Abstract
Patients with inflammatory bowel disease (IBD) are particularly susceptible to behavioral diagnoses, and the microbiome has been repeatedly implicated in the pathogenesis of IBD. The intestinal microbiome’s ability to affect behavior has become increasingly recognized and studied. The so-called ‘psychobiome’ has been linked to a plethora of neurological and psychological diagnoses, including autism and Parkinson’s disease. Despite the ability of many bacterial species within the human intestinal microbiome to synthesize neurotransmitters, it has never been previously reported that a single bacterial species is sufficient to induce depression. Here, we demonstrate that our mouse model of Crohn’s disease (CD)-like ileitis, the SAMP1/YitFc (SAMP1), does not exhibit baseline behavioral abnormalities. By comparison, SAMP6 mice develop depressive-like behavior that is associated with a rise in the GABA-producing bacterial genus Parabacteroides. We finally demonstrate that administration of Parabacteroides distasonis into our SAMP1 mice induces depressive-like behavior. Colonization with P. distasonis was not associated with increased intestinal inflammation or alterations in other measures of behavior. The intestinal environment of CD may be particularly conducive to colonization with P. distasonis and subsequent induction of depressive-like behavior. To our knowledge, this is the first report of a bacterial species specifically inducing depressive-like behavior.
Keywords: Crohn’s disease, inflammatory bowel disease, microbiome, psychobiome, microbiome-gut-brain axis, Parabacteroides distasonis, major depressive disorder
1. Introduction
Inflammatory bowel disease (IBD) is a debilitating intestinal disorder that is categorized into two broad classifications: Crohn’s disease (CD) and ulcerative colitis (UC). Patients with IBD exhibit approximately a six times greater rate of developing major depressive disorder (MDD) when compared to the general population, which exceeds the rates reported in other chronic disorders, such as cancer (Kochar et al., 2017; Patten et al., 2005).
The intestinal microbiome is a key factor in the pathogenesis of IBD. The ability of the microbiome to influence host behavior as a part of the bi-directional ‘microbiome-gut-brain axis’ or ‘psychobiome’ has only recently been appreciated (Bravo et al., 2011; Collins et al., 2012; De Palma et al., 2017). To our knowledge, it has never been previously reported that a single bacterial species is sufficient to induce depression. The bacterial genus Parabacteroides has been identified in some non-IBD patients with MDD, and has also been shown to produce gamma-aminobutyric acid (GABA) (Barandouzi et al., 2020; Strandwitz, 2018; Strandwitz et al., 2018; Valles-Colomer et al., 2019). Interestingly, the species Parabacteroides distasonis has also been identified in two environmental niches specific to CD: cavernous fistulae and creeping mesenteric fat (Ha et al., 2020; Rodriguez-Palacios et al., 2015; Yang et al., 2019).
Here, we use the SAMP1/YitFc (SAMP1) mouse model of CD-like ileitis. The SAMP1 mouse is an extensively characterized congenic mouse model that develops spontaneous ileitis in a predictable time course (Pizarro et al., 2011). Despite extensive characterization of the inflammatory profile, prior to this study, no behavioral characterization was previously documented for the SAMP1.
Here, we demonstrate that despite profound intestinal inflammation, SAMP1 mice do not exhibit behavioral aberrancies. The SAMP6 mouse, which initially served as a negative control for depressive-like behavior at young age, surprisingly developed spontaneous depressive phenotypes at 30 weeks, contrary to previous findings (Niimi and Takahashi, 2014). Onset of depressive-like behavior in our SAMP6 mice was associated with a concurrent rise in the genus Parabacteroides. We show here that administration of P. distasonis to naïve specific pathogen-free (SPF) mice is sufficient to induce depressive-like behavior in the SAMP1 ileitis model.
2. Materials and Methods
2.1. Animals
SAMP1/YitFc, AKR, and SAMP6 mice were maintained at Case Western Reserve University and provided through core services supported by the Animal and Mouse Models Cores. Tissue was collected from euthanized mice and processed with Bouin’s fixative for 24 hours, then washed with 70% EtOH prior to paraffinization, sectioning, and staining.
2.3. Behavioral assays
Mouse behavior was assessed using standardized protocols detailed in supplemental materials. Behavioral tests were conducted at the same time each day to remove circadian variation, and tests were performed in the same order for each cohort.
