Abstract
Light and electron microscopy were used to describe the mode of penetration by the entomopathogenic fungus Beauveria bassiana (Balsamo) Vuillemin into corn, Zea mays L. After inoculation with a foliar spray of conidia, germinating hyphae grew randomly across the leaf surface. Often a germ tube formed from a conidium and elongated only a short distance before terminating its growth. Not all developing hyphae on the leaf surface penetrated the cuticle. However, when penetration did occur, the penetration site(s) was randomly located, indicating that B. bassiana does not require specific topographic signals at an appropriate entry site as do some phytopathogenic fungi. Long hyphal structures were observed to follow the leaf apoplast in any direction from the point of penetration. A few hyphae were observed within xylem elements. Because vascular bundles are interconnected throughout the corn plant, this may explain how B. bassiana travels within the plant and ultimately provides overall insecticidal protection. Virulency bioassays demonstrate that B. bassiana does not lose virulence toward the European corn borer, Ostrinia nubilalis (Hübner), once it colonizes corn. This endophytic relationship between an entomopathogenic fungus and a plant suggests possibilities for biological control, including the use of indigenous fungal inocula as insecticides.
The fungus Beauveria bassiana (Balsamo) Vuillemin (Deuteromycotina: Hyphomycetes) has been reported as a suppressive agent for several insect species worldwide (1, 15). The use of biological control with microorganisms, including fungi, against plant pests and diseases has been the major content of several reviews (9, 15, 40). In North America, B. bassiana is a natural agent of control for the European corn borer, Ostrinia nubilalis (Hübner) (1, 10), a serious pest of corn, Zea mays L. The potential for suppression of O. nubilalis larvae by B. bassiana persisting on the phylloplane or within the corn plant has been proposed (24). Bing and Lewis (4–6, 23) showed that B. bassiana, applied to whorl-stage corn by foliar application or injection, colonized, translocated, and persisted in corn plants to provide season-long suppression of O. nubilalis (26). Field studies have demonstrated that B. bassiana forms an endophyte with corn, but the potential insecticidal application of this relationship and the method of colonization of corn by B. bassiana have not been investigated. Thus, in the present study, we used light microscopic (LM) and electron microscopic techniques to elucidate penetration and colonization of corn by B. bassiana.
MATERIALS AND METHODS
The first two leaves from germinated corn seeds of the inbred cultivar B73 were inoculated with B. bassiana conidia. This inbred cultivar is susceptible to whorl, leaf sheath, and collar feeding by O. nubilalis (18). Plants were grown in environmental chambers (12-h light/dark cycle; 80% rH) located in the Department of Botany, Bessey Hall, Iowa State University, Ames. The micrographs are representative of approximately 25 leaf samples.
The B. bassiana strain (ARSEF 3113; USDA-ARS Entomopathogenic Fungi Collection, Ithaca, N.Y.) was originally isolated from the soil on an Iowa State University research farm in Ankeny and is virulent to O. nubilalis. Fungal cultures, started from dry conidia, were grown on Sabouraud's dextrose agar in the dark at 22°C. Approximately every 6 weeks, dry conidia were transferred to fresh media and allowed to grow to maturity. Every 6 months, the fungal culture was passed back through O. nubilalis to verify virulency. Conidia were either hand brushed onto the leaf surfaces or applied by spraying of a conidial solution (final concentration, 104 spores/ml) by an atomizer. At 12- to 48-h intervals following inoculation, leaves were chosen for microscopic examination.
For LM and transmission electron microscopy (TEM), young leaves inoculated with B. bassiana conidia were selected, cut into small pieces, and initially fixed in 2.5% glutaraldehyde–2% paraformaldehyde in phosphate buffer (0.1 M; pH 7.2) for 2 h at 22 to 24°C. Subsequently, the leaves were fixed in a fresh fixative solution (for 12 to 24 h) at 4°C. Specimens were rinsed in three changes of buffer, 10 ml each, and then postfixed in 1% OsO4 for 2 h. The leaves were washed twice in distilled water for 30 min, dehydrated in a graded ethanol series, and immersed in a transition fluid of propylene oxide or acetone. Specimens were infiltrated with an epoxy resin (37) according to the manufacturers' instructions for a hard block, cast in aluminum weighing pans, and placed in a 60°C oven overnight.
