Abstract
A long-chain aldehyde dehydrogenase, Ald1, was found in a soluble fraction of Acinetobacter sp. strain M-1 cells grown on n-hexadecane as a sole carbon source. The gene (ald1) was cloned from the chromosomal DNA of the bacterium. The open reading frame of ald1 was 1,512 bp long, corresponding to a protein of 503 amino acid residues (molecular mass, 55,496 Da), and the deduced amino acid sequence showed high similarity to those of various aldehyde dehydrogenases. The ald1 gene was stably expressed in Escherichia coli, and the gene product (recombinant Ald1 [rAld1]) was purified to apparent homogeneity by gel electrophoresis. rAld1 showed enzyme activity toward n-alkanals (C4 to C14), with a preference for longer carbon chains within the tested range; the highest activity was obtained with tetradecanal. The ald1 gene was disrupted by homologous recombination on the Acinetobacter genome. Although the ald1 disruptant (ald1Δ) strain still had the ability to grow on n-hexadecane to some extent, its aldehyde dehydrogenase activity toward n-tetradecanal was reduced to half the level of the wild-type strain. Under nitrogen-limiting conditions, the accumulation of intracellular wax esters in the ald1Δ strain became much lower than that in the wild-type strain. These and other results imply that a soluble long-chain aldehyde dehydrogenase indeed plays important roles both in growth on n-alkane and in wax ester formation in Acinetobacter sp. strain M-1.
The microbial degradation of petrochemicals has attracted much interest due to its potential for the development of bioremediation processes for oil spill environments, as well as for the production of fine and moderately priced chemicals. Acinetobacter spp. are known to utilize long-chain n-alkanes and to accumulate intracellular wax esters (8), which are enveloped by a single membrane (25) and serve as cell reserves. The composition of intracellular wax esters as to carbon chain length or the degree of unsaturation can be controlled by altering the growth substrate or temperature (5, 16, 17). Therefore, Acinetobacter spp. are expected to have great potential for industrial utilization.
Recently, the hydroxylation of n-alkanes, involving rubredoxin and rubredoxin reductase, has been found to be indispensable for n-alkane degradation by Acinetobacter sp. strain ADP1 (9, 19). From these facts, together with this evidence of the existence of a membrane-bound aldehyde dehydrogenase (2, 12, 27), the main pathway of n-alkane oxidation to acyl coenzyme A (acyl-CoA) via carboxylic acid is assumed to proceed through the membrane-bound enzymes in these Acinetobacter strains. This pathway is analogous to that confirmed in the alkBAC operon of Pseudomonas oleovorans (29), although the genetic organization of the alk genes is different from that in Acinetobacter spp. (9, 19). On the other hand, a variety of enzymes that catalyze the oxidation of n-alkanes and related compounds have also been found in soluble fractions of cell extracts of Acinetobacter spp. (1, 11, 7, 30), but their physiological significance in n-alkane metabolism has not been confirmed.
In this study, we found a long-chain aldehyde dehydrogenase, Ald1, in a soluble fraction of Acinetobacter sp. strain M-1 cells. In order to determine the physiological role of Ald1 in Acinetobacter sp. strain M-1, we cloned the Ald1-encoding gene, ald1, and expressed and characterized the recombinant enzyme. From these results together with those of investigation of an ald1 disruptant strain, Ald1 was confirmed to play a significant role in n-alkane utilization, especially in intracellular wax ester synthesis.
MATERIALS AND METHODS
Chemicals and enzymes.
Tetradecanal was purchased from Aldrich Chemical Co., Inc. (Milwaukee, Wis.). Achromobacter lysyl endopeptidase (EC 3.4.21.50) was from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Restriction enzymes, alkaline phosphatase (calf intestine), T4 DNA ligase, and Ex Taq DNA polymerase were products of Takara Shuzo Co. (Kyoto, Japan). A dye deoxy terminator cycle sequencing kit was purchased from Applied Biosystems, Inc. (Foster City, Calif.), and [α-32P]dCTP was from Amersham Corp. (Arlington Heights, Ill.).
Microorganisms, culture conditions, and vectors.
Acinetobacter sp. strain M-1, which utilizes n-alkanes of carbon chain lengths from C13 to C44 as sole carbon sources, was cultured in a salt medium containing an n-alkane as described previously (22). Escherichia coli JM109 (23) was used for gene cloning and expression. E. coli cells are usually grown on 2× yeast-Tryptone (YT) medium (pH 7.0) containing Bacto Yeast extract (10 g/liter), Bacto Tryptone (16 g/liter), and NaCl (5 g/liter), in the presence of ampicillin (10 mg/ml), 1.0 mM isopropyl-β-d-(−)-thiogalactopyranoside, and 0.05 mM 5-bromo-4-chloro-3-indolyl-β-d-galactoside when necessary. pT7Blue (Novagen, Madison, Wis.) was used for the subcloning of PCR products. pBluescript II SK(+) (Toyobo, Osaka, Japan) was used as a cloning vector, pUC118 (Takara Shuzo Co.) was used as an expression vector, and pKT231 (3) was used for construction of the ald1 disruptant.
Analysis.