2.4. Fecal microbiome homogenization and P. distasonis administration
2 weeks prior to initial gavage, fecal samples from all mice were collected and homogenized with 1.0mm sterilized glass silica beads (BioSpec Products, Bartlesville, OK) in PBS with 7% DMSO (Sigma-Aldrich, St. Louis, MO). The homogenate was passed through sterile gauze to remove large particulates and prevent blockage of the gavage needle. The homogenate was then orally gavaged three times over the course of a week (10mL/kg). After each gavage, mice were returned to a clean cage to prevent influence of soiled bedding material and coprophagia (Rodriguez-Palacios et al., 2018). 200 uL P. distasonis was administered in PBS with 5% DMSO at a concentration of 5x106 CFUs/mL. Propagation of P. distasonis is detailed in supplemental.
2.5. Statistical Analysis
Data were analyzed using Prism 9.1 (GrapdPad software) and RStudio. Selection of appropriate statistical tests was based on variance and underlying distribution and is described in each figure legend. Statistical significance is indicated on graphs, with a p-value ≤ 0.05 considered significant. 16s data was analyzed using the Rhea pipeline (Lagkouvardos et al., 2017) and visualized with R.
2.6. Rigor and reproducibility
Experiments were conducted at a minimum in duplicate and presented as unique data points. Age and sex-matched littermates were used at all times. Behavioral assays were scored using investigator-independent computer analysis and investigators were blinded to mice identities. Histological samples were scored by blinded pathologists. Key biological resources, data, and software used are available upon request.
3. Results and discussion
The SAMP1/YitFc (SAMP1) mouse develops spontaneous Crohn’s disease (CD) like ileitis. SAMP1 and SAMP6 mice are derived from the parental AKR/J (AKR) mouse strain. Unlike SAMP1, however, SAMP6 is a model of senile osteoporosis. Despite a shared genetic background, neither AKR nor SAMP6 mice develop ileitis or any other intestinal inflammation (supplemental figure 1). The behavioral profiles of both SAMP6 and AKR mice have been previously characterized. SAMP6 have been previous reported not to exhibit depressive-like behavior, whereas AKR mice do (Niimi and Takahashi, 2014; Nikulina et al., 1991). SAMP6 and AKR were therefore used as negative and positive controls, respectively, for depressive-like behavior for the initial behavioral characterization of the SAMP1 mouse.
