Seizure. 2021;92:2–9. doi: 10.1016/j.seizure.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Wrede R., Pukropski J., Moskau-Hartmann S., Surges R., Baumgartner T. COVID-19 vaccination in patients with epilepsy: First experiences in a German tertiary epilepsy center. Epilepsy Behav. 2021;122:108160. doi: 10.1016/j.yebeh.2021.108160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Özdemir H.N., Dere B., Gökçay F., Gökçay A. Are COVID-19 vaccines safe for people with epilepsy? A cross-sectional study. Neurol Sci. 2022;27:1–8. doi: 10.1007/s10072-022-05956-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood H. Safety and efficacy of COVID-19 vaccines in people with neurological disorders. Nat Rev Neurol. 2022; 18: 66. https://doi.org/10.1038/s41582-021-00603-8. [DOI] [PMC free article] [PubMed]
- 22.Fisher R.S., Cross J.H., French J.A., Higurashi N., Hirsch E., Jansen F.E., et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522–530. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 23.Verbeek N.E., Jansen F.E., Vermeer-de Bondt P.E., de Kovel C.G., van Kempen M.J.A., Lindhout D., et al. Vaccination triggers, rather than causes, seizures. Pediatrics. 2014;134:658–666. doi: 10.1542/peds.2014-0690. [DOI] [PubMed] [Google Scholar]
- 24.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forsgren L., Beghi E., Õun A., Sillanpää M. The epidemiology of epilepsy in Europe - A systematic review. Eur J Neurol. 2005;12:245–253. doi: 10.1111/j.1468-1331.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- 26.Maloney E.M., Chaila E., O'Reilly É.J., Costello D.J. Incidence of first seizures, epilepsy, and seizure mimics in a geographically defined area. Neurology. 2020;95:e576–e590. doi: 10.1212/WNL.0000000000009980. [DOI] [PubMed] [Google Scholar]
- 27.Lv R.J., Wang Q., Cui T., Zhu F., Shao X.Q. Status epilepticus-related etiology, incidence and mortality: A meta-analysis. Epilepsy Res. 2017;136:12–17. doi: 10.1016/j.eplepsyres.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Clayton L.M., Balestrini S., Cross J.H., Wilson G., Eldred C., Evans H., et al. The impact of SARS-CoV-2 vaccination in Dravet syndrome: A UK survey. Epilepsy Behav. 2021;124:108258. doi: 10.1016/j.yebeh.2021.108258. [DOI] [PMC free article] [PubMed] [Google Scholar]