Sections for LM were cut at 2 μm with glass knives on a Reichert Ultracut E or S ultramicrotome. Sections of ∼70 to 85 nm were cut with a diamond knife on a Reichert Ultracut E or S ultramicrotome and collected on 200-mesh copper grids. Sections were stained with aqueous uranyl acetate (39), followed by lead citrate (31), and viewed on a JEOL 1200EX scanning TEM (SEM) at an 80-kV accelerating voltage.
Specimens for conventional SEM were fixed according to the preceding procedure and then dehydrated in an ethanol series to 100% ethanol. Leaves were critical point dried in a DCP-1 critical drying unit (Denton Vacuum Inc., Cherry Hill, N.J.) with CO2. Specimens were coated with gold-palladium (20:80) in a Polaron E5100 sputter coating unit. Photographs were taken with a JEOL JSM-35 SEM at 20 kV.
For viewing of leaf surfaces with the wax layer intact, leaf samples previously inoculated and incubated with aerial conidia or conidia in a solution were dissected from the corn plant, immediately frozen in liquid nitrogen slush with an EMSCOPE SP2000A cryounit, and subsequently viewed in the SEM. Cleared leaves were stained with the fluorescent dye calcofluor white MR2 (27) for observation of the fungal cell walls. The fungal hyphae on the leaf surfaces were stained with fluorescent dyes. Excitation wavelengths of 340 to 380 nm and a barrier filter (430 nm) were used for photography.
Isolate ARSEF 3113 and an isolate from the corn stem proper were grown on Sabouraud's dextrose agar to produce dry conidia for a comparison of virulence to O. nubilalis before and after colonization. Diapausing fifth-instar O. nubilalis organisms from a laboratory colony were contaminated with dry conidia and then placed on a laboratory diet in 19-ml plastic cups (25). Four replicates with 30 larvae each were prepared. Cups with larvae were held at 27°C and 70% rH until a mycosis developed or until insects pupated or died without evidence of a mycosis. Insects not showing a mycosis were dissected and examined for hyphae. Data were subjected to analysis of variance (MSTAT microcomputer statistical program; Michigan State University, East Lansing, Mich.), and differences between means were separated with the Student t test (JMP statistics and graphic guide, version 3.1; SAS Institute, Inc., Cary, N.C.).
RESULTS
Germination of dry conidia occurs after rehydration on the corn leaf surface. Germ tubes gradually elongated into hyphae and randomly spread across the leaf epidermal cells (Fig. 1A). Figure 1B and C illustrate early germination and the formation of a germ tube from a single conidium. Penetration sites were observed with both conventionally prepared and cryoprepared specimens. Some hyphae penetrated the epidermal cell layer. Often a germ tube elongated only a short distance before terminating its growth and penetrating the leaf surface (Fig. 1D). A higher-magnification view of a penetration site shows the hyphae penetrating the epidermis between two cells (Fig. 1E).