To measure behavior over the natural course of disease, behavioral assays were performed at 4 weeks (pre-disease), 10 weeks (acute inflammation), and 30 weeks (chronic inflammation). Mice were not reused for different time points, and distinct assay-naïve mice were used for all behavioral experiments.
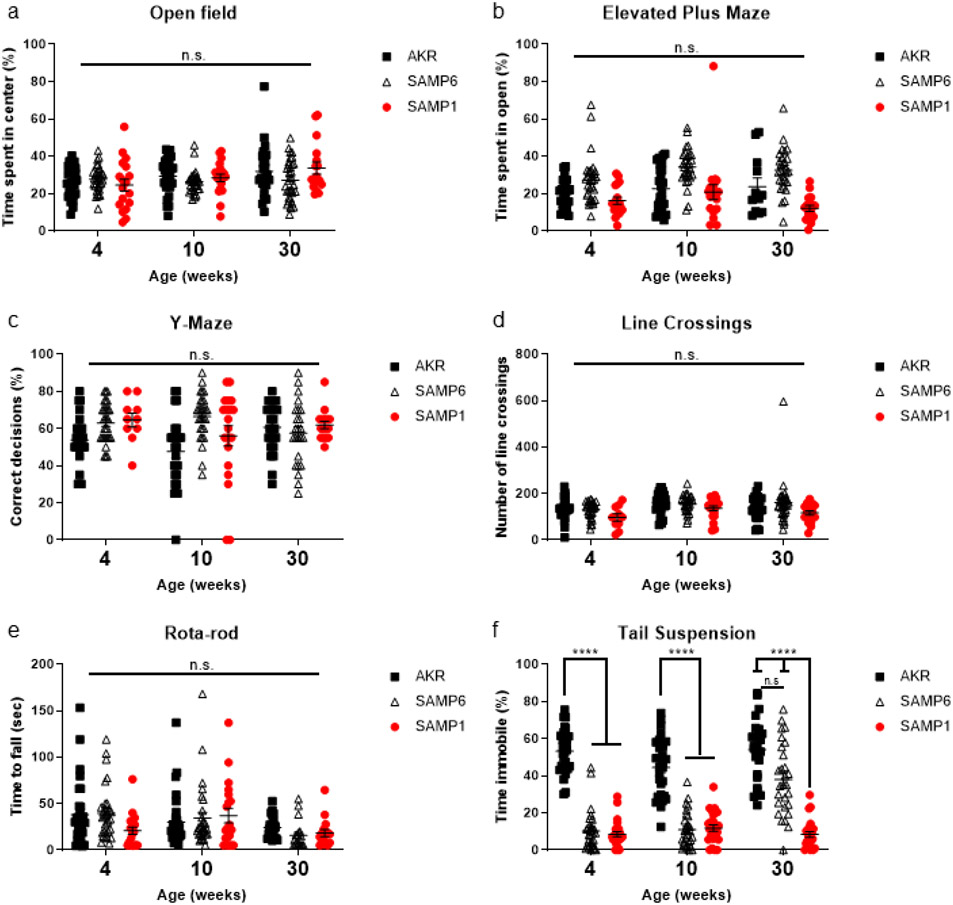
As expected, none of the experimental mouse groups exhibited anxiety-like behavior in either the open field (OF) or elevated plus maze (Fig. 1a and 1b). No strain exhibited spatial memory deficits during Y-maze (Fig. 1c). Similarly, we did not observe decreased locomotor activity, measured by quantifying computer-defined line crossings during the OF assay, or impaired motor coordination during the rota-rod test (Fig. 1d and 1e).
Figure 1. SAMP6, but not SAMP1, develop depressive-like behavior at later time points without anxiety-like behavior, motor, or spatial memory deficits.
Anxiety-like behavior was measured using (a) open field and (b) elevated plus maze. (c) Short-term spatial memory was measured by novel arm exploration in Y-Maze. (d) Gross locomotor activity was measured using computer-defined line crossings during the open field test. (e) Motor coordination was assessed by measuring fall latency during rota-rod. (f) Depressive-like behavior was assessed by measuring immobility time during tail suspension. Each time point is representative of individual mice and behavioral assays were performed in a consistent order. Mice did not perform behavioral assays more than once. Data compared with two-way ANOVA (strain and age as independent variables). Symbols represent individual mice (n=30) from three technical replicates, bars indicate mean ± S.E.M. (n.s. p > 0.05, ****p < 0.0001).
We used the tail suspension (TS) test as a measure of depressive-like behavior (Fig. 1f). TS was chosen over the forced-swim test and the sucrose-preference test, other measures of depressive-like behavior, to minimize effects that the assays would exert on the microbiome. As expected, at all time points, AKR mice exhibited depressive-like behavior, and at 4 and 10 weeks, SAMP6 mice did not exhibit depressive-like behavior.
Interestingly, at 30 weeks, SAMP6 mice developed a spontaneous depressive phenotype (Fig. 1f). This late onset of depressive-like behavior was in contrast to previous reports where SAMP6 showed no signs of depressive-like behavior at any time points measured (Niimi and Takahashi, 2014). Even with vigilant husbandry, the microbiome of mouse strains can differ dramatically between institutions (Alegre, 2019). We therefore hypothesized that these observed differences were likely due to environmental factors, such as the microbiome.