For growth measurement, Acinetobacter sp. strain M-1 cells were washed with a solvent mixture (ethanol-butanol-chloroform, 10:10:1 [vol/vol]) to remove the residual oily substance and were suspended in 0.85% NaCl; then the optical density at 610 nm (OD610) was measured. The protein concentration was determined with a protein assay kit (Japan Bio-Rad Laboratories, Tokyo, Japan), with bovine serum albumin as the standard. The relative molecular mass of the native enzyme was determined by gel filtration with a Fast Protein Liquid Chromatography system (Pharmacia Biotech, Uppsala, Sweden), using a Superdex 200 column equilibrated with 50 mM Tris-Cl buffer (pH 7.5) containing 0.1 M KCl. The standard protein markers were from Oriental Yeast Co., Ltd. (Tokyo, Japan). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on a 12.5% polyacrylamide gel (13), and Bio-Rad standard proteins (Low Range) were used for molecular mass measurement.
Extraction and analysis of wax esters.
Acinetobacter sp. strain M-1 was cultured on a nitrogen-limiting medium (20) containing n-hexadecane (0.5%, vol/vol). After a 72-h cultivation, the cells were collected by centrifugation, washed three times with 0.85% NaCl, and then resuspended in 10 mM Tris-Cl (pH 8.0) containing 1.0 mM EDTA. The cells were disrupted with zirconia-silica beads (diameter, 0.5 mm; Biospec Products, Bartlesville, Okla.), and then wax esters were extracted with an equal volume of chloroform-methanol (2:1, vol/vol). After centrifugation at 2,000 × g at room temperature for 10 min, the chloroform phase was subjected to gas chromatography (with a GC7-A; Shimadzu, Kyoto, Japan) under the following conditions: glass column (0.5 m by 3.2 mm) packed with Silicone OV-17 (Shimadzu); gas phase, He (50 ml/min); column temperature, 250°C; injection port temperature, 280°C; flame ionization detector temperature, 280°C.
Enzyme assay.
The reaction mixture was composed of 50 mM glycine-NaOH buffer (pH 9.5), 2.5 mM NAD+, and 1.0 mM tetradecanal. Before addition to the mixture, the substrate, i.e., tetradecanal or another insoluble substrate, was homogenized in 50 mM glycine-NaOH buffer containing 3% (wt/wt of substrate) Plysurf A210G (a detergent; Daiichi Kogyo Seiyaku, Tokyo, Japan) by heating in boiling water for 3 min and subsequent sonication at 20 kHz for 1 min. An appropriate quantity of the enzyme was added to initiate the reaction. The mixture without the substrate was used as a reference. One unit of enzyme was defined as the amount of the enzyme that catalyzed the reaction of 1.0 μmol of NAD+ per min at 43°C. Calculations were based on a molar extinction coefficient at 340 nm for NADH of 6.22 × 103.
Aldehyde dehydrogenase activity was localized in situ on native PAGE by the method described by Singer and Finnerty (26), using the reaction mixture described above.
Enzyme purification and amino acid sequence analysis.
All the procedures for enzyme purification were performed at 4°C. An NAD+-dependent long-chain aldehyde dehydrogenase was partially purified from Acinetobacter sp. strain M-1 as described previously (22) and was then further chromatographed on a Resource Q column (Amersham Pharmacia Biotech, Tokyo, Japan). The purified preparation was subjected to SDS-PAGE and then electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Millipore Corp., Bedford, Mass.). The band corresponding to the enzyme was cut out, subjected to fragmentation with lysyl endopeptidase, and then fractionated by high-performance liquid chromatography (HPLC) (33). The amino acid sequence of each fraction was determined by Edman's method with a Perkin-Elmer Protein Sequencer 476A.
Recombinant Ald1 (rAld1) was purified from cells of the transformant E. coli JM109(pCM1) (see below) as follows. The cells were harvested by centrifugation, washed twice with 0.85% NaCl, disintegrated with a Kubota Isonator model 200M at 150 W for 30 min, and then centrifuged at 18,000 × g for 10 min. After further centrifugation at 100,000 × g for 60 min, the clear supernatant was applied to a DEAE-Toyopearl column (Tosoh Co., Tokyo, Japan) equilibrated with buffer A (50 mM Tris-Cl, pH 8.5, containing 0.5 mM EDTA and 2 mM dithiothreitol). The enzyme was eluted with a linear gradient of increasing KCl concentration (0.1 to 0.3 M). The active fractions were collected, dialyzed against buffer A, and then applied to a Q-Sepharose column (Pharmacia Biotech) equilibrated with buffer A. The enzyme was eluted with a linear gradient of increasing KCl concentration (0.1 to 0.5 M). The active fractions were dialyzed against buffer A and then concentrated. The purified preparation, which gave a single protein band on SDS-PAGE and a single activity band on native PAGE, was stored at 0°C until use.
Cloning of the ald1 gene from the genome of Acinetobacter sp. strain M-1.