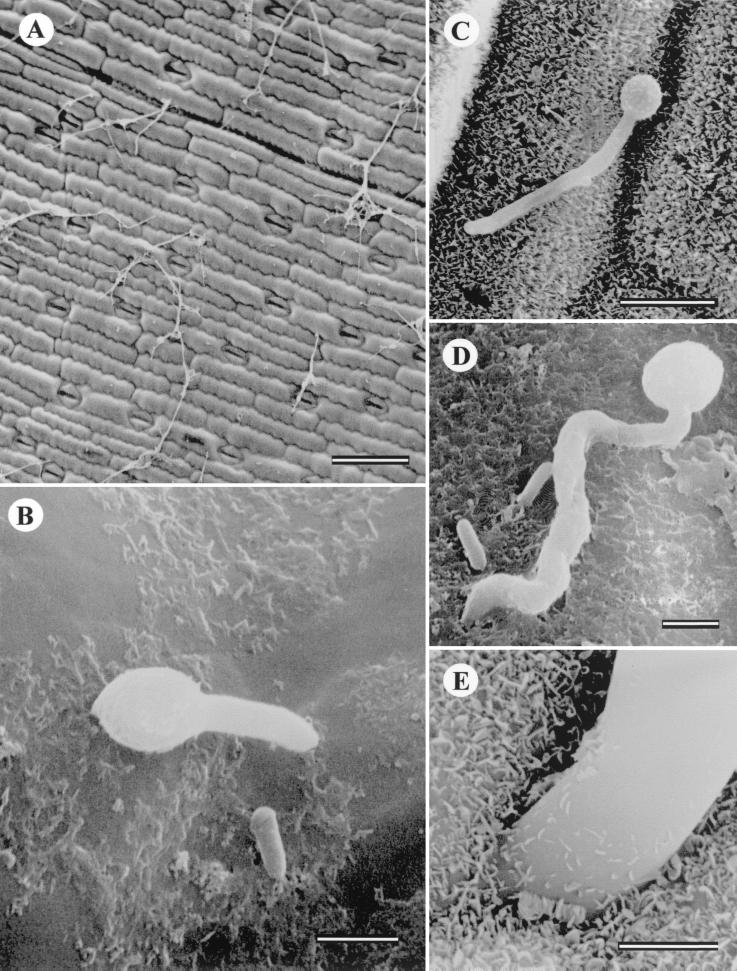
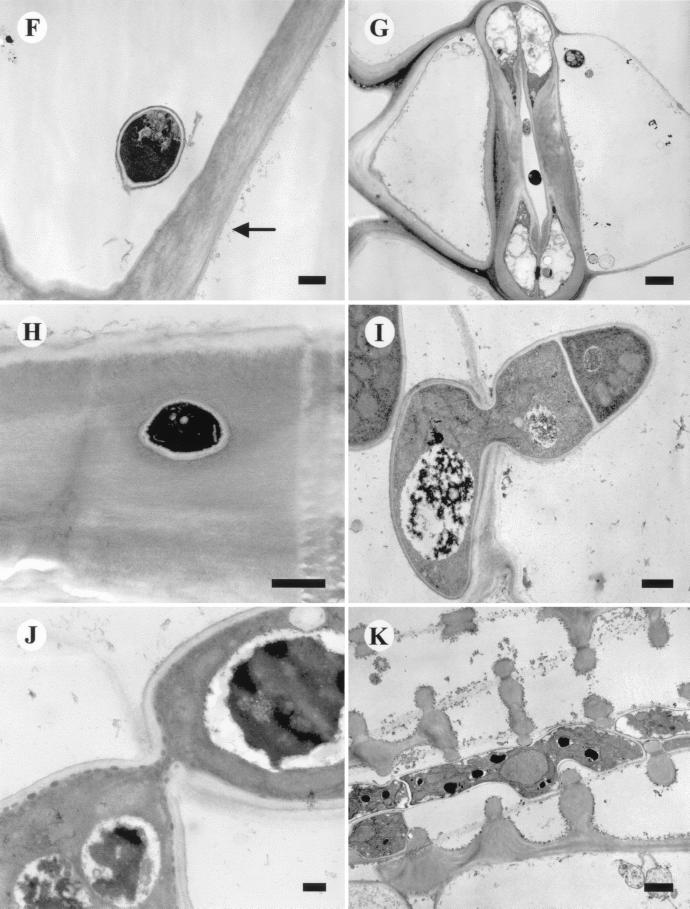
FIG. 1.