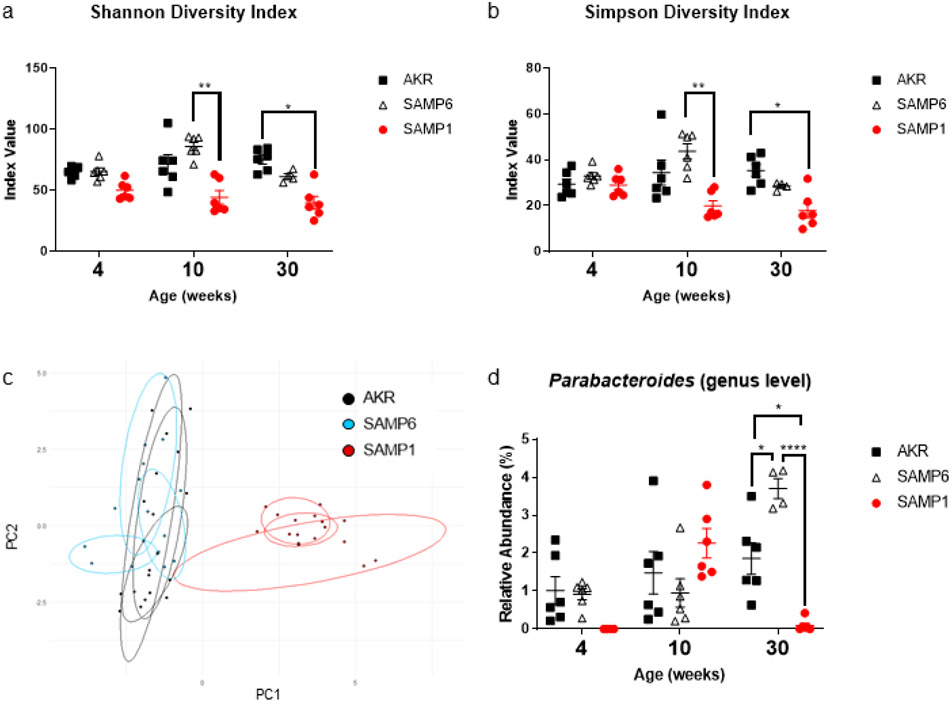
To test the potential impact of the microbiome on depressive-like behavior, we performed 16s rRNA analysis of fecal samples. SAMP1 mice had decreased richness and evenness as measured by Shannon (Fig. 2c) and Simpson (Fig. 2d) diversity indices. Inflammatory states are well recognized to be associated with diminished richness and evenness in the microbiome (Basson et al., 2016; Boulangé et al., 2016). Principal component analysis (PCA) of the overall composition of the microbiome demonstrated that the microbiomes of the SAMP6 and AKR mice were largely similar when compared to that of the SAMP1 mice (Fig. 2b). This observation is consistent with our previous findings that show the microbiome of SAMP1 mice is distinct and more often associated with inflammatory states (Basson et al., 2019).
Figure 2. Depressive-like behavior in SAMP6 mice is associated with expansion of Parabacteroides.
16s rRNA analysis of the fecal microbiome revealed some changes in the richness and evenness of the samples as measured by the (a) Shannon and (b) Simpson diversity indices. (c) Principal component analysis (PCA) of the fecal microbiomes over time. Principal component 1 and 2 shown here accounting for ~39% of explained variance. (d) 16s rRNA measured relative abundance of Parabacteroides. Pairwise Wilcoxon Rank Sum tests performed to analyze data; bars indicate mean ± S.E.M. (*p < 0.05, **p < 0.01, and ****p < 0.0001).
16s rRNA analysis of fecal samples also revealed that SAMP6 mice had an expansion of the genus Parabacteroides at 30 weeks, which coincided with the onset of depressive-like behavior (Fig 2a). 16s rRNA sequencing also revealed that Parabacteroides was present at all time points in AKR mice, our positive control for depressive-like behavior. In SAMP1 mice, which did not exhibit depressive-like behavior, Parabacteroides was increased at 10 weeks only. Importantly, the relative abundance of Parabacteroides observed in the 10-week old SAMP1 mice was comparable to the abundance of Parabacteroides in SAMP6 mice at earlier time points (Fig 2d). Parabacteroides has been previously shown to be abundant in patients with MDD (Jiang et al., 2015). In addition, the species Parabacteroides distasonis has been identified in two intestinal niches specific to CD: cavernous fistulae and creeping mesenteric fat (Ha et al., 2020; Yang et al., 2019). Interestingly, P. distasonis is capable of synthesizing GABA, a neurotransmitter known to influence the pathogenesis of MDD (Luscher et al., 2011; Olson et al., 2018; Strandwitz et al., 2018). We therefore reasoned that P. distasonis may be capable of inducing depressive-like behavior.