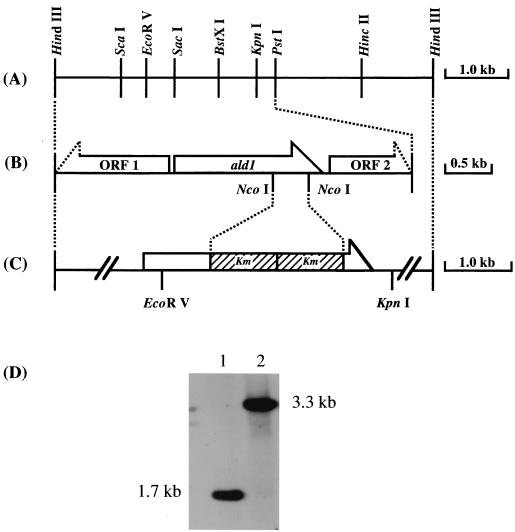
To amplify the fragment of DNA encoding Ald1 from the chromosomal DNA of Acinetobacter sp. strain M-1 by PCR, upstream and downstream primers were designed from the internal amino acid sequences of K-5 and K-12 (see Results). The primers used were K-12N [5′-TT(T/C)AT(A/C/T)GG(A/C/T)GG(A/C/T)CA(A/G)TGGGT-3′] and K-5C [5′-TT(A/G/T/C)GT(T/C)TG(T/C)TG(A/G)TA(A/G)TG(A/G)TC-3′]. The conditions for PCR were principally the same as described by Sakai et al. (21). The PCR product was approximately 1.4 kb in length and was used as a probe. Through Southern hybridization (28) and colony hybridization (21), one positive clone which contained the 5.7-kb HindIII fragment of pBluescript II SK(+) was isolated; this plasmid was named pSH6. A restriction map of pSH6 is shown in Fig. 1A. The 3.5-kb PstI fragment was subcloned from pSH6 into pBluescript II SK(+) and sequenced.
FIG. 1.
Genetic organization of the cloned region including ald1 and that of disrupted ald1. (A) Restriction map of the 5.7-kb HindIII cloned fragment. (B) The ORFs within the sequenced region of the 3.5-kb PstI fragment. ORF1 showed homology with a hypothetical 47.3-kDa protein in the thcA 5′ region (ORF3) from Rhodococcus sp. strain NI86/21 (GenBank accession number U17129) (15), and ORF2 showed homology with ethanolamine permease from Rhodococcus sp. strain NI86/21 (GenBank accession number L24492) (4). (C) Construction of the gene disruption vector pDALD1, derived from pSH6. The hatched box represents the kanamycin resistance gene. (D) Genomic Southern analysis of EcoRV-KpnI double-digested total DNAs (5.0 μg each) from the wild-type strain (lane 1) and the ald1Δ strain (lane 2), with the 32P-labeled ald1 fragment as a probe.
Nucleotide sequencing.
Nucleotide sequencing was performed by the dideoxy chain termination method of Sanger et al. (24) using a DNA Sequencing Kit and an ABI 373A Sequencer (Applied Biosystems, Inc.). The sequencing reaction was performed as described in the manual. The sequence data were analyzed with the BLAST program (by using the GenBank, EMBL, and Swissprot databases).
Expression of the ald1 gene in E. coli JM109.
To obtain the open reading frame (ORF) of the Acinetobacter sp. strain M-1 ald1 gene, PCR primers with an EcoRI site and the ribosomal binding sequence were synthesized as follows: aldN, 5′ - GGAAT TCC TAAGGAGG T T T T TATATGCACTATGT TGATCCGAAT - 3′ (the translation start codon is underlined), and aldC, 5′-GGAATTCCTTAGAAAAAGCCCATGGCTTT-3′. The PCR product was ligated with the pT7Blue vector and then with pUC118. A pUC118-derived plasmid, designated pCM1, was introduced into E. coli JM109. Expression of ald1 in the E. coli transformant cells was confirmed by the activity and protein bands on SDS-PAGE.
Northern hybridization.
Acinetobacter sp. strain M-1 was grown on a salt medium containing various carbon sources, i.e., sodium acetate (1.0%) or an n-alkane (0.5%, vol/vol). The total RNA of the cells in the early-exponential growth phase was obtained by the acid-guanidinium thiocyanate-phenol-chloro-form (AGPC) method using ISOGEN (Nippon Gene Co., Ltd.), and total RNA was electrophoresed on a 0.7% agarose gel in 20 mM morpholinepropanesulfonic acid (MOPS) buffer containing 1.0 mM EDTA and 2.2 M formaldehyde and was then transferred to a nylon membrane filter (Gene Screen Plus; NEN Life Science Products, Boston, Mass.) in 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Hybridization was carried out using AlkPhos DIRECT (Amersham Pharmacia Biotech) according to the manufacturer's protocol, using the pCM1-derived ald1 gene as a probe. rRNA was used as a standard for loaded total RNA and was visualized by ethidium bromide staining.
Transformation method.
E. coli was transformed by the method of Hanahan (10). Acinetobacter sp. strain M-1 was transformed by electroporation using a Gene Pulser II (Bio-Rad Laboratories, Richmond, Calif.) under the following conditions: a 0.1-cm cuvette, 200 Ω, 50 μF, and 16 kV/cm. Competent cells for electroporation were prepared by the method of Leahy (14). After the pulse, 800 μl of prewarmed 2× YT was added immediately, followed by incubation at 30°C for 4 h and then plating.
Construction of the ald1 disruptant.