(A) Leaf surface and growing hyphae. Bar, 100 μm. (B) Conventionally prepared leaf with conidia and developing germ tube. Bar, 2 μm. (C) Conventionally prepared leaf with elongating germ tube. Bar, 10 μm. (D) Germinating conidia with short germ tube and penetration site. Bar, 2 μm. (E) Frozen preparation illustrating hyphal penetration site at cell wall junction and waxy surface of leaf cuticle. Bar, 1 μm. (F) TEM section parallel to leaf surface showing conidium inside epidermal cell. Arrow indicates epidermal leaf surface (wax cuticular layer has been removed during processing). Bar, 500 nm. (G) Cross section parallel to leaf surface illustrating stomata with invading hyphae. Bar, 2 μm. (H) Perpendicular section of epidermal cell wall showing cross section of hyphae within wall. Epidermal cell surface at top. Bar, 500 nm. (I) Penetration site showing hypha moving through cell wall. Bar, 500 nm. (J) Penetration site showing thin hyphal neck and fungal cytoplasm. Bar, 200 nm. (K) Hyphae in corn leaf xylem vessel. Bar, 1 μm.
Epidermal cells of corn have very dense walls, a large central vacuole, and typically little or no cytoplasm. Conventional preparation techniques remove the surface wax, and the conidia on the leaf surface appear to be separated from the cell wall by a small distance (Fig. 1F). It is important to ascertain the number of conidia actually germinating and penetrating the corn leaf surface. An experiment on infection efficiency was conducted to count the number of conidia placed on the leaf surface and the number germinating and also to record the percentage of developing hyphae actually penetrating the surface. A 20-μl droplet containing on average 35 conidia was applied to the abaxial surfaces of the leaves. The numbers of germinating and penetrating conidia were measured after 48 h. Approximately 3% of the conidia germinated, and less than 1% of these penetrated the leaf surface directly. These results mirror the many observations with LM and SEM, where although numerous conidia were observed on the leaf surface, very few actually germinated and fewer still penetrated the surface. It was rare to observe hyphae growing into the stomates. Hyphae may gain access to the leaf interior through stomatal openings (Fig. 1G). The typical method of invasion is directly through the epidermal cell wall and into the leaf interior, most often at the junction of two epidermal cells. Figure 1H illustrates the complex ultrastructure of a corn leaf epidermal cell wall around a penetration hole formed by a B. bassiana hypha. All penetration sites observed had a narrow neck region, and distortion of the plant cell wall cuticular ultrastructure was visible. Penetration of the epidermal cell wall shows that the plant cell wall is completely breached and the hyphae grow through the hole (Fig. 1I and J). Examination of hyphae inside the leaf shows that they grow through the air spaces between parenchyma cells. No instances of haustorium formation were observed during this study.
B. bassiana hyphae also were observed in the xylem vessels with TEM (Fig. 1K). Following this continuous path throughout the plant, hyphae may travel the length of the plant and invade other areas not accessible from the primary inoculation point. B. bassiana may be able to grow from the leaf to the stalk via the vascular tissues and, therefore, colonize the entire plant.
Virulence of B. bassiana against O. nubilalis was evaluated prior to the inoculation of the corn leaves by exposing fifth-instar O. nubilalis to unlimited numbers of conidia and rearing the larvae in the laboratory. The mortality rate for fifth-instar O. nubilalis was greater for larvae that were exposed to the original soil isolate of B. bassiana (25.7%) before colonization than for larvae exposed to the isolate from corn stems (18.2%) after colonization. These differences, however, were not statistically significant (F = 1.41; df = 1, 6; P = 0.28).