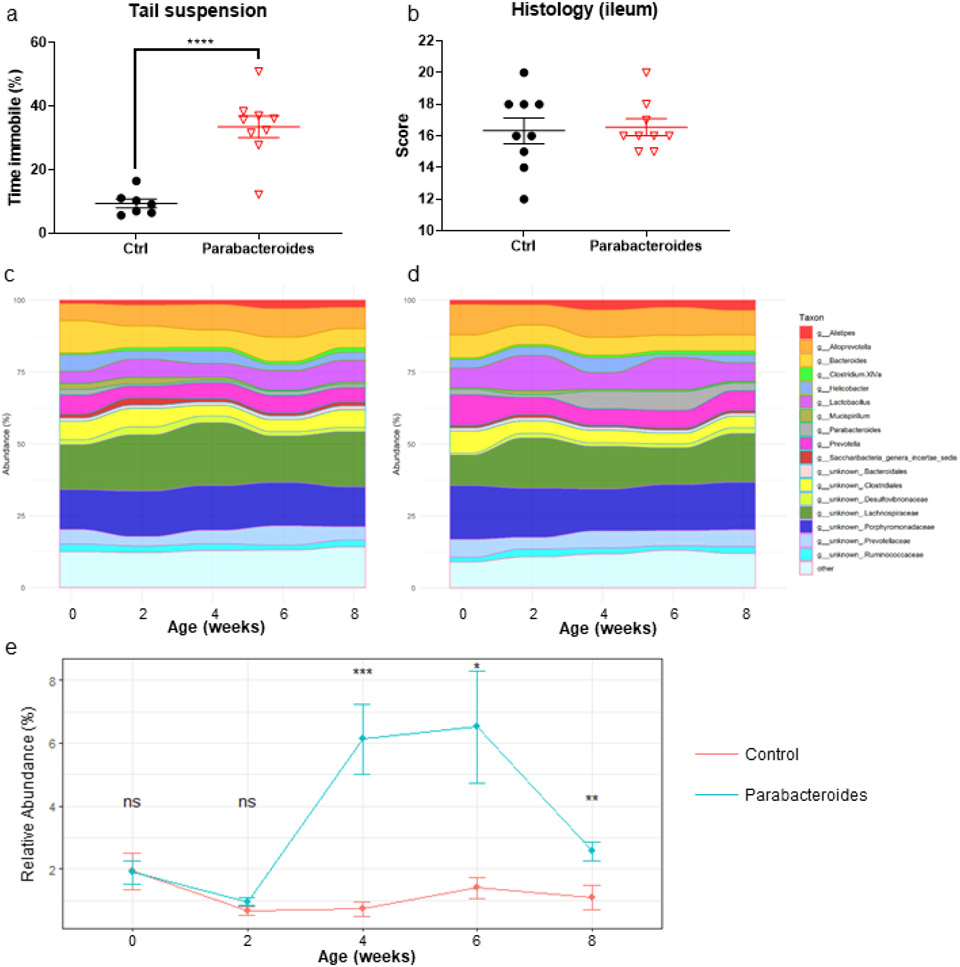
To determine if P. distasonis is sufficient to induce depressive-like behavior, we inoculated SPF SAMP1 mice with Parabacteroides distasonis ATCC®8503™ via oral gavage. Mice were gavaged with 106 CFUs of P. distasonis versus sham (PBS + 5% DMSO) once per week for 3 weeks (3 total gavages) after fecal homogenization. Behavioral analyses were performed 4 weeks after the final inoculation (week 7) and tissue collection was performed the following week (week 8). SAMP1 mice inoculated with P. distasonis did not exhibit anxiety-like behavior, locomotor deficits, or spatial memory deficits (supplemental figure 3a-e). However, administration of P. distasonis did result in the development of depressive-like behavior (Fig 3a). Importantly, intestinal inflammation was unchanged (Fig 3b) implying that the altered behavior was not due to chronic inflammatory illness or ‘sickness behavior’ (Holmes and Miller, 1963).
Figure 3. Inoculation with Parabacteroides distasonis induces depressive-like behavior in SAMP1 mice.
(a) Administration of P. distasonis did increase time immobile during tail suspension (b) but did not worsen ileitis. Alluvial plot of 16s rRNA data for (c) sham and (d) P. distasonis gavaged groups reveals largely similar microbiomes. (e) Relative abundance of Parabacteroides in sham versus inoculated group. Tail suspension was analyzed using student’s t-test, histology was analyzed with Mann-Whitney test, and relative abundance was measured using Wilcoxon Rank Sum test. Symbols represent individual mice (n=10) from two technical replicates, bars indicate mean ± S.E.M. (n.s. p > 0.05, *p < 0.05, **p < 0.01, ***p< 0.001 and ****p < 0.0001).