The kanamycin resistance gene (Km) including its own promoter was amplified by PCR using pKT231 as a template. The primer sequences flanking the NcoI site were as follows: Km-N, CCATGGGGACCAGTTGGTGATTTT, and Km-C, CCATGGTTAGAAAAACTCATCGAGC. The amplified Km gene replaced a 500-bp NcoI fragment within the ald1 gene of pSH6 (Fig. 1C), yielding pDALD1. The ald1 disruption plasmid, pDALD1, was linearized, dephosphorylated, and then introduced into Acinetobacter sp. strain M-1 by electroporation.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper will appear in the DDBJ/EMBL/GenBank nucleotide sequence databases under accession number AB042203.
RESULTS
Nucleotide and deduced amino acid sequences of ald1.
An NAD+-dependent long-chain aldehyde dehydrogenase, which showed activity toward tetradecanal, was purified from a soluble cell-free fraction of n-hexadecane-grown Acinetobacter sp. strain M-1 cells. We obtained the amino acid sequence information on two internal peptide sequences, K-5 (MMLDHYQQTK) and K-12 (DQYENFIGGQWVAPVK), from limited proteolytic digests of the purified enzyme. Based on these amino acid sequences, we synthesized two PCR primers, K-12N and K-5C, as described in Materials and Methods. A DNA fragment of about 1.4 kb was amplified by PCR using Acinetobacter sp. strain M-1 chromosomal DNA as the template with these primers. Through Southern hybridization and colony hybridization selection using the 32P-labeled 1.4-kb PCR-amplified product, one positive clone containing a 5.7-kb HindIII fragment in pBluescript II SK(+) was isolated. The ORF corresponding to Ald1 was found in the 3.5-kb PstI region, and ald1 was not clustered with other genes for enzymes related to n-alkane oxidation (Fig. 1B). This is unlike the placement of the aldehyde dehydrogenase gene (alkH) in the alk operon of the OCT plasmid in P. oleovorans (29). The ald1 gene was composed of 1,512 bp corresponding to 503 amino acid residues with a predicted molecular mass of 55,496 Da. A database search revealed that Ald1 showed considerable similarity to various aldehyde dehydrogenases, despite the differences in their origin and in their physiological and catalytic properties, including, e.g., AcoD of Alcaligenes eutrophus (18) (70% identity and 85% similarity) and AldB of E. coli (32) (68% identity and 83% similarity). Although AcoD is involved in the catabolism of acetoin and ethanol, Ald1 of our strain prefers longer aldehydes and is not active toward such short-chain aldehydes (see below). The following sequences are presumed to be functional motifs: GXGXXXG (positions 218 to 224; X represents any amino acid) as an AMP-binding site; VTLELGGKSP (positions 259 to 268) as a glutamic acid active-site motif (31); and GYKKSGVG (positions 467 to 474; Y represents an aromatic residue) as a putative conserved sequence among various aldehyde dehydrogenases (32).
Purification of rAld1 from E. coli JM109(pCM1).
Acinetobacter sp. strain M-1 has several types of enzyme that catalyzed the oxidation of n-alkanals (22). Since the amount of the purified Ald1 from Acinetobacter sp. strain M-1 was too small for study of its biochemical properties, ald1 was overexpressed in E. coli. The cell extract of E. coli JM109(pCM1) showed aldehyde dehydrogenase activity toward tetradecanal, 0.156 U/mg of protein, but no activity was observed for the control strains, E. coli JM109 and E. coli JM109(pUC118). The enzyme was purified up to 70-fold from a cell extract of the transformant cells, as mentioned in Materials and Methods. The specific activity of the purified enzyme toward tetradecanal was 10.9 U/mg of protein. The purified enzyme preparation showed apparent homogeneity on SDS-PAGE.
General properties of the purified rAld1.
The relative molecular mass of the purified enzyme was estimated to be 55 kDa on SDS-PAGE and 232 kDa on gel filtration; these values were identical to those obtained with the preparation from the native Acinetobacter strain. The molecular mass of the subunit is in close agreement with the value calculated from the deduced amino acid sequence, as mentioned above. Only one N-terminal amino acid sequence, MHYVDPNQSGSKIHFKDQYE, was obtained upon Edman degradation. Judging from these results, the enzyme is homotetrameric.
The enzyme showed an optimum temperature of 43°C, and 90% of its activity remained after incubation at 60°C for 30 min. The optimum pH was 9.5, and more than 90% of the activity was retained after incubation for 30 min at 30°C within pH 7.5 to 9.0.
The enzyme activity was completely inhibited by p-chloromercuribenzoate (1.0 mM) and N-ethylmaleimide (1.0 mM) and was strongly inhibited by iodoacetate (1.0 mM), suggesting that a sulfhydryl group was essential for the activity. The enzyme activity was completely inhibited by Pb2+, Fe3+, Ag+, and Hg2+ at a concentration of 1.0 mM and was partially inhibited by several other metal ions at 1.0 mM: Mn2+ (37% inhibition), Zn2+ (44%), and Cu2+ (63%). Mg2+ (1.0 mM) slightly activated the enzyme (135% activation).
Substrate specificity.