DISCUSSION
Nicholson and Epstein (28) stated that attachment of a fungus to a host plant cuticle is essential for penetration, development, and successful infection. Many fungal pathogens of plants and insects produce dry, windborne conidia that must attach to the hydrophobic outer surfaces of their intended host before germination and colonization. With B. bassiana, conidia are deposited on the leaf surface, and germination may follow two characteristic forms: relatively short hyphal growth followed by penetration or extensive mycelial growth and branching, which may or may not terminate in penetration sites. In the present study, after initial attachment, the shrunken and dry conidia imbibed moisture over a 24- to 48-h period. At the second stage of development (swollen conidia), no adhesive material was observed attaching the conidia to the corn cuticular surface. The final stage of conidia development is delineated by the emergence of the germ tube. Stomata-penetrating fungi represent a group of phytopathogens that have evolved extremely sensitive and precise mechanisms (thigmotropism and thigmodifferentiation) for perceiving the correct site at which to develop infection structures. The most notable morphological requirement described to date is that of the height of the guard cell lip of a bean, which triggers the initiation of appressorium formation in the rust fungus Uromyces appendiculatus (Pers.:Pers.) Unger (17, 41). Most phytopathogenic fungi gain access into their host by penetration of unwounded tissue, although some, such as rusts, invade the host via stomata (11). In contrast, data from this study suggest that B. bassiana does not require precise orientation, as do rust fungi, to invade a corn plant. Instead, B. bassiana hyphae grow randomly across the surfaces of corn leaves. However, if a natural opening (e.g., stomata) is encountered, B. bassiana may enter and invade the plant. Infection sites are indicated by these apparently terminal hyphae.
B. bassiana exhibited no appressorium-like structures at the penetration site on corn leaves in this study. Furthermore, no evidence was seen of a host response to these penetration sites. Pekrul and Grula (29) also depicted direct penetration by B. bassiana of the integument of a corn earworm, Helicoverpa zea (Boddie), without appressorial formation. However, B. bassiana has been shown to form appressoria on the cuticles of some host insects, such as O. nubilalis, Melolontha melolontha L. (21), and Leptinotarsa decemlineata (Say) (12, 38). B. bassiana penetrates directly through the corn leaf with the infection hyphae sharply constricted in the passage area through the cuticle, suggesting that penetration occurs through a small hole produced, in part, by enzymatic activity. Penetration of the plant cuticle may be aided by the mechanical force exerted by the infection structure (3) or may require the enzymatic dissolution of the cuticle, or a combination of both (14, 19). Some evidence for both models has been based on ultrastructural observations (20).
Although the histopathology of B. bassiana on insects has been investigated for more than a century, it is only recently that the underlying mechanisms involved have begun to be understood, primarily because of electron microscopy studies of surface-inoculated material (21). Infection by B. bassiana has been shown to require direct penetration of the insect host integument by growing hyphae, apparently facilitated by both mechanical and enzymatic activity (22, 29, 30, 38). Several studies have shown that a spectrum of enzymatic activities against the insect cuticle are detected in conidial preparations of B. bassiana (8, 16, 29, 32–36). Potentially, the primary function of many of the enzymes associated with conidia, including those of B. bassiana, is to hydrolyze the epicuticular wax layer and provide nutrients required for germ tube formation. The disappearance of the wax layer beneath appressoria of Metarhizium anisopliae on the wireworm cuticle indicates enzymatic activity (42), as do the circular holes around germ tubes of B. bassiana at the point of entry into H. zea larvae (29). Distortions of the corn cell wall around B. bassiana penetration sites suggest similar enzymatic activity (Fig. 1H and I).
After B. bassiana penetrates corn, the primary hyphae develop rapidly into a branched, multicellular mycelial network. Hyphae may grow directly into neighboring epidermal cells and subtending palisade parenchyma and/or grow into intercellular spaces. No conidial formation or emergence of B. bassiana in or from corn plant tissues was observed. Whether B. bassiana completes a life cycle in the corn plant was not evident, since no conidia formation was observed during this study either within the leaves and stems or on the leaf surfaces.
Studies have shown that when B. bassiana is injected into the corn stem, it colonizes and moves within the plant (4, 6). In these studies, most of the plants were colonized at the injection site and B. bassiana was isolated from the node above the injection site. These data support the idea proposed by Bartlett and Lefebvre (2) that the succulent pith inside corn is ideal for growth of B. bassiana. B. bassiana applied to plants by foliar application or by injection colonized, translocated, and persisted within the plants to provide season-long suppression of O. nubilalis. Perhaps fungal movement within corn can be attributed to passive movement of B. bassiana within the xylem. In this study, B. bassiana hyphae were seen within the vascular elements of the xylem and theoretically could move throughout the plant via the interconnecting xylem tissues to colonize the entire plant. No adverse effects on the corn plants colonized by B. bassiana could be ascertained during this study. Subsequent experiments have confirmed that B. bassiana is not a plant pathogen (Leslie C. Lewis, unpublished data).