At all time points following inoculation, apart from Parabacteroides, bacterial genera were largely equal between sham (Fig. 3c) and inoculated group (Fig 3d). As expected, Parabacteroides was consistently elevated after inoculation in comparison with the sham group (Fig 3e). Luminal GABA levels, measured from collected stool, was not significantly elevated (supplemental figure 3f). However, given the lack of any other consistently altered bacteria (supplemental figure 4) and the persistent elevation of Parabacteroides, it seems more likely that the observed behavioral changes are a result of direct action from Parabacteroides.
4. Conclusion
Here we have identified a candidate for microbially-induced depression in a mouse model of CD-like ileitis. To our knowledge, this is the first demonstration of bacterially-induced depression from a specific strain. Remarkably, despite profound inflammation, our mouse model does not exhibit spontaneous anxiety or depressive-like behaviors. It is uncommon to find an animal model of significant, persistent inflammation that does not result in behavioral deficits. Given the well-established connection between inflammation and psychological symptoms (Dantzer, 2009), these findings provide an important platform for future studies into the mechanisms by which behavioral symptoms and inflammation are related.
Our findings with P. distasonis are amplified by previous reports that P. distasonis specifically colonizes two CD-specific intestinal niches: cavernous fistulae and ‘creeping fat’. It has been reported that cavernous fistulae have a distinct microbiome, which includes P. distasonis (Rodriguez-Palacios et al., 2015; Yang et al., 2019). ‘Creeping fat,’ a phenomenon only found in CD patients, also has a distinct microbiome that includes P. distasonis. Importantly, P. distasonis was not identified in the adipose tissue of ulcerative colitis patients. (Ha et al., 2020) Further corroborating our findings, previous work has shown that Parabacteroides, and other GABA producing bacteria, are present in human patients with MDD (Barandouzi et al., 2020). While luminal GABA was not significantly altered upon inoculation with P. distasonis, it is still possible that the GABA from P. distasonis communicates with the vagus nerve, instead of entering the lumen. Alternatively, P. distasonis may induce depressive-like behavior through activation of Toll-like receptors (TLRs) within the nervous system (Gárate et al., 2011; Hines et al., 2013).
In conclusion we have identified a candidate for a microbially-induced depressive phenotype. Given the presence of P. distasonis within two previously identified CD-specific intestinal niches, as well as human MDD patients, we propose a model in which P. distasonis opportunistically colonizes the intestinal niches of susceptible CD patients and exerts depressogenic effects. The proposition that an antibiotic may one day treat patients with MDD is an exciting one to consider.
Supplementary Material
Highlights.
SAMP1/YitFc, a model of Crohn’s disease, exhibits no behavioral abnormalities at baseline.
SAMP1/YitFc can be used for future studies of stress and inflammation.
SAMP6 mice display depressive-like behavior by 30 weeks.
Parabacteroides increases concurrently with depressive-like behavior in SAMP6 mice.
Parabacteroides distasonis administration induces depressive-like behavior in SAMP1.