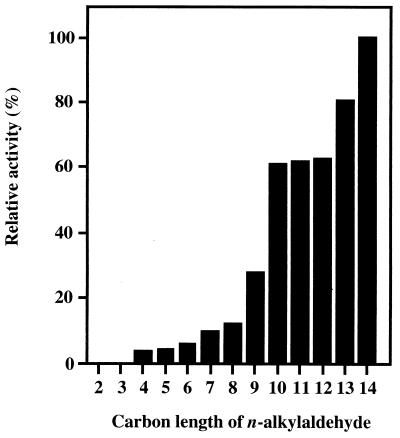
The enzyme was active toward n-alkanals with carbon chains longer than four carbons but was not active toward ethanal and propanal. The enzyme activity became higher with increasing substrate carbon chain length. Among the substrates examined, the n-alkanal most preferred was tetradecanal, which is the longest n-alkanal commercially available (Fig. 2). This means that the enzyme can be categorized as a long-chain aldehyde dehydrogenase. The enzyme catalyzed the dehydrogenation of benzaldehyde (66% activity toward tetradecanal), o-, m-, and p-fluorobenzaldehyde (76, 136, and 84%, respectively), p-chlorobenzaldehyde (95%), m-methylbenzaldehyde (36%), trans-2-decenal (32%), trans-cinnamaldehyde (38%), and cis-9-hexadecenal (31%). The purified enzyme utilized only NAD+ as a cofactor, i.e., it did not use NADP+.
FIG. 2.
Substrate specificity of rAld1 purified from E. coli(pCM1). Enzyme activity was measured under standard conditions. One hundred percent corresponds to 10.9 U/mg of protein. Numbers on the x axis indicate n-alkanals as follows: 2, ethanal (100 mM); 3, propanal (100 mM); 4, butanal (20 mM); 5, pentanal (10 mM); 6, hexanal (5 mM); 7, heptanal (5 mM); 8, octanal (2 mM); 9, nonanal (2 mM); 10, decanal (2 mM); 11, undecanal (1 mM); 12, dodecanal (1 mM); 13, tridecanal (1 mM); 14, tetradecanal (1 mM).
Northern analysis.
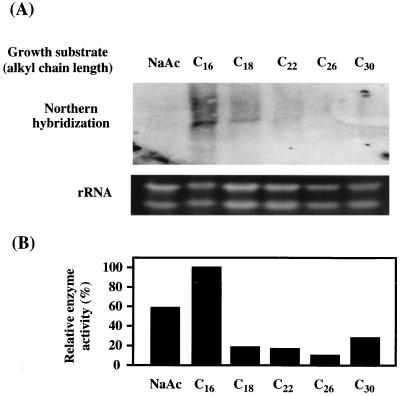
To examine the expression and regulation of ald1, Northern blot analysis was performed with the total RNA of Acinetobacter sp. strain M-1 cells grown on a salt medium with an n-alkane ranging from C16 to C30, and with sodium acetate as a control carbon source (Fig. 3A). The most intensive band was detected with the mRNA from n-hexadecane-grown cells. The level of mRNA became lower with increasing carbon chain length of the n-alkane and became almost negligible with n-hexacosane, indicating that ald1 mRNA was most abundant when the cells were grown on C16. In spite of no signal on Northern hybridization, the cells grown on n-hexacosane, n-triacontane, and sodium acetate showed significant levels of enzyme activity toward tetradecanal (Fig. 3B). This means that an alternative aldehyde dehydrogenase(s) active toward tetradecanal is induced with these carbon sources. The existence of isozymes was also indicated by gene disruption analysis (see below).
FIG. 3.
(A) Northern analysis of the ald1 gene. A 20-μg portion of total RNA was loaded on each lane, and ald1 transcription was detected by hybridization with the 32P-labeled ald1 fragment as a probe. RNA was prepared from Acinetobacter sp. strain M-1 cells grown on a salt medium containing 1% (wt/vol) sodium acetate (NaAc) or 0.5% (vol/vol) n-alkane, the carbon length of which is indicated. (B) Relative enzyme activity of aldehyde dehydrogenase in cells grown on each substrate. The enzyme activity was measured under standard conditions using tetradecanal as a substrate. One hundred percent corresponds to 0.76 U/mg of protein.
Gene disruption of ald1 in Acinetobacter sp. strain M-1.
To determine the physiological role of Ald1 in Acinetobacter sp. strain M-1, the chromosomal ald1 gene was destroyed by homologous recombination with the disruption vector pDALD1 as described in Materials and Methods. The transformant was selected for the kanamycin resistance phenotype. The ald1 gene disruption was confirmed by Southern analysis (Fig. 1D). The chromosomal DNAs from both the wild-type strain and the ald1Δ strain were double digested with EcoRV and KpnI. The signal of the wild-type strain corresponded to the size of 1.7 kb. This shifted to 3.3 kb for the ald1Δ strain, the size expected to result from insertion of two copies of the Km gene.
The activity of aldehyde dehydrogenase toward tetradecanal was compared between 2× YT-grown cells of the wild-type strain and the ald1Δ strain. The cell extract of the ald1Δ strain showed about half of the aldehyde dehydrogenase activity (0.16 U/mg of protein) toward tetradecanal that the wild-type strain showed.
Wax ester accumulation and growth rate of the ald1Δ strain.