Virulency of B. bassiana (measured as the mortality rate of fifth-instar O. nubilalis) did not change significantly once the fungus colonized the corn plant. Percent mortality was 25 and 18, respectively, for the original isolate and one from corn. There is a paucity of data on the dose response of O. nubilalis to isolates of B. bassiana. It is known that first instars are the most susceptible, with percent mortality ranging up to 100 depending on the dosage and the isolate (13). Results of other research on virulency of B. bassiana isolates from corn are reported as percentages of isolates causing mortality of fifth instars, not as percent mortality of the larvae (7).
Classification of entomopathogenic fungi based on morphological characteristics requires further knowledge of virulence and specificity. Electrophoresis and immunoelectrophoresis studies associated with characteristic reactions of enzymatic activity are being used to elucidate the mechanisms involved with host identification, adhesion, and penetration. Understanding this unique relationship will be invaluable in the continued development and use of such fungi to manage insect pests of corn and other food plants. Given the production of well-known extracellular antimicrobial metabolites by B. bassiana, some protection against plant pathogens may exist because of this endophytic relationship, suggesting possibilities for biological control, including the use of indigenous fungal inocula as “symbiotic insecticides.”
ACKNOWLEDGMENTS
We thank the Bessey Microscopy Facility at Iowa State University for use of the electron microscopes and other ancillary equipment and Tracy Pepper for preparing the figure plates.
Footnotes
Joint contribution from the U.S. Department of Agriculture, Agricultural Research Service, and the Iowa Agriculture and Home Economics Experiment Station, Ames, Iowa, Project 3543, journal paper J-17700.
REFERENCES
- 1.Baird R B. The artificial control of insects by means of entomogenous fungi, a compilation of references with abstracts. Nova Scotia, Canada: Canadian Agricultural Research Station, Kentrille; 1958. [Google Scholar]
- 2.Bartlett K A, Lefebvre C L. Field experiments with Beauveria bassiana (Bals.) Vuill., a fungus attacking the European corn borer. J Econ Entomol. 1934;27:1147–1157. [Google Scholar]
- 3.Bidochka M J, Khachatourians C G. The implication of metabolic acids produced by Beauveria bassiana in pathogenesis of the migratory grasshopper, Melanoplus sanguinipes. J Invertebr Pathol. 1991;58:106–117. [Google Scholar]
- 4.Bing L A, Lewis L C. Suppression of Ostrinia nubilalis (Hübner) (Lepidoptera: Pyralidae) by endophytic Beauveria bassiana (Balsamo) Vuillemin. Environ Entomol. 1991;20:1207–1211. [Google Scholar]
- 5.Bing L A, Lewis L C. Endophytic Beauveria bassiana (Balsamo) Vuillemin in corn: the influence of the plant growth stage and Ostrinia nubilalis (Hübner) Biocontrol Sci Technol. 1992;2:39–47. [Google Scholar]
- 6.Bing L A, Lewis L C. Temporal relationships between Zea mays, Ostrinia nubilalis (Lep.: Pyralidae) and endophytic Beauveria bassiana. Entomophaga. 1992;37:525–536. [Google Scholar]
- 7.Bing L A, Lewis L C. Occurrence of the entomopathogen Beauveria bassiana (Balsamo) Vuillemin in different tillage regimes and in Zea mays L. and virulence towards Ostrinia nubilalis (Hübner) Agric Ecosys Environ. 1993;45:147–156. [Google Scholar]
- 8.Boucias D G, Pendland J C. Attachment of mycopathogens to cuticle: the initial event of mycosis in arthropod hosts. In: Cole G T, Hoch H C, editors. The fungal spore and disease initiation in plants and animals. New York, N.Y: Plenum Press; 1991. pp. 101–127. [Google Scholar]