5. Acknowledgments
This work was supported by National Institutes of Health Grants DK042191, DK055812, and DK091222 awarded to Fabio Cominelli and DK122695 awarded to Adrian Gomez-Nguyen. We also acknowledge the Mouse Models Core and the Histology/Imaging Core of the Cleveland Digestive Disease Research Core Center (DK097948). We also thank Gina Ponzani and Dr. Minh Lam for their assistance, Valeriy Poroyko for his data analysis, and Drs. Zengbiao Li and Qibo Zhang at Drumetix Laboratories for their GABA quantification.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- Alegre M-L, 2019. Mouse microbiomes: overlooked culprits of experimental variability. Genome Biol. 20, 108. 10.1186/s13059-019-1723-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barandouzi ZA, Starkweather AR, Henderson WA, Gyamfi A, Cong XS, 2020. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front. Psychiatry 11, 541. 10.3389/fpsyt.2020.00541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson A, Trotter A, Rodriguez-Palacios A, Cominelli F, 2016. Mucosal Interactions between Genetics, Diet, and Microbiome in Inflammatory Bowel Disease. Front. Immunol 7, 290. 10.3389/fimmu.2016.00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson AR, Gomez-Nguyen A, Menghini P, Buttó LF, Di Martino L, Aladyshkina N, Osme A, LaSalla A, Fischer D, Ezeji JC, Erkkila HL, Brennan CJ, Lam M, Rodriguez-Palacios A, Cominelli F, 2019. Human Gut Microbiome Transplantation in Ileitis Prone Mice: A Tool for the Functional Characterization of the Microbiota in Inflammatory Bowel Disease Patients. Inflamm. Bowel Dis. 26, 347–359. 10.1093/ibd/izz242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein CN, Singh S, Graff LA, Walker JR, Miller N, Cheang M, 2010. A Prospective Population-Based Study of Triggers of Symptomatic Flares in IBD. Am. J. Gastroenterol 105, 1994–2002. 10.1038/ajg.2010.140 [DOI] [PubMed] [Google Scholar]
- Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas M-E, 2016. Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med. 8, 42. 10.1186/s13073-016-0303-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF, 2011. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U. S. A 108, 16050–16055. 10.1073/pnas.1102999108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P, 2012. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol 10, 735–42. 10.1038/nrmicro2876 [DOI] [PubMed] [Google Scholar]
- Colombel J-F, 2015. Targeting the Preclinical Phase of Inflammatory Bowel Disease. Gastroenterol. Hepatol 11, 711–713. [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, 2009. Cytokine, Sickness Behavior, and Depression. Immunol. Allergy Clin. North Am 29, 247–264. 10.1016/j.iac.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma G, Lynch MD, Lu J, Dang VT, Deng Y, Jury J, Umeh G, Miranda PM, Pigrau Pastor M, Sidani S, Pinto-Sanchez MI, Philip V, McLean PG, Hagelsieb MG, Surette MG, Bergonzelli GE, Verdu EF, Britz-McKibbin P, Neufeld JD, Collins SM, Bercik P, 2017. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med 9. 10.1126/scitranslmed.aaf6397 [DOI] [PubMed] [Google Scholar]
- Gárate I, García-Bueno B, Madrigal JL, Bravo L, Berrocoso E, Caso JR, Micó JA, Leza JC, 2011. Origin and consequences of brain Toll-like receptor 4 pathway stimulation in an experimental model of depression. J. Neuroinflammation 8, 151. 10.1186/1742-2094-8-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracie DJ, Guthrie EA, Hamlin PJ, Ford AC, 2018. Bi-directionality of Brain–Gut Interactions in Patients With Inflammatory Bowel Disease. Gastroenterology 154, 1635–1646.e3. 10.1053/j.gastro.2018.01.027 [DOI] [PubMed] [Google Scholar]
- Gracie DJ, Williams CJM, Sood R, Mumtaz S, Bholah MH, Hamlin PJ, Ford AC, 2017. Negative Effects on Psychological Health and Quality of Life of Genuine Irritable Bowel Syndrome–type Symptoms in Patients With Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol 15, 376–384.e5. 10.1016/j.cgh.2016.05.012 [DOI] [PubMed] [Google Scholar]