The major determinant of wax ester composition during growth on alkanes is the chain length of the alkane substrate. The main component of intracellular wax esters in Acinetobacter sp. strain M-1 and the ald1Δ strain, which were grown under nitrogen-limiting conditions with 0.5% n-hexadecane for 72 h, was hexadecyl-hexadecanoate, i.e., no other significant components of wax esters were observed under the experimental conditions used. This finding suggested that C16 is the main carbon chain length species in intracellular wax esters in this strain.
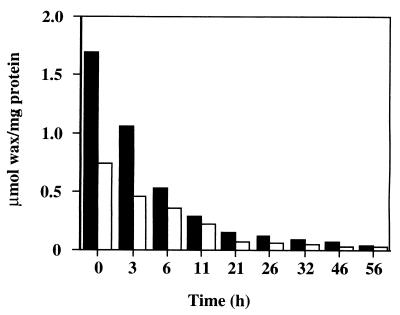
The wax ester content was compared between the wild-type and ald1Δ strains grown on n-hexadecane under nitrogen-limiting conditions. As expected, the amount of wax esters in the cells of the ald1Δ strain, 0.73 μmol/mg of protein, was less than half of that in the wild-type strain, 1.7 μmol/mg of protein. Next, wax ester degradation was compared by shifting the cells to a salt medium without a carbon source. The accumulated wax esters were degraded at similar rates in the wild-type and the ald1Δ strain (Fig. 4). These results suggest that Ald1 plays an important role in the synthesis of wax esters but not in their degradation.
FIG. 4.
Degradation of intracellular wax esters in the wild-type and ald1Δ strains. The cells, which were grown on a nitrogen-limiting medium containing 0.5% (vol/vol) n-hexadecane, were shifted to a salt medium without a carbon source, and then the amount of intracellular wax esters was measured at intervals. Solid bars, wild-type strain; open bars, ald1Δ strain.
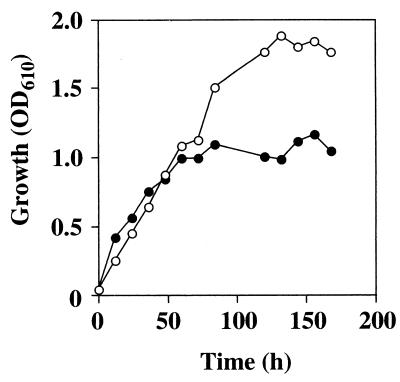
As shown in Fig. 5, the ald1Δ strain still retained the ability to grow on n-hexadecane as a sole carbon source. The initial growth rate was the same as that of the wild-type strain, but the final cell yield was less than half that of the wild type strain. The same result was obtained when n-tridecane was used as the carbon source instead of n-hexadecane (data not shown). We did not attempt to detect the intermediates, such as corresponding aldehydes or free fatty acids, in growth medium of the ald1Δ strain on n-alkane.
FIG. 5.
Growth of the wild-type and ald1Δ strains on n-hexadecane. Cells were cultured in a salt medium containing 0.5% (vol/vol) n-hexadecane, and growth was measured as described in Materials and Methods. Open circles, wild-type strain; solid circles, ald1Δ strain.
DISCUSSION
In this study, we investigated the physiological role of a long-chain aldehyde dehydrogenase, Ald1, which was found in a soluble fraction of a cell extract of n-alkane-grown Acinetobacter sp. strain M-1 cells. Through genetic and biochemical analyses, Ald1 was shown to play a significant role in intracellular wax ester synthesis and in n-alkane utilization by the following results: (i) the purified Ald1 from recombinant E. coli cells was active toward a long-chain n-alkanal; (ii) the ald1 transcript was induced by long-chain n-alkanes, C16 to C22; (iii) disruption of ald1 caused a remarkable decrease in the amount of intracellular wax esters; and (iv) the cell yield of the ald1Δ strain was much lower than that of the wild-type strain. The remaining ability of the ald1Δ strain to accumulate some amount of wax esters suggests the participation of an alternative aldehyde dehydrogenase(s) or the existence of an alternative wax synthesis pathway. Indeed, the soluble fraction of the ald1Δ strain still exhibited some NAD+-dependent aldehyde dehydrogenase activity (50% that of the wild-type strain toward tetradecanal and 80% toward n-decanal or n-hexanal).
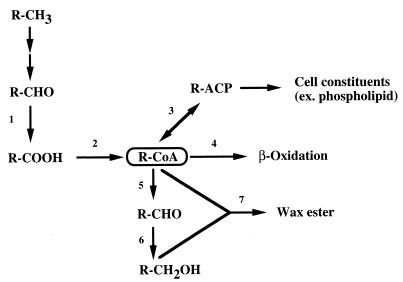
Ratajczak et al. reported that alkM, encoding integral-membrane terminal alkane hydroxylase, is essential for n-alkane degradation by Acinetobacter sp. strain ADP1 (19). (We recently obtained genes encoding the enzymes involved in the n-alkane hydroxylation system in Acinetobacter sp. strain M-1 [unpublished data]). These findings suggest that the oxidation of an n-alkane to the corresponding acyl-CoA proceeds through membrane-bound enzymes as in P. oleovorans. This was supported by the existence of membrane-bound alcohol and aldehyde dehydrogenases in Acinetobacter spp. (1). On the other hand, acyl-CoA reductase has been reported to be essential for wax ester synthesis in Acinetobacter calcoaceticus BD413 (20), indicating that the resulting aldehyde would be further catalyzed by aldehyde reductase and acyl-CoA:alcohol transacylase for wax ester synthesis. As judged from these facts, acyl-CoA is the common intermediate for β-oxidation and wax ester synthesis (as well as phospholipid synthesis [1]) (Fig. 6). The principal role of aldehyde dehydrogenase might be to supply a fatty acid which is a precursor of acyl-CoA, and a variety of aldehyde dehydrogenases have been found in the membrane and cytosolic fractions of Acinetobacter spp. (1). As described above, a membrane-bound enzyme would mainly participate in the carbon flow of n-alkanes to cell constituent synthesis and to energy production through β-oxidation. In contrast, our present results, i.e., (i) retarded growth on n-hexadecane and impaired wax ester synthesis in the ald1Δ strain, (ii) inducibility of ald1 by n-hexadecane, and (iii) the substrate specificity of rAld1, supported the possibility that soluble aldehyde dehydrogenases (one of them being Ald1) mainly participate in wax ester synthesis.
FIG. 6.
Proposed carbon flow of n-alkanes in Acinetobacter sp. strain M-1. Enzymes: 1, aldehyde dehydrogenase; 2, acyl-CoA synthetase; 3, acyl-CoA:ACP transacylase; 4, acyl-CoA dehydrogenase; 5, acyl-CoA reductase; 6, aldehyde reductase; 7, acyl-CoA:alcohol transacylase. R, acyl group; ACP, acyl carrier protein.
The growth of the ald1Δ strain on n-hexadecane showed the same rate as that of the wild-type strain until the mid-exponential phase; then it ceased at a growth level less than half that of the wild-type strain. In an Acinetobacter sp., wax esters began to accumulate at the mid-exponential phase (6). Judging from these observations, wax ester synthesis might be related to the growth of Acinetobacter sp. strain M-1 on n-alkanes after the mid-exponential phase.
Although n-alkane metabolism in Acinetobacter spp. seemed to be complicated due to the diversity and overlapping functions of enzymes, determination of the carbon flow from n-alkanes to various metabolites, such as wax esters, is of both academic and applied interest and is a challenging problem which should be solved through enzymological and genetic studies.
REFERENCES
- 1.Asperger O, Kleber H. The biology of Acinetobacter. New York, N.Y: Plenum Press; 1991. pp. 323–350. [Google Scholar]
- 2.Aurich H, Eitner G. Induktion der NADP+-abhängigen Aldehyddehydrogenase durch Kohlenwasserstoffe bei Acinetobacter calcoaceticus. Z Allg Mikrobiol. 1977;17:263–266. doi: 10.1002/jobm.3630170402. [DOI] [PubMed] [Google Scholar]
- 3.Bagdasarian M, Lurz R, Rückert B, Franklin F C H, Bagdasarian M M, Frey J, Timmis K N. Specific-purpose plasmid cloning vectors. II. Broad-host-range, high-copy-number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981;16:237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- 4.De Mot R, Nagy I, Schoofs G, Vanderleyden J. Sequence of a Rhodococcus gene cluster encoding the subunits of ethanolamine ammonia-lyase and an APC-like permease. Can J Microbiol. 1994;40:403–407. doi: 10.1139/m94-066. [DOI] [PubMed] [Google Scholar]
- 5.Dewitt S, Erbin J L, Howes-Orchison D, Dalietos D, Neidleman S L. Saturated and unsaturated wax esters produced by Acinetobacter sp. HO1-N grown on C16–C20n-alkanes. J Am Oil Chem Soc. 1982;59:69–74. [Google Scholar]
- 6.Fixter L M, Nagi M N, McCormack J G, Fewson C A. Structure, distribution and function of wax esters in Acinetobacter calcoaceticus. J Gen Microbiol. 1986;132:3147–3157. [Google Scholar]
- 7.Fox M G A, Dickinson F M, Ratledge C. Long-chain alcohol and aldehyde dehydrogenase activities in Acinetobacter calcoaceticus strain HO1-N. J Gen Microbiol. 1992;138:1963–1972. doi: 10.1099/00221287-138-9-1963. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher I H C. Occurrence of waxes in Acinetobacter. J Gen Microbiol. 1971;68:245–247. doi: 10.1099/00221287-68-2-245. [DOI] [PubMed] [Google Scholar]
- 9.Geissdörfer W, Frosch S C, Haspel G, Ehrt S, Hillen W. Two genes encoding proteins with similarities to rubredoxin and rubredoxin reductase are required for conversion of dodecane to lauric acid in Acinetobacter calcoaceticus ADP1. Microbiology. 1995;141:1425–1432. doi: 10.1099/13500872-141-6-1425. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Biol Chem. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 11.Hidalgo E, Chen Y, Lin E C C, Aguilar J. Molecular cloning and DNA sequencing of the Escherichia coli K-12 ald gene encoding aldehyde dehydrogenase. J Bacteriol. 1991;173:6118–6123. doi: 10.1128/jb.173.19.6118-6123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klossek P, Kirchner M, Kurth J. Immobilisierung partikelgebundener Aldehyddehydrogenase aus Acinetobacter calcoaceticus EB104. J Basic Microbiol. 1985;25:429–435. [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Leahy J G. Transformation of Acinetobacter calcoaceticus RAG-1 by electroporation. Can J Microbiol. 1994;40:233–236. [Google Scholar]
- 15.Navy I, Schoofs G, Compernolle F, Proost P, Vanderleyden J, De Mot R. Degradation of the thiocarbamate herbicide EPTC(S-ethyl dipropylcarbamothioate) and biosafening by Rhodococcus sp. strain NI86/21 involve an inducible cytochrome P-450 system and aldehyde dehydrogenase. J Bacteriol. 1995;177:676–687. doi: 10.1128/jb.177.3.676-687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neidleman, S. L., and J. Geigert. September 1983. U.S. patent 4,404,283.
- 17.Neidleman S L, Geigert J. Biotechnology and oleochemicals: changing patterns. J Am Oil Chem Soc. 1984;61:290–297. [Google Scholar]
- 18.Priefert H, Krüger N, Jendrossek D, Schmidt B, Steinbüchel A. Identification and molecular characterization of the gene coding for acetaldehyde dehydrogenase II (acoD) of Alcaligenes eutrophus. J Bacteriol. 1992;174:899–907. doi: 10.1128/jb.174.3.899-907.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ratajczak A, Geißdörfer W, Hillen W. Alkane hydroxylase from Acinetobacter sp. strain ADP1 is encoded by alkM and belongs to a new family of bacterial integral-membrane hydrocarbon hydroxylases. Appl Environ Microbiol. 1998;64:1175–1179. doi: 10.1128/aem.64.4.1175-1179.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiser S, Somerville C. Isolation of mutants of Acinetobacter calcoaceticus deficient in wax ester synthesis and complementation of one mutation with a gene encoding a fatty acyl coenzyme A reductase. J Bacteriol. 1997;179:2969–2975. doi: 10.1128/jb.179.9.2969-2975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakai Y, Ishikawa J, Fukasaka S, Yurimoto H, Mitsui R, Yanase H, Kato N. A new carboxylesterase from Brevibacterium linens IFO 12171 responsible for the conversion of 1,4-butanediol diacrylate to 4-hydroxybutyl acrylate: purification, characterization, gene cloning, and gene expression in Escherichia coli. Biosci Biotechnol Biochem. 1999;63:688–697. doi: 10.1271/bbb.63.688. [DOI] [PubMed] [Google Scholar]
- 22.Sakai Y, Maeng J H, Kubota S, Tani A, Tani Y. A non-conventional dissimilation pathway for long chain n-alkanes in Acinetobacter sp. M-1 that starts with a dioxygenase reaction. J Ferment Bioeng. 1996;81:286–291. [Google Scholar]
- 23.Sambrook J, Fritsh E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 24.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott C C L, Finnerty W R. Characterization of intracytoplasmic hydrocarbon inclusions from the hydrocarbon-oxidizing Acinetobacter species HO1-N. J Bacteriol. 1976;127:481–189. doi: 10.1128/jb.127.1.481-489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer M E, Finnerty W R. Alcohol dehydrogenases in Acinetobacter sp. strain HO1-N: role in hexadecane and hexadecanol metabolism. J Bacteriol. 1985;164:1017–1024. doi: 10.1128/jb.164.3.1017-1024.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer M E, Finnerty W R. Fatty aldehyde dehydrogenases in Acinetobacter sp. strain HO1-N: role in hexadecane and hexadecanol metabolism. J Bacteriol. 1985;164:1011–1016. doi: 10.1128/jb.164.3.1011-1016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 29.van Beilen J B, Wubbolts M G, Witholt B. Genetics of alkane oxidation by Pseudomonas oleovorans. Biodegradation. 1994;5:161–174. doi: 10.1007/BF00696457. [DOI] [PubMed] [Google Scholar]
- 30.Wales M R, Fewson C A. Constitutive NADP-dependent alcohol dehydrogenase of Acinetobacter sp. strain HO1-N. Curr Microbiol. 1994;29:273–277. [Google Scholar]
- 31.Weretilnyk E A, Hanson A D. Molecular cloning of a plant betaine-aldehyde dehydrogenase, an enzyme implicated in adaptation to salinity and drought. Proc Natl Acad Sci USA. 1990;87:2745–2749. doi: 10.1073/pnas.87.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu J, Johnson R C. aldB, an RpoS-dependent gene in Escherichia coli encoding an aldehyde dehydrogenase that is repressed by Fis and activated by Crp. J Bacteriol. 1995;177:3166–3175. doi: 10.1128/jb.177.11.3166-3175.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanase H, Ikeyama K, Mitsui R, Ra S, Kita K, Sakai Y, Kato N. Cloning and sequence analysis of the gene encoding 3-hexulose-6-phosphate synthase from the methylotrophic bacterium, Methylomonas aminofaciens 77a, and its expression in Escherichia coli. FEMS Microbiol Lett. 1996;135:201–205. doi: 10.1111/j.1574-6968.1996.tb07990.x. [DOI] [PubMed] [Google Scholar]