- 9.Burges H D. Strategy for microbial control of pests in 1980 and beyond. In: Burges H D, editor. Microbial control of pests and diseases 1970–1980. New York, N.Y: Academic Press; 1981. pp. 797–836. [Google Scholar]
- 10.Charles V K. A preliminary checklist of the entomogenous fungi of North America. US Dep. Agric., Bur. Entomol. Plant Q., Insect Pest Surv. Bull. 1941;21:707–785. [Google Scholar]
- 11.Epstein L, Laccetti C B, Staples R C, Hoch H C. Cell-substratum adhesive protein involved in surface contact responses of the bean rust fungus. Physiol Mol Plant Pathol. 1987;30:373–388. [Google Scholar]
- 12.Fargues J, Vey A. Modalités d'infection des larves de Leptinotarsa decemlineata par Beauveria bassiana au cours de la mue. Entomophaga. 1974;19:311–323. [Google Scholar]
- 13.Feng Z, Carruthers R I, Roberts D W, Robson D S. Age-specific dose-mortality effects of Beauveria bassiana (Deuteromycotina: Hyphomycetes) on the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae) J Invertebr Pathol. 1985;46:259–264. [Google Scholar]
- 14.Ferron P. Biological control of insect pests by entomogenous fungi. Annu Rev Entomol. 1978;23:409–442. [Google Scholar]
- 15.Gillespie A T. Use of fungi to control pests of agricultural importance. In: Burge M N, editor. Fungi in biological control systems. Manchester, England: Manchester University Press; 1988. pp. 37–60. [Google Scholar]
- 16.Grula E A, Burton R C, Smith R, Mapes T L, Cheung P Y K, Pekrul S, Champlin F R, Grula M, Abegaz B. Biochemical basis for the pathogenicity of Beauveria bassiana. In: Ignoffo C M, editor. Proceedings of the 1st Joint USA/USSR Conference on the Production, Selection, and Standardization of Entomopathogenic Fungi. Washington, D.C.: American Society for Microbiology; 1978. pp. 192–216. [Google Scholar]
- 17.Hoch H C, Staples R C. Signaling for infection structure formation in fungi. In: Cole G T, Hoch H C, editors. The fungal spore and disease initiation in plants and animals. New York, N.Y: Plenum Press; 1991. pp. 25–46. [Google Scholar]
- 18.Kim S, Hallauer A R, Guthrie W D, Barry D, Lamkey K R, Hong C S. Genetic resistance of tropical corn inbreds to second-generation European corn borer (Lepidoptera: Pyralidae) J Econ Entomol. 1989;82:1207–1211. [Google Scholar]
- 19.Kolattukudy P E. Fungal penetration of defensive barriers in plants. In: Dugger W M, Bartnicki-Garcia S, editors. Structure, function, and biosynthesis of plant cell walls. Rockville, Md: American Society of Plant Physiologists; 1984. pp. 31–37. [Google Scholar]
- 20.Köller W. The plant cuticle. In: Cole G T, Hoch H C, editors. The fungal spore and disease initiation in plants and animals. New York, N.Y: Plenum Press; 1991. pp. 219–246. [Google Scholar]
- 21.Lecuona R, Riba G, Cassier P, Clement J L. Alterations of insect epicuticular hydrocarbons during infection with Beauveria bassiana or B. brongniartii. J Invertebr Pathol. 1991;58:10–18. [Google Scholar]
- 22.Lefebvre C L. Penetration and development of the fungus Beauveria bassiana, in the tissues of the corn borer. Ann Bot. 1934;48:441–452. [Google Scholar]
- 23.Lewis L C, Bing L A. Bacillus thuringiensis Berliner and Beauveria bassiana (Balsamo) Vuillemin for European corn borer control: potential for immediate and season-long suppression. Can Entomol. 1991;123:387–393. [Google Scholar]
- 24.Lewis L C, Cossentine J E. Season long interplant epizootics of entomopathogens, Beauveria bassiana and Nosema pyrausta, in a corn agroecosystem. Entomophaga. 1986;31:363–369. [Google Scholar]
- 25.Lewis L C, Lynch R E. Rearing of European corn borer, Ostrinia nubilalis (Hübner), on diets containing corn leaf and wheat germ. Iowa State J Sci. 1969;44:9–14. [Google Scholar]
- 26.Lewis L C, Berry E C, Obrycki J J, Bing L A. Aptness of insecticides (Bacillus thuringiensis and carbofuran) with endophytic Beauveria bassiana, in suppressing larval populations of the European corn borer. Agric Ecosys Environ. 1996;57:27–34. [Google Scholar]
- 27.Monheit J E, Cowan D F, Moore D G. Rapid detection of fungi in tissues using calcofluor white and fluorescence microscopy. Arch Pathol Lab Med. 1984;108:616–618. [PubMed] [Google Scholar]
- 28.Nicholson R L, Epstein L. Adhesion of fungi to the plant surface: prerequisite for pathogenesis. In: Cole G T, Hoch H C, editors. The fungal spore and disease initiation in plants and animals. New York, N.Y: Plenum Press; 1991. pp. 3–23. [Google Scholar]
- 29.Pekrul S, Grula E A. Mode of infection of the corn earworm (Heliothus zea) by Beauveria bassiana as revealed by scanning electron microscopy. J Invertebr Pathol. 1979;34:238–247. [Google Scholar]
- 30.Pendland J C, Boucias D G. Lectin binding characteristics of several entomophagous Hyphomycetes: possible relationship to insect hemagglutinins. Mycologia. 1986;78:818–824. [Google Scholar]
- 31.Reynolds E S. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St. Leger R J, Charnley A K, Cooper R M. Cuticle-degrading enzymes of entomopathogenic fungi: mechanisms of interaction between pathogen enzymes and insect cuticle. J Invertebr Pathol. 1986;47:295–302. [Google Scholar]
- 33.St. Leger R J, Charnley A K, Cooper R M. Cuticle-degrading enzymes of entomopathogenic fungi: synthesis in culture on cuticle. J Invertebr Pathol. 1986;48:85–95. [Google Scholar]
- 34.St. Leger R J, Cooper R M, Charnley A K. Distribution of chymoelastases and trypsin-like enzymes in five species of entomopathogenic Deuteromycetes. Arch Biochem Biophys. 1987;258:121–131. doi: 10.1016/0003-9861(87)90329-8. [DOI] [PubMed] [Google Scholar]
- 35.St. Leger R J, Staples R C, Roberts D W. Entomopathogenic isolates of Metarhizium anisopliae, Beauveria bassiana, and Aspergillus flavus produce multiple extracellular chitinase isozymes. J Invertebr Pathol. 1993;61:81–84. [Google Scholar]
- 36.Samsináková A, Misikova S, Leopold J. Action of enzymatic systems of Beauveria bassiana on the cuticle of the greater wax moth larvae (Galleria melonella) J Invertebr Pathol. 1971;18:322–330. [Google Scholar]
- 37.Spurr A R. A low viscosity epoxy resin embedding medium of electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 38.Vey A, Fargues J. Histological and ultrastructural studies of Beauveria bassiana infection in Leptinotarsa decemlineata larvae during ecdysis. J Invertebr Pathol. 1977;30:207–215. [Google Scholar]
- 39.Watson M C. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958;4:475–479. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Windels C E, Lindow S E, editors. Biological control on the phylloplane. Symposium, book no. 3. St. Paul, Minn: American Phytopathological Society; 1985. [Google Scholar]
- 41.Wynn W K, Staples R C. Tropisms of fungi in host recognition. In: Staples R C, Toenniessen G H, editors. Plant disease control: resistance and susceptibility. New York, N.Y: John Wiley & Sons; 1981. pp. 45–69. [Google Scholar]
- 42.Zacharuk R Y. Fungal diseases of terrestrial insects. In: Davidson E D W, editor. Pathogenesis of invertebrate microbial diseases. Totowa, N.J: Allanheld, Osmun Publishers; 1981. pp. 367–402. [Google Scholar]