- Ha CWY, Martin A, Sepich-Poore GD, Shi B, Wang Y, Gouin K, Humphrey G, Sanders K, Ratnayake Y, Chan KSL, Hendrick G, Caldera JR, Arias C, Moskowitz JE, Ho Sui SJ, Yang S, Underhill D, Brady MJ, Knott S, Kaihara K, Steinbaugh MJ, Li H, McGovern DPB, Knight R, Fleshner P, Devkota S, 2020. Translocation of Viable Gut Microbiota to Mesenteric Adipose Drives Formation of Creeping Fat in Humans. Cell 183, 666–683.e17. 10.1016/j.cell.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines DJ, Choi HB, Hines RM, Phillips AG, MacVicar BA, 2013. Prevention of LPS-Induced Microglia Activation, Cytokine Production and Sickness Behavior with TLR4 Receptor Interfering Peptides. PLOS ONE 8, e60388. 10.1371/journal.pone.0060388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes JE, Miller NE, 1963. EFFECTS OF BACTERIAL ENDOTOXIN ON WATER INTAKE, FOOD INTAKE, AND BODY TEMPERATURE IN THE ALBINO RAT. J. Exp. Med 118, 649–658. 10.1084/jem.118.4.649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B, 2015. Altered fecal microbiota composition in patients with major depressive disorder. Brain. Behav. Immun 48, 186–194. 10.1016/j.bbi.2015.03.016 [DOI] [PubMed] [Google Scholar]
- Kochar B, Barnes EL, Long MD, Cushing KC, Galanko J, Martin CF, Raffals LE, Sandler RS, 2017. Depression Is Associated With More Aggressive Inflammatory Bowel Disease. Am. J. Gastroenterol 113, 80. 10.1038/ajg.2017.423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagkouvardos I, Fischer S, Kumar N, Clavel T, 2017. Rhea: a transparent and modular R pipeline for microbial profiling based on 16S rRNA gene amplicons. PeerJ 5, e2836. 10.7717/peerj.2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N, 2011. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry 16, 383–406. 10.1038/mp.2010.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi K, Takahashi E, 2014. Characterization of Senescence-Accelerated Mouse Prone 6 (SAMP6) as an Animal Model for Brain Research. Exp. Anim 63, 1–9. 10.1538/expanim.63.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina EM, Skrinskaya JA, Popova NK, 1991. Role of genotype and dopamine receptors in behaviour of inbred mice in a forced swimming test. Psychopharmacology (Berl.) 105, 525–529. 10.1007/BF02244374 [DOI] [PubMed] [Google Scholar]
- Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY, 2018. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 173, 1728–1741.e13. 10.1016/j.cell.2018.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten SB, Beck CA, Kassam A, Williams JV, Barbui C, Metz LM, 2005. Long-Term Medical Conditions and Major Depression: Strength of Association for Specific Conditions in the General Population. Can. J. Psychiatry 50, 195–202. 10.1177/070674370505000402 [DOI] [PubMed] [Google Scholar]
- Pizarro TT, Pastorelli L, Bamias G, Garg RR, Reuter BK, Mercado JR, Chieppa M, Arseneau KO, Ley K, Cominelli F, 2011. The SAMP1/YitFc Mouse Strain: A Spontaneous Model of Crohn’s Disease-Like Ileitis. Inflamm. Bowel Dis 17, 2566–2584. 10.1002/ibd.21638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Palacios A, Aladyshkina N, Ezeji JC, Erkkila HL, Conger M, Ward J, Webster J, Cominelli F, 2018. ‘Cyclical Bias’ in Microbiome Research Revealed by A Portable Germ-Free Housing System Using Nested Isolation. Sci. Rep 8. 10.1038/s41598-018-20742-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Palacios A, Kodani T, Kaydo L, Pietropaoli D, Corridoni D, Howell S, Katz J, Xin W, Pizarro TT, Cominelli F, 2015. Stereomicroscopic 3D-pattern profiling of murine and human intestinal inflammation reveals unique structural phenotypes. Nat. Commun 6, 7577. 10.1038/ncomms8577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandwitz P, 2018. Neurotransmitter modulation by the gut microbiota. Brain Res. 1693, 128–133. 10.1016/j.brainres.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, McDonald D, Dietrich D, Ramadhar TR, Lekbua A, Mroue N, Liston C, Stewart EJ, Dubin MJ, Zengler K, Knight R, Gilbert JA, Clardy J, Lewis K, 2018. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol 10.1038/s41564-018-0307-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles-Colomer M, Falony G, Darzi Y, Tigchelaar EF, Wang J, Tito RY, Schiweck C, Kurilshikov A, Joossens M, Wijmenga C, Claes S, Van Oudenhove L, Zhernakova A, Vieira-Silva S, Raes J, 2019. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol, 10.1038/s41564-018-0337-x [DOI] [PubMed] [Google Scholar]
- Yang F, Kumar A, Davenport KW, Kelliher JM, Ezeji JC, Good CE, Jacobs MR, Conger M, West G, Fiocchi C, Cominelli F, Dichosa AEK, Rodriguez-Palacios A, 2019. Complete Genome Sequence of a Parabacteroides distasonis Strain (CavFT hAR46) Isolated from a Gut Wall-Cavitating Microlesion in a Patient with Severe Crohn’s Disease. Microbiol. Resour. Announc 8, e00585–19. 10.1128/MRA.00585